Abstract
Purpose of review:
This review focuses on the cerebrospinal fluid (CSF) findings in connection to the central nervous system (CNS) reservoir in treatment-naïve and virally-suppressed PLWH, followed by the findings in CSF HIV −1 escape and analytical treatment interruption studies.
Recent Findings:
Compared to chronic infection, initiating antiretroviral therapy (ART) during acute HIV-1 infection results in more homogeneous longitudinal benefits in the CNS. Viral variants in CSF HIV-1 escape are independently linked to infected cells from the systemic reservoir and in the CNS, highlighting the phenomenon as a consequence of different mechanisms. HIV-infected cells persist in CSF in nearly half of the individuals on stable ART and are associated with worse neurocognitive performance.
Summary:
Future studies should probe into the origin of the HIV-infected cells in the CSF. Examining the capacity for viral replication would provide new insight into the CNS reservoir and identify strategies to eradicate it or compensate for the insufficiency of ART.
Keywords: HIV cure, central nervous system, compartmentalization, antiretroviral therapy, HIV reservoir
Introduction
Although modern antiretroviral therapy (ART) effectively suppresses HIV-1 replication and restores considerable T-cell immunity in people living with HIV (PLWH), ART is inadequate to eradicate the infection due to the persistence of HIV-1 in the cellular reservoir. Within days after ART interruption, viral replication reignites with measurable plasma HIV-1 RNA, highlighting the need for consistent ART adherence to maintain HIV-1 suppression. Initially, a small subset of CD4+ resting T-cells was considered the main source of the HIV-1 latent reservoir. Years of research confirms that HIV-1 persists in multiple subsets of T-lymphocytes [1] and potentially other cell types that reside in various anatomical compartments, including the lymph nodes, bone marrow, gut and the central nervous system (CNS) [2]. Similar to other compartments, HIV-1 DNA remains detectable in the brain tissue despite years of suppressive ART [3, 4]. Further, the CNS is distinct from other compartments because of the presence of the blood brain barrier (BBB) that partially limits the entry of antiretroviral agents, a unique immune-surveillance system that limits the trafficking of immune cells [5], and most importantly, the presence of long-lived glial cells susceptible to HIV-1 infection and supportive of replication. These characteristics make the CNS a potential sanctuary and reservoir of HIV-1.
The benefit of ART is incomplete in the CNS. HIV-1 RNA escape in the cerebrospinal fluid (CSF), defined by a measurable CSF HIV-1 RNA level during plasma HIV-1 suppression, or a CSF HIV-1 RNA level higher than that in plasma in the context of partially-effective treatment [6], occurs in 5–20% of PLWH on ART [7–10]. Although CSF HIV-1 escape is relatively uncommon, frequently transient, and mostly asymptomatic, it can manifest with clinical complications including cognitive decline and even overt encephalitis. More importantly, a spectrum of neurological and neuropsychiatric symptoms (currently termed HIV-associated neurocognitive disorder, or HAND) manifests in some PLWH despite stable ART [12]. Those with a longer duration of infection and worse pre-ART immunodeficiency, denoted by a lower nadir CD4+ T-cell level, are at a higher risk of cognitive impairment. It has recently been appreciated that HIV-infected cells are detected in the CSF in almost half of the people living with HIV (PLWH) on long-term ART and their detection in the CSF is associated with worse neurocognitive performance [11]. These observations raise the question of whether the pool of HIV-1 infected cells in the CNS continues to impact long-term neurological outcomes despite plasma viral suppression.
In addition to the pragmatic need to achieve HIV-1 cure, understanding the cellular reservoirs of HIV-1 in the CNS provides important insight towards the persistence of HAND and CSF HIV-1 escape. Moreover, this understanding will help stratify the neurological risk of intervening agents in cure studies and during subsequent analytical treatment interruption (ATI) that tests for HIV-1 remission. Unfortunately, conventional brain imaging does not reveal the activity of cellular reservoirs of HIV-1. Repeated brain biopsy for research is unquestionably impractical, making CSF examination the only means to examine the HIV-1 reservoir in the CNS in living humans. Though CSF is produced within the brain by the choroid plexus, components within the CSF may reflect products originating in the blood as well as components derived from brain and other CNS tissue (meninges, spinal cord). Comparing plasma and CSF samples at different points of infection thus offers a unique opportunity to interrogate the process of HIV-1 compartmentalization in the CNS. The timing of CSF sampling is important for outcome interpretation. In early infection before the presence of a productive source infected cells in the brain, HIV-1 RNA in the CSF mainly originates from the free virus or infected CD4+ T-cells transmigrating from the circulation. During chronic infection, infected CNS cells, namely, perivascular macrophages, microglia [13], and possibly astrocytes [14] and tissue resident CD4+ T-cells are an additional source of infected cells in CSF. This review focuses on the CSF findings in connection to the CNS reservoir in treatment-naïve and virally-suppressed PLWH, followed by the findings in CSF HIV-1 escape and ATI studies.
Examining CNS Compartmentalization of HIV-1 through pre-ART CSF Samples
HIV-1 invades the CNS within days after transmission. HIV-1 RNA is measurable in over 90% of CSF samples collected during later Fiebig stages (III-V) of acute HIV infection (AHI) [15]. Identification of HIV-1 quasispecies in the CSF that are absent in the plasma provides evidence of local HIV-1 replication and hence compartmentalization within the CNS. In a deep sequencing study based on paired samples collected during AHI, different proportions of mostly minor variants were only detected in participants infected with multiple Transmitted/Founder viruses [16]. In contrast, phylogenetic studies identified unique quasispecies in up to 20% of CSF samples during primary HIV infection (PHI, i.e. within the first year of infection), indicating the presence of CNS compartmentalization [17–19]. Of note, compartmentalized quasispecies in CSF studies of early infection are all R5 T-cell tropic in origin [18]. This is different from the frequent detection of macrophage-tropic HIV-1 populations in PLWH of later stages of chronic infection or with HIV-associated dementia (HAD) [20]. These studies indicate that CNS compartmentalization emerges within the first years after HIV acquisition, likely reflecting local infection established in CNS cells over time, paralleling early disease progression.
