Abstract
Background
Clinical outcomes and quality of life (QoL) indices are not well described after transcatheter aortic valve replacement (TAVR) in patients aged ≥ 90 years.
Methods
We conducted a retrospective cohort study of TAVR among nonagenarian patients between 2008 and 2020. The survival of TAVR patients among nonagenarians was compared to the provincial estimated survival for an age- and sex-matched general population. QoL was assessed up to 1 year postintervention, using standardized questionnaires.
Results
During the study period, n = 268 patients aged ≥ 90 years were evaluated for severe aortic stenosis. TAVR was performed in n = 171 (48% female; median [IQR] Rockwood Clinical Frailty Scale score: 4 [3-4]); n = 84 underwent medical therapy; and n = 13 underwent surgical aortic valve replacement. Survival was significantly better following TAVR, compared to that after MT (adjusted hazard ratio [95% CI]: 1.99 [1.37-2.88], P < 0.001). TAVR patients demonstrated a survival advantage compared with the general population, with an estimated relative mortality of 0.86 (0.75-0.87). TAVR patients showed sustained improvements in functional status and QoL up to 1 year compared to baseline (all P < 0.05): the 6-minute walk test results improved from 192 to 252 m; the Kansas City Cardiomyopathy Questionnaire score improved from 64 to 81; the Duke Activity Status Index score improved from 13 to 16; and the health state scale result of the Euro Quality of life - 5 Dimensions improved from 63% to 74%.
Conclusions
Nonagenarians undergoing TAVR experience a slightly better survival rate, compared to that of an age- and sex-matched general population, and they have significant improvements in functional status and several QoL indices following the procedure.
Graphical abstract
Graphical abstract of this study
Résumé
Introduction
Les résultats cliniques et les indices de la qualité de vie (QdV) après le remplacement valvulaire aortique par cathéter (RVAC) chez les patients ≥ 90 ans ne sont pas bien décrits.
Méthodes
Nous avons réalisé une étude de cohorte rétrospective sur le RVAC chez les patients nonagénaires entre 2008 et 2020. Nous avons comparé la survie des patients nonagénaires qui avaient subi un RVAC à la survie provinciale estimée d’une population générale appariée selon l’âge et le sexe. Nous avons évalué la QdV jusqu’à 1 an après l’intervention au moyen de questionnaires standardisés.
Résultats
Durant la période étudiée, nous avons inclus des patients (n =268) âgés de ≥ 90 ans et évalués pour une sténose aortique sévère. Le RVAC a été réalisé chez 171 patients (48 % de sexe féminin ; score médian [écart interquartile] à l’échelle de fragilité clinique de Rockwood [Rockwood Clinical Frailty Scale] : 4 [3-4]) ; 84 ont reçu un traitement médical (TM) ; 13 ont subi un remplacement valvulaire aortique chirurgical. La survie était significativement meilleure à la suite du RVAC, comparativement à celle des patients TM (rapport de risque ajusté [IC à 95 %] : 1,99 [1,37-2,88], P < 0,001). Par rapport à la population générale, les patients ayant subi un RVAC ont démontré un avantage sur le plan de la survie, soit une mortalité relative estimée de 0,86 (0,75-0,87). Les patients ayant subi un RVAC ont montré des améliorations continues de l’état fonctionnel et de la QdV jusqu’à 1 an par rapport au début (toutes les valeurs P < 0,05) : les résultats à l’épreuve de marche de 6 minutes sont passés de 192 à 252 m ; les scores au questionnaire Kansas City Cardiomyopathy Questionnaire (KCCQ) sont passés de 64 à 81 ; les scores au questionnaire Duke Activity Status Index (DASI) sont passés de 13 à 16 ; les résultats à l’échelle de l’état de santé de l’EQ-5D (de l’EuroQol Group) sont passés de 63 % à 74 %.
Conclusions
Les nonagénaires qui subissent un RVAC ont un taux de survie légèrement meilleur à celui d’une population générale appariée selon l’âge et le sexe, et montrent des améliorations significatives de leur état fonctionnel et de plusieurs indices de la QdV à la suite de l’intervention.
