Abstract
Purpose
To characterize changes in subfoveal choroidal thickness in preterm infants from 30 to 60 weeks' postmenstrual age (PMA).
Design
The prospective, observational Study of Eye Imaging in Preterm infantS (BabySTEPS) enrolled infants eligible for retinopathy of prematurity screening per the American Association of Pediatrics guidelines.
Subjects
Infants imaged with an investigational, handheld OCT at ≥ 4 distinct imaging sessions between 30 to 60 weeks' PMA as part of BabySTEPS.
Methods
Average choroidal thickness across the central subfoveal 1 mm in each eye at each time point was measured using custom segmentation software, and errors were manually corrected by a trained grader. We prospectively collected birth history data. A segmented mixed model was used to analyze the change in choroidal thickness as a function of PMA, birth weight, and gestational age (GA).
Main Outcome Measures
Characterization of normative subfoveal choroidal thickness values and choroidal growth rate between 30 to 60 weeks' PMA.
Results
We included 592 imaging sessions of 79 preterm infants (152 eyes). Mean (± standard deviation) GA was 27.5 ± 2.5 weeks. Mean choroidal thickness was 141.4 ± 34.5 μm at 30 weeks, 272.2 ± 83.9 μm at 38 weeks, and 306.2 ± 77.4 μm between 56 and 60 weeks. Between 30 and 60 weeks' PMA, choroidal growth followed a biphasic model, with a linear growth rate of 14.8 μm per week (95% confidence interval [CI], 13.6–16.0) from 30 until 38.4 weeks, then cessation of growth, with a growth rate of 0.3 μm per week (95% CI, −1.1 to 1.6) from 38.4 to 60 weeks. Infants with extremely low birth weight (ELBW; < 1000 g) and extremely preterm (GA < 28 weeks) infants had significantly slower initial growth rates compared with very low and low birth weight and very preterm and preterm infants (ELBW 13.0 vs. 21.0 μm per week; P < 0.0001 and extremely preterm 13.2 vs. 18.0 μm per week; P = 0.003).
Conclusions
Preterm infant choroidal thickness experiences rapid linear growth from 30 to 38 weeks' PMA, at which time growth nearly stops. These foundational measurements and identification of the impact of extremes of low birth weight and prematurity on choroidal development will be essential as researchers begin to understand the role of choroidal development in ocular and retinal health in human infants.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Key Words: Choroid, Optical coherence, Premature infant, Tomography
The choroid is a vascular layer that serves as the sole supplier of oxygen to the photoreceptors, making successful choroidal development critical for visual development.1 However, in comparison to our understanding of retinal vascular development in preterm infants, very little is known about choroidal development. When retinal vascular development goes awry, as in the case of retinopathy of prematurity (ROP), we intervene to optimize the chances of an excellent visual outcome for these infants. Although differences in lateral displacement of choroidal and retinal vessel landmarks in infants with ROP have been recognized,2 the thickness of the choroid does not currently factor into the vascular developmental assessment of the preterm infant’s eye due to historical difficulties in imaging the choroid and the inability to assess choroidal health with indirect ophthalmoscopy.
Portable, investigational, handheld, swept-source OCT has recently allowed noncontact, high-resolution imaging of the choroid at the bedside in 59% to 94% of newborn infants in research studies.3,4 This has begun to improve our understanding of the choroid and how it is impacted by ocular and systemic factors. However, although we can now image the choroid, there has been only one study on longitudinal choroidal growth in preterm infants during the newborn phase. That first-in-human study used spectral domain OCT centered at a wavelength of 840 nm and found a decrease in visibility of the choroidoscleral junction with age, as the pigmentation of the choroid increased; this limited the longitudinal analysis to 6 infants in whom they found choroidal thickness increased linearly between 32 and 44 weeks' postmenstrual age (PMA).5
The purpose of the present study is to characterize the longitudinal change in subfoveal choroidal thickness (SFCT) from 30 to 60 weeks' PMA in preterm infants undergoing OCT imaging as part of the Study of Eye Imaging in Preterm infantS (BabySTEPs). Retinal nerve fiber layer (RNFL) thickness follows a bimodal distribution, with more rapid growth peaking at 38 weeks and then slowly thinning.6 Axial length follows a parabolic curve, growing rapidly until around 38 to 42 weeks, then much more slowly thereafter.7,8 Given the previous findings by Moreno et al,5 we predicted the choroid would grow linearly until at least 44 weeks' PMA but were unsure if the choroidal thickness would continue to increase after that or would slow, similar to the growth curves we had observed for other ocular structures.