Comparing the viral load in plasma and CSF throughout the course of infection evaluates the viral dynamics from a quantitative perspective. In untreated infection, the level of HIV-1 RNA in plasma is generally higher than that of CSF but the difference in HIV-1 RNA levels reduces over time. Numerically, the median viral load differences were 2.36 log10 (copies/ml) during AHI [15], 1.77 log10 during PHI, and one log10 or below during chronic infection [21]. While plasma invariably has a higher level of HIV-1 RNA than CSF during AHI, inter-individual variation exists and around 6% of individuals showed a reduced viral load difference <1 log10 during acute HIV [15], highlighting an increased transmigration of HIV-1 from the systemic circulation into the intrathecal space. Further, CSF HIV-1 RNA is higher than that in plasma in up to 13% of paired samples collected after AHI. The phenomenon is rare during PHI (1%) but becomes more common upon disease progression, ranging from 10% during chronic infection to 30% in HAD. While it is plausible that the phenomenon links to an accelerated HIV-1 replication and thus an expanded reservoir within the CNS, the underlying mechanism remains elusive. One study evaluated the viral characteristics in CSF in HAD during which HIV-1 replication within the CNS is most abundant [20]. It found that genetically compartmentalized R5 T cell-tropic and macrophage-tropic HIV-1 populations were independently detected in the CSF of subjects diagnosed with HAD. Closer inspection reveals different features of compartmentalization between the two variants of different tropism. The R5 T cell-tropic variants were clonally amplified and associated with CSF pleocytosis, whereas macrophage-tropic variants were genetically diverse and presumably represented infection among CNS-resident cells [20].
Examining the chronological changes of immune activation markers in the CSF may hint at the evolution of HIV-1 CNS compartmentalization. A multi-centre study measuring 10 functionally diverse soluble inflammatory biomarkers in paired blood and CSF samples revealed a broadly compartmentalized inflammatory response in the CSF throughout the course of infection [22]. The immunological response consisted of two distinctive clusters of immune activation markers. During early infection with robust lymphocytic activation, there was a distinctive clustering of immune activation markers that consisted of CSF TNF-α, MMP9, CXCL10, sCD14 and neopterin. The cluster of markers rose rapidly during PHI, peaked at blood CD4+ T-cell levels of 200–350 cells/ul, and fell steadily thereafter, forming an inverted U-shape pattern. The second cluster of markers were associated with monocyte/macrophage activation and consisted of sCD163 and CCL2. This cluster of markers rose steadily since early infection throughout the decline of CD4+ T-cells and were better associated with the degree of neuronal damage, represented by the level of CSF neurofilament light chain protein (NfL), than others. Intriguingly, HAD was associated with an exponential elevation in both clusters of markers that are well above the measurements in neuroasymptomatic individuals with comparable CD4+ T-cell level. The findings indicates that HAD is a distinctive inflammatory disorder with extensive activation of T-cells and monocytes/microglia.
To summarize, HIV-1 compartmentalization in the CNS can emerge within the first year after acute infection but inter-individual variation exists. Intrathecal inflammation during early infection is predominantly driven by T-cell activation that declines upon more severe CD4+ T-cell depletion. Immune responses driven by monocyte/macrophage lineage progressively heightens and parallels disease progression. The evolution of immune activation markers perhaps also coincide with the transition of HIV-1 tropism (macrophage- vs. T-cell tropism) and their cellular targets within the CNS. During early infection, R5 T-cell tropic virus accounts for the transmitted/founder viruses and most circulating virus. R5 T-cell tropic viruses can effectively infect CD4+ T-cell high expression of CD4 and CCR5 surface co-receptors. Macrophage-tropic variants arise in advanced HIV-1 infection in T-cell depleted environments such as in the CNS. Macrophage-tropic variants are more capable of infecting and replicating in myeloid cells with low CD4 expression on the surface. The factor of viral tropism thus highlights that HIV compartmentalization in the CNS is a dynamic process that extends beyond PHI into later infection, evolving over time. Most importantly, the independent association between HAD and clonally amplified R5 T-cell tropic variants and genetically diverse macrophage-tropic variants [20] points to the possibility that HIV encephalitis, and potentially HAND, may be driven through different mechanisms involving changes in viral tropism and targets of infection (Figure 1).
Figure 1. Outcomes of CSF Studies in Connection with the Timing of ART Initiation.
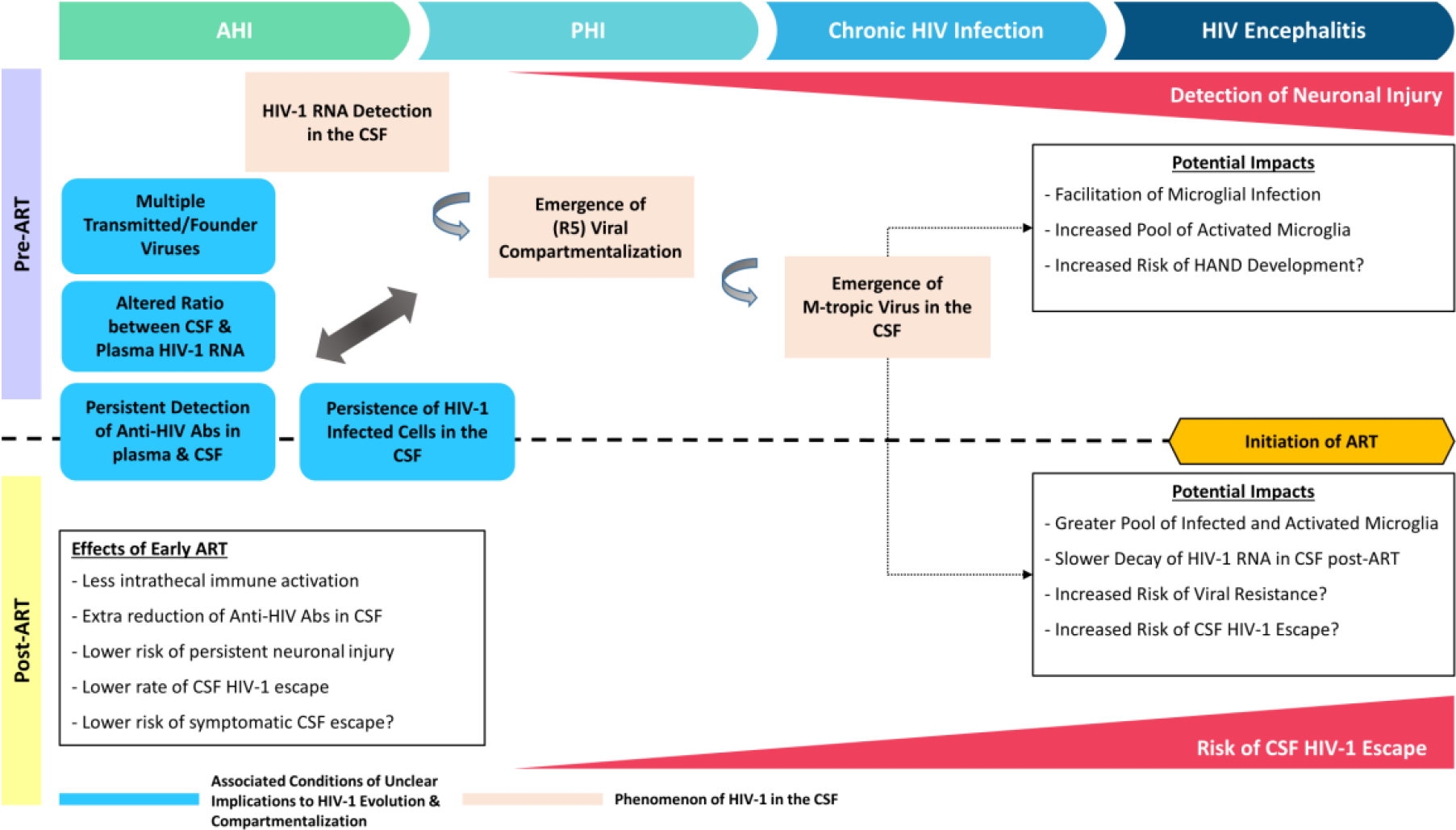