A 4-fold increase in the number of people aged 85 years and older is expected between 2006 and 2045 in most industrialized countries.1 The incidence of age-related diseases, such as aortic stenosis (AS), is also expected to rise. Transcatheter aortic valve replacement (TAVR) is a safe and effective therapeutic option for patients with severe AS.2, 3, 4 Interest is growing in clinical outcomes and quality of life following TAVR in very elderly patients with promising data previously published.5 Patients aged 90 years and above have been traditionally underrepresented or excluded in prospective and observational TAVR studies,6, 7, 8 with some data suggesting that TAVR among nonagenerians is superior to medical therapy for severe symptomatic AS.7 However, these previous studies had small numbers of nonagenarian patients and were mainly post hoc subanalyses of larger studies conducted in younger populations. Moreover, the long-term survival benefit following TAVR, compared to survival in the general nonagenarian population without severe AS, remains less certain. Accordingly, if TAVR were to be expanded broadly to patients ≥ 90 years, this would have important implications regarding healthcare resource allocation and cost-effectiveness. Thus, patient-centred outcomes (ie, quality of life, functional status) in these patients could provide complementary insights regarding the survival benefit of TAVR observed in previous studies, to guide treatment options in this specific population. Our objectives were therefore to describe clinical outcomes, quality of life, and changes in function after TAVR in a cohort of Canadian nonagenarians and to compare these factors to the estimated survival of the age- and sex-matched general population.
Patients and Methods
Study design and population
We conducted a retrospective study including all nonagenarian patients evaluated for severe AS and referred to TAVR or who remained on medical treatment (MT), between 2008 and 2020, at our institution. No specific exclusion criteria were predefined. We thus include all patients ≥ 90 years of age with severe AS, and all patients were evaluated by the multidisciplinary heart team. Demographic, baseline clinical and echocardiographic characteristics, and postoperative data were retrieved from our TAVR database, which collects patient data prospectively for all consecutive patients. The study was approved by our institutional research ethics board, which waived individual patient consent, owing to the retrospective nature of the study (approval number 2021–3627, 22050).
During the study period, n = 268 patients aged 90 years and above were referred for TAVR at our institution. Of these, n = 171 (64%) underwent TAVR, n = 84 (31%) were treated medically, and n = 13 (5%) were treated with surgical AVR (these patients were excluded from the analysis).
Cognitive, frailty, functional status, and quality-of-life assessments
TAVR patients had several predefined cognitive, frailty, and functional status tests prior to intervention. However, due to various reasons related to clinical considerations, not all patients underwent these tests. Cognitive assessment was accomplished in 70% of patients (n = 120) with the use of the Folstein Mini-Mental State Examination on a scale of 0 to 30. An abnormal Folstein examination result was established by any of the following: (i) a score under the predicted value; (ii) according to the following classification—normal (ie, a score of 26-30); mild impairment (ie, a score of 20-25); moderate (ie, a score of 10-19); and severe (ie, a score of 0-9); and (iii) according to level of education (ie, a score < 22 if 0-4 years of education; a score < 26 if 5-8 years of education; and a score < 29 if ≥ 9 years of education).9 Frailty was assessed by the “eye-ball” (n = 132; 77%) and the Rockwood Clinical Frailty Scale tests (n = 85; 49%).10,11 Also, the 6-minute walk test (6MWT) was conducted in 65% of patients (n = 111).