Methods
A total of 118 infants were enrolled between August 2016 and November 2019 as part of the longitudinal, prospective, National Institutes of Health–funded BabySTEPS (clinicaltrials.gov, NCT02887157). BabySTEPS enrolled infants eligible for ROP screening according to the guidelines set forth by the American Association of Pediatrics.9 The described research adhered to the Declaration of Helsinki, and all data were obtained with the approval of the institutional review board at Duke University. Informed consent was obtained from the parent or legal guardian. Both eyes of infants were dilated then imaged with a bedside investigational swept-source OCT system at multiple time points during their stay in the intensive care nursery, corresponding with their ROP screening examinations, and at predetermined research-imaging time points after discharge (corrected gestational age [GA] of 1 month [i.e,. ∼44 weeks' PMA], 4 months [i.e., ∼56 weeks' PMA], and 9 months). Longitudinal analysis of the subfoveal choroid was prospectively planned as part of the study. Our investigational swept-source OCT uses a source centered around a wavelength of 1050 nm, which allows deeper signal penetration into the choroid, compared with the more commonly used 850 nm central wavelength in spectral domain OCT, in which the wavelength limits the ability to visualize a deep chorioscleral junction of thick choroid, resulting in falsely thin choroidal measurements.10
For this longitudinal analysis, we included only infants who had gradable choroidal imaging from ≥ 4 different time points between 30 and 60 weeks' PMA. We excluded all images posttreatment for infants treated for ROP, because the purpose of this study was to characterize the natural history of longitudinal development of the choroid in preterm infants. Detailed demographic data, imaging, and image processing techniques used for each of the BabySTEPS study participants have previously been reported.3
We used an updated custom segmentation software, Baby version 2.0 of the Duke OCT Retinal Analysis Program,11 to segment the OCT scans and delineate the central 1 mm subfoveal choroid for each eye of each subject at each visit.3 The average choroid thickness across the central 1 mm centered on the fovea was selected because the contour of the outer border of the choroid varied across the outer choroidal vessels, which made averaging across 1 mm more appropriate than a single subfoveal measurement.
Intergrader agreement for measuring choroid thickness at 2-week intervals from 31 to 41 weeks was assessed using limits of agreement and intraclass correlation. After confirmation of excellent intergrader agreement, errors in OCT segmentation were manually corrected by a trainer grader. The foveal frame was selected by the graders from the macular volume (scan parameters in Mangalesh et al12), from b-scans spaced < 40 microns apart. Selection was based on the inner retinal divot as well as the tenting of the ellipsoid zone. There were 2 graders (S.M., K.P.W.) with a process of adjudication to ensure both graders were selecting the same outer boundary for segmentation. All graders were masked to study subject. Infants’ birth histories were extracted from the medical record and stored securely in Research Electronic Data Capture. All data entry was independently audited by members of the BabySTEPS study group. Birth weight and GA were grouped into 2 groups each: extremely low birth weight (ELBW; < 1000 g) versus very low birth weight and low birth weight (1000–2500 g) and extremely preterm (EPT; < 28 weeks) versus very preterm and preterm (28–37 weeks) (categories commonly used by the World Health Organization) to assess the impact of these factors on choroidal growth rate.13,14
Statistical analysis was performed using R Statistical Software (Version 4.1.0, R Foundation for Statistical Computing). All continuous measures were summarized using mean and standard deviation (SD). For the weekly averages, both eyes imaged from each infant that week were included because right and left eyes were previously shown to have similar choroidal thicknesses in our cohort at 36 weeks.12 Similarly, for the segmented mixed model, both eyes were included, and this was controlled for on an infant level in the model. Change in choroidal thickness as a function of PMA, GA, and birth weight was analyzed using a segmented mixed model developed by Muggeo et al,15,16 with the eye as the unit of analysis, and data from both eyes were included for analysis. In the model, the intereye correlation of an infant and longitudinal correlation among measures from the same eye were accounted for using eye-level random intercept and infant-level random intercept, transition point, and slope difference. The fixed effect in the model included time in terms of PMA (as a continuous variable), the factor of interest (as a categorical variable, such as birth weight group), and its interaction with time. The segmented mixed model provided estimates for the transition time, the slope before and after the transition time point for each level of the factor of interest. We have previously applied this segmented mixed model to analyze the change of RNFL thickness.6 Statistical significance was defined as 2-sided P < 0.05.
Results
Longitudinal Change of SFCT
Of the 118 infants enrolled in BabySTEPS, 79 infants (152 eyes) had ≥ 4 eligible imaging sessions and were included in the analysis, for a total of 592 imaging sessions from 30.0 to 60.7 weeks' PMA (Table 1). Among these 592 imaging sessions, 525 (88.7%) of the sessions had choroidal thickness measurements from both eyes of the same infant. The mean (± SD) GA was 27.5 ± 2.5 weeks, and the mean birth weight was 928.7 ± 273.5 grams.
Table 1.