This schematic diagram illustrates neurologic and virologic observations in people living with HIV (PLWH) before and after antiretroviral therapy (ART). Elevation of markers of neuronal injury in the cerebrospinal fluid (CSF) is observed in a subset of untreated PLWH during the first year of HIV infection and becomes more frequent towards chronic infection. In addition, compartmentalization of R5 T-cell tropic HIV-1 can be observed in the CSF during the first year of infection, whereas the emergence of macrophage-tropic HIV-1 in CSF occurs during more advanced infection. To date, early initiation of ART is associated with less intrathecal inflammation, lower levels of anti-HIV antibodies and neuronal injury marker in the CSF, and reduced risk of CSF viral escape as compared to treatment initiated in later stage infection. A number of conditions (boxes in blue) may impact neurological outcomes, including infection with multiple transmitted/founder HIV-1 variants and an altered ratio of HIV-1 RNA between CSF pre-ART, and levels of anti-HIV antibodies and detection of HIV-infected cells in the CSF post-ART.
The Neurological Benefit of Early ART
Immediate ART initiation significantly reduces the mortality and morbidities in PLWH with chronic infection [23]. However, the neurological benefit of immediate ART during chronic infection is less clear. The neurology sub study of the START trial reported no neurological advantage for initiating ART at CD4+ T-cells level >500cells/ul compared to a deferred treatment until CD4+ T-cells <350cells/ul [24]. Despite stable viral suppression, elevations of CSF immune activation and neuronal damage markers are often observed in PLWH who initiated ART during chronic infection (Table 1). In one study, over 40% of virally suppressed participants showed elevated NfL in the CSF, suggesting an ongoing neuronal injury despite ART [25]. Another study reported an elevated concentration of CSF neopterin in 50% of PLWH despite over 10 years of effective ART [26]. Of note, the presence of macrophage-tropic variants in the CSF may contribute to a protracted course of intrathecal inflammation in treated PLWH due to slower decay in the CSF HIV-1 RNA [20]. Whether a low grade but persistent elevation of CSF neopterin is benign remains questionable. In CNS positron emission tomography (PET) studies based on macrophage/microglia-specific ligand, virally suppressed, neuroasymptomatic PLWH demonstrated persistent microglial activation compared to HIV-uninfected control [27–29]. In addition, the intensity of microglial activation was associated with worse cognitive performance.
Table 1.
CSF Features of HIV-1 Infection Before and After ART
| Acute HIV Infection | Primary HIV Infection | Chronic HIV Infection | ||||
|---|---|---|---|---|---|---|
| Pre-ART | Post-ART | Pre-ART | Post-ART | Pre-ART | Post-ART | |
| Neopterin Elevation | √ | X | √ | Absent/Rare | √ | √ |
| NfL elevation | X | X | ~40% | Unknown | 10–75% | 16% |
| HIV-1 Antibodies | Unknown | Unknown | √ | √, 43-fold reduction from pre-ART | √ | √, 3- to 6- fold reduction from pre-ART |
| Evidence of HIV-1 Compartmentalization | X | N/A | Uncommon | N/A | V | N/A |
| ΔHIV-1 RNA between Plasma & CSF (log10 cps/ml) | ≤2 | N/A | 1–2 | N/A | 1 or below | N/A |
| CSF HIV-1 Escape | N/A | Rare (1%) | N/A | Unknown | N/A | 5–20% |
Abbreviations: ART: antiretroviral therapy; CSF: cerebrospinal fluid; NfL: neurofilament light chain; N/A: not available
Compared to incomplete results of treatment in chronic infection, ART initiation within the first year of infection results in consistently better neurological outcomes. PLWH who initiated ART during AHI show stable cognitive performance years after ART [30, 31]. CSF neopterin, CXCL10, CCL2, and interleukin 6 were within the normal range 96 weeks after ART initiation during AHI [32]. The level of CSF NfL remained normal before and after ART initiation during AHI [25]. One study compared the levels of anti-HIV Abs in CSF and blood before and after ART. Suppressive ART in chronic infection resulted in modest and parallel decreases (3- to 6-fold) in antibody levels in CSF and blood, compared to 43- and 7-fold lower levels respectively if ART was initiated within the first year of infection [33]. Notably, the antibody levels in CSF and blood in treated chronic infection are similar to untreated early infection [33]. Generally speaking, antibody response corresponds to the levels of viral antigens. The diminished reduction of antibody response in post-ART chronic infection indicates a greater production and presentation of viral antigens, likely viral proteins, by the corresponding systemic and CNS reservoir, in line with the observation that HIV reservoir persistently produces HIV-encoded proteins including transactivator of transcription (Tat), negative factor (Nef) and envelope protein gp120 through low-level transcription during plasma viral suppression [34–36]. Conversely, the additional reduction of the antibody level in the CSF with respect to the blood implies that the CNS reservoir is disproportionally less-established than the systemic reservoir during early infection. Indeed, CSF HIV-1 escape, a phenomenon believed to be a consequence of HIV-1 release from the CNS reservoir, is rare if ART is initiated during AHI. The rate of CSF HIV-1 escape is 1% in individuals who started ART during AHI [37], compared to 5–20% in chronic infection [7–10].