TAVR patients were also evaluated systematically at 1 to 3 months and 1 year after intervention with functional status assessment (ie, 6MWT) and with various standardized quality-of-life questionnaires, such as the Kansas City Cardiomyopathy Questionnaire (KCCQ),12 the Duke Activity Status Index (DASI),13 and the Euro Quality of Life - 5 Dimensions (EuroQoL-5D).14
Study endpoints
Early and late clinical outcomes were compared between nonagenarian TAVR patients and medically treated patients. Long-term survival data of nonagenarians undergoing TAVR were compared to the age- and sex-matched general nonagenarian population survival for the specific year of the TAVR procedure, using data retrieved from Statistics Canada.15
Late deaths were ascertained by linking the institutional database with vital statistics compiled by the single provincial government healthcare insurer; thus, follow-up was 100% complete. The clinical outcomes of interest in this study among TAVR patients were defined according to the Valve Academic Research Consortium-2 (VARC-2) consensus definitions.16
Statistical analysis
Categorical variables are described as counts and percentages. Continuous data were tested for normality of distribution and homogeneity of variances with the Shapiro-Wilk and Levene tests, respectively, and then expressed as mean ± standard deviation or median (interquartile range [IQR]), as appropriate. For baseline characteristics, comparisons between groups were made using either χ2 tests or Fisher’s exact tests, and the Student t test or the Wilcoxon Mann-Whitney U test, as appropriate. Survival curves using the Kaplan-Meier test and the log-rank test were obtained to compare TAVR patients to MT patients and to nonagenerians from the general population. For the latter, survival was compared to age- and sex-specific life expectancy of the general provincial population at the year of the TAVR procedure. Univariate and multivariate Cox proportional hazard models of late mortality were performed among TAVR and MT patients, and results are presented as hazard ratio (HR) and 95% confidence interval (CI). Multivariate adjustment included all variables with a P < 0.10 in univariate analysis for the prediction of mortality. Analysis of quality of life (QoL) in TAVR patients at follow-up was performed with either a paired t-test or a Wilcoxon signed-rank test, for continuous variables, and the McNemar-Bowker test, for categorical variables. Patients with missing data were excluded from these analyses. A P-value of < 0.05 was considered statistically significant. All analyses were performed using SPSS (V.27, IBM, Chicago, IL) and SAS (V.9.4, SAS Institute, Cary, NC) software.
Results
Among the 84 patients who did not undergo TAVR and instead were redirected toward MT, the breakdown is as follows: 45 patients were offered TAVR but refused the procedure, 17 patients were considered to be too frail for the procedure after a standardized assessment, and 22 patients had prohibitive noncardiovascular comorbidities. No patients had important life expectancy–limiting illnesses, such as active cancer. Baseline characteristics of the study population are presented in Table 1. TAVR patients are 48% of female patients with several comorbidities, a median (IQR) European System for Cardiac Operative Risk Evaluation II (EuroSCORE II) of 6.0% (4.4%-9.8%), and 11% had mild cognitive impairment. Acording to the Rockwood Clinical Frailty Scale, TAVR patients were considered mildly frail (median [IQR]: 4 [3-4]). The TAVR and MT groups were comparable, except for a significantly higher prevalence of dyslipidemia (71% vs 57%, P = 0.031), and a significantly lower prevalence of coronary artery disease (57% vs 70%, P = 0.037), among the TAVR patients. Echocardiographic data were similar in the 2 groups, with the exception of higher systolic pulmonary artery pressure in the TAVR patients (48 ± 15 vs 42 ± 16 mm Hg, P < 0.001).
Table 1.