Characteristics of the Study Cohort on Infant Level
| Study Cohort Characteristics |
Study Cohort Numbers |
|---|---|
| N | |
| Number of infants | 79 |
| Number of OCT imaging sessions∗ | 592 |
| Number of OCT imaging sessions per infant, median (Q1, Q3) | 6 (5, 10) |
| Gestational age, mean ± SD, wk | 27.5 ± 2.5 |
| Birth weight, mean ± SD, g | 928.7 ± 273.5 |
| Sex, n (%) | |
| Female | 37 (46.8%) |
| Race, n (%) | |
| African American | 35 (44.3%) |
| Asian | 5 (6.3%) |
| White | 36 (45.6%) |
| > 1 | 3 (3.8%) |
| Ethnicity, n (%) | |
| Non-Hispanic | 72 (91.1%) |
| PMA at the first imaging session, median (Q1, Q3), wk | 32.7 (31.0, 34.1) |
| PMA at the last imaging session, median (Q1, Q3), wk† | 49.0 (42.3, 58.2) |
| Gestational age | |
| Extremely preterm (< 28 wk) | 40 (75 eyes; 50.6%) |
| Very preterm and preterm (28–37 weeks) | 39 (77 eyes, 49.4%) |
| Birth weight | |
| Extremely low birth weight (< 1000 g) | 43 (81 eyes; 53.3 54.4%) |
| Very low birth weight and low birth weight (1000–2500 g) | 36 (71; 46.7 45.6%) |
PMA = postmenstrual age; Q1 = first quartile; Q3 = third quartile; ROP = retinopathy of prematurity; SD = standard deviation.
Among 592 OCT imaging sessions, 525 (88.7%) sessions have choroidal thickness measurements from both eyes of the same infants.
The last imaging session before or on 60 weeks and 6 days' PMA. Imaging sessions after the treatment for retinopathy of prematurity were excluded from the analysis, which contributed to the large interquartile range of PMA at the last imaging session.
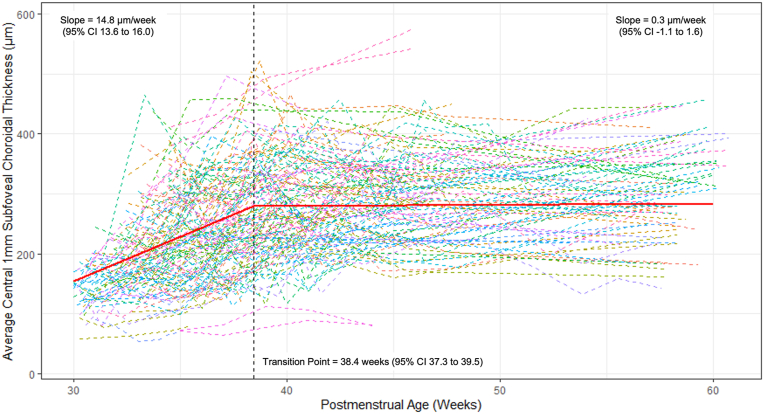
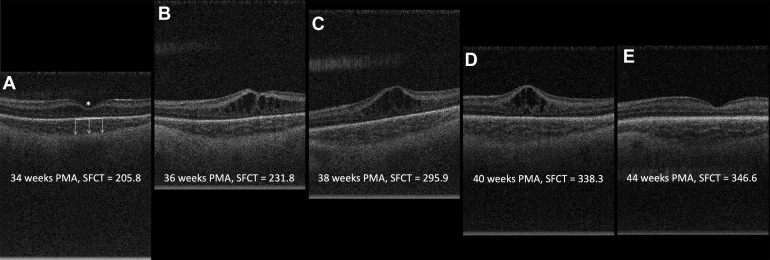
Choroidal thickness increased rapidly and linearly with a growth rate from 30.0 weeks of 14.8 (95% confidence interval [CI], 13.6–16.0) μm per week and a transition point at 38.4 (95% CI, 37.7–39.5) weeks (Fig 1). After 38.4 weeks, the growth rate slowed significantly to 0.3 (95% CI, −1.1 to 1.6) μm per week (P < 0.0001 for comparison with slope before PMA of 38.4 weeks). Figure 2 illustrates the change in SFCT on OCT scans from 34 weeks to 44 weeks for a single infant’s left eye. Mean (SD) of choroidal thickness measurements were calculated for weekly intervals from 30 to 40 weeks' PMA and 5-week averaged bins from 41 to 60 weeks' PMA to provide a normative data set of SFCT measurements from 30 to 60 weeks' PMA (Table 2).
Figure 1.
Longitudinal change in average subfoveal choroidal thickness for all 152 eyes of the 79 infants who were imaged at ≥ 4 time points from 30 to 60 weeks' postmenstrual age. Each dashed line represents the data of an eye. The regression line from the segmented mixed regression model is illustrated by the red line, showing a linear growth rate of 14.8 μm per week from 30 weeks until a transition point at 38.4 weeks, after which growth slows to 0.3 μm per week until 60 weeks. CI = confidence interval.
Figure 2.
OCT b-scans from the left eye of a representative infant illustrating the changes in subfoveal choroidal thickness (SFCT) in μm from 34–44 weeks' postmenstrual age (PMA). Panel A illustrates the selection of the fovea (∗) and measurement of the choroidal thickness from the retinal pigment epithelium (solid white line) to the choroidal-scleral junction (tip of the arrows) at several points across the subfoveal 1 mm. Each of these measurements were then averaged to calculate the average choroidal thickness across the central subfoveal 1 mm SFCT.
Table 2.
