Summary
Approved fibroblast growth factor receptor (FGFR) inhibitors include erdafitinib, pemigatinib, and futibatinib. We review the most common toxicities associated with FGFR inhibitors and provide practical advice regarding their management. Hyperphosphatemia can be managed with careful monitoring, dose reduction or interruption, a prophylactic low-phosphate diet, and phosphate-lowering therapy. Ocular adverse events (AEs) are managed by withholding or adjusting the dose of the FGFR inhibitor. Dermatologic AEs include alopecia, which can be managed with minoxidil, and dry skin, which can be treated with moisturizers. Hand-foot syndrome can be prevented by lifestyle changes and managed with moisturizing creams, urea, or salicylic acid. Among gastrointestinal AEs, diarrhea may be managed with loperamide; stomatitis can be managed with baking soda rinses, mucosa-coating agents, and topical anesthetics; and dry mouth may be alleviated with salivary stimulants. Most FGFR inhibitor-associated toxicities are manageable with prophylactic measures and treatments; proactive monitoring is key to ensuring optimal clinical benefits.
Keywords: fibroblast growth factor receptor, FGFR1, FGFR2, FGFR3, FGFR4, adverse events, management, erdafitinib, pemigatinib, futibatinib
Graphical abstract
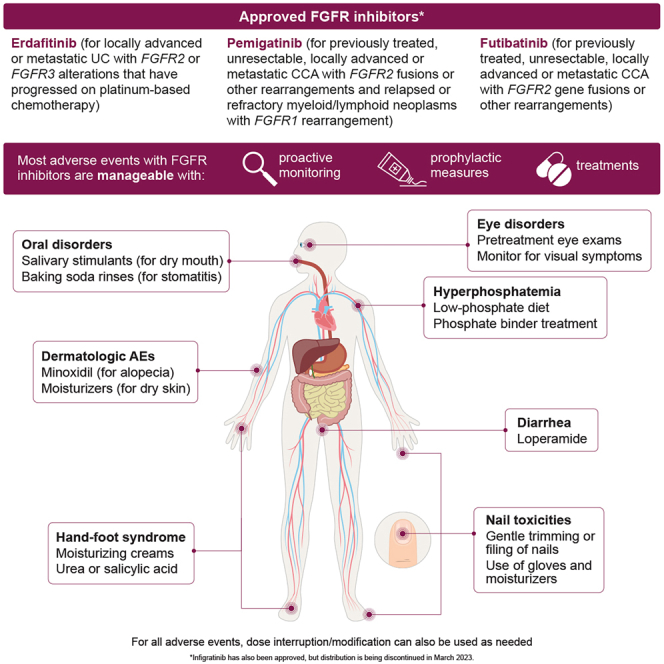
Erdafitinib, pemigatinib, and futibatinib are FGFR inhibitors approved for a number of malignancies but are associated with characteristic toxicities. Vivek Subbiah et al. review the most common adverse events with FGFR inhibitors and provide practical advice on how to manage them, allowing patients to stay on therapy for longer.
Introduction
Genetic alterations in the fibroblast growth factor receptor (FGFR) gene family, including activating fusions or rearrangements, amplifications, and mutations, are associated with oncogenesis in a wide variety of malignancies.1,2,3 The oncogenic potential of FGFR alterations has prompted development of several small-molecule FGFR inhibitors for treatment of neoplasms, including urothelial carcinoma (UC) and cholangiocarcinoma (CCA). Erdafitinib is approved for treatment of locally advanced or metastatic UC with FGFR2 or FGFR3 alterations that has progressed on platinum-based chemotherapy.4 Pemigatinib and futibatinib are approved for previously treated, unresectable, locally advanced or metastatic CCA with FGFR2 fusions or other rearrangements (futibatinib is approved only for intrahepatic CCA).5,6 In August 2022, pemigatinib was additionally approved for treatment of relapsed or refractory myeloid/lymphoid neoplasms (MLNs) with FGFR1 rearrangement,5,7 based on promising and durable clinical activity in the FIGHT-203 study.8 Infigratinib was approved by the US Food and Drug Administration (FDA) for treatment of CCA with an FGFR2 fusion or other rearrangement;9 however, distribution of infigratinib was discontinued in March 2023.10 Other FGFR inhibitors, including derazantinib, rogaratinib, and RLY-4008, are being investigated for a variety of cancers.11,12,13
For any therapeutic agent, demonstrated efficacy must be balanced against associated on-target toxicities to optimize clinical benefits. Commonly encountered on-target toxicities associated with FGFR inhibitors include hyperphosphatemia and nail, eye, dermatologic, and gastrointestinal toxicities.14,15,16,17,18 Close monitoring of and addressing adverse events (AEs) associated with FGFR inhibitors in the clinical trial setting has been reviewed previously;19,20 however, there remains an unmet need for practical insights into AE management in a real-world setting. This review provides a comprehensive assessment of AEs associated with FGFR inhibitors and best practices for monitoring and managing AEs to maintain efficacy and improve patient outcomes according to our real-world clinical experience and review of the literature and prescribing information.
Overview of FGFR alterations in cancer
An analysis of 355,813 solid tumors using next-generation sequencing found that FGFR1–FGFR4 short variants and gene rearrangements were present in 2.7% and copy number alterations in 4.2% of tumor samples.2 FGFR1 was the most frequently altered gene (3.6%), followed by FGFR2 (1.7%), FGFR3 (1.3%), and FGFR4 (0.2%). The cancers with the highest frequency of FGFR alterations include CCA (short variants, 2.3%; gene rearrangements, 8.6%), bladder cancer (short variants, 14.0%; gene rearrangements, 2.8%), and urinary cancer (short variants, 14.4%; gene rearrangements, 2.6%).2 Among patients with CCA, FGFR alterations are almost always found in intrahepatic CCA (iCCA). One molecular profiling study found FGFR2 alterations in 20 of 158 iCCA tumor samples and 0 of 37 extrahepatic CCA tumor samples, whereas another found alterations in genes in the FGF pathway, including FGFR2 and FGFR3, in 13% of iCCA and 5% of extrahepatic CCA samples.21,22 Another study in 6,130 patients with iCCA found FGFR2 alterations present in 11.6% of patient samples.23
FGFR inhibitors approved or in clinical development
Several FGFR inhibitors are approved for use in UC or CCA and now in FGFR1-rearranged MLN; more are in clinical development. Treatment-emergent AEs for these compounds are presented in Table 1 (approved compounds) and Table 2 (compounds in development).
Table 1.
