Abstract
Background
Vector control is an important approach in the control of most parasitic and vector-borne diseases including malaria, and schistosomiasis. Distribution of these two infections often overlaps and in such areas it’s more economically viable to employ an integrated approach in the control of their vectors which largely shares the same breeding ecosystem. We carried out a baseline epidemiological and vector surveys for malaria and schistosomiasis in Mwea, Kirinyaga County, in preparation for the upscaling of integrated vector management (IVM) for the two diseases.
Methods
This was a repeated cross sectional survey, where mosquito and snails were sampled during dry and wet seasons in three different ecological zones, Kiamaciri, Thiba and Murinduko to identify possible breeding sites. Mosquito larvae were collected using standard dippers, adults using CDC miniature light traps while snail vectors were sampled using standard snail scoops in different breeding habitats. A total of 1200 pupils from 12 primary schools were tested for malaria using rapid diagnostic tests (Malaria Pf/PAN Ag combo). Stool samples were processed using the Kato Katz technique for intestinal schistosomiasis.
Results
The overall prevalence of intestinal schistosomiasis was 9.08 % (95 % CI: 07.00–11.00), with Kiamaciri zone recording the highest prevalence at 19 % (95%CI: 15.00–23.00) and Murinduko zone the least at 0.17 % (95%CI: 0.00–0.01). Majority of the infections were of light intensity 78.9 % (95%CI: 70.04–86.13). There was no positive malaria case detected in this study. Of the 3208 adult mosquitoes sampled during the dry season, 20.6 % (95 % CI: 19.25–22.08) were Anopheles gambiae s.l while 79.4 % (95 % CI: 77.92–80.75) were culicines. During the wet season, 3378 adult mosquitoes were collected, of which 14.7 % (95 % CI: 13.56–15.98) were Anopheles gambiae s.l and 85.3 % (95 % CI: 84.02–86.44) culicines. Overall, 4085 mosquito larvae were collected during the two seasons, of which, 57.3 % and 42.7 % were anopheles and culicine respectively. Majority of the larvae (85.1 % (95%CI: 84.01–86.10) were collected during the wet season, with only 14.9 % (95%CI: 14.10–16.00) being collected during the dry season. A total of 2292 fresh water vector snails were collected with a majority (69.6 % (95%CI: 68.00–71.10) being Biomphalaria pffeiferi responsible for transmission of intestinal schistosomiasis.
Conclusion
This study demonstrates that intestinal schistosomiasis is prevalent in Kiamaciri and Thiba zones, and points to the possibility of active transmission of schistosomiasis in Murinduko zone. Malaria vectors were predominantly observed in all sites despite there being no malaria positive case. Culex quinquefaciatus responsible for the spread of several arboviruses was also observed. The presence of these vectors may lead to future disease outbreaks in the area if concerted control initiatives are not undertaken. The disease vectors shared the same breeding sites and thus its economical and feasible to adopt an integrated vector management approach in control efforts for these disease in the study area.
Keywords: Schistosomiasis, Malaria, Disease vectors, Integrated, control
1. Introduction
Schistosomiasis is a parasitic disease caused by a trematode worm of the genius Schistosoma [1]. The disease has a high global burden with an estimated over 240 million people being infected with one or more of the Schistosoma species [2], and over 280,000 deaths annually [3]. The bulk of schistosomiasis global transmission occurs in sub-Saharan Africa [4]. In Kenya, recent data estimates that approximately nine million people are infected with schistosomiasis and about eighteen million are at risk of infection [5].
Malaria remains a public health problem globally with an estimated 247 million cases and 619,000 deaths being reported in 2021. Sub-Saharan countries carries the greater burden with an estimated 234 million cases (95 %) and 593,000 deaths (96 %) [6]. In Kenya, nearly 6.7 million clinical cases of malaria are reported annually, with 70 % of the population being at risk of malaria [7]. It is estimated that approximately 4000 people die from malaria annually, most of them being children. Besides, malaria is responsible for 13–15 % of outpatient consultations [8]. Malaria is transmitted through bites of an infected female Anopheles mosquito which breeds in water.
The distribution of schistosomiasis and malaria often overlaps due to various conditions that favour multiple parasitic species survival and transmission [9]. Studies have shown that, the two infections do occur together [[10], [11], [12]]. The two infections have also been shown to have complex immunological and pathological interactions [12,13]. People with heavy intestinal schistosomiasis tend to have increased malaria susceptibility [14], suggesting that having an integrated approach in the control for both diseases could yield significant additive benefits. Currently, the control for schistosomiasis in Kenya is through preventive chemotherapy which involves periodic mass drug administration (MDA) of praziquantel to populations at risk [15]. While this approach has resulted in reduction of long-term morbidities of schistosomiasis and other helminths especially among school going children [16], there is still ongoing active local transmission [17]. Children typically suffer from high rates of reinfection, and the broader community (preschool children, adolescents, and adult populations) remains affected with schistosomiasis [18,19]. There is now growing interest in improving morbidity control through reduction or elimination of schistosomiasis [20]. In addition to MDA, one complementary approach is the local control of intermediate host to interrupt the non-human phase of the Schistosoma lifecycle [20,21]. In the recent past, there has been very little effort in vector control for schistosomiasis in the country.
The control of malaria in Kenya on the other hand is focused on a combination of several primary vector control interventions. These interventions include use of long lasting insecticide nets (LLINs) which are given for free during the mass net distribution campaigns, indoor residual spraying (IRS) [22,23], accelerated adoption of universal parasitological diagnosis in health facilities, and administration of artemisinin-based combination therapies (ACTs) to at risk populations [22,24].
Vector control has been an instrumental and effective tool in the control and elimination of most parasitic and vector-borne diseases including malaria [25,26], and schistosomiasis [20,27]. Implementation of the control measures has always been disease specific and mostly with different actors. However, in areas where the two diseases are co endemic, it may be more economically viable to employ an integrated approach to the vector control, which also brings forth collaborations of different actors.
Integrated vector management (IVM) strategy is defined by World Health Organization (WHO) as a rational decision-making process, that encourages optimal use of resources for efficient, cost-effective and sustainable vector control [28]. The strategy is based on the premise that effective control is not the sole preserve of the health sector but of various public and private agencies, including communities. IVM calls for strengthening vector control through increased capacity to community members as part of intervention so that they become key players in disease prevention and control. It also involves improved surveillance, better coordination and integrated action across sectors and diseases.
We carried out a baseline epidemiological and vector surveys for malaria and schistosomiasis in Mwea, Kirinyaga county, to understand the current burden of malaria and schistosomiasis, to identify vector breeding sites for the two infections and to explore the feasibility of implementation of an integrated vector management (IVM) for the two diseases.
2. Material and methods
2.1. Study area
This study was carried out in Mwea, Kirinyaga county. The County covers an area of 1478.1 square kilometers and lies between 1158 m and 5380 m above sea level in the South and at the Peak of Mt. Kenya, respectively. There are six major rivers namely; Sagana, Nyamindi, Rupingazi, Thiba, Rwamuthambi and Ragati, all of which drain into the Tana River. Water from these rivers has been harnessed through canals to support rice irrigation farming at the lower areas of the county. The county has five sub counties namely Kirinyaga East, Kirinyaga Central, Kirinyaga West, Mwea East and Mwea West [29]. The mean annual rainfall in the county is 950 mm with long rains in March to May (wet season) and short rains in October to December (dry season). The average temperatures are in the range of 16–26.5 °C and relative humidity varies from 52 to 67 % [29].
