Abstract
Multiple isolates of Cryptococcus neoformans, including those with fluconazole resistance, were tested to assess the in vitro activity of the new triazole TAK-187. MICs of TAK-187 were at least eightfold lower than those of fluconazole, and fungicidal concentrations for most isolates were 4 μg/ml or less. TAK-187 also was evaluated as intermittent therapy using two dosages in a rabbit model of experimental cryptococcal meningitis. Compared to daily treatment with fluconazole, as little as two doses of TAK-187 given 7 days apart were found to be effective. Plasma and cerebrospinal fluid TAK-187 concentrations were many times higher than MICs and fungicidal concentrations. Based upon its therapeutic efficacy and long half-life in the rabbit model, TAK-187 should be investigated for intermittent dosing in treatment or suppression of cryptococcal infections in humans.
Cryptococcosis occurs worldwide and has rapidly increased in frequency among immunocompromised individuals, particularly as a result of the pandemic of human immunodeficiency virus (HIV) infection. Significant improvements in the management of cryptococcal meningitis have been made over the last decade. Amphotericin B, fluconazole, and amphotericin B in combination with flucytosine all have been carefully studied for efficacy in the treatment of cryptococcal meningitis with and without coexisting HIV infection (2, 5, 20). Most recently, use of amphotericin B with or without flucytosine for 2 weeks of induction therapy followed by consolidation therapy with fluconazole or itraconazole for 8 weeks has been found to be effective in the management of AIDS patients with cryptococcal meningitis (22). However, daily fluconazole is still required for long-term continuous suppressive therapy (4) in patients with HIV infection. Intermittent amphotericin B dosing has been shown to be significantly less effective for suppression of cryptococcosis, and currently no antifungal agent has efficacy for treatment when given on less than a daily basis (18). Development of an antifungal compound that is effective for treatment when used intermittently could offer significant advantages, such as improved patient compliance and reduced costs, particularly for patients whose conditions require life-long treatment with multiple medications.
The new triazole TAK-187 was examined for in vitro and in vivo activity against Cryptococcus neoformans (7, 21). TAK-187 was compared in vitro to fluconazole against 10 isolates of C. neoformans strains and found to be extremely potent against all isolates tested, including those for which fluconazole MICs are relatively high. Based on in vitro test results, a rabbit model of cryptococcal meningitis (10–16, 24) was used to determine the pharmacokinetics and in vivo efficacy of TAK-187. The efficacy of TAK-187 was compared to that of fluconazole, an agent for which there is significant experience in the prophylaxis, suppression, and treatment of cryptococcosis (4, 8, 17, 20). In this experimental model, pulse dosing with as little as 2 doses of TAK-187 was found to have therapeutic activity similar to treatment with 12 daily doses of fluconazole.
MATERIALS AND METHODS
Animals.
New Zealand White rabbits (weight, 2 to 3 kg) were housed in separate cages and given rabbit chow (Purina) and water ad libitum. Immunosuppression was induced by daily intramuscular injection of 5 mg of cortisone acetate (Merck Sharpe & Dohme, West Point, Pa.) per kg of body weight. Intramuscular injections of 46.4 mg of ketamine (Ketaset; Bristol Laboratories, Syracuse, N.Y.) per kg plus 5.4 mg of xylazine (Rompum; Mobay Corp., Shawnee, Kans.) per kg were given for all invasive procedures. Animals were sacrificed with an intravenous injection of sodium pentobarbital (Lethalis; Barber Veterinary Supply, Fayetteville, N.C.) at experiment termination.
Antifungal agents.