Summary of TEAEs: Approved FGFR inhibitors
| Erdafitinib BLC2001 (N = 99)a,14 |
Pemigatinib FIGHT-202 (N = 146)b,24 |
Futibatinib TPU-TAS-120-101 (N = 170)c,17 |
||||||
|---|---|---|---|---|---|---|---|---|
| AE, n (%) | Any grade | Grade ≥ 3 | AE, n (%) | Any grade | Grade ≥ 3 | AE, n (%) | Any grade | Grade ≥ 3 |
| Hyperphosphatemia | 76 (77) | 2 (2) | hyperphosphatemia | 88 (60) | 0 | hyperphosphatemia | 138 (81) | 38 (22) |
| Stomatitis | 57 (58) | 10 (10) | alopecia | 72 (49) | 0 | diarrhea | 56 (33) | 1 (1) |
| Diarrhea | 50 (51) | 4 (4) | diarrhea | 68 (47) | 4 (3) | constipation | 54 (32) | 2 (1) |
| Dry mouth | 45 (46) | 0 | fatigue | 62 (42) | 7 (5) | nausea | 48 (28) | 0 |
| Decreased appetite | 38 (38) | 0 | dysgeusia | 59 (40) | 0 | fatigue | 43 (25) | 9 (5) |
| Dysgeusia | 37 (37) | 1 (1) | nausea | 58 (40) | 3 (2) | vomiting | 43 (25) | 2 (1) |
| Fatigue | 32 (32) | 2 (2) | constipation | 51 (35) | 1 (1) | AST increased | 41 (24) | 9 (5) |
| Dry skin | 32 (32) | 0 | stomatitis | 51 (35) | 8 (5) | ALT increased | 40 (24) | 17 (10) |
| Alopecia | 29 (29) | 0 | dry mouth | 49 (34) | 0 | abdominal pain | 33 (19) | 5 (3) |
| Constipation | 28 (28) | 1 (1) | decreased appetite | 48 (33) | 2 (1) | alopecia | 33 (19) | 0 |
| Hand-foot syndrome | 23 (23) | 5 (5) | vomiting | 40 (27) | 2 (1) | decreased appetite | 32 (19) | 3 (2) |
| Anemia | 20 (20) | 4 (4) | dry eye | 37 (25) | 1 (1) | dry mouth | 30 (18) | 0 |
| Asthenia | 20 (20) | 7 (7) | arthralgia | 36 (25) | 9 (6) | asthenia | 27 (16) | 7 (4) |
| Nausea | 20 (20) | 1 (1) | abdominal pain | 33 (23) | 7 (5) | stomatitis | 26 (15) | 5 (3) |
| Dry eye | 19 (19) | 1 (1) | hypophosphatemia | 33 (23) | 18 (12) | anemia | 23 (14) | 9 (5) |
| Onycholysis | 18 (18) | 2 (2) | back pain | 29 (20) | 4 (3) | dry skin | 22 (13) | 0 |
| ALT increased | 17 (17) | 2 (2) | dry skin | 29 (20) | 1 (1) | PPES | 22 (13) | 6 (4) |
| Paronychia | 17 (17) | 3 (3) | pain in extremity | 28 (19) | 3 (2) | increased blood creatinine | 20 (12) | 0 |
| Blurred vision | 17 (17) | 0 | edema, peripheral | 26 (18) | 1 (1) | arthralgia | 19 (11) | 0 |
| Nail dystrophy | 16 (16) | 6 (6) | weight decreased | 24 (16) | 3 (2) | hypercalcemia | 19 (11) | 2 (1) |
| Urinary tract infection | 16 (16) | 5 (5) | headache | 23 (16) | 0 | dysgeusia | 18 (11) | 0 |
| Vomiting | 13 (13) | 2 (2) | urinary tract infection | 23 (16) | 4 (3) | decreased weight | 17 (10) | 1 (1) |
| Hyponatremia | 12 (12) | 11 (11) | dehydration | 22 (15) | 5 (3) | |||
| Hematuria | 10 (10) | 2 (2) | hypercalcemia | 22 (15) | 3 (2) | |||
| Dyspnea | 8 (8) | 2 (2) | PPES | 22 (15) | 6 (4) | |||
| Nail disorder | 8 (8) | 3 (3) | anemia | 21 (14) | 5 (3) | |||
| Acute kidney injury | 6 (6) | 2 (2) | epistaxis | 20 (14) | 0 | |||
| Cataract | 6 (6) | 2 (2) | pyrexia | 20 (14) | 1 (1) | |||
| Colitis | 5 (5) | 2 (2) | asthenia | 19 (13) | 2 (1) | |||
| General deterioration in physical health | 5 (5) | 4 (4) | dizziness | 19 (13) | 1 (1) | |||
| Keratitis | 5 (5) | 3 (3) | myalgia | 18 (12) | 2 (1) | |||
| Aphthous ulcer | 4 (4) | 2 (2) | hyponatremia | 16 (11) | 8 (5) | |||
| GGT increased | 3 (3) | 2 (2) | blood creatinine increased | 16 (11) | 2 (1) | |||
| Urosepsis | 3 (3) | 3 (3) | gastroesophageal reflux disease | 16 (11) | 1 (1) | |||
| musculoskeletal pain | 15 (10) | 0 | ||||||
| blood alkaline phosphatase increased | 14 (10) | 5 (3) | ||||||
| onychomadesis | 14 (10) | 0 | ||||||
| dyspnea | 14 (10) | 1 (1) | ||||||
| nail discoloration | 14 (10) | 1 (1) | ||||||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; FGFR, fibroblast growth factor receptor; GGT, γ-glutamyltransferase; PPES, palmar-plantar erythrodysesthesia syndrome; TEAE, treatment-emergent adverse event.
TEAEs occurring in 15% or more of patients (N = 99) with locally advanced or metastatic UC with susceptible FGFR3 or FGFR2 genetic alterations receiving erdafitinib (8 mg or 9 mg) on a continuous dosing regimen.14
TEAEs occurring in 10% or more of patients (N = 146) with locally advanced or metastatic CCA and FGF/FGFR alterations receiving pemigatinib (13.5 mg once daily). MedDRA preferred terms related to hyperphosphatemia were combined: blood phosphorus increased, hyperphosphatemia. MedDRA preferred terms related to hypophosphatemia were combined: blood phosphorus decreased, hypophosphatemia.24
TEAEs occurring in 10% or more of patients (N = 170) receiving futibatinib (20 mg) for advanced solid tumors and FGF/FGFR aberrations.17
Table 2.
Summary of TEAEs: FGFR inhibitors not yet approved
| Derazantinib ARQ 087–101 (N = 29)a,25 |
Rogaratinib 16443 (N = 126)b,18 |
||||
|---|---|---|---|---|---|
| AE, n (%) | Any grade | Grade ≥ 3 | AE, n (%) | Any grade | Grade ≥ 3 |
| Dry mouth | 13 (44.8) | 0 | hyperphosphatemia | 77 (61) | 1 (1) |
| Nausea | 13 (44.8) | 0 | diarrhea | 65 (52) | 3 (2) |
| Fatigue | 10 (34.5) | 1 (3.4) | decreased appetite | 48 (38) | 3 (2) |
| Asthenia | 10 (34.5) | 2 (6.9) | fatigue | 42 (33) | 12 (10) |
| Dysgeusia | 9 (31.0) | 0 | nausea | 37 (29) | 2 (2) |
| Vomiting | 9 (31.0) | 1 (3.4) | constipation | 33 (26) | 1 (1) |
| Alopecia | 7 (24.1) | 0 | anemia | 26 (21) | 9 (7) |
| Vision blurred | 7 (24.1) | 1 (3.4) | dry mouth | 26 (21) | 0 |
| Diarrhea | 6 (20.7) | 0 | alopecia | 25 (20) | 0 |
| ALT increased | 6 (20.7) | 1 (3.4) | arthralgia | 25 (20) | 0 |
| Dry eye | 5 (17.2) | 1 (3.4) | dysgeusia | 25 (20) | 0 |
| Decreased appetite | 5 (17.2) | 0 | urinary tract infection | 22 (17) | 8 (6) |
| AST increased | 5 (17.2) | 1 (3.4) | increased AST | 21 (17) | 1 (1) |
| Conjunctivitis | 4 (13.8) | 0 | back pain | 21 (17) | 2 (2) |
| Anemia | 3 (10.3) | 0 | dyspnea | 20 (16) | 8 (6) |
| Dry skin | 3 (10.3) | 0 | stomatitis | 19 (15) | 0 |
| Pruritus | 3 (10.3) | 0 | increased ALT | 18 (14) | 2 (2) |
| Visual acuity reduced | 3 (10.3) | 0 | increased blood creatinine | 18 (14) | 2 (2) |
| Dizziness | 2 (6.9) | 0 | dry skin | 18 (14) | 0 |
| Dermatitis | 2 (6.9) | 0 | increased lipase | 18 (14) | 10 (8) |
| Flatulence | 2 (6.9) | 0 | cough | 17 (13) | 1 (1) |
| Headache | 2 (6.9) | 0 | hypercalcemia | 17 (13) | 3 (2) |
| Neuropathy peripheral | 2 (6.9) | 0 | pyrexia | 17 (13) | 0 |
| Photophobia | 2 (6.9) | 0 | vomiting | 16 (13) | 2 (2) |
| Somnolence | 2 (6.9) | 0 | dry eye | 14 (11) | 0 |
| Thrombocytopenia | 2 (6.9) | 0 | insomnia | 14 (11) | 0 |
| Stomatitis | 2 (6.9) | 1 (3.4) | abdominal pain | 13 (10) | 1 (1) |
| Leukopenia | 1 (3.4) | 1 (3.4) | increased amylase | 13 (10) | 3 (2) |
| Upper gastrointestinal hemorrhage | 1 (3.4) | 1 (3.4) | asthenia | 13 (10) | 1 (1) |
| hemoptysis | 13 (10) | 3 (2) | |||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; FGFR, fibroblast growth factor receptor; TEAE, treatment-emergent adverse event.
TEAEs related to treatment occurring in patients (N = 29) receiving derazantinib for unresectable iCCA with FGFR2 fusion.25
TEAEs occurring in patients (N = 126) receiving rogaratinib for advanced cancers.18
Erdafitinib, an inhibitor of FGFR1–FGFR4,14 has been approved for treatment of locally advanced or metastatic UC with susceptible FGFR2 or FGFR3 genetic alterations that progressed during or following prior platinum-based chemotherapy.4 In a phase II study of erdafitinib in patients with locally advanced and unresectable or metastatic UC and FGFR alterations (N = 101), the objective response rate (ORR) was 40% (including 4 complete responses [CRs]), median progression-free survival (PFS) was 5.5 months, and median overall survival (OS) was 11.3 months.26
Pemigatinib is a potent inhibitor of FGFR1, FGFR2, and FGFR327 approved for previously treated, unresectable, locally advanced or metastatic CCA with FGFR2 fusion or other rearrangement and relapsed or refractory MLNs with FGFR1 rearrangement.5,28,29 In the primary analysis of the FIGHT-202 study (N = 146),15,24 ORR in patients with CCA and FGFR2 fusions or rearrangements was 35.5% (including 3 CRs), median PFS was 6.9 months, and median OS was 21.1 months.15 In a recent analysis of pemigatinib for treatment of MLN with FGFR1 rearrangements in FIGHT-203 (N = 34), the CR rate per investigator was 64.5%, and the complete cytogenic response rate per investigator was 72.7%.8
The FGFR1–FGFR4 inhibitor infigratinib30 is approved for adults with previously treated, unresectable, locally advanced or metastatic CCA with FGFR2 fusion or other rearrangement9 but has been withdrawn from distribution for CCA.10 In the pivotal phase II trial of infigratinib in patients with FGFR2 fusion or other rearrangement (N = 108), ORR was 23.1% (including 1 CR), median PFS was 7.3 months, and median OS was 12.2 months.15
The irreversible FGFR1–FGFR4 inhibitor futibatinib is also approved for adults with previously treated, unresectable, locally advanced or metastatic iCCA with FGFR2 gene fusions or other rearrangements.6 In the phase II FOENIX-CCA2 study in patients with previously treated, FGFR inhibitor-naive, locally advanced or metastatic unresectable iCCA and FGFR2 fusions or rearrangements (N = 103), the ORR was 41.7%, median PFS was 9.0 months, and median OS was 21.7 months.31 Other FGFR inhibitors, such as derazantinib,12 rogaratinib,18 and RLY-4008,11 are in clinical development (Table S1).