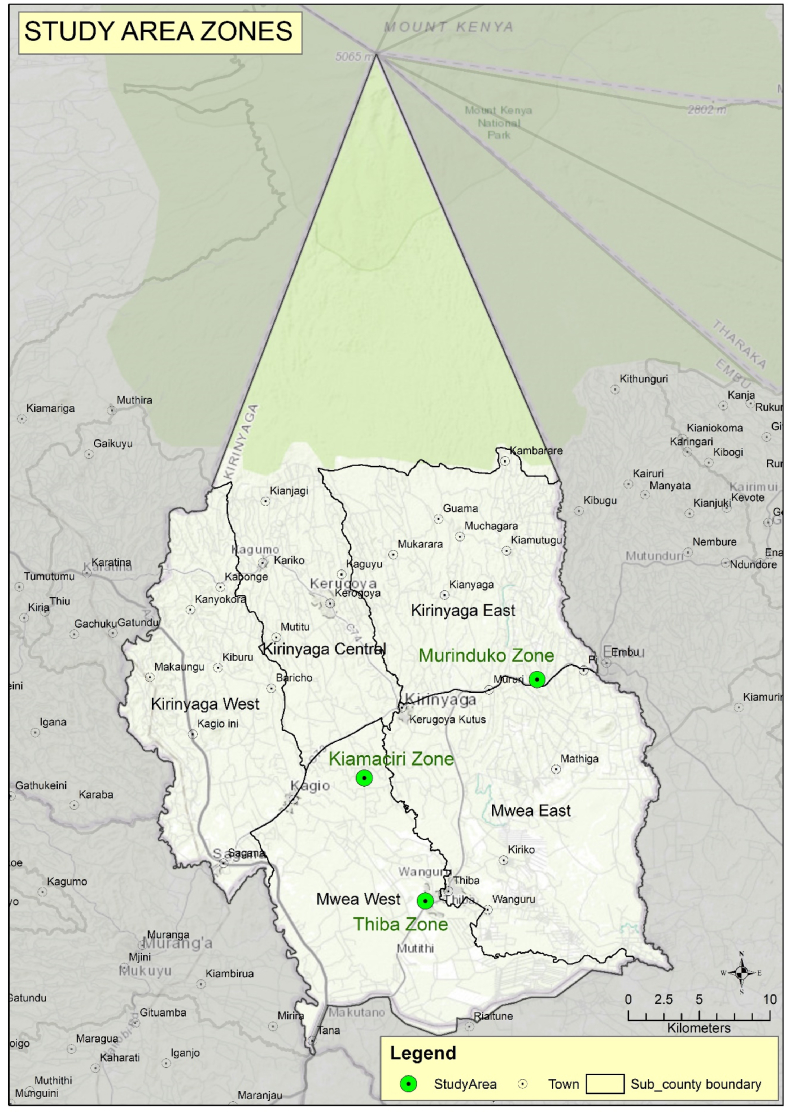
Mwea West and Mwea East sub counties were purposively selected for the study for various reasons. The giant Mwea irrigation scheme (MIS) is located within these two counties. In the irrigation scheme, over 50 % of the scheme area is used for irrigated rice cultivation while the remaining area is used for subsistence farming, grazing, and community activities [30]. Secondly, this area is endemic for both intestinal schistosomiasis and malaria. The area has many shallow water canals and water drainage systems that surround rice farms and settlement villages. The water bodies are breeding places for snails and mosquito vectors. Previous studies have reported prevalence of 23.1 % for intestinal schistosomiasis [18]. The transmission of malaria in the area has been reported to be meso-endemic with Anopheles Arabiensis being the main mosquito sustaining malaria parasite transmission with prevalence of 10 % [31]. Control of malaria in this area involves use of LLINs at the households [28,32], while that of schistosomiasis is through mass drug administration of praziquantel to school going children through the School based deworming program (SBDP) [ 16, 18]. The study was carried out in three ecological zones represented by Kiamaciri (lowlands), Thiba (midland) and Murinduko (highlands area) (Fig. 1).
Fig. 1.
Map of Kirinyaga County showing the study areas.
2.2. Study design
This was a repeated cross sectional study. Data was collected during the wet and dry seasons to allow for vector density comparisons. For wet seasons were between the months of March and May with dry seasons covering the months of October and November.
2.3. Study populations
The study population involved heads of households for the houses where adult mosquitoes were collected and school going children aged between 5 and 16 years in 12 selected primary schools.
2.4. Sampling and sample size determination
For epidemiological data, a two stage sampling design was employed to sample schools: (i) schools (primary sampling units) were selected using probability proportional to size (PPS); (ii) In each school, a fixed number of children (n = 100) (secondary sampling units) were randomly selected using a Microsoft Excel generated table of random numbers [33]. Houses for adult mosquito sampling were selected purposively based on the following criteria, within a radius of 2 km from a participating school and consenting by the household head. House characteristics including the roof type (grass thatch and iron sheet), wall type (mud) and proximity to the water bodies was collected. Each participating village was divided into four sub-sectors (East, west, North & South) in which 5 representative houses were selected per sub-sector.
2.4.1. Mosquito vector sampling
Due to the sustained use of vector control interventions in the area, mosquito abundance was likely to be low. In order for the study to achieve a good coverage and increase the collections, 20 houses were selected from each of three areas.
2.4.2. Parasite prevalence
The sampling frame was based on the primary schools within each area. e The unit of analysis was prevalence of infection among children aged 5–16 years within a school. In each school, 100 children were randomly selected, 20 from each of grade 2–6 using computer-generated random table numbers [34]. Age-corrected, population-weighted parasite prevalence (PfPR2-10) predicted from modelled data in 2010 was used as a mean estimate of risk for Kirinyaga county which was given as 3.75 %. Assuming a design effect of 2 as per the previous school surveys that have been undertaken in Kenya [35] and considering that Kirinyaga county had low malaria prevalence and high prevalence of schistosomiasis, we estimated a sample size of 1200. In total 12 primary schools were enrolled in the study, 6 schools in Murinduko, 4 schools in Kiamaciri and 2 schools in Thiba.
2.5. Inclusion and exclusion criteria
For epidemiologic data, we included selected schools and in those schools, children whose parents gave signed consent for their children to participate and children who assented to the study. For households where mosquito was collected, we included households whose heads or an adult person gave written consent to participate in the study. The study excluded schools which were not selected, children whose parents did not consent and also children who did not assent to participate in the study. It also excluded households whose heads did not give written consent to the study.
2.6. Data collection procedures
2.6.1. Malaria and schistosomiasis epidemiological data collection
In each school, 100 children were sampled randomly, 20 children from each of grade 2–6. They were asked to provide a stool sample, and a finger prick blood sample. The stool sample was used to assess for intestinal schistosomiasis while blood sample was used to assess malaria infection (Plasmodium species) using rapid diagnostic tests (RDTs). The RDT used in the study was CareStart™ Malaria Pf/PAN Ag combo (Access Bio, Inc65 Clyde Rd., Suite A, Somerset, NJ 08873, USA), which uses monoclonal antibodies (HRP2/pLDH). Malaria tests were done in school, while the stool samples were transported to Kimbimbi sub county hospital laboratory. In the laboratory, two slides were prepared from each stool sample and examined microscopically for presence and intensity of intestinal Schistosomiasis using the Kato-katz technique [36]. For quality checks, 10 % of all the slides were re-examined by a senior technologist. Children positive for intestinal Schistosomiasis were treated with praziquantel.
