Summary
Wet age-related macular degeneration (AMD), characterized by leaky neovessels emanating from the choroid, is a main cause of blindness. As current treatments for wet AMD require regular intravitreal injections of anti-vascular endothelial growth factor (VEGF) biologics, there is a need for the development of less invasive treatments. Here, we designed an allosteric inhibitor of end binding-3 (EB3) protein, termed EBIN, which reduces the effects of environmental stresses on endothelial cells by limiting pathological calcium signaling. Delivery of EBIN via eye drops in mouse and non-human primate (NHP) models of wet AMD prevents both neovascular leakage and choroidal neovascularization. EBIN reverses the epigenetic changes induced by environmental stresses, allowing an activation of a regenerative program within metabolic-active endothelial cells comprising choroidal neovascularization (CNV) lesions. These results suggest the therapeutic potential of EBIN in preventing the degenerative processes underlying wet AMD.
Keywords: choroidal neovascularization, calcium signaling, endothelial barrier, rational drug design, single nuclei sequencing, angiogenesis
Graphical abstract

Highlights
-
•
EBIN prevents VEGF-induced pathological calcium signaling
-
•
EBIN reduces neovascularization in mice and an NHP model of AMD
-
•
EBIN promotes regeneration of endothelial cells through epigenetic changes
Using both mouse and NHP models, this study demonstrates the therapeutic promise of EBIN, an allosteric inhibitor of EB3, against wet AMD. EBIN inhibits pathological calcium signaling and reverses stress-induced epigenetic changes, allowing activation of a regenerative program in metabolic-active endothelial cells within choroidal neovascularization lesions.
Introduction
The sprouting of aberrant blood vessels in the choroid layer beneath the retina, and in the retina itself, is a key factor responsible for vision impairment in wet age-related macular degeneration (AMD).1 Common underlying factors of this disease include ocular inflammation and ischemia associated with hypertension and aging.2,3,4 These factors contribute to tissue hypoxia and stabilize hypoxia-induced factors (HIFs) in the pigment epithelium and photoreceptors.5,6 HIFs, in turn, trigger the production of pro-angiogenic signaling molecules such as vascular endothelial growth factor-A (VEGF-A), causing aberrant angiogenesis and neovascular leakage.7 VEGF-A, generated in the posterior segment of the eye as a result of these chronic conditions, is a key factor contributing to choroidal neovascularization (CNV) in humans.8 VEGF-A-mediated signaling induces proliferation, migration, and survival of endothelial cells (ECs) and promotes the unchecked growth of neovessels. These neovessels, growing from underneath the macula, leak protein-rich fluids causing localized edema and disturbed central vision. VEGF-A functions, in part, through activating intracellular Ca2+ signaling in ECs,9 leading to a diverse set of responses ranging from migration and proliferation10,11 to hyperpermeability of the endothelial vessel wall.12 The primary therapies targeting defective angiogenesis and hyperpermeability are recombinant decoy VEGF receptor (EYLEA) and humanized anti-VEGF antibodies (LUCENTIS and MACUGEN). Although these agents represent the standard of care, regular lifetime intravitreal (ITV) injections and potential refractoriness to blocking VEGF signaling are downsides of this therapy,13,14,15,16,17,18,19,20,21 indicating the need for novel therapies for this blinding disease.
Our previous work demonstrates that the microtubule cytoskeleton is a crucial regulator of pathological Ca2+ signaling in ECs.22 We showed that the microtubule-accessory factor end binding-3 (EB3) interacts with inositol 1,4,5-trisphosphate receptor 3 (IP3R3) in the endoplasmic reticulum (ER) membrane and augments IP3-evoked Ca2+ release from ER stores.22 Importantly, the disruption of this interaction through a single-point mutation within the EB-binding motif of IP3Rs or targeting the EB3-IP3R3 interaction with a cognate peptide prevents pathological Ca2+ signaling in response to a variety of pro-inflammatory factors.22,23 Furthermore, the cognate peptide blocks pulmonary microvascular leakage and mitigates inflammation-induced lung injury,23 suggesting a promising approach to treating hyperpermeability in microvessels. In the present study, we show the results of an allosteric EB3 inhibitor, which suppresses both vascular leakage and aberrant CNV in murine and non-human primate (NHP) models of wet AMD. This inhibitor reverses the epigenetic changes induced by environmental stresses, allowing regenerative programs to take their course.
Results
Design of EB3 allosteric inhibitor
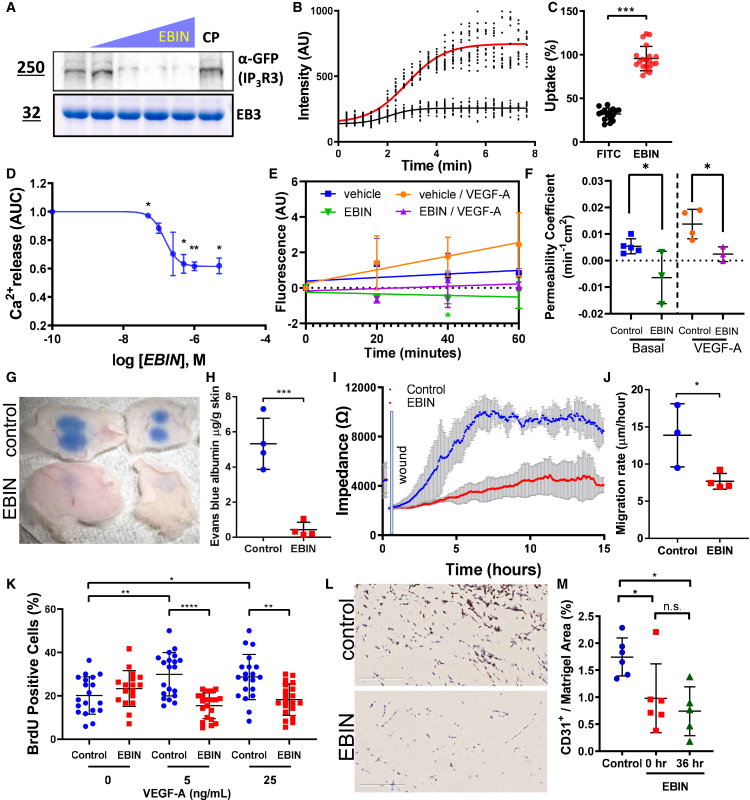
The design of the EB3 allosteric inhibitor was based on computational alanine scanning24 of the IP3R3 sequence identified as the binding domain for EB3.22,23 Alanine scanning showed contributions of each of the amino acids surrounding the EB-binding motif (TxIP) within the IP3R3 sequence in stabilizing or destabilizing the EB3-IP3R3 complex (Figure S1A). We identified the 820 Da peptide, named end-binding inhibitor (EBIN), using this approach (Figure S1A) and mapped the binding interface of EB3 and EBIN (Figure 1). We assigned backbone resonances using a suite of standard three-dimensional nuclear magnetic resonance experiments.25 The spectrum of EB3 alone showed a well-folded protein (Figure 1A). EBIN, added at a 1:1 M ratio, caused significant spectral changes due to an exchange between the free and bound species, as annotated on the protein 15N heteronuclear single quantum coherence spectroscopy (HSQC) spectra of EB3 (Figure 1A). The most significant spectral changes were identified within the binding interface presented by the hydrophobic groove proximal to the C terminus of EB3 (Figure 1B). EBIN binding also caused long-range conformational changes emanating beyond the peptide-binding interface (Figure 1C). EBIN’s interference with EB3’s structure upon binding was confirmed with a nano-format of differential scanning fluorimetry, demonstrating EBIN’s ability to lower the melting temperature of EB3 (Figure S1B).
Figure 1.
Structural analysis of EBIN binding to C-terminal domain of EB3
(A) Overlay of 1H15N HSQC spectra of EB3 (red) and EB3 with EBIN at a 1:1 M ratio (blue).
(B) A homology model of EB3 generated in the I-TASSER web server. Two EB3 chains are colored in red and orange. The amino acid residues of EB3 shown in the inset of (A) are highlighted in blue and numerically labeled on the model.
(C) The docked binding model of EBIN (depicted as a white line, with carbon and oxygen shown in gray and red, respectively) with C-terminal EB3 homodimer (depicted as surfaces colored in red and orange, respectively). The inset shows a 2D map of EB3 amino acids involved in the interaction with EBIN based on the model.
See also Figures S1A and S1B.
EBIN blocks responses of ECs to VEGF-A
We next investigated the therapeutic effects of EBIN in blocking VEGFR-mediated Ca2+ signaling in endothelial monolayers. EBIN prevented the interaction of recombinant EB3 with IP3R3 in a concentration-dependent manner in a cell-free assay (Figure 2A). Uptake of myristoylated (Myr)-EBIN by ECs reached 95.8% and occurred at a half life (t1/2) of 3.18 ± 0.63 min (Figures 2B and 2C). Myr-EBIN mitigated Ca2+ release from ER stores (Figure 2D) in response to VEGF-A, which activates VEGFR2 at the EC surface.26 The half-maximal inhibitory concentration (IC50) of Myr-EBIN was 164.4 ± 0.3 nM following stimulation of ECs with VEGF-A (Figure 2D). However, Myr-EBIN had no effect on PDGF-mediated Ca2+ release in human lens epithelial (HEL-B3) cells expressing negligible levels of EB327 (Figure S1C). Consistent with previous results in EB3-deficient ECs,22 Myr-EBIN did not suppress Ca2+ release from ER stores in response to thapsigargin (Figure S1D), an inhibitor of ER Ca2+-ATPase,28,29 suggesting that EBIN did not alter calcium concentrations inside the ER. Furthermore, treatment with Myr-EBIN caused no cytotoxicity or apoptosis of ECs at maximal soluble concentration (Figure S1E).
Figure 2.
EBIN disrupts interaction of EB3 with IP3R3 and prevents VEGF-A-induced vascular endothelial permeability
(A) EB3 interaction with GFP-IP3R3 assessed by increasing amounts of EBIN (left to right: no peptide and 1:0.1; 1:1; 1:2, and 1:5 M ratios of EB3:EBIN). Top: western blots for GFP; bottom: Coomassie brilliant blue-stained gels. n = 3 independent experiments.
(B and C) Measurements of Myr-5-FAM-EBIN accumulation inside cells, as changes in intracellular fluorescent intensities (B), and percentage of uptake of Myr-5-FAM-conjugated EBIN (red) compared to negative control fluorescein (black; C) in endothelial cells (ECs). n = 15–20 cells cumulatively from 3 independent experiments. Statistics by two-tailed t test.
(D) Functional evaluation of EBIN effects in ECs through determining increases in cytosolic [Ca2+]i. Concentration-dependent curve showing changes in intracellular [Ca2+]i vs. Myr-EBIN concentration plotted in log scale. n = 3 independent experiments. Statistics by two-tailed t test.
(E and F) The effects of Myr-EBIN on the permeability of human endothelial monolayers to 70 kDa dextran tracer after challenge with 50 ng/mL human (h)VEGF-A. Changes in endothelial permeability over time (E) and the permeability coefficient (F) under basal condition and after stimulating with VEGF-A. n = 2 independent experiments. Statistics using two-tailed t test between control peptide- and EBIN-treated groups.
(G and H) Image (G) and quantification (H) of Evans blue-labeled albumin tracer permeability following hVEGF-A intradermal injection. n = 4 mice per group. Statistics using two-tailed t test.
(I and J) Changes in impedance (I) and wound closure rates (J) after the application of an electrical wound to endothelial monolayers stimulated with 1 ng/mL hVEGF-A and treated with 1 μM Myr-control or Myr-EBIN peptide. n = 3 per group. Statistics using t test.
(K) Percentage of proliferating ECs following stimulation with indicated concentrations of hVEGF-A, treated with 1 μM Myr-control or Myr-EBIN peptide. n = 20 fields per group from 2 independent experiments.
(L) Representative images of Matrigel implants stained for CD31 (brown) excised from mice receiving daily intravenous (i.v.) treatment with 1 μmol/kg BW Myr-control or Myr-EBIN peptide. Scale bar, 200 μm.
(M) Percentage of area of CD31-positive cells normalized to the implant area in mice that received Myr-control or Myr-EBIN peptide administrated at 0 or 36 h post-implant. n = 6 implants from 3 mice per group.
Data as mean ± SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; and ∗∗∗∗p < 0.0001 using one-way ANOVA unless otherwise indicated.
See also Figures S1C–S1M.
Consistent with our published results,22,23 inhibition of EB3 function with Myr-EBIN significantly reduced permeability to 70 kDa dextran in endothelial monolayers challenged with VEGF-A as compared to the control condition, the Myr-control (Thr2 to Ala mutant of EBIN) peptide, used in all in vitro and mouse experiments (Figures 2E and 2F). Administration of Myr-EBIN through the intravenous route reduced VEGF-A-induced hyperpermeability of skin capillaries in mice (Figures 2G and 2H), signifying the benefits of EBIN in vivo. Mechanistically, Myr-EBIN had no effect on the cell surface expression or recycling of VEGFR2 (Figures S1F and S1G) but prevented the disruption of VE-cadherin adherens junctions (AJs) and reduced the formation of interendothelial gaps following VEGF-A challenge (Figures S1H–S1J). Myr-EBIN had no effect on VE-cadherin total protein expression (Figure S1K).
Next, we determined the effects of Myr-EBIN on biological processes underlaying angiogenesis.30 As VEGFR-dependent Ca2+ signaling converts quiescent ECs to an active phenotype associated with cell migration and proliferation,11 we surmised that inhibiting VEGF-induced Ca2+ signaling with EBIN would also inhibit those processes. Indeed, Myr-EBIN significantly reduced both migration and proliferation of ECs in response to VEGF-A (Figures 2I–2K) and prevented endothelial tube formation both in vitro (Figures S1L and S1M) and in mice (Figures 2L and 2M).
Topical application of EBIN attenuates CNV in mice and NHPs
We next assessed the therapeutic benefits of EBIN in treating capillary leakage and aberrant neovascularization (Figures 3A–3E) in laser-induced CNV in mice.31,32 Compared to ITV injection of Myr-control peptide, Myr-EBIN (1 μg/eye) or an antibody against mouse VEGF-A (2 μg/eye; LEAF) used as a positive control significantly reduced the area of CNV lesions assessed by retinal thickness on both days 7 and 14 post-CNV induction (Figures 3A–3D), by fluorescent angiogram (Figures S2A and S2B), and by post-mortem staining of retinal and choroidal tissue for isolectin B4 (IB4) (Figures 3B and 3E). The latter staining visualizing neovascular ECs, macrophages, and monocytes31,33,34,35,36 was used to determine the size of CNV lesions (Figures 3B and 3E).
Figure 3.
EBIN abrogates choroidal neovascularization in the murine model of wet AMD
(A) CNV lesions determined by optical coherence tomography (OCT) of choroid and retinal layers at days 7 and 14 after laser photocoagulation. Scale bar, 100 (height) × 500 μm (width).
(B) Images of CNV lesions detected by flat-mount staining of retinal pigment epithelium/choroid/sclera for isolectin B4 (IB4) after 14 days of laser photocoagulation in mice treated as in (A). Scale bar, 50 μm.
(C and D) Changes in CNV thickness of choroid and retinal layers, quantified at 7 and 14 days post-laser injury, in mice treated as in (A). n = 8–12 eyes per group; individual data points are mean thickness of all lesions per eye.
(E) Area of CNV lesions detected by flat-mount staining as in (B). n = 7–12 eyes per group; individual data points are mean area of all lesions per eye.
(F) 3D-reconstructed images of CNV lesions detected by flat-mount staining of retinal pigment epithelium/choroid/sclera for IB4 (magenta), desmin (yellow), and VE-cadherin (green) from indicated treatment groups. Scale bar, 50 μm.
(G–I) Quantification of VE-cadherin projected area (G), volume of VE-cadherin-positive vessels normalized to volume of IB4-positive lesion (H), and pericyte coverage (I) from images in (F). n = 9–12 lesions per group.
Data as mean ± SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; and ∗∗∗∗p < 0.0001 using one-way ANOVA unless otherwise indicated.
See also Figure S2.
Because EBIN has a small molecular weight of 820 Da, we also determined if topical administration of Myr-EBIN via eye drops (5 μg twice daily) would be an effective alternative to ITV injection. Topical application of Myr-EBIN twice a day for 5 days only transiently saturated the posterior segments of the eye, including the sclera, choroid, retina, and optic nerve at 3 h following application (Figure S2C). Myr-EBIN was lost from eye tissues by 4 h post-topical dosing (Figure S2C), suggesting prompt clearance. Nevertheless, topical application of Myr-EBIN was shown to be as effective at reducing the area of CNV as the ITV route (Figures 3C–3E), producing comparable therapeutic effects to the anti-VEGF-A antibody. In these experiments, mice that received topical application of Myr-EBIN also received one dose of control immunoglobulin G2α (IgG2α) antibody through the ITV route. As ITV injection itself causes a transient increase in intraocular pressure,13,14 this was an important control to other groups that received ITV treatment. Unexpectedly, the combination of anti-VEGF-A antibody and Myr-EBIN did not further reduce the lesion size as compared to Myr-EBIN alone (Figures 3C–3E and S2B).
Further analysis of CNV lesions showed that both anti-VEGF therapy and Myr-EBIN reduced the size of VE-cadherin-positive choroidal neovessels (Figures 3F, 3G, and S2D), although the accumulation of VE-cadherin at AJs was significantly greater in the EBIN-treated group (Figures 3F, 3G, and S2D). These data support the protective effects of Myr-EBIN on VE-cadherin junctions, consistent with the results in endothelial monolayers (Figures S1H and S1I). Treatment with Myr-EBIN, however, had no effect on neovessel pericyte coverage (Figures 3F, 3I, and S2D), indicating that EBIN did not have an effect on the interactions of neovascular ECs with pericytes.
We next carried out studies on Chlorocebus sabaeus, in which the eye anatomy more closely resembles humans. Topical dosing of 150 μg Myr-EBIN per eye for 6 days twice daily produced a significantly greater accumulation of Myr-EBIN in both the posterior and anterior segments of the eye after 2 h of the last dosing as compared to ITV route (Figures S3A and S3B). No traces of Myr-EBIN were observed in the blood at either 2 or 6 h post-topical treatment (data not shown).
Therefore, we compared the efficacy of topical Myr-EBIN to vehicle (negative control) or single ITV administration of aflibercept in the NHP model of AMD. Treatment with Myr-EBIN significantly reduced the incidence of grade IV lesions, a clinically relevant measurement of AMD in humans (Figures 4A, 4B, and S3C; Table S1) at both days 14 and 21. There was no significant difference in the incidence of grade IV lesions between the Myr-EBIN and aflibercept groups (Figures 4B and 4C). Myr-EBIN also reduced the total area of CNV lesions (Figures 4C and S3D) at day 14 as compared to topical vehicle treatment. Although no difference in lesion area between vehicle- and EBIN-treated groups was observed at day 21, this might be explained by the spontaneous regression of neovessels in the vehicle-treated group (p = 0.0018 between days 14 and 21; Figure 4C). Nevertheless, a decrease in neovascular leakage correlated with an increase in VE-cadherin junction area in the EBIN-treated group even at day 21 (Figures 4D, 4E, and S3E). Similar to aflibercept, Myr-EBIN caused neither gross changes at the histological level (Figure S3E) nor led to inflammation nor neurotoxicity, as evidenced by the clinical scores over the duration of the study and by normal intraocular pressures (Figures S3F and S3G; Table S2).
