Abstract
Background
Adherence to secondary preventive pharmacotherapy after an acute coronary syndrome (ACS) is generally poor and is associated with recurrent cardiovascular events. Patients’ beliefs about their medications are a strong predictor of intentional nonadherence.
Methods
This prospective, observational study assessed adult patients’ beliefs about their post-ACS medications, using the Beliefs About Medicines Questionnaire (BMQ), and adherence, using the Medication Adherence Report Scale (MARS-5) at St. Paul’s Hospital in Vancouver, Canada during May-December, 2022. The BMQ and MARS-5 were administered in-hospital and at 4 weeks after discharge. Outcomes included difference in BMQ necessity-concerns differential (BMQ-NCD) from hospitalization to 4-week follow-up and factors associated with the BMQ-NCD.
Results
Forty-seven participants completed the 4-week follow-up. The mean age was 64 years, and 83% were male. Most presented with a non-ST-segment-elevation ACS. No difference occurred in BMQ-NCD (7.3 vs 6.6, P = 0.29) or MARS-5 scores from discharge to 4 weeks (22.8 vs 23.7, P = 0.06); however, the BMQ specific-necessity subscale score decreased significantly (20.3 vs 18.8, P = 0.002). South Asian and Middle Eastern ethnic origins, compared to European, were associated with a higher BMQ-NCD. Part-time employment and male sex were associated with a lower BMQ-NCD.
Conclusions
Participants held favourable beliefs about their post-ACS medications, which were largely unchanged from hospitalization to 4 weeks postdischarge, except for beliefs about the necessity of taking their medications. Those of European descent, those with part-time employment, and males had the lowest BMQ-NCD. Self-reported adherence was high. Ongoing reassessment of patients’ beliefs about the necessity of taking their post-ACS medications may be warranted to mitigate further decline in BMQ-NCD.
Résumé
Contexte
L’adhésion à une pharmacothérapie préventive secondaire après la survenue d’un syndrome coronarien aigu (SCA) est généralement faible et associée à des manifestations cardiovasculaires récurrentes. Les croyances du patient au sujet de ses médicaments représentent un facteur prédictif majeur de la non-adhésion intentionnelle.
Méthodologie
Cette étude observationnelle prospective avait pour objectif d’évaluer les croyances des patients au sujet des médicaments à prendre après la survenue d’un SCA, au moyen du questionnaire BMQ (Beliefs About Medicines), ainsi que l’adhésion thérapeutique, à l’aide de l’échelle de rapport sur l’adhésion aux médicaments MARS-5 (Medication Adherence Report Scale), à l’hôpital St. Paul de Vancouver, au Canada, de mai à décembre 2022. Les questionnaires BMQ et MARS-5 ont été administrés pendant l’hospitalisation, puis 4 semaines après le congé de l’hôpital. Les résultats comprenaient la différence du score BMQ-NCD (necessity-concerns differential – écart nécessité-inquiétudes), entre l’hospitalisation et le suivi à 4 semaines, et les facteurs associés au score BMQ-NCD.
Résultats
Au total, 47 participants ont terminé l’étude, jusqu’au suivi à 4 semaines. L’âge moyen était de 64 ans, et 83 % des sujets étaient de sexe masculin. La plupart des sujets présentaient un SCA sans élévation du segment ST. Aucune variation du score BMQ-NCD (7,3 vs 6,6; p = 0,29) ou MARS-5 (22,8 vs 23,7; p = 0,06) n’a été observée entre le congé de l’hôpital et le suivi à 4 semaines; cependant, le score BMQ spécifique à la nécessité avait significativement diminué (20,3 vs 18.8; p = 0,002). Les origines ethniques sud-asiatiques et moyen-orientales étaient associées à des scores BMQ-NCD plus élevés que les origines européennes. L’occupation d’un emploi à temps partiel et le sexe masculin étaient associés à des scores BMQ-NCD inférieurs.
Conclusions
Les participants entretenaient des croyances favorables envers leurs médicaments à prendre après la survenue d’un SCA, qui sont demeurées largement les mêmes entre l’hospitalisation et le suivi, 4 semaines après le congé de l’hôpital, à l’exception des croyances au sujet de la nécessité de prendre les médicaments. Les sujets d’origine européenne, ceux occupant un emploi à temps partiel et les sujets masculins ont eu les scores BMQ-NCD les plus faibles. L’adhésion thérapeutique autosignalée était élevée. Des réévaluations constantes des croyances des patients au sujet de la nécessité de prendre leurs médicaments après la survenue d’un SCA pourraient être justifiées afin d’éviter que les scores BMQ-NCD diminuent davantage.