What Have We Learned from CSF HIV-1 Escape by far?
The phenomenon of CSF HIV-1 escape was initially reported in an individual who presented with acute meningoencephalitis despite stable ART and ongoing plasma viral suppression [38]. Subsequent studies informed the infrequent presence of CSF HIV-1 escape, with and without neurological symptoms, in PLWH [8, 39–42]. Symptomatic escape can present acutely or chronically [41], with a diverse spectrum of neurological complications from headache, cognitive decline, to overt encephalitis and coma [41]. In a large series of over 100 cases of CSF HIV-1 escape, cognitive decline was the most common manifestation in symptomatic escape of chronic presentation [41]. The rate of asymptomatic CSF HIV-1 escape ranges from 5–20% in different studies [7–10]. In a longitudinal study with repeated CSF sampling (median number of and duration between lumbar punctures: 5 and 374 days), 36% of neurologically asymptomatic PLWH on stable ART demonstrated at least one episode of detectable HIV-1 RNA in the CSF [42]. Persistent CSF HIV-1 escape, defined by a repeated detection of HIV-1 RNA in the CSF without a concurrent blip in plasma, was rarely found in 3% of participants [42]. Both symptomatic and asymptomatic CSF HIV-1 escape appear to share a similar set of risk factors, including prolonged duration of HIV-1 infection, low CD4+ T-cell nadir <200cells/μL, persistent low-level plasma viremia, poor ART adherence, and pre-existing ART resistance with sub-optimal ART regimens such as simplified ART regimens or regimens with low CNS penetration effectiveness (CPE) [10, 43].
Asymptomatic viral escape is generally considered benign and the current consensus is to modify the ART only in the context of symptoms [6]. Whether asymptomatic CSF escape connects to symptomatic escape or other adverse neurological outcomes remains uncertain. In a cross-sectional, multi-cohort study, the rates of cognitive impairment were similar between participants with or without CSF viral escape [10]. In another study, however, the rates of symptomatic cognitive impairment were 35% and 20% in participants with and without CSF viral escape, respectively [9]. Studies based on single-copy assay similarly reported an association between detectable CSF HIV-1 RNA and worse cognitive performance or HAND [44, 45]. Moreover, asymptomatic CSF escape is associated with higher levels of neopterin [8, 42], CXCL10(IP-10) [10, 46], and several inflammatory markers [46]. Lymphocytic pleocytosis and elevated CSF protein level are common in CSF HIV-1 escape [10]. Of note, CD8+ T-cells are the predominant cell type contributing to lymphocytic pleocytosis in symptomatic escape [47]. Nearly 70% of reported cases of CD8 encephalitis, a potentially life-threatening condition that requires immediate medical attention, is associated with CSF HIV-1 escape [48], suggesting an overlap between the two conditions pathologically.
The origin of CSF HIV-1 escape viruses and their relationship with ART regimens are areas under active investigation. Due to observed associations between protease inhibitor (PI) use and viral resistance of CSF HIV-1 escape strains, reduced ART concentration in the CNS due to limited drug penetration has been considered a facilitator of the development of CSF viral escape. A recent study examining the cellular source of escape viruses and their relationship with ART concentration in the CSF [49] did not observe an association between reduced ART concentration in the CSF and CSF HIV-1 escape. CD26 T-cell host surface marker was detected in the CSF HIV-1 escape viruses, implying that at least a subset of these came from infected CD4+ T-cells [49]. Another study examined the relationship between viral tropism and persistent CSF escape by examining the sequence of the viral envelope [50]. Among the three participants with CSF viral escape and serial CSF sampling, one participant showed persistent CSF escape. The CSF HIV-1 escape variants in that participant were macrophage-tropic, partially drug-resistant, and genetically diverse. Phylogenetic analysis suggested that the macrophage-tropic population originated from a minor M-tropic lineage pre-existing in the blood prior to ART. Another two only had transient CSF escape and the CSF HIV-1 escape virus demonstrated R5 T-cell tropism of little diversity [50], implying a recent clonal expansion without significant evolution of virus. These variants in CSF HIV-1 escape indicate distinct associations with R5 T-cell and macrophage-tropic viral replication, suggesting diverse pathogenic origins.
CSF Analysis in HIV Intervention and Treatment Interruption Trials
Given vulnerability of the CNS, neurological safety is one of the key concerns in HIV-1 intervention trials. In one non-human primate (NHP) study, despite ongoing suppressive ART, one out of two SIV-infected macaques developed SIV encephalitis after the administration of Ingenol-B and vorinostat as latency-reversing agents (LRAs) [51]. Subsequent examination revealed a CSF viral load 10-fold higher than that in plasma, resembling symptomatic CSF HIV-1 escape [51]. Post necropsy investigations suggested that CSF viral transcripts originated from resident CD68+ macrophages/microglia. Further, replication-competent virus was isolated from infected brain macrophages in seven out of eight macaques under similar experimental conditions [52]. The finding proves the concept that different compartments and cell-types may respond differently to intervening agents. In another in-vitro study, HIV-1 variants isolated from the CSF demonstrated greater resistance to broadly neutralizing antibodies (bNAbs) than their blood counterpart in certain PLWH with advanced disease [53]. These outcomes highlight a practical need of CSF sampling in intervention studies in order to understanding the effects across reservoirs. These studies indicate the importance of testing for CSF HIV-1 escape even if plasma viral suppression is sustained post-intervention.
Several NHP studies examined the neurological outcomes in analytic treatment interruption (ATI) studies without intervention. In one study, ATI in five macaques that initially received ART during early simian-human immunodeficiency virus (SHIV) infection was associated with mild, localized T-cell infiltration in the brain at 12 weeks after viral rebound [54]. However, it was not associated with detectable SHIV-RNA in the brain parenchyma or CSF, nor elevation of CSF immune activation markers [54]. In contrast, ATI in simian immunodeficiency (SIV)-infected macaques in another study was associated with a progressive increase in the mean standardized uptake values (SUV) in FDG-based positive emission tomography (PET) and a general increase in CSF cytokines over a 6-month period [55]. The values of SUVmean and SUVmax were positively associated with CSF viral load. Early ART initiation or use of a SHIV rather than a more neurovirulent SIV strain in the former study may have limited the establishment of the CNS reservoir and led to a milder inflammatory response during treatment interruption and viral rebound.