Figure 4.
Topical administration of EBIN prevents choroidal neovascularization in the NHP (Chlorocebus sabaeus) model of wet AMD
(A) Representative color fundus photography and fluorescein angiography images of monkey eyes at day 21 post-laser induced photocoagulation. Numbers indicate the grades of the CNV lesions; the clinically relevant grade IV lesions are circled in red. Scale bar, 2 mm.
(B) Incidence of grade IV lesions on days 14 and 21 after laser-induced injury. n = 11–12 eyes from 6 monkeys per group; individual data points are mean percentage of the grade IV lesions per eye.
(C) Neovascular complex area was analyzed by the mixed-effects model followed by multiple comparisons using OCT images. n = 11–12 eyes from 6 monkeys per group; individual data points are mean area of all lesions per eye.
(D) Immunofluorescent staining of CNV lesions from vehicle- and EBIN-treated NHP for collagen IV (yellow) used as a marker of neovessels and VE-cadherin (blue); dashed line indicates lesion area; scale bar, 50 μm.
(E) VE-cadherin area normalized to vessel area from images in (D). n = 3–4 lesions per group; statistics using two tailed t test.
Data as mean ± SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; and ∗∗∗∗p < 0.0001 using one-way ANOVA unless otherwise indicated.
EBIN mitigates hypoxic stress in retinal ECs
Hypoxia is a known risk factor for AMD pathogenesis.37,38 Using bulk RNA sequencing (RNA-seq) analysis, we determined the effects of EBIN on the gene expression profile of human retinal microvascular ECs cultured under normoxic or acute hypoxic (1% O2) conditions (Tables S3 and S4). Acute hypoxia led to the downregulation of 4,662 genes and the upregulation of 4,368 genes in vehicle-treated monolayers (Figures 5A and S4A). EBIN markedly reduced these changes, normalizing the expression levels of 5,822 genes to baseline normoxic conditions (Figure 5A). Using QIAGEN’s Ingenuity Pathway Analysis (IPA), we have determined the signaling pathways to be either reversed (Figure 5B) or unaffected by Myr-EBIN (Figure S4B; Table S5). Treatment with Myr-EBIN prevented the downregulation of genes involved in cell metabolism, protein synthesis, and defense system against reactive oxygen species (Figure 5B). Myr-EBIN also blocked the upregulation of senescence, renin-angiotensin, and adrenomedullin signaling pathways (Figure 5B). However, Myr-EBIN treatment had no effect on the genes responsible for cell survival and regeneration (Figure S4B). Cumulatively, these data suggest that EBIN reversed maladaptive cellular responses, such as inhibition of cell metabolism and protein synthesis, without altering adaptive changes including cell survival.
Figure 5.
EBIN reverses maladaptive response of ECs to environmental stresses in vitro and in NHPs
(A) The effects of the Myr-EBIN treatment on the transcriptome of human retinal ECs (HRECs). Each row represents a gene. The color key shows whether gene expression was upregulated (red) or downregulated (blue) using Z scored log2 counts per million reads mapped (CPM). n = 2–3 independent experiments.
(B) Chart graph showing the top 5 relevant pathways affected by hypoxia from data in (A), sorted based on logarithmic adjusted p values and negative (blue) or positive (red) Z score values for each pathway. The highlighted pathways are pathways involved in cellular metabolism.
(C) qRT-PCR results showing the effect of Myr-EBIN and chronic hypoxia on genes comprising the OXPHOS pathway in HRECs. The color scale represents the relative fold change of each gene respective to the normoxia vehicle group and the loading control TOMM20. n = 3 independent experiments.
(D and E) Western blot (D) validating the protective effects of Myr-EBIN on the mitochondrial genes within the OXPHOS pathway and quantification (E). n = 3 independent experiments.
(F and G) Western blot of choroid tissue from vehicle- and EBIN-treated NHP eyes for MT-CYB, SDHB, and TOMM20 (F) and quantification (G). NHP ID and corresponding treatment groups. Blue and green IDs refer to active (leaky) and healed lesions, respectively. n = 2 NHPs per condition.
Data as mean ± SD. ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001 using one-way ANOVA unless otherwise indicated.
See also Figures S4 and S5 and Tables S3, S4, and S5.
Of all genes affected, Myr-EBIN had the most effect on the oxidative phosphorylation (OXPHOS) pathway that was downregulated in response to acute hypoxic stress in vehicle-treated ECs, further confirmed by qRT-PCR (Figures S4C–S4E). Interestingly, Myr-EBIN also reversed the effects of chronic (over 24 h) hypoxia, which, in contrast to acute hypoxia, upregulated the expression of mitochondria genes at both the mRNA and protein levels (Figures 5C–5E). Therefore, we analyzed the effects of Myr-EBIN on protein expression of the OXPHOS pathways in the choroid tissue of NHPs at 21 days after laser-induced photocoagulation (Figures 5F, 5G, and S5). Myr-EBIN increased the level of mitochondria proteins as compared to the vehicle-treated group in the choroid tissue containing grade IV lesions (Figures 5F, 5G, and S5). However, it did not have an effect on the healed, not leaky lesions (Figures 5F, 5G, and S5), suggesting that the treatment preserved mitochondria function in choroid tissue containing active CNV lesions. Furthermore, these effects of Myr-EBIN were not observed in the cornea (Figures S5D and S5E). Together, these data suggest that EBIN elicited a therapeutic benefit by protecting eye tissue from environmental stresses, in part, through the normalization of mitochondrial function.
EBIN alters transcriptomes in metabolic-active ECs comprising CNV lesions
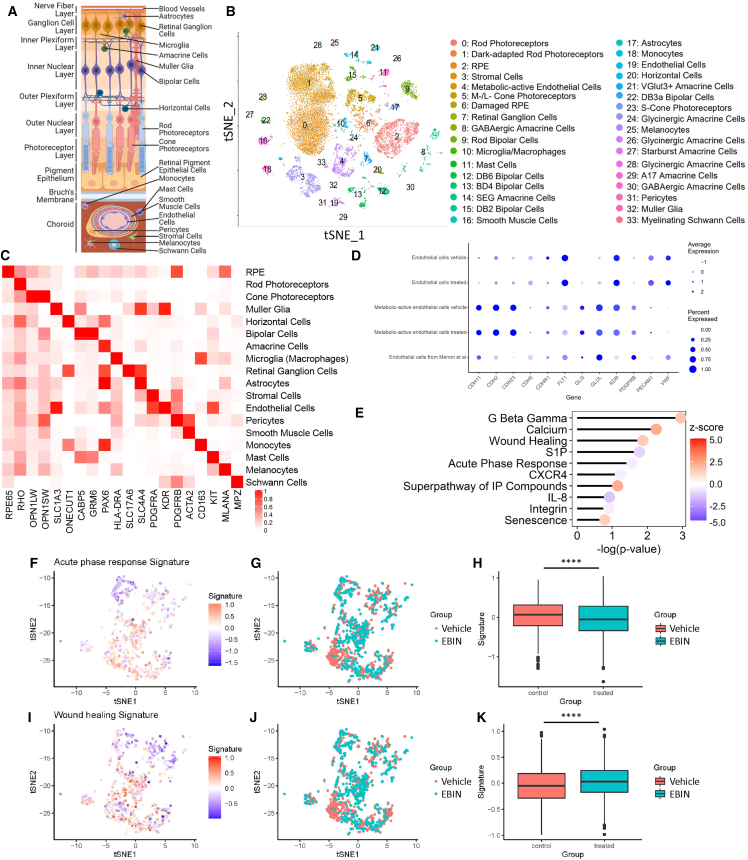
To gain an unbiased picture of the therapeutic benefits of Myr-EBIN in the choroid neovessels at the transcriptomic level, we next investigated changes in the gene expression profiles of cells comprising the choroid and retinal tissues of NHP at a single-nucleus (sn) level (Figures 6A–6C and S6; Tables S3 and S4). Using snRNA-seq analysis of tissues dissected from laser-induced CNV, we distinguished 33 transcriptome clusters (Figure 6B), which were then characterized into 18 broader retinal cell types (Figure 6C) using well-established markers.39,40 Cell subtypes were also assigned based on subtype-specific markers (Figures 6D, S6B, and S6C).
Figure 6.
EBIN reverses gene expression changes in the metabolic-active ECs of CNV
(A) Schematic cross-section diagram of the retina displaying major retinal cell types.
(B) tSNE projection of snRNA-seq data from 4 NHP retina and choroid tissues. Each single point represents an individual cell; each color and number represent a unique cluster. The cell types assigned to each cluster are shown on the right. n = 2 NHP eyes per group.
(C) Heatmap showing average expression of known cell markers in association with major retinal and choroidal cell types. Each row represents the corresponding cell types. Each column represents the gene marker used for cell-type identification. The color scale bar represents the average gene expression.
(D) A dot plot depicting the expression of markers used to identify metabolic-active ECs (cluster 4) in NHPs. The expression of these markers in the macula of patients with AMD39 is shown for comparison. The size of the dot represents the percentage of cells expressing the gene within the cluster. The color of the dot represents the average expression level of the gene within the cluster.
(E) Graph showing the top pathways affected by Myr-EBIN treatment in metabolic-active ECs of NHP CNV lesions. Positive (red) and negative (blue) Z scores depict activated and inhibited pathways, respectively.
(F–K) A series of graphs showing the effects of Myr-EBIN on the acute phase response pathway (F–H) or the wound-healing pathway (I–K) in metabolic-active ECs (cluster 4).
(F and I) A tSNE projection shows changes in gene expression. Each dot represents a single cell. Cells with an upregulated pathway have a positive signature score (red), whereas cells with an inhibited pathway have a negative signature score (blue).
(G and J) A tSNE projection of the metabolic-active ECs categorized into vehicle (red) or Myr-EBIN (cyan) treatment groups.
(H and K) Quantification of the pathway signature score from (F) or (I), respectively, for each treatment group.
Mean ± SD, ∗∗∗∗p < 0.0001 using Wilcoxon ranked test.
We identified two distinct transcriptome clusters in ECs (Figures 6B and 6D): mature (cluster 19) and metabolic active (cluster 4). Similar to the ECs of patients with AMD,39 the latter subtype was characterized by high expression of glutamate-ammonia ligase (GLUL), fms-related receptor tyrosine kinase 1 (FLT1), and kinase insert domain receptor (KDR) (Figure 6D). Upregulation of GLUL in cluster 4 suggested an increase in glutamine metabolism, which has been previously linked to angiogenesis.41,42 However, metabolic-active ECs showed low expression of cadherin 5 (CDH5) encoding VE-cadherin, platelet and EC adhesion molecule 1 (PECAM1), and von Willebrand factor (VWF) (Figures 6D and S6A), the signatures of mature ECs. Consistent with our findings, ECs of laser-induced CNV in mice also expressed low expression of vWF.43 ECs in cluster 4 was also negative for mesenchymal markers (APLNR, NGFR),44,45 retinal progenitor markers (SOX2, HES5),46 microglia markers (P2RY12, TMEM119, AIF1),47 monocyte markers (CCL2, ITGAX, CX3CR1),47 and macrophage markers (HLA-DRA, CTSS)48,49 (Figure S6A; Table S4), suggesting that these cells were distinct from retinal progenitor or immune cells. Interestingly, metabolic-active ECs also showed expression of Muller glial cell markers DKK3, RLBP1, and SLC1A3 but not GFAP or S100A16 (Figure S6A; Table S6). Previous studies showed that choroidal ECs have higher expression of CLU when compared with other ECs within the eye and that increased expression of CLU promotes angiogenesis and is associated with Fuchs’ endothelial dystrophy.40,50,51 It also has been shown that DKK3, a marker of neovascular AMD, is a potent inducer of the endothelial-to-mesenchymal transition.52,53,54,55 SLC1A3 expression is upregulated in ECs co-cultured with astrocytes, suggesting that the tissue environment might contribute to tissue-specific signatures of ECs.56,57 Based on these findings, we speculate that laser photocoagulation promotes genetic changes in choroidal neovessels that favor metabolism and modify the capacity of these cells to adopt a phenotype of mature ECs.
Treatment of NHP eyes with Myr-EBIN caused changes in the transcriptome profile of rod (clusters 0/1) and cone photoreceptors (cluster 5), retinal pigment epithelium (RPE; clusters 2/6), stromal (cluster 3), and metabolic-active ECs (cluster 4) (Figure S6D). The majority of these cell types expressed MAPRE3, the gene encoding EB3, at high levels, except for photoreceptors and stromal cells (Figure S6E). Consistent with the proposed anti-stress action of Myr-EBIN on retinal ECs (Figures 5A and 5B), we observed marked inhibition of acute phase response and inflammatory pathways (Figures 6E–6H), whereas the wound-healing pathway was activated within metabolic-active ECs (Figures 6E and 6I–6K; Table S5). Myr-EBIN treatment also reduced the expression of GLUL to 15% (Table S4) but had no effect on squalene monooxygenase (SQLE) or aldehyde dehydrogenase (ALDH18A1), metabolic angiogenic targets characterizing murine CNV ECs.43 While Myr-EBIN altered the gene expression profile in metabolic-active ECs (Figure S6D; cluster 4), it induced minimal changes in mature ECs (Figure S6D; cluster 19), consistent with the proposed mechanism of action. Within the RPE (clusters 2 and 6 assigned to healthy and damaged RPE), Myr-EBIN promoted cell-to-cell and cell-to-matrix adhesion by upregulating epithelial AJ, integrin, and actin cytoskeleton signaling pathways, whereas it decreased cell migration and proliferation through the downregulation of wingless-related integration site (WNT)/β-catenin and Rho GDP-dissociation inhibitor (RHOGDI) pathways (Figure S6F; Table S5). In addition, Myr-EBIN also promoted cell survival through the upregulation of the p72S6K pathway, as it functions to protect cells against oxidative stress-induced apoptosis.58 In rod photoreceptors (clusters 0 and 1), Myr-EBIN upregulated many neuroprotective genes comprising ciliary neurotrophic factor (CNTF), protein kinase A, Janus kinase (JAK), signal transducer and activator (STAT), and 5′ AMP-activated protein kinase (AMPK) signaling pathways (Figure S6G; Table S5). Within the M-/L-cone photoreceptors (cluster 5), there was an increase in genes responsible for the synthesis and metabolism of inositol phosphate compounds (Figure S6H; Table S5), suggesting an enhancement in phototransduction.59 These compounds can also promote retinal cell homeostasis and survival by triggering PI3K/Akt activation.60 Furthermore, neuroprotective genes like arrestin β1 (ARRB1) and protein tyrosine phosphatase non-receptor type 1 (PTPN1) were found to be increased in the EBIN group (Table S4).61,62 These data together suggest that not only did Myr-EBIN mitigate stress on metabolic-active neovessels, thereby improving regeneration of eye tissue after laser photodamage (Figures 3A and S3D), but it also protected the photoreceptors and RPE from environmental stresses such as hypoxia and inflammation.
EBIN globally increases chromatin accessibility across different cell types
Previous findings demonstrate a progressive decrease in chromatin accessibility in the cells of the macula in patients with late-stage AMD.63 Thus, we investigated the effects of EBIN on the epigenetic landscape of choroidal and retinal cells using sn-assay for transposase-accessible chromtain (ATAC)-seq. 22 clusters were identified using Louvain clustering and viewed via t-distributed stochastic neighbor embedding (tSNE) (Figure S7A). These 22 clusters were assigned to specific cell subtypes using the Pearson’s correlation between average gene expression and the peak of each snRNA-seq and snATAC-seq cluster, respectively (Figure S7B). Comparison between vehicle- and EBIN-treated groups showed an increase of promoter peaks in rod photoreceptors (ATAC cluster 0′), RPE (ATAC cluster 1′), metabolic-active ECs (ATAC cluster 7′), mast cells (ATAC cluster 8′), amacrine cells (ATAC cluster 10′), and bipolar cells (ATAC clusters 11′ and 17′) (Figure S7C), indicating the widespread changes in chromatin architecture caused by Myr-EBIN. These changes in the chromatin structure were specific to cell subtypes affected by Myr-EBIN at the transcriptomic level (Figure S7A).
Since Myr-EBIN caused an effect on chromatin structure, we were concerned about the unwanted activation of genes involved in AMD progression. Thus, we further compared snATAC-seq data obtained from Myr-EBIN-treated choroid and retinal tissues with published databases of patients with AMD.63 The results of this analysis showed that Myr-EBIN did not increase the chromatin accessibility of genes involved in the pathogenesis and progression of AMD (Figure S7D). Thus, we concluded that EBIN will be unlikely to elicit progression of the disease in AMD patients.
Previous work showed that the widespread decrease in the chromatin landscape in patients with AMD was triggered by histone deacetylases.63 Acetylation of histones H3 and H4 inhibits DNA-nucleosome interaction64 and thereby increases chromatin accessibility.64 Therefore, we used the levels of H3 and H4 histone acetylation as the indicator of epigenetically driven changes within the CNV lesions. In the EBIN-treated group, immunofluorescent staining of CNV lesions showed an increased acetylation of lysine 27 of H3 (H3K27ac; Figures 7A and 7B), a target of histone deacetylases HDAC1, HDAC2,65 HDAC7,66 and HDAC11.63,67,68 However, Myr-EBIN did not increase the H4K8ac (Figures S7E and S7F), which is controlled by histone deacetylase HDAC3.69 These data suggest that Myr-EBIN increased the chromatin landscape through the epigenetic control of H3, but not H4, acetylation. This differential regulation of histone acetylation by Myr-EBIN might be attributed to inhibition of specific histone deacetylases.
Figure 7.
EBIN increases global chromatin accessibility and activates the MEIS2-PAX6 pathway in metabolic-active ECs of CNV
(A) Immunofluorescent staining of CNV from vehicle- and EBIN-treated NHP eyes for H3K27ac (yellow) and DAPI (blue). Scale bars, 60 and 10 μm (inset).
(B) Quantification of the average intensity of nuclear-localized H3K27ac. n = 4 lesions from 4 NHPs per group.
(C) Heatmap showing the number of genes concordantly altered at both the transcriptome and promoter accessibility states across major cell types. snATAC-seq and snRNA-seq clusters are shown on the x and y axes and numerically labeled by (1′) and (1), respectively. Scale bar shows how many concordant genes are present in each cluster. Black boxes highlight cell type of interest.
(D) Motif enrichment analysis of the known DNA-binding motifs for PAX6 and MEIS2 in vehicle- and EBIN-treated metabolic-active ECs. n = 2 NHP eyes per group.
(E–H) Immunofluorescent staining of CNV lesions from vehicle- and EBIN-treated NHP eyes for MEIS2 (E and F; yellow), PAX6 (G and H; yellow), and DAPI (blue). Scale bars, 100 and 10 μm (insets).
(F and H) Quantification of the average intensity of nuclear-localized MEIS2 and PAX6. n = 3 NHPs per group.
(I and J) Western blot of choroid tissue from vehicle- and EBIN-treated NHP eyes for MEIS2, PAX6, and vinculin (I) and quantification (J). NHP ID and corresponding treatment groups are shown. Blue and green IDs refer to active (leaky) and healed lesion. n = 2 NHPs per condition; statistics using one-way ANOVA with Tukey’s post hoc test.