Cardiovascular disease (CVD) is the leading cause of death in North America, and approximately 40% of those deaths are secondary to coronary heart disease.1 In the US, the age-adjusted rate of death secondary to CVD-related causes decreased by approximately 11% from 2009 to 2019, in part due to advances in medical therapy.1 Several medications are recommended for secondary prevention of cardiovascular events in patients with coronary heart disease, including renin-angiotensin-aldosterone system (RAAS) inhibitors (eg, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers), beta-blockers, hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins), and one or more antiplatelet agents (eg, acetylsalicylic acid with or without a P2Y12 inhibitor).2, 3, 4 However, patients must actually take these medications as prescribed in order to realize the benefits. Nonadherence to medications after an acute coronary syndrome (ACS) is common.5, 6, 7, 8, 9, 10, 11, 12 Studies have shown that 34% of post-myocardial infarction (MI) patients discontinued taking ≥ 1 of their medications at 1 month,8 and the level of nonadherence ranged from 8% to 20% at 6 months among patients who experienced an ACS.6 The level of nonadherence to taking beta-blockers at 1-year post-MI has been shown to be 39%-55%.5,9 Patients with a lower level of adherence to medications after an ACS have an increased risk of mortality, recurrent cardiovascular events, and hospitalization.8,10,11
Many factors contribute to intentional medication nonadherence, but a strong predictor is patients’ beliefs about their condition and its treatment. The Beliefs About Medicines Questionnaire (BMQ) is a validated instrument used in various chronic medical conditions to assess patients’ cognitive representation of overuse, harm, necessity, and concerns associated with medications.13, 14, 15 BMQ scores have been correlated with medication adherence and used to guide motivational interviewing and tailored interventions to improve adherence.16 The Medication Adherence Report Scale (MARS-5) is a 5-item questionnaire with established reliability and validity to measure self-reported medication adherence in various chronic medical conditions.14,17,18
Data are limited regarding patients’ beliefs about their medications after an ACS. The purpose of this study was to prospectively evaluate patients’ beliefs about and adherence to their secondary preventive medications after an ACS during their index hospitalization, and 4 weeks later, and to identify patient characteristics associated with positive or negative beliefs about their post-ACS medications.
Methods
This was a prospective, observational cohort study of patients admitted for an ACS to a cardiology unit at a quaternary referral centre for cardiac care (St. Paul’s Hospital in Vancouver, British Columbia, Canada). The study was approved by the University of British Columbia and Providence Health Care Research Ethics Boards (H19-02301).
Study population
Participants were screened for eligibility based on the following criteria: (i) age > 18 years; (ii) admission to the hospital with an ACS (ST-segment-elevation ACS or non-ST-segment-elevation ACS) coded as their primary diagnosis; (iii) being prescribed one or more secondary cardiovascular prevention medications (RAAS inhibitor, beta-blocker, statin, or antiplatelet agent); (iv) having a planned or high likelihood of discharge within 7 days of admission; and (v) being a resident of Canada. Patients were excluded if they died during their index hospitalization, presented with a type II MI (eg, demand ischemia, severe aortic stenosis), were unable to understand or communicate in English, received direct administration of their medications by a caregiver or medical professional, or did not have a telephone. Patients who were readmitted to the hospital within 4 weeks, or after completion of the 4-week follow-up, were not re-enrolled into the study. Eligible patients were required to provide written informed consent to participate, which was obtained by a study investigator (L.Z.) who was not involved in the patient’s care.
Data collection
The 18-question BMQ and its computed subscales were utilized as described by its authors.13 For the 10 “BMQ-specific” questions, respondents were asked to answer in reference to their post-ACS cardiovascular preventive medications (RAAS inhibitor, beta-blocker, statin, or antiplatelet agent) only. Each question used a 5-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). Scores for the general-harm and general-overuse subscales range from 4 to 20, and specific-necessity and specific-concerns subscales range from 5 to 25, with higher values indicating stronger beliefs about harm, overuse, necessity, and concerns. To assess an individual’s relative perception of medication necessity vs their concerns, the necessity-concerns differential (NCD) was calculated by subtracting the specific-necessity score from the specific-concerns score. The NCD ranges from -20 to 20, with positive scores indicating a perception that the benefits outweigh the risks, and negative scores indicating a perception that the risks outweigh the benefits. The MARS-5 was administered per its instructions, with a 5-point unipolar scale, as follows: 1 = never; 2 = rarely; 3 = sometimes; 4 = often; and 5 = always.14 Participants were asked to answer in reference to only their post-ACS cardiovascular preventive medications. MARS-5 scores range from 5 to 25, with higher scores indicating greater self-reported adherence. The complete questionnaires are included in Appendices 1 and 2.
Data were collected by the same study investigator at 2 different time points—in-person during the patient’s hospital admission and via telephone approximately 4 weeks after discharge. In most cases, patients completed the surveys while they were in the hospital, prior to receiving discharge medication education. For the 4-week follow-up, participants were called 4 times without success in reaching them before they were considered to be lost to follow-up. All data were recorded using the Research Electronic Data Capture (REDCap) tool (Vanderbilt University, Nashville, TN), hosted by the University of British Columbia.
Outcomes
The primary outcome was difference in the NCD from the patient’s index hospitalization to the 4-week follow-up. Secondary outcomes were differences in each of the 4 BMQ subscale scores and the MARS-5 score from hospitalization to 4 weeks postdischarge.
Sample size
A 2-point or greater change in the NCD was assumed to correlate with a clinically meaningful difference in adherence.19 Based on this, a sample size of 24 participants was estimated to be required, using a paired-samples t-test, with an assumed standard deviation of 3.2, a type I error of 5%, and a power of 80%. The sample size was increased to 48 based on an expected attrition rate of 50%.
Data analysis
Descriptive statistics were utilized to report participant baseline characteristics, and BMQ and MARS-5 scores. Paired t-tests were used to assess the difference in the NCD, BMQ subscale scores, and MARS-5 scores between hospitalization and 4 weeks postdischarge. A linear regression model was used to identify participant characteristics that were associated with NCD score during hospitalization and at 4 weeks postdischarge using the following covariates as determined by the research team: age, sex, ethnic origin, highest level of education, employment status, type of ACS, and type of coronary revascularization. A P-value of < 0.05 was considered to be statistically significant. Analyses were performed using IBM SPSS Statistics version 28 (IBM Corporation, Armonk, NY) and R v4.0.5 (R Core Team, Vienna, Austria) and RStudio v1.3.1039 (RStudio Team, Boston, MA).
Results
Data were collected from May 1 to December 31, 2022. A study flow diagram is depicted in Figure 1. In total, 85 patients completed the questionnaires during their index hospitalization, and 47 patients completed the 4-week follow-up. Participant baseline characteristics are shown in Table 1. The mean age was 64 years, and most participants were male (83%) and of self-reported European ethnic origin (70%). Most patients presented with an non-ST-segment-elevation ACS (79%). At the 4-week follow-up, 42 respondents (89%) had seen their primary care provider, and 22 respondents (47%) had seen their cardiologist. A total of 31 respondents (66%) stated that they received education from their community-based pharmacist. Most respondents (87%) stated that they received full or partial insurance coverage for their medications.
Figure 1.
Patient enrollment flow diagram. ACS, acute coronary syndrome.
Table 1.
Patient characteristics (N = 47)
| Age, y | 63.9 ± 9.5 |
| Male sex | 39 (83.0) |
| Ethnic origin | |
| European | 33 (70.2) |
| South Asian | 5 (10.6) |
| First Nations or Métis | 3 (6.4) |
| Middle Eastern | 3 (6.4) |
| African | 1 (2.1) |
| East Asian | 1 (2.1) |
| Southeast Asian | 1 (2.1) |
| Number of people in household | 2.4 ± 1.3 |
| Area of residence | |
| Urban | 29 (61.7) |
| Rural | 18 (38.3) |
| Highest level of education | |
| Secondary | 20 (42.6) |
| Postsecondary | 22 (46.8) |
| Postgraduate | 5 (10.6) |
| Employment status | |
| Retired | 25 (53.2) |
| Full-time | 17 (36.2) |
| Part-time | 2 (4.3) |
| Not employed or long-term disability | 3 (6.4) |
| Number of medications prior to admission | 5.4 ± 5.9 |
| Hypertension | 18 (38.3) |
| Dyslipidemia | 17 (36.2) |
| Diabetes mellitus | 11 (23.4) |
| CAD | 11 (23.4) |
| Smoker (current or former) | 10 (21.3) |
| Stroke or TIA | 6 (12.8) |
| Atrial fibrillation | 6 (12.8) |
| Heart failure | 2 (4.3) |
| Type of ACS | |
| NSTEACS | 37 (78.7) |
| STEACS | 10 (21.3) |
| STEACS revascularization | |
| Primary PCI | 5 (50.0) |
| Fibrinolysis with early PCI | 3 (30.0) |
| Fibrinolysis with rescue PCI | 1 (10.0) |
| CABG surgery | 1 (10.0) |
| NSTEACS revascularization | |
| PCI | 20 (54.1) |
| CABG surgery | 17 (45.9) |
Values are n (%) or mean ± standard deviation.