Baseline characteristics of the study population
| Variable | TAVR (n = 171) | Medical treatment (n = 84) | P |
|---|---|---|---|
| Clinical data | |||
| Age, y | 91 ± 2 | 92 ± 2 | 0.11 |
| Female | 82 (48) | 46 (55) | 0.31 |
| BMI, kg/m2 | 25 ± 4 | 25 ± 5 | 0.70 |
| BSA, m2 | 1.71 ± 0.17 | 1.71 ± 0.18 | 0.76 |
| History of stroke/TIA | 22 (13) | 18 (21) | 0.08 |
| Diabetes | 26 (15) | 20 (24) | 0.09 |
| Dyslipidemia | 121 (71) | 48 (57) | 0.031 |
| Arterial hypertension | 151 (88) | 69 (82) | 0.18 |
| COPD | 22 (13) | 14 (17) | 0.41 |
| Coronary artery disease | 97 (57) | 59 (70) | 0.037 |
| History of myocardial infarction | 48 (28) | 23 (27) | 0.91 |
| < 90 d | 18 (11) | 9 (11) | 0.96 |
| History of atrial fibrillation/flutter | 75 (44) | 31 (37) | 0.29 |
| Peripheral vascular disease | 34 (20) | 12 (14) | 0.28 |
| Cognitive impairment | 4 (2) | 5 (6) | 0.16 |
| EuroSCORE II, % | 6.0 (4.4–9.8) | 5.7 (3.3–8.8) | 0.08 |
| Cognitive status | |||
| Folstein absolute score (n = 120) | 28 (27–29) | — | — |
| Normal | 107 (89) | — | — |
| Mild impairment | 13 (11) | — | — |
| Moderate/severe impairment | 0 (0) | — | — |
| Abnormal vs predicted Folstein | 6 (2) | — | — |
| Abnormal vs education level (n = 101) |
27 (27) | — | — |
| Frailty assessment | |||
| Eyeball test (n = 132) | 76 (58) | — | — |
| Rockwood Clinical Frailty Scale score (n = 85) | 4 (3–4) | — | — |
| Score ≥ 5 | 16 (19) | — | — |
| Functional status | |||
| 6-min walk test distance, m (n = 111) |
187 ± 86 | — | — |
| Laboratory data | |||
| Creatinine clearance (Cockcroft-Gault, mL/min) | 36 (30–45) | 37 (29–52) | 0.54 |
| < 60 mL/min | 165 (97) | 56 (89) | 0.05 |
| Creatinine level, μmol/L | 96 (75–119) | 103 (73–132) | 0.31 |
| Echocardiographic data | |||
| LVEF, % | 52 ± 13 | 50 ± 15 | 0.58 |
| Indexed AVA, cm2/m2 | 0.36 ± 0.12 | 0.38 ± 0.17 | 0.37 |
| SPAP, mm Hg | 48 ± 15 | 42 ± 16 | < 0.001 |
| ≥ moderate AR | 30 (18) | 13 (16) | 0.68 |
| ≥ moderate MR | 45 (26) | 27 (32) | 0.33 |
| ≥ moderate TR | 41 (24) | 17 (20) | 0.50 |
Values are mean ± standard deviation, median (25th-75th percentile), or n (%). Bold text highlights the statistically significant associations.
AR, aortic regurgitation; AVA, aortic valve area; BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; EuroSCORE II, European system for cardiac operative risk evaluation; Folstein, Folstein Mini-Mental State Examination; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; SPAP, systolic pulmonary artery pressure; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack; TR, tricuspid regurgitation.
TAVR early outcomes
The periprocedural data and early outcomes, including postprocedural echocardiographic data, are depicted in Table 2. In-hospital mortality was observed in n = 11 patients (6%), and n = 9 (5%) had an early stroke or transient ischemic attack. The transfemoral approach was performed in n = 123 patients (72%), and in n = 48 patients (28%) an alternative approach was used, mostly transcarotid. Balloon-expandable prostheses were used in n = 128 patients (75%), and self-expandable protheses were used in n = 43 (25%). Aortic valve predilatation was performed in n = 76 patients (51%), and n = 21 (12%) had postdilatation.
Table 2.
Procedural data and postoperative outcomes in the transcatheter aortic valve replacement (TAVR) population
| Variable | TAVR (n = 171) |
|---|---|
| Access | |
| Transfemoral | 123 (72) |
| Alternative | 48 (28) |
| Predilatation | 76 (51) |
| Postdilatation | 21 (12) |
| TAVR valve-in-valve | 4 (2) |
| Valve type | |
| Balloon-expandable | 128 (75) |
| Self-expanding | 43 (25) |
| Valve size, mm | |
| 20–23 | 51 (30) |
| 25–26 | 77 (45) |
| 27–29 | 38 (22) |
| 34 | 5 (3) |
| Hospital LOS, d | 5 (3–7) |
| Postoperative echocardiographic data | |
| LVEF, % | 53 ± 12 |
| AVA, m2 (n = 137) | 1.62 ± 0.51 |
| Mean gradient, mm Hg | 10 ± 5 |
| Pulmonary systolic arterial pressure, mm Hg | 45 ± 14 |
| Complications at 30 d | |
| Death | 11 (6) |
| Stroke/TIA | 9 (5) |
| Surgical conversion | 5 (3) |
| Significant periprosthetic insufficiency | 5 (3) |
| Pacemaker implantation | 7 (5) |
| Acute kidney insufficiency | 14 (8) |
| Pulmonary embolus | 3 (2) |
| Pleural effusion | 4 (2) |
| Heart conduction deficits/arrythmias | |
| Atrioventricular block | 15 (9) |
| Atrial fibrillation de novo | 11 (6) |
| Left bundle block | 22 (13) |
Values are mean ± standard deviation, median (25th-75th percentile), or n (%).