For in vitro testing, 4.8 mg of TAK-187 (Takeda Chemical Industries, Ltd., Osaka, Japan) was dissolved in 100% dimethyl sulfoxide (DMSO) in a volumetric flask and diluted and stored according to standard protocol (6). For in vivo testing, TAK-187 was suspended in sterile 2% carboxymethyl cellulose (medium viscosity) by grinding with mortar and pestle and then dilution in a graduated cylinder (1:4) with sterile distilled water, to yield a final vehicle concentration of 0.5% carboxymethyl cellulose, and stored at 2 to 5°C in the dark. For in vivo studies, the TAK-187 suspension was administered by oral gavage with a 3-in. gavage needle. Fluconazole 100-mg tablets (Pfizer-Roerig, New York, N.Y.) were given orally in daily doses of 80 mg/kg.
Organisms.
C. neoformans H99 (= DUMC 135.97) is a clinical isolate which has been used in previous experiments (10, 13). Nine additional C. neoformans isolates for susceptibility testing consisted of isolates from AIDS and non-AIDS patients; serotypes A, B, and C; and isolates for which fluconazole MICs are both low and high, as follows: DUMC 114.95 (serotype B) and DUMC 119.95 (serotype C), obtained from M. R. McGinnis as N32 and N34, respectively; DUMC 133.95; DUMC 109.97; DUMC 123.96; DUMC 124.96; DUMC 125.96; DUMC 251.86; and DUMC 114.96.
In vitro susceptibility testing.
The approved method for yeast susceptibility testing, as modified for C. neoformans, was followed (6). This broth macrodilution method specifies an inoculum of approximately 103 CFU/ml in RPMI 1640 medium with MOPS (3-[N-morpholino]propanesulfonic acid) incubated at 35°C for 72 h. The endpoint for azole compounds is defined as 80% growth reduction compared to a drug-free control tube. For determination of fungicidal concentrations, 100-μl aliquots from tubes with growth inhibition were plated on Sabouraud agar plates and the lowest drug concentration that yielded 30 or fewer yeast colonies was recorded as the fungicidal concentration.
Antimicrobial assay.
Concentrations of TAK-187 in plasma and cerebrospinal fluid (CSF) were measured by bioassay with yeast nitrogen base agar, agar diffusion bioassay plates, and Candida kefyr (ATCC 46764). Known concentrations of TAK-187 dissolved in DMSO were prepared in rabbit plasma and CSF. Controls included normal rabbit plasma and CSF and aqueous DMSO at 1.25 and 0.625%. Data were plotted manually and by using SigmaPlot scientific graphing software (Jandel Corp.).
Production of cryptococcal meningitis.
Beginning 1 day prior to inoculation and for the duration of the experiment, all animals received a daily intramuscular injection of cortisone acetate, 5.0 mg/kg. Four-day-old growth of C. neoformans (H99) from Sabouraud with chloramphenicol agar plates was suspended in 0.015 M phosphate-buffered saline at pH 7.4 to a density of 1.2 × 109, as verified by hemocytometric analysis. On day 0, rabbits were sedated and inoculated intracisternally with 0.3 ml of the yeast suspension. On days 2, 6, 11, and 14, intracisternal taps were performed and approximately 0.5 ml of CSF was aspirated from each animal. The CSF was diluted in phosphate-buffered saline and cultured on Sabouraud with chloramphenicol agar plates. The results are expressed as log10 CFU per milliliter of CSF.
Treatment regimens.
Three treatment regimens were used. (i) Treatment with 40 mg of TAK-187 per kg consisted of single doses given on days 2, 7, and 12. (ii) Treatment with 80 mg of TAK-187 per kg consisted of single doses given on days 2 and 9. (iii) Fluconazole at 80 mg/kg was given daily from day 2 through day 14. Four rabbits were assigned to the fluconazole group, and five each were assigned to the TAK-187 groups and the untreated group.
Statistical methods.
Analysis of variance was used to test for an overall difference in mean log concentrations between drug groups at each time. Dunnett’s one-sided multiple comparison test was used to compare the mean log concentrations of each treatment with the control group. The overall slopes from linear regressions of mean log concentration versus time were estimated (with 95% confidence limits).