Overview of tolerability and AEs in clinical trials of FGFR inhibitors
Clinical studies for each of the FGFR inhibitors were reviewed for safety and tolerability data to provide an overview of AEs requiring dose adjustments and leading to study discontinuation. Available recommendations regarding management of these AEs from the prescribing information of licensed FGFR inhibitors are shown in Table 3.
Table 3.
Dose modification schemes for adverse reactions
| Adverse reaction | Dose modification |
|---|---|
| Erdafitinib4 | |
| Hyperphosphatemia | |
| Limit daily phosphate intake to 600–800 mg for all patients | |
| Serum phosphate 5.6–6.9 mg/dL | Maintain current dose of erdafitinib. |
| Serum phosphate 7.0–9.0 mg/dL | Withhold erdafitinib and assess serum phosphate concentration weekly. When the concentration is <5.5 mg/dL (or ≤ the patient’s baseline concentration), restart the same dose of erdafitinib. If the hyperphosphatemia lasted >1 week, then erdafitinib dose may be reduced. |
| Serum phosphate >9.0 mg/dL | Withhold erdafitinib and assess serum phosphate concentration weekly. When the concentration is <5.5 mg/dL (or ≤ the patient’s baseline concentration), restart erdafitinib 1 dose level lower than the previous dosage. |
| More than 10.0 mg/dL or significant alteration in baseline renal function or grade 3 hypercalcemia | Withhold erdafitinib and assess serum phosphate concentration weekly. When the concentration is <5.5 mg/dL (or ≤ the patient’s baseline concentration), restart erdafitinib 2 dose levels below the previous dosage. |
| Central serous retinopathy (CSR)/retinal pigment epithelial detachment (RPED) | |
| Grade 1: asymptomatic; clinical, or diagnostic observations only | Withhold erdafitinib until resolution. Resume at 1 dose level lower if CSR/RPED resolves within 4 weeks. Consider re-escalating dose if no CSR/RPED recurrence for a month. If CSR/RPED remains stable for 2 consecutive eye exams but has not resolved, then resume erdafitinib at the next lower dose level. |
| Grade 2: visual acuity 20/40 or better or ≤3 lines of decreased vision from baseline | Withhold erdafitinib until resolution. May resume at 1 dose level lower if CSR/RPED resolves within 4 weeks |
| Grade 3: visual acuity worse than 20/40 or >3 lines of decreased vision from baseline | Withhold erdafitinib until resolution. May resume at 2 dose levels lower if CSR/RPED resolves within 4 weeks. Consider permanent discontinuation if CSR/RPED recurs. |
| Grade 4: visual acuity 20/200 or worse in the affected eye | Permanently discontinue erdafitinib. |
| Other adverse reactions | |
| Grade 3 | Withhold erdafitinib until resolution to grade 1 or baseline. Then, erdafitinib may be resumed at 1 dose level lower. |
| Grade 4 | Permanently discontinue erdafitinib. |
| Pemigatinib5 | |
| Hyperphosphatemia | |
| Serum phosphate >7 to ≤10 mg/dL | Start phosphate-lowering therapy; measure serum phosphate levels weekly. Withhold pemigatinib if levels do not return to <7 mg/dL within 2 weeks of initiating phosphate-lowering therapy. After the first occurrence, resume pemigatinib at the same dose when phosphate levels are <7 mg/dL; after subsequent recurrences, resume pemigatinib at a lower dose level. |
| Serum phosphate >10 mg/dL | Start phosphate-lowering therapy; measure serum phosphate levels weekly. Withhold pemigatinib if levels do not return to ≤10 mg/dL within 1 week of initiating phosphate-lowering therapy. When phosphate levels are <7 mg/dL, resume pemigatinib at 1 dose level lower. If serum phosphate >10 mg/dL recurs following 2 dose reductions, permanently discontinue pemigatinib. |
| Retinal pigment epithelial detachment (RPED) | |
| Continue pemigatinib if RPED is stable on serial examination and asymptomatic. Withhold pemigatinib if RPED is worsening on serial examination or symptomatic. Resume pemigatinib at a lower dose if RPED is improved on subsequent examination and asymptomatic. Consider permanently discontinuing pemigatinib, based on clinical status, if examination does not improve or symptoms persist. | |
| Other adverse reactions | |
| Grade 3 | Withhold pemigatinib until resolution to grade 1 or baseline. If resolution occurs within 2 weeks, resume pemigatinib at 1 dose lower. If resolution does not occur within 2 weeks, permanently discontinue pemigatinib. If grade 3 AEs recur after 2 dose reductions, permanently discontinue pemigatinib. |
| Grade 4 | Permanently discontinue pemigatinib. |
| Futibatinib6 | |
| Hyperphosphatemia | |
| Serum phosphate ≥5.5 to ≤7 mg/dL | Initiate phosphate-lowering therapy and continue futibatinib at the current dose. Monitor serum phosphate levels weekly. |
| Serum phosphate >7 to ≤10 mg/dL | Initiate or adjust phosphate-lowering therapy. Monitor serum phosphate levels weekly and reduce futibatinib to the next lower dose. If the serum phosphate concentration resolves to ≤7 mg/dL within 2 weeks after dose reduction, continue at this reduced dose. If serum phosphate concentration does not reach ≤7 mg/dL within 2 weeks, further reduce futibatinib to the next lower dose. If serum phosphate concentration does not reach ≤7 mg/dL within 2 weeks after the second dose reduction, withhold futibatinib until serum phosphate concentration is ≤ 7 mg/dL and resume at the dose prior to treatment interruption. |
| Serum phosphate >10 mg/dL | Withhold futibatinib until serum phosphate concentration is ≤7 mg/dL and resume futibatinib at the next lower dose; initiate or adjust phosphate-lowering therapy and monitor serum phosphate levels weekly. Permanently discontinue futibatinib if serum phosphate concentration does not reach ≤7 mg/dL within 2 weeks following 2 dose interruptions and reductions. |
| RPED | |
| Continue futibatinib at the current dose and continue periodic ophthalmic evaluation. If RPED resolves within 14 days, continue futibatinib at the current dose. If RPED does not resolve within 14 days, withhold futibatinib until RPED resolves, then resume futibatinib at previous or lower dose. | |
| Other adverse reactions | |
| Grade 3 | Withhold futibatinib until toxicity resolves to grade 1 or baseline. For hematologic toxicities resolving within 1 week, resume futibatinib at the dose prior to dose interruption. For other adverse reactions, resume futibatinib at the next lower dose. |
| Grade 4 | Permanently discontinue futibatinib. |
| Starting dose | Dose reductions |
|||||
|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | ||
| Erdafitiniba,4 | 9 mg QD (three 3-mg tablets) | 8 mg QD (two 4-mg tablets) | 6 mg QD (two 3-mg tablets) | 5 mg QD (one 5-mg tablet) | 4 mg QD (one 4-mg tablet) | discontinue erdafitinib |
| Pemigatinib5 | 13.5 mg (one 13.5-mg tablet) QD for first 14 days of each 21-day cycle | 9 mg (one 9-mg tablet) QD for first 14 days of each 21-day cycle | 4.5 mg (one 4.5-mg tablet) QD for first 14 days of each 21-day cycle | discontinue pemigatinib | ||
| Futibatinib6 | 20 mg QD (five 4-mg tablets) | 16 mg QD (four 4-mg tablets) | 12 mg QD (three 4-mg tablets) | discontinue futibatinib | ||
QD, once daily.
Patients may also start at 8 mg QD and, if needed, decrease dosage from there.
In the BLC2001 phase II study of erdafitinib in 99 patients with previously treated advanced UC and alterations in FGFR3 or FGFR2, 13% of patients discontinued because of an AE.14 Dose reductions occurred in 56% of patients, most frequently because of stomatitis (16%) and hyperphosphatemia (9%). AEs followed a similar pattern in the phase I study of erdafitinib in patients with advanced solid tumors,32 with the most commonly reported treatment-emergent AEs (TEAEs) being hyperphosphatemia (65%), asthenia (55%), dry mouth (45%), nail toxicity (35%), constipation (34%), decreased appetite (32%), and dysgeusia (31%). Dose interruptions were required in 45% of patients, 17% had dose reductions, and 5% discontinued treatment because of TEAEs.
In the FIGHT-202 phase II study of pemigatinib among 146 patients with previously treated, locally advanced or metastatic CCA, 9% discontinued treatment because of an AE, most frequently intestinal obstruction (n = 2) and acute kidney injury (n = 2).24 Dose interruptions because of AEs were reported in 42% of patients, most frequently for stomatitis (n = 11), palmar-plantar erythrodysesthesia syndrome (PPES; n = 8), arthralgia (n = 7), fatigue (n = 6), and abdominal pain (n = 4). Dose reductions because of AEs occurred in 14% of patients, most frequently for stomatitis (n = 5), PPES (n = 5), arthralgia (n = 5), asthenia (n = 2), and onychomadesis (n = 2).24 TEAEs were generally similar among patients with FGFR1-rearranged MLN treated with pemigatinib in FIGHT-203 and patients in the FIGHT-202 study: hyperphosphatemia (68% and 60%, respectively), alopecia (59% and 49%, respectively), diarrhea (50% and 47%, respectively), and stomatitis (44% and 35%, respectively).8,24 Anemia was reported in 35% of patients in FIGHT-203 versus 14% in FIGHT-202,24 which may be related to the patients’ underlying hematologic malignancy. Grade 3 or greater TEAEs occurring in 10% or more of patients in FIGHT-203 were anemia (18%) and pain in an extremity and stomatitis (both 12%).8 Hypophosphatemia (12%) was the only grade 3 or greater TEAE occurring in 10% or more of patients in FIGHT-202.24
In the CBGJ398X2204 phase II study of infigratinib among 108 patients with previously treated, unresectable, locally advanced or metastatic CCA with an FGFR2 fusion or other rearrangement, 15% permanently discontinued treatment because of an AE, most commonly because of subretinal fluid, fatigue, or increased blood creatinine concentration (2 patients each).15 Dose interruptions because of AEs were reported in 64% of patients. Dose reductions because of AEs occurred in 60% of patients, most frequently hyperphosphatemia (n = 28) and stomatitis (n = 13). The AE profile of infigratinib was similar in the phase I study in patients with solid tumors harboring FGFR alterations, where hyperphosphatemia (74%), constipation (40%), and decreased appetite (40%) were the most frequently reported TEAEs, 59% of patients experienced a dose adjustment or interruption because of an AE, and 14% had an AE leading to treatment discontinuation.33
Dose interruptions and reductions because of treatment-related AEs (TRAEs) were reported in 50% and 54% of 103 patients with iCCA receiving futibatinib in FOENIX-CCA2, respectively; 2% of patients discontinued because of TRAEs.31 In the phase Ib FIDES-02 study of derazantinib in patients with solid tumors, 19% of patients had dose interruptions or reductions because of TRAEs, and 8% discontinued because of TRAEs.34 In the phase I study of rogaratinib in 126 patients with advanced FGFR-altered cancers, 32% had a dose reduction and 6 discontinued treatment because of TRAEs.18 In preliminary results from a phase I/II study of RLY-4008 in patients with advanced solid tumors, the most common AEs were stomatitis (48%), PPES (46%), and dry mouth (31%).11 In a phase I study of RLY-4008, minimal hyperphosphatemia and diarrhea were observed, which is consistent with the FGFR2-selective mechanism of RLY-4008.35
Common AEs associated with licensed FGFR inhibitors and their practical management
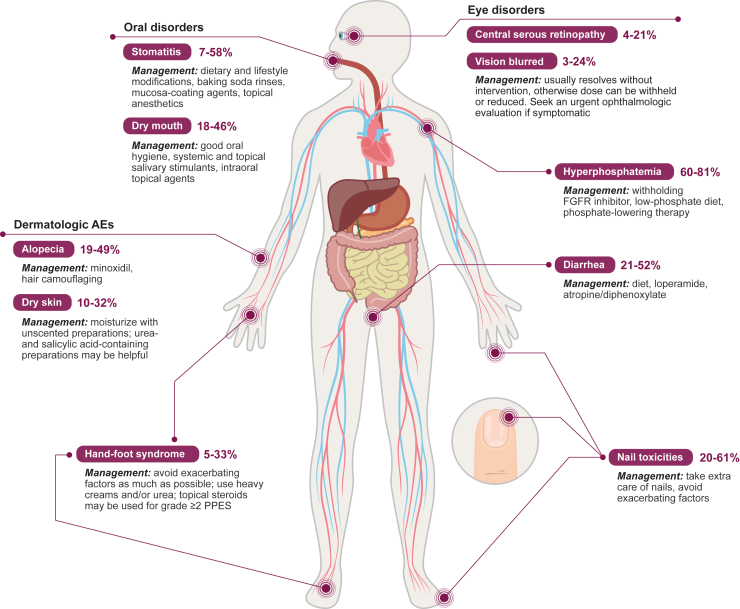
Tables 1 and 2 show the most common TEAEs associated with erdafitinib,14 pemigatinib,24 futibatinib,17 derazantinib,25 and rogaratinib.18 Some toxicities particularly common to FGFR inhibitors include hyperphosphatemia, nail toxicities, eye toxicities, dermatologic AEs (including PPES), and gastrointestinal AEs (Figure 1). Photographic examples of some of these common toxicities are shown in Figure S1.
Figure 1.
Common AEs associated with FGFR inhibitors and their management
AE, adverse event; FGFR, fibroblast growth factor receptor; PPES, palmar-plantar erythrodysesthesia syndrome.
Hyperphosphatemia and hypophosphatemia
Hyperphosphatemia is one of the most frequently reported AEs in clinical trials of FGFR inhibitors, occurring as a TEAE in 77% of patients receiving erdafitinib in BLC2001,14 60% of patients receiving pemigatinib in FIGHT-202,24 and 77% of patients receiving infigratinib in NCT0215096715 and as a TRAE in 85% of patients receiving futibatinib in FOENIX-CCA2.31 Considering FGFR involvement in phosphate homeostasis via feedback mechanisms involving FGF23, klotho, and 1,25-dihydroxyvitamin D,19,36 the high incidence of hyperphosphatemia is an expected on-target AE of FGFR inhibition and an FGFR inhibitor class effect.36,37 Median time to onset of hyperphosphatemia was 8 days with infigratinib (interquartile range, 8–15)15 and 15 days for pemigatinib (95% confidence interval, 8–47) and derazantinib (confidence interval not available).24,25
Whereas hyperphosphatemia was the most common toxicity in all FGFR inhibitor trials, in most cases it was mitigated with appropriate management. In FIGHT-202, although hyperphosphatemia was the most frequently occurring TEAE (60%), all events were grade 1–2; patients were managed with a low-phosphate diet and received concomitant phosphate binders (18%) and diuretics (1%), 1 patient (1%) had a dose reduction, and 2 patients (1%) had a dose interruption.24 In the CBGJ398X2204 study with infigratinib, no patients discontinued treatment because of hyperphosphatemia. Most patients (81%) took a phosphate binder either prophylactically (48%) or after the first dose of infigratinib (32%).15 Hyperphosphatemia was the most frequent grade 3 TRAE in the FOENIX-CCA2 study of futibatinib in CCA, reported in 30% of patients, and was resolved with adequate management over a median of 7 days.31 Differences in hyperphosphatemia severity may possibly be attributed to the different inhibitory profiles and mechanisms of action of FGFR inhibitors; erdafitinib, pemigatinib, and infigratinib are reversible FGFR inhibitors,27,30,38 whereas futibatinib is an irreversible inhibitor.39
Hypophosphatemia was a notable TEAE in FIGHT-202 (pemigatinib) and CBGJ398X2204 (infigratinib), occurring in 10% and 22% of patients, respectively.15,24 This could possibly be a result of treatment for management of hyperphosphatemia symptoms (e.g., continued use of a low-phosphate diet or phosphate binders during the pemigatinib off-treatment week or negative feedback on phosphate homeostasis).24
Given the frequency of hyperphosphatemia and hypophosphatemia seen with FGFR inhibitors, proactive management may be key to minimize the need for dose reductions and drug discontinuation.
