Abstract
Beta-lactam resistance by Streptococcus pneumoniae is becoming a significant threat to public health worldwide. However, data concerning antibiotic susceptibility patterns in China have not been published. In this study, a total of 79 clinical isolates and 244 nasopharyngeal isolates of S. pneumoniae were recovered between June and November 1997 in Beijing. The agreement between the MICs (±1 log2 dilution) of penicillin and ceftriaxone obtained by the agar dilution and E-test methods for the 79 clinical strains was very good (97.5 and 93.7%, respectively). Of these 79 strains, 9 (11.4%) were intermediate and 2 (2.5%) were resistant to penicillin. Of the 244 nasopharyngeal strains, 32 (13.1%) were intermediate and 3 (1.2%) were resistant to penicillin. The total of 277 penicillin-susceptible clinical and nasopharyngeal isolates of Streptococcus pneumoniae were 100% susceptible to amoxicillin-clavulanic acid, cefuroxime, ceftriaxone, and cefotaxime. In the 35 penicillin-intermediate and -resistant nasopharyngeal strains, elevated MICs of amoxicillin-clavulanic acid, cefuroxime, ceftriaxone, and cefotaxime were seen for ≤4 isolates. Of 244 nasopharyngeal isolates, the overall percentages of tetracycline, erythromycin, chloramphenicol, ofloxacin, and trimethoprim-sulfamethoxazole resistance were 87.6, 74.0, 47.8, 3.7 and 63.3, respectively. Vancomycin and rifampin resistance were not detected. These findings demonstrate that the rate of penicillin-resistant pneumococci is relatively low in China compared to those of other Asian countries. Resistance to non-beta-lactams was much higher than to beta-lactams. The E-test and agar dilution methods appeared to be comparable in identifying resistant strains.
The emergence of penicillin resistance and multidrug-resistant pneumococcal strains has become a global concern. Since the late 1980s, antibiotic-resistant pneumococci have markedly increased worldwide and have been recognized as globally spread pathogens. Penicillin-resistant pneumococci are particularly common in Spain and South Africa (1). In the United States, resistance to penicillin was <5% before 1989 (including <0.02% of isolates for which MICs were ≥2.0 μg/ml) but increased to 6.6% in 1991 to 1992 (MICs of 2.0 μg/ml for 1.3% of isolates) (2, 3). Resistant pneumococci may spread from country to country, and widened and intensified surveillance is needed in all areas, including those countries where resistance is relatively uncommon (4, 6).
Treatment of pneumococcal infections is often based on historical data and studies on patients with fully susceptible strains. However, the emergence of penicillin resistance and resistance to other antimicrobials has important implications for treatment (7–9, 11, 14). Ceftriaxone and cefotaxime, alone or in combination with vancomycin, are often used for serious systemic infections (including meningitis) caused by pneumococcal strains with reduced penicillin susceptibility.
No published data are currently available on pneumococcal resistance in China. However, information on the susceptibility of pneumococci is important because any shift in resistance will have great impact on antimicrobial therapy. In order to obtain information on resistance of pneumococci to commonly used antimicrobial agents in China, a microbiological survey was performed on 79 pneumococcal strains from four teaching hospitals and on 244 nasopharyngeal strains from day care centers in 1997. This study investigated the in vitro activities of 12 antimicrobial agents, including penicillin, cephalosporins, and non-beta-lactams.
MATERIALS AND METHODS
Bacterial strains.
Seventy-nine clinical strains were recovered from outpatients and inpatients at four teaching hospitals from January to June 1997, and 244 nasopharyngeal strains were taken from children under 5 years old in eight day care centers between September and November 1997 in Beijing, China. These strains were reidentified by conventional tests after arriving at the laboratory of Peking Union Medical College Hospital. For testing, isolates were removed from storage, streaked onto Columbia agar plates containing 5% sheep blood, and incubated for 24 h at 35°C in a 5% CO2 incubator. One single colony was picked and streaked onto a new Columbia plate supplemented with blood and incubated for 18 to 24 h. All inocula were prepared from this subculture. Streptococcus pneumoniae ATCC 49619 and Staphylococcus aureus ATCC 29213 were used as quality control strains.
Antimicrobial agents.
Antimicrobial standard powders were obtained from Sigma Chemical Company (St. Louis, Mo.), and solutions were freshly prepared for each test. Penicillin and ceftriaxone MICs were determined and compared simultaneously by agar dilution and E test for the 79 clinical isolates. For these strains, seven other antibiotics, including amoxicillin-clavulanic acid, cefuroxime, cefotaxime, vancomycin, tetracycline, erythromycin, and chloramphenicol, were tested by the agar dilution method. For the 244 nasopharyngeal isolates, MICs of penicillin, ceftriaxone, amoxicillin-clavulanic acid, cefuroxime, cefotaxime, and trimethoprim-sulfamethoxazole (TMP-SMZ) were determined by E test, and MICs of tetracycline, erythromycin, chloramphenicol, vancomycin, rifampin, and ofloxacin were determined by agar dilution.
E test.
E test was performed according to the instructions of the manufacturer. Mueller-Hinton medium containing 5% sheep blood was used for all susceptibility testing except for that of TMP-SMZ, which was performed on lysed horse blood agar plates.
Agar dilution test.
Agar dilution was performed as described by the National Committee for Clinical Laboratory Standards (13a). The MICs of the antimicrobial agents obtained by E test and agar dilution were all within the recommended ranges for the two quality control strains.
Statistics and analysis.
The MIC results were compared and analyzed by WHONET-4 software, provided kindly by J. Steeling.
RESULTS
Table 1 shows the comparison of E-test- and agar dilution-determined MICs of penicillin and ceftriaxone for the 79 clinical isolates. E-test-determined penicillin MICs were within ±1 dilution for 77 of the 79 (97.5%) isolates, and ceftriaxone MICs were within ±1 dilution for 74 of the 79 (93.7%) isolates. The agreement between the MICs (±1 log2 dilution) obtained by the two methods was acceptable for both penicillin and ceftriaxone (>90%). No very major or major errors were found for the two antibiotics. Minor errors were 2.5% for penicillin and 1.0% for ceftriaxone.
TABLE 1.
Comparison of E-test- and agar dilution-determined MICs of penicillin and ceftriaxone for the 79 S. pneumoniae clinical isolates
| Antimicrobial agent | No. of isolates for which E-test MICs differed from agar dilution MICs by indicated no. of log2 dilutionsa
|
% Agreementb | % Interpretive errorc
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | −1.5 | −1 | −0.5 | 0 | +0.5 | +1 | +1.5 | +2 | Very major | Major | Minor | ||
| Penicillin | 2 | 11 | 11 | 21 | 28 | 6 | 97.5 | 0 | 0 | 2.5 | |||
| Ceftriaxone | 1 | 1 | 7 | 13 | 23 | 20 | 11 | 3 | 93.7 | 0 | 0 | 1.0 | |
A difference of 0 indicates that the MICs were identical; −1 and +1 indicate a ±1 log2 dilution difference, etc.
Percentage of isolates for which MICs of the indicated agent differed by the two methods within ±1 log2 dilution.
Very major error, number of false-susceptible results/number of resistant isolates × 100; major error, number of false-resistant results/number of susceptible isolates × 100; minor error, number of susceptible-to-intermediate, intermediate-to-susceptible, resistant-to-intermediate, or intermediate-to-resistant results/total number of isolates tested × 100.
By the agar dilution method, 2 of the 79 (2.5%) clinical isolates were resistant to penicillin (MICs, 2 μg/ml) and 9 of the 79 (11.4%) were intermediate (MICs, 0.125 to 1 μg/ml). Penicillin MICs for the 68 susceptible strains ranged from 0.008 to 0.064 μg/ml. MICs of each antibiotic for penicillin-susceptible strains and nonsusceptible strains are presented in Table 2. Amoxicillin-clavulanic acid, cefuroxime, ceftriaxone, and cefotaxime MICs at which 90% of the isolates were inhibited (MIC90s) for strains with raised penicillin MICs (MIC ≥ 0.125 μg/ml) were greater than those for penicillin-susceptible strains. The resistance frequencies of penicillin-susceptible strains to tetracycline, erythromycin, and chloramphenicol were 52.9, 39.7, and 17.6%, respectively, while those of penicillin-intermediate and -resistant strains were 27.3, 45.5, and 9.1%.
TABLE 2.