2.6.2. Sampling of mosquito larvae
Water bodies surrounding the selected villages were identified. The water bodies included canals, rice paddies, man-made dams, water collection points, drainage channels and temporary stagnant water points. Mosquito larvae were sampled during the wet and dry seasons using standard dippers. The water bodies included rice canals, man-made dams and rice paddies. Mosquito immatures were grouped to early instars (L1& L2), late instars (L3 & L4), and pupae. All pupae were kept in emergent cages in the insectary and identified as per taxonomic keys [37].
2.6.3. Adult mosquitoes sampling
Adult mosquitoes were collected inside homesteads using Center for Disease Control (CDC) miniature light trap (Model 512) (John W. Hock Co., Gainesville, FL, USA). The traps were set in the evening at 1800hrs, and collected the following morning at 0600hrs and taken to Kimbimbi sub county hospital where they were sorted and identified to species on the basis of morphologic characters according to identification keys [38,39].
2.6.4. Collection and identification of snails
Snails were sampled from streams, rice canals, water pools (rice paddies) and man-made dams. This was done by two trained field collectors using standard snail scoops. Each site was sampled for 30 min between 08:30 h and 10:30 h. Each sampling site consisted of an area of approximately 5 m2. The collected snails were sorted and identified to species level based on shell morphological characteristics using standard keys [40,41]. Biomphalaria and Bulinus species were labelled per site and time of collection and transported in separate perforated plastic containers to Kimbimbi sub county health center laboratory for processing. All the other species were returned back to the water after identification. In the laboratory, the snails were rinsed and placed individually in 24-well culture plates containing 1 ml of clear, filtered water (same source as site of collection) and exposed to indirect sunlight for 4 h to induce cercarial shedding. The time of cercariae shedding was carefully selected to coincide with the early peak shedding time (midday) [42]. The wells of the plates were then examined for presence of cercariae under a dissecting microscope. Snails that did not shed cercariae on the first exposure were re-exposed on the second day.
2.7. Data analysis
Data was entered and stored in an excel spreadsheet, and checked for accuracy to ensure completeness. Statistical analyses were carried out using STATA version 14.0 (Stata Corporation, College Station, TX, USA). Snail and mosquito abundance and prevalence of S. mansoni 95 % confidence interval (95 % CI) were calculated using binomial logistic regression taking into account clustering by schools and zone level. The Kato-katz method assumes evaluation of 41.7 mg of stool sample; to calculate eggs per gram of feaces (EPG) from the stool sample, a multiplication factor of 24 was applied to the number of parasite eggs counted, also known as fecal egg count (FEC) as 24 × 47.1 mg = 1000.8 mg or 1 g [[43], [44]]. These were then classified into light, moderate and heavy infections, according to WHO guidelines [44].
2.8. Ethical consideration
The study was reviewed and approved by the Scientific and Ethical Review Unit (SERU) of the Kenya Medical Research Institute (KEMRI), number (KEMRI/SERU/CGMR-C/068/3468. Prior to the beginning of data collection, meetings with the community gate keepers were held in the three study areas where objectives of the study were explained, followed with public community meetings (Barazas) for the same purpose. Household heads for houses where mosquitoes were collected provided written consent as well as parents to school children who participated in the study. Children were also asked for verbal assent.
3. Results
3.1. Parasitological surveys
There was no malaria positive case among the 1200 school children who participated in the study (Table 1). The overall prevalence of intestinal schistosomiasis was 9.08 % (95 % CI: 07.00–11.00). In Kiamaciri 19 % (95%CI: 15.49–23.25) of the participants were infected with intestinal schistosomiasis, while 16.5 % (0.11–0.22) of those who participated in Thiba had the infection. Only 0.17 % (95%CI: 0.00–0.01) were infected in Murinduko. Intestinal schistosomiasis was reported in seven of the twelve participating schools. Of the six schools in Murinduko, only one school reported a positive case of intestinal schistosomiasis. All the participating schools in Kiamaciri and Thiba reported cases of intestinal schistosomiasis. Wakaniu primary school in Kiamaciri reported the highest prevalence of intestinal schistosomiasis overall at 31.0 %, (95 % CI:22.12–40.31), followed by Mwea central primary school at 21 % (95%CI: 0.13–0.29). A total of five schools did not record any positive case of intestinal schistosomiasis (Table 1).
Table 1.
Prevalence of malaria and intestinal schistosomiasis among school going children aged 5–16 years.
| Zone | Schools | Number testeda | Malaria Prevalence | Intestinal schistosomiasis cases | Prevalence (95 % CI) | Intestinal schistosomiasis prevalence by zone |
|---|---|---|---|---|---|---|
| Murinduko | Mugamba ciura | 100 | 0.0 | 0 | 0.0 | |
| St Joseph | 100 | 0.0 | 0 | 0.0 | ||
| Karuangi | 100 | 0.0 | 0 | 0.0 | ||
| Gathigi-Ini | 100 | 0.0 | 1 | 1.0 % (0.00–0.03) | ||
| Murinduko | 100 | 0.0 | 0 | 0.0 | ||
| Mumbu-Ini | 100 | 0.0 | 0 | 0.0 | ||
| Total | 600 | 0.0 | 1 | 0.17 % (0.00–0.01) | ||
| Kiamaciri | Wakaniu | 100 | 0.0 | 31 | 31.0 % (0.22–0.40) | |
| Destiny | 100 | 0.0 | 6 | 6.0 % (0.01–0.11) | ||
| Gitoo-ini | 100 | 0.0 | 19 | 19.0 % (0.11–0.27) | ||
| Kangai | 100 | 0.0 | 19 | 19.0 % (0.11–0.27) | ||
| Total | 400 | 0.0 | 75 | 19.0 % (15.49–23.25) | ||
| Thiba | Mwea central | 100 | 0.0 | 21 | 21.0 % (0.13–0.29) | |
| Thiba | 100 | 0.0 | 12 | 12.0 % (0.06–0.18) | ||
| Total | 200 | 0.0 | 33 | 16.5 % (11.12–22.00) | ||
| Overall | 1200 | 109 | 9.08 % (07.00–11.00) |
Number sampled per school.
Of all the 9.08 % (109) (95 % CI: 07.00–11.00) cases of intestinal schistosomiasis, 17.4 % (95%CI: 10.83–25.87) were of heavy intensity infections (>400 eggs per gram), 3.7 % (95%CI: 01.01–09.13) moderate infection (100–399 eggs per gram) with the majority being of light intensity infection (1–99 eggs per gram) 78.9 % (95%CI: 70.04–86.13). Majority of the infections 80.7 % (95%CI: 72.07–87.66) were among children of age group 11–16 years. Children of age group 5–10 reported the least infections at 19.3 % (95%CI: 12.34–27.93).
3.2. Abundance of adult mosquito vectors
A total 6586 adult mosquitoes were collected of which 3208 and 3378 were collected during the dry and wet season respectively. Of the mosquitoes collected during the dry season, 20.6 % (95 % CI: 19.25–22.08) were Anopheles and 79.4 % (95 % CI: 77.92–80.75) were culicine. During the wet season surveys, 14.7 % (95 % CI: 13.56–15.98) of the adult mosquitoes collected were Anopheles and 85.3 % (95 % CI: 84.02–86.44) culicine. The population of Anopheles and culicine species differed significantly across the two seasons (p < 0.001, α = 0.05). Overall, Kiamaciri recorded the highest abundance of mosquitoes 57.15 % (95%CI: 55.96–58.35). The distribution of adult mosquitoes in the three study area is tabulated in Table 2. Anopheles gambiae s.l was the most predominant species across the three ecological zones. Other Anopheles species observed included An. coustani, An. pharoensis, An. funestus and An. pretoriensis. However, Culex quinquefasciatus was the most prevalent culicine mosquito found in the three zones occurring during both dry and wet seasons.
Table 2.
Summary of distribution of adult mosquito species collected inside houses during the wet and dry season.
| Mosquito species | Dry season | Wet season | Total | |
|---|---|---|---|---|
| Murinduko | An. gambiae | 76 | 87 | 163 |
| An. coustani | 5 | 7 | 12 | |
| An. pharoensis | 0 | 0 | 0 | |
| An. funestus | 7 | 2 | 9 | |
| An. pretoriensis | 0 | 2 | 2 | |
| Total Anopheles (%) | 88 (41.9) | 98 (66.2) | 186 (51.9) | |
| Cx. quinquefasciatus (%) | 122 (58.1) | 50 (33.8) | 172 (48.1) | |
| Total Murinduko (%) | 210 (100) | 148 (100) | 358 (100) | |
| Kiamaciri | An. gambiae | 399 | 103 | 502 |
| An. coustani | 63 | 48 | 111 | |
| An. pharoensis | 0 | 0 | 0 | |
| An. funestus | 49 | 0 | 49 | |
| An. pretoriensis | 4 | 4 | 8 | |
| Total Anopheles (%) | 515 (21.3) | 155 (11.60 | 670 (17.8) | |
| Cx. quinquefasciatus (%) | 1908 (78.7) | 1186 (88.4) | 3094 (82.2) | |
| Total Kiamaciri (%) | 2423 (100) | 1341 (100) | 3764 (100) | |
| Thiba | An. gambiae | 48 | 209 | 257 |
| An. coustani | 5 | 12 | 17 | |
| An. pharoensis | 0 | 18 | 18 | |
| An. funestus | 5 | 3 | 8 | |
| An. pretoriensis | 1 | 3 | 4 | |
| Total Anopheles (%) | 59 (10.3) | 245 (13.0) | 304 (12.3) | |
| Cx. quinquefasciatus (%) | 516 (89.7) | 1644 (87.0) | 2160 (87.7) | |
| Total Thiba (%) | 575 (100) | 1889 (100) | 2464 (100) | |
| Totals | Total Anopheles (%) | 662 (20.6) | 498 (14.7) | 1160 (17.6) |
| Total culicine (%) | 2546 (79.4) | 2880 (85.3) | 5426 (82.7) | |
| Total mosquitoes (%) | 3208 (100) | 3378 (100) | 6586 (100) |
An- Anopheles Cx- Culex.
3.3. Mosquito larvae
A total of 4085 mosquito immatures were sampled during the wet and dry seasons. Of these, 14.9 % (607) (95%CI: 14.32–16.60) were collected during the dry season and 85.1 % (3,478) (95%CI: 84.13–86.52) during the wet season. During the dry season, 89.6 % (544) (95%CI: 87.46–92.10) were Anopheles’ larvae while 10.4 % (63) (95%CI: 08.31–13.17) were culicine. During the wet season, 51.7 % (1798) (95%CI: 50.43–53.23) of all the mosquitoes collected were Anopheles while 48.3 % (1680) (95%CI: 47.11–49.32) were culicine. Anopheles larvae were significantly abundant across the two seasons (p < 0.001). The distribution of larvae in the three study zones is tabulated in Table 3.
Table 3.
Summary of the number of mosquito larvae species distribution during different season in the study area.
| Larval stage | Anopheles (dry) | Culicine (dry) | Anopheles (wet) | Culicine (wet) | Total anopheles | Total culicine | Total | |
|---|---|---|---|---|---|---|---|---|
| Murinduko | LI (n, %) | 68 (29.7) | 31 (49.2) | 243 (41.5) | 299 (47.2) | 311 (38.1) | 330 (47.3) | 641 (42.4) |
| L2 (n, %) | 37 (16.0) | 16 (25.4) | 121 (20.7) | 210 (33.1) | 158 (19.4) | 226 (32.4) | 384 (25.4) | |
| L3 (n, %) | 64 (27.6) | 10 (15.9) | 89 (15.2) | 57 (9.0) | 153 (18.8) | 67 (9.6) | 220 (14.5) | |
| L4 (n, %) | 56 (24.1) | 2 (3.2) | 101 (17.3) | 49 (7.7) | 157 (19.2) | 51 (7.4) | 208 (13.7) | |
| Pupae (n, %) | 6 (2.6) | 4 (6.3) | 31 (5.3) | 19 (3.0) | 37 (4.50 | 23 (3.3) | 60 (4.0 | |
| Total (n, %) | 232 (100) | 63 (100) | 585 (100) | 634 (100) | 816 (100) | 697 (100) | 1513 (100) | |
| Kiamaciri | LI (n, %) | 74 (45.5) | 0 | 171 (56.1)) | 102 (37.5) | 245 (52.8) | 102 (37.5) | 347 (47.1) |
| L2 (n, %) | 54 (34.0) | 0 | 98 (32.1) | 99 (36.4) | 152 (32.8) | 99 (36.4) | 251 (34.1) | |
| L3 (n, %) | 21 (13.2) | 0 | 16 (5.2) | 41 (15.1) | 37 (8.0) | 41 (15.1) | 78 (10.6) | |
| L4 (n, %) | 10 (80.3) | 0 | 18 (5.9) | 21 (7.7) | 28 (6.0) | 21 (7.7) | 49 (6.6) | |
| Pupae (n, %) | 0 | 0 | 2 (0.7) | 9 (3.3) | 2 (0.4) | 9 (3.3) | 12 (1.6) | |
| Total (n, %) | 159 (100) | 0 | 305 (100) | 272 (100) | 464 (100) | 272 (100) | 737 (100) | |
| Thiba | LI (n, %) | 63 (41.2) | 0 | 299 (32.9) | 341 (44.1) | 362 (34.1) | 341 (44.1) | 703 (38.3) |
| L2 (n, %) | 43 (28.1) | 0 | 301 (33.1) | 107 (13.8) | 344 (32.4) | 107 (13.8) | 451 (25.6) | |
| L3 (n, %) | 33 (21.6) | 0 | 177 (19.5) | 162 (20.9) | 210 (19.8) | 162 (21.0) | 372 (20.3) | |
| L4 (n, %) | 14 (9.1) | 0 | 99 (10.9) | 111 (14.4) | 113 (10.7) | 111 (14.3) | 224 (12.2) | |
| Pupae (n, %) | 0 | 0 | 32 (3.5) | 53 (6.8) | 32 (3.0) | 53 (6.8) | 85 (4.6) | |
| Total (n, %) | 153 (100) | 0 | 908 (100) | 774 (100) | 1061 (100) | 774 (100) | 1835 (100) | |
| Total | 544 | 63 | 1798 | 1680 | 2341 | 1743 | 4085 | |
L-larvae Instar, Dry- Dry season, Wet- Wet season.