Practical strategies for hyperphosphatemia and hypophosphatemia management
Hyperphosphatemia can be managed via monitoring, a low-phosphate diet, phosphate-lowering therapy, and withholding or reducing the FGFR inhibitor. Information on low-phosphate diets is widely available and should be discussed with the patient using a multidisciplinary approach.40 The phosphate pyramid is a useful tool to illustrate the phosphate content of various foods to patients.41 Examples of high-phosphate foods and low-phosphate alternatives are shown in Table S2. Patients should be educated to look for “hidden” phosphates in additives as well as concomitant medications.42 Boiling, which reduces the phosphate content of food, can also be suggested as the preferred cooking technique.40
Serum phosphate concentrations should be monitored throughout treatment at regular intervals.4,5,6 For serum phosphate levels greater than 7 mg/dL, the FGFR inhibitor may be withheld or the dose reduced or permanently discontinued, and phosphate-lowering therapy should be introduced.4,5,6 For serum phosphate levels greater than 5.5 mg/dL for pemigatinib, a low-phosphate diet should be initiated;5 for futibatinib, phosphate-lowering therapy should be started.6 Readily available and traditional phosphate-lowering therapies include antacids, such as calcium carbonate (500 mg [chewable] or 600 mg [elemental]) tablets 3 times per day, and sevelamer hydrochloride.43 If a patient has hypercalcemia and hyperphosphatemia, then sevelamer is preferred. If a patient has only hyperphosphatemia with no elevation in calcium levels, then calcium carbonate may be used. When initiating phosphate binders, the lowest possible dose should be used to mitigate development of hypophosphatemia.19 Acetazolamide may also be used to reduce hyperphosphatemia.44
To manage hypophosphatemia in patients with cancer, focus on the underlying cause first.45 Hypophosphatemia is postulated to arise because of prophylactic low-phosphate diets or phosphate binders,24 and thus first steps may be eliminating phosphate binders and returning to a higher-phosphate diet. If this approach fails, then oral phosphate formulations with either sodium or potassium phosphate salts can be used. In chronic cases where phosphate is not being absorbed through the gastrointestinal tract, parenteral phosphate replacement may be necessary.45
Nail toxicities
Changes in the nails are a known on-target toxicity of FGFR inhibitors and were reported in the pivotal trials of erdafitinib, pemigatinib, infigratinib, and futibatinib.14,15,24 In the BLC2001 study of erdafitinib in patients with advanced UC, the following nail toxicities were reported (grade ≥3 events in parentheses): onycholysis 18% (2%), paronychia 17% (3%), nail dystrophy 16% (6%), and nail disorder 8% (3%).14 In FIGHT-202 in patients with CCA treated with pemigatinib, nail discoloration occurred in 10% of patients (1% grade ≥3), onychoclasis in 6% (1%), paronychia in 7% (1%), and nail disorder in 3% (1%).24 In the CBGJ398X2204 study of infigratinib in patients with CCA, nail discoloration occurred in 18% of patients (0% grade ≥3), onychomadesis in 16% (0%), nail disorder in 15% (0%), and onycholysis in 12% (0%).15 In the phase I dose-expansion study of futibatinib, onycholysis occurred in 6% of patients, nail disorder in 5%, and paronychia in 4%; all nail toxicities were grade 1 or 2 in severity, apart from 1 case of grade 3 onychalgia.17
Practical strategies for nail toxicity management
Physicians should advise patients receiving FGFR inhibitors that they may experience changes to their nails while on treatment. Nails may darken or develop white streaks or ridges, or they may become brittle, dry, and cracked and may lift up from the nailbed.
Patients should be advised to take special care of nails to reduce the chance of infection and nail loss. They should gently trim or file nails and not trim too close to the nail bed. Patients should not obtain professional manicures or pedicures unless approved by their health care team. Moisturizing lotions and creams should be used to keep nails and cuticles healthy. Gloves should be worn while working around the house or yard. Patients should not use nail-strengthening products because they may irritate their skin or nails. Artificial nails may contribute to the growth of fungal infections and may mask nail changes caused by cancer treatment and thus should be avoided. Patients should be asked to report any redness, pain, or other changes that occur around their cuticles to the health care team. If nails appear infected, then cultures should be sent for bacterial sensitivity, and patients should initiate antibiotics and obtain a dermatology referral.
Eye toxicities
Ocular AEs, ranging from dry eye to serious retinal disorders, are other on-target toxicities of FGFR inhibitors. FGFR inhibition interferes with downstream mitogen-activated protein kinase (MAPK)-mediated signaling, dysregulation of which has been associated with retinopathy.46 Therefore, the mechanism underlying FGFR inhibitor-associated retinopathy may, in part, involve abrogation of MAPK signaling. Supporting this, retinopathy is commonly associated with inhibitors of the MAPK pathway, including extracellular signal-regulated kinase (ERK) inhibitors and MAPK kinase (MEK) inhibitors.46 Central serous retinopathy occurred in 21% of patients (3% grade ≥3) receiving 8 mg continuous erdafitinib in BLC2001 (N = 99).14 Most (76%) central serous retinopathy AEs resolved following dose interruption or reduction or administration of concomitant medications; all unresolved events were grade 1 or 2. Three patients discontinued because of central serous retinopathy. Ocular AEs other than central serous retinopathy were reported in 52% of patients (5% grade ≥3). The most common other ocular AEs were dry eye (19%), blurry vision (16%), increased lacrimation (11%), and conjunctivitis (9%).
In the FIGHT-202 study of pemigatinib, 4% of patients had AEs related to serous retinal detachment because of subretinal fluid accumulation.24 Most events were grade 1 or 2; the sole grade 3 event was considered unrelated to treatment. One patient experienced treatment dose interruption related to serous retinal detachment. Other ocular AEs reported in FIGHT-202 included dry eye in 25% of patients (1% grade ≥3) and keratitis and blurred vision in 2% (1%) each.24 Among 466 patients in clinical trials of pemigatinib, retinal pigment epithelial detachment (RPED) occurred in 6% of patients (1% grade ≥3).5 Among patients whose pemigatinib dose was reduced because of RPED, 87.5% experienced resolution or improvement to grade 1.
In CBGJ398X2204 with infigratinib, treatment-emergent central serous retinopathy-like and RPED-like events occurred in 17% of patients (1% grade ≥3).15 Other ocular TEAEs included dry eye in 34% (1% grade ≥3), blurred vision in 21% (0%), blepharitis in 11% (0%), and trichiasis in 11% (0%).15 In the NCT02052778 phase I dose-expansion study with futibatinib, retinal disorders were reported in 8% of patients; all were grade 1–2.31
In a recent retrospective case series of 146 patients with solid tumors treated with FGFR inhibitors, 20 (13.7%) showed FGFR inhibitor-associated retinopathy.46 Median time to subretinal fluid detection was 21 days; median time to resolution was 64 days. Retinopathy occurred at the same time for both eyes, and the FGFR inhibitor dose was not associated with subretinal fluid detection. No patient interrupted or discontinued treatment solely because of retinopathy. In all cases, the fluid was self-limiting and did not require intervention. Analysis of the location and number of fluid foci per eye showed that they were predominantly bilateral, unifocal, and relatively symmetric between both eyes (Figure S2). Six of 20 patients (30%) reported symptoms at the time of fluid accumulation. At fluid accumulation, 11 eyes (28%) had decreased vision; the median change from baseline in best-corrected visual acuity was −0.1. The median change in best-corrected visual acuity from baseline to resolution of fluid was 0.46 The case series authors recommended that patients receiving FGFR inhibitors with new visual symptoms should obtain an ophthalmic assessment with optical coherence tomography (OCT).
Practical strategies for eye toxicity management
Prescribing information for erdafitinib, pemigatinib, and futibatinib recommends comprehensive eye examinations, including OCT, before treatment initiation and frequently for the first few months thereafter.4,5,6 Patients should be urgently referred for ophthalmologic evaluation when they develop visual symptoms, and the FGFR inhibitor may be withheld, its dose modified, or discontinued according to the prescribing information for each drug. Follow-up every 3 weeks is recommended for patients with symptoms who continue FGFR inhibitor treatment.4,5,6
A baseline ophthalmologic evaluation before starting FGFR inhibitors is recommended for all patients, along with education regarding the potential for ocular toxicities. Because these AEs are on-target toxicities associated with FGFR inhibitors, they require proactive management. Ocular toxicities can be managed by dose modification with the goal of maintaining treatment compliance and, thus, benefit. Although some serious ocular toxicities require discontinuation of FGFR inhibitor treatment (e.g., grade 4 RPED), others (e.g., subretinal fluid accumulation) can typically be resolved by dose interruption or reduction.
Dermatologic AEs: Hand-foot syndrome/PPES
PPES is a common toxicity associated with FGFR inhibitors and other tyrosine kinase inhibitors and is distinct from the hand-foot syndrome observed with cytotoxic agents such as capecitabine and doxorubicin. PPES occurring with FGFR inhibitors is characterized by focal calluses, hyperkeratosis, erythema, and fissures found mostly on fingers and toes.47 PPES occurred in 23% of patients (5% grade ≥3) in the BLC2001 study of erdafitinib,14 in 11% in the FIGHT-202 study of pemigatinib (4% grade ≥3),24 and in 33% of patients (6% grade ≥3) in the CBGJ398X2204 study of infigratinib.15 As of May 2019, 13% of patients (4% grade ≥3) in the phase I dose-expansion study with futibatinib had reported PPES.17 Two patients discontinued erdafitinib treatment,14 5 patients had a pemigatinib dose reduction,24 and 5% had a futibatinib dose reduction because of PPES (not reported for infigratinib).17
Practical strategies for PPES management
Patients receiving FGFR inhibitors who develop PPES should limit exposure of hands and feet to heat sources, keep showers or baths in warm water short, and use warm instead of hot water when washing dishes. Cold water may be applied to hands and feet to provide relief. Patients should try to avoid pressure and rubbing of the skin and should avoid wearing tight socks or gloves. Patients should avoid contact of the hands and feet with hard surfaces, such as while walking barefoot or playing sports, or using their hands with tools such as knives, hammers, or gardening tools. Patients should take short walks and alter their exercise routine so that they do not spend extended time on their feet.