AVA, aortic valve area; LOS, length of stay; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack.
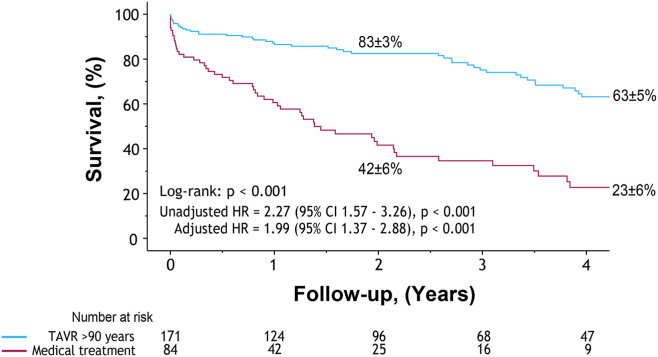
TAVR vs MT late survival
Survival up to 5 years was compared between the TAVR and MT groups, demonstrating a survival benefit of TAVR at 5 years (63% vs 23%, log-rank P < 0.001) (Figure 1). In univariate analysis, MT vs TAVR (HR 2.27 [95% CI: 1.57-3.26], P < 0.001), age (HR 1.29 [95% CI: 1.10-1.52], P = 0.002), diabetes (HR 1.75 [95% CI: 1.14-2.69], P = 0.011), and arterial hypertension (HR 1.61 [95% CI: 1.03-2.52], P = 0.038) were associated with an increased risk of late mortality (Table 3). After multivariate adjustment, MT was associated with an increased risk of late mortality in these patients (HR 1.99 [95% CI: 1.37-2.88], P < 0.001; Table 3).
Figure 1.
Survival benefit of transcatheter aortic valve replacement (TAVR), compared with medical treatment, in nonagenarian patients. Kaplan-Meier survival curves of nonagenarian patients who underwent TAVR (blue), compared to medical treatment (burgundy). CI, confidence interval; HR, hazard ratio.
Table 3.
Cox proportional hazard models
| Variable | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Medical treatment vs TAVR, age ≥ 90 y | 2.27 (1.57–3.26) | < 0.001 | 1.99 (1.37–2.88) | < 0.001 |
| Age, increment 2 y | 1.29 (1.10–1.52) | 0.002 | 1.32 (1.10–1.57) | 0.002 |
| Female sex | 0.92 (0.64–1.32) | 0.64 | 0.98 (0.67–1.42) | 0.90 |
| NYHA class III/IV vs I/II | 1.34 (0.89–2.00) | 0.16 | — | — |
| History of stroke/TIA | 1.26 (0.81–1.96) | 0.30 | — | — |
| Dyslipidemia | 1.26 (0.86–1.85) | 0.23 | — | — |
| Diabetes | 1.75 (1.14–2.69) | 0.011 | 2.06 (1.29–3.29) | 0.003 |
| Arterial hypertension | 1.61 (1.03–2.52) | 0.038 | 1.89 (1.17–3.03) | 0.009 |
| COPD | 1.41 (0.88–2.26) | 0.16 | — | — |
| Coronary artery disease | 1.07 (0.73–1.54) | 0.75 | — | — |
| Atrial fibrillation/flutter | 0.94 (0.65–1.36) | 0.73 | — | — |
| Creatinine clearance < 60 mL/min | 0.86 (0.43–2.01) | 0.86 | — | — |
| Carotid stenosis | 0.83 (0.39–1.80) | 0.64 | — | — |
| SPAP, increment 5 mm Hg | 0.96 (0.90–1.02) | 0.20 | — | — |
Text in bold highlights the statistically significant associations.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; NYHA, New York Heart Association; SPAP, systolic pulmonary artery pressure; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack.