RESULTS
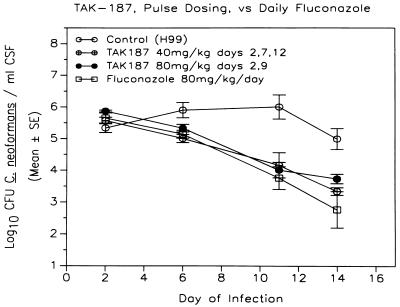
MICs of TAK-187 for all isolates from both AIDS and non-AIDS patients, including two isolates of C. neoformans var. gatti (DUMC 114.95, DUMC 119.95), were low (≤0.03 to 0.5 μg/ml) and relatively uniform (Table 1). Yeasts were inhibited at concentrations that were at least eightfold lower than those of fluconazole, and five isolates having relatively high fluconazole MICs (8 to 16 μg/ml) showed low TAK-187 MICs (≤0.03 to 0.5 μg/ml). Concentrations of TAK-187 in plasma and CSF were measured to determine the amount of drug delivered to these body sites in treated animals. Pulse dosing with either low or high doses of TAK-187 yielded plasma drug concentrations at 2 to 5 days postdose that were more than 100 times greater than the MIC of TAK-187 for the most resistant C. neoformans isolate found in vitro. Concentrations of TAK-187 in CSF ranged from 4.8 to 6.9 μg/ml at 2 to 5 days postdose (Table 2). Figure 1 shows that fluconazole and TAK-187 at either low or high doses of treatment both resulted in continuous and significant drops in yeast counts in CSF during the first and second weeks of treatment, compared to untreated animals (P < 0.05). Pulse therapy with either dose of TAK-187 was found to have efficacy of treatment equal to daily dosing with fluconazole in this model.
TABLE 1.
MICs and fungicidal concentrations of fluconazole and TAK-187 for 10 isolates of C. neoformans
| Isolate | MIC (μg/ml) of fluconazole | MIC/FCa (μg/ml) of TAK-187 |
|---|---|---|
| DUMC 125.96 | 0.5 | ≤0.0313/>16.0 |
| DUMC 251.86 | 1.0 | 0.125/>16.0 |
| DUMC 124.96 | 2.0 | 0.0625/0.5 |
| DUMC 119.95 | 4.0 | ≤0.0313/0.5 |
| DUMC 135.97 (H99) | 4.0 | 0.125/0.5 |
| DUMC 114.95 | 8.0 | ≤0.0313/1.0 |
| DUMC 109.97 | 8.0 | 0.125/4.0 |
| DUMC 133.95 | 8.0 | 0.5/1.0 |
| DUMC 114.96 | 16.0 | 0.0625/2.0 |
| DUMC 123.96 | 16.0 | 0.0625/>16.0 |
FC, fungicidal concentration.
TABLE 2.
TAK-187 in plasma and CSF of rabbits with cryptococcal meningitis
| Rabbit | Dose (mg/kg) | Days on which treatment was administered | Time (h) after last dose | TAK-187 concn (μg/ml) in:
|
|
|---|---|---|---|---|---|
| Serum | CSF | ||||
| 1 | 40 | 2, 7, 12 | 48 | 66.4 | 6.6 |
| 2 | 40 | 2, 7, 12 | 48 | 58.9 | 6.4 |
| 3 | 40 | 2, 7, 12 | 48 | 75.0 | 6.9 |
| 4 | 40 | 2, 7, 12 | 48 | 75.0 | 4.8 |
| 5 | 80 | 2, 9 | 120 | 62.5 | 5.8 |
| 6 | 80 | 2, 9 | 120 | 62.5 | 5.4 |
| 7 | 80 | 2, 9 | 120 | 66.4 | 6.1 |
| 8 | 80 | 2, 9 | 120 | 62.5 | 5.7 |
FIG. 1.