Patients may use thick creams, including those containing lanolin, to keep their skin moisturized. Moisturizing lotions may be used, but creams are better.
Management of PPES involves use of keratolytic agents, such as 40% urea and/or salicylic acid, to exfoliate hyperkeratotic calluses in the case of grade 1 or higher symptoms, along with cushioning of the affected areas with gel inserts or use of soft shoes. Grade 3 or higher PPES can be managed with supportive measures such as cortisone creams and topical antibiotics.
Other dermatologic AEs
Dermatologic changes have been frequently reported in clinical studies of FGFR inhibitors.14,15,17,24 However, the mechanisms underlying FGFR inhibitor-associated dermatologic toxicities are not fully understood.47 In the BLC2001 study of erdafitinib, dry skin was reported in 32% of patients (0% grade ≥3) and alopecia in 29% of patients (0% grade ≥3).14 Two patients discontinued treatment because of skin events.14 In the FIGHT-202 study with pemigatinib, alopecia was reported in 49% of patients (0% grade ≥3) and dry skin in 20% of patients (1% grade ≥3).24 In the phase II study of infigratinib, alopecia was reported in 38% of patients (0% grade ≥3) and dry skin in 23% of patients (0% grade ≥3).15 In the phase I dose-expansion study of futibatinib, 19% of patients experienced alopecia (0% grade ≥3), and 13% had dry skin (0% grade ≥3); 1 patient discontinued because of grade 2 eczema.17
Practical strategies for dermatologic AE management
Patients treated with FGFR inhibitors are not helped by the preventive measures often used for alopecia during chemotherapy treatment, such as scalp cooling or compression.48 Prophylactic or reactive use of topical minoxidil is recommended instead. Hair camouflaging methods also may be used. Alopecia typically reverses when treatment is discontinued.
Dry skin can be ameliorated with moisturizing lotions and creams. Patients should be advised to minimize exposure to fragranced detergents and soaps. Urea preparations and salicylic acid preparations may be helpful. Low-potency topical steroids may be used for grade 3 dry skin.
Specific guidance on managing alopecia and dry skin is not addressed in the prescribing information of any of the licensed FGFR inhibitors. Patients should consult with a health care professional when they experience progressive or intolerable skin disorders. When needed, the generic advice on managing grade 3 or 4 AEs by withholding doses, reducing the dose, or discontinuing treatment for each drug should be followed.4,5,6
Gastrointestinal AEs
Gastrointestinal AEs are common with FGFR inhibitors. Diarrhea may be mediated by FGFR4 signaling, for which FGFR inhibitors have varying degrees of selectivity.19 Inhibition of FGF19/FGFR4-mediated signaling upregulates conversion of cholesterol to bile acid in the liver, leading to modified bile acid metabolism.49 Bile acid metabolism imbalance has been demonstrated to increase intestinal water secretion, increase mucosal permeability, and stimulate peristalsis, which therefore may result in diarrhea.50 In the BLC2001 study of erdafitinib, stomatitis was reported in 58% of patients (10% grade ≥3), diarrhea in 51% (4%), dry mouth in 46% (0%), decreased appetite in 38% (0%), dysgeusia in 37% (1%), constipation in 28% (1%), nausea in 20% (1%), and vomiting in 13% (2%).14 Stomatitis was one of the most common grade ≥3 AEs with erdafitinib and led to a dose reduction in 16 patients.14 In the FIGHT-202 study of pemigatinib, dysgeusia was reported in 40% of patients (0% grade ≥3), diarrhea in 47% (3%), stomatitis in 35% (5%), dry mouth in 34% (0%), nausea in 40% (2%), decreased appetite in 33% (1%), and constipation in 35% (1%).24 Five patients had a dose reduction and 11 had a dose interruption because of stomatitis.24 In the CBGJ398X2204 study of infigratinib, treatment-emergent stomatitis was reported in 55% of patients (15% grade 3), dysgeusia in 31% (0%), constipation in 30% (1%), dry mouth in 25% (0%), diarrhea in 24% (3%), decreased appetite in 22% (1%), and vomiting in 21% (1%).15 In the dose-expansion study with futibatinib as of June 2019, diarrhea was reported in 33% of patients (1% grade 3), constipation in 32% (1%), nausea in 28% (0%), vomiting in 25% (1%), abdominal pain in 19% (3%), decreased appetite in 19% (2%), dry mouth in 18% (0%), stomatitis in 15% (3%), and dysgeusia in 11% (0%).17 Three patients discontinued treatment because of gastrointestinal TRAEs (one with grade 3 oral mucositis, one with grade 3 vomiting and grade 1 diarrhea and nausea, and one with grade 2 diarrhea, fatigue, anorexia, and nail detachment).17
Practical strategies for gastrointestinal AE management
Baking soda rinses can be used to ameliorate stomatitis: half a teaspoon (about 2.5 g) baking soda mixed with 8 fluid ounces (about 240 mL) of warm water, swished around the mouth for 30 s, then spat out. The rinse may also be gargled for several seconds prior to spitting. Patients should rinse every 2 or 3 h while they are awake, including after meals and at bedtime, but not more than 6 times a day. Mucosa-coating agents are available by prescription. These should be swished around the inside of the patient’s mouth to coat it. Swallowing these agents may help soothe the throat, but they may be spat out if the patient feels nauseous. Topical anesthetics in the forms of thick liquids, gels, or sprays may also be useful.
Dietary and lifestyle modifications can also help. Patients should choose soft, moist foods that are easy to swallow and avoid rough-textured, acidic, tart, and spicy foods that may cause irritation. They should also avoid extremely hot and cold foods. Patients should cut foods into small bites to reduce chewing. If use of spoons or forks causes pain, blended meals may be drunk from a cup. Foods can be pureed or liquefied using a blender. Drinking liquids through a straw can help avoid painful areas in the mouth.
For patients with diarrhea, loperamide is available without a prescription and is usually the medication used to treat diarrhea. Patients should take 2 capsules at the first sign of diarrhea, followed by 1 capsule after each loose stool. Unless directed otherwise, patients should not exceed 16 mg per day and should reduce or eliminate treatment if they experience constipation. Second-choice treatment is atropine/diphenoxylate, which requires a prescription. Patients should take 1 tablet by mouth every 6 h as needed. Common adverse effects include drowsiness, constipation, and dizziness. Patients may alternate between loperamide and atropine/diphenoxylate because they do not interact and can be used together safely. Patients should contact their care team if they are taking these medicines as directed and symptoms are not controlled or if they have trouble swallowing pills. While having diarrhea, patients should stop taking stool softeners and laxatives and should maintain a low-fiber diet. Patients could start with the BRAT (bananas, rice, applesauce, toast) diet and then progress to foods low in fiber, such as skinless chicken, scrambled eggs, crackers, and white bread.
For patients with dry mouth, emphasize the importance of good oral hygiene and regular dental visits. Systemic and topical salivary stimulants and intraoral topical agents, such as chewing gums, and saliva stimulants and substitutes can ameliorate symptoms. High-fluoride toothpaste may be helpful to prevent cavities.47
Dosing schedules
During the development of a number of these agents, attempts were made to find the optimal balance between efficacy and tolerability by altering the dosing schedule, with varying outcomes. In the phase I study of erdafitinib, continuous daily dosing and intermittent dosing (7 days on/7 days off) were investigated.32 Erdafitinib at 10 mg daily on the intermittent dosing schedule was found to have clinical activity with a manageable safety profile. The phase II study in patients with advanced UC was initiated with the 10-mg intermittent regimen and a 6-mg continuous regimen, which was amended during an interim analysis to just a continuous dosing regimen of 8 mg per day,14 and this dose was approved.4 Pemigatinib was also investigated at continuous and intermittent dosing schedules (14 days on/7 days off) in its first-in-human study.48,51 Pharmacokinetics, pharmacodynamics, and clinical data were combined to determine that 13.5 mg once daily on the intermittent schedule should be investigated in phase II,24,48,51 and this dose was approved.5 The phase I study of infigratinib examined dosing on a continuous or 3-weeks-on/1-week-off schedule. Safety profiles were similar between the groups, but fewer patients experienced AEs requiring dose adjustments or interruptions with the intermittent schedule (50%) than the continuous schedule (74%).33 The intermittent dosing schedule was taken into the phase II study and is the approved schedule.9,15 Futibatinib was investigated in a once-daily continuous and 3-times-a-week continuous dosing regimen in its phase I study. Although the safety profile was consistent across the dosing schedules, once-daily dosing was selected for phase II studies based on a closer correlation between serum pharmacokinetics and pharmacodynamics (serum phosphate levels) with this schedule compared with the thrice-weekly schedule.52 Clearly, each FGFR inhibitor requires individualized dosing to balance efficacy, pharmacokinetics, and safety.