In human HIV-1 infection, plasma HIV-1 RNA invariably rebounds within days to weeks after ART interruption [56–58]. Neither commencing ART during early infection nor a smaller HIV-1 reservoir size pre-ATI significantly defers the timing of plasma viral rebound [56, 57, 59]. Comparing the sequence between HIV-1 strains from plasma and different anatomical tissues suggested that the rebound HIV-1 could originate from a number of cellular and anatomical compartments instead of a central or single source [60]. Of note, the viral load in the rebound CSF samples in studies of ATI after early treatment has been insufficient for sequencing [60]. Another ATI study identified a minor population of macrophage-tropic HIV-1 variant from the rebound HIV-1 in plasma [61]. A proportion of the macrophage-tropic viruses responded to CD14 macrophage-specific antibodies but not to T-cell specific CD3 antibodies, signifying a macrophage origin of the variants. Molecular clock analysis indicated the existence of these variants prior to ART interruption, implying the persistence of replication-competent virus in infected macrophages despite suppressive ART, though the tissue distribution of these macrophages is unknown.
To date, ATI with a brief period of low-level plasma viremia (<1000 copies/ml) followed by ART resumption appears to be safe. Serious adverse events and emergence of ART resistance have been absent [57–59, 62]. After ART resumption, the HIV-1 reservoir size and plasma immune activation markers returned to their pre-ATI levels in 6–12 months [57, 59, 62, 63]. Similarly, irreversible neurological changes were absent in brief ATI trials [63, 64]. A study that combined neurological outcomes from 4 ATI trials with or without intervention reported insignificant change in neurocognitive performance, levels of CSF immune activation markers, and diffusion tensor imaging (DTI) parameters before and after ATI. In this study the only detected CNS perturbation after ATI was a mild elevation of choline level in the basal ganglia in magnetic resonance spectroscopy (MRS) [64]. Another study reported unchanged CSF markers of neuronal injury and neuroinflammation (NfL and YKL-40 respectively) at the time of viral rebound [63]. Of note, no ATI studies to date have report HIV-1 rebound in the CSF ahead of the plasma. Taken together, recent ATI studies with close monitoring of plasma HIV-1 RNA level and strict criteria of ART resumption appear to be uneventful for the nervous system in small studies. However, the design of these studies do not inform as to whether viral replication in infected CNS cells or neuroinflammatory processes might reignite in the setting of prolonged interruption of ART.
Perspective: Bridging the Knowledge Gaps in Understanding CNS HIV-1 Persistence and Reservoirs
CSF studies from the past years confirm early invasion of HIV-1 into the CNS during acute infection. However, the timing of viral compartmentalization in the CNS varies individually, evident in up to 20% of people during the first two years of infection and increasing over time. The emergence of macrophage-tropic variants in the CNS, a feature of more advanced infection, is perhaps another important milestone of compartmentalization of clinical importance. Macrophage-tropic variants are presumed to have predilection to infect CNS resident cells of monocyte/macrophage lineage, potentially accelerating the degree of neuronal injury in ART-naïve PLWH. In this setting, the consequential larger viral CNS reservoir may retard the decay of CSF HIV-1 RNA after ART initiation due to the limited access of ART into the CNS. Whether the emergence of macrophage-tropic variants contributes to the diminished reduction in anti-HIV antibodies in the CNS, persistent elevation of CSF immune activation and neuronal injury markers, and higher risk of CSF viral escape remains to be determined.
Nonetheless, examination of the variant populations in CSF HIV-1 escape has indicated that T-cell derived HIV-1 [49, 50] independently contributes to HIV-1 escape. Phylogenetic analysis revealed that variant populations were R5 T-cell tropic with little genetic diversity, suggesting that the escape virus was produced by trafficking and transient expansion of infected T-cells in the CNS [50]. The findings highlight the possibility that continuous trafficking of infected cells between the CNS and other reservoirs continues during ART. Importantly, recent studies assessing for cell-associated HIV-1 DNA demonstrated that HIV-infected cells can be detected in CSF in almost half of virally-suppressed individuals [11, 65]. Moreover, the median value of cell-associated DNA was 2.1 copies per thousand cells in CSF, versus 0.5 copies per one thousand CD4 T-cells in PBMC [11]. The unexpectedly high level of cell-associated HIV-1 DNA in the CSF questions if there is facilitated trafficking of HIV-infected cell into the CNS, or a local CNS source maintaining infection in these cells. Future studies should examine whether early ART reduces the frequency of detectable HIV-infected cell in CSF, and probe their identity, characterize the proviruses found in CSF cells, and examine their potential relationship with CSF HIV-1 escape.
In recent years, the application of single-cell technology offers an unprecedented opportunity to analyse cellular properties. Single-cell RNA sequencing (scRNA-seq) interrogating transcriptomic profiles at a single-cell level provides not only unique insight into rare cells types but also quantifies the cellular subsets within samples. For example, a scRNA-seq study revealed a rare microglia-like cell type in CSF in virally suppressed PLWH. This rare subset of cells showed genetic signatures similar to neurodegenerative disease-associated microglia [66], highlighting the usefulness of this surface-marker-free approach to potentially identify important novel cell subsets, including those that may harbor HIV infection in CSF. PET imaging using anti-HIV antibody-based radiomarkers also has the potential to improve our understanding of the distribution of the HIV-1 reservoir. While a recent pilot study using 64Cu-radiolabeled antibody was unable to detect HIV-1 env expression in vivo [67], another study detected correlation between increased uptake of 89Zr-radiolabeled antibody and HIV-1 translational activity in lymph nodes and other tissues [68, 69]. Compared to antibody-based ligands that have limited penetration through the BBB, 18F-FAraG PET that tracked activated T-cells could be particularly useful for CNS disease with T-cell driven pathogenesis [69, 70].
Conclusion
Taken together, years of research reveal highly diverse neurological outcomes in both treatment-naïve and virally-suppressed PLWH. CSF studies focusing on viral compartmentalization and tropism identify factors that may alter clinical outcomes. In addition to the effect of infected CNS resident cells, the observation of T-cell derived viruses in CSF escape points to a complex relationship between infected cells from the systemic reservoir and neurological outcomes during stable ART. The application of single-cell techniques opens up a new avenue to characterize the infected cells across anatomical tissues, and allow the discovery of strategies that compensate for the shortcomings of ART.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Phillip Chan declares that he has no conflict of interest.