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CAD, coronary artery disease; NSTEACS, non-ST-segment-elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment-elevation acute coronary syndrome; TIA, transient ischemic attack.
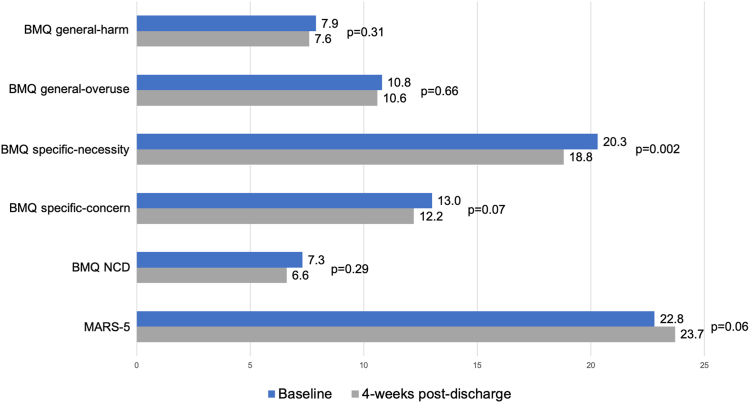
The results of the BMQ and the MARS-5 are shown in Figure 2. The primary outcome is difference in NCD was not significant from hospitalization to 4 weeks postdischarge (7.3 vs 6.6, P = 0.29). Also, no significant differences occurred in the general-harm, general-overuse, or specific-concerns subscales. However, a significant decrease occurred in the specific-necessity subscale from hospitalization to 4 weeks postdischarge (20.3 vs 18.8, P = 0.002), indicating a decline in participants’ perception of the necessity of taking their secondary prevention medications. Among the 31 participants who were taking one or more medications prior to their hospitalization, no significant difference occurred in their MARS-5 score from baseline to 4 weeks postdischarge (22.8 vs 23.7, P = 0.06).
Figure 2.
Beliefs About Medicines Questionnaire (BMQ) and 5-item Medication Adherence Rating Scale (MARS-5) responses. Reported as mean ± standard deviation, determined with a paired t-test. NCD, necessity-concerns differential.
For the NCD at 4 weeks postdischarge, self-reported South Asian and Middle Eastern ethnic origin were associated with a higher NCD, via regression analysis, compared to NCD for those of European origin (Table 2). Furthermore, part-time employment (vs full-time employment) and male sex (vs female sex) were associated with a lower NCD. The variables of age, level of education, type of ACS, and type of coronary revascularization were not significant. No covariates were significant for the in-hospital NCD regression analysis.
Table 2.
Linear regression analysis for necessity-concerns differential at 4 weeks postdischarge
| Covariate | Coefficient estimate | P |
|---|---|---|
| Age, y (deciles) | 1.45 | 0.22 |
| Sex | ||
| Female | Reference | |
| Male | –4.5 | 0.04 |
| Ethnic origin | ||
| European | Reference | |
| First Nations or Métis | 1.1 | 0.75 |
| South Asian | 8.8 | 0.006 |
| Middle Eastern | 6.4 | 0.0495 |
| African | 9.3 | 0.17 |
| Southeast Asian | 2.2 | 0.71 |
| East Asian | –5.1 | 0.34 |
| Employment status | ||
| Full-time | Reference | |
| Part-time | –12.8 | 0.004 |
| Retired | –1.3 | 0.53 |
| Not employed or long-term disability | –8.4 | 0.06 |
| Highest level of education | ||
| Secondary | Reference | |
| Postsecondary | 0.86 | 0.65 |
| Postgraduate | –0.52 | 0.85 |
| Type of ACS | ||
| STEACS | Reference | |
| NSTEACS | –1.8 | 0.46 |
| Type of coronary revascularization | ||
| PCI | Reference | |
| CABG surgery | 0.45 | 0.80 |
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; NSTEACS, non-ST-segment-elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment-elevation acute coronary syndrome.