Conclusions
FGFR inhibitors have similar but distinct safety profiles, depending on their activity toward specific FGFRs; differential inhibition of specific FGFRs may influence the frequency and severity of side effects. For example, the degree of hyperphosphatemia may be related to the strength of inhibition of FGFR1, while the incidence and severity of diarrhea may be a function of FGFR4 inhibition.19 Therefore, adverse management strategies need to be individualized to each patient and the treatment they are receiving. Early consultation and involvement of specialists (e.g., ophthalmologists, dermatologists) during treatment to manage and mitigate FGFR inhibitor-associated AEs may benefit patients’ quality of life and outcomes. Most FGFR inhibitor-associated AEs are manageable with appropriate prophylactic measures and treatment; however, proactive monitoring is key to minimizing the need for dose reduction and allowing patients to continue treatment.
Acknowledgments
The authors thank all motivated patients and caregivers who enrolled in the clinical trials and the clinical trial support staff at all enrollment sites. This manuscript was sponsored by Incyte Corporation (Wilmington, DE, USA). Medical writing assistance was provided by Peijia (Jessica) Yuan, PhD, and Simon Slater, PhD, of Envision Pharma Group (Philadelphia, PA, USA), funded by Incyte Corporation.
Author contributions
Conceptualization, V.S. and S.V.; writing – original draft, V.S. and S.V.; writing – review & editing, V.S. and S.V.
Declaration of interests
V.S. was affiliated with UT MD Anderson Cancer Center at the time of submission. At the time of submission, V.S. reports a consulting or advisory role with Incyte Corporation; grants from Eli Lilly/Loxo Oncology, Blueprint Medicines Corporation, Turning Point Therapeutics, and Boston Pharmaceuticals; research funding and a consulting or advisory role with Eli Lilly/Loxo Oncology; research funding from Roche/Genentech, Bayer, GlaxoSmithKline, Helsinn Pharmaceuticals, NanoCarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berg Health, Incyte, Fujifilm, D3, Pfizer, MultiVir, Amgen, AbbVie, Alfasigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum Q10, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCICTEP, University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, Pharmamar, and MedImmune; an advisory board/consultant position with Helsinn, Incyte Corporation, QED Pharma, Daiichi-Sankyo, Signant Health, Novartis, Janssen, Relay Therapeutics, Roche, and MedImmune; travel funds from Pharmamar, Incyte Corporation, ASCO, and ESMO; and other support from Medscape. S.V. has received honoraria and research support from Incyte Corporation.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101204.
Supplemental information
References
- 1.Brooks A.N., Kilgour E., Smith P.D. Molecular pathways: fibroblast growth factor signaling: a new therapeutic opportunity in cancer. Clin. Cancer Res. 2012;18:1855–1862. doi: 10.1158/1078-0432.CCR-11-0699. [DOI] [PubMed] [Google Scholar]
- 2.Murugesan K., Necchi A., Burn T.C., Gjoerup O., Greenstein R., Krook M., López J.A., Montesion M., Nimeiri H., Parikh A.R., et al. Pan-tumor landscape of fibroblast growth factor receptor 1-4 genomic alterations. ESMO Open. 2022;7:100641. doi: 10.1016/j.esmoop.2022.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krook M.A., Reeser J.W., Ernst G., Barker H., Wilberding M., Li G., Chen H.Z., Roychowdhury S. Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br. J. Cancer. 2021;124:880–892. doi: 10.1038/s41416-020-01157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen Oncology . Highlights of Prescribing Information; 2022. BALVERSA® (Erdafitinib) Tablets, for Oral Use. [Google Scholar]
- 5.Incyte Corporation . Highlights of Prescribing Information; 2022. PEMAZYRE® (Pemigatinib) Tablets, for Oral Use. [Google Scholar]
- 6.Taiho Oncology . 2022. LYTGOBI® (Futibatinib) Tablets, for Oral Use. Highlights of Prescribing Information. [Google Scholar]
- 7.US Food & Drug Administration . 2022. FDA Approves Pemigatinib for Relapsed or Refractory Myeloid/lymphoid Neoplasms with FGFR1 Rearrangement. [Google Scholar]
- 8.Gotlib J., Kiladjian J.-J., Vannucchi A., Rambaldi A., Reiter A., Shomali W., George T.I., Patel J.L., Colucci P., Walker C., et al. A phase 2 study of pemigatinib (FIGHT-203; INCB054828) in patients with myeloid/lymphoid neoplasms (MLNs) with fibroblast growth factor receptor 1 (FGFR1) rearrangement (MLN FGFR1) Blood. 2021;138:385. [Google Scholar]
- 9.QED Therapeutics Inc . Highlights of Prescribing Information; 2021. TRUSELTIQ (Infigratinib) Capsules, for Oral Use. [Google Scholar]
- 10.Helsinn Healthcare SA . NOTICE OF PERMANENT DISCONTINUATION OF DISTRIBUTION; 2022. TRUSELTIQ® (INFIGRATINIB) CAPSULES. [Google Scholar]
- 11.Park J.O., Wai Meng D.T., Hollebecque A., Borad M., Goyal L., Schram A., Cassier P., Kamath S.D., Dotan E., Kim R., et al. 76MO Efficacy of RLY-4008, a highly selective FGFR2 inhibitor in patients (pts) with an FGFR2-fusion or rearrangement (f/r), FGFR inhibitor (FGFRi)-naïve cholangiocarcinoma (CCA): ReFocus trial. Ann. Oncol. 2022;33:S1461–S1462. [Google Scholar]
- 12.Javle M.M., Shaib W.L., Braun S., Engelhardt M., Borad M.J., Abou-Alfa G.K., Boncompagni A., Friedmann S., Gahlemann C.G. FIDES-01, a phase II study of derazantinib in patients with unresectable intrahepatic cholangiocarcinoma (iCCA) and FGFR2 fusions and mutations or amplifications (M/A) J. Clin. Oncol. 2020;38:TPS597. [Google Scholar]
- 13.Quinn D.I., Petrylak D.P., Bellmunt J., Necchi A., Gurney H., Lee J.-L., Van Der Heijden M.S., Rosenbaum E., Penel N., Pang S.-T., et al. FORT-1: phase II/III study of rogaratinib versus chemotherapy (CT) in patients (pts) with locally advanced or metastatic urothelial carcinoma (UC) selected based on FGFR1/3 mRNA expression. J. Clin. Oncol. 2020;38:489. doi: 10.1200/JCO.21.02303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loriot Y., Necchi A., Park S.H., Garcia-Donas J., Huddart R., Burgess E., Fleming M., Rezazadeh A., Mellado B., Varlamov S., et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2019;381:338–348. doi: 10.1056/NEJMoa1817323. [DOI] [PubMed] [Google Scholar]
- 15.Javle M., Roychowdhury S., Kelley R.K., Sadeghi S., Macarulla T., Weiss K.H., Waldschmidt D.T., Goyal L., Borbath I., El-Khoueiry A., et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021;6:803–815. doi: 10.1016/S2468-1253(21)00196-5. [DOI] [PubMed] [Google Scholar]
- 16.Subbiah V., Iannotti N.O., Gutierrez M., Smith D.C., Féliz L., Lihou C.F., Tian C., Silverman I.M., Ji T., Saleh M. FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann. Oncol. 2022;33:522–533. doi: 10.1016/j.annonc.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meric-Bernstam F., Bahleda R., Hierro C., Sanson M., Bridgewater J., Arkenau H.T., Tran B., Kelley R.K., Park J.O., Javle M., et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 2022;12:402–415. doi: 10.1158/2159-8290.CD-21-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuler M., Cho B.C., Sayehli C.M., Navarro A., Soo R.A., Richly H., Cassier P.A., Tai D., Penel N., Nogova L., et al. Rogaratinib in patients with advanced cancers selected by FGFR mRNA expression: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1454–1466. doi: 10.1016/S1470-2045(19)30412-7. [DOI] [PubMed] [Google Scholar]
- 19.Kommalapati A., Tella S.H., Borad M., Javle M., Mahipal A. FGFR inhibitors in oncology: insight on the management of toxicities in clinical practice. Cancers. 2021;13:2968. doi: 10.3390/cancers13122968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahipal A., Tella S.H., Kommalapati A., Yu J., Kim R. Prevention and treatment of FGFR inhibitor-associated toxicities. Crit. Rev. Oncol. Hematol. 2020;155:103091. doi: 10.1016/j.critrevonc.2020.103091. [DOI] [PubMed] [Google Scholar]
- 21.Lowery M.A., Ptashkin R., Jordan E., Berger M.F., Zehir A., Capanu M., Kemeny N.E., O'Reilly E.M., El-Dika I., Jarnagin W.R., et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin. Cancer Res. 2018;24:4154–4161. doi: 10.1158/1078-0432.CCR-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Churi C.R., Shroff R., Wang Y., Rashid A., Kang H.C., Weatherly J., Zuo M., Zinner R., Hong D., Meric-Bernstam F., et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendre G., Murugesan K., Brummer T., Segatto O., Saborowski A., Vogel A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J. Hepatol. 2023;78:614–626. doi: 10.1016/j.jhep.2022.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Abou-Alfa G.K., Sahai V., Hollebecque A., Vaccaro G., Melisi D., Al-Rajabi R., Paulson A.S., Borad M.J., Gallinson D., Murphy A.G., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzaferro V., El-Rayes B.F., Droz Dit Busset M., Cotsoglou C., Harris W.P., Damjanov N., Masi G., Rimassa L., Personeni N., Braiteh F., et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer. 2019;120:165–171. doi: 10.1038/s41416-018-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siefker-Radtke A.O., Necchi A., Park S.H., García-Donas J., Huddart R.A., Burgess E.F., Fleming M.T., Rezazadeh Kalebasty A., Mellado B., Varlamov S., et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study. Lancet Oncol. 2022;23:248–258. doi: 10.1016/S1470-2045(21)00660-4. [DOI] [PubMed] [Google Scholar]
- 27.Liu P.C.C., Koblish H., Wu L., Bowman K., Diamond S., DiMatteo D., Zhang Y., Hansbury M., Rupar M., Wen X., et al. INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS One. 2020;15:e0231877. doi: 10.1371/journal.pone.0231877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Medicines Agency . 2021. Pemazyre (Pemigatinib) Summary of Product Characteristics. [Google Scholar]
- 29.Health Canada . 2021. Qualifying Notice: Pemazyre. [Google Scholar]
- 30.Guagnano V., Kauffmann A., Wöhrle S., Stamm C., Ito M., Barys L., Pornon A., Yao Y., Li F., Zhang Y., et al. FGFR genetic alterations predict for sensitivity to NVP-BGJ398, a selective pan-FGFR inhibitor. Cancer Discov. 2012;2:1118–1133. doi: 10.1158/2159-8290.CD-12-0210. [DOI] [PubMed] [Google Scholar]
- 31.Goyal L., Meric-Bernstam F., Hollebecque A., Valle J.W., Morizane C., Karasic T.B., Abrams T.A., Furuse J., Kelley R.K., Cassier P.A., et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N. Engl. J. Med. 2023;388:228–239. doi: 10.1056/NEJMoa2206834. [DOI] [PubMed] [Google Scholar]
- 32.Tabernero J., Bahleda R., Dienstmann R., Infante J.R., Mita A., Italiano A., Calvo E., Moreno V., Adamo B., Gazzah A., et al. Phase 1 study of JNJ-42756493, a pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2015;33:3401–3408. doi: 10.1200/JCO.2014.60.7341. [DOI] [PubMed] [Google Scholar]
- 33.Nogova L., Sequist L.V., Perez Garcia J.M., Andre F., Delord J.P., Hidalgo M., Schellens J.H.M., Cassier P.A., Camidge D.R., Schuler M., et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J. Clin. Oncol. 2017;35:157–165. doi: 10.1200/JCO.2016.67.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdul-Karim R.M., Chaudhry A., Patrikidou A., Falcon Gonzalez A., Racca F., Loriot Y., Pouessel D., Deville J.-L., Lee H.J., Cantero F., et al. Derazantinib (DZB) in combination with atezolizumab (AZB) in patients with solid tumors: results from the dose-finding phase Ib substudy of FIDES-02. J. Clin. Oncol. 2021;39:437. [Google Scholar]
- 35.Goyal L., Borad M., Subbiah V., Mahipal A., Kamath S., Mody K., Kelley R.K., Kim R., Sahai V., El-Khoueiry A., et al. Abstract P02-02: First results of RLY-4008, a potent and highly selective FGFR2 inhibitor in a first-in-human study in patients with FGFR2-altered cholangiocarcinoma and multiple solid tumors. Mol. Cancer Ther. 2021;20:P02-02–P02. P02-02. [Google Scholar]
- 36.Wöhrle S., Bonny O., Beluch N., Gaulis S., Stamm C., Scheibler M., Müller M., Kinzel B., Thuery A., Brueggen J., et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J. Bone Miner. Res. 2011;26:2486–2497. doi: 10.1002/jbmr.478. [DOI] [PubMed] [Google Scholar]
- 37.Lyou Y., Grivas P., Rosenberg J.E., Hoffman-Censits J., Quinn D.I., Petrylak D.P., Galsky M., Vaishampayan U., De Giorgi U., Gupta S., et al. Hyperphosphatemia secondary to the selective fibroblast growth factor receptor 1-3 inhibitor infigratinib (BGJ398) is associated with antitumor efficacy in fibroblast growth factor receptor 3-altered advanced/metastatic urothelial carcinoma. Eur. Urol. 2020;78:916–924. doi: 10.1016/j.eururo.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perera T.P.S., Jovcheva E., Mevellec L., Vialard J., De Lange D., Verhulst T., Paulussen C., Van De Ven K., King P., Freyne E., et al. Discovery and pharmacological characterization of JNJ-42756493 (erdafitinib), a functionally selective small-molecule FGFR family inhibitor. Mol. Cancer Ther. 2017;16:1010–1020. doi: 10.1158/1535-7163.MCT-16-0589. [DOI] [PubMed] [Google Scholar]
- 39.Sootome H., Fujita H., Ito K., Ochiiwa H., Fujioka Y., Ito K., Miura A., Sagara T., Ito S., Ohsawa H., et al. Futibatinib is a novel irreversible FGFR 1-4 inhibitor that shows selective antitumor activity against FGFR-deregulated tumors. Cancer Res. 2020;80:4986–4997. doi: 10.1158/0008-5472.CAN-19-2568. [DOI] [PubMed] [Google Scholar]
- 40.Vallée M., Weinstein J., Battistella M., Papineau R., Moseley D., Wong G. Multidisciplinary perspectives of current approaches and clinical gaps in the management of hyperphosphatemia. Int. J. Nephrol. Renovasc. Dis. 2021;14:301–311. doi: 10.2147/IJNRD.S318593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Alessandro C., Piccoli G.B., Cupisti A. The “phosphorus pyramid”: a visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015;16:9. doi: 10.1186/1471-2369-16-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benini O., D'Alessandro C., Gianfaldoni D., Cupisti A. Extra-phosphate load from food additives in commonly eaten foods: a real and insidious danger for renal patients. J. Ren. Nutr. 2011;21:303–308. doi: 10.1053/j.jrn.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Stormont R., McCoy R., Bashir K., Malesker M.A. New pharmacotherapy options for hyperphosphatemia. U.S. Pharm. 2016;41:HS18-. HS. [Google Scholar]
- 44.Landini-Enríquez V., Escamilla M.A., Soto-Vega E., Chamizo-Aguilar K. Response to acetazolamide in a patient with tumoral calcinosis. Nefrologia. 2015;35:503–505. doi: 10.1016/j.nefro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Adhikari S., Mamlouk O., Rondon-Berrios H., Workeneh B.T. Hypophosphatemia in cancer patients. Clin. Kidney J. 2021;14:2304–2315. doi: 10.1093/ckj/sfab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francis J.H., Harding J.J., Schram A.M., Canestraro J., Haggag-Lindgren D., Heinemann M., Kriplani A., Jhaveri K., Voss M.H., Bajorin D., et al. Clinical and morphologic characteristics of fibroblast growth factor receptor inhibitor-associated retinopathy. JAMA Ophthalmol. 2021;139:1126–1130. doi: 10.1001/jamaophthalmol.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lacouture M.E., Sibaud V., Anadkat M.J., Kaffenberger B., Leventhal J., Guindon K., Abou-Alfa G. Dermatologic adverse events associated with selective fibroblast growth factor receptor inhibitors: overview, prevention, and management guidelines. Oncologist. 2021;26:e316–e326. doi: 10.1002/onco.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saleh M., Gutierrez M., Subbiah V., Smith D.C., Féliz L., Zhen H., Ji T., Nemunaitis J. Preliminary results from a phase 1/2 study of INCB054828, a highly selective fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced malignancies. Mol. Cancer Ther. 2018;17:A098. [Google Scholar]
- 49.Moreau F., Brunao B.B., Liu X.-Y., Tremblay F., Fitzgerald K., Avila-Pacheco J., Clish C., Kahn R.C., Softic S. Liver-specific FGFR4 knockdown in mice on an HFD increases bile acid synthesis and improves hepatic steatosis. J. Lipid Res. 2023;64:100324. doi: 10.1016/j.jlr.2022.100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mottacki N., Simrén M., Bajor A. Review article: bile acid diarrhoea – pathogenesis, diagnosis and management. Aliment. Pharmacol. Ther. 2016;43:884–898. doi: 10.1111/apt.13570. [DOI] [PubMed] [Google Scholar]
- 51.Subbiah V., Barve M., Iannotti N.O., Gutierrez M., Smith D.C., Roychowdhury S., Papadopoulos K.P., Mettu N., Edenfield W.J., Morgensztern D., et al. Abstract A078: FIGHT-101: A phase 1/2 study of pemigatinib, a highly selective fibroblast growth factor receptor (FGFR) inhibitor, as monotherapy and as combination therapy in patients with advanced malignancies. Mol. Cancer Ther. 2019;18:A078. [Google Scholar]
- 52.Bahleda R., Meric-Bernstam F., Goyal L., Tran B., He Y., Yamamiya I., Benhadji K.A., Matos I., Arkenau H.T. Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1-4 inhibitor in patients with advanced solid tumors. Ann. Oncol. 2020;31:1405–1412. doi: 10.1016/j.annonc.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.