In vitro activities of eight antimicrobial agents against the 79 clinical isolates of S. pneumoniae by agar dilutiona
| Antimicrobial agent | Penicillin-susceptible strains (n = 68)b
|
Penicillin-intermediate and -resistant strains (n = 11)c
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R (%) | I (%) | MIC50 | MIC90 | MIC range | R (%) | I (%) | MIC50 | MIC90 | MIC range | |
| Amoxicillin-clavulanate | 0 | 0 | 0.064 | 0.064 | ≤0.064–0.125 | 9.1 | 0 | 0.064 | 0.25 | ≤0.064–2 |
| Cefuroxime | 0 | 0 | 0.064 | 0.25 | ≤0.064–0.5 | 18.2 | 0 | 0.125 | 4 | ≤0.064–8 |
| Cefotaxime | 0 | 0 | 0.064 | 0.064 | ≤0.064–0.25 | 0 | 9.1 | 0.064 | 0.25 | ≤0.064–1 |
| Ceftriaxone | 0 | 0 | 0.064 | 0.064 | ≤0.064–0.5 | 0 | 9.1 | 0.064 | 0.25 | ≤0.064–1 |
| Vancomycin | 0 | 0 | 0.5 | 1 | ≤0.125–1 | 0 | 0 | 0.5 | 1 | ≤0.5–1 |
| Tetracycline | 52.9 | 1.5 | 8 | 32 | ≤0.25–32 | 27.3 | 9.1 | 0.25 | 32 | ≤0.25–32 |
| Erythromycin | 39.7 | 0 | 0.032 | 16 | ≤0.032–16 | 45.5 | 0 | 0.125 | 16 | ≤0.032–16 |
| Chloramphenicol | 17.6 | 0 | 2 | 16 | ≤0.5–16 | 9.1 | 0 | 0.5 | 4 | ≤0.5–16 |
All MICs given are in micrograms per milliliter. R, resistant strains; I, intermediate strains.
Susceptible, MIC ≤ 0.064 μg/ml.
Intermediate, MIC = 0.125 to 1 μg/ml; resistant, MIC ≥ 2 μg/ml.
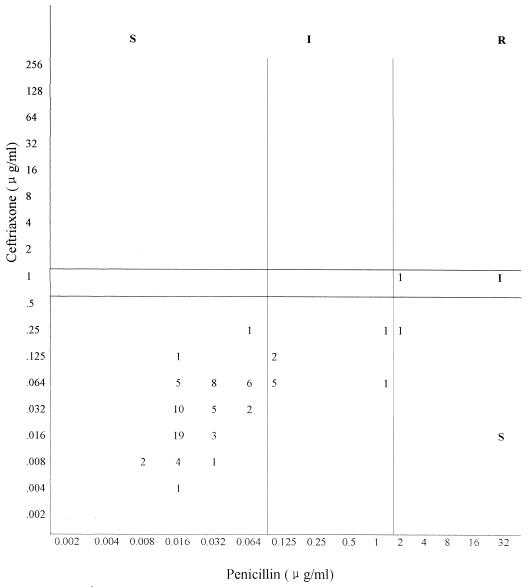
A comparison of MICs by agar dilution of penicillin and ceftriaxone for the 79 clinical strains demonstrated that 9 strains classified as intermediate to penicillin were susceptible to ceftriaxone, and 2 penicillin-resistant strains (MICs, 2 μg/ml) were susceptible (MIC, 0.25 μg/ml) and intermediate (MIC, 1 μg/ml) to ceftriaxone (Fig. 1).
FIG. 1.
Scatterplot of penicillin and ceftriaxone MICs for the 79 S. pneumoniae strains, as determined by the agar dilution method. S, susceptible; I, intermediate; R, resistant.
MICs of 11 antimicrobials for the 244 nasopharyngeal isolates are listed in Tables 3 and 4. By E test, 32 of the 244 (13.1%) isolates were intermediate to penicillin (MICs, 0.094 to 0.25 μg/ml), and 3 of the 244 (1.2%) were resistant (MICs, 3 μg/ml). The MIC range of penicillin for the 209 susceptible strains was 0.008 to 0.064 μg/ml. There were not any strains resistant to four tested beta-lactams among penicillin-susceptible isolates. Resistance to other beta-lactams existed among strains intermediately and fully resistant to penicillin. Reduced susceptibilities to other beta-lactams among strains for which penicillin MICs were raised were as follows: amoxicillin-clavulanate, 5.7% resistant and 2.9% intermediate; cefuroxime, 8.6% resistant and 2.9% intermediate; cefotaxime, 2.9% resistant and 5.7% intermediate; ceftriaxone, 2.9% resistant and 5.7% intermediate. No differences in resistance to erythromycin, tetracycline, or TMP-SMZ were found between these two groups of strains, while a significant difference existed for chloramphenicol (51% versus 29%). All of the strains were susceptible to vancomycin and rifampin. Resistance to ofloxacin was uncommon.
TABLE 3.