Average Subfoveal Choroidal Thickness across the Central 1 mm for Each Postmenstrual Age from 30 to 40 Weeks Then Binned in 5-Week Intervals until 60 Weeks
| Postmenstrual Age (wk) | Number of Infants | Number of Eyes | Number of Measurements | Mean Choroidal Thickness Central 1 mm (μm) | Standard Deviation (μm) |
|---|---|---|---|---|---|
| 30 | 18 | 36 | 36 | 141.4 | 34.5 |
| 31 | 26 | 46 | 46 | 160.2 | 40.3 |
| 32 | 28 | 52 | 52 | 175.5 | 54.8 |
| 33 | 39 | 74 | 74 | 209.5 | 76.1 |
| 34 | 49 | 91 | 95 | 214.0 | 80.6 |
| 35 | 41 | 78 | 78 | 215.8 | 86.4 |
| 36 | 49 | 91 | 93 | 236.2 | 73.0 |
| 37 | 37 | 72 | 74 | 230.2 | 88.6 |
| 38 | 42 | 78 | 78 | 272.2 | 83.9 |
| 39 | 34 | 66 | 70 | 255.4 | 82.1 |
| 40 | 35 | 69 | 69 | 275.0 | 77.3 |
| 41–45 | 53 | 103 | 199 | 283.0 | 84.0 |
| 46–50 | 25 | 48 | 66 | 296.7 | 72.4 |
| 51–55 | 10 | 19 | 23 | 275.2 | 84.1 |
| 56–60 | 35 | 64 | 64 | 306.2 | 77.4 |
Impact of Birth Weight and GA on Choroidal Growth Rate
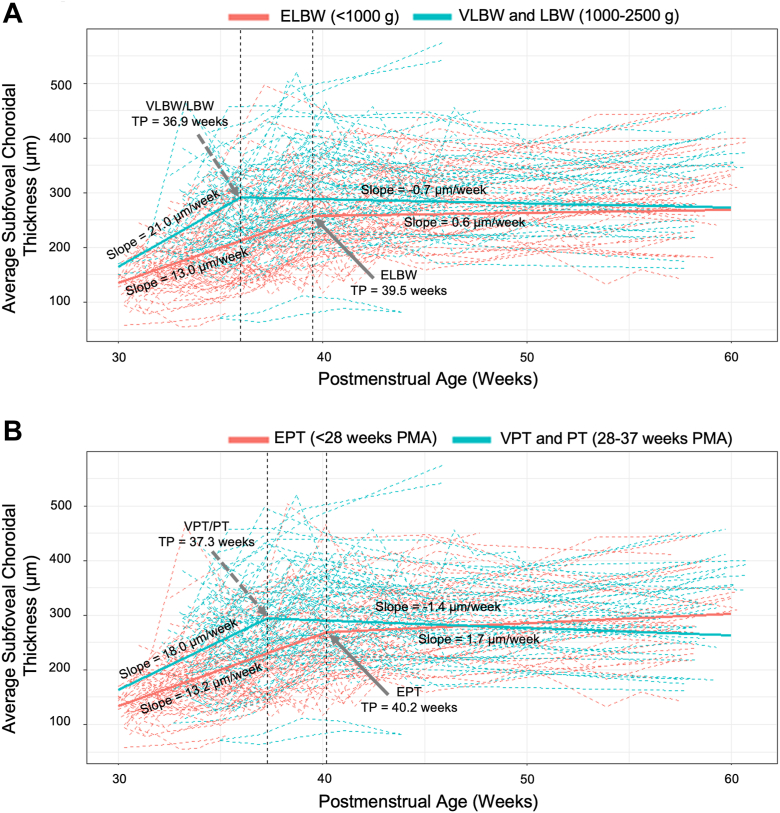
Of the 79 infants with ≥ 4 imaging sessions, about one-half (43 infants, 81 eyes) had extremely low birthweight (ELBW, < 1000 g). We found that infants with ELBWs had a thinner choroid starting from the time measurements were first obtained at 30 weeks and persisting through 60 weeks (Fig 3A). In addition, infants with ELBW had a significantly lower initial growth rate (13.0; 95% CI, 11.9–14.0) μm per week and later transition point (TP) until 39.5 (95% CI, 38.1–40.9) weeks' TP versus 21.0 (95% CI, 17.6–24.4) μm per week until 36.0 (95% CI, 34.8–37.2) weeks TP; both P <0.0001 left slope comparison and P = 0.0002 TP comparison. For both groups, the slope after the TP was not significantly different from 0 (0.6; 95% CI, −1.2 to 2.3) for infants with ELBW versus infants with very low and low birth weight (−0.7; 95% CI, −2.4 to 0.9; P = 0.28).
Figure 3.
Longitudinal change in average subfoveal choroidal thickness for (A) infants with extremely low birth weight (ELBW; 81 eyes of 43 infants) versus very low birth weight (VLBW) and low birth weight (LBW; 71 eyes of 36 infants) and (B) infants born extremely preterm (EPT; 75 eyes of 40 infants) versus very preterm and preterm (VPT and PT; 77 eyes of 39 infants). Each dashed line represents the data of an eye. The solid blue and red lines represent the regression lines from the segmented mixed regression models.
GA had a similar impact on choroidal thickness and growth rate. Infants who were born EPT (< 28 weeks; 40 infants, 75 eyes) had a slower growth rate (13.2 [95% CI, 12.0–14.6] μm per week vs. 18.0 [95% CI, 15.2–20.8] μm per week; P = 0.003) and later TP (40.2 [95% CI, 38.6–41.8] weeks vs. 37.3 [95% CI, 36.1–38.5] weeks; P = 0.005) than those born 28 weeks or later (Fig 3B). The slope after the TP was not significantly different than 0 for either EPT or very preterm/preterm infants (1.7 [95% CI, −0.1 to 3.6] vs. −1.4 [95% CI, −3.0 to 0.3], respectively).
Intergrader Reproducibility
The intergrader agreement of the subfoveal central 1-mm choroidal thickness was found to be excellent across all cross-sectional timepoints (Table 3; intraclass correlation coefficient range, 0.89–0.97). Overall, the 2 graders had a mean difference of 9.8 ± 26.3 μm (intraclass correlation coefficient, 0.94) for choroidal thickness measures taken between 31 to 41 weeks' PMA.
Table 3.
Intergrader Reproducibility of Choroidal Thickness Measurements across the Central Subfoveal 1 mm
| Postmenstrual Age (wk) | Number of Eyes | Mean Choroidal Thickness Central 1 mm (μm) | Mean ± SD Difference between 2 Graders (μm) | 95% Limits of Agreement between 2 Graders (μm) | Intraclass Correlation Coefficient (95% CI) |
|---|---|---|---|---|---|
| 31 | 20 | 165.8 | 10.5 ± 16.8 | (-22.3, 43.4) | 0.89 |
| 33 | 18 | 210.1 | 8.6 ± 17.3 | (-25.3, 42.6) | 0.97 |
| 36 | 15 | 243.6 | 3.5 ± 14.4 | (-24.8, 31.7) | 0.97 |
| 39 | 17 | 231.8 | 16.9 ± 39.7 | (-60.8, 94.6) | 0.89 |
| 41 | 19 | 274.4 | 9.0 ± 34.0 | (-57.8, 75.7) | 0.92 |
| Overall | 89 | 223.7 | 9.8 ± 26.3 | (-41.7, 61.4) | 0.94 |
CI = confidence interval.
Discussion
In this prospective study using handheld OCT images obtained for 79 preterm infants across 592 imaging sessions, we characterized the longitudinal change in choroidal thickness during a critical period of development from 30 to 60 weeks' PMA and provided a robust data set of mean SFCTs from 30 to 60 weeks' PMA. Choroidal measurements were highly reproducible between graders. We found that the choroid grows rapidly from 30 to 38 weeks' PMA, after which time growth slows significantly (growth rate of 14.8 μm per week from 30–38.4 weeks and 0.3 μm per week from 38.4–60 weeks; P < 0.0001). We also found that infants born with ELBW and infants born extremely prematurely have significantly lower initial growth rates and later transition points compared with their peers at equivalent PMAs.
Interestingly, the choroidal growth curve timeline parallels the timeline of other ocular structures, including RNFL thickness and axial length. Central retinal thickness is difficult to compare due to the prevalence of macular edema in preterm infants, which ranges from 40% to 60%.12,17,18 Both RNFL thickness and axial length undergo rapid growth until approximately 38 to 42 weeks or around the time an infant would be considered full-term, after which grow slows dramatically.6, 7, 8 Similarly, the retinal vasculature grows rapidly until 36 weeks nasally and 40 weeks temporally, at which time the retina is considered fully vascularized. The signaling molecules underlying this abrupt TP near term followed by slower growth rate post 40 weeks remain unclear. Furthermore, we found this TP can occur later in EPT infants or those with ELBW.
The present study found a slightly different growth curve than the previous 2013 study by Moreno et al,5 which followed 6 infants longitudinally from 32 to 44 weeks' PMA and found that SFCT increased linearly during that timeframe.19 There are several strengths to the present study compared with the one by Moreno et al.5 Our study used swept-source OCT, which has a longer wavelength (1050 nm compared with 850 nm), allowing for continued visualization of the choroidoscleral junction at later ages, even as pigmentation of the choroid increases with age.10 In addition, this study follows a greater number of infants (79) longitudinally. Therefore, we believe the growth curve extrapolated from this larger cohort over a longer PMA range more accurately estimates the changes in choroidal thickness during the newborn period.