Data as mean ± SD. ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗∗p < 0.0001 using two-tailed t test unless otherwise indicated.
EBIN activates MEIS2-PAX6 endothelial developmental pathway in neovascular cells
Increase in chromatin accessibility acts as a switch of transcriptional regulation, allowing transcriptional complexes to activate cell- and context-specific transcriptional programs.70 Therefore, we determined whether Myr-EBIN caused concordant changes in both the gene promoter and transcriptional activity. The concordant analysis of upregulated genes and genes with increased chromatin accessibility demonstrated the most prominent effects of Myr-EBIN on rod photoreceptors, RPE, and metabolic-active ECs (Figures 7C; Table S7). The number of these concordant genes, however, was several folds lower compared with the number of upregulated genes in each cell type (Figure 7C; Table S4).
Using the Gene Transcription Regulation Database (GTRD; see STAR Methods), we thus investigated whether Myr-EBIN might increase chromatin accessibility through epigenetic control of specific transcription regulators to alter the gene expression profile of the larger subset of genes in metabolic-active ECs (RNA cluster 4 and ATAC cluster 7′). Myr-EBIN induced concordant changes of 10 genes, while it changed the expression of 355 genes (Figure S7G; Table 7). Two transcriptional factors, meis homeobox 2 (MEIS2) and paired box 6 (PAX6), were among the 10 concordant genes affected by Myr-EBIN at both the epigenetic and transcriptome levels (Figure S7G; Table S7). Analysis of snATAC-seq data showed significant enrichment of MEIS2 and PAX6 motifs in the Myr-EBIN-treated monkey eye (Figure 7D). Further analysis of MEIS2 and PAX6 target genes using the GTRD suggested that these two transcriptional factors might function by upregulating transcription factors 4 and 12 (TCF4 and TCF12), affecting the expression profile of 230 genes (Figure S7G).
To further validate these findings, we analyzed both the protein expression and intracellular localization of MEIS2 and PAX6 (Figures 7E–7J). Immunofluorescent staining showed greater nuclear accumulation of both transcriptional factors in cells within but not outside of CNV lesions of the Myr-EBIN-treated group (Figures 7E–7H, S7H, and S7I). We also observed an increase in MEIS2 and PAX6 protein expression in healed lesions of the Myr-EBIN group (Figures 7I and 7J). However, the expression level of these proteins in the retinal tissue was unaffected (Figures S7J and S7K). Consistent with the proposed role of PAX6 in upregulating the expression of junctional proteins,71 we observed increased expression of N-cadherin (CDH2) and CDH23 in metabolic-active ECs (cluster 4; Figure S6D) of NHPs treated with Myr-EBIN. Additionally, our results showed that Myr-EBIN upregulated VE-cadherin in mature ECs (cluster 19; Figure S6D). These data are consistent with increased VE-cadherin expression in neovascular ECs (Figures 4D and 4E). These results might suggest that Myr-EBIN mitigated the response of metabolic-active ECs to environmental stresses, allowing maturation of neovascular ECs through activation of the MEIS2-PAX6 pathway.
Discussion
In the present study, we show the therapeutic benefits of an EB3 allosteric inhibitor that is designed to disrupt the EB3-IP3R3 binding interphase and mitigate pathological Ca2+ signaling in ECs. This peptide functions by destabilizing the ternary structure of the EB3 homodimer72,73 through long-range effects on the C terminus. EBIN mimics EB3 deficiency in ECs22 and prevents endothelial barrier disruption induced by VEGF-A and other environmental stresses, and it maintained barrier integrity by increasing junctional VE-cadherin in the neovessels of CNV lesions. Whereas vascular leakage and neovascularization are two convergent events emanating at the level of VEGFR2,74 our data suggest that EB3 concurrently regulates both events through positive feedback regulation of EB3 function by Ca2+ signaling.22,75 Thus, blocking EB3’s pathological function with EBIN provides an efficient strategy to reduce both CNV leakage and lesion size.
Mechanistically, EBIN treatment protects neovascular cells from the effects of environmental stresses and activates the MEIS2-PAX6 transcriptional program. From this perspective, EBIN shows therapeutic potential to promote this developmental program through the epigenetic regulation of the promoter regions of MEIS2 and PAX6 genes, the key TFs involved in retinal development.76 Consistent with the proposed protective role of PAX6 in corneal endothelial injury through the increased expression of junctional proteins,71 our data demonstrate upregulation of N-cadherin (Cdh2 gene), which promotes the recruitment of VE-cadherin to AJs,77 in metabolic-active ECs after EBIN treatment. EBIN also induces the expression of MEIS2, which not only functions as a co-factor of PAX678 but transcriptionally activates PAX6 expression through binding to its enhancer.76 In addition, MEIS2 is a known cell-cycle regulator79,80,81,82 acting upstream of p15,79 a cell-cycle-dependent kinase that induces G1 cell-cycle arrest.83 This MEIS2 regulatory network is critical in cell differentiation and maturation and thus may be important to mitigate aberrant neovascularization and improve wound healing in eye tissue without apparent scarring. This is in contrast to the current standard of care, aflibercept and ranibizumab, which are used to inhibit aberrant neovascular growth and hyperpermeability by eliminating VEGF in eye.84,85 These differences in the mechanism of action between EBIN and anti-VEGF may explain why EBIN shows no additional therapeutic benefits when both interventions are combined. EBIN mitigates unwanted effects of VEGF, “the stressor,” on ECs, whereas anti-VEGF eliminates “the stressor” itself.
Our data also show that EBIN preserves mitochondrial function in activated endothelial monolayers subjected to both acute and chronic hypoxic stresses. While acute and chronic hypoxia are driven by HIF1α and HIF2α signaling pathways,86,87 respectively, both alter mitochondrial function.88,89,90,91 In this context, EBIN’s ability to protect mitochondrial biogenesis from the disrupting effects of acute and chronic hypoxia as evidenced by the reversal in the expression of mitochondrial genes of the OXPHOS pathway back to normoxic levels highlights the significance of endothelial metabolism in aberrant neovascularization.43 How EBIN reverses the effects of hypoxia on the OXPHOS pathway remains unclear. One potential mechanism might involve a direct effect of EBIN on hypoxia-mediated increases in cytosolic calcium and a dampening HIF1α signaling in this manner. Indeed, EBIN downregulated HIF1α signaling in cultured ECs subjected to acute hypoxia (Table S5). In this context, hypoxia is known to induce prolonged release of Ca2+ from IP3-evoked ER stores92 and to rapidly stabilize HIF1α by calcineurin, the Ca2+/calmodulin-dependent serine/threonine phosphatase.93 Since hypoxia promotes the HIF-1-mediated expression of MAX interactor 1 (MXI), which, in turn, inhibits transcription of peroxisome proliferator-activated receptor gamma coactivator 1β (PGC-1β) and thereby decreases mitochondrial biogenesis,94 EBIN can potentially reverse these effects of hypoxia on mitochondria genes through MXI. Taken together, these data suggest that EBIN protects against hypoxia, a core concept in neovascularization and angiogenesis.
Genomic data also show the marked effects of Myr-EBIN on gene expression changes in the RPE and photoreceptors. While we find no effect of Myr-EBIN on PDGF-induced calcium release from ER stores in human lens epithelial cells, which express low levels of the MAPRE3 gene,27 we cannot be certain that Myr-EBIN does not interfere with pathological calcium signaling that resulted from laser-induced injury in the RPE, as these cells express substantial levels of MAPRE3 transcripts. Furthermore, Myr-EBIN had a marked effect on gene expression in photoreceptors, the cell type expressing low levels of MAPRE3. Per our analysis, these effects of EBIN on the RPE and photoreceptors are also protective. One potential explanation of these data is that these changes are the result of improved regeneration and wound healing of injured macular tissue through normalization of tissue-fluid homeostasis.
As environmental factors alter genetic markers,95 they are considered the major cause of pathological changes associated with AMD.63,96,97 snATAC-seq analysis of patients with early and late atrophic stages of AMD reveal a global decrease in chromatin accessibility.63 Similar changes are also induced by the treatment of cultured RPE cells with cigarette smoke or overexpression of HDAC11, a molecular marker upregulated in the RPE during the early stage of AMD.63 Our data presented here demonstrate that EBIN reverses these pathological epigenetic changes in various cell types. The treatment of EBIN causes a widespread opening of the promoter regions of various genes across rod photoreceptors, the RPE, and metabolic-active ECs in the choroid and retina. Our results also suggest that EBIN induces these changes in affected ECs through H3K27ac, a post-translational modification that is regulated by the opposing actions of histone acetyltransferases98 and HDAC enzymes.66,99 Interestingly, intracellular calcium finely tunes histone acetylation.100 Physiological calcium levels favor histone acetylation through engaging the activity of histone acetyltransferase CBP’s co-factor, CREB, as well as inducing HDAC heterodimerization101 or the nuclear export of HDACs.102,103 However, pathological calcium signaling triggered by hypoxia blocks CREB activity,104 which could potentially shift the balance toward histone deacetylation. Thus, we speculate that EBIN might regulate H3K27 acetylation through blocking pathological Ca2+ signaling. In addition, similar to EBIN, pharmacological inhibition of histone deacetylases leads to a global increase in chromatin accessibility,105 protects against ischemic damage,106,107 and prevents photoreceptor degeneration.108 These data further characterize the stress-reducing effects of EBIN and the therapeutic promise in treating both atrophic and neovascular forms of AMD.
Further, we showed that topical EBIN penetrates the sclera, reaching the choroid and retina tissues of both mouse and NHP eyes. The small size of EBIN and the attachment of a myristoyl group73 ensure rapid uptake of EBIN by ECs and possibly other cells residing within the retina and choroid tissues. This widespread distribution of topically administered EBIN in the posterior segments of the eye is in contrast to larger biologics, which are unable to accumulate in the retina due to the limited passage across the RPE barrier.109 Although EBIN is promptly cleared from both the interior and the posterior segments of the eye, even a short exposure of these tissues to EBIN provides therapeutic benefits by normalizing neovascularization and tissue healing. Compared with the topical route, ITV injection leads to accumulation of EBIN in the lens and vitreous humor with much lower concentrations of the peptide in the retina and choroid. Whereas these two routes provide different pharmacokinetics of EBIN in eye tissues, both are effective at delivering EBIN to the choroid and retina tissues and lead to similar pharmacological effects.
In conclusion, we describe an alternative approach for the treatment of CNV, which is different in the mechanism of action from the current standard of care eliminating VEGF. EBIN mitigates pathological calcium signaling in ECs and protects the endothelial barrier from the damaging effects of environmental stresses. Based on our genetic data, we conclude that EBIN activates regenerative processes in the eye through the epigenetic control of the chromatin architecture. These results suggest the potential therapeutic value of EBIN in reversing the degenerative processes associated with both the neovascular and atrophic forms of AMD.
Limitations of the study
The current study investigates the therapeutic potential of the EB3 allosteric inhibitor EBIN in treating wet AMD. EBIN reverses the effects of environmental stresses on metabolic-active ECs comprising CNV lesions and epigenetically activates a regenerative program. Although we identify and characterize this subtype of ECs based on gene signatures, the use of spatial transcriptomics would better illustrate the heterogeneity and metabolic plasticity of these ECs. Another limitation of our work is the animal model used to study wet AMD. Whereas laser ablation is the common method to induce CNV in mice and NHPs, it only recapitulates acute responses to injury. Transgenic models such as CXCR5110 or NRF2 knockout (KO)111 mice, which exhibit age-dependent development of CNV, could provide further insights of EBIN effectiveness in treating chronic and progressive pathologies in eye.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse beta-actin, monoclonal | Santa Cruz Biotechnology | Cat# sc-47778; RRID: AB_626632 |
| Mouse OXPHOS antibody cocktail: ATP5A, UQCRC2, MT-CO1, SDHB | Invitrogen | Cat# 45–8099; RRID:AB_2533835 |
| Mouse VEGFR2, monoclonal | Fitzgerald Industries | Cat# 10R-V106a; RRID:AB_1289468 |
| Rabbit CYTB, polyclonal | Proteintech | Cat# 55090-1-AP; RRID:AB_2881266 |
| Rabbit desmin, polyclonal | Abcam | Cat# ab15200; RRID:AB_301744 |
| Rabbit histone H3 acetyl K27 (H3K27ac), polyclonal | Abcam | Cat# ab4729; RRID:AB_2118291 |
| Rabbit histone H4 acetyl K8 (H4K8ac), monoclonal | Abcam | Cat# ab45166; RRID:AB_732937 |
| Rabbit IgG2, monoclonal | Abcam | Cat# ab134050 |
| Rabbit MEIS2, polyclonal | Novus Biologicals | Cat# NBP1-81669; RRID:AB_11026529 |
| Rabbit MT-ATP6, polyclonal | ABClonal | Cat# A8193; RRID:AB_2768510 |
| Rabbit MT-CO2, polyclonal | Proteintech | Cat# 55070-1-AP; RRID:AB_10859832 |
| Rabbit PAX6, polyclonal | Proteintech | Cat# 12323-1-AP; RRID:AB_2159695 |
| Rabbit pVEGF receptor 2 (Tyr1175), monoclonal | Cell Signaling Technology | Cat# 2478; RRID:AB_331377 |
| Rabbit TOM20, polyclonal | Proteintech | Cat# 11802-1-AP; RRID: AB_2207530 |
| Rabbit Vinculin, monoclonal | Abcam | Cat #ab129002; RRID:AB_11144129 |
| Rat VE-Cadherin (CD144), monoclonal | BD Biosciences | Cat# 555289; RRID:AB_395707 |
| Rat VEGF-A, monoclonal | BioLegend | Cat# 512809; RRID:AB_2814439 |
| Bacterial and virus strains | ||
| Escherichia coli BL21 DE3 | Stratagene | 200131 |
| Chemicals, peptides, and recombinant proteins | ||
| (6xHis)-tagged EB3 and EB3-Ct | Geyer et al.22 | N/A |
| 1,4-dithiothreitol (DTT) | Roche | 10197777001 |
| 1-ethyl-3-carbodiimide | Thermo Fisher | 22980 |
| 2-mercaptoethanol | Sigma-Aldrich | 63689 |
| 4′,6-diamidino-2-phenylindole (DAPI) | Invitrogen | D1306 |
| Ammonium chloride (NH4Cl) | Sigma-Aldrich | A4514 |
| Bovine serum albumin | Sigma-Aldrich | A7906 |
| Calcium chloride (CaCl2) | Sigma-Aldrich | 21115 |
| Digitonin | Promega | G9441 |
| Dimethylformamide (DMF) | Supelco | PHR1553 |
| EBIN, 5′6-FAM-EBIN, Myr-EBIN, control peptide, Myr-control peptide | This manuscript | N/A |
| Ethanol | Sigma-Aldrich | E7023 |
| Ethylenediaminetetraacetic acid (EDTA) | Sigma-Aldrich | 798681 |
| Evans Blue | Sigma-Aldrich | E2129 |
| Fluo-4, AM | Invitrogen | F14201 |
| Fluorescein isothiocyanate (FITC)-dextran | Sigma-Aldrich | FD70S |
| Formaldehyde | Sigma-Aldrich | 252549 |
| Fura-2, AM | Invitrogen | F1221 |
| Human PDGF-BB | Biotechne | 220-BB |
| Human VEGF-A | Miltenyi Biotec | 130-094-029 |
| IGEPAL CA-630 | Sigma-Aldrich | I8896 |
| Imidazole | Sigma-Aldrich | I2399 |
| Ionomycin | Sigma-Aldrich | I9657 |
| Isolectin, Alexa Fluor 647 Conjugate | Invitrogen | I32450 |
| Isopropyl 1-thio-β-D-galactopyranoside (IPTG) | Millipore Sigma | I5502 |
| Kanamycin | Millipore Sigma | K0254 |
| LB medium | Thermo Fisher | 10855001 |
| Lectin, Alexa Fluor 594 Conjugate | Invitrogen | L21416 |
| Magnesium acetate | Sigma-Aldrich | M0631 |
| Magnesium chloride hexahydrate (MgCl2) | Sigma-Aldrich | M0250 |
| Manganese chloride (MnCl2) | Sigma-Aldrich | M1787 |
| N-hydroxysuccinimide | Sigma-Aldrich | 130672 |
| NP-40 | Thermo Scientific | 85124 |
| Paraformaldehyde | Sigma-Aldrich | 158127 |
| Phenylmethylsulfonyl fluoride (PMSF) | Roche | 10837091001 |
| Potassium acetate | Sigma-Aldrich | P1147 |
| Recombinant tobacco etch virus (TEV) protease | Biotechne | 4469-TP |
| Sodium Chloride (NaCl) | Sigma-Aldrich | S9888 |
| Sodium citrate dihydrate | Sigma-Aldrich | 567446 |
| Sodium dodecyl sulfate (SDS) | Sigma-Aldrich | L4509 |
| Sodium fluoride (NaF) | Sigma-Aldrich | 201154 |
| Sodium orthovanadate (Na3VO4) | Sigma-Aldrich | S6508 |
| Triton X-100 | Sigma-Aldrich | X100 |
| TRIzol reagent | Invitrogen | 15596026 |
| Tween 20 | Sigma-Aldrich | P1379 |
| Xylene | Sigma-Aldrich | 214736 |
| Critical commercial assays | ||
| ApoTox-Glo triplex assay | Promega | G6320 |
| High-capacity cDNA reverse transcription kit | Applied Biosystems | 4368814 |
| Pierce bicinchoninic acid (BCA) protein assay | Thermo Scientific | 23225 |
| Qubit dsDNA HS and BR assay | Thermo Fisher | Q32851 |
| RNeasy plus kit | Qiagen | 74034 |
| X-tremeGENE HP DNA transfection kit | Roche Life Science | 6366244001 |
| Deposited data | ||
| Bulk and single nuclei (sn)RNA- and ATAC-sequencing data | This manuscript; Gene Expression Omnibus | GEO: GSE228276 |
| EB3 NMR chemical shift assignments | This manuscript; Biological Magnetic Resonance DataBank | BMRB: 50003 |
| Experimental models: Cell lines | ||
| Human embryonic kidney 293 cells (HEK293) | ATCC | CRL-1573 |
| Human lens epithelial B3 cells (BEL-B3) | ATCC | CRL-11421 |
| Human lung microvascular endothelial cells (HLMEC) | Cell Systems | ACBRI 468 |
| Human retinal microvascular endothelial cells (HREC) | Cell Systems | ACBRI 181 |
| Experimental models: Organisms/strains | ||
| Mouse: Crl:CD1 (ICR) | Charles River Laboratories | 022 |
| Non-human primate: African Green Monkey (Chlorocebus sabaeus) | St. Kitts Biomedical Research Foundation | N/A |
| Oligonucleotides | ||
| Primer: MT-ATP6 forward, 5′-ACCACAAGGCACACCTACAC-3′ |
This manuscript | N/A |
| Primer: MT-ATP6 reverse, 5′-GGCCAGGGCTATTGGTTGAA-3′ |
This manuscript | N/A |
| Primer: MT-CYB forward, 5′-CCCATCCAACATCTCCGCAT-3′ |
This manuscript | N/A |
| Primer: MT-CYB reverse, 5′-GATGAAAAGGCGGTTGAGGC-3′ |
This manuscript | N/A |
| Primer: MT-CO1 forward, 5′-GGAGGAGGAGACCCCATTCT-3′ |
This manuscript | N/A |
| Primer: MT-CO1 reverse, 5′-AGTGGAAGTGGGCTACAACG-3′ |
This manuscript | N/A |
| Primer: MT-CO2 forward, 5′-GTACTCCCGATTGAAGCCCC-3′ |
This manuscript | N/A |
| Primer: MT-CO2 reverse, 5′-TCGTGTAGCGGTGAAAGTGG-3′ |
This manuscript | N/A |
| Primer: MT-CO3 forward, 5′-ACCCCGCTAAATCCCCTAGA-3′ |
This manuscript | N/A |
| Primer: MT-CO3 reverse, 5′-ATGTTGAGCCGTAGATGCCG-3′ |
This manuscript | N/A |
| Primer: MT-ND1 forward, 5′-GGCTATATACAACTACGCAAAGGC-3′ |
This manuscript | N/A |
| Primer: MT-ND1 reverse, 5′-GGTAGATGTGGCGGGTTTTAGG-3′ |
This manuscript | N/A |
| Primer: MT-ND2 forward, 5′-AGCACCACGACCCTACTACT-3′ |
This manuscript | N/A |
| Primer: MT-ND2 reverse, 5′-TGGTGGGGATGATGAGGCTA-3′ |
This manuscript | N/A |
| Primer: MT-ND3 forward, 5′-GCGGCTTCGACCCTATATCC-3′ |
This manuscript | N/A |
| Primer: MT-ND3 reverse, 5′-AGGGCTCATGGTAGGGGTAA-3′ |
This manuscript | N/A |
| Primer: MT-ND4 forward, 5′-CCCTCGTAGTAACAGCCATTCTC-3′ |
This manuscript | N/A |
| Primer: MT-ND4 reverse, 5′-CGACTGTGAGTGCGTTCGTAGT-3′ |
This manuscript | N/A |
| Primer: MT-ND5 forward, 5′-CGGAAGCCTATTCGCAGGAT-3′ |
This manuscript | N/A |
| Primer: MT-ND5 reverse, 5′-TGGAGGTGGAGATTTGGTGC-3′ |
This manuscript | N/A |
| Primer pair: TOMM20 forward, 5′-CGAGAACGAAGAAAGAAACAGAAG-3’; TOMM20 reverse, 5′-CCTTGAGCTAGTAACTCTTCACC-3′ |
Integrated DNA Technologies | Hs.PT.58.2753511 |
| Software and algorithms | ||
| Aperio ImageScope v12.4.6 | Leica Biosystems | https://www.leicabiosystems.com/us/digital-pathology/manage/aperio-imagescope/ |
| BEDtools v2.18 | Quinlan and Hall112 | https://bedtools.readthedocs.io/en/latest/ |
| Cell Ranger v3.0.2 | 10X Genomics | https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger |
| edgeR v3.17 | McCarthy, Chen et al.113 | https://bioconductor.org/packages/release/bioc/html/edgeR.html |
| FeatureCounts v2.0.0 | Liao, Smyth et al.114 | https://sourceforge.net/projects/subread/files/subread-2.0.0/ |
| FIMO v5.5.2 | Bailey, Boden et al.115 | https://meme-suite.org/meme/tools/fimo |
| Ggplot2 v3.4.2 | Tidyverse | https://ggplot2.tidyverse.org |
| Gplot v3.1.3 | Tal Galili | https://github.com/talgalili/gplots |
| ImageJ (FIJI) | National Institutes of Health | https://fiji.sc/ |
| Imaris v9.5 | Oxford Instruments | https://imaris.oxinst.com/versions/9-5 |
| Ingenuity Pathway Analysis | Qiagen | https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa/ |
| JASPAR CORE | JASPAR | https://jaspar.genereg.net |
| MACS2 v3.0.0 | Zhang, Liu et al.116 | https://github.com/macs3-project/MACS |
| Metamorph v7.7 | Molecular Devices | https://www.moleculardevices.com/products/cellular-imaging-systems/acquisition-and-analysis-software/metamorph-microscopy |
| Picard v3.0.0 | Broad Institute of MIT and Harvard | https://broadinstitute.github.io/picard/ |
| Prism 9 | Graph Pad | https://www.graphpad.com/scientific-software/prism/ |
| Seurat v4.3.0 | Stuart et al.117 | https://github.com/satijalab/seurat |
| STAR v2.7.10 | Dobin, Davis et al.118 | https://github.com/alexdobin/STAR |
| Zen v3.6 | Carl Zeiss | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html |
| Other | ||
| Dulbecco’s Phosphate Buffered Saline | Corning | 21-030-CM |
| HEPES | Sigma-Aldrich | H0887 |
| NEBNext High-fidelity 2X PCR Master Mix | New England Biolabs | M0541 |
| PowerUp SYBR Green Master Mix | Applied Biosystems | A25742 |
| Protease inhibitor cocktail | Sigma-Aldrich | P8340 |
| RNase inhibitor | Promega | N211B |
| Tris-HCl buffer, pH 7.5 | Invitrogen | 15567027 |
Resource availability
Lead contact
Any additional information and requests for resources and materials should be addressed and fulfilled by the lead contact, Yulia A. Komarova (ykomarov@uic.edu).
Materials availability
Any request for resources and materials utilized in this paper should be addressed and fulfilled by the lead contact, Yulia A. Komarova (ykomarov@uic.edu).
Experimental model and study participant details
Cell culture
Human embryonic kidney 293 cells (HEK293; cat #CRL-1573) and human lens epithelial B3 cells (HEL-B3; cat #CRL-11421) were sourced from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle’s medium (cat #10-017-CV). Human lung microvascular endothelial cells (cat #ACBRI 468) and human retinal microvascular endothelial cells (cat #ACBRI 181) were sourced from Cell Systems and cultured in EGM-2 media (cat #CC-4176; Lonza) supplemented with EGM2 Bulletkit (cat #CC-3162; Lonza) and 10% fetal bovine serum. Cells were cultured at 37°C with 5% CO2. The sources for these cell lines provided authenticated cells that test negative for mycoplasma.
Mouse studies
All CD1 mice used in this study were sourced from Charles River Laboratories and were 7-8-weeks old and weights 18–20 g. All mice were housed in the Biological Resource Laboratory at the University of Illinois at Chicago (UIC) under University of Illinois’ Animal Care and Use Committee approved conditions accredited by the American Association for the Accreditation of Laboratory Animal Care (ACC 18–218 and 18–244). The animals were maintained under a surveillance program implemented by the College of Medicine Animal Care Disease and Diagnostic Laboratory and under the supervision of well-trained University of Illinois certified veterinary doctors.
Non-human primate studies
All African green monkeys (Chlorocebus sabaeus) used in this study were sourced from the St. Kitts Biomedical Research Foundation. All studies were conducted at Virscio, Inc. in compliance with the facility’s Standard Operating Procedures and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Study animals were housed in Virscio, Inc. primate enclosures at the facility under the approved conditions of the University of Illinois’ Animal Care and Use Committee (ACI 7166). An equal number of adult male monkeys (with a body weight range of 4.92–6.64 kg) and female monkeys (with a body weight range of 2.82–4.18 kg) were used in the study.
Method details
Computational alanine scanning
Computational alanine scanning uses a simple free energy function to calculate the effects of alanine mutations on the binding free energy of a protein-protein complex. The function consists of a linear combination of a Lennard-Jones potential to describe atomic packing interactions, an implicit solvation model, an orientation-dependent hydrogen-bonding potential derived from high-resolution protein structures, statistical terms approximating the backbone-dependent amino acid-type and rotamer probabilities, and an estimate of unfolded reference state energies. The algorithm automatically identifies all interface residues in a protein-protein interface. An interface residue is defined as (i) a residue that has at least one atom within a sphere with a 4 Å radius of an atom belonging to the other partner in the protein complex, or (ii) a residue that becomes significantly buried upon complex formation, as measured by an increase in the number of Cβ atoms within a sphere with a radius of 8 Å around the Cβ atom of the residue of interest. The program then replaces each of the interface residues individually with alanine residues and computes the effect of this mutation on the binding free energy of the complex. Rosetta based computational alanine scan was performed using the EB3-IP3R3 peptide complex. Hot-spot residues can be defined operationally as those for which alanine mutations have destabilizing effects on ΔΔG bind of more than 1 kcal/mol.
Expression of EB3
Preparation of (6xHis)-tagged EB3 and EB3-Ct was described previously by us (Geyer et al., 2015). (6xHis)-tagged recombinant proteins were expressed in Escherichia coli strain BL21 (DE3) (Stratagene). The expression construct contains a tobacco etch virus (TEV) protease cleavage site immediately following the 6xHis tag for efficient removal. Bacteria were grown at 37°C in LB medium containing 50 μg/mL kanamycin. For NMR studies, bacteria were grown in minimal media containing 15N and 13C stable isotopes. When the OD600 reached 0.6–0.7, protein production was induced by addition of isopropyl 1-thio-β-D-galactopyranoside (IPTG) to a final concentration of 250 μM. After 4 h at 37°C, bacterial pellets were isolated and sonicated (4 × 1 min) in medium comprising 150 mM NaCl, 5 mM 2-mercaptoethanol, 2 mM CaCl2, 10 mM imidazole, 2 mM PMSF, 25 mM Tris-HCl, pH 7.4.
Affinity Purification
(Geyer et al., 2015): 6xHis-tagged-EB3 protein were purified using Ni-NTA beads (Thermo Scientific). Ni-NTA beads (1 mL) in a 20 mL column (Bio-Rad) were equilibrated with 50 bed-volumes of binding buffer (25 mM Tris-HCl, pH 7.4, 300 mM NaCl, 5 mM 2-mercaptoethanol, 2 mM PMSF). Bacterial lysate (50 mL) was then added to the column, followed by washing (150 bed-volumes of wash buffer, ∼75 mL). The protein-bound beads were washed with phosphate-buffered saline (PBS) and stored in the same buffer.
Gel-filtration
After washing the Ni-NTA beads, recombinant 6xHis-tagged EB3 was eluted by addition of wash buffer containing 150 mM imidazole. Peak elution fractions were pooled, exchanged to imidazole-free buffer using PD-10 desalting columns (GE Life Sciences), and concentrated with an Amicon Ultra-15 with 10 kDa cut-off concentrator unit (Millipore, Inc.). The 6xHis tag was removed by addition of 1.5% (w/w) recombinant TEV protease (Kapust & Waugh, 2000) and incubation at 0°C for 16 h. For further polishing, EB3 protein was then subjected to gel filtration chromatography over tandem Superdex 200 HR 10/30 columns connected in series and controlled by an AKTA FPLC (GE Life Sciences). Peak fractions containing EB3 protein was then pooled and concentrated as described above.
Cross-linking of EB3 to beads
For cross-linking of 6xHis-tagged-EB3 to the Ni-NTA beads, 40–50 μL of (6xHis)-tagged protein bound to beads were incubated with N-hydroxysuccinimide (75 mM) and 1-ethyl-3-carbodiimide (50 mM) in 20 mM HEPES buffer, pH ∼7.0 (1 mL for 1 h at 4°C). The beads were washed with PBS (2 × 1 mL) and incubated with PBS containing 5% bovine serum albumin (BSA) for 1 h to block non-specific binding before use in pull-downs.
Pull-down analysis
(Geyer et al., 2015, Komarova et al., 2012). For pull-down analyses, HEK 293 cells expressing GFP-tagged proteins were lysed in medium comprising 150 mM NaCl, 50 mM Tris-HCl, pH 8.0, 1% NP40 (Sigma), and 1% protease inhibitor cocktail (Sigma). The lysates (∼300 μL/sample) containing equal amounts of GFP-tagged proteins were incubated with the 6xHis-tagged-EB3/Ni-NTA beads (50 μL) pre-treated with various concentration of EBIN analogs in the presence of 10 mM imidazole at 4°C for 2 h. Bound proteins were eluted by heating in sample buffer at 95°C for 5 min before SDS-PAGE using 4–20% tris-glycine gels (Life Technologies). After transfer to a nitrocellulose membrane (Bio-Rad), the blots were probed with anti-GFP antibodies.
For analysis of protein expression in cell culture, cell samples were lysed with radioimmunoprecipitation assay (RIPA) buffer (#R027, Sigma-Aldrich) with protease and phosphatase inhibitor cocktails (#P8340, #P5726, #P0044, Sigma-Aldrich). For in vivo experiments, NHP tissue samples were lysed with a 1% SDS lysis buffer with 10 mM NaF, 20 mM HEPES, 2 mM Na3VO4, and protease inhibitor cocktail. The samples were measured of total protein concentration using the Pierce bicinchoninic acid (BCA) protein assay kit (#23225, Thermo Scientific) and prepared in Laemmli sample buffer (#1610747, Bio-Rad). The protein samples were separated using SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% BSA, the samples were incubated with primary antibodies at 4°C overnight for ATP5A, UQCRC2, MT-CO1, SDHB using an OXPHOS cocktail antibody (1:10,000, #45–8099, Invitrogen), MT-CYB (1:1000, #55090-1-AP, ProteinTech), MT-CO2 (1:1000, #55070-1-AP, ProteinTech), ATP6 (1:1000, #A8193, ABclonal), TOMM20 (1:1000, #11802-1-AP, ProteinTech), Meis2 (1:1000, #NBP1-81669, Novus), Pax6 (1:1000, #12323-1-AP, ProteinTech), vinculin (1:5000, #ab129002, Abcam), pY1175 VEGFR2 (1:1000, #2478, Cell Signaling), and/or β-Actin (1:2000, sc-47778, Santa Cruz). After washing, the samples were incubated with corresponding horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature and detected using electrochemiluminescence.
qRT-PCR
RNA samples were isolated using TRIzol Reagent (#15596026, Invitrogen) per the manufacturer’s instructions. Afterward, the RNA samples underwent reverse transcription using the High-Capacity cDNA Reverse Transcription Kit (#4368814, Applied Biosystems). The cDNA of the samples was then used in qRT-PCR with the Viia7 Real-Time PCR System (Applied Biosystems) using PowerUp Sybr Green Master Mix (#A25742, Applied Biosystems) with appropriate primers (listed in the key resources table). Relative gene fold change was quantified using the ΔΔCT method.
Nano differential scanning fluorimetry (DSF)
Unfolding of EB3 alone or in presence of EBIN was measured by detecting the temperature-dependent change in tryptophan fluorescence at λ = 330 nm and 350 nm using the Prometheus NT.Plex (NanoTemper). The temperature gradient was set in a range from 200°C to 900°C. Melting temperatures were calculated from the maximum of the first derivative of the fluorescence ratios (F350/F330). For this, an eighth order polynomial fit was calculated for the transition region and then the first derivative of the fit was formed. The peak position at melting temperature was determined from the first derivative.
Molecular docking
The interaction between EB3’s C-terminus (200–281) and EBIN was modeled using Zdock (Pierce, Wiehe et al., 2014). The structure of EBIN was sketched using the ChemOffice software suite and optimized using MM2. At least 10 models were generated and assessed based on spectral changes observed in 1H-15N HSQC experiments. Using Molecular operating environment (MOE) software, we have generated a 2D map showing EBIN interaction with EB3.
NMR Spectroscopy
1H-15N Heteronuclear Single Quantum Coherence (HSQC) experiments were performed on 350 μM recombinant human EB3 C-terminus in PBS, pH 7.4. Experiments were carried out in the presence and absence of 350 μM EBIN resuspended in PBS, pH 7.4. Backbone 1H, 15N, 13C assignments were carried out using double and triple resonance experiments of 2D 1H–15N HSQC, 1H-13C HSQC, HNCA, HN(CO)CA, HNCACB and HN(CO)CACB (Kay, Ikura et al., 2011). Assignments were deposited to the Biological Magnetic Resonance DataBank. All NMR experiments were performed on an 800-MHz Bruker Avance Spectrometer equipped with a cryogenic probe. Data processing and analysis were carried out using NMRPipe (Delaglio, Grzesiek et al., 1995).
Cell culture Transfections
HEK 293 cells are transfected at ∼80% confluence using X-tremeGENE HP according to the manufacturer’s protocol (Roche) and used after 24–48 h.
EBIN uptake in endothelial cells
The rate of EBIN uptake by primary human microvascular endothelial cells was measured using 5′6-FAM (Fluorescein)-labeled Myr-EBIN and confocal fluorescence live-cell imaging, as previously described (Procter et al., 2018). Fluorescein alone was used as a negative control. The changes in intracellular fluorescence were monitored over time and saponin, an amphipathic glycoside that interacts with cholesterol and phospholipids of the plasma membrane, was added by the end of the experiment to induce full uptake of Myr-EBIN by cells. The fluorescent intensity inside of the cells were plotted over time and the t1/2 uptake and max uptake (relative to maximum intracellular concentration of Myr-EBIN after treatment of cells with saponin) were determined.
Cytosolic Ca2+ measurements
Intracellular Ca2+ concentrations were measured with Ca2+-sensitive fluorescent dye Fluo-4 using a robotically integrated platform for high content screening (FlexStation-II, Molecular Devices). Primary human microvascular endothelial cells and human lens epithelial cell line HEL-B3 grown on a 96-well microplate (Molecular Device) were loaded with Fluo-4 a.m. (Life Technologies) for 20 min at 37°C in culture medium without supplements. Treatments with Myr-EBIN were applied during loading with Fluo-4 a.m. After incubation, cells were washed with Hank’s Balanced Salt Solution (HBSS) containing Ca2+ and Mg2+ and replaced with Ca2+ free HBSS containing 2 mM Mg2+ before imaging. Endothelial cells were stimulated with 50 ng/mL VEGF-A; epithelial cells were stimulated with 20 ng/mL PDGF-bb at 37°C. The Fluo-4 fluorescent intensities were measured at excitation λ = 494 nm and emission λ = 506 nm every 2 s with FlexStation-II platform. The relative changes in fluorescence in treated cells were determined by measuring area under the curve and used to determine the relative cytosolic Ca2+ concentration. Six different concentrations of Myr-EBIN: 50 nM, 100 nM, 250 nM, 500 nM, 1 μM, 5 μM, were tested. The cytosolic calcium concentration was plotted as a function of peptide concentration in logarithmic scale and EC50 was determined from the dose-response curve.
To validate the overall results with Fluo-4 dye, intracellular Ca2+ was also measured using the Ca2+-sensitive ratiometric dye Fura 2-AM, as previously described (Geyer et al., 2015). These data are presented in Fig. EV1A-B. Human microvascular endothelial cells grown on glass-bottom dishes (Becton Dickinson) were treated with different concentrations of Myr-EBIN or 5 μM Myr-control peptide (the max concentration used for Myr-EBIN) for 15 min at 37°C in culture medium without supplements. Cells were loaded with Fura-2 a.m. (Life Technologies) at the same time. Fura-2 fluorescence was excited at 340 and 380 nm and collected at 510 ± 80 nm using an Axiovert 100 inverted microscope (Carl Zeiss) equipped with Plan-Apo 60× with the numerical aperture (NA) 1.4 oil immersion objective, Lambda DG-4 switcher illumination system (Sutter Instruments), AxioCom Hsm camera (Zeiss), fura-2 filter set (Chroma), and AxioVision Physiology Acquisition module. Images were collected at 2 s intervals. Fluorescence ratios (F340/F380) were calculated within a circular region of interest (radius 3 μm) for each cell after subtraction of intracellular background fluorescence, determined by quenching Fura-2a.m. fluorescence with a mixture of 3 μM ionomycin and 5 mM MnCl2.