3.4. Vector snails
A total of 2292 fresh water snails were collected. Of these, 67.32 % (95%CI: 65.36–69.24) were collected during the dry season while 32.68 % (95 % CI: 30.76–34.64) were collected during the wet season. Overall, Biomphalaria pffeiferi, were significantly predominant snails across the dry and wet seasons (p < 0.001). During the dry season, majority of the Biomphalaria pffeiferi snails were collected in Murinduko 56.1 % (689) (95%CI: 53.24–58.86), followed by Thiba 35.7 % (439) (95%CI: 33.04–38.47), with Kiamaciri recording the least 8.2 % (101) (95%CI: 6.74–9.90). (Table 4).
Table 4.
Summary of snail vectors collected and their distribution in the three study zones.
| Murinduko | Snail species | dry season | wet season | Total |
|---|---|---|---|---|
| Biomphalaria (n, %) | 689 (81.2) | 219 (50.1) | 908 | |
| Bulinus (n, %) | 80 (9.4) | 52 (11.9) | 132 | |
| Other (n, %) | 80 (9.4) | 166 (38.0) | 246 | |
| Total (n, %) | 849 (100) | 437 (100) | 1286 | |
| Kiamaciri | Biomphalaria (n, %) | 101 (87.8) | 1 (4.3)) | 102 |
| Bulinus (n, %) | 11 (9.6) | 0 | 11 | |
| Other (n, %) | 3 (2.6) | 22 (95.7) | 25 | |
| Total (n, %) | 115 (100) | 23 (100) | 138 | |
| Thiba | Biomphalaria (n, %) | 439 (75.8) | 143 (49.4) | 582 |
| Bulinus (n, %) | 120 (20.7) | 73 (25.3) | 193 | |
| Other (n, %) | 20 (3.5) | 73 (25.3) | 93 | |
| Total (n, %) | 579 (100) | 289 (100) | 868 | |
| Total | 1543 | 749 | 2292 |
4. Discussion
This study presents baseline epidemiological and disease vector data for malaria and schistosomiasis in Mwea. The results show that there was no positive malaria case despite abundance of malaria vectors. Previous studies have reported decreased cases of malaria [31] in the study area., However, malaria is still perceived as the most common disease [45], attributed to the high densities of mosquitoes which are present throughout the year. These findings could be attributed to the ongoing malaria control efforts which includes regular mass distribution of LLINs [22] and the high uptake and use of the distributed LLINs at the household level [46].
Although there was no positive malaria case reported, high abundance of both adult and larval stages of malaria vectors were collected from all the three study zones. Previous studies in the area have also reported high abundance of the malaria vectors in the three study sites [47].
The mosquito species collected included Anopheles gambiae sensu lato (s.l.) and Anopheles funestus., known vectors for malaria transmission [48,49]. Culex quinquefasciatus, was reported occurring in abundance in the study area. This specie of mosquito has been incriminated in the spread of several arboviruses including Rift Valley fever virus, West Nile virus and Chikungunya fever virus [[50], [51], [52]]. These mosquito species Anopheles gambiae sensu lato (s.l.), Anopheles funestus and Culex quinquefasciatus are also known important vectors of bancroftian filariasis [53,54].
The number of mosquitoes collected did not vary across the dry and wet season, suggesting little variation in environmental conditions across the two seasons in the study area. Similar findings have been reported [55]. However, there was significant difference in species composition across the two seasons. More Anopheles were collected during the dry season with culicine mosquitoes being more during the wet season. Anopheles mosquitoes has previously been shown to persist during the dry season [56] which has been attributed to aestivation [57,58] and migration [59]. Anopheles larval were more abundant during the dry season than wet season. This could be explained by the fact that during the wet seasons, there is formation of favorable larval breeding sites which dries up during the dry season. Similar findings have been reported in Kenya [60]. The presence of these important disease vectors in Mwea could lead to future disease outbreaks, and thus the need for concerted initiatives to control them.
Our study shows that intestinal schistosomiasis is a public health problem in the area, with Kiamaciri zone reporting the highest rates of infection followed closely by Thiba zone. High prevalence of intestinal schistosomiasis across all age groups have been observed in the area [ 18] and among preschool going children [61]. Thiba zone is within the rice growing irrigation scheme where crop cultivation is carried out in a planned manner, and the water canals are managed by the irrigation scheme management. Kiamaciri zone is located outside the planned irrigation scheme where rice cultivation is uncontrolled and thus the water canals are solely managed by individual farmers. In these two zones, people reside in settlement aggregated villages and work in the expansive rice fields which are far from the homesteads. Water canals that serves the rice fields were shown to be important breeding habitats for both mosquito and snail vectors. Similar findings have been reported previously [62]. There are no toilets in the rice fields and thus there is possibility that open defecation in the rice canals is rampant. Open water bodies and fields have been shown to be particularly attractive sites for open defecation [63]. Studies have demonstrated that schistosome reproduces exponentially within the host snail, and therefore even small numbers of eggs entering freshwater may give rise to high risk of infection to people encountering the water [64]. The presence of ready breeding habitats for snail vectors, and the possibility of open defecation in the area may be the reason why the two zones reported high prevalence of intestinal schistosomiasis even when Kiamaciri had low densities of Biomphalaria snails.
Murinduko which reported only one case of intestinal schistosomiasis is not within the traditional rice growing belt of Mwea irrigation scheme. However, rice cultivation has been introduced along the river valleys which may lead to a rise in schistosomiasis transmission if no control measures are put in place. Interestingly, Murinduko zone reported the highest abundance of Biomphalaria pfeifferi snails, and also the highest number of snails that shed cercariae suggesting a potential high risk of disease outbreak. The cercariae that transmits intestinal schistosomiasis are diurnal and are released during daylight hours peaking during midday and dawn [42] corresponding to the time when people are actively in the rice farms. This therefore means that there is already active transmission of intestinal schistosomiasis in this zone, probably among the adult population who are more involved in rice cultivation. The low numbers of snails that shed cercariae in our study was consistent with previous studies carried out in Lake Victoria, Kenya [42,65], and in Uganda [66].
Our study shows abundance of important disease causing vectors (malaria, filariasis, schistosomiasis, arboviruses) causing vectors (mosquitoes and snails). The vectors shared similar breeding habitats and therefore which makes it feasible for implementation of an integrated vector control strategy to achieve greater results using minimal resources. It also provides a platform for inter sectoral and intra sectoral collaborations in tackling different diseases at the same time, and using minimal resources. Integrated vector management (IVM) focuses on utilizing preventative methods to control or eliminate vector populations in a sustainable manner [67,68]. It involves employment of a range of proven vector control methods, either alone or in combination where several methods may be used against a single disease, or a single or several methods may be used against several diseases [69]. It is a strategy that leads to sustainable control of the vectors and reduction in disease transmission. The strategy has previously been successfully implemented in Malindi Kenya [70] and Tanzania [71], and we recommend the same in the study area.
One of the limitations of this study was that we did not study the quality of water found at the snail and larvae breeding sites and its possible association with abundance. Also the seasonal mosquito and snail abundance was studied over one season cycle which may not be sufficient enough to provide robust data on the ecological activities of these vectors, which is an opportunity for further studies on the same.
5. Conclusion
This study demonstrates that intestinal schistosomiasis is a public health problem in Kiamaciri and Thiba zones. There is great risk of active schistosomiasis transmission in Murinduko zone owing to reported infective vector snails and a positive reported case. Murinduko zone has previously been non-endemic for intestinal schistosomiasis. The study also shows that there was no positive case of malaria in all the three study zones despite abundance in malaria vectors. Both malaria and intestinal schistosomiasis vectors predominates all the three study zones although they vary from within the small geographical areas. The disease vectors were found to be sharing the same breeding habitats and therefore an integrated approach towards the control of these vector borne disease will yield better results while ensuring minimal use of available resources.
Funding
This study was supported by funding from Kenya Medical Research Institute (KEMRI) Internal Research Grant (IRG) ref no: KEMRI/IRG/071/1. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Ethical approval
The study received ethical approval from the Kenya Medical Research Institute - Scientific and Ethics Review Unit reference number (KEMRI/SERU/CGMR-C/068/3468). Permission to conduct the study was obtained from Kirinyaga County Health Management Team, local administration, and village elders. Prior to the survey, stakeholders’ meetings were held at the county and sub-county levels, after which sensitization meetings were held with chief and assistant chiefs from all the three study zones. Village meetings were held at the villages led by the village elders. Written informed consent was obtained from all participants before they were enrolled in the study. All study participants who after testing were positive for intestinal schistosomiasis were treated with praziquantel (40 mg/kg), and those who turned positive for STHs were treated with albendazole (400 mg) after the study.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Paul M. Gichuki: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Lydia Kibe: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Conceptualization. Cassian Mwatele: Writing – review & editing, Writing – original draft, Investigation. Joseph Mwangangi: Writing – review & editing, Writing – original draft, Validation, Resources, Methodology, Formal analysis, Conceptualization. Charles M. Mbogo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Paul M. Gichuki, Email: paulmgichuki@gmail.com, pgichuki@kemri.go.ke.
Lydia Kibe, Email: lkibe@kemri.go.ke.
Cassian Mwatele, Email: cmwatele@kemri.go.ke.
Joseph Mwangangi, Email: jmwangangi@kemri.go.ke.
Charles M. Mbogo, Email: CMbogo@kemri-wellcome.org.
References
- 1.Nelwan M.L. Schistosomiasis: life cycle, diagnosis, and control. Curr. Ther. Res. Clin. Exp. 2019;91:5–9. doi: 10.1016/j.curtheres.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization https://www.who.int/news-room/fact-sheets/detail/schistosomiasis Schistosomiasis [Internet]. Fact-Sheets. 2022. Available from:
- 3.Savioli L., Albonico M., Colley D.G., Correa-Oliveira R., Fenwick A., Green W., et al. Building a global schistosomiasis alliance: an opportunity to join forces to fight inequality and rural poverty. Infect Dis Poverty [Internet] 2017;6(1):65. doi: 10.1186/s40249-017-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotez P.J., Alvarado M., Basáñez M.G., Bolliger I., Bourne R., Boussinesq M., et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8(7) doi: 10.1371/journal.pntd.0002865. pmid:25058013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GAHI . 2010. Global Atlas of Helminth Infections.http://www.thiswormyworld.org [Google Scholar]
- 6.WHO. Weekly epidemiological record. 2021;48(96):585–595. https://www.who.int/publications/i/item/who-wer9748-621-632 [Google Scholar]
- 7.Sultana M., Sheikh N., Mahumud R.A., Jahir T., Islam Z., Sarker A.R. Prevalence and associated determinants of malaria parasites among Kenyan children. Trop. Med. Health. 2017;45:25. doi: 10.1186/s41182-017-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Division of National Malaria Programme . Kenya Ministry of Health; Nairobi: 2022. Kenya Malaria Programme: Mid-term Review 2022. [Google Scholar]
- 9.Kinung’hi S.M., Magnussen P., Kaatano G.M., Kishamawe C., Vennervald B.J. Malaria and helminth co-infections in school and preschool children: a cross-sectional study in Magu district, North-Western Tanzania. PLoS One. 2014;9(1):1–8. doi: 10.1371/journal.pone.0086510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazigo H.D., Waihenya R., Lwambo N.J., Mnyone L.L., Mahande A.M., Seni J., et al. Co-infections with Plasmodium falciparum, Schistosoma mansoni and intestinal helminths among schoolchildren in endemic areas of northwestern Tanzania. Parasit Vectors. 2010;3:44. doi: 10.1186/1756-3305-3-44. PMID: 20482866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabatereine N.B., Standley C.J., Sousa-Figueiredo J.C., Fleming F.M., Stothard J.R., Talisuna A., et al. vol. 4. BioMed Central Ltd; 2011. p. 232. (Integrated Prevalence Mapping of Schistosomiasis, Soil-Transmitted Helminthiasis and Malaria in Lakeside and Island Communities in Lake Victoria, Uganda. Parasit Vectors). 10.1186/1756-3305-4-232 PMID: 22166365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abay S.M., Tilahun M., Fikrie N., Habtewold A. Plasmodium falciparum and Schistosoma mansoni coinfection and the side benefit of artemether-lumefantrine in malaria patients. J Infect Dev Ctries. 2013;7:468–474. doi: 10.3855/jidc.2658. 10.3855/jidc.2658 PMID: 23771290. [DOI] [PubMed] [Google Scholar]
- 13.Getie S., Wondimeneh Y., Getnet G., Workineh M., Worku L., Kassu A., et al. Prevalence and clinical correlates of Schistosoma mansoni co-infection among malaria infected patients, Northwest Ethiopia. BMC Res Notes. BioMed Central. 2015;8:480. doi: 10.1186/s13104-015-1468-2. 10.1186/s13104-015-1468-2 PMID: 26415939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokhna C., Le Hesran J.-Y., Mbaye P a, Akiana J., Camara P., Diop M., et al. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar. J. 2004;3:43. doi: 10.1186/1475-2875-3-43. 10.1186/1475-2875-3-43 PMID: 15544703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schistosomiasis W.H.O. World Health Organization; Geneva: 2013. Progress Report 2001–2011, Strategic Plan 2012–2020. [Google Scholar]
- 16.Mwandawiro C., Okoyo C., Kihara J., et al. Results of a national school-based deworming programme on soil-transmitted helminths infections and schistosomiasis in Kenya: 2012–2017. Parasites Vectors. 2019;12:76. doi: 10.1186/s13071-019-3322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson R., Farrell S., Turner H., et al. Assessing the interruption of the transmission of human helminths with mass drug administration alone: optimizing the design of cluster randomized trials. Parasites Vectors. 2017;10:93. doi: 10.1186/s13071-017-1979-x. 10.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gichuki P.M., Kepha S., Mulewa D., et al. Association between Schistosoma mansoni infection and access to improved water and sanitation facilities in Mwea, Kirinyaga County, Kenya. BMC Infect. Dis. 2019;19:503. doi: 10.1186/s12879-019-4105-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lelo A.E., et al. No apparent reduction in schistosome burden or genetic diversity following four years of school-based mass drug administration in mwea, central Kenya, a heavy transmission area. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo N.C., et al. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Infect. Dis. 2017;17:e64–e69. doi: 10.1016/S1473-3099(16)30535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO . WHO; Geneva): 2017. Field Use of Molluscicides in Schistosomiasis Control Programmes: an Operational Manual for Programme Managers. [Google Scholar]
- 22.Division of National Malaria Programme (DNMP) [Kenya] and ICF. 2021. Kenya Malaria Indicator Survey. DNMP and ICF; Nairobi, Kenya and Rockville, Maryland, USA: 2020. [Google Scholar]
- 23.Tiono A.B., Pinder M., N'Fale S., Faragher B., Smith T., Silkey M., et al. The AvecNet trial to assess whether addition of pyriproxyfen, an insect juvenile hormone mimic, to long-lasting insecticidal mosquito nets provides additional protection against clinical malaria over current best practice in an area with pyrethroid-resist. Trials. 2015;16:113. doi: 10.1186/s13063-015-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onchiri F.M., Pavlinac P.B., Singa B.O., Naulikha J.M., Odundo E.A., Farquhar C., et al. Low bacteremia prevalence among febrile children in areas of differing malaria transmission in rural Kenya: a cross-sectional study. J Pediatric Infect Dis Soc. 2016;5:385–394. doi: 10.1093/jpids/piv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingabire C.M., Hakizimana E., Kateera F., Rulisa A., Van Den Borne B., Nieuwold I., et al. Using an intervention mapping approach for planning, implementing and assessing a community-led project towards malaria elimination in the Eastern Province of Rwanda. Malar. J. 2016;15(1):594. doi: 10.1186/s12936-016-1645-3. pmid:27986094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng X.Y., Xia Z.G., Vong S., Yang W.Z., Zhou S.S. Surveillance and response to drive the national malaria elimination program. Adv. Parasitol. 2014;86:81–108. doi: 10.1016/B978-0-12-800869-0.00004-4. pmid:25476882. [DOI] [PubMed] [Google Scholar]
- 27.Wang X., Wang W., Wang P. Long-term effectiveness of the integrated schistosomiasis control strategy with emphasis on infectious source control in China: a 10-year evaluation from 2005 to 2014. Parasitol. Res. 2017;116(2):521–528. doi: 10.1007/s00436-016-5315-8. pmid:27812902. [DOI] [PubMed] [Google Scholar]
- 28.Global W.H.O. World Health Organization; Geneva: 2004. Strategic Framework for Integrated Vector Management.http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_PVC_2004_10.pdf WHO/CDS/CPE/PVC/2004.10. [Google Scholar]
- 29.Kenya National Bureau of Statistics . KNBS; Nairobi: 2009. Population and Housing Census. 2010. [Google Scholar]
- 30.Gichuki P.M., Kamau L., Njagi K., Karoki S., Muigai N., Matoke-Muhia D., Bayoh N., Mathenge E., Yadav R.S. Bioefficacy and durability of Olyset® Plus, a permethrin and piperonyl butoxide-treated insecticidal net in a 3-year long trial in Kenya. Infect Dis Poverty. 2021 Dec 20;10(1):135. doi: 10.1186/s40249-021-00916-2. PMID: 34930459; PMCID: PMC8691082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okech B.A., Mwobobia I.K., Kamau A., Muiruri S., Mutiso N., Nyambura J., et al. Use of integrated malaria management reduces malaria in Kenya. PLoS One. 2008;3(12) doi: 10.1371/journal.pone.0004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Malaria Control Programme (Nmcp) NMCP, KNBS, and ICF International; Nairobi, Kenya, and Rockville, Maryland, USA: 2016. Kenya National Bureau of Statistics (KNBS) and I, International. Kenya Malaria Indicator Survey 2015. [Google Scholar]
- 33.Mwandawiro C.S., Nikolay B., Kihara J.H., Ozier O., Mukoko D.A., et al. Monitoring and evaluating the impact of national school-based deworming in Kenya: study design and baseline results. Parasit Vectors. 2013;6:198. doi: 10.1186/1756-3305-6-198. pmid:23829767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gitonga C.W., Kihara J.H., Njenga S.M., Awuondo K., Noor A.M., et al. Use of rapid diagnostic tests in malaria school surveys in Kenya: does their under-performance matter for planning malaria control? Am. J. Trop. Med. Hyg. 2012;87(6):1004–1011. doi: 10.4269/ajtmh.2012.12-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gitonga C.W., Karanja P.N., Kihara J., Mwanje M., Juma E., Snow R.W., Noor A.M., Brooker S.J. Implementing school malaria surveys in Kenya: towards a national surveillance system. Malar. J. 2010;9:306. doi: 10.1186/1475-2875-9-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz N., Chavas A., Pellegrino J. A simple device for quantitative stool thick-smears technique in Schistosoma mansosni. Rev. Inst. Med. Trop. Sao Paulo. 1972;14:379–400. [PubMed] [Google Scholar]
- 37.Snell Amy E. Identification keys to larval and adult female mosquitoes (Diptera: Culicidae) of New Zealand. N. Z. J. Zool. 2005;32(2):99–110. doi: 10.1080/03014223.2005.9518401. [DOI] [Google Scholar]
- 38.Brown D.S. 2 edition. Taylor and Francis; London: 1994. Freshwater Snails of Africa and Their Medical Importance. [Google Scholar]
- 39.DBL-WHO: A Field Guide to African Freshwater Snails. Danish Bilharziasis Laboratory. WHO collaborating Centre for Applied Malacology; Charlottenlund, Denmark: 1998. [Google Scholar]
- 40.Gillies M.T., Coetzee M. vol. 55. Publication of the South Africa Institute of Medical Research; 1987. pp. 1–143. (A Supplement to Anophelinae of Africa South of Sahara (Afro-Tropical Region)). [Google Scholar]
- 41.Edwards F. British Museum (Nat. Hist.; London: 1941. Mosquitoes of the Ethiopian Region. III. Culicine Adults and Pupae. [Google Scholar]
- 42.Steinauer M.L., Mwangi I.N., Maina G.M., Kinuthia J.M., Mutuku M.W., Agola E.L., Mungai B., Mkoji G.M., Loker E.S. Interactions between natural populations of human and rodent schistosomes in the Lake Victoria region of Kenya: a molecular epidemiological approach. PLoS Negl Trop Dis. 2008;2:e222. doi: 10.1371/journal.pntd.0000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization . World Health Organization; Geneva: 1991. Basic Laboratory Methods in Medical Parasitology; pp. 25–28. [Google Scholar]
- 44.Montresor A., Crompton D.W., Hall A., Bundy D.A., Savioli L. World Heal Organ Geneva, Switz; 1998. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level : a Guide for Managers of Control Programmes.https://apps.who.int/iris/handle/10665/63821 [Internet] Available from: [Google Scholar]