Birth weight and GA are well known risk factors for abnormal vascular development, so much so that they define the current screening guidelines for ROP.9 We found that EPT infants (< 28 weeks) had a significantly slower initial growth rate (13.2 μm per week vs. 18.0 μm per week for very preterm and preterm infants; P = 0.003) and later TP (40.2 weeks vs. 37.3 weeks; P = 0.005). Similarly, we found that infants who had an ELBW (< 1000 g) had a significantly slower initial growth rate (13.0 μm/week vs. 21.0 μm/week for infants with very low and low birth weight; P < 0.0001) and later TP (39.5 weeks vs. 36.0 weeks; P = 0.0002). There are 2 leading hypotheses for why the choroid may grow more slowly in infants with lower birth weight and/or earlier GA: (1) early extrauterine growth restriction related to additional systemic stresses in the intensive care nursery and (2) decreased levels of VEGF from an immature retinal pigment epithelium. Previous studies have shown that preterm infants often grow at a slower rate than term infants, partially due to periods of inadequate nutrition and partially due to other morbidities associated with preterm birth and that these size differences can last into adolescence and even adulthood.20, 21, 22 Secondly, the retinal pigment epithelium, which expresses VEGF essential for choroidal vascular development, is not fully developed in infants born prematurely. A decrease in the levels of VEGF from an immature retinal pigment epithelium may lead to a slower initial choroidal growth rate.23
After discovering the slower growth rates in EPT infants and infants with low birth weight, we attempted to investigate whether a significant difference in choroidal thickness could be seen as early as 30 weeks. In Figure 3A and 3B, it seems that the choroid is initially thinner in EPT infants and infants with ELBW but then catches up around 45 weeks for EPT infants (Fig 3B) and 60 weeks in infants with ELBW (Fig 3A). However, the numbers of infants with measurements at 30 and 60 weeks' PMA were too small in each group to statistically verify this observation. We previously showed in our cross-sectional analysis that the SFCT at 36 weeks is significantly thinner in EPT infants and infants with ELBW.3 A 2016 study by Erol et al19 analyzed OCT images taken between 36 and 42 weeks' PMA for 80 preterm infants and also found a thinner subfoveal choroid was significantly correlated with lower birth weight.
Other factors that may have influenced choroidal thickness in this study include systemic health variables. Our previous study3 that investigated the impact of systemic health factors on choroidal thickness at 36 weeks found that growth velocity and oxygen supplementation were associated with a thinner choroid. In general, the number of babies receiving oxygen supplementation decreased with increasing GA, and, by 36 weeks, < 25% were receiving supplemental oxygen. Thus, longitudinal trends in choroidal thickness growth may be impacted by the number of babies receiving oxygen supplementation over time.
We presented weekly means (SD) of SFCT for each postmenstrual age 30 to 40 weeks and then in 5-week intervals until 60 weeks (Table 3). This forms the first normative data set for choroidal thickness in preterm infants during the nursery period. Furthermore, the mean choroidal thickness of 275.0 ± 77.3 μm at 40 weeks in these preterm infants was much thinner than that found by Huang et al4 in term-age infants (455.5 ± 93.9 μm at the mean GA of 39.5 weeks and within 72 hours of birth). In the future, we hope to learn whether and for how long these differences persist as these babies grow into childhood. Li et al24 found in children 10 to 12 years of age that those with low birth weight had significantly thinner choroids than normal birth weight children (P = 0.001), but the difference in choroidal thickness for preterm versus term-age infants was not significant. Other studies have also not found a significant difference in choroidal thickness of former full-term versus preterm infants based on GA alone once they reach 4 to 10 years old.25,26 We will follow the choroidal growth, refractive error, and visual acuity in our cohort at early school age as part of BabySTEPS2.
This study has several limitations. The primary limitation comes from inherent difficulties with research in preterm infants in an intensive care nursery; data were captured corresponding to the timing of ROP examinations, and return for outpatient eye care visits were not always at our clinic. The high-speed, investigational OCT technology allows us to capture images of the choroid in most infants; however, we have not delineated choroidal layers or determined the infant choroidal vascularity index to assess further how this vasculature is evolving. Another consideration is that not as many infants were imaged between 46 and 55 weeks. Infants who were doing well began to be discharged home around 40 weeks and returned for OCT imaging visits at 44 and 56 weeks' PMA. Therefore, we decided to include weekly SFCT averages for only 30 to 40 weeks in Table 2 and binned in 5-week intervals thereafter. Although fluctuations in choroidal thickness due to diurnal variation and mydriatic use have been well documented in prior studies in older children and adults,27,28 unlike adults and older children, preterm infants are not upright during the day, which may limit fluctuation. Because infants in this study were consistently imaged in the morning and with mydriatics for standard care ROP examination, with rare exceptions, such fluctuations have low likelihood of impacting this study. Choroidal thicknesses in undilated infants, however, may vary from those reported in this study.
In conclusion, we found that, in preterm infants, the choroid undergoes rapid linear growth from 30 to 38 weeks' PMA, after which the growth rate slows considerably. We also found that the initial growth rate in infants with ELBW and EPT infants was significantly slower than their peers. Although it remains unclear if choroidal thickness is related to long-term visual outcomes, it is becoming clear that systemic and ocular health factors are correlated with choroidal thickness.3,12,19 Now that we have described how the central choroidal thickness changes over the nursery course in a large cohort of preterm infants, we are excited for future studies comparing these choroidal growth curves to those of infants who develop diseases, such as ROP or bronchopulmonary dysplasia, to delineate possible OCT biomarkers for a variety of ocular and systemic conditions. We also look forward to additional studies needed to confirm our findings, evaluate changes in choroidal thickness in multiple quadrants, elucidate how the choroid continues to change after 60 weeks' PMA, and determine how changes in the choroid may impact visual development and function.
Manuscript no. XOPS-D-22-00256R1.
Footnotes
This abstract was presented at the American Academy of Ophthalmology Annual Meeting in Chicago, Illinois, October 2022, where it received the best paper award for the pediatrics section. It was also presented at the Retina Society meeting in Pasadena, California, November 2022, where it received the Raymond R. Margherio award. A portion of this data was also presented at the 2022 Association for Research in Vision and Ophthalmology Annual Meeting, Denver, Colorado, May 2022.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s):
G.Y.: Research support – National Eye Institute.
L.L.S.: Consultant – Acuta Capital Partners, LLC.
C.A.T.: Research support – Research to Prevent Blindness; Royalties – Alcon; Consultant – EMMES Inc; Patents pending – related to OCT imaging methods and technology; Advisory board – EMMEs, Inc; Leadership – International Retinal Research Foundation, The Robert Machemer Foundation; Equity – Theia Imaging; Employment – Theia Imaging.
L.V.: Research support – AGTC, Alcon Laboratories, Inc, Aldeyra, Gyroscope/Orbit Biomedical, Heidelberg Engineering, Janssen, Novartis, Regenxbio, Roche/Genentech; Consultant – Abbvie/Allergan, Alcon, Alimera Sciences, Apellis, Bausch & Lomb, Beaver-Visitec International, BMC, Coherus Biosciences, DORC, Guidepoint, Gyroscope/Orbital Biomedical, Iveric Bio, Ocugen Inc, OcuTerra, RegenxBio, Roche/Genentech, Vindico Medical Education; Advisory board – Clearside Biomedical; Leadership – Women in Ophthalmology Executive Board, AAO Councilor for Retina Society; Receipt of materials – Optos Inc.
Supported by Grants R01 EY025009 (C.A.T.); P30 EY005722, and K23 EY02827 from the National Eye Institute (NEI) and Heed Ophthalmic Foundation Grant (S.M.M.). The contents in this manuscript are solely the responsibility of the authors and do not represent the official view of NEI, NIH, or Heed Ophthalmic Foundation. The sponsor or funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. The described research adhered to the Declaration of Helsinki, and all data were obtained with the institutional review board at Duke University’s approval. Informed consent was obtained from the parent or legal guardian.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Michalak, Mangalesh, Chen, Shen, Tai, Winter, Sarin, Ying, Toth, Vajzovic
Data collection: Michalak, Mangalesh, Tai, Winter, Sarin, Toth, Vajzovic
Analysis and interpretation: Michalak, Mangalesh, Chen, Shen, Tai, Winter, Sarin, Ying, Toth, Vajzovic
Obtained funding: Michalak, Toth, Vajzovic
Overall responsibility: Michalak, Toth, Vajzovic
Contributor Information
Lejla Vajzovic, Email: lejla.vajzovic@duke.edu.
BabySTEPS Group:
Xi Chen, Charles M. Cotten, Mays Antioine El-Dairi, Sina Farsiu, Sharon Freedman, Hesham Gabr, Sara Grace, Kathryn E. Gustafson, Madja Hadziahmetovic, Joseph Izatt, Ramiro Maldonado, Suzanne Michalak, Matthew O’Sullivan, Sally Ong, Miroslav Pajik, Carolyn Pizoli, S. Grace Prakalapakorn, Joan Roberts, Alabi Rolake, Cynthia Toth, Nita Valikodath, Lejla Vajzovic, Christian Viehland, David Wallace, Xiao Yi Zhou, Michelle McCall, Joanne Finkle, Neeru Sarin, Alexandria Dandridge, Ryan Imperio, Shwetha Mangalesh, William Raynor, Du Tran-Viet, Stephanie Chiu, Heena Divecha, Vincent Tai, Katrina P. Winter, Kimberly Fisher, Lacey Andrews, Melissa Babilonia-Rosa, Anne Baez Love, Lucy DeStefano-Pearce, Jessicka Hamilton, Grace Jefferson, Amanda Marion, Isabella Pallotto, Marito Passero, Caitlin Stone, Michelle Sunico, Caelan Eckard, Karthik Ganesan, Xiao Tang, Kira Wang, Brittany Wong, Mark Draelos, Francesco LaRocca, Amit Narawane, Qitong Gao, Isaac Bleicher, Pujan Patel, Jay Rathinavelu, Kai Seely, Mason Seely, Maureen G. Maguire, Gui-Shuang Ying, Brendan McGeehan, Joshua Shimony, Dimitrios Alexopoulos, Sydney Kaplan, Jeanette Kenley, Kayla Hannon, Brian P. Smith, Michael O’Shea, Subashri Kurgatt, Daniel X. Hammer, and William Good
References
- 1.Nickla D.L., Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S.J., Campbell J.P., Ostmo S., et al. Changes in relative position of choroidal versus retinal vessels in preterm infants. Invest Ophthalmol Vis Sci. 2017;58:6334–6341. doi: 10.1167/iovs.17-22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalak S.M., Mangalesh S., Shen L.L., et al. Systemic factors associated with a thinner choroid in preterm infants. Ophthalmol Sci. 2021;1 doi: 10.1016/j.xops.2021.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L.C., Zhou H., Legocki A.T., et al. Choroidal thickness by handheld swept-source optical coherence tomography in term newborns. Transl Vis Sci Technol. 2021;10:27. doi: 10.1167/tvst.10.2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno T.A., O’Connell R.V., Chiu S.J., et al. Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci. 2013;54:4140–4147. doi: 10.1167/iovs.12-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen L.L., Mangalesh S., McGeehan B., et al. Biphasic change in retinal nerve fibre layer thickness from 30 to 60 weeks postmenstrual age in preterm infants. Br J Ophthalmol. 2022 Sep 16 doi: 10.1136/bjo-2022-321621. bjophthalmol-2022-321621. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groot A.L.W., Lissenberg-Witte B.I., van Rijn L.J., Hartong D.T. Meta-analysis of ocular axial length in newborns and infants up to 3 years of age. Surv Ophthalmol. 2022;67:342–352. doi: 10.1016/j.survophthal.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Prakalapakorn S.G., Sarin N., Sarin N., et al. Evaluating the association of clinical factors and optical coherence tomography retinal imaging with axial length and axial length growth among preterm infants. Graefes Arch Clin Exp Ophthalmol. 2021;259:2661–2669. doi: 10.1007/s00417-021-05158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fierson W.M., Chiang M.F., Good W., et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142 doi: 10.1542/peds.2018-3061. [DOI] [PubMed] [Google Scholar]
- 10.Unterhuber A., Považay B., Hermann B., et al. In vivo retinal optical coherence tomography at 1040 nm-enhanced penetration into the choroid. Opt Express. 2005;13:3252–3258. doi: 10.1364/opex.13.003252. [DOI] [PubMed] [Google Scholar]
- 11.Chiu S.J., Li X.T., Nicholas P., et al. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010;18:19413–19428. doi: 10.1364/OE.18.019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangalesh S., McGeehan B., Tai V., et al. Macular OCT characteristics at 36 weeks’ postmenstrual age in infants examined for retinopathy of prematurity. Ophthalmol Retina. 2021;5:580–592. doi: 10.1016/j.oret.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . 10th revision, 2nd ed. World Health Organization Inc; 2004. International Statistical Classification of Diseases and Related Health Problems. [Google Scholar]
- 14.World Health Organization . World Health Organization Inc; 2015. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. [PubMed] [Google Scholar]
- 15.Muggeo V.M., Atkins D.C., Gallop R.J., Dimidjian S. Segmented mixed models with random changepoints: a maximum likelihood approach with application to treatment for depression study. Stat Model. 2014;14:293–313. [Google Scholar]
- 16.Muggeo M. Segmented mixed models with random changepoints in R. Working Paper. February 2016. https://www.researchgate.net/publication/292629179
- 17.Dubis A.M., Subramaniam C.D., Godara P., et al. Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1665–1671. doi: 10.1016/j.ophtha.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee A.C., Maldonado R.S., Sarin N., et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011;31:1470–1482. doi: 10.1097/IAE.0b013e31821dfa6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erol M.K., Coban D.T., Ozdemir O., et al. Choroidal thickness in infants with retinopathy of prematurity. Retina. 2016;36:1191–1198. doi: 10.1097/IAE.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 20.Hack M., Flannery D.J., Schluchter M., et al. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 21.Ford G.W., Doyle L.W., Davis N.M., Callanan C. Very low birth weight and growth into adolescence. Arch Pediatr Adolesc Med. 2000;154:778–784. doi: 10.1001/archpedi.154.8.778. [DOI] [PubMed] [Google Scholar]
- 22.Peila C., Spada E., Giuliani F., et al. Extrauterine growth restriction: definitions and predictability of outcomes in a cohort of very low birth weight infants or preterm neonates. Nutrients. 2020;12:1224. doi: 10.3390/nu12051224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marneros A.G., Fan J., Yokoyama Y., et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167:1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X.Q., Munkholm A., Copenhagen Child Cohort 2000 Study Group, et al. Choroidal thickness in relation to birth parameters in 11-to 12-year-old children: the Copenhagen Child Cohort 2000 Eye Study. Invest Ophthalmol Vis Sci. 2015;56:617–624. doi: 10.1167/iovs.14-15016. [DOI] [PubMed] [Google Scholar]
- 25.Fieß A., Christian L., Kölb-Keerl R., et al. Peripapillary choroidal thickness in former preterm and full-term infants aged from 4 to 10 years. Invest Ophthalmol Vis Sci. 2016;57:6548–6553. doi: 10.1167/iovs.16-20128. [DOI] [PubMed] [Google Scholar]
- 26.Acar D.E., Acar U., Tunay Z.O., et al. Retinal choroidal and retinal nerve fiber layer thickness in former preterm and full-term infants aged 4 to 8 years. Int Ophthalmol. 2021;41:1071–1079. doi: 10.1007/s10792-020-01666-0. [DOI] [PubMed] [Google Scholar]
- 27.Tan C.S., Ouyang Y., Ruiz H., Sadda S.R. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 28.Öner V., Bulut A., Öter K. The effect of topical anti-muscarinic agents on subfoveal choroidal thickness in healthy adults. Eye (Lond) 2016;30:925–928. doi: 10.1038/eye.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]