Pulse therapy with either of two dosing schedules of TAK-187 or daily dosing with fluconazole resulted in a continuous significant drop in yeast counts in CSF during the first and second weeks of treatment, compared to untreated animals (P < 0.05). Pulse therapy with either dosing regimen of TAK-187 was also found to have efficacy of treatment equal to daily dosing with fluconazole.
DISCUSSION
TAK-187 demonstrated potent in vitro activity against all isolates of C. neoformans tested, including isolates from HIV-seropositive patients and two isolates of C. neoformans var. gatti. Furthermore, five isolates having relatively high fluconazole MICs were found to be susceptible to TAK-187 (Table 1). These in vitro results are similar to those found with another new azole, SCH56592 (10). The efficacy of TAK-187 against such isolates could become more important if fluconazole-resistant C. neoformans strains in immunocompromised hosts continue to be encountered (1, 3, 19, 23). Although in vitro fungicidal activity has yet to be correlated with in vivo outcome, it is hypothesized that antifungal compounds must exhibit in vitro fungicidal activity to consistently eliminate fungi from severely immunocompromised hosts. TAK-187 showed in vitro fungicidal activity for 7 of the 10 isolates tested in vitro.
Based on in vitro susceptibility test results, experiments were designed to study the characteristics of TAK-187 in a rabbit model of experimental cryptococcal meningitis. The model uses corticosteroid immunosuppression to produce severe CSF leukopenia (13), which is pertinent to cryptococcal meningitis in humans with AIDS. The experiment compared fungicidal activities of two drugs in the subarachnoid space over a defined period of time. Fluconazole was chosen as the comparative triazole because of significant experience with its use in treatment and suppression of cryptococcal infections in humans and animals. Preliminary pharmacokinetic studies of TAK-187 showed very long persistence of the compound in biological fluids, indicating that intermittent administration may have efficacy comparable to daily dosing with presently available triazoles. The pharmacokinetics of TAK-187 for the low-dose (40 mg/kg given on days 2, 7, and 12) and high-dose (80 mg/kg given on days 2 and 9) regimens both yielded serum drug concentrations 2 to 4 days after drug administration that were more than 500 times that of the MIC for C. neoformans H99. CSF drug concentrations measured from the same time points were more than 30 times that of the MIC for H99. Although concurrent levels of drug in CSF were approximately 10% of those in plasma, the data show that the drug does penetrate and persist in rabbit CSF during infection. These pharmacokinetic data suggest that intermittent dosing with TAK-187 also could be very effective for suppression of cryptococcal meningitis. If similar pharmacokinetics are found in humans, TAK-187 should be studied for use in prolonged therapy of coccidioidal meningitis as well.
Fluconazole treatment was compared to two regimens of intermittent treatment with TAK-187. No differences were found in the rates of yeast killing between the two triazoles, but there was significant killing of yeast in the CSF by both drugs compared to untreated control animals. Analysis of the data suggests that at the doses tested, TAK-187 and fluconazole have equivalent therapeutic activities in this animal model. There was consistent killing of C. neoformans over the 2 weeks of treatment, but the subarachnoid spaces of most animals were not sterilized at the end of 2 weeks by either treatment regimen. Prior experience with amphotericin B found more rapid sterilization of CSF than with fluconazole alone (9). The finding of more rapid sterilization by amphotericin B and its combination with flucytosine has been confirmed in humans with cryptococcal meningitis (2, 20). We expect that in comparison to regimens containing amphotericin B, TAK-187 will have a delay in CSF sterilization in humans similar to that seen with fluconazole. However, TAK-187 offers the potential advantages of lower doses and an intermittent dosing schedule, which may be extremely useful in the suppression phase of treatment for cryptococcal meningitis. The rabbit model of cryptococcal meningitis generally has been an accurate predictor of antifungal drug activity in humans. Results from this study support further investigation of triazole TAK-187 for the management of cryptococcosis in humans.
ACKNOWLEDGMENT
This work was supported by a grant from Takeda Chemical Industries, Ltd., Osaka, Japan.
REFERENCES
- 1.Armengou A, Porcar C, Mascaro J, Garcia-Bragado F. Possible development of resistance to fluconazole during suppressive therapy for AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1996;23:1337–1338. doi: 10.1093/clinids/23.6.1337-a. [DOI] [PubMed] [Google Scholar]
- 2.Bennett J E, Dismukes W, Duma R J, Medoff G, Sande M A, Gallis H, Leonard J, Fields B T, Bradshaw M, Haywood H, McGee Z A, Cate T R, Cobbs C G, Warner J F, Alling D W. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N Engl J Med. 1979;301:126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 3.Birley H D, Johnson E M, McDonald P, Parry C, Carey P B, Warnock D W. Azole drug resistance as a cause of clinical relapse in AIDS patients with cryptococcal meningitis. Int J Sex Transm Dis AIDS. 1995;6:353–355. doi: 10.1177/095646249500600510. [DOI] [PubMed] [Google Scholar]
- 4.Bozzette S A, Larsen R A, Chin J. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:580–584. doi: 10.1056/NEJM199102283240902. [DOI] [PubMed] [Google Scholar]
- 5.Dismukes W E, Cloud G, Gallis H A, Kerkering T M, Medoff G, Craven P L, Kaplowitz L G, Fisher J F, Gregg C R, Bowles C A, Shadomy S, Stamm A M, Diasio R B, Kaufman L, Soong S-J, Blackwelder W the National Institute of Allergy and Infectious Diseases Mycoses Study Group. Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared with six weeks. N Engl J Med. 1987;317:334–341. doi: 10.1056/NEJM198708063170602. [DOI] [PubMed] [Google Scholar]
- 6.Galgiani J N, Bartlett M S, Ghannoum M A, Espinel-Ingroff A, Lancaster M V, Odds F C, Pfaller M A, Rinaldi M G, Walsh T J. Reference method for broth dilution antifungal susceptibility testing of yeasts. 1997. pp. 1–29. . Approved standard 17-9. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 7.Itoh K, Okonogi K, Tasaka A, Hayashi R, Tamura N, Tsuchimori N, Kitazaki T, Matsushita Y, Obita J. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. TAK-187, a new antifungal triazole: synthesis and antifungal activity, abstr. F74; p. 112. [Google Scholar]
- 8.Pappas P, Perfect J, Henderson H, Kauffman C, Saccente M, Haas D, Pankey G, Lancaster D, Holloway M, Cloud G, Dismukes W. Thirty-fifth Infectious Disease Society of America. 1997. Cryptococcosis in non-HIV infected patients: a multicenter survey, abstr. 128. [Google Scholar]
- 9.Perfect, J. R. 1990. Fluconazole therapy for experimental cryptococcosis and candidiasis in the rabbit. Rev. Infect. Dis. 12(Suppl. 3):299–302. [DOI] [PubMed]
- 10.Perfect J R, Cox G M, Dodge R K, Schell W A. In vitro and in vivo efficacies of the azole SCH56592 against Cryptococcus neoformans. Antimicrob Agents Chemother. 1996;40:1910–1913. doi: 10.1128/aac.40.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perfect J R, Durack D T. Treatment of experimental cryptococcal meningitis with amphotericin B, 5-fluorocytosine and ketoconazole. J Infect Dis. 1982;146:429–435. doi: 10.1093/infdis/146.3.429. [DOI] [PubMed] [Google Scholar]
- 12.Perfect J R, Durack D T. Comparison of amphotericin B and N-d-ornithyl amphotericin B methyl ester in experimental cryptococcal meningitis and Candida albicans endocarditis with pyelonephritis. Antimicrob Agents Chemother. 1985;28:751–755. doi: 10.1128/aac.28.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perfect J R, Lang S D R, Durack D T. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol. 1980;101:177–194. [PMC free article] [PubMed] [Google Scholar]
- 14.Perfect J R, Savani D V, Durack D T. Comparison of itraconazole and fluconazole in treatment of cryptococcal meningitis and Candida pyelonephritis in rabbits. Antimicrob Agents Chemother. 1986;29:579–583. doi: 10.1128/aac.29.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perfect J R, Wright K A. Amphotericin B lipid complex in the treatment of experimental cryptococcal meningitis and disseminated candidiasis. J Antimicrob Chemother. 1994;33:73–81. doi: 10.1093/jac/33.1.73. [DOI] [PubMed] [Google Scholar]
- 16.Perfect J R, Wright K A, Hobbs M M, Durack D T. Treatment of experimental cryptococcal meningitis and disseminated candidiasis with SCH 39304. Antimicrob Agents Chemother. 1989;33:1735–1740. doi: 10.1128/aac.33.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powderly W G, Finkelstein D M, Feinberg J, Frame P, He W, van der Horst C, Koletar S L, Eyster M E, Carey J, Waskin H, Hooton T M, Hyslop N, Spector S A, Bozzette S A. A randomized trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. N Engl J Med. 1995;332:700–705. doi: 10.1056/NEJM199503163321102. [DOI] [PubMed] [Google Scholar]
- 18.Powderly W G, Saag M S, Cloud G A, Robinson P, Meyer R, Jacobson J M, Graybill J R, Sugar A M, McAuliffe V J, Follansbee S E, Tuazon C U, Stern J J, Feinberg J, Hafner R, Dismukes W E. A controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:793–798. doi: 10.1056/NEJM199203193261203. [DOI] [PubMed] [Google Scholar]
- 19.Prugam A, Dupouy-Camet J, Blanche P, Gangneux J P, Tourte-Schaefer C, Sicard D. Increased fluconazole resistance of Cryptococcus neoformans isolated from a patient with AIDS and recurrent meningitis. Clin Infect Dis. 1994;19:975–976. doi: 10.1093/clinids/19.5.975-a. [DOI] [PubMed] [Google Scholar]
- 20.Saag M S, Powderly W G, Cloud G A, Robinson P, Grieco M H, Sharkey P K, Thompson S E, Sugar A, Tuazon C U, Fisher J F, Hyslop N, Jacobson J M, Hafner R, Dismukes W F. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. N Engl J Med. 1992;326:83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 21.Tasaka A, Kitazaki T, Tsuchimori N, Matsushita Y, Hayashi R, Okonogi K, Itoh K. Optically active antifungal azoles. VII. Synthesis and antifungal activity of stereoisomers of 2-[(1R,2R)-2-(2,4-difluorophe-nyl)-2-hydroxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]-4-[4-(2,2,3,3-tetra-fluoropropoxy)phenyl]-3(2H,4H)-1,2,4-triazolone (TAK-187) Chem Pharm Bull. 1997;45:321–326. doi: 10.1248/cpb.45.321. [DOI] [PubMed] [Google Scholar]
- 22.van der Horst C, Saag M S, Cloud G A, Hamill R J, Graybill J R, Sobel J D, Johnson P C, Tuazon C U, Kerkering T, Moskovitz B L, Powderly W G, Dismukes W E. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 23.Viard J P, Hennequin C, Fortineau N, Pertuiset N, Rothschild C, Zylberberg H. Fulminant cryptococcal infections in HIV-infected patients on oral fluconazole. Lancet. 1995;346:118. doi: 10.1016/s0140-6736(95)92139-7. [DOI] [PubMed] [Google Scholar]
- 24.Wright K A, Perfect J R, Ritter W. The pharmacokinetics of BAY R3783 and its efficacy in the treatment of experimental cryptococcal meningitis. J Antimicrob Chemother. 1990;26:387–397. doi: 10.1093/jac/26.3.387. [DOI] [PubMed] [Google Scholar]