Cytotoxicity studies
To determine the toxicity of Myr-EBIN, cellular metabolic activity of human microvascular endothelial cells was measured using Promega ApoTox-Glo Triplex Assay system. Cells seeded onto 96 well black wall clear bottom plates were incubated with different concentrations of Myr-EBIN (200 nM, 1 μm, 10 μm, 100 μm and 200 μM) for 24 h. The viability, cytotoxicity, and apoptosis events were assessed in the same well according to the manufacturer’s protocol.
Transwell endothelial permeability measurements
Endothelial barrier function was also assessed by measuring FITC-dextran 70 kDa (Sigma Aldrich) tracer flow-through of human endothelial monolayers. These monolayers were grown on Corning 12 well Transwell Polyester Membrane Cell Culture Inserts (12 mm with 0.4 μm pore) (Millipore Sigma) for 3 days. After serum-starving with endothelial cell growth basal medium, phenol red free (EBM; Lonza) in the insert and bottom chamber for 2 h, they were incubated with 1 μM Myr-EBIN or Myr-control peptides for an hour. A 0.5 μg/mL solution of FITC-dextran 70 kDa was added to the insert before starting 20 μL triplicate collection in 96-well black solid-bottom microplates (Greiner) of the flow-through at 30-min intervals to establish initial baseline rate. With each collection, the total collected volume was replaced in the insert with 3% BSA. After an hour, the cells were left without challenge or challenged with 50 ng/mL of human VEGF-A (Miltenyi Biotec) with flow-through collection every 20 min. Fluorescence intensity was measured against standards established with varying concentrations of FITC-dextran 70 kDa in the EBM phenol red free media using PHERAstar fluorescent.
VEGFR2 surface expression
To measure the surface expression of VEGFR2, human endothelial cells were immunostained as previously described (Yamada, Nakajima et al., 2014). Cells were serum-starved in EBM with 0.1% BSA for 2 h, then treated with either 1 μM control or Myr-EBIN peptide for 1 h and simulated with 50 ng/mL hVEGF-A. Cells were fixed with 4% paraformaldehyde, washed with PBS, and quenched with 50 mM ammonium chloride. After blocking with 3% BSA in PBS, cells were stained with an antibody against the extracellular domain of VEGFR2 (1:100, #10R-V106A, Fitzgerald) and DAPI. Samples were imaged using a Zeiss LSM-880 confocal microscope equipped with a GaAsP detector and a 63 ×1.4 NA Plan-Apochromat oil immersion objective. Integrated intensity of VEGFR2 expression was measured using ImageJ (NIH) software and was normalized to the cell area.
EBIN action on capillary permeability in vivo
Vascular permeability was quantified using the Miles assay as described elsewhere (Miles & Miles, 1952). The back of the animal was shaved to remove the fur. Mice were treated with IV injection of Myr-EBIN or Myr-control peptide at 1 μM/kg body weight. 30 min later, mice were injected with 100 mL of 0.5% Evans blue into the tail vein, followed immediately by intradermal hVEGF-A (50 ng/kg BW) in the back skin. Mice were euthanized 30 min later, perfused with saline, and tissue was collected with an 8-mm skin punch. Evans blue was extracted by incubating tissue in formamide at 56°C for 48 h, and dye concentration was measured by spectrophotometer at λ = 610 nm, corrected for hemoglobin (450 nm) and skin weight.
Induction, monitoring, and analysis of choroidal neovascularization in mice
We adopted a new Micron IV platform image-guided laser system for CNV induction. The fundus was viewed with an imaging camera, and laser photocoagulation was induced using the image-guided laser system (Micron IV, Phoenix Research Laboratories, Pleasanton, CA). Four laser burns at equal distance from the optic nerve were induced one by one in the right eye of the mouse by a green Argon laser pulse with a wavelength of 532 nm, a fixed diameter of 50 μm, duration of 70 ms, and power levels from 210 to 250 mW. Appearance of a bubble or small subretinal hemorrhage (diameter <1 mm) at the laser spot served as an indication of rupture of the Bruch’s membrane.
Treatment with Myr-control or Myr-EBIN peptides (1μg/eye) and antibody against mouse VEGF-A (2μg/eye; LEAF; Low Endotoxin, Azide-Free) were administrated once to the right eye via intravitreal injection (2 μL) after laser photocoagulation. The eyes were gently rinsed with sterile saline to remove the lubricating eye drops and treated with antibiotic ointment erythromycin (Fougera, Melville, NY). Mice were then placed on a pre-warmed warming plate at 35°C until they awakened. Another cohort was treated with twice daily topical application of Myr-EBIN at 5 μg per eye in combination with intravitreal injection (2 μL) of antibody against control IgG2α antibody. Treatment with Myr-EBIN or Myr-control peptide started one-day prior to laser photocoagulation.
The Myr-EBIN anti-angiogenic efficacy was evaluated by fluorescein angiography and ocular coherence tomography at days 7 and 14. The experiments were terminated at day 14 and the flat mount preparations of retina/choroid/sclera were used for staining with Alexa 594-labled lectin from Bandeiraea simplicifolia (B4) for postmortem analysis of the CNV area. Data analysis was performed using exclusion criteria established elsewhere (Gong et al., 2015). The cases of severe hemorrhages, excessive laser burns that damage not only Bruch’s membrane but also the choroid and retinal pigment epithelium, fused lesions, and lesions more than 5 times greater than the mean of the lesions under the same experimental conditions were excluded from analysis. The area of vascular leakage and CNV were quantified using fluorescein angiography images and confocal images of CNV staining for lectin B4 (IB4) and endothelial cell marker VE-cadherin using MetaMorph software.
For immunostaining of other markers, the flat mount preparations of retina/choroid/sclera were fixed and permeabilized using 0.1% PBS-X for at least 24 h at 4°C. Samples were incubated with primary antibodies for pericyte marker desmin (1:200, #ab15200, Abcam), VE-cadherin (1:50, #555289, BD Biosciences), and/or Alexa Fluor 647 conjugated Isolectin B4 (1:100, #I32450, Invitrogen). After washing, the samples were incubated with corresponding secondary antibodies for 2 h at room temperature. Lesions were imaged using a Zeiss LSM-880 confocal microscope with a 40 ×1.3 NA EC Plan-Neofluar oil immersion objective. The immunolabeled lesion images were reconstructed using Imaris 3D software (Bitplane) after creating a mask that excluded surfaces outside of the lesion area. The volume of desmin and VE-cadherin was normalized to the volume of the IB4-positive lesion.
EBIN biodistribution in the eye
Three adult St. Kitts African green monkeys (Chlorocebus sabaeus) received 30 μL topical instillation of 150 μg Myr-EBIN twice daily for 6 days in both eyes. Dosing was performed under ketamine/xylazine sedation using a positive displacement pipette (Gilson Microman) between 8:00 and 9:30 a.m. each morning and 4:00 and 5:00 p.m. in the afternoon. The formulation was instilled into the conjunctival sac, and the eyelids were gently closed for ∼5 s post-dosing.
To compare Myr-EBIN biodistribution between topical and ITV administration, one monkey received ITV injection of 150 μg Myr-EBIN in each eye on day one and eye tissue was collected on day 6. For ITV dosing, topical local anesthesia was administered (0.5% proparacaine) and eyes were disinfected with 5% Betadine and rinsed with sterile normal saline. ITV injections were administered using a 31-gauge 6/15-inch needle (Ulticare Vet RX, #A60801) placed 2 mm posterior to the limbus in the inferior temporal quadrant, targeting the central vitreous. Injections were followed by topical administration of triple antibiotic ointment (neomycin/polymyxin B sulfates/bacitracin zinc).
At the end of day 6, monkeys were euthanized with sodium pentobarbital (25 mg/kg IV to effect) while under anesthesia and eyes immediately enucleated. Excess orbital tissue was trimmed, and globes flash frozen in liquid nitrogen then dissected along frozen tissue planes at room temperature. Bulbar conjunctiva, cornea, lens, iris/ciliary body, retina, choroid, sclera and optic nerve were collected into pre-tared, pre-labeled vials, weighed and flash frozen in liquid nitrogen.
Myr-EBIN concentration was analyzed with the LC-MS/MS method. Myr-EBIN was extracted with 90% acetonitrile and 10 μL of supernatant was injected into the LC-MS/MS system consisting of the Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA) coupled with the QTRAP 6500 mass spectrometer (Sciex, Framingham, MA). Chromatography was performed using a binary solvent system consisting of A: 0.1% formic acid and 5% ACN and B: 0.1% formic acid and 95% ACN at a flow rate of 200 μL/min and the mobile phase and the reversed-phase column SB-C18 (2.1 mm × 50 mm, particle size 1.8 μm, Agilent Technologies). A gradient was run from 5% B to 100% B over 10 min. The multiple reaction monitoring was operated in the positive ion mode monitoring the 515.8–330.2 m/z transition. Concentration of Myr-EBIN was determined using the standard curve and expressed as ng per g of dry tissue.
Induction, monitoring, and analysis of choroidal neovascularization in AMD model in non-human primates
Laser-induced CNV
On Day 0, CNV were induced between temporal vascular arcades by laser photocoagulation targeting the Bruch’s membrane. Six laser spots were symmetrically placed within the perimacular region in each eye by an ophthalmologist employing an Iridex Oculight TX 532 nm laser with a laser duration of 100 ms, spot size of 50 μm, and power of 750 mW. Laser spots were applied using a contact laser lens. Any spots demonstrating severe retinal/subretinal hemorrhage immediately post-laser and not resolved by the time of follow-up examinations were excluded from analyses. If the hemorrhaging encompassed all target lesion areas within the central retina, then the animal was substituted with another screened monkey, with up to six monkeys across all treatment groups, taking measures to assure balanced assignment to treatment. Immediately post-laser treatment, monkeys underwent color fundus imaging to document the appearance of the laser lesions and to allow balanced randomization to treatment assignment (balanced incidence of well-defined versus mildly hemorrhagic lesions per treatment regimen). Laser treatments were distributed across two consecutive days in 3 treatment cohorts (3 monkeys from each group to ensure that control and test groups are analyzed on the same day) to accommodate imaging at ophthalmic exam intervals.
EBIN and vehicle delivery
Monkeys received topical formulations of Myr-EBIN or vehicle in each eye on study day 0 after laser outcome assessment to randomize eyes by achieved laser photocoagulation spot quality within 2 h of laser photocoagulation. Monkeys received 30 μL topical instillation of Myr-EBIN or vehicle formulations under ketamine/xylazine sedation using a positive displacement pipette (Gilson Microman, or equivalent) twice daily for 7 days and once a day for another 14 days.
Aflibercept delivery
Monkeys received ITV injections of 1 mg Aflibercept. For ITV dosing, topical local anesthesia was administered (0.5% proparacaine) and eyes were disinfected with 5% Betadine and rinsed with sterile normal saline. ITV injections were administered using a 31-gauge 0.5-inch needle placed 2 mm posterior to the limbus in the inferior temporal quadrant, targeting the central vitreous. Injections were followed by topical administration of 0.3% ciprofloxacin ophthalmic solution or equivalent antibiotic ointment.
Slit lamp biomicroscopy and fundoscopy
Anterior segment inflammation was examined with slit lamp biomicroscopy at days 0, 14 and 21. Evaluation of the posterior wall and vitreous inflammation was performed by a posterior segment slit lamp exam with a 90-diopter lens. Scoring was applied to qualitative clinical ophthalmic findings using a modified McDonald-Shadduck scoring system.
Color fundus photography and fluorescein angiography (FA)
At days 0, 14, and 21, bilateral color fundus images of the retina were captured with 50 degrees of view centered on the fovea using a Topcon TRC-50EX retinal camera with Canon 6D digital imaging hardware and New Vision Fundus Image Analysis System software. FA was performed with IV administration of 0.1 mL/kg of 10% sodium fluorescein. Fluorescein leakage in angiograms of CNV lesions was graded (I-IV; Table S1) by a masked ophthalmologist, assessing image composites generated after uniform adjustment of image intensity.
FA lesion grading
Graded scoring of angiograms was performed on FA series collected on days 0, 14 and 21. Prior to graded scoring, all ∼30 s (early phase), ∼3-min (mid-transitional phase) and ∼6-min (late phase) angiograms were enhanced in Adobe Photoshop by auto brightness and contrast, then by slightly adjusting the brightness and contrast to a similar level to minimize the impact of the inherent variability of image intensity associated with different pupil size and ocular media clarity on visual scoring. Each angiogram series was reviewed to confirm overall consistency in the quality and sequence of images collected. The extent of fluorescein leakage at each lesion site, an angiographic change associated with CNV, was rated on a I to IV scale (Table S1).
Optical coherence tomography (OCT)
At days 0, 14, and 21, OCT was performed using a Heidelberg Spectralis OCT Plus with eye tracking and HEYEX image capture and analysis software. At baseline examination, a cross-sectional display image and an overall volume scan of the entire macula were obtained. At post-laser examinations, star-shaped scans, centered on each lesion, were performed, as well as an overall volume scan of the entire macula encompassing the laser spots at a dense scan interval. The principal axis of maximal CNV complex formation within each star-shaped scan at each laser lesion was defined, and the CNV complex area measured by a masked evaluator using the freehand tool within ImageJ to delineate the CNV complex boundary and calculate maximum complex area in square microns (μm2).
CNV complex area measurement
OCT was performed using a Heidelberg Engineering Spectralis OCT Plus system (Vista, CA) and Heidelberg Eye Explorer (HEYEX) software (v1.6.1.). At baseline examinations prior to laser, the retinal thickness map and cross-sectional display image were obtained of each eye. At post-laser examinations, 6 star-shaped scans per eye, centered on each lesion, were performed. The principal axis of maximal CNV complex size within each star-shaped scan at each laser lesion was defined, and the CNV complex area in the OCT image in that axis was measured by an evaluator masked to the treatment using the freehand tool within ImageJ to delineate the CNV complex boundary and calculate maximum complex area in square microns (μm2).
Immunofluorescent staining
At the end of the study, half of the NHP eyes were fixed in formaldehyde for further immunohistochemical analysis, and the other half was flash frozen for downstream biochemical and bioinformatic analyses. Fixed eyes were then embedded in paraffin and sectioned. For immunostaining NHP eye tissues, the sections were deparaffinized and rehydrated sequentially in 100% xylene, 50% xylene:ethanol (EtOH), 100% EtOH, 95% EtOH:H2O, 70% EtOH, 50% EtOH, and 100% H2O. Samples underwent antigen retrieval with 10 mM sodium citrate buffer (pH 6.0) at 95°C for 20 min. Samples were then washed, permeabilized in 0.25% PBS-X, and blocked with a 5% BSA solution. Samples were then incubated with primary antibodies for H3K27ac (1:200, #ab4729, abcam), H4K8ac (1:200, #ab45166, abcam), Meis2 (1:100, #NBP1-81669, Novus), Pax6 (1:100, #12323-1-AP, ProteinTech), and/or DAPI (1:500, #D1306, Invitrogen) overnight at 4°C. After washing, the samples were incubated with corresponding secondary antibodies for 2 h at room temperature. Lesions were imaged using a Zeiss LSM-880 confocal microscope with a 40 ×1.3 NA EC Plan-Neofluar oil immersion objective. Using ImageJ, the intensity of H3K27ac, H4K8ac, Meis2, or Pax6 was measured after masking to exclude signal outside of the nuclei and then normalized to the nuclei.
Bulk in vitro RNA-seq studies
RNA isolation
Total RNA from HRECs challenged with normoxia or acute (3 h) hypoxia treated with vehicle or EBIN were isolated by RNeasy Plus kit (Qiagen, 74034). Concentrations of isolated RNA samples were measured using the Nanodrop 1000 (Thermo Fisher) at 260 nm. The quality of the isolated RNA was assessed by 260/280 optical density ratio to check for purity. Lastly, the RNA samples were reverse transcribed to cDNAs using a high-capacity cDNA reverse transcription kit (Thermo Fischer, 4368814). The resulting cDNAs were sent to the University of Chicago for bulk RNA sequencing.
Bulk RNA sequencing
The RNA quality and quantity was assessed using the Agilent bio-analyzer. All samples showed RNA integrity number >8. Strand-specific RNA-SEQ libraries were prepared using a TruSEQ mRNA RNA-SEQ library protocol (Illumina provided). Library quality and quantity was assed using the Agilent bio-analyzer and libraries were sequenced using an Illumina NovaSEQ6000 (Illumina provided reagents and protocols).
Bulk RNA-seq analysis
Raw reads were aligned to reference genome hg38 using STAR (Dobin, Davis et al., 2013). ENSEMBL gene expression was quantified using FeatureCounts (Liao, Smyth et al., 2014). Differential expression statistics were computed using edgeR (McCarthy, Chen et al., 2012) using the exactTest() function for each pairwise comparison, and p values were adjusted for multiple testing using the false discovery rate (FDR) correction of Benjamini and Hochberg (Benjamini & Hochberg, 1995).
Genes that were differentially expressed in any pairwise comparison based on FDR<0.05 were selected for clustering analysis. We performed k-means clustering with ten random initializations on a range of cluster numbers k (2–20). For each k, we will evaluate the reproducibility of the repeated clustering runs by comparing the pairwise distance between clustering results: we compute the fraction of co-clustered feature pairs across two clustering results relative to the number of co-clustered feature pairs within each result individually. This difference was averaged across all result pairs for each value of k, and the largest k with an average distance less than 1e-5 was selected as the largest k with highly reproducible clusters, which resulted in 5 gene clusters. Patterns from clusters will be visualized using heatmaps and boxplots to aid in interpretation. Pathway analysis of the genes in each cluster was performed using the core analysis function in Ingenuity Pathway Analysis (IPA).
Single-nucleus RNA-seq studies
Single nucleus RNA-seq was provided by the Center for Epigenomics, UC San Diego using the Droplet-based Chromium Single-Cell 3′ solution (10x Genomics, v3 chemistry (Zheng, Terry et al., 2017). To ensure reproducibility, two monkey eyes from each condition were sequenced. In addition, technical replicates were generated for one sample (2.1 and 2.2) from each group (Appendix Figure S6B). An average of 3,635 cells with an average of 154,227,954 reads per sample were sequenced (Appendix Figure S6B).