Serena Spudich reports grants from NIH - NIMH & NINDS, during the conduct of the study; non-financial support from ViiV Healthcare, Inc., outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Fromentin R, Chomont N. HIV persistence in subsets of CD4+ T cells: 50 shades of reservoirs. Semin Immunol. 2021;51:101438. doi: 10.1016/j.smim.2020.101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong JK, Yukl SA. Tissue reservoirs of HIV. Curr Opin HIV AIDS. 2016;11(4):362–70. doi: 10.1097/COH.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamers SL, Rose R, Maidji E, Agsalda-Garcia M, Nolan DJ, Fogel GB, et al. HIV DNA Is Frequently Present within Pathologic Tissues Evaluated at Autopsy from Combined Antiretroviral Therapy-Treated Patients with Undetectable Viral Loads. J Virol. 2016;90(20):8968–83. doi: 10.1128/JVI.00674-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaillon A, Gianella S, Dellicour S, Rawlings SA, Schlub TE, De Oliveira MF, et al. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J Clin Invest. 2020;130(4):1699–712. doi: 10.1172/JCI134815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croese T, Castellani G, Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat Immunol. 2021;22(9):1083–92. doi: 10.1038/s41590-021-00994-2. [DOI] [PubMed] [Google Scholar]
- 6.Winston A, Antinori A, Cinque P, Fox HS, Gisslen M, Henrich TJ, et al. Defining cerebrospinal fluid HIV RNA escape: editorial review AIDS. AIDS. 2019;33 Suppl 2:S107–S11. doi: 10.1097/QAD.0000000000002252. [DOI] [PubMed] [Google Scholar]
- 7.Joseph J, Cinque P, Colosi D, Dravid A, Ene L, Fox H, et al. Highlights of the Global HIV-1 CSF Escape Consortium Meeting, 9 June 2016, Bethesda, MD, USA. J Virus Erad. 2016;2(4):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202(12):1819–25. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukerji SS, Misra V, Lorenz DR, Uno H, Morgello S, Franklin D, et al. Impact of Antiretroviral Regimens on Cerebrospinal Fluid Viral Escape in a Prospective Multicohort Study of Antiretroviral Therapy-Experienced Human Immunodeficiency Virus-1-Infected Adults in the United States. Clin Infect Dis. 2018;67(8):1182–90. doi: 10.1093/cid/ciy267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Valero I, Ellis R, Heaton R, Deutsch R, Franklin D, Clifford DB, et al. Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. AIDS. 2019;33(3):475–81. doi: 10.1097/QAD.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11. Spudich S, Robertson KR, Bosch RJ, Gandhi RT, Cyktor JC, Mar H, et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J Clin Invest. 2019;129(8):3339–46. doi: 10.1172/JCI127413. This work describes an unexpectedly high frequency of HIV-infected cells in the cerebrospinal fluid despite stable suppressive antiretroviral therapy.
- 12.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallet C, De Rovere M, Van Assche J, Daouad F, De Wit S, Gautier V, et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front Cell Infect Microbiol. 2019;9:362. doi: 10.3389/fcimb.2019.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Harti L, Joseph J, Nath A. Astrocytes as an HIV CNS reservoir: highlights and reflections of an NIMH-sponsored symposium. J Neurovirol. 2018;24(6):665–9. doi: 10.1007/s13365-018-0691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan P, Patel P, Hellmuth J, Colby DJ, Kroon E, Sacdalan C, et al. Distribution of HIV RNA in CSF and Blood is linked to CD4/CD8 Ratio During Acute HIV. J Infect Dis. 2018. doi: 10.1093/infdis/jiy260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tovanabutra S, Sirijatuphat R, Pham PT, Bonar L, Harbolick EA, Bose M, et al. Deep Sequencing Reveals Central Nervous System Compartmentalization in Multiple Transmitted/Founder Virus Acute HIV-1 Infection. Cells. 2019;8(8). doi: 10.3390/cells8080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol. 2010;84(5):2395–407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog. 2015;11(3):e1004720. doi: 10.1371/journal.ppat.1004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gega A, Kozal MJ, Chiarella J, Lee E, Peterson J, Hecht FM, et al. Deep sequencing of HIV-1 variants from paired plasma and cerebrospinal fluid during primary HIV infection. J Virus Erad. 2015;1(4):264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7(10):e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulfhammer G, Eden A, Antinori A, Brew BJ, Calcagno A, Cinque P, et al. Cerebrospinal Fluid viral load across the spectrum of untreated HIV-1 infection: a cross-sectional multi-center study. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22. Gisslen M, Keating SM, Spudich S, Arechiga V, Stephenson S, Zetterberg H, et al. Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression. PLoS One. 2021;16(5):e0250987. doi: 10.1371/journal.pone.0250987. This work examines the evolution of the elevation of immune activation markers in the CSF across different stages of HIV infection.