TAVR patients vs general population late survival
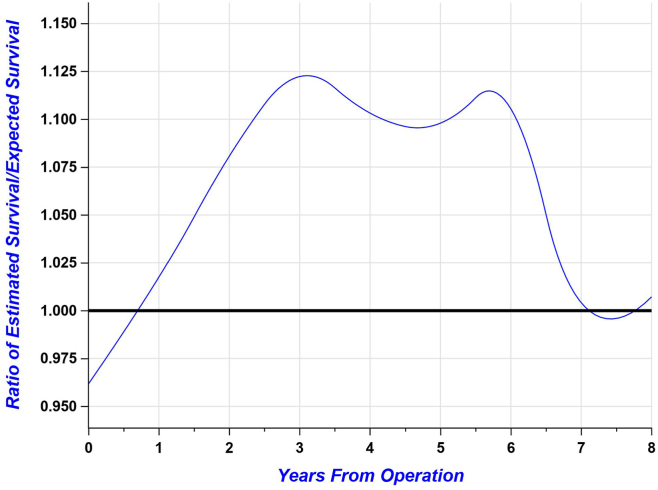
In the secondary analysis comparing TAVR to the general population of nonagenarians in the Canadian province of Quebec, the survival rate post-TAVR was higher than the expected survival rate for the general population when adjusted for year of procedure, age, and gender (Fig. 2), with a cumulative relative mortality of 0.86 (0.75-0.87). Thus, this difference represents the relative “excess” mortality compared to general population, which is thereby < 1. The ratios of the estimated (ie, observed in TAVR patients) survival rate over the expected one (ie, for the general population) according to the years after TAVR are shown in Figure 3.
Figure 2.
Survival comparison of nonagenarian patients undergoing transcatheter aortic valve replacement with the age- and sex-matched general provincial population. Observed survival (black) after the procedure, compared to the expected mortality (blue) of the general population.
Figure 3.
Survival ratio of the observed survival (TAVR treated patients) over expected survival (age- and sex-matched provincial general population) in severe AS patients.
Improvements in quality of life of TAVR nonagenarian patients
In paired analyses, TAVR nonagenarian patients showed significant improvements from baseline to 1-3 months in functional status in the 6MWT (192 ± 84 vs 259 ± 102 m, P < 0.05), and in QoL, as assessed by the KCCQ (64 [52-73] vs 79 [68-91], P < 0.05), the DASI (13 ± 7 vs 16 ± 10, P < 0.05), and various aspects of the EuroQoL-5D questionnaire and the global health state scale (63% ± 16% vs 71% ± 15%, P < 0.05; Table 4). These observations of functional status and QoL improvements were significantly maintained up to 1 year without being significantly different than those at the 1-3 month follow-up.
Table 4.
Functional status and quality of life during follow-up in nonagenarians who underwent TAVR.
| Variable | Baseline | 1–3 mo | 1 y |
|---|---|---|---|
| Functional status | |||
| 6-min walk test distance, m | 192 ± 84 (n = 96) | 259 ± 102∗ (n = 63) | 252 ± 97∗ (n = 56) |
| Quality of life | |||
| KCCQ score | 64 (52–73) (n = 109) |
79 (68–91)∗ (n = 74) |
81 (70–94)∗ (n = 53) |
| DASI score | 13 ± 7 (n = 131) | 16 ± 10∗ (n = 101) | 16 ± 9∗ (n = 108) |
| Euro Quality of Life - 5 Dimensions questionnaire | (n = 131) | (n = 103) | (n = 101) |
| Mobility ≥ 2 | 51 (39) | 46 (45) | 52 (52)∗ |
| Self-care ≥ 2 | 22 (17) | 18 (17) | 21 (21) |
| Activities ≥ 2 | 76 (58) | 44 (43)∗ | 42 (42)∗ |
| Pain ≥ 2 | 47 (36) | 39 (38) | 38 (38) |
| Anxiety ≥ 2 | 28 (21) | 26 (25) | 16 (16) |
| Health state scale, % | 63 ± 16 | 71 ± 15∗ | 74 ± 15∗ |
Values are mean ± standard deviation, median (25th-75th percentile), or EuroQoL-5D score (n).