Discussion
To our knowledge, this study was the first to evaluate the association between BMQ scores and self-reported medication adherence, both in-hospital and after 4 weeks postdischarge, in patients admitted with an ACS. We found that on average, patients’ beliefs about their cardiovascular prevention medications were positive and fairly stable during this early post-ACS period.
No significant difference in the NCD occurred between hospitalization and 4 weeks after discharge, and the overall positive mean NCD values at both time points suggest that participants’ beliefs in the necessity of taking their secondary cardiovascular medications outweighed their concerns. These generally favourable and stable views about their post-ACS medications were reflected across the BMQ subscales. Although the specific-necessity score significantly decreased from hospitalization to the 4-week follow-up, whether this decrease would predict a decline in adherence is unclear, given its small magnitude and the lack of corresponding changes in NCD and MARS-5 scores. Although of borderline statistical significance, the higher self-reported adherence at follow-up may be attributable to increased motivation following an ACS and/or medication education provided to patients by their in-hospital and outpatient providers.
The mean NCD, specific-necessity subscale, and specific-concerns subscale scores were similar to those in a previous study involving patients hospitalized with an ACS, which show that a lower perceived necessity score and higher perceived concerns score were associated with a significantly higher likelihood of nonadherence to post-ACS medications 3 months after hospital discharge.19
Patients of self-identified South Asian and Middle Eastern ethnic origin held stronger beliefs about the necessity of their medications than those of European origin. This finding signals a potential need for clinicians to provide culturally appropriate education and resources regarding medication-taking post-ACS.20,21 Our finding that patients of male sex had a lower NCD than those of female sex diverges from results of a Norwegian studying showing that women had significantly higher concerns scores than men, with no difference in necessity scores.22 However, this finding may have been due to the small number of respondents of female sex. Patients who had part-time employment (vs full-time) had a significantly lower NCD score, which may have been due to a sampling bias, as only 2 patients (4%) in the study cohort had part-time employment.
Our results identify a potential unmet need for interventions to improve patients’ beliefs about the necessity of taking their medications after an ACS. Most respondents had seen their primary care provider in the 4 weeks after discharge, but only two-thirds reported receiving education from their community-based pharmacist, and less than half had seen their cardiologist. Studies have demonstrated that interventions aimed at increasing adherence need to be multifaceted and tailored to the patient’s specific barriers to adherence.16,23, 24, 25 In a study of 56 patients admitted to hospital with an ACS, a pharmacist-led personalized intervention designed to address perceptual and practical barriers to adherence improved BMQ specific-necessity scores at 6 weeks, although the effect did not persist at 12 weeks of follow-up.23 Another pharmacist-led intervention, using motivational interviewing and support tailored to each patient’s beliefs about medications, improved adherence among 316 patients with coronary heart disease.24 Further studies are warranted to evaluate the effect of different interventions on CVD patients’ beliefs about their medications and long-term adherence.
This study utilized a prospective, observational design to assess patients’ beliefs about their medications, both in and out of hospital, using validated instruments; however, it has limitations that warrant discussion. Patients were recruited from a single quaternary referral centre and may not be reflective of those in other practice settings, although a variety of patients from urban and rural areas of the province participated. The results may be less generalizable to female patients, as the majority of patients enrolled in this study were men, reflecting the disproportionately higher rate of ACS in men vs women.26 Although this study assessed patients’ beliefs and self-reported adherence at 2 different time points, the 4-week follow-up may have been too short to detect meaningful changes in beliefs and adherence. Further, although the MARS-5 is known to reliably reflect actual adherence,14,17,18 inclusion of additional objective measures of adherence in this study was not feasible. As with all prospective studies requiring consent, selection bias toward patients who held more favourable views about their medications may have occurred. Further selection bias may have resulted from the rapid turnover of patients on the cardiology units, preventing some patients from being approached to participate in this study. Finally, although we did predict the loss to follow-up of a significant proportion of participants, this may have resulted in unknown biases. Despite the loss of 45% of participants to follow-up, and the potential internal and external validity concerns this prompts, the baseline questionnaire responses of participants who completed the 4-week follow-up were similar to responses of those who did not (Supplemental Table S1).
Conclusions
Among a cohort of patients admitted to the hospital with an ACS, most held generally favourable beliefs about their secondary cardiovascular preventive therapy. Although patients’ concerns about their post-ACS medications were stable from their index hospitalization to 4 weeks after discharge, their beliefs about the necessity of taking their medications significantly declined. However, the overall difference in their perceived necessity of their medications vs their concerns, as well as their self-reported adherence, remained positive. Reassessment of patients’ beliefs about the necessity of taking their post-ACS medications may be warranted to identify any further decline in their NCD, and continued educational interventions from patients’ healthcare team members (eg, family physician, cardiologist, pharmacist) may be needed to address this decline.
Acknowledgments
Ethics Statement
The study was approved by the University of British Columbia and Providence Health Care Research Ethics Boards (H19-02301). Eligible patients were required to provide written informed consent to participate, which was obtained by a study investigator (L.Z.) who was not involved in the patient’s care.
Patient Consent
The authors confirm that patient consent forms have been obtained for this article.
Funding Sources
This research project was supported by internal start-up funding at the Faculty of Pharmaceutical Sciences at the University of British Columbia, Vancouver, British Columbia, Canada.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 750 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.07.004.
Appendix 1. In-hospital Beliefs About Medication Questionnaire (BMQ) and 5-Item Medication Adherence Rating Scale (MARS-5)
To be collected from electronic medical record:
-
1.
Age.
-
2.
Sex.
-
3.
Patient’s city or town.
-
4.
Number of prescribed medications prior to admission.
-
5.
Comorbid medical conditions (if applicable).
-
6.Type of ACS:
-
•STEACS
-
•NSTEACS
-
•
-
7.For STEACS patients, type of revascularization procedure during index admission:
-
•Primary PCI
-
•Fibrinolysis and early PCI
-
•Fibrinolysis and rescue PCI
-
•CABG surgery
-
•
-
8.For NSTEACS patients, type of revascularization procedure during index admission:
-
•PCI
-
•CABG surgery
-
•
To be collected from patient:
-
1.
What is your ethnicity?
-
2.
What is the number of people in your household (including yourself)?
-
3.What is your highest level of education completed?
-
•Secondary (ie, high school)
-
•Postsecondary (ie, university/college)
-
•Advanced (ie, postgraduate)
-
•Would rather not say
-
•
-
4.What was your employment status prior to admission?
-
•Full-time
-
•Part-time
-
•Retired
-
•Not employed/long-term disability
-
•Would rather not say
-
•
-
5.When discharged from hospital, do you have a pharmacy in mind that you’ll go to to get these prescriptions filled?
-
•At a pharmacy close to home
-
•At a pharmacy near hospital (as a first-time visitor)
-
•
Beliefs About Medicines Questionnaire (BMQ)
The following questions are regarding your view on medications in general . Please provide a response that best relates to your view—strongly disagree, disagree, unsure, agree, or strongly agree .
BMQ General-Harm
-
6.
People who take medicine should stop their treatment for a period of time once in a while.
-
7.
Most medicines are addictive.
-
8.
Medicines do more harm than good.
-
9.
All medicines are toxic.
BMQ G eneral-Overuse
-
10.
Doctors prescribe too many medicines.
-
11.
Natural remedies are safer than medicines.
-
12.
Doctors place too much trust on medicines.
-
13.
If doctors spent more time with patients, they would prescribe fewer medicines.
The following questions are regarding your view on your heart medications . Please provide a response that best relates to your view—strongly disagree, disagree, unsure, agree, or strongly agree.
BMQ Specific-Necessity
-
14.
Currently, my health depends on my heart medicines.
-
15.
My life would be impossible without my heart medicines.
-
16.
Without my heart medicines, I would be very ill.
-
17.
My health in the future will depend on my heart medicines.
-
18.
My heart medicines prevent my condition from worsening.
BMQ Specific-Concern s
-
19.
Having to take my heart medicines worries me.
-
20.
I sometimes worry about the long-term effects of my heart medicines.