- 45.Ng'ang'a P.N., Jayasinghe G., Kimani V., et al. Bed net use and associated factors in a rice farming community in Central Kenya. Malar. J. 2009;8:64. doi: 10.1186/1475-2875-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nyangi M., Kigondu E., Irungu B., et al. Integrity, use and care of long-lasting insecticidal nets in Kirinyaga County, Kenya. BMC Publ. Health. 2021;21:856. doi: 10.1186/s12889-021-10882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mwangangi J.M., Shililu J., Muturi E.J., Muriu S., Jacob B., Kabiru E.W., Mbogo C.N.M., Githure J., Novak R.J. Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malar. J. 2010;9:228. doi: 10.1186/1475-2875-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coetzee M., Fontenille D. Advances in the study of Anopheles funestus, a major vector of malaria in Africa. Insect Biochem. Mol. Biol. 2004;34:599–605. doi: 10.1016/j.ibmb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Ogola E.O., et al. Insights into malaria transmission among Anopheles funestus mosquitoes Kenya. Parasit. Vectors. 2018;11:577. doi: 10.1186/s13071-018-3171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goddard L.B., Roth A.E., Reisen W.K., Scott T.W. Vertical transmission of West Nile virus by three California Culex (Diptera: Culicidae) species. J. Med. Entomol. 2003;40:743–746. doi: 10.1603/0022-2585-40.6.743. [DOI] [PubMed] [Google Scholar]
- 51.Lutomiah J.L., Koka H., Mutisya J., Yalwala S., Muthoni M., Makio A., Limbaso S., Musila L., Clark J.W., Turell M.J., et al. Ability of selected Kenyan mosquito (Diptera: Culicidae) species to transmit West Nile virus under laboratory conditions. J. Med. Entomol. 2011;48:1197–1201. doi: 10.1603/me11062. [DOI] [PubMed] [Google Scholar]
- 52.LaBeaud A.D., Banda T., Brichard J., Muchiri E.M., Mungai P.L., Mutuku F.M., Borland E., Gildengorin G., Pfeil S., Teng C.Y., et al. High rates of o’nyong nyong and Chikungunya virus transmission in coastal Kenya. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derua Y.A., Rumisha S.F., Batengana B.M., Max D.A., Stanley G., Kisinza W.N., Mboera L.E.G. Lymphatic filariasis transmission on Mafia Islands, Tanzania: evidence from xenomonitoring in mosquito vectors. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mwangangi J.M., Midega J., Kahindi S., Njoroge L., Nzovu J., Githure J., Mbogo C.M., Beier J.C. Mosquito species abundance and diversity in Malindi, Kenya and their potential implication in pathogen transmission. Parasitol. Res. 2012;110:61–71. doi: 10.1007/s00436-011-2449-6. [DOI] [PubMed] [Google Scholar]
- 55.Mayi M.P.A., Bamou R., Djiappi-Tchamen B., Fontaine A., Jeffries C.L., Walker T., Antonio-Nkondjio C., Cornel A.J., Tchuinkam T. Habitat and seasonality affect mosquito community composition in the west region of Cameroon. Insects. 2020 May 15;11(5):312. doi: 10.3390/insects11050312. PMID: 32429075; PMCID: PMC7291174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magombedze G., Ferguson N.M., Ghani A.C. A trade-off between dry season survival longevity and wet season high net reproduction can explain the persistence of Anopheles mosquitoes. Parasites Vectors. 2018;11:576. doi: 10.1186/s13071-018-3158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adamou A., Dao A., Timbine S., Kassogué Y., Diallo M., Traoré S.F., et al. The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar. J. 2011;10:151. doi: 10.1186/1475-2875-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehmann T., Dao A., Adamou A., Kassogue Y., Diallo M., Sékou T., et al. Aestivation of the african malaria mosquito, Anopheles gambiae in the sahel. Am. J. Trop. Med. Hyg. 2010;83:601–606. doi: 10.4269/ajtmh.2010.09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dao A., Yaro A., Diallo M., Timbiné S., Huestis D., Kassogué Y., et al. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nature. 2014;516:387–390. doi: 10.1038/nature13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mala A.O., Irungu L.W., Shililu J.I., Muturi E.J., Mbogo C.C., Njagi J.K., Githure J.I. Dry season ecology of Anopheles gambiae complex mosquitoes at larval habitats in two traditionally semi-arid villages in Baringo, Kenya. Parasit Vectors. 2011 Feb 28;4:25. doi: 10.1186/1756-3305-4-25. PMID: 21352608; PMCID: PMC3060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sifuna Wefwafwa Sakari Stephen, Mbugua Amos K., Mkoji Gerald M. Prevalence of soil-transmitted helminthiases and schistosomiasis in preschool age children in mwea division, Kirinyaga South district, Kirinyaga county, and their potential effect on physical growth. J. Trop. Med. 2017;2017:12. doi: 10.1155/2017/1013802. Article ID 1013802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loker E.S., Hofkin B.V., Mkoji G.M., Mungai B., Kihara J., Koech D.K. Distributions of freshwater snails in southern Kenya with implications for the biological control of schistosomiasis and other snail-mediated parasites. J Med Appl Malacol. 1993;5:1–20. [Google Scholar]
- 63.Tayo M.A., Pugh R.N.H., Bradley A.K. Malumfashi endemic diseases Research project, XI: water-contact activities in the schistosomiasis study area. Ann. Trop. Med. Parasitol. 1980 doi: 10.1080/00034983.1980.11687351. [DOI] [PubMed] [Google Scholar]
- 64.Secor W.E. 2012. The Effects of Schistosomiasis on HIV/AIDS Infection, Progression and Transmission. Current Opinion in HIV and AIDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Opisa S., Odiere M.R., Jura W.G., et al. Malacological survey and geographical distribution of vector snails for schistosomiasis within informal settlements of Kisumu City, western Kenya. Parasites Vectors. 2011;4:226. doi: 10.1186/1756-3305-4-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Standley C.J., Adriko M., Arinaitwe M., Atuhaire A., Kazibwe F., Fenwick A., Kabatereine N.B., Stothard J.R. Epidemiology and control of intestinal schistosomiasis on the Sesse Islands, Uganda: integrating malacology and parasitology to tailor local treatment recommendations. Parasit Vectors. 2010;3:64. doi: 10.1186/1756-3305-3-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Who . 2014. A Global Brief on Vector-Borne Diseases. [Google Scholar]
- 68.Upadhyay A.K., Maansi Parmar T., Singh P., Pathak A.P. Vector-borne zoonotic diseases. J Anim Health Behav Sci. 2018;2:1–6. Benelli G., Jeffries L. C., Walker T. (2016): Biological Control of Mosquito Vectors: Past, Present, and Future. insects 7: 1-18. [Google Scholar]
- 69.Oda Gizaw, Gebisa Gelaye, Bogale Biruk, Wondosen Zewdu, Geda Regassa. Control of vector borne diseases of public health importance: the role of integrated vector management and indigenous knowledge. J. Nat. Sci. Res. 2019;9(22) www.iiste.org ISSN 2224-3186 (Paper) ISSN 2225-0921 Online. [Google Scholar]
- 70.Mutero C.M., Mbogo C., Mwangangi J., Imbahale S., Kibe L., Orindi B., Girma M., Njui A., Lwande W., Affognon H., Gichuki C., Mukabana W.R. An assessment of participatory integrated vector management for malaria control in Kenya. Environ. Health Perspect. 2015 Nov;123(11):1145–1151. doi: 10.1289/ehp.1408748. Epub 2015 Apr 10. PMID: 25859686; PMCID: PMC4629737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaki P.P., Dongus S., Fillinger U., Kelly A., Killeen G.F. Community-owned resource persons for malaria vector control: enabling factors and challenges in an operational programme in Dar es Salaam, United Republic of Tanzania. Hum. Resour. Health. 2011;921 doi: 10.1186/1478-4491-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.