Briefly, 30 mg pulverized retina/choroid tissue was resuspended in 500 μL of nuclei permeabilization buffer (0.1% Triton X-100 (Sigma-Aldrich, T8787), 1X protease inhibitor, 1 mM DTT, and 0.2 U/μL RNase inhibitor (Promega, N211B), 2% BSA (Sigma-Aldrich, SRE0036) in PBS). Samples were incubated on a rotator for 5 min at 4°C and then centrifuged at 500 rcf for 5 min (4°C, run speed 3/3). Supernatant was removed and pellet was resuspended in 400 μL of sort buffer (1 mM EDTA 0.2 U/μL RNase inhibitor (Promega, N211B), 2% BSA (Sigma-Aldrich, SRE0036) in PBS) and stained with DRAQ7 (1:100; Cell Signaling, 7406). 75,000 nuclei were sorted using an SH800 sorter (Sony) into 50 μL of collection buffer consisting of 1 U/μL RNase inhibitor in 5% BSA; the FACS gating strategy sorted based on particle size and DRAQ7 fluorescence. Sorted nuclei were then centrifuged at 1000 rcf for 15 min (4°C, run speed 3/3) and supernatant was removed. Nuclei were resuspended in 35 μL of reaction buffer (0.2 U/μL RNase inhibitor (Promega, N211B), 2% BSA (Sigma-Aldrich, SRE0036) in PBS) and counted on a hemocytometer. 12,000 nuclei were loaded onto a Chromium Controller (10x Genomics). Libraries were generated using the Chromium Single-Cell 3′ Library Construction Kit v3 (10x Genomics, 1000075) with the Chromium Single-Cell B Chip Kit (10x Genomics, 1000153) and the Chromium i7 Multiplex Kit for sample indexing (10x Genomics, 120262) according to manufacturer specifications. CDNA was amplified for 12 PCR cycles. SPRISelect reagent (Beckman Coulter, B23319) was used for size selection and clean-up steps. Final library concentration was assessed by Qubit dsDNA HS Assay Kit (Thermo-Fischer Scientific) and fragment size was checked using Tapestation High Sensitivity D1000 (Agilent) to ensure that fragment sizes were distributed normally about 500 bp. Libraries were sequenced using the NextSeq500 and a NovaSeq6000 (Illumina) with these read lengths: 28 + 8 + 91 (Read1 + Index1 + Read2).
snRNA-seq analysis
Single-cell gene expression was quantified from raw reads using CellRanger (10X Genomics), using a reference built from Chlorocebus_sabaeus (ChlSab1), with gene sequences annotated based on the complete gene sequence (including exons and introns). Further analysis was performed within the Seurat R package (Stuart, Butler et al., 2019). Cells with >10% mitochondrial expression, <500 genes expressed, or <1000 total counts were removed. Gene expressions were normalized using the NormalizeData function with default parameters, and the top 4000 variable genes were obtained using the FindVariableFeatures function with default parameters. Dimensionality reduction was run using principal component analysis (PCA), and the top 83 principal components were retained, assessed based on statistical significance (JackStraw) and heatmap visualizations of PC signal across the top cells and genes. Cell sub-populations were identified using Louvain clustering in Seurat at resolution 0.25; one cluster at that resolution (cluster 6) was further divided into two clusters based on a clustering analysis at resolution 0.5 due to the presence of conflicting cell type markers within the cluster.
Individual clusters were then manually characterized into 18 broad retinal cell types based on expression of the cell specific markers (Figure 6C). These markers were: retinal pigment epithelium specific protein 65 (RPE65) for retinal pigmented epithelium, rhodopsin (RHO) for rod photoreceptors, long wave and short length sensitive opsin (OPN1LW/OPN1SW) for cone photoreceptors, solute carrier family 1 member 3 (SLC1A3) for Muller Glia, one cut homeobox 1 (ONECUT1) for horizontal cells, calcium binding protein 5 and glutamate metabotropic receptor 6 (CABP5/GRM6) for bipolar cells, paired box 6 (PAX6) for amacrine cells, major histocompatibility complex class II, DR alpha (HLA-DRA) for microglia/macrophages, solute carrier family 17 member 6 (SLC17A6) for retinal ganglion cells, solute carrier family 4 member 4(SLC4A4) for astrocytes, platelet-derived growth factor receptor alpha (PDGFRA) for stromal cells, vascular endothelial growth factor receptor 2 (KDR) for endothelial cells, platelet-derived growth factor receptor beta (PDGFRB) for pericytes, smooth muscle actin alpha 2 (ACTA2) for smooth muscle cells, hemoglobin scavenger receptor (CD163) for monocytes, tyrosine-protein kinase kit (KIT) for mast cells, melan-A (MLANA) for melanocytes, and myelin protein zero (MPZ) for Schwann cells.
snRNA-seq cell sub-type analysis
Subtype-specific gene expressions were used to further characterize sub cell populations (Figures 6A and 6B; Appendix Figure S7) as described in Single-cell clustering. With high gene expression of OPN1LW and OPN1SL, we further characterized cone photoreceptors in M-, L- and S-cone photoreceptors for cluster 5 and 23, respectively.
Clusters associated with bipolar cells were further characterized into rod bipolar cells using carbonic anhydrase 8 (CA8, cluster 9) as a specific marker, DB2 bipolar cells were distinguished using germ cell-specific gene 1 protein (GSG1, cluster 15), DB3a bipolar cells were characterized based on calbindin 1 (CALB1, cluster 22), DB4 bipolar cells were identified based on expression of LIM domain only protein 4 (LMO4, cluster 13), and DB6 bipolar cells were characterized using Delta and Notch-like epidermal growth factor-related receptor (DNER, cluster 12). (Peng, Shekhar et al., 2019, Shekhar, Lapan et al., 2016) (Appendix Figure S7A)
Clusters associated with amacrine cells were further characterized into glycinergic amacrine cells (cluster 24/26/28) based on expression of DAB adaptor protein 1 and solute carrier family 6 member 9 (DAB1/SLC6A9); SEG amacrine cells (cluster 14) were identified based on expression of SATB homeobox 2, EBF transcription factor 3, and sodium- and chloride-dependent glycine transporter 1 (SATB2/EBF3/SLC6A9); VGlut3+ amacrine cells (cluster 21) were identified based on the expression of vesicular glutamate transporter 3 (SLC17A8); starburst amacrine cells (cluster 27) were detected based on expression of choline O-acetyltransferase (CHAT); A17 amacrine cells (cluster 29) were identified from sidekick cell adhesion molecular 1 (SDK1); and GABAergic amacrine cells (cluster 8, 30) were characterized based on expression of glutamate decarboxylase 1 (GAD1) (Cherry, Trimarchi et al., 2009) (Appendix Figure S7B).
Interestingly, we also found two distinct clusters for each of the following cell types: RPE, rod photoreceptors, and endothelial cells. Cluster 2 and 6 represented RPE and damaged RPE, respectively (Appendix Figure S7C). Damaged RPE were characterized by the low expression level of the RPE signature genes (BEST1, RPE65). Low expression of RPE65 causes an accumulation of retinyl esters in the epithelium and have been linked to blinding diseases. (Maeda, Maeda et al., 2006, Sheridan, Boyer et al., 2018) The expression of BEST1 is also essential for volume regulation in the RPE. (Milenkovic, Brandl et al., 2015) The cluster 0 and 1were characterized as rod photoreceptors and dark-adapted rod photoreceptors, respectively. Apart from expression of the classical marker RHO, the dark-adapted rod photoreceptors have increased expression of recoverin (RCVRN; Appendix Figure S7D). The increased gene expression of RCVRN have been associated with increased light sensitivity and cell responsiveness to illumination. (Morshedian, Woodruff et al., 2018) As for the two clusters of endothelial cells (cluster 4 and 19), they both were characterized by the high expression of KDR. Cluster 19 have higher expression of von Willebrand Factor (vWF), platelet and endothelial cell adhesion molecule 1 (PECAM1), and vascular endothelial cadherin (CDH5), indicating that this cluster presented mature endothelial cells (Figure 6D) whereas cluster 4 have a higher expression of glutamate-ammonia ligase (GLUL), glioma-associated oncogene family zinc finger 3 (GLI3), and platelet-derived growth factor receptor beta (PDGFRB; Figure 6D). It has been shown that glutamine catabolism regulates endothelial cell proliferation (Kim, Li et al., 2017) and that GLUL plays a role in endothelial cell migration during pathological angiogenesis. (Eelen, Dubois et al., 2018a) Overexpression of GLI3 in endothelial cells have been shown to contribute to cell migration and angiogenesis. (Renault, Roncalli et al., 2009a) In addition, an increase expression in PDGFRB promotes endothelial cells angiogenesis in vivo and in vitro. (Magnusson, Looman et al., 2007) Thus cluster 4 was characterized as metabolic-active endothelial cells.
IPA pathway analysis
Single cell sequencing pathway enrichment was performed using QIAGEN Ingenuity Pathway Analysis (IPA) on differentially expressed genes to obtain canonical pathways within each cluster. Analysis utilized calculated Z score and q-value generated from EdgeR in R programming, to perform predictive and literature prediction of regulations.
Single-nucleus ATAC-seq study
Combinatorial barcoding single-nucleus ATAC-seq was provided by the Center for Epigenomics, UC San Diego and performed as previously describe (Hocker, Poirion et al., 2021, Preissl, Fang et al., 2018, Wang, Chiou et al., 2020). Two laser-induced CNV monkey eyes were sequenced from each vehicle and EBIN-treated NHPs. 135,837,688 raw reads with an average alignment percent of 98.5723 were obtained an average from each eye. After quality control and peak calling, an average of 129,433 peaks were obtained (Appendix Figure S6C). Briefly, for each sample, retina/choroid tissues were homogenized using mortar and pestle on liquid nitrogen. 1 mL nuclei permeabilization buffer (10 mM Tris-HCL [pH 7.5], 10 mM NaCl, 3 mM MgCl2, 0.1% Tween 20 [Sigma], 0.1% IGEPAL-CA630 [Sigma] and 0.01% Digitonin [Promega] in water; Corces et al., 2017) was added to 30 mg of ground retina/choroid tissue and tissue was resuspended by pipetting for 8–15 times. Nuclei suspension was incubated for 10 min at 4°C and filtered with 30 μm filter (CellTrics). Nuclei were pelleted with a swinging bucket centrifuge (500 x g, 5 min, 4°C; 5920R, Eppendorf), resuspended in 500 μL high salt tagmentation buffer (36.3 mM Tris-acetate (pH = 7.8), 72.6 mM potassium-acetate, 11 mM Mg-acetate, 17.6% DMF) and counted using a hemocytometer. Concentration was adjusted to 2000 nuclei/9 μL, and 2000 nuclei were dispensed into each well of one 96-well plate. For tagmentation, 1 μL barcoded Tn5 transposomes [4] was added using a BenchSmart 96 (Mettler Toledo), mixed five times and incubated for 60 min at 37°C with shaking (500 rpm). To inhibit the Tn5 reaction, 10 μL of 40 mM EDTA were added to each well with a BenchSmart 96 (Mettler Toledo) and the plate was incubated at 37°C for 15 min with shaking (500 rpm). Next, 20 μL 2 x sort buffer (2% BSA, 2 mM EDTA in PBS) was added using a BenchSmart 96 (Mettler Toledo). All wells were combined into a FACS tube and stained with 3 μM Draq7 (Cell Signaling). Using an SH800 (Sony), 20 2 n nuclei were sorted per well into eight 96-well plates (total of 768 wells) containing 10.5 μL EB (25 pmol) primer i7, 25 pmol primer i5, 200 ng BSA (Sigma). Preparation of sort plates and all downstream pipetting steps were performed on a Biomek i7 Automated Workstation (Beckman Coulter). After addition of 1 μL 0.2% SDS, samples were incubated at 55°C for 7 min with shaking (500 rpm). 1 μL 12.5% Triton X- was added to each well to quench the SDS. Next, 12.5 μL NEBNext High-Fidelity 2 × PCR Master Mix (NEB) were added, and samples were PCR-amplified (72°C 5 min, 98°C 30 s, (98°C 10 s, 63°C 30 s, 72°C 60 s)×12 cycles, held at 12°C). After PCR, all wells were combined. Libraries were purified according to the MinElute PCR Purification Kit manual (Qiagen) using a vacuum manifold (QIAvac 24 plus, Qiagen) and size selection was performed with SPRI Beads (Beckmann Coulter, 0.55x and 1.5x). Libraries were purified one more time with SPRI Beads (Beckmann Coulter, 1.5x). Libraries were quantified using a Qubit fluorimeter (Life technologies) and the nucleosomal pattern was verified using a Tapestation (High Sensitivity D1000, Agilent). The library was sequenced on a NovaSeq6000 or nextSeq500 sequencer (Illumina) using custom sequencing primers with following read lengths: 50 + 10 + 12 + 50 (Read1 + Index1 + Index2 + Read2).
snATAC-seq analysis
Peak calling
Peak calling analysis was performed separately for all reads in each single-cell capture. Raw reads were aligned to the reference genome for Chlorocebus sabaeus (ChlSab1) with BWA MEM (Li, 2013), and cell barcodes were added as tags in the BAM file. Apparent PCR duplicates were removed using Picard MarkDuplicates (Wysoker, Tibbets et al., 2019), performed with respect to cell barcode using the BARCODE TAG option, so that duplicate reads were only removed if they came from the same cell barcode. Read alignments were first adjusted to account for TAC transposon binding: +4bp for + strand alignments, -5bp for – strand alignments. Open chromatin peaks were called using Macs2 (Zhang, Liu et al., 2008) with nomodel set and no background provided (flags “–nomodel –nolambda”); peaks with a score >5 (-log10 q-value) were retained.
Single cell quantification
A union set of peak calls from all captures was obtained using bedtools merge (Quinlan & Hall, 2010), and formatted as a reference file for featureCounts. Read-based annotations were obtained for each read using featureCounts (Liao et al., 2014) with “-R CORE” to obtain read-based peak assignments. Peak counts for individual cells were obtained through the combination of the cell barcode and peak assignment for each read in the peak assignment output from featureCounts.
Single-cell clustering
Further analysis was performed with R packages Seurat (Stuart et al., 2019), ggplot2, and gplot. Dimensionality reduction was performed using LSI (Latent Semantic Indexing) on all peaks that were observed in at least 200 cells, retaining the top 50 dimensions but discarding the 1st dimension, which typically correlates with sequencing depth rather than biological variability. Cell sub-populations were identified using Louvain clustering in Seurat at resolution 0.5. As we were unable to assign each ATAC-seq cluster to the specific cell type by comparing the differential peaks (see RNA-seq and ATAC-seq differential analysis), we used RNA-seq and ATAC-seq integration analysis described below.
snRNA-seq and snATAC-seq analysis
Differential abundance of features, e.g., genes or peaks for snRNA-seq and snATAC-seq, respectively, across cell clusters, and between control and treated cells within each cluster, was computed using the Wilcox test using FindMarkers in Seurat. For tests between clusters, all genes with log2FC > 0.25 and expressed in at least 25% of cells were tested; the Bonferroni correction was used to adjust for multiple testing. For tests between control and treated cells within each cluster, all genes expressed in at least 25% of cells were tested regardless of the logFC, and the FDR correction was used to correct for multiple testing.
snRNA-seq and snATAC-seq Integrative analysis
ATAC-seq peaks were annotated based on genic context, in particular identifying peaks within gene promoter regions (<2kb from TSS). To associate ATAC clusters with RNA clusters, we obtained peak-gene pairs based on these promoter associations among the top 4000 variable genes and the top peaks that were present in at least 200 cells. We computed the average expression of each gene within each RNA cluster, and the average normalized abundance of each peak within each ATAC cluster and computed the Pearson correlation between each pair of RNA and ATAC clusters, using the averaged values for each promoter-based peak-gene pair.
A heatmap showing the Pearson correlation between the snRNA-seq clusters and snATAC-seq clusters was used to correlate the snRNA-seq cluster cell type to snATAC-seq clusters (Fig. EV5B). Since there are fewer ATAC clusters when compared to the RNA clusters, some RNA clusters will have multiple ATAC cluster correlation. Microglia/macrophages (RNA cluster 10ATAC cluster 6), endothelial cells (RNA cluster 19ATAC cluster 16), melanocytes (RNA cluster 25ATAC cluster 20), myelinating Schwann cells (RNA cluster 33ATAC clusters 5, 7), smooth muscle cells (RNA cluster 16ATAC cluster 21), stromal cells (RNA cluster 3ATAC cluster 5), and pericytes (RNA cluster 31ATAC cluster 5, 21) all have similar gene expression and chromatin accessibility as most of these cells are cells residing in the choroid. Rod photoreceptors (RNA cluster 0, 1ATAC cluster 0) and cone photoreceptors (RNA cluster 5, 23ATAC cluster 2) have similar functions and thus similar gene expression and chromatin accessibility. Mast cells (RNA cluster 11ATAC cluster 8), bipolar cells (RNA cluster 9, 12, 13, 15, 22ATAC cluster 8, 11, 13, 14, 17), horizontal cells (RNA cluster 20ATAC cluster 9), monocytes (RNA cluster 18ATAC cluster 12), amacrine cells (RNA cluster 8, 14, 2, 24, 26, 27, 28, 29, 30ATAC cluster 10, 12, 18, 19), and retinal ganglion cell (RNA cluster 7ATAC cluster 18) have similar gene expression and chromatin accessibility as they are all mostly neuronal cells. The RPE (RNA cluster 2, 6ATAC cluster 1), metabolic active endothelial cells (RNA cluster 4ATAC cluster 7), astrocytes (RNA cluster 17ATAC cluster 5, 20, 21), and Muller Glia cells (RNA cluster 32ATAC cluster 5, 7, 20, 21) have distinct gene expression and chromatin accessibility among all of the cells.
snATAC-seq motif enrichment analysis
We searched for instances of known transcription factor motifs from the JASPAR core vertebrate database (Khan, Fornes et al., 2018) using FIMO (Bailey, Boden et al., 2009) in the union set of ATAC peak sequences. Motif enrichment statistics for up-regulated peaks (adjusted p value <0.05 and log fold-change>0) in (1) each cluster, and (2) control or treated groups within each cluster against the background distribution of all peaks was computed using Fisher’s Exact test. Here, we compared the fraction of peaks containing a given motif over all peaks to the fraction of up-regulated peaks containing the motif. We also directly compared the motif enrichments between control and treated up-regulated peaks within each cluster by comparing the fraction of peaks containing a motif, also using Fisher’s Exact test. In both cases, p values were adjusted for multiple testing using the FDR correction over all motifs tested.
snATAC-seq comparison to AMD-Associated differentially enriched peaks
AMD-associated differentially enriched peaks were obtained from Wang et al., 2018. All AMD-associated peaks were converted from the hg19 genome to the ChlSab1.1 genome before intersection with the differential peaks found between the EBIN-treated and vehicle per ATAC cluster based on binding in the promoter for those peaks. The AMD fitted coefficient was computed as a measurement of the effect size in AMD patients using the AMD-associated peaks. Spearman’s rank correlation was computed across the AMD fitted coefficient vs. the EBIN/vehicle fold change of the AMD-associated peaks within the ATAC clusters.
Quantification and statistical analysis
Statistical significance was calculated using the GraphPad Prism program unless indicated otherwise. Statistical details of experiments can be found in the figure legends. All data shown as mean ± SEM unless otherwise noted. Student’s t-test was used for data comparison between two experimental groups. To analyze more than two groups, one-way ANOVA with Tukey’s Post Hoc. A Fisher’s exact test was used to evaluate incidence of different lesion grades. two-way ANOVA with repeated measures followed by Tukey-Kramer test was used to analyze the OCT CNV complex area. Non-parametric tests were applied if the data were not normally distributed and had unequal variance. To ensure adequate power to detect pre-specified effect size, data were collected from two or more independent experiments in triplicates and the sample size were determined from what is commonly used in similar experiments, unless indicated otherwise. Mice were selected to be similar in age, sex, and weight. Monkeys of both sexes were used and with equal number of males and females across the treatment groups. Immediately after post-laser treatment, both mice and monkeys underwent color fundus imaging to document the appearance of the laser lesions and to allow balanced randomization to treatment assignment. The investigators were blinded during data collection and analysis when calculating the leakage and lesion areas to laser-induced photocoagulation. All data were tested for outliers and removed from analysis using ROUT’s method.
Acknowledgments
We thank Wyatt Strutz and NanoTemper Technologies, Inc., for their assistance with the use of NanoDSF. We also thank Dr. Kadur Lakshminarasimha Murthy for discussion of snRNA-seq data. The authors would like to thank the Center for Epigenomics at UC San Diego for performing the snRNA-seq and snATAC-seq experiments. Work at the Center for Epigenomics was supported in part by the UC San Diego School of Medicine. This publication includes data generated at the UC San Diego IGM Genomics Center utilizing an Illumina NovaSeq 6000 that was purchased with funding from a National Institutes of Health SIG grant (#S10 OD026929). Bioinformatics analysis in the project described was performed by the UIC Research Informatics Core, supported in part by NCATS through grant UL1TR002003. All NHP studies were conducted at Virscio, Inc. Portions of this work were carried out in the Fluorescence Imaging Core via the Research Resource Center at the University of Illinois at Chicago. Certain diagrams and figures were made using BioRender.com. This project has been supported in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract nos. HHSN268201700007C, R01HL103922, R01HL045638, and UL1TR002003; the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust; Laurel Therapeutics, Inc.; and the American Cancer Society (ACS) Postdoctoral Fellowship 132722-PF-18-196-01-DMC.
Author contributions
Y.A.K. conceived the project; Y.A.K., V. Gaponenko, and M.I.R. interpreted data; and Y.A.K. and V. Gaponenko wrote the manuscript. W.C.C., Q.L., X.Q., K.K., J.L., and M.M.-C. analyzed data and prepared figures; U.S. performed computational scanning; B.H. and A.B. performed HSQC experiments; V. Gaponenko analyzed NMR data; H.A. performed in silico docking of EBIN to EB3; Q.L. performed analysis of data obtained from cell culture and mouse studies; W.C.C. and Q.L. performed analysis of the effects of EBIN on expression of VE-cadherin in NHP lesions; Q.L., W.C.C., and J.L. performed in vitro studies on EBIN’s affect in mitochondrial gene and protein expression; W.C.C. and Q.L. performed analysis of the effects of EBIN on histone acetylation and PAX6 and MEIS2 expression on NHP lesions; X.Q. purified EB3, performed calcium experiments, determined the rate of EBIN uptake by ECs, and determined EBIN’s effect on cytotoxicity, cell viability, and apoptosis; F.H. performed pull-down experiments; M.G. performed angiogenesis assays in vivo; V. Guaiquil supervised CNV experiments in mice; M.S.L. designed and supervised NHP experiments; M.Y.S. performed the cell culture experiments related to VEGF-A-evoked permeability; Y.S. performed CNV experiments in mice; M.M.-C. performed all bioinformatics analysis; A.A.M. and A.B.M. edited the manuscript; and M.S. designed NHP studies and contributed to discussion of data.
Declaration of interests
Y.A.K., U.S., M.I.R., and A.B.M. have two international patents on EBIN; Y.A.K., M.I.R., and A.B.M. had served the role of consultants for Laurel Therapeutics, Inc.; and M.S. is the CEO of Laurel Therapeutics, Inc. M.S.L. is the CEO of Virscio, Inc.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: October 3, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101223.
Supplemental information
A table showing the ophthalmic data and clinical scores for NHP eyes during the pre-screen, and days 14 and 21 after laser-induced photocoagulation.
A table showing the processing statistics for all bulk RNA-, single nuclei (sn)RNA- and snATAC-sequencing experiments separated by different tabs.
A table showing the gene expression changes caused by Myr-EBIN in the bulk RNA-seq of endothelial cells and snRNA-seq in the rod photoreceptors (cluster 0, 1), RPE (cluster 2, 6), stromal cells (cluster 3), metabolic-active endothelial cells (cluster 4), and cone photoreceptors (cluster 5) separated by different tabs. The table includes the gene ensembl ID, p-value, average log fold change, and gene symbol for each gene significantly affected by MyrEBIN.
A table showing the IPA canonical pathway analysis of the gene expression changes caused by Myr-EBIN in the bulk RNA-seq of endothelial cells and snRNA-seq of rod photoreceptors (cluster 0), RPE (cluster 2), and metabolic active endothelial cells (cluster 4) separated by different tabs. The table includes the Ingenuity Canonical Pathways, p-value, ratio, z-scores, and the molecules within the pathway. Note, the changes in bulk RNA-seq are computed as “hypoxia vehicle” over “normoxia vehicle” and presented as “maladaptive responses” which are reversed by EBIN or “adaptive responses” not affected by EBIN.
A table showing expression levels of the positive and negative cell-type markers for the metabolic-active endothelial cells (cluster 4) and mature endothelial cells for comparison (cluster 19). The table includes the gene symbol, the endothelial cluster number, mean expression (Expression.mean), mean standard deviation (Expression.sd), and the percentage of cells where the gene is expressed (Expression.pct.expr).
A table showing a list of concurrent changes in gene expression and increased in peak for rod photoreceptors, cone photoreceptors, RPE, stromal cells, metabolic-active endothelial cells, RGC, amacrine cells, and bipolar cells. Transcription factors are highlighted in yellow. The table includes the gene ensembl ID and gene symbol, as well as the respective snRNA-seq and snATAC-seq defined cluster numbers, p-values, and average log fold change for each concurrent gene within the same cell type.
Data and code availability
NMR assignments have been deposited at the Biological Magnetic Resonance DataBank and are publicly available as of the date of publication. Entry numbers are listed in the key resources table. Bulk and single nuclei RNA and ATAC sequencing have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Any additional information and data reported in this paper is available from the lead contact, Yulia A. Komarova (ykomarov@uic.edu), upon request.
References
- 1.Congdon N., O'Colmain B., Klaver C.C.W., Klein R., Muñoz B., Friedman D.S., Kempen J., Taylor H.R., Mitchell P., Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Beatty S., Koh H., Phil M., Henson D., Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 3.Fritsche L.G., Igl W., Bailey J.N.C., Grassmann F., Sengupta S., Bragg-Gresham J.L., Burdon K.P., Hebbring S.J., Wen C., Gorski M., et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016;48:134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasiak J., Petrovski G., Veréb Z., Facskó A., Kaarniranta K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. BioMed Res. Int. 2014;2014:768026. doi: 10.1155/2014/768026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barben M., Ail D., Storti F., Klee K., Schori C., Samardzija M., Michalakis S., Biel M., Meneau I., Blaser F., et al. Hif1a inactivation rescues photoreceptor degeneration induced by a chronic hypoxia-like stress. Cell Death Differ. 2018;25:2071–2085. doi: 10.1038/s41418-018-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelby S.J., Angadi P.S., Zheng Q.D., Yao J., Jia L., Zacks D.N. Hypoxia inducible factor 1alpha contributes to regulation of autophagy in retinal detachment. Exp. Eye Res. 2015;137:84–93. doi: 10.1016/j.exer.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulaki V., Qin W., Joussen A.M., Hurlbut P., Wiegand S.J., Rudge J., Yancopoulos G.D., Adamis A.P. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J. Clin. Invest. 2002;109:805–815. doi: 10.1172/JCI13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Y.Z. VEGF production and signaling in Muller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vis. Res. 2017;139:108–114. doi: 10.1016/j.visres.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.E G., Cao Y., Bhattacharya S., Dutta S., Wang E., Mukhopadhyay D. Endogenous vascular endothelial growth factor-A (VEGF-A) maintains endothelial cell homeostasis by regulating VEGF receptor-2 transcription. J. Biol. Chem. 2012;287:3029–3041. doi: 10.1074/jbc.M111.293985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearnley G.W., Bruns A.F., Wheatcroft S.B., Ponnambalam S. VEGF-A isoform-specific regulation of calcium ion flux, transcriptional activation and endothelial cell migration. Biol. Open. 2015;4:731–742. doi: 10.1242/bio.201410884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noren D.P., Chou W.H., Lee S.H., Qutub A.A., Warmflash A., Wagner D.S., Popel A.S., Levchenko A. Endothelial cells decode VEGF-mediated Ca2+ signaling patterns to produce distinct functional responses. Sci. Signal. 2016;9:ra20. doi: 10.1126/scisignal.aad3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates D.O., Curry F.E. Vascular endothelial growth factor increases microvascular permeability via a Ca(2+)-dependent pathway. Am. J. Physiol. 1997;273:H687–H694. doi: 10.1152/ajpheart.1997.273.2.H687. [DOI] [PubMed] [Google Scholar]
- 13.Avery R.L. What is the evidence for systemic effects of intravitreal anti-VEGF agents, and should we be concerned? Br. J. Ophthalmol. 2014;98:i7–i10. doi: 10.1136/bjophthalmol-2013-303844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falavarjani K.G., Nguyen Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye. 2013;27:787–794. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keir L.S., Firth R., Aponik L., Feitelberg D., Sakimoto S., Aguilar E., Welsh G.I., Richards A., Usui Y., Satchell S.C., et al. VEGF regulates local inhibitory complement proteins in the eye and kidney. J. Clin. Invest. 2017;127:199–214. doi: 10.1172/JCI86418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li A.L., Wykoff C.C., Wang R., Chen E., Benz M.S., Fish R.H., Wong T.P., Major J.C., Jr., Brown D.M., Schefler A.C., et al. Endophthalmitis after intravitreal injection: role of prophylactic topical ophthalmic antibiotics. Retina. 2016;36:1349–1356. doi: 10.1097/IAE.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 17.Okabe K., Kobayashi S., Yamada T., Kurihara T., Tai-Nagara I., Miyamoto T., Mukouyama Y.-s., Sato T.N., Suda T., Ema M., Kubota Y. Neurons limit angiogenesis by titrating VEGF in retina. Cell. 2014;159:584–596. doi: 10.1016/j.cell.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Saint-Geniez M., Maharaj A.S.R., Walshe T.E., Tucker B.A., Sekiyama E., Kurihara T., Darland D.C., Young M.J., D'Amore P.A. Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enslow R., Bhuvanagiri S., Vegunta S., Cutler B., Neff M., Stagg B. Association of Anti-VEGF Injections with Progression of Geographic Atrophy. Ophthalmol. Eye Dis. 2016;8:31–32. doi: 10.4137/OED.S38863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunwald J.E., Daniel E., Huang J., Ying G.-S., Maguire M.G., Toth C.A., Jaffe G.J., Fine S.L., Blodi B., Klein M.L., et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rofagha S., Bhisitkul R.B., Boyer D.S., Sadda S.R., Zhang K., SEVEN-UP Study Group Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Geyer M., Huang F., Sun Y., Vogel S.M., Malik A.B., Taylor C.W., Komarova Y.A. Microtubule-Associated Protein EB3 Regulates IP3 Receptor Clustering and Ca(2+) Signaling in Endothelial Cells. Cell Rep. 2015;12:79–89. doi: 10.1016/j.celrep.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwok M.L., Geyer M., Chan W.C., Zhao S., Gu L., Huang F., Vogel S.M., Petukhov P.A., Komarova Y. Targeting EB3-IP3R3 interface with cognate peptide protects from acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 2023 doi: 10.1165/rcmb.2022-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison K.L., Weiss G.A. Combinatorial alanine-scanning. Curr. Opin. Chem. Biol. 2001;5:302–307. doi: 10.1016/s1367-5931(00)00206-4. [DOI] [PubMed] [Google Scholar]
- 25.H Abdelkarim, B.H., X Qu, A Banerjee, YA Komarova, V Gaponenko NMR Resonance Assignment and Structure Prediction of the C-Terminal Domain of the Microtubule End-Binding Protein 3. PLoS One. [DOI] [PMC free article] [PubMed]
- 26.Terman B.I., Dougher-Vermazen M., Carrion M.E., Dimitrov D., Armellino D.C., Gospodarowicz D., Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 1992;187:1579–1586. doi: 10.1016/0006-291X(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan V., Rowan-Carroll A., Gagné R., Kuo B., Williams A., Yauk C.L. The use of in vitro transcriptional data to identify thresholds of effects in a human lens epithelial cell-line exposed to ionizing radiation. Int. J. Radiat. Biol. 2019;95:156–169. doi: 10.1080/09553002.2019.1539883. [DOI] [PubMed] [Google Scholar]
- 28.Thastrup O., Cullen P.J., Drøbak B.K., Hanley M.R., Dawson A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor C.W., Broad L.M. Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol. Sci. 1998;19:370–375. doi: 10.1016/s0165-6147(98)01243-7. [DOI] [PubMed] [Google Scholar]
- 30.DeCicco-Skinner K.L., Henry G.H., Cataisson C., Tabib T., Gwilliam J.C., Watson N.J., Bullwinkle E.M., Falkenburg L., O'Neill R.C., Morin A., Wiest J.S. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J. Vis. Exp. 2014;91:51312. doi: 10.3791/51312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong Y., Li J., Sun Y., Fu Z., Liu C.H., Evans L., Tian K., Saba N., Fredrick T., Morss P., et al. Optimization of an Image-Guided Laser-Induced Choroidal Neovascularization Model in Mice. PLoS One. 2015;10:e0132643. doi: 10.1371/journal.pone.0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua J., Spee C., Kase S., Rennel E.S., Magnussen A.L., Qiu Y., Varey A., Dhayade S., Churchill A.J., Harper S.J., et al. Recombinant human VEGF165b inhibits experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2010;51:4282–4288. doi: 10.1167/iovs.09-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinores S.A., Derevjanik N.L., Vinores M.A., Okamoto N., Campochiaro P.A. Sensitivity of different vascular beds in the eye to neovascularization and blood-retinal barrier breakdown in VEGF transgenic mice. Adv. Exp. Med. Biol. 2000;476:129–138. doi: 10.1007/978-1-4615-4221-6_11. [DOI] [PubMed] [Google Scholar]
- 34.Kuemmel T.A., Thiele J., Hafenrichter E.G., Varus E., Fischer R. Distribution of lectin binding sites in human bone marrow. Identification by use of an ultrastructural postembedding technique. J. Submicr. Cytol. Pathol. 1996;28:537–551. [PubMed] [Google Scholar]
- 35.Caballero S., Kent D.L., Sengupta N., Li Calzi S., Shaw L., Beli E., Moldovan L., Dominguez J.M., 2nd, Moorthy R.S., Grant M.B. Bone Marrow-Derived Cell Recruitment to the Neurosensory Retina and Retinal Pigment Epithelial Cell Layer Following Subthreshold Retinal Phototherapy. Invest. Ophthalmol. Vis. Sci. 2017;58:5164–5176. doi: 10.1167/iovs.16-20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maddox D.E., Shibata S., Goldstein I.J. Stimulated macrophages express a new glycoprotein receptor reactive with Griffonia simplicifolia I-B4 isolectin. Proc. Natl. Acad. Sci. USA. 1982;79:166–170. doi: 10.1073/pnas.79.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L., Cui X., Han Y., Park K.S., Gao X., Zhang X., Yuan Z., Hu Y., Hsu C.W., Li X., et al. Hypoxic drive caused type 3 neovascularization in a preclinical model of exudative age-related macular degeneration. Hum. Mol. Genet. 2019;28:3475–3485. doi: 10.1093/hmg/ddz159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mammadzada P., Correidoira P.M., André H. The role of hypoxia-inducible factors in neovascular age-related macular degeneration: a gene therapy perspective. Cell. Mol. Life Sci. 2020;819:833. doi: 10.1007/s00018-019-03422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menon M., Mohammadi S., Davila-Velderrain J., Goods B.A., Cadwell T.D., Xing Y., Stemmer-Rachamimov A., Shalek A.K., Love J.C., Kellis M., Hafler B.P. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat. Commun. 2019;10:4902. doi: 10.1038/s41467-019-12780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voigt A.P., Mulfaul K., Mullin N.K., Flamme-Wiese M.J., Giacalone J.C., Stone E.M., Tucker B.A., Scheetz T.E., Mullins R.F. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc. Natl. Acad. Sci. USA. 2019;116:24100–24107. doi: 10.1073/pnas.1914143116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eelen G., Dubois C., Cantelmo A.R., Goveia J., Brüning U., DeRan M., Jarugumilli G., van Rijssel J., Saladino G., Comitani F., et al. Role of glutamine synthetase in angiogenesis beyond glutamine synthesis. Nature. 2018;561:63–69. doi: 10.1038/s41586-018-0466-7. [DOI] [PubMed] [Google Scholar]
- 42.Renault M.A., Roncalli J., Tongers J., Misener S., Thorne T., Jujo K., Ito A., Clarke T., Fung C., Millay M., et al. The Hedgehog transcription factor Gli3 modulates angiogenesis. Circ. Res. 2009;105:818–826. doi: 10.1161/CIRCRESAHA.109.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohlenova K., Goveia J., García-Caballero M., Subramanian A., Kalucka J., Treps L., Falkenberg K.D., de Rooij L.P.M.H., Zheng Y., Lin L., et al. Single-Cell RNA Sequencing Maps Endothelial Metabolic Plasticity in Pathological Angiogenesis. Cell Metabol. 2020;31:862–877.e14. doi: 10.1016/j.cmet.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Vodyanik M.A., Yu J., Zhang X., Tian S., Stewart R., Thomson J.A., Slukvin I.I. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lv F.J., Tuan R.S., Cheung K.M.C., Leung V.Y.L. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cell. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 46.Schmitt S., Aftab U., Jiang C., Redenti S., Klassen H., Miljan E., Sinden J., Young M. Molecular characterization of human retinal progenitor cells. Invest. Ophthalmol. Vis. Sci. 2009;50:5901–5908. doi: 10.1167/iovs.08-3067. [DOI] [PubMed] [Google Scholar]
- 47.Ronning K.E., Karlen S.J., Miller E.B., Burns M.E. Molecular profiling of resident and infiltrating mononuclear phagocytes during rapid adult retinal degeneration using single-cell RNA sequencing. Sci. Rep. 2019;9:4858. doi: 10.1038/s41598-019-41141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes C.S., Colhoun L.M., Bains B.K., Kilgour J.D., Burden R.E., Burrows J.F., Lavelle E.C., Gilmore B.F., Scott C.J. Extracellular cathepsin S and intracellular caspase 1 activation are surrogate biomarkers of particulate-induced lysosomal disruption in macrophages. Part. Fibre Toxicol. 2016;13:19. doi: 10.1186/s12989-016-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costabel U., Bross K.J., Andreesen R., Matthys H. HLA-DR antigens on human macrophages from bronchoalveolar lavage fluid. Thorax. 1986;41:261–265. doi: 10.1136/thx.41.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu Y., Lai Y., Wang Q., Liu X., He W., Zhang H., Fan C., Yang G. Overexpression of clusterin promotes angiogenesis via the vascular endothelial growth factor in primary ovarian cancer. Mol. Med. Rep. 2013;7:1726–1732. doi: 10.3892/mmr.2013.1436. [DOI] [PubMed] [Google Scholar]