- 23.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright EJ, Grund B, Robertson KR, Cysique L, Brew BJ, Collins GL, et al. No neurocognitive advantage for immediate antiretroviral treatment in adults with greater than 500 CD4+ T-cell counts. AIDS. 2018;32(8):985–97. doi: 10.1097/QAD.0000000000001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peluso MJ, Valcour V, Ananworanich J, Sithinamsuwan P, Chalermchai T, Fletcher JL, et al. Absence of Cerebrospinal Fluid Signs of Neuronal Injury Before and After Immediate Antiretroviral Therapy in Acute HIV Infection. J Infect Dis. 2015;212(11):1759–67. doi: 10.1093/infdis/jiv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulfhammer G, Eden A, Mellgren A, Fuchs D, Zetterberg H, Hagberg L, et al. Persistent central nervous system immune activation following more than 10 years of effective HIV antiretroviral treatment. AIDS. 2018;32(15):2171–8. doi: 10.1097/QAD.0000000000001950. [DOI] [PubMed] [Google Scholar]
- 27.Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28(1):67–72. doi: 10.1097/01.aids.0000432467.54003.f7. [DOI] [PubMed] [Google Scholar]
- 28.Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, et al. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology. 2016;86(15):1425–32. doi: 10.1212/WNL.0000000000002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin LH, Sacktor N, Creighton J, Du Y, Endres CJ, Pomper MG, et al. Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS. 2018;32(12):1661–7. doi: 10.1097/QAD.0000000000001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evering TH, Applebaum A, La Mar M, Garmon D, Dorfman D, Markowitz M. Rates of non-confounded HIV-associated neurocognitive disorders in men initiating combination antiretroviral therapy during primary infection. AIDS. 2016;30(2):203–10. doi: 10.1097/QAD.0000000000000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan P, Kerr SJ, Kroon E, Colby D, Sacdalan C, Hellmuth J, et al. Cognitive trajectories after treatment in acute HIV infection. AIDS. 2021;35(6):883–8. doi: 10.1097/QAD.0000000000002831. [DOI] [PubMed] [Google Scholar]
- 32.Hellmuth J, Slike BM, Sacdalan C, Best J, Kroon E, Phanuphak N, et al. Very Early Initiation of Antiretroviral Therapy During Acute HIV Infection Is Associated With Normalized Levels of Immune Activation Markers in Cerebrospinal Fluid but Not in Plasma. J Infect Dis. 2019;220(12):1885–91. doi: 10.1093/infdis/jiz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **33. Burbelo PD, Price RW, Hagberg L, Hatano H, Spudich S, Deeks SG, et al. Anti-Human Immunodeficiency Virus Antibodies in the Cerebrospinal Fluid: Evidence of Early Treatment Impact on Central Nervous System Reservoir? J Infect Dis. 2018;217(7):1024–32. doi: 10.1093/infdis/jix662. This work demonstrated the association between early ART and significant level reductions of anti-HIV antibodies in blood and CSF. Given the level of anti-HIV antibodies correlates with the extent of antigen exposure, the findings suggest that early ART may reduce the long-term immune activation by limiting the size of the HIV reservoir.
- 34.DeMaster LK, Liu X, VanBelzen DJ, Trinite B, Zheng L, Agosto LM, et al. A Subset of CD4/CD8 Double-Negative T Cells Expresses HIV Proteins in Patients on Antiretroviral Therapy. J Virol. 2015;90(5):2165–79. doi: 10.1128/JVI.01913-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imamichi H, Smith M, Adelsberger JW, Izumi T, Scrimieri F, Sherman BT, et al. Defective HIV-1 proviruses produce viral proteins. Proc Natl Acad Sci U S A. 2020;117(7):3704–10. doi: 10.1073/pnas.1917876117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imamichi H, Dewar RL, Adelsberger JW, Rehm CA, O’Doherty U, Paxinos EE, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci U S A. 2016;113(31):8783–8. doi: 10.1073/pnas.1609057113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handoko R, Chan P, Jagodzinski L, Pinyakorn S, Ubolyam S, Phanuphak N, et al. Minimal detection of cerebrospinal fluid escape after initiation of antiretroviral therapy in acute HIV-1 infection. AIDS. 2021;35(5):777–82. doi: 10.1097/QAD.0000000000002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wendel KA, McArthur JC. Acute meningoencephalitis in chronic human immunodeficiency virus (HIV) infection: putative central nervous system escape of HIV replication. Clin Infect Dis. 2003;37(8):1107–11. doi: 10.1086/378300. [DOI] [PubMed] [Google Scholar]
- 39.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194(12):1686–96. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 40.Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- **41. Chan TY, De Zan V, Gregg A, Alagaratnam J, Gerevini S, Antinori A, et al. The symptomatology of cerebrospinal fluid HIV RNA escape: a large case-series. AIDS. 2021;35(14):2341–6. doi: 10.1097/QAD.0000000000002992. This large case series illustrates the diversity in symptom onset and clinical manifestations of HIV CSF escape.
- 42.Eden A, Nilsson S, Hagberg L, Fuchs D, Zetterberg H, Svennerholm B, et al. Asymptomatic Cerebrospinal Fluid HIV-1 Viral Blips and Viral Escape During Antiretroviral Therapy: A Longitudinal Study. J Infect Dis. 2016;214(12):1822–5. doi: 10.1093/infdis/jiw454. [DOI] [PubMed] [Google Scholar]
- 43.Ferretti F, De Zan V, Gerevini S, Turrini F, Boeri E, Gianotti N, et al. Relapse of Symptomatic Cerebrospinal Fluid HIV Escape. Curr HIV/AIDS Rep. 2020;17(5):522–8. doi: 10.1007/s11904-020-00526-x. [DOI] [PubMed] [Google Scholar]
- 44.Anderson AM, Munoz-Moreno JA, McClernon DR, Ellis RJ, Cookson D, Clifford DB, et al. Prevalence and Correlates of Persistent HIV-1 RNA in Cerebrospinal Fluid During Antiretroviral Therapy. J Infect Dis. 2017;215(1):105–13. doi: 10.1093/infdis/jiw505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson AM, Tang B, Vaida F, McClernon D, Deutsch R, Cherner M, et al. Low-Level HIV RNA in Cerebrospinal Fluid and Neurocognitive Performance: A Longitudinal Cohort Study. J Acquir Immune Defic Syndr. 2021;87(5):1196–204. doi: 10.1097/QAI.0000000000002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nightingale S, Michael BD, Fisher M, Winston A, Nelson M, Taylor S, et al. CSF/plasma HIV-1 RNA discordance even at low levels is associated with up-regulation of host inflammatory mediators in CSF. Cytokine. 2016;83:139–46. doi: 10.1016/j.cyto.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mastrangelo A, Turrini F, de Zan V, Caccia R, Gerevini S, Cinque P. Symptomatic cerebrospinal fluid escape. AIDS. 2019;33 Suppl 2:S159–S69. doi: 10.1097/QAD.0000000000002266. [DOI] [PubMed] [Google Scholar]
- **48. Lucas SB, Wong KT, Nightingale S, Miller RF. HIV-Associated CD8 Encephalitis: A UK Case Series and Review of Histopathologically Confirmed Cases. Front Neurol. 2021;12:628296. doi: 10.3389/fneur.2021.628296. This work illustrates the possible link between CD8 encephalitis and CSF HIV escape through the detailed work in histopathology.
- **49. Lustig G, Cele S, Karim F, Derache A, Ngoepe A, Khan K, et al. T cell derived HIV-1 is present in the CSF in the face of suppressive antiretroviral therapy. PLoS Pathog. 2021;17(9):e1009871. doi: 10.1371/journal.ppat.1009871. This work illustrates that latently-infected T-cells remains an important source of HIV escape in CSF in addition to the viral latency in residential monocyte/macrophage in the CNS.