In vitro activities of five antimicrobial agents against the 244 nasopharyngeal isolates of S. pneumoniae by E testa
| Antimicrobial agent | Penicillin-susceptible strains (n = 209)b
|
Penicillin-intermediate and -resistant strains (n = 35)c
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R (%) | I (%) | MIC50 | MIC90 | MIC range | R (%) | I (%) | MIC50 | MIC90 | MIC range | |
| Amoxicillin-clavulanate | 0 | 0 | 0.016 | 0.023 | 0.016–0.125 | 5.7 | 2.9 | 0.023 | 0.125 | 0.016–4 |
| Cefuroxime | 0 | 0 | 0.032 | 0.125 | 0.016–0.38 | 8.6 | 2.9 | 0.19 | 0.75 | 0.064–6 |
| Cefotaxime | 0 | 0 | 0.016 | 0.032 | 0.004–0.19 | 2.9 | 5.7 | 0.064 | 0.125 | 0.023–1.5 |
| Ceftriaxone | 0 | 0 | 0.016 | 0.047 | 0.003–0.19 | 2.9 | 5.7 | 0.064 | 0.125 | 0.023–1.5 |
| TMP-SMZ | 62.4 | 11.9 | 3 | 32 | 0.016–32 | 65.7 | 22.9 | 4 | 32 | 0.094–32 |
All MICs given are in micrograms per milliliter. R, resistant strains; I, intermediate strains.
Susceptible, MIC ≤ 0.064 μg/ml.
Intermediate, MIC = 0.125 to 1 μg/ml; resistant, MIC ≥ 2 μg/ml.
TABLE 4.
In vitro activities of six antimicrobial agents against the 244 nasopharyngeal isolates of S. pneumoniae by agar dilutiona
| Antimicrobial agent | Penicillin-susceptible strains (n = 209)b
|
Penicillin-intermediate and -resistant strains (n = 35)c
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R (%) | I (%) | MIC50 | MIC90 | MIC range | R (%) | I (%) | MIC50 | MIC90 | MIC range | |
| Erythromycin | 72.1 | 0.5 | 64 | 128 | 0.023–≥128 | 85.3 | 0 | 64 | 128 | 0.023–≥128 |
| Chloramphenicol | 51.0 | 0.5 | 8 | 16 | 1–32 | 29.4 | 0 | 4 | 8 | 2–16 |
| Tetracycline | 86.4 | 3.0 | 32 | 32 | 0.125–64 | 94.1 | 0 | 32 | 64 | 0.125–64 |
| Vancomycin | 0 | 0 | 0.25 | 0.25 | 0.023–0.25 | 0 | 0 | 0.25 | 0.25 | 0.064–0.25 |
| Rifampin | 0 | 0 | ≤0.125 | ≤0.125 | ≤0.125 | 0 | 0 | ≤0.125 | ≤0.125 | ≤0.125 |
| Ofloxacin | 0.5 | 3.5 | 1 | 2 | 0.25–2 | 0 | 2.9 | 1 | 2 | 0.5–4 |
All MICs given are in micrograms per milliliter. R, resistant strains; I, intermediate strains.
Susceptible, MIC ≤ 0.064 μg/ml.
Intermediate, MIC = 0.125 to 1 μg/ml; resistant, MIC ≥ 2 μg/ml.
DISCUSSION
In China there have been no detailed reports of the incidence of pneumococcal resistance. In view of the increasing frequency worldwide, it is important that studies are conducted and reported on a regular basis. High resistance has been reported in Spain and South Africa and recently in South Korea (73.4%), Japan (67.7%), Thailand (63%), and Vietnam (53.4%) (4, 6, 15, 16).
Our findings indicate that penicillin resistance is relatively low in Beijing, with a resistance rate considerably lower than that of neighboring Asian countries and many other areas.
This study also compared two methods (agar dilution and E test) for determining the MICs of penicillin and ceftriaxone. The results demonstrated a good agreement of E-test results for these two antibiotics against 79 clinical strains compared with those of the agar dilution method. Others have also reported good correlations between E-test and agar dilution MICs in the assessment of pneumococcal susceptibility to penicillin (12). E test is simple to perform, and the MICs are relatively easy to read.
In common with other studies, susceptibility patterns in Beijing demonstrated that the majority of penicillin-intermediate strains retained full susceptibility to ceftriaxone, which may have important implications for therapy in the future. Fortunately, in this study there was no evidence of the emergence of cephalosporin resistance. Although these results are highly encouraging, it is well known that pneumococcal resistance can spread across countries and even continents. Hence, it is important that susceptibility patterns be recorded from various regions in China on a regular basis so that trends can be monitored closely and treatment guidelines can be modified as appropriate.
In our study, resistance to tetracycline, erythromycin, chloramphenicol, and TMP-SMZ was significantly higher (47.8 to 87.6%) than in many European countries and the United States. In Greece, 19 to 29% of the isolates recovered from day care centers were resistant to chloramphenicol, tetracycline, and erythromycin (17). A report from the United States showed that the overall level of resistance to these four antibiotics was less than 18% (5). In South Korea, however, of 105 clinical isolates, 56 to 83% were resistant to chloramphenicol, tetracycline, and erythromycin (13). In Taiwan, more than 60% of isolates were resistant to erythromycin and tetracycline (10). High resistance to non-beta-lactams may be related to the fact that these drugs have been prescribed widely in China.
Many studies have demonstrated that penicillin-resistant strains are more frequently resistant to non-beta-lactams, such as tetracycline, erythromycin, chloramphenicol, and TMP-SMZ, than are penicillin-susceptible strains. Our data failed to support this conclusion. Perhaps this was due to the low numbers of penicillin-resistant strains included in the study.
ACKNOWLEDGMENTS
We thank Xiuli Xie, Yingchun Xu, and their group for technical assistance.
This work was supported by grants from Shanghai Roche, Glaxo Wellcome China, Hoechst China, and SmithKline Beecham Hong Kong.
REFERENCES
- 1.Appelbaum P C. Worldwide development of antibiotic resistance in pneumococci. Eur J Clin Microbiol. 1987;6:367–377. doi: 10.1007/BF02013089. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 4.Doern G V. Susceptibility tests of fastidious bacteria. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 1342–1349. [Google Scholar]
- 5.Doern G V, Brueggemann A, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowson C G, Johnson A P, Cercenado E. Genetics of oxacillin resistance in clinical isolates of Streptococcus pneumoniae that are oxacillin resistant and penicillin susceptible. Antimicrob Agents Chemother. 1994;38:49–53. doi: 10.1128/aac.38.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedland I R, Istre G S. Management of penicillin-resistant pneumococcal infections. Pediatr Infect Dis J. 1992;11:433–435. doi: 10.1097/00006454-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Friedland I R, McCracken G H., Jr Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 9.Friedland I R, Paris M, Ehrett S, Hickey S, Olsen K, McCracken G H., Jr Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1993;37:1630–1636. doi: 10.1128/aac.37.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsueh P R, Wu J J, Hsiue T R. Invasive Streptococcus pneumoniae infection associated with rapidly fatal outcome in Taiwan. J Formos Med Assoc. 1996;95:364–371. [PubMed] [Google Scholar]
- 11.Jacobs M R. Treatment and diagnosis of infections caused by drug resistant Streptococcus pneumoniae. Clin Infect Dis. 1992;15:119–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs M R, Bajaksouzian S, Appelbaum P C, Bolmstrom A. Evaluation of the E-test for susceptibility testing of pneumococci. Diagn Microbiol Infect Dis. 1992;15:473–478. doi: 10.1016/0732-8893(92)90093-9. [DOI] [PubMed] [Google Scholar]
- 13.Kim S N, Kim S W, Choi I H, Pyo S N, Rhee D K. High incidence of multidrug-resistant Streptococcus pneumoniae in South Korea. Microb Drug Resist. 1996;2:401–406. doi: 10.1089/mdr.1996.2.401. [DOI] [PubMed] [Google Scholar]
- 13a.National Committee for Clinical Laboratory Standards. Approved standard M7-A4. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 14.Paris M M, Ramilo O, McCracken G H., Jr Management of meningitis caused by penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1995;39:2171–2175. doi: 10.1128/aac.39.10.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J H, Yang J W, Jin J H, Peck K R, Kim S, Lee N Y, Jacobs M R, Appelbaum P C. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Molecular epidemiology of multidrug-resistant pneumococci in Korea, abstr. C-170; p. 75. [Google Scholar]
- 16.Spika J S, Facklam R R, Plikaytis B D, Oxtoby M J the Pneumococcal Surveillance Working Group. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979–1987. J Infect Dis. 1991;163:1273–1278. doi: 10.1093/infdis/163.6.1273. [DOI] [PubMed] [Google Scholar]
- 17.Syrogiannopoulos G A, Grivea I N, Beratis N G, Spiliopoulou A E, Fasola E L, Bajaksouzian S, Appelbaum P C, Jacobs M R. Resistance patterns of Streptococcus pneumoniae from carriers attending day-care centers in Southwestern Greece. Clin Infect Dis. 1997;25:188–194. doi: 10.1086/514526. [DOI] [PubMed] [Google Scholar]