- 51.Jurkunas U.V., Bitar M.S., Rawe I., Harris D.L., Colby K., Joyce N.C. Increased custerin expression in Fuchs’ endothelial dystrophy. Invest. Ophthalmol. Vis. Sci. 2008;49:2946–2955. doi: 10.1167/iovs.07-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen T., Karamariti E., Hong X., Deng J., Wu Y., Gu W., Simpson R., Wong M.M., Yu B., Hu Y., et al. DKK3 (Dikkopf-3) transdifferentiates fibroblasts into functional endothelial cells—brief report. Atertio. Thromb. Vasc. Biol. 2019;39:765–773. doi: 10.1161/ATVBAHA.118.311919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipphardt M., Dihazi H., Jeon N.L., Dadafarin S., Ratliff B.B., Rowe D.W., Müller G.A., Goligorsky M.S. Dickkopf-3 in aberrant endothelial secretome triggers renal fibroblast activation and endothelial–mesenchymal transition. Nephrol. Dial. Transplant. 2019;34:49–62. doi: 10.1093/ndt/gfy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park K.H., Choi A.J., Yoon J., Lim D., Woo S.J., Park S.J., Kim H.C., Chung H. Wnt modulators in the aqueous humor are associated with outer retinal damage severity in patients with neovascular age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2014;55:5522–5530. doi: 10.1167/iovs.14-14566. [DOI] [PubMed] [Google Scholar]
- 55.Untergasser G., Steurer M., Zimmermann M., Hermann M., Kern J., Amberger A., Gastl G., Gunsilius E. The Dickkopf-homolog 3 is expressed in tumor endothelial cells and supports capillary formation. Int. J. Cancer. 2008;122:1539–1547. doi: 10.1002/ijc.23255. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Lozada Z., Robinson M.B. Reciprocal communication between astrocytes and endothelial cells is required for astrocytic glutamate transporter 1 (GLT-1) expression. Neurochem. Int. 2020;139:104787. doi: 10.1016/j.neuint.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jambusaria A., Hong Z., Zhang L., Srivastava S., Jana A., Toth P.T., Dai Y., Malik A.B., Rehman J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife. 2020;9:e51413. doi: 10.7554/eLife.51413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faghiri Z., Bazan N.G. PI3K/Akt and mTOR/p70S6K Pathways Mediate Neuroprotectin D1-Induced Retinal Pigment Epithelial Cell Survival during Oxidative Stress-Induced Apoptosis. Exp. Eye Res. 2010;90:718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt S.Y. Light- and cytidine-dependent phosphatidylinositol synthesis in photoreceptor cells of the rat. J. Cell Biol. 1983;97:832–837. doi: 10.1083/jcb.97.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li G., Rajala A., Wiechmann A.F., Anderson R.E., Rajala R.V.S. Activation and Membrane Binding of Retinal Protein Kinase Bα/Akt1 is Regulated through Light-Dependent Generation of Phosphoinositides. J. Neurochem. 2008;107:1382–1397. doi: 10.1111/j.1471-4159.2008.05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown B.M., Ramirez T., Rife L., Craft C.M. Visual Arrestin 1 Contributes to Cone Photoreceptor Survival and Light Adaptation. Invest. Ophthalmol. Vis. Sci. 2010;51:2372–2380. doi: 10.1167/iovs.09-4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajala R.V.S., Rajala A. Neuroprotective role of protein tyrosine phosphatase-1B in rod photoreceptor neurons. Protein Cell. 2013;4:890–892. doi: 10.1007/s13238-013-3063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J., Zibetti C., Shang P., Sripathi S.R., Zhang P., Cano M., Hoang T., Xia S., Ji H., Merbs S.L., et al. ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration. Nat. Commun. 2018;9:1364. doi: 10.1038/s41467-018-03856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norton V.G., Marvin K.W., Yau P., Bradbury E.M. Nucleosome linking number change controlled by acetylation of histones H3 and H4. J. Biol. Chem. 1990;265:19848–19852. doi: 10.1016/S0021-9258(17)45450-0. [DOI] [PubMed] [Google Scholar]
- 65.Johnson D.P., Spitz G.S., Tharkar S., Quayle S.N., Shearstone J.R., Jones S., McDowell M.E., Wellman H., Tyler J.K., Cairns B.R., et al. HDAC1,2 inhibition impairs EZH2- and BBAP- mediated DNA repair to overcome chemoresistance in EZH2 gain-of-function mutant diffuse large B-cell lymphoma. Oncotarget. 2015;6:4863–4887. doi: 10.18632/oncotarget.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caslini C., Hong S., Ban Y.J., Chen X.S., Ince T.A. HDAC7 regulates histone 3 lysine 27 acetylation and transcriptional activity at super-enhancer-associated genes in breast cancer stem cells. Oncogene. 2019;38:6599–6614. doi: 10.1038/s41388-019-0897-0. [DOI] [PubMed] [Google Scholar]
- 67.Mishra V.K., Wegwitz F., Kosinsky R.L., Sen M., Baumgartner R., Wulff T., Siveke J.T., Schildhaus H.U., Najafova Z., Kari V., et al. Histone deacetylase class-I inhibition promotes epithelial gene expression in pancreatic cancer cells in a BRD4- and MYC-dependent manner. Nucleic Acids Res. 2017;45:6334–6349. doi: 10.1093/nar/gkx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan Y., Zhao K., Yao Y., Liu C., Chen Y., Li J., Wang Y., Pei R., Chen J., Hu X., et al. HDAC11 restricts HBV replication through epigenetic repression of cccDNA transcription. Antivir. Res. 2019;172:104619. doi: 10.1016/j.antiviral.2019.104619. [DOI] [PubMed] [Google Scholar]
- 69.McQuown S.C., Barrett R.M., Matheos D.P., Post R.J., Rogge G.A., Alenghat T., Mullican S.E., Jones S., Rusche J.R., Lazar M.A., Wood M.A. HDAC3 Is a Critical Negative Regulator of Long-Term Memory Formation. J. Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tazi J., Bird A. Alternative chromatin structure at CpG islands. Cell. 1990;60:909–920. doi: 10.1016/0092-8674(90)90339-G. [DOI] [PubMed] [Google Scholar]
- 71.Yu F., Zhang W., Yan C., Yan D., Zhou M., Chen J., Zhao X., Zhu A., Zhou J., Liu H., et al. PAX6, modified by SUMOylation, plays a protective role in corneal endothelial injury. Cell Death Dis. 2020;11:683. doi: 10.1038/s41419-020-02848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leśniewska K., Warbrick E., Ohkura H. Peptide aptamers define distinct EB1- and EB3-binding motifs and interfere with microtubule dynamics. Mol. Biol. Cell. 2014;25:1025–1036. doi: 10.1091/mbc.E13-08-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Procter D.J., Banerjee A., Nukui M., Kruse K., Gaponenko V., Murphy E.A., Komarova Y., Walsh D. The HCMV Assembly Compartment Is a Dynamic Golgi-Derived MTOC that Controls Nuclear Rotation and Virus Spread. Dev. Cell. 2018;45:83–100.e7. doi: 10.1016/j.devcel.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith R.O., Ninchoji T., Gordon E., André H., Dejana E., Vestweber D., Kvanta A., Claesson-Welsh L. Vascular permeability in retinopathy is regulated by VEGFR2 Y949 signaling to VE-cadherin. Elife. 2020;9:e54056. doi: 10.7554/eLife.54056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komarova Y.A., Huang F., Geyer M., Daneshjou N., Garcia A., Idalino L., Kreutz B., Mehta D., Malik A.B. VE-cadherin signaling induces EB3 phosphorylation to suppress microtubule growth and assemble adherens junctions. Mol. Cell. 2012;48:914–925. doi: 10.1016/j.molcel.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X., Friedman A., Heaney S., Purcell P., Maas R.L. Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis. Genes Dev. 2002;16:2097–2107. doi: 10.1101/gad.1007602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kruse K., Lee Q.S., Sun Y., Klomp J., Yang X., Huang F., Sun M.Y., Zhao S., Hong Z., Vogel S.M., et al. N-cadherin signaling via Trio assembles adherens junctions to restrict endothelial permeability. J. Cell Biol. 2019;218:299–316. doi: 10.1083/jcb.201802076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agoston Z., Heine P., Brill M.S., Grebbin B.M., Hau A.C., Kallenborn-Gerhardt W., Schramm J., Götz M., Schulte D. Meis2 is a Pax6 co-factor in neurogenesis and dopaminergic periglomerular fate specification in the adult olfactory bulb. Development. 2014;141:28–38. doi: 10.1242/dev.097295. [DOI] [PubMed] [Google Scholar]
- 79.Bjerke G.A., Hyman-Walsh C., Wotton D. Cooperative Transcriptional Activation by Klf4, Meis2, and Pbx1. Mol. Cell Biol. 2011;31:3723–3733. doi: 10.1128/MCB.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alam P., Haile B., Arif M., Pandey R., Rokvic M., Nieman M., Maliken B.D., Paul A., Wang Y.G., Sadayappan S., et al. Inhibition of Senescence-Associated Genes Rb1 and Meis2 in Adult Cardiomyocytes Results in Cell Cycle Reentry and Cardiac Repair Post–Myocardial Infarction. J. Am. Heart Assoc. 2019;8:e012089. doi: 10.1161/JAHA.119.012089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heine P., Dohle E., Bumsted-O'Brien K., Engelkamp D., Schulte D. Evidence for an evolutionary conserved role of homothorax/Meis1/2 during vertebrate retina development. Development. 2008;135:805–811. doi: 10.1242/dev.012088. [DOI] [PubMed] [Google Scholar]
- 82.Dupacova N., Antosova B., Paces J., Kozmik Z. Meis homeobox genes control progenitor competence in the retina. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2013136118. e2013136118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hannon G.J., Beach D. pl5INK4B is a potentia| effector of TGF-β-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 84.Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y., MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen Q.D., Shah S.M., Browning D.J., Hudson H., Sonkin P., Hariprasad S.M., Kaiser P., Slakter J.S., Haller J., Do D.V., et al. A Phase I Study of Intravitreal Vascular Endothelial Growth Factor Trap-Eye in Patients with Neovascular Age-Related Macular Degeneration. Ophthalmology. 2009;116:2141–2148.e1. doi: 10.1016/j.ophtha.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 86.Reiterer M., Colaço R., Emrouznejad P., Jensen A., Rundqvist H., Johnson R.S., Branco C. Acute and chronic hypoxia differentially predispose lungs for metastases. Sci. Rep. 2019;9:10246. doi: 10.1038/s41598-020-58616-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koh M.Y., Lemos R., Liu X., Powis G. The Hypoxia-Associated Factor Switches Cells from HIF-1α- to HIF-2α-Dependent Signaling Promoting Stem Cell Characteristics, Aggressive Tumor Growth and Invasion. Cancer Res. 2011;71:4015–4027. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li H.S., Zhou Y.N., Li L., Li S.F., Long D., Chen X.L., Zhang J.B., Feng L., Li Y.P. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019;25:101109. doi: 10.1016/j.redox.2019.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koziel A., Jarmuszkiewicz W. Hypoxia and aerobic metabolism adaptations of human endothelial cells. Pflügers Archiv. 2017;469:815–827. doi: 10.1007/s00424-017-1935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Natarajan B., Arige V., Khan A.A., Reddy S.S., Barthwal M.K., Mahapatra N.R. Hypoxia-mediated regulation of mitochondrial transcription factors in renal epithelial cells: implications for hypertensive renal physiology. Hypertens. Res. 2021;44:154–167. doi: 10.1038/s41440-020-00539-4. [DOI] [PubMed] [Google Scholar]
- 91.Manalo D.J., Rowan A., Lavoie T., Natarajan L., Kelly B.D., Ye S.Q., Garcia J.G.N., Semenza G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 92.Kanatous S.B., Mammen P.P.A., Rosenberg P.B., Martin C.M., White M.D., DiMaio J.M., Huang G., Muallem S., Garry D.J. Hypoxia reprograms calcium signaling and regulates myoglobin expression. Am. J. Physiol. Cell Physiol. 2009;296:393–402. doi: 10.1152/ajpcell.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y.V., Hubbi M.E., Pan F., McDonald K.R., Mansharamani M., Cole R.N., Liu J.O., Semenza G.L. Calcineurin promotes hypoxia-inducible factor 1alpha expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J. Biol. Chem. 2007;282:37064–37073. doi: 10.1074/jbc.M705015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang H., Gao P., Fukuda R., Kumar G., Krishnamachary B., Zeller K.I., Dang C.V., Semenza G.L. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Feinberg A.P. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 96.van Leeuwen R., Boekhoorn S., Vingerling J.R., Witteman J.C.M., Klaver C.C.W., Hofman A., de Jong P.T.V.M. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005;294:3101–3107. doi: 10.1001/jama.294.24.3101. [DOI] [PubMed] [Google Scholar]
- 97.Tan J.S.L., Wang J.J., Flood V., Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch. Ophthalmol. 2009;127:656–665. doi: 10.1001/archophthalmol.2009.76. [DOI] [PubMed] [Google Scholar]
- 98.Tie F., Banerjee R., Stratton C.A., Prasad-Sinha J., Stepanik V., Zlobin A., Diaz M.O., Scacheri P.C., Harte P.J. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development. 2009;136:3131–3141. doi: 10.1242/dev.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bagchi R.A., Ferguson B.S., Stratton M.S., Hu T., Cavasin M.A., Sun L., Lin Y.H., Liu D., Londono P., Song K., et al. HDAC11 suppresses the thermogenic program of adipose tissue via BRD2. JCI Insight. 2018;3:e120159. doi: 10.1172/jci.insight.120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma A., Nguyen H., Geng C., Hinman M.N., Luo G., Lou H. Calcium-mediated histone modifications regulate alternative splicing in cardiomyocytes. Proc. Natl. Acad. Sci. USA. 2014;111:4920–4928. doi: 10.1073/pnas.1408964111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Backs J., Backs T., Bezprozvannaya S., McKinsey T.A., Olson E.N. Histone Deacetylase 5 Acquires Calcium/Calmodulin-Dependent Kinase II Responsiveness by Oligomerization with Histone Deacetylase 4. Mol. Cell Biol. 2008;28:3437–3445. doi: 10.1128/MCB.01611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hardingham G.E., Arnold F.J., Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 103.Chawla S., Vanhoutte P., Arnold F.J.L., Huang C.L.H., Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J. Neurochem. 2003;85:151–159. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 104.Hardingham G.E., Fukunaga Y., Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 105.Görisch S.M., Wachsmuth M., Tóth K.F., Lichter P., Rippe K. Histone acetylation increases chromatin accessibility. J. Cell Sci. 2005;118:5825–5834. doi: 10.1242/jcs.02689. [DOI] [PubMed] [Google Scholar]
- 106.Fan J., Alsarraf O., Dahrouj M., Platt K.A., Chou C.J., Rice D.S., Crosson C.E. Inhibition of HDAC2 protects the retina from ischemic injury. Invest. Ophthalmol. Vis. Sci. 2013;54:4072–4080. doi: 10.1167/iovs.12-11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crosson C.E., Mani S.K., Husain S., Alsarraf O., Menick D.R. Inhibition of histone deacetylase protects the retina from ischemic injury. Invest. Ophthalmol. Vis. Sci. 2010;51:3639–3645. doi: 10.1167/iovs.09-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sancho-Pelluz J., Paquet-Durand F. HDAC inhibition prevents Rd1 mouse photoreceptor degeneration. Adv. Exp. Med. Biol. 2012;723:107–113. doi: 10.1007/978-1-4614-0631-0_15. [DOI] [PubMed] [Google Scholar]
- 109.de Cogan F., Hill L.J., Lynch A., Morgan-Warren P.J., Lechner J., Berwick M.R., Peacock A.F.A., Chen M., Scott R.A.H., Xu H., Logan A. Topical Delivery of Anti-VEGF Drugs to the Ocular Posterior Segment Using Cell-Penetrating Peptides. Invest. Ophthalmol. Vis. Sci. 2017;58:2578–2590. doi: 10.1167/iovs.16-20072. [DOI] [PubMed] [Google Scholar]
- 110.Huang H., Liu Y., Wang L., Li W. Age-related macular degeneration phenotypes are associated with increased tumor necrosis-alpha and subretinal immune cells in aged Cxcr5 knockout mice. PLoS One. 2017;12:e0173716. doi: 10.1371/journal.pone.0173716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao Z., Chen Y., Wang J., Sternberg P., Freeman M.L., Grossniklaus H.E., Cai J. Age-Related Retinopathy in NRF2-Deficient Mice. PLoS One. 2011;6:e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quinlan A.R., Hall I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCarthy D.J., Chen Y., Smyth G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 115.Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. MEME SUITE: tools for motif discovery and searching. Nuclei Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Berstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., Liu X.S. Model-based Analysis of ChIP-Seq (MACS) Genome Biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., 3rd, Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A table showing the ophthalmic data and clinical scores for NHP eyes during the pre-screen, and days 14 and 21 after laser-induced photocoagulation.
A table showing the processing statistics for all bulk RNA-, single nuclei (sn)RNA- and snATAC-sequencing experiments separated by different tabs.
A table showing the gene expression changes caused by Myr-EBIN in the bulk RNA-seq of endothelial cells and snRNA-seq in the rod photoreceptors (cluster 0, 1), RPE (cluster 2, 6), stromal cells (cluster 3), metabolic-active endothelial cells (cluster 4), and cone photoreceptors (cluster 5) separated by different tabs. The table includes the gene ensembl ID, p-value, average log fold change, and gene symbol for each gene significantly affected by MyrEBIN.
A table showing the IPA canonical pathway analysis of the gene expression changes caused by Myr-EBIN in the bulk RNA-seq of endothelial cells and snRNA-seq of rod photoreceptors (cluster 0), RPE (cluster 2), and metabolic active endothelial cells (cluster 4) separated by different tabs. The table includes the Ingenuity Canonical Pathways, p-value, ratio, z-scores, and the molecules within the pathway. Note, the changes in bulk RNA-seq are computed as “hypoxia vehicle” over “normoxia vehicle” and presented as “maladaptive responses” which are reversed by EBIN or “adaptive responses” not affected by EBIN.
A table showing expression levels of the positive and negative cell-type markers for the metabolic-active endothelial cells (cluster 4) and mature endothelial cells for comparison (cluster 19). The table includes the gene symbol, the endothelial cluster number, mean expression (Expression.mean), mean standard deviation (Expression.sd), and the percentage of cells where the gene is expressed (Expression.pct.expr).
A table showing a list of concurrent changes in gene expression and increased in peak for rod photoreceptors, cone photoreceptors, RPE, stromal cells, metabolic-active endothelial cells, RGC, amacrine cells, and bipolar cells. Transcription factors are highlighted in yellow. The table includes the gene ensembl ID and gene symbol, as well as the respective snRNA-seq and snATAC-seq defined cluster numbers, p-values, and average log fold change for each concurrent gene within the same cell type.
Data Availability Statement
NMR assignments have been deposited at the Biological Magnetic Resonance DataBank and are publicly available as of the date of publication. Entry numbers are listed in the key resources table. Bulk and single nuclei RNA and ATAC sequencing have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Any additional information and data reported in this paper is available from the lead contact, Yulia A. Komarova (ykomarov@uic.edu), upon request.