- 50.Joseph SB, Kincer LP, Bowman NM, Evans C, Vinikoor MJ, Lippincott CK, et al. Human Immunodeficiency Virus Type 1 RNA Detected in the Central Nervous System (CNS) After Years of Suppressive Antiretroviral Therapy Can Originate from a Replicating CNS Reservoir or Clonally Expanded Cells. Clin Infect Dis. 2019;69(8):1345–52. doi: 10.1093/cid/ciy1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gama L, Abreu CM, Shirk EN, Price SL, Li M, Laird GM, et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS. 2017;31(1):5–14. doi: 10.1097/QAD.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, et al. Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir. MBio. 2017;8(4). doi: 10.1128/mBio.01186-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefic K, Chaillon A, Bouvin-Pley M, Moreau A, Braibant M, Bastides F, et al. Probing the compartmentalization of HIV-1 in the central nervous system through its neutralization properties. PLoS One. 2017;12(8):e0181680. doi: 10.1371/journal.pone.0181680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu DC, Silsorn D, Inthawong D, Kuncharin Y, Sopanaporn J, Im-Erbsin R, et al. Impact of analytical treatment interruption on the central nervous system in a simian-HIV model. AIDS. 2019;33 Suppl 2:S189–S96. doi: 10.1097/QAD.0000000000002270. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber-Stainthorp W, Sinharay S, Srinivasula S, Shah S, Wang J, Dodd L, et al. Brain (18)F-FDG PET of SIV-infected macaques after treatment interruption or initiation. J Neuroinflammation. 2018;15(1):207. doi: 10.1186/s12974-018-1244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colby DJ, Trautmann L, Pinyakorn S, Leyre L, Pagliuzza A, Kroon E, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24(7):923–6. doi: 10.1038/s41591-018-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pannus P, Rutsaert S, De Wit S, Allard SD, Vanham G, Cole B, et al. Rapid viral rebound after analytical treatment interruption in patients with very small HIV reservoir and minimal on-going viral transcription. J Int AIDS Soc. 2020;23(2):e25453. doi: 10.1002/jia2.25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castagna A, Muccini C, Galli L, Bigoloni A, Poli A, Spagnuolo V, et al. Analytical treatment interruption in chronic HIV-1 infection: time and magnitude of viral rebound in adults with 10 years of undetectable viral load and low HIV-DNA (APACHE study). J Antimicrob Chemother. 2019;74(7):2039–46. doi: 10.1093/jac/dkz138. [DOI] [PubMed] [Google Scholar]
- 59.Huiting ED, Gittens K, Justement JS, Shi V, Blazkova J, Benko E, et al. Impact of Treatment Interruption on HIV Reservoirs and Lymphocyte Subsets in Individuals Who Initiated Antiretroviral Therapy During the Early Phase of Infection. J Infect Dis. 2019;220(2):270–4. doi: 10.1093/infdis/jiz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **60. De Scheerder MA, Vrancken B, Dellicour S, Schlub T, Lee E, Shao W, et al. HIV Rebound Is Predominantly Fueled by Genetically Identical Viral Expansions from Diverse Reservoirs. Cell Host Microbe. 2019;26(3):347–58 e7. doi: 10.1016/j.chom.2019.08.003. The study outcomes indicate that a successful HIV cure possibly needs to cover a broad range of tissue reservoirs with latent HIV infection.
- 61.Andrade VM, Mavian C, Babic D, Cordeiro T, Sharkey M, Barrios L, et al. A minor population of macrophage-tropic HIV-1 variants is identified in recrudescing viremia following analytic treatment interruption. Proceedings of the National Academy of Sciences. 2020;117(18):9981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clarridge KE, Blazkova J, Einkauf K, Petrone M, Refsland EW, Justement JS, et al. Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLoS Pathog. 2018;14(1):e1006792. doi: 10.1371/journal.ppat.1006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Scheerder MA, Van Hecke C, Zetterberg H, Fuchs D, De Langhe N, Rutsaert S, et al. Evaluating predictive markers for viral rebound and safety assessment in blood and lumbar fluid during HIV-1 treatment interruption. J Antimicrob Chemother. 2020;75(5):1311–20. doi: 10.1093/jac/dkaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hellmuth J, Muccini C, Colby DJ, Kroon E, de Souza M, Crowell TA, et al. Central Nervous System Safety During Brief Analytic Treatment Interruption of Antiretroviral Therapy Within 4 Human Immunodeficiency Virus Remission Trials: An Observational Study in Acutely Treated People Living With Human Immunodeficiency Virus. Clin Infect Dis. 2021;73(7):e1885–e92. doi: 10.1093/cid/ciaa1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliveira MF, Chaillon A, Nakazawa M, Vargas M, Letendre SL, Strain MC, et al. Early Antiretroviral Therapy Is Associated with Lower HIV DNA Molecular Diversity and Lower Inflammation in Cerebrospinal Fluid but Does Not Prevent the Establishment of Compartmentalized HIV DNA Populations. PLoS Pathog. 2017;13(1):e1006112. doi: 10.1371/journal.ppat.1006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farhadian SF, Mehta SS, Zografou C, Robertson K, Price RW, Pappalardo J, et al. Single-cell RNA sequencing reveals microglia-like cells in cerebrospinal fluid during virologically suppressed HIV. JCI insight. 2018;3(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMahon JH, Zerbato JM, Lau JSY, Lange JL, Roche M, Tumpach C, et al. A clinical trial of non-invasive imaging with an anti-HIV antibody labelled with copper-64 in people living with HIV and uninfected controls. EBioMedicine. 2021;65:103252. doi: 10.1016/j.ebiom.2021.103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vera DB, Schulte B, Henrich T, Flavell R, Seo Y, Abdelhafez Y, et al. First-in-human total-body PET imaging of HIV with 89Zr-VRC01 on the EXPLORER. Soc Nuclear Med; 2020. [Google Scholar]
- 69.VanBrocklin H, Vera DB, Schulte B, Flavell R, Seo Y, Levi J, et al. Imaging viral load and T cell activation in HIV: Tools for cure development. Soc Nuclear Med; 2020. [Google Scholar]
- 70.Guglielmetti C, Levi J, Huynh TL, Tiret B, Blecha J, Tang R, et al. Longitudinal imaging of T-cells and inflammatory demyelination in a preclinical model of multiple sclerosis using 18F-FAraG PET and MRI. Journal of Nuclear Medicine. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