-
21.
My heart medicines are a mystery to me.
-
22.
My heart medicines disrupt my life.
-
23.
I sometimes worry about being too dependent on my heart medicines.
-
24.In the last few weeks before being admitted to St. Paul’s Hospital, I was not prescribed any medicines at home.
-
•If yes, please go to the final question.
-
•If no, please go to the next question.
-
•
Medication Adherence Rating Scale (MARS-5)
Please provide a response that best relates to your view— never, rarely, sometimes, often, or always.
-
25.
I forgot to take my medication at home.
-
26.
I altered the dose of my medication at home.
-
27.
I stopped taking my medication for a while at home.
-
28.
I decided to miss out on a dose of my medication.
-
29.
I took less medication than instructed at home.
-
30.
Is there anything you want to tell us about your medication?
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; NSTEACS, non-ST-segment-elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment-elevation acute coronary syndrome.
Appendix 2. Beliefs About Medication Questionnaire (BMQ) and Medication Adherence Rating Scale (MARS-5) 4-Week Follow-up
Beliefs About Medicines Questionnaire (BMQ)
The following questions are regarding your view on medications in general . Please provide a response that best relates to your view—strongly disagree, disagree, unsure, agree, or strongly agree.
BMQ General-Harm
-
1.
People who take medicine should stop their treatment for a period of time once in a while.
-
2.
Most medicines are addictive.
-
3.
Medicines do more harm than good.
-
4.
All medicines are toxic.
BMQ General-Overuse
-
5.
Doctors prescribe too many medicines.
-
6.
Natural remedies are safer than medicines.
-
7.
Doctors place too much trust on medicines.
-
8.
If doctors spent more time with patients, they would prescribe fewer medicines.
The following questions are regarding your view on your heart medications . Please provide a response that best relates to your view—strongly disagree, disagree, unsure, agree, or strongly agree.
BMQ Specific-Necessity
-
9.
Currently, my health depends on my heart medicines.
-
10.
My life would be impossible without my heart medicines.
-
11.
Without my heart medicines, I would be very ill.
-
12.
My health in the future will depend on my heart medicines.
-
13.
My heart medicines prevent my condition from worsening.
BMQ Specific-Concern s
-
14.
Having to take my heart medicines worries me.
-
15.
I sometimes worry about the long-term effects of my heart medicines.
-
16.
My heart medicines are a mystery to me.
-
17.
My heart medicines disrupt my life.
-
18.
I sometimes worry about being too dependent on my heart medicines.
Medication Adherence Rating Scale (MARS-5)
Please provide a response that best relates to your view—never, rarely, sometimes, often, or always.
-
19.
I forgot to take my medication at home.
-
20.
I altered the dose of my medication at home.
-
21.
I stopped taking my medication for a while at home.
-
22.
I decided to miss out on a dose of my medication.
-
23.
I took less medication than instructed at home.
The following questions relate to the time since you have been discharged from hospital .
-
24.Have you been re-admitted to a hospital?
-
•Yes
-
•No
-
•
-
25.Did you fill your prescriptions?
-
•All
-
•Most
-
•Some
-
•None
-
•
-
26.Have you seen your primary care provider (family doctor or nurse practitioner)?
-
•Yes
-
•No
-
•I don’t have one
-
•
-
27.Have you seen your cardiologist?
-
•Yes
-
•No
-
•I don’t have one
-
•
-
28.Did you receive education from your community pharmacist?
-
•Yes
-
•No
-
•
-
29.
Are you taking the following medications (examples to be provided, as needed): an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, beta-blocker, statin, acetylsalicylic acid, and P2Y12 inhibitor? If not, why?
-
30.
What was the approximate cost of your medications when you filled them at your pharmacy?
-
31.Did you receive any coverage for your medications?
-
•Yes, from the British Columbia PharmaCare drug plan
-
•Yes, from an extended (private) drug plan
-
•Yes, from the British Columbia PharmaCare and extended drug plans
-
•Yes, but I don’t know from whom
-
•Unsure or I don’t know
-
•
-
32.
Is there anything you want to tell us about your medication?
Supplementary Material
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145:153–639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.O’Gara P.T., Kushner F.G., Ascheim D.D., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 3.Amsterdam E.A., Wenger N.K., Brindis R.G., et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 4.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:18–114. doi: 10.1161/CIR.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 5.Butler J., Arbogast P.G., BeLue R., et al. Outpatient adherence to beta-blocker therapy after acute myocardial infarction. J Am Coll Cardiol. 2002;40:1589–1595. doi: 10.1016/s0735-1097(02)02379-3. [DOI] [PubMed] [Google Scholar]
- 6.Eagle K.A., Kline-Rogers E., Goodman S.G., et al. Adherence to evidence-based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med. 2004;117:73–81. doi: 10.1016/j.amjmed.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 7.Simpson S.H., Eurich D.T., Majumdar S.R., et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho P.M., Spertus J.A., Masoudi F.A., et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 9.Kramer J.M., Hammill B., Anstrom K.J., et al. National evaluation of adherence to beta-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J. 2006;152:454.e1–454.e8. doi: 10.1016/j.ahj.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen J.N., Chong A., Alter D.A. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 11.Jackevicius C.A., Li P., Tu J.V. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–1036. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- 12.Shang P., Liu G.G., Zheng X., et al. Association between medication adherence and 1-year major cardiovascular adverse events after acute myocardial infarction in China. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horne R., Weinman J., Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1–24. [Google Scholar]
- 14.Horne R., Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health. 2002;17:17–32. [Google Scholar]
- 15.Horne R., Chapman S.C., Parham R., et al. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen T.M., La Caze A., Cottrell N. Validated adherence scales used in a measurement-guided medication management approach to target and tailor a medication adherence intervention: a randomised controlled trial. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tommelein E., Mehuys E., Van Tongelen I., Brusselle G., Boussery K. Accuracy of the Medication Adherence Report Scale (MARS-5) as a quantitative measure of adherence to inhalation medication in patients with COPD. Ann Pharmacother. 2014;48:589–595. doi: 10.1177/1060028014522982. [DOI] [PubMed] [Google Scholar]
- 18.Lee C.S., Tan J.H.M., Sankari U., Koh Y.L.E., Tan N.C. Assessing oral medication adherence among patients with type 2 diabetes mellitus treated with polytherapy in a developed Asian community: a cross-sectional study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen LaPointe N.M., Ou F.S., Calvert S.B., et al. Association between patient beliefs and medication adherence following hospitalization for acute coronary syndrome. Am Heart J. 2011;161:855–863. doi: 10.1016/j.ahj.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Jamil A., Jonkman L.J., Miller M., Jennings L., Connor S.E. Medication adherence and health beliefs among South Asian immigrants with diabetes in the United States: a qualitative study. J Am Coll Clin Pharm. 2022;5:829–836. [Google Scholar]
- 21.Kumar K., Greenfield S., Raza K., Gill P., Stack R. Understanding adherence-related beliefs about medicine amongst patients of South Asian origin with diabetes and cardiovascular disease patients: a qualitative synthesis. BMC Endocr Disord. 2016;16:24. doi: 10.1186/s12902-016-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viktil K.K., Frøyland H., Rogvin M., Moger T.A. Beliefs about medicines among Norwegian outpatients with chronic cardiovascular disease. Eur J Hosp Pharm. 2014;21:118–120. doi: 10.1136/ejhpharm-2013-000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawshaw J., Weinman J., McRobbie D., Auyeung V. Initial evaluation of a brief pharmacy-led intervention to modify beliefs about medicines and facilitate adherence among patients hospitalised with acute coronary syndrome. Eur J Hosp Pharm. 2022;29:18–25. doi: 10.1136/ejhpharm-2019-002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Östbring M.J., Eriksson T., Petersson G., Hellström L. Effects of a pharmaceutical care intervention on clinical outcomes and patient adherence in coronary heart disease: the MIMeRiC randomized controlled trial. BMC Cardiovasc Disord. 2021;21:367. doi: 10.1186/s12872-021-02178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shehab A., Elnour A.A., Swaidi S.A., et al. Evaluation and implementation of behavioral and educational tools that improves the patients’ intentional and unintentional non-adherence to cardiovascular medications in family medicine clinics. Saudi Pharm J. 2016;24:182–188. doi: 10.1016/j.jsps.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haider A., Bengs S., Luu J., et al. Sex and gender in cardiovascular medicine: presentation and outcomes of acute coronary syndrome. Eur Heart J. 2020;41:1328–1336. doi: 10.1093/eurheartj/ehz898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.