DASI, Duke Activity Status Index; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Significant paired difference vs baseline, P < 0.05.
Discussion
TAVR improvements observed during the past decades have led to increasing use of this procedure in many subgroups of patients with severe AS. The survival benefit of TAVR in very elderly patients, such as nonagenarians, has been established previously, but questions remain, as advanced age inherently is related to a lower life expectancy, and current guidelines do not provide definitive recommendations regarding this specific population. Although previous studies have demonstrated superiority of TAVR compared to MT in nonagenarians, they were limited by small numbers and the inherent limitations of national databases. Other studies have shown that nonagenarians undergoing TAVR are at increased risk of mortality during hospital admission and at 30 days, compared with younger patients.5,17 Thus, the potential futility of TAVR in the very elderly has been a concern, even as iterative improvements in both TAVR technology and patients’ postintervention outcomes continue to be noted.18 Moreover, QoL improvement and functional status of patients who undergo TAVR remain subjects of interest, especially in elderly patients.19, 20, 21 In fact, these patient-centred outcomes of health status are very important in this elderly population, sometimes as important as survival benefit, and thus could help guide clinicians in their preoperative evaluation.
Survival with TAVR vs MT
This study demonstrated that TAVR is associated with improved survival among nonagenerians, compared to MT, after multivariate adjustment. These results observed in a “real-life” single-centre cohort are consistent with those of previous studies of TAVR in high-risk surgical patients and nonagenarians.6,7,22,23 Nevertheless, nonagenarians remain at higher risk of complications than younger patients, reinforcing the need for a careful patient selection process,7,17,24, 25, 26, 27, 28 including preoperative frailty assessment.29, 30, 31
Survival with TAVR vs in the general population
When comparing survival of TAVR nonagenarian patients with the life expectancy of the general population of the same age, gender, and according to the year the TAVR was performed, we demonstrated a survival benefit of up to 12.5% at approximately 3 years. These results suggest that TAVR may confer an improvement in cardiovascular health status that translates into better long-term functional capacity and survival. Zadrozny et al. previously showed similar 2-year survival in the general population vs nonagenarians, but they used an average mortality and not a direct age-, sex-, and year of referral-matched comparison.32 This difference is a potential explanation for the current results, as improvements in TAVR technology during the years may have contributed to creating this survival benefit. Another possible explanation for these results may be related to the fact that the nonagenarians undergoing TAVR are highly selected and are those with few life expectancy–limiting comorbidities in addition to the AS. As shown by a number of studies, nonagenarians undergoing TAVR have lower surgical risk scores (EuroSCORE II and Society of Thoracic Surgery Predicted Risk of Mortality [STS-PROM]) than younger patients undergoing TAVR.7,17 Nonagenarians undergoing TAVR have a lower prevalence of lung disease, diabetes, coronary artery disease, and obesity than younger patients, but they have a higher rate of heart failure and slightly more severe echocardiographic criteria for AS.7,17 Further studies are required to confirm these observations in other populations, but nonetheless, nonagenarians seem to have reasonable survival with TAVR if selected appropriately by a multidisciplinary heart team.
QoL improvements
We have shown an improvement in QoL objective indices among nonagenerians undergoing TAVR. Few studies have demonstrated an improvement in either KCCQ score or an adapted version of the EuroQol-5D questionnaire, but these were performed in smaller numbers of patients.21,33 Moreover, as suggested in a study on cardiac resynchronization therapy,34 older patients should not be refused an optimal therapy based solely on their age and the limited potential survival benefit associated with advanced age. For nonagenarians, QoL and functional status improvements may result in a longer time period with autonomy. Improvements in mobility and general state of health, as demonstrated in our study, are therefore of foremost importance in nonagenