Abstract
The intestinal epithelial barrier plays an important role during human immunodeficiency virus (HIV) disease progression. However, the extent to which the intestinal epithelial barrier is damaged in immunological non-responders (INRs) and immunological responders (IRs) is largely unknown. In this study, we investigated and compared the levels of intestinal gland damage and related molecules, including the tight junction protein claudin-1, apoptosis marker caspase-3, HIV DNA, CD4+ T cell count, and inflammation marker tumor necrosis factor-α (TNF-α) among the IRs (n = 10), INRs (n = 8), and healthy controls (HCs, n = 7). Intestinal damage was not completely restored in both INRs and IRs and was more serious in INRs than that in IRs. Moreover, intestinal damage was positively correlated with HIV DNA levels and negatively correlated with CD4+ T cell counts. These results provide insight into understanding the characteristics of intestinal epithelial barrier damage between IRs and INRs.
Keywords: HIV, Intestinal damage, Immunological responders, Immunological non-responders
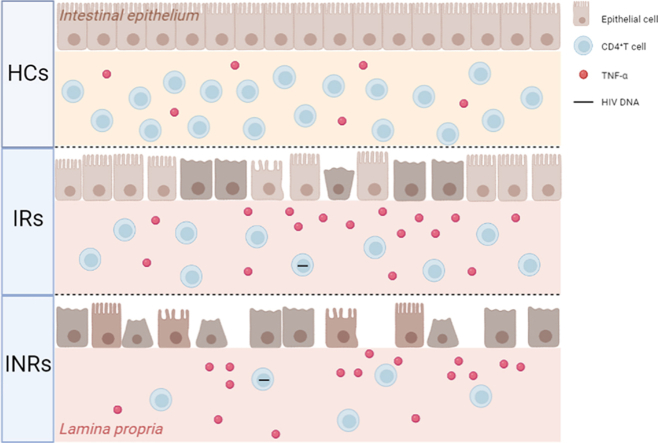
Graphical abstract
Intestinal damage was more severe in INRs than that in IRs. Intestinal damage was positively correlated with HIV DNA levels and negatively correlated with CD4+ T cell counts. Intestinal TNF-α was higher in IRs and INRs than that in HCs.
1. Introduction
Successful antiretroviral therapy (ART) can fully suppress human immunodeficiency virus 1 (HIV-1) replication to undetectable levels and greatly reduce the morbidity and mortality of patients infected with HIV. However, CD4+ T-cell restoration remains <200 cells/μL in approximately 10%–40 % of patients infected with HIV with more than 2 years of ART [[1], [2], [3]]. These individuals were referred to as immunological non-responders (INRs). Acquired immunodeficiency syndrome (AIDS)-related and non-AIDS-related events are more likely to occur in INRs than that in patients infected with HIV with immune reconstitution (peripheral CD4+ T-cell count >500 cells/μL after more than 2 years of ART, immunological responders [IRs]) [4]. Immune reconstitution in the peripheral blood of patients with HIV during ART has been well described. The gastrointestinal mucosa is considered the central compartment of viral replication and CD4+ T-cell depletion [5]. The intestinal epithelial barrier is severely damaged during disease progression [6]. Although patients infected with HIV can achieve immune reconstitution in peripheral blood after long-term ART [7], the immune reconstitution of the intestinal tract occurs much more slowly [8] since both structural injury and immune disorders persist in the intestinal tract [9]. Gut injury leads to imbalance in the composition of gut microbiota and microbial translocation, which induces chronic immune activation and inflammation [[10], [11], [12]]. Therefore understanding the extent of intestinal damage in INR patients is important for developing effective strategies to improve the persistence of chronic immune activation and inflammation. Although several studies have indicated a defect in the intestinal epithelial barrier during chronic HIV infection [13,14], the extent to which the mucosal barrier defects among INRs and IRs are largely unknown.
Epithelial integrity of the intestine requires a complex protein structure of intercellular tight junctions responsible for paracellular barriers and pores [13]. Claudins are the main constituents of intercellular tight junctions and determine the permeability of the intestinal endothelial and epithelial cells. Claudin-1, an intestinal link protein of claudins, is mainly situated in the apical region of intestinal epithelial cells [15] and helps seal the epithelial monolayer. Decreased claudin-1 levels result in increased intestinal permeability [16], which may lead to systemic inflammation in patients with HIV infected. Therefore, the degree of claudin-1 reduction in the intestine is often used to evaluate the severity of intestinal injury [17]. Furthermore, the decrease in claudin-1 expression is closely related to the apoptosis of epithelial cells in HIV-positive individuals [13,18], and caspase-3 is a representative marker of apoptosis.
HIV DNA level is a marker of HIV reservoir size [19] and CD4+ T cell count is an important indicator of immune recovery after long-term ART. The intestinal tract is enriched with T cells and is the main HIV reservoir [20]. There are some reports on HIV reservoirs in the peripheral blood of both IRs and INRs [21]. HIV DNA has been detected in the intestines of patients with ART suppression. However, there has been no comparative analysis of HIV DNA in the intestines between IRs and INRs. CD4+ T cell depletion persists in the intestinal tract during HIV infection. Some studies have reported that CD4+ T cell restoration in the peripheral blood is equivalent to that in the intestinal tract during ART [22]. Other studies showed that gut and intestinal resident CD4+ T cells could not be restored to the normalized level in the intestinal tract of most patients, although peripheral CD4+ T cells have been shown to normalize through long-term ART [22,23]. Most of these studies are based on flow cytometry methods. CD4+ T cells can be intuitively observed by immunostaining of CD4+ T cells in the intestine. In this study, we used immunohistochemistry to analyze the recovery of CD4+ T cells in the intestine after long-term ART. Intestinal injury and inflammation are closely related to HIV DNA levels and CD4 recovery [20]. The inflammatory cytokine tumor necrosis factor-alpha (TNF-α) in intestinal cells is associated with claudin-1 expression and epithelial apoptosis [13]. Therefore, we also analyzed TNF-α levels in the intestinal tract of the enrolled patients in this study.
In this study, we compared intestinal injury, apoptosis, HIV DNA levels, CD4 + T cell counts, and TNF-α levels of the ileocecal tissues of healthy controls (HCs), IRs, and INRs. Furthermore, we assessed whether changes in intestinal damage were associated with CD4+ T cell counts and HIV DNA levels.
2. Results
2.1. Characterization of HCs, IRs, and INRs of the intestinal mucosa
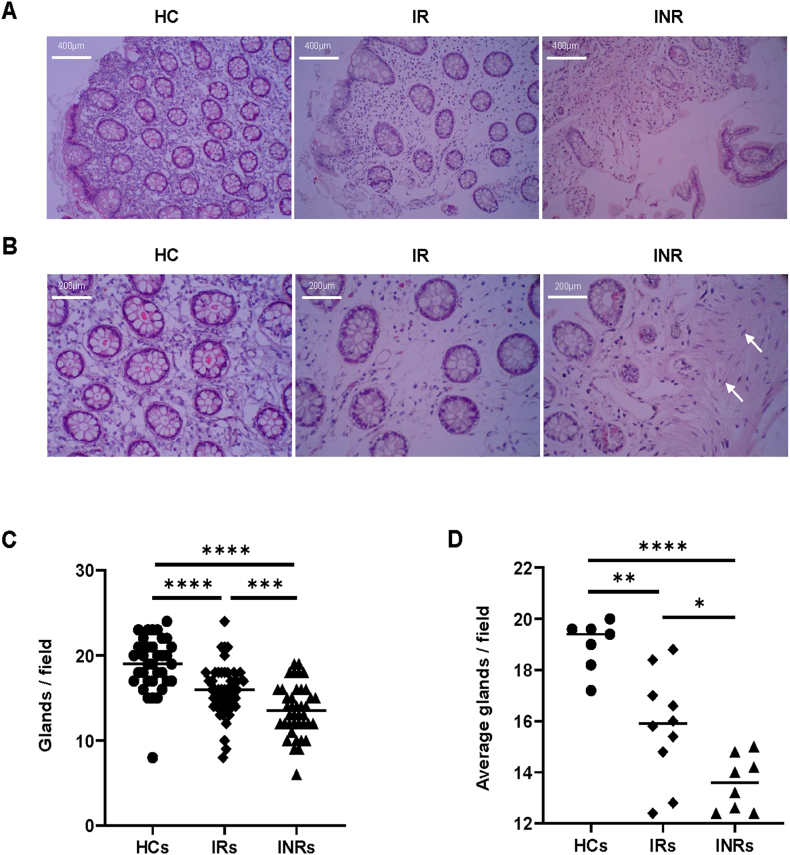
To characterize the structure of the intestinal mucosal barrier, we used scaffold staining with hematoxylin and eosin (H&E) to show the general outline (Fig. 1A). The intestinal glands extend downward from the epithelial cell surface and are usually simple, unbranched tubules composed mainly of mucus cells and smaller absorptive, endocrine and undifferentiated cells. The glands are known for secreting digestive fluids into the alimentary canal [24]. Compared with that of IRs and INRs, the intestinal glands arrangement in HCs appeared relatively tight (Fig. 1A and B). In the HCs group, the intestinal mucosal epithelium structure was intact, whereas there was minor damage in IRs, and obvious damage and partial sloughing in INRs (Fig. 1A). The interstitial space among intestinal glands in HCs was more compact than that in IRs, and edematous changes in the stroma were noted in IRs(Fig. 1A and B), which suggested that there might be an inflammatory reaction. In addition, fibroblasts can be seen in INRs, which suggests to us that fibrosis may be present in these samples (such as the white arrows in Fig. 1B). To quantitatively evaluate intestinal mucosal damage, five randomly visual fields per section were selected to count the number of glands. The number of glands in HCs was higher than that in IRs (Fig. 1C, P < 0.0001) and INRs (Fig. 1C, P < 0.0001). The number of glands was directly counted under a microscope. The number of glands in IRs was higher than that in INRs (Fig. 1C, P < 0.001). Each section gland quantification result was averaged from five randomly selected visual field counts (Fig. 1D), which indicated that the HCs’ average glands were more than those in IRs (Fig. 1D, P < 0.01) and INRs (Fig. 1D, P < 0.0001), and the average number of glands in the IRs was higher than that in the INRs (Fig. 1D, P < 0.05).
Fig. 1.
Characterization of the intestinal mucosa using hematoxylin and eosin (H&E) staining (A) Representative photograph in the three groups of hematoxylin and eosin (H&E)-stained intestinal sections, scale bars = 400 μm. (B) Visualization of the H&E-stained intestinal sections focused on the stroma and glands scale bars = 200 μm. Fibroblasts are indicated by white arrows. (C) Glands are counted in five randomly selected microscopic fields per section. (D) Statistical graph of average all field glands per section. Horizontal bars represent median values. Significance values were calculated by Kruskal-Wallis non-parametric test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
2.2. Comparison of claudin-1 expression levels among HCs, IRs, and INRs
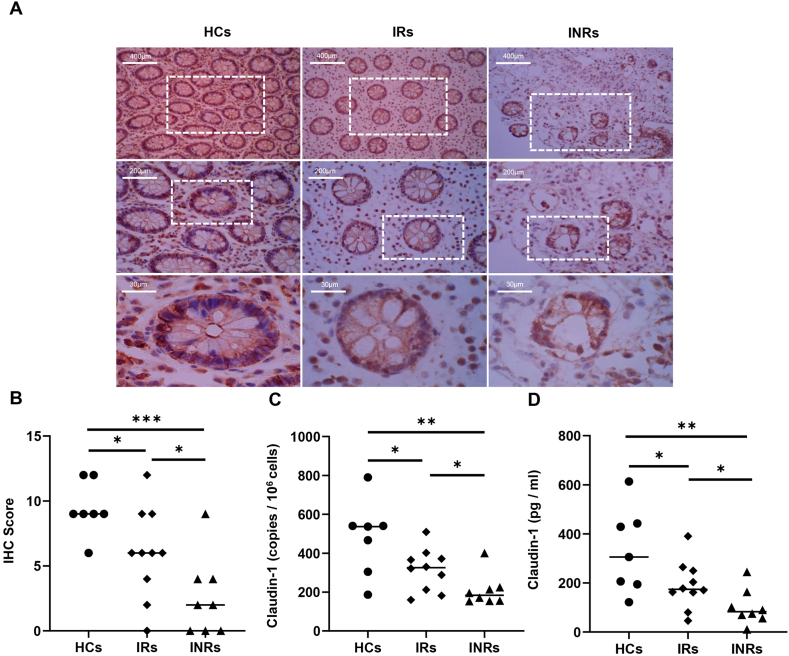
To visualize the expression of claudin-1 in the intestinal mucosa, antibodies against claudin-1 (brownish red) were used for immunohistochemical staining. The intestinal mucosal claudin-1 level in HCs was the highest, followed by IRs and INRs (Fig. 2A). The IHC scores of claudin-1 in HCs were higher than those in IRs (P < 0.05) and INRs (P < 0.001), and the INRs scores were lower (Fig. 2B). We also quantified claudin-1 mRNA in the intestinal mucosa by Quantitative Real-time PCR (qPCR); HCs claudin-1 mRNA was higher than that in IRs and INRs (Fig. 2C, P < 0.05 and P < 0.01, respectively). Furthermore, claudin-1 mRNA in INRs was lower than that in IRs (Fig. 2C, P < 0.05). Thus, claudin-1 expression in the intestinal mucosa of both INRs and IRs was significantly reduced. The claudin-1 levels in the peripheral blood also showed the same trend among the three groups as in the intestinal mucosa (Fig. 2D).
Fig. 2.
Comparison of claudin-1 levels among health controls (HCs), immunological responders (IRs), and immunological non-responders (INRs) (A) 3 Typical images of sections staining of claudin-1. (B) Comparison of immunohistochemistry (IHC) score of claudin-1 among the three groups. (C) Comparison of claudin-1 levels of the intestinal mucosa among the three groups. (D) Comparison of claudin-1 levels in the peripheral blood among the three groups. Horizontal bars represent median values. *P < 0.05, **P < 0.01, ***P < 0.001.
2.3. Comparison of caspase-3 levels among HCs, IRs, and INRs
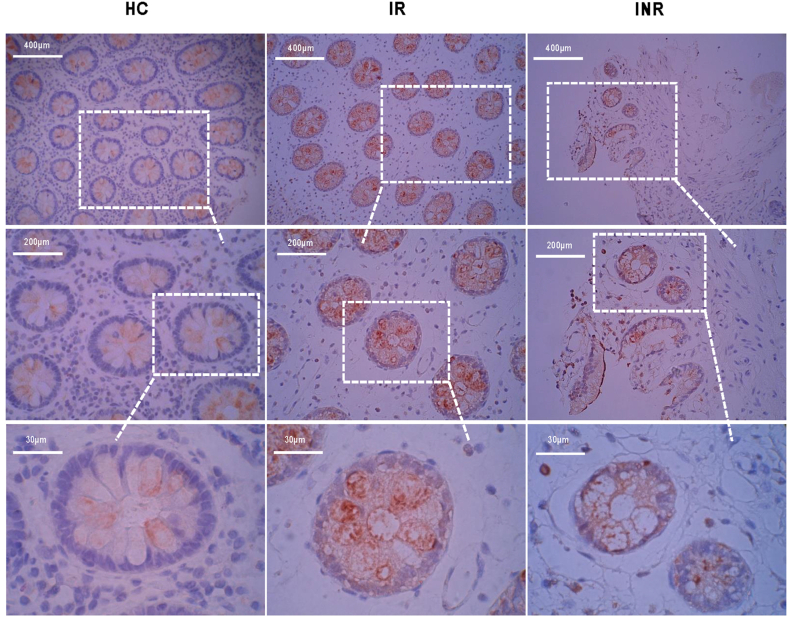
Caspase −3 is a marker of apoptosis. Sections from the three groups were also stained with caspase-3 antibody in our study (Fig. 3). We found that it was remarkably low in the HCs sections, and some sections showed no staining. The outline of the intestinal glands was clear, with no caspase-3 expression in HCs. The sections in IRs with obvious red can be viewed from inside the intestinal glands, suggesting that epithelial apoptosis occurred in the intestinal mucosa. INRs sections had fewer glands but were more deeply stained with caspase-3. Additionally, we noticed that apoptosis occurred in the inner glands and even reached the space between them.
Fig. 3.
Comparison of caspase-3 levels among HCs, IRs, and INRs The sections in the three groups (HCs, IRs, and INRs) are immunohistochemically stained with caspase-3 as illustrated from three scale bars field (scale bar = 400 μm, scale bar = 200 μm, scale bar = 30 μm).
2.4. Characterization of CD4+ T cells, HIV DNA, and TNF-α of the intestinal mucosa
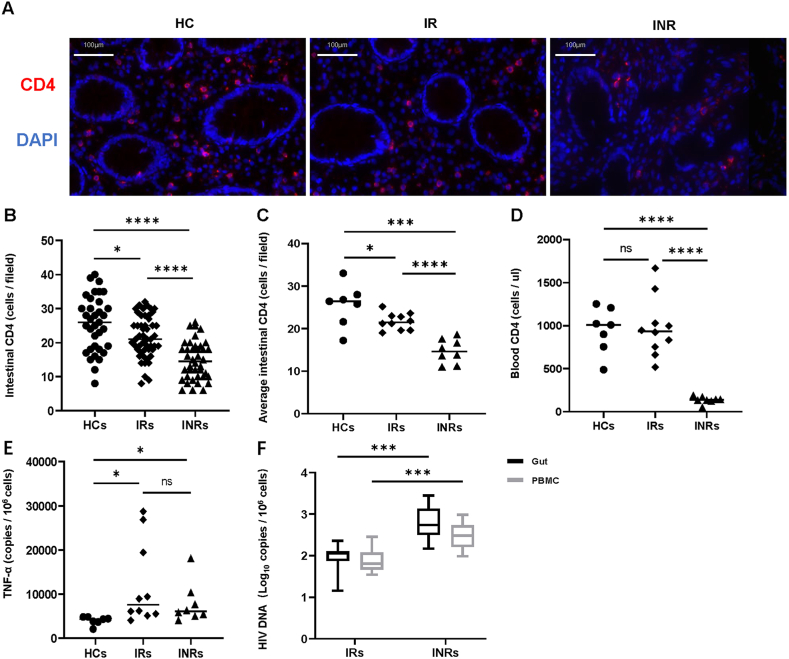
There were obvious differences among the three groups based on immunofluorescence images to determine intestinal mucosa CD4+ T cells (Fig. 4A). CD4+ T cell counts in HCs was higher than that in IRs, and IRs has a higher CD4+ T cell counts than that in INRs. For better quantification, we randomly selected five fields in each section at the same magnification to determine the CD4+ T cell counts. According to the graph, the highest CD4+ T cell counts in HCs were greater than those in IRs and INRs (Fig. 4B, P < 0.05 and P < 0.0001, respectively). The CD4+ T cell counts in IRs were higher than those in INRs (P < 0.05). The average CD4+ T cell counts, calculated using five randomly selected visual fields (Fig. 4C), were consistent with CD4+ T cell counts. In addition, we found that the peripheral blood CD4+ T cells levels of participants in HCs, compared with that in IRs, were not significant, but in INRs, compared with those in HCs and IRs, were significantly different (Fig. 4D). To examine the degree of inflammation in the intestinal mucosa, we investigated TNF-α mRNA expression. Compared with that in HCs, the TNF-α mRNA measured in IRs and INRs was significantly higher (Fig. 4E). Next, we assessed intestinal mucosal and PBMC HIV DNA levels in INRs and IRs. HIV DNA measured in IRs from PBMCs was significantly lower than that in INRs (P < 0.001), and the intestinal mucosal HIV DNA determined in INRs was higher than that in the IRs group (P < 0.001) (Fig. 4F). This revealed that INRs had a higher reservoir than that in IRs in PBMCs or the intestinal tract.
Fig. 4.
Characterization of CD4+ T cells, HIV DNA, and TNF-α of the intestinal mucosa (A) The intestinal tissue sections staining with CD4 (red) and DAPI (blue) by immunofluorescence are shown in the figure (scale bar = 200 μm). (B) Quantification of CD4+ T cell counts by five randomly selected visual fields counting per section at the same magnification. (C) Average of five visual fields CD4+ T cell counts in intestinal mucosa from HCs, IRs, INRs participants. (D) Peripheral blood CD4+ T cells of all participants are tabulated and subjected to statistical analysis. (E) TNF-α mRNA in the intestinal mucosa from the three groups of participants. (F) Comparisons of HIV DNA in PBMCs and intestinal mucosa between IRs and INRs. Horizontal bars represent median values. Kruskal–Wallis and Mann–Whitney test values. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
2.5. Intestinal impairment is positively correlated with HIV DNA and negatively correlated with the CD4+ T cell counts
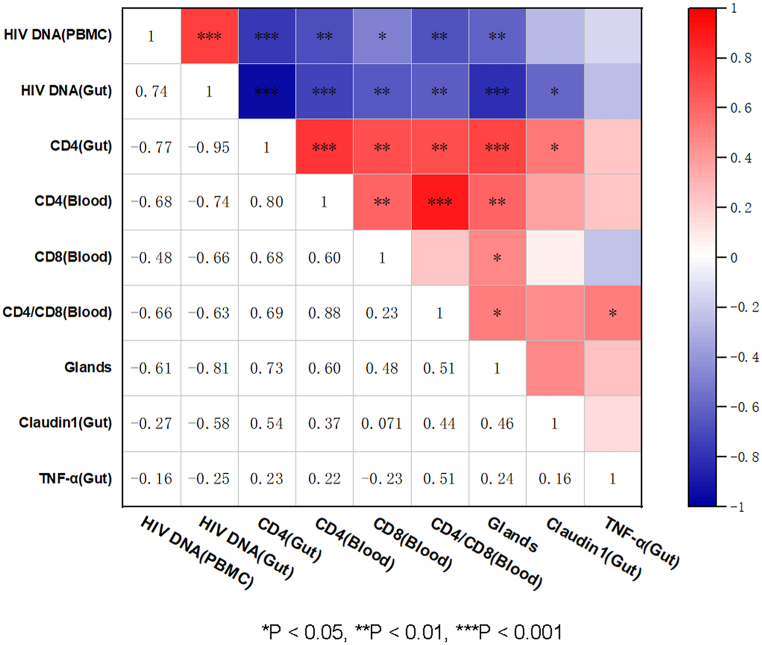
We then compared and analyzed correlations between each indicator and HIV DNA, CD4+ T cell counts, CD8+ T cell counts, and CD4/CD8 ratio determined from PBMCs, as well as HIV DNA, CD4+ T cell counts, intestinal glands, claudin-1 mRNA and TNF-α mRNA were measured from intestinal tissue (Fig. 5). On this map, several notable observations can be drawn from this analysis: intestinal impairment was associated with the number of intestinal glands, and claudin-1 was positively correlated with intestinal CD4+ T cells, blood CD4+ T cells, blood CD8+ T cells, and blood CD4/CD8 ratio, and negatively correlated with HIV DNA from PBMCs and intestinal HIV DNA. HIV DNA was negatively correlated with CD4+ T cell counts in the blood and intestines. These results demonstrate that intestinal impairment was positively correlated with HIV DNA and negatively correlated with CD4+ T cell counts.
Fig. 5.
Impairment of the intestine is positively correlated with HIV DNA and negatively correlated with CD4+ T cells HIV DNA, CD4+ T cell counts, CD8+ T cell counts, and CD4/CD8 ratio are determined from PBMCs, and HIV DNA, CD4+ T cell counts, claudin-1 mRNA, TNF-α mRNA, and gland counts measured from intestinal tissue are correlatedly analyzed and processed into a heat map. Spearman correlation is adopted between every two indicators. *P < 0.05, **P < 0.01, ***P < 0.001.
3. Discussion
To the best of our knowledge, this is the first study to report the comparison of intestinal epithelial barrier damage among INRs, IRs, and HCs using indicators including number of intestinal glands, claudin-1, and caspase-3 levels. Our results showed that IRs and INRs have lower levels of intestinal glands and claudin-1 but higher epithelial apoptosis than that in HCs. A previous study found significantly reduced expression of junctional complex proteins in intestinal biopsies and dilated intercellular spaces in INRs [25]. Somsouk et al. shown that neutrophil infiltration and mucosal apoptosis remain abnormally high despite ART, and mucosal apoptosis may be associated with systemic inflammation [26]. Consistent with previous reports [13,23,27], our results indicated that intestinal epithelial damage persisted even after long-term ART in patients infected with HIV. However, A study have reported complete recovery of intestinal mucosal injury in rhesus macaques with simian immunodeficiency virus(SIV) infection under ART [28]. This difference may be due to the different timing of treatment. The report indicated that the intestinal tract can recover completely in patients infected with HIV who are treated in the acute phase without obvious intestinal injury [28]. In particular, INRs have a relatively low nadir CD4+ T cell compared to IRs, which may have implications for the extent of intestinal damage and the ability to restore it. In addition to our mention of apoptosis in relation to intestinal injury, recent studies by our team have identified pyroptosis as an important form of immune cell death during the course of HIV disease [29,30]. Pyroptosis can be further investigated in the intestinal mucosa. In addition, we found that INRs had more severe intestinal injuries and fibroblasts. Previous studies have shown that lymph node fibrosis has been found to be associated with immune cell imbalance in HIV infection, affecting the immune response [31,32]. Therefore, whether intestinal injury or fibrosis is the cause of immune reconstitution failure is still worth studying, and monitoring intestinal injury may be important for the correct evaluation of immune reconstitution. Thus, treatment aimed at enhancing gastrointestinal recovery may be beneficial for immune reconstitution.
As one of the main components of tight junctions in epithelial cells, claudin-1 plays an important role in maintaining the integrity of intestinal epithelial tight junctions. Reducing claudin-1 expression increases the permeability of the intestinal epithelium and is linked to residual and systemic inflammation [[33], [34], [35], [36], [37]].
The increase in the inflammatory cytokine TNF-α has been shown to affect claudin-1 expression [38], epithelial apoptosis [39,40], and intestinal injury [[41], [42], [43], [44], [45]]. Our results showed that the TNF-α levels in the intestine were not statistically different between the INRs and IRs, although both were higher than those in HCs. These results are consistent with a report that there is no difference in TNF-α levels between inflamed and non-inflamed intestinal tissues [20]. Moreover, there is no correlation between TNF-α levels and claudin-1 and intestinal glands. Intestinal injury may be due to the influence of other cytokines in addition to TNF-α [1,[46], [47], [48]]. In contrast to our findings, Epple et al. reported that TNF-α levels were not increased in patients with suppressed HIV infection [13]. This difference may be due to the different methods used; we quantified TNF-α in tissue cells, and Epple et al. quantified TNF-α levels in each gram of tissue.
Other mechanisms included virus replication, which may affect the intestinal epithelial barrier. HIV DNA is positively correlated with HIV replication [[49], [50], [51]] and is easily detectable. We detected HIV DNA in the intestine and analyzed its association with glands and claudin-1. We found that, in both the peripheral blood and intestinal tract, HIV DNA levels were higher in INRs than that in IRs. The more severe intestinal injury in INRs may be due to the greater amount of virus DNA. HIV Gp120, Tat, and Vpr have been found to induce epithelial barrier and epithelial apoptosis [52,53]. The results of this study also showed a negative correlation between HIV DNA and the glands and claudin-1 levels. These results suggest that the reduction of intestinal infection can be taken into account in the design of treatment strategies to clear the HIV reservoirs. In contrast to reports that HIV DNA in the blood was higher than that in the gut in ART-suppressed patients with HIV [54,55], we did not find a statistical difference between HIV DNA levels in the peripheral blood and intestine. This difference may be due to the differences in the denominator. CD4+ T cells have been used as the denominator in other studies [54,55], and PBMCs and total intestinal cells were used as the denominator in this study.
CD4+ T cells from the gastrointestinal mucosa are important targets of HIV infection, and the depletion of gastrointestinal mucosal CD4+ T cells is thought to be responsible for HIV-related gastrointestinal barrier defects [14,56,57]. In this study, we observed an obvious decrease in CD4+ T cells in the intestines of both IRs and INRs compared with that in HCs, although CD4+ T cells in the peripheral blood did not differ between IRs and HCs. These results are consistent with those of the previous studies. In addition, Guadalupe et al. found that CD4+ T cell restoration in the gastrointestinal tract was slower than that in peripheral blood during ART [58]. Several studies have also reported a significant depletion of CD4+ T cells in the intestinal tract, even after the successful reconstitution of CD4+ T cells in the peripheral blood [9]. Our results indicate that CD4+ T cell restoration in the gastrointestinal tract was slower in INRs than that in IRs. CD4+ T cell counts were positively correlated with claudin-1 levels and the glands. Multiple mechanisms may contribute to the slow restoration of CD4+ T cells in the intestinal tract, especially INRs. These include persistent viral replication in the intestinal tract despite undetectable viral loads in the plasma and inflammation microenvironment in the intestinal tract. In addition, other studies have reported that CD4+ T cells complete reconstitution in the intestine; these patients mainly initiated treatment during early infection [22,23].
Our study has several limitations. First, the sample sizes were relatively small. Further studies with large sample size are required to verify our conclusion. Second, the detection of HIV DNA did not sort out CD4+ T cells and CD4+ T cell subpopulations were not evaluated in the intestinal mucosa, which could be achieved by increasing the amount of intestinal tissue in the future. In addition, caspase 3 is a marker for apoptosis, but more indicators such as TUNEL assay should be added to validate our conclusions. Finally, our study explored intestinal fibrosis, but did not explore the relationship between fibrosis in other tissues such as lymph nodes and HIV immune reconstitution because the tissue was difficult to obtain.
In summary, we showed that the intestinal injury of both INRs and IRs could not be restored to normal after long-term ART, and it was more severe in INRs. Therefore, whether taking interventions to improve intestinal repairment is beneficial to the intestinal immune recovery deserves further study in large cohort samples [1].
4. Materials and methods
4.1. Ethics statement
This study was approved by the research ethics committee of the Fifth Medical Center of the PLA General Hospital. Informed consent was obtained from all the participants.
4.2. Study participants
Eighteen patients with HIV, including ten immune responders (IRs) with peripheral CD4+ T-cell counts >500 cells/μL and eight immune non-responders (INRs) with CD4+ T-cell counts <200 cells/μL with undetectable plasma HIV RNA levels for more than 2 years after ART were enrolled in this study. In addition, seven HIV negative individuals who have undergone physical examination were included as HCs. Intestinal tissues were collected from the ileocecal region and processed into paraffin blocks, derived from the enrolled individuals who voluntarily donated during their routine colonoscopy examination at the Fifth Medical Center of PLA General Hospital. Matched peripheral blood samples were also collected. General information on the HCs, IRs, and INRs is shown in Table 1.
Table 1.
Demographic characteristics of enrolled participants in this study.
| HCs | IRs | INRs | P value | |
|---|---|---|---|---|
| Gender (male/female) | 7/0 | 10/0 | 8/0 | NA |
| Age (years) | 47 (43–49) | 36.5 (29–42.5) | 45.5 (35.5–57.5) | 0.142a |
| CD4+ T (cells/ul) | 1008 (754–1207) | 935.5 (730–1128) | 137.5 (126–164) | <0.001a |
| CD8+ T (cells/ul) | 894 (664–1053) | 1175.5 (756.5–1422.5) | 726.5 (476.75–958.75) | 0.112a |
| CD4/CD8 ratio | 1.15 (0.62–1.50) | 0.81 (0.69–1.24) | 0.19 (0.15–0.26) | <0.001a |
| Viral load (log10 copies/ml) | NA | <20 | <20 | NA |
| Nadir CD4+ T (cells/ul) | NA | 351.5 (257–516.5) | 74 (10–88) | <0.0001b |
| ART time (years) | NA | 4.5 (4–6.5) | 3.5 (3–5) | 0.173b |
| ART regimens | 0.520c | |||
| 2NRTIs+1NNRTIs | NA | 6 | 4 | |
| 2NRTIs+1INSTIs | NA | 4 | 4 |
All indicators except gender are shown as median (interquartile range).
HCs, healthy controls; IRs, immune responders; INRs, immune non-responders; NA, not applicable; NRTIs, nucleoside reverse transcriptase inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; INSTIs, integrase strand transfer inhibitors.
Kruskal-Wallis test;
Mann-Whitney test;
Fisher's Exact test.
4.3. Hematoxylin-eosin (H&E) staining of the intestinal mucosa
H&E staining were performed as previous description [59,60]. Paraffin sections were baked at 65 °C, dewaxed in xylene, rehydrated using decreasing concentrations of ethanol, and washed in distilled water. After staining with hematoxylin and eosin (ZSGB-BIO, China), the sections were dehydrated, cleared, and mounted. Images were acquired using a confocal microscope (Olympus, Japan).
4.4. Claudin-1 and Caspase-3 immunohistochemistry of the intestinal mucosa
Formalin-fixed tissue sections were chosen for baking, deparaffinization, blocking endogenous peroxidase activity with 0.3 % hydrogen peroxidase (H2O2), and heat-induced epitope retrieval. Following a brief wash and rinsing in phosphate-buffered saline (PBS), sections were incubated with primary antibodies against claudin-1 (ZSGB-BIO, China) or caspase-3 (Abcam, UK) separately overnight at 4 °C, followed by anti-rabbit-horse radish peroxidase (HRP) (ZSGB-BIO, China) secondary antibody at room temperature for 1 h. The human caspase-3 antibody is specific for the pro form and the p17 cleaved form. Tissue sections were stained using 3,3′-diaminobenzidine (DAB) (ZSGB-BIO, China) for another 15 min and were counterstained with hematoxylin for cell nuclei detection. Representative images were captured using a Leica camera (Leica, Germany). To assess the degree of claudin-1, the tissue sections were reviewed and scored by three pathologists with over 20 years of experience independently in a blinded manner. The immunohistochemistry (IHC) score determined by the percentage score and the intensity score of five visual fields from each slide. The percentage score was calculated according to the area of staining: ≤20.0 % = 0 points, 20.1–40.0 % = 1 point, 40.1–60.0 % = 2 points, 60.1–80.0 % = 3 points, and ≥80 % = 4 points. The intensity score was judged by the color depth: no staining = 0 point, light red = 1 point, brown red = 2 points, and brown = 3 points. The final IHC score was calculated as percentage score × intensity score [61,62].
5. Detection of Claudin-1 in plasma using ELISA
An enzyme-linked immunosorbent assay (ELISA) (YGYR-Biotech, China) was used to measure claudin-1 levels in plasma. Human claudin-1 antibodies coated on ELISA 96-well plates were incubated with plasma samples and standards at 37 °C for 2 h. After washing three times with PBS in a 96-well plate, the HRP-conjugated antibody was added to the wells and incubated for an additional 1 h at 37 °C. After washing completely, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution was added, followed by TMB chromogen solution, until it turned blue under HRP enzyme catalysis. Adding an acidic stop solution to TMB produced a blue-colored product that turned yellow. Optical density (OD) was measured at 450 nm using an EPOCH plate reader (BioTek, USA). The standard curve data were fitted to a seven-parameter curve using GraphPad Prism 8.0 (GraphPad Software, USA), and the absorbance values were used to back-calculate the concentration of claudin-1 in blood plasma samples from the absorbance at 450 nm.
5.1. Quantification of HIV DNA
Total DNA was separately extracted from human peripheral blood mononuclear cells (PBMCs) using QIAsymphony DNA Mini Kits (Qiagen, Germany) and from intestinal mucosa using Qiagen DNeasy Blood & Tissue Kits (Qiagen, Germany). In addition, a real-time fluorescence-based HIV DNA quantitative detection kit (SUPBIO, China) was used to quantify the HIV DNA [63]. In parallel, total cell numbers were determined for each sample by quantifying actin gene DNA copy numbers [64]. Copy numbers of each gene were generated using standard curves and HIV DNA copy number per 106 cells was calculated.
5.2. Quantification of Claudin-1 and TNF-α of the intestinal mucosa
Quantitative polymerase chain reaction (qPCR) primers and probe sequences were designed using the OLIGO 7.0 (https://www.oligo.net). Claudin-1 forward primer sequence was 5′-GTC TTT GAC TCC TTG CTG AAT CTG-3′, the reverse primer sequence was 5′-TTG CTA TCA CTC CCA GGA GGA T-3′, and probe sequence was 5′- CAG CAC ATT GCA AGC AAC CCG TG -3’. TNF-α forward primer sequence was 5′- CTC TTC AAG GGC CAA GGC T-3′, the reverse primer sequence was 5′-GAT GCG GCT GAT GGT GTG-3′, and probe sequence was 5′- CCC CTC CAA CCA TGT GCT CCT CA -3’. RNase-free water (Qiagen, Germany), 5X Colorless GoTaq ®Flexi® Buffer (Promega, USA), deoxynucleotide triphosphates (dNTPs, Promega, USA), GoTaq ®Hot Start Polymerase (Promega, USA), GoScriptTM Reverse Transcriptase (Promega, USA), RNase inhibitor (Takara, Japan), probe of claudin-1 (Sangon Biotech, China), and TNF-α (Sangon Biotech, China) were prepared for the reaction solutions that were subjected to qRCR. PCR conditions were as follows: initial denaturation (45 °C for 30 min, 95 °C for 10 min), followed by 45 cycles of denaturation (95 °C for 30 s,60 °C for 45 s), and final extension (72 °C for 15 min). To create standard curves for claudin-1 and TNF-α, 1.53 × 106 copies of the plasmid were serially diluted to one copy. Cell numbers were quantified by the real-time PCR measurement of the actin gene numbers. We performed quantitative real-time PCR using the SLAN96P PCR Detection System (Hongshi, China) calibrated with FAM and VIC fluorescent dyes.
5.3. CD4+ T cell detection of the intestinal mucosa by immunofluorescence
Paraffin sections were selected for baking, dewax, blocking endogenous peroxidase, and repair antigen. After incubation with anti-human CD4 rabbit monoclonal antibody (Abcam), goat anti-HRP conjugated to Cy5 (Panovue, China) was used at a 1:200 dilution. Finally, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (ZSGB-BIO, China) and observed under a fluorescence microscope (Leica, Germany).
5.4. Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, USA). Origin 2021 software (OriginLab, Massachusetts, USA) was used to perform correlation analysis and generate heat maps. The Mann-Whitney and the Kruskal–Wallis nonparametric test were used to compare the two and multiple groups outcomes, respectively. Spearman's rank correlation tests were used to determine the relationships among variables. Statistical significance was set at P < 0.05.
Author contributions
Y.J., F.S.W., P.M., Y.C., X.G. and Y.G. conceived the study and designed the experiments; Y.J., Y.G., X.G. and Z.W. performed the experiments; Y.J., F.S.W., P.M., Y.C., X.G., Y.G., Z.W., R.C., L.F. and Y.H. participated in subject recruitment and sample collection; F.S.W., P.M., Y.C., Z.W., R.C., Y.H., L.F., M.Q., C.Z., J.S., R.X., X.F., W.X., J.Z., B.B. and E.L. contributed to reagents and materials; Y.J., F.S.W., X.G. and Y.G. wrote the article. All authors participated in data acquisition and analysis. All authors reviewed and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82272317, 82171732), the Health Science and Technology Project of Tianjin Health Commission (ZC20037), the Tianjin Key Medical Discipline (Specialty) Construction Project (Infectious Diseases ZD02, ZD10), Chongqing medical scientific research project (2020FYYX118), Chongqing Talent Cultivation Program (cstc2021ycjh-bgzxm0275), the Joint Medical Research Projects of Chongqing Municipal Health Committee and Chongqing Municipal Science and Technology Bureau (2022QNXM032), Chongqing Science and Technology Bureau (cstc2020jscx-cylhX0001). We acknowledge pathological support from Prof. Yanhong Tai (Department of Pathology, the Fifth Medical centre of Chinese PLA General Hospital, Beijing, China.)
Contributor Information
Yan-Mei Jiao, Email: jiaoyanmei@sina.com.
Yao-Kai Chen, Email: yaokaichen@hotmail.com.
Ping Ma, Email: mapingtianjin@163.com.
Fu-Sheng Wang, Email: fswang302@163.com.
References
- 1.Yang X., Su B., Zhang X., Liu Y., Wu H., Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J. Leukoc. Biol. 2020;107:597–612. doi: 10.1002/JLB.4MR1019-189R. http://10.1002/JLB.4MR1019-189R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchetti G., Gazzola L., Trabattoni D., Bai F., Ancona G., Ferraris L., Meroni L., Galli M., Clerici M., Gori A., D'Arminio Monforte A. Skewed T-cell maturation and function in HIV-infected patients failing CD4+ recovery upon long-term virologically suppressive HAART. AIDS (London, England) 2010;24:1455–1460. doi: 10.1097/QAD.0b013e328339cf40. 10.1097/QAD.0b013e328339cf40. [DOI] [PubMed] [Google Scholar]
- 3.Gaardbo J.C., Hartling H.J., Ronit A., Springborg K., Gjerdrum L.M., Ralfkiær E., Thorsteinsson K., Ullum H., Andersen Å.B., Nielsen S.D. Regulatory T cells in HIV-infected immunological nonresponders are increased in blood but depleted in lymphoid tissue and predict immunological reconstitution. J. Acquir. Immune Defic. Syndr. 2014;66(1999):349–357. doi: 10.1097/QAI.0000000000000173. 10.1097/qai.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 4.Hou Y., Liu J., Zhao Y., Wu Y., Ma Y., Zhao D., Dou Z., Liu Z., Shi M., Jiao Y., et al. Epidemiological trends of severely immunosuppressed people living with HIV at time of starting antiretroviral treatment in China during 2005-2018. J. Infect. 2022;84:400–409. doi: 10.1016/j.jinf.2021.12.034. http://10.1016/j.jinf.2021.12.034 [DOI] [PubMed] [Google Scholar]
- 5.Estes J., Baker J.V., Brenchley J.M., Khoruts A., Barthold J.L., Bantle A., Reilly C.S., Beilman G.J., George M.E., Douek D.C., et al. Collagen deposition limits immune reconstitution in the gut. J. Infect. Dis. 2008;198:456–464. doi: 10.1086/590112. http://10.1086/590112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng J., Banerjee S., Zhang L., Sindberg G., Moidunny S., Li B., Robbins D.J., Girotra M., Segura B., Ramakrishnan S., Roy S. Opioids impair intestinal epithelial repair in HIV-infected humanized mice. Front. Immunol. 2019;10:2999. doi: 10.3389/fimmu.2019.02999. http://10.3389/fimmu.2019.02999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayarsaikhan S., Jagdagsuren D., Gunchin B., Sandag T. Survival, CD4 T lymphocyte count recovery and immune reconstitution pattern during the first-line combination antiretroviral therapy in patients with HIV-1 infection in Mongolia. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247929. http://10.1371/journal.pone.0247929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer-Myklestad M.H., Medhus A.W., Lorvik K.B., Seljeflot I., Hansen S.H., Holm K., Stiksrud B., Troseid M., Hov J.R., Kvale D., et al. Human immunodeficiency virus-infected immunological nonresponders have colon-restricted gut mucosal immune dysfunction. J. Infect. Dis. 2022;225:661–674. doi: 10.1093/infdis/jiaa714. http://10.1093/infdis/jiaa714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mudd J.C., Brenchley J.M. Gut mucosal barrier dysfunction, microbial dysbiosis, and their role in HIV-1 disease progression. J. Infect. Dis. 2016;214(Suppl 2):S58–S66. doi: 10.1093/infdis/jiw258. http://10.1093/infdis/jiw258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zevin A.S., McKinnon L., Burgener A., Klatt N.R. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr. Opin. HIV AIDS. 2016;11:182–190. doi: 10.1097/COH.0000000000000234. 10.1097/coh.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abad-Fernández M., Vallejo A., Hernández-Novoa B., Díaz L., Gutiérrez C., Madrid N., Muñoz M.A., Moreno S. Correlation between different methods to measure microbial translocation and its association with immune activation in long-term suppressed HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 2013;64(1999):149–153. doi: 10.1097/QAI.0b013e31829a2f12. 10.1097/QAI.0b013e31829a2f12. [DOI] [PubMed] [Google Scholar]
- 12.Vázquez-Castellanos J.F., Serrano-Villar S., Latorre A., Artacho A., Ferrús M.L., Madrid N., Vallejo A., Sainz T., Martínez-Botas J., Ferrando-Martínez S., et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2015;8:760–772. doi: 10.1038/mi.2014.107. 10.1038/mi.2014.107. [DOI] [PubMed] [Google Scholar]
- 13.Epple H.J., Schneider T., Troeger H., Kunkel D., Allers K., Moos V., Amasheh M., Loddenkemper C., Fromm M., Zeitz M., Schulzke J.D. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut. 2009;58:220–227. doi: 10.1136/gut.2008.150425. http://10.1136/gut.2008.150425 [DOI] [PubMed] [Google Scholar]
- 14.Stockmann M., Fromm M., Schmitz H., Schmidt W., Riecken E.O., Schulzke J.D. Duodenal biopsies of HIV-infected patients with diarrhoea exhibit epithelial barrier defects but no active secretion. AIDS. 1998;12:43–51. doi: 10.1097/00002030-199801000-00006. 10.1097/00002030-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Gunzel D., Yu A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. http://10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L., Zhou C., Li T., Xiao W., Yu M., Yang H. Interleukin-28A maintains the intestinal epithelial barrier function through regulation of claudin-1. Ann. Transl. Med. 2021;9:365. doi: 10.21037/atm-20-5494. 10.21037/atm-20-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nazli A., Chan O., Dobson-Belaire W.N., Ouellet M., Tremblay M.J., Gray-Owen S.D., Arsenault A.L., Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000852. http://10.1371/journal.ppat.1000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barmeyer C., Schulzke J.D., Fromm M. Claudin-related intestinal diseases. Semin. Cell Dev. Biol. 2015;42:30–38. doi: 10.1016/j.semcdb.2015.05.006. http://10.1016/j.semcdb.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Liu R., Catalano A.A., Ho Y.C. Measuring the size and decay dynamics of the HIV-1 latent reservoir. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100249. 10.1016/j.xcrm.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng X., Isnard S., Lin J., Fombuena B., Bessissow T., Chomont N., Routy J.P. Differences in HIV burden in the inflamed and non-inflamed colon from a person living with HIV and ulcerative colitis. J Virus Erad. 2021;7 doi: 10.1016/j.jve.2021.100033. 10.1016/j.jve.2021.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L.X., Song J.W., Zhang C., Fan X., Huang H.H., Xu R.N., Liu J.Y., Zhang J.Y., Wang L.F., Zhou C.B., et al. Dynamics of HIV reservoir decay and naïve CD4 T-cell recovery between immune non-responders and complete responders on long-term antiretroviral treatment. Clin. Immunol. (Orlando, Fla. 2021;229 doi: 10.1016/j.clim.2021.108773. 10.1016/j.clim.2021.108773. [DOI] [PubMed] [Google Scholar]
- 22.Mattapallil J.J., Smit-McBride Z., Dailey P., Dandekar S. Activated memory CD4(+) T helper cells repopulate the intestine early following antiretroviral therapy of simian immunodeficiency virus-infected rhesus macaques but exhibit a decreased potential to produce interleukin-2. J. Virol. 1999;73:6661–6669. doi: 10.1128/jvi.73.8.6661-6669.1999. 10.1128/JVI.73.8.6661-6669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehandru S., Poles M.A., Tenner-Racz K., Jean-Pierre P., Manuelli V., Lopez P., Shet A., Low A., Mohri H., Boden D., et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh S.K., Nathanael Johannes. Lieberkühn (1711-1756): luminary eighteenth century anatomist and his illuminating discovery of intestinal glands. Anatomy Cell Biol. 2023;56:25–31. doi: 10.5115/acb.22.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tincati C., Merlini E., Braidotti P., Ancona G., Savi F., Tosi D., Borghi E., Callegari M.L., Mangiavillano B., Barassi A., et al. Impaired gut junctional complexes feature late-treated individuals with suboptimal CD4+ T-cell recovery upon virologically suppressive combination antiretroviral therapy. AIDS (London, England) 2016;30:991–1003. doi: 10.1097/QAD.0000000000001015. 10.1097/qad.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 26.Somsouk M., Estes J.D., Deleage C., Dunham R.M., Albright R., Inadomi J.M., Martin J.N., Deeks S.G., McCune J.M., Hunt P.W. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS (London, England) 2015;29:43–51. doi: 10.1097/QAD.0000000000000511. 10.1097/qad.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao Y.M., Hayes P.J., Gotch F.M., Barrett M.C., Francis N.D., Gazzard B.G. Elevated mucosal addressin cell adhesion molecule-1 expression in acquired immunodeficiency syndrome is maintained during antiretroviral therapy by intestinal pathogens and coincides with increased duodenal CD4 T cell densities. J. Infect. Dis. 2002;185:1043–1050. doi: 10.1086/340235. 10.1086/340235. [DOI] [PubMed] [Google Scholar]
- 28.George M.D., Reay E., Sankaran S., Dandekar S. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J. Virol. 2005;79:2709–2719. doi: 10.1128/JVI.79.5.2709-2719.2005. 10.1128/JVI.79.5.2709-2719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C., Song J.W., Huang H.H., Fan X., Huang L., Deng J.N., Tu B., Wang K., Li J., Zhou M.J., et al. NLRP3 inflammasome induces CD4+ T cell loss in chronically HIV-1-infected patients. J. Clin. Investig. 2021;131 doi: 10.1172/JCI138861. 10.1172/jci138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia P., Xing X.D., Yang C.X., Liao X.J., Liu F.H., Huang H.H., Zhang C., Song J.W., Jiao Y.M., Shi M., et al. Activation-induced pyroptosis contributes to the loss of MAIT cells in chronic HIV-1 infected patients. Military Medical Research. 2022;9:24. doi: 10.1186/s40779-022-00384-1. 10.1186/s40779-022-00384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yero A., Farnos O., Rabezanahary H., Racine G., Estaquier J., Jenabian M.A. Differential dynamics of regulatory T-cell and Th17 cell balance in mesenteric lymph nodes and blood following early antiretroviral initiation during acute simian immunodeficiency virus infection. J. Virol. 2019;93 doi: 10.1128/JVI.00371-19. 10.1128/jvi.00371-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kityo C., Makamdop K.N., Rothenberger M., Chipman J.G., Hoskuldsson T., Beilman G.J., Grzywacz B., Mugyenyi P., Ssali F., Akondy R.S., et al. Lymphoid tissue fibrosis is associated with impaired vaccine responses. J. Clin. Investig. 2018;128:2763–2773. doi: 10.1172/JCI97377. 10.1172/jci97377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derdeyn C.A., Silvestri G. Viral and host factors in the pathogenesis of HIV infection. Curr. Opin. Immunol. 2005;17:366–373. doi: 10.1016/j.coi.2005.06.001. 10.1016/j.coi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Epple H.J., Allers K., Tröger H., Kühl A., Erben U., Fromm M., Zeitz M., Loddenkemper C., Schulzke J.D., Schneider T. Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology. 2010;139:1289–1300. doi: 10.1053/j.gastro.2010.06.065. 10.1053/j.gastro.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 35.Giorgi J.V., Hultin L.E., McKeating J.A., Johnson T.D., Owens B., Jacobson L.P., Shih R., Lewis J., Wiley D.J., Phair J.P., et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 1999;179:859–870. doi: 10.1086/314660. http://10.1086/314660 [DOI] [PubMed] [Google Scholar]
- 36.Hazenberg M.D., Otto S.A., van Benthem B.H., Roos M.T., Coutinho R.A., Lange J.M., Hamann D., Prins M., Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. http://10.1097/00002030-200309050-00006 [DOI] [PubMed] [Google Scholar]
- 37.Keating J., Bjarnason I., Somasundaram S., Macpherson A., Francis N., Price A.B., Sharpstone D., Smithson J., Menzies I.S., Gazzard B.G. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995;37:623–629. doi: 10.1136/gut.37.5.623. http://10.1136/gut.37.5.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruewer M., Luegering A., Kucharzik T., Parkos C.A., Madara J.L., Hopkins A.M., Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. http://10.4049/jimmunol.171.11.6164 [DOI] [PubMed] [Google Scholar]
- 39.Schmitz H., Rokos K., Florian P., Gitter A.H., Fromm M., Scholz P., Ullrich R., Zeitz M., Pauli G., Schulzke J.D. Supernatants of HIV-infected immune cells affect the barrier function of human HT-29/B6 intestinal epithelial cells. AIDS. 2002;16:983–991. doi: 10.1097/00002030-200205030-00004. http://10.1097/00002030-200205030-00004 [DOI] [PubMed] [Google Scholar]
- 40.Heller F., Florian P., Bojarski C., Richter J., Christ M., Hillenbrand B., Mankertz J., Gitter A.H., Burgel N., Fromm M., et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. http://10.1016/j.gastro.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 41.Fish S.M., Proujansky R., Reenstra W.W. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45:191–198. doi: 10.1136/gut.45.2.191. http://10.1136/gut.45.2.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gitter A.H., Bendfeldt K., Schulzke J.D., Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 2000;14:1749–1753. doi: 10.1096/fj.99-0898com. http://10.1096/fj.99-0898com [DOI] [PubMed] [Google Scholar]
- 43.Grotjohann I., Schmitz H., Fromm M., Schulzke J.D. Effect of TNF alpha and IFN gamma on epithelial barrier function in rat rectum in vitro. Ann. N. Y. Acad. Sci. 2000;915:282–286. [PubMed] [Google Scholar]
- 44.Schmitz H., Fromm M., Bentzel C.J., Scholz P., Detjen K., Mankertz J., Bode H., Epple H.J., Riecken E.O., Schulzke J.D. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J. Cell Sci. 1999;112(Pt 1):137–146. doi: 10.1242/jcs.112.1.137. http://10.1242/jcs.112.1.137 [DOI] [PubMed] [Google Scholar]
- 45.Zeissig S., Bojarski C., Buergel N., Mankertz J., Zeitz M., Fromm M., Schulzke J.D. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. http://10.1136/gut.2003.036632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maartens G., Celum C., Lewin S.R. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet (London, England) 2014;384:258–271. doi: 10.1016/S0140-6736(14)60164-1. http://10.1016/s0140-6736(14)60164-1 [DOI] [PubMed] [Google Scholar]
- 47.Margolis D.M., Archin N.M., Cohen M.S., Eron J.J., Ferrari G., Garcia J.V., Gay C.L., Goonetilleke N., Joseph S.B., Swanstrom R., et al. Curing HIV: seeking to target and clear persistent infection. Cell. 2020;181:189–206. doi: 10.1016/j.cell.2020.03.005. http://10.1016/j.cell.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Autran B., Carcelain G., Li T.S., Blanc C., Mathez D., Tubiana R., Katlama C., Debre P., Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. http://10.1126/science.277.5322.112 [DOI] [PubMed] [Google Scholar]
- 49.Orlandi C., Canovari B., Bozzano F., Marras F., Pasquini Z., Barchiesi F., De Maria A., Magnani M., Casabianca A. A comparative analysis of unintegrated HIV-1 DNA measurement as a potential biomarker of the cellular reservoir in the blood of patients controlling and non-controlling viral replication. J. Transl. Med. 2020;18:204. doi: 10.1186/s12967-020-02368-y. http://10.1186/s12967-020-02368-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiselinova M., De Spiegelaere W., Buzon M.J., Malatinkova E., Lichterfeld M., Vandekerckhove L. Integrated and total HIV-1 DNA predict ex vivo viral outgrowth. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005472. http://10.1371/journal.ppat.1005472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rouzioux C., Avettand-Fenoël V. Total HIV DNA: a global marker of HIV persistence. Retrovirology. 2018;15:30. doi: 10.1186/s12977-018-0412-7. http://10.1186/s12977-018-0412-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clayton F., Kotler D.P., Kuwada S.K., Morgan T., Stepan C., Kuang J., Le J., Fantini J. Gp120-induced Bob/GPR15 activation: a possible cause of human immunodeficiency virus enteropathy. Am. J. Pathol. 2001;159:1933–1939. doi: 10.1016/S0002-9440(10)63040-4. http://10.1016/S0002-9440(10)63040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buccigrossi V., Laudiero G., Nicastro E., Miele E., Esposito F., Guarino A. The HIV-1 transactivator factor (Tat) induces enterocyte apoptosis through a redox-mediated mechanism. PLoS One. 2011;6 doi: 10.1371/journal.pone.0029436. http://10.1371/journal.pone.0029436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brenchley J.M., Schacker T.W., Ruff L.E., Price D.A., Taylor J.H., Beilman G.J., Nguyen P.L., Khoruts A., Larson M., Haase A.T., Douek D.C. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004;200:749–759. doi: 10.1084/jem.20040874. http://10.1084/jem.20040874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guadalupe M., Reay E., Sankaran S., Prindiville T., Flamm J., McNeil A., Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. http://10.1128/jvi.77.21.11708-11717.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brenchley J.M., Price D.A., Schacker T.W., Asher T.E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. http://10.1038/nm1511 [DOI] [PubMed] [Google Scholar]
- 57.Sankaran S., George M.D., Reay E., Guadalupe M., Flamm J., Prindiville T., Dandekar S. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J. Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. http://10.1128/JVI.01449-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guadalupe M., Sankaran S., George M.D., Reay E., Verhoeven D., Shacklett B.L., Flamm J., Wegelin J., Prindiville T., Dandekar S. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J. Virol. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. http://10.1128/JVI.00120-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao P., Zhou W.C., Li D.L., Mo X.T., Xu L., Li L.C., Cui W.H., Gao J. Total glucosides of Danggui buxue Tang attenuate BLM-induced pulmonary fibrosis via regulating oxidative stress by inhibiting NOX4. Oxid. Med. Cell. Longev. 2015 doi: 10.1155/2015/645814. 2015. 10.1155/2015/645814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z., Qu J., Zheng C., Zhang P., Zhou W., Cui W., Mo X., Li L., Xu L., Gao J. Nrf2 antioxidant pathway suppresses Numb-mediated epithelial-mesenchymal transition during pulmonary fibrosis. Cell Death Dis. 2018;9:83. doi: 10.1038/s41419-017-0198-x. http://10.1038/s41419-017-0198-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu Y., Wu T., Sheng Y., Dai Y., Xia B., Xue Y. Correlation between Claudin-18 expression and clinicopathological features and prognosis in patients with gastric cancer. J. Gastrointest. Oncol. 2020;11:1253–1260. doi: 10.21037/jgo-20-463. http://10.21037/jgo-20-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Z., Zhang X., Zhu H., Zhong N., Luo X., Zhang Y., Tu F., Zhong J., Wang X., He J., Huang L. TELO2 induced progression of colorectal cancer by binding with RICTOR through mTORC2. Oncol. Rep. 2021;45:523–534. doi: 10.3892/or.2020.7890. http://10.3892/or.2020.7890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitney J.B., Hill A.L., Sanisetty S., Penaloza-MacMaster P., Liu J., Shetty M., Parenteau L., Cabral C., Shields J., Blackmore S., et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. http://10.1038/nature13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai X.P., Wu F.Y., Cui C., Liao X.J., Jiao Y.M., Zhang C., Song J.W., Fan X., Zhang J.Y., He Q., Wang F.S. Increased platelet-CD4(+) T cell aggregates are correlated with HIV-1 permissiveness and CD4(+) T cell loss. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.799124. http://10.3389/fimmu.2021.799124 [DOI] [PMC free article] [PubMed] [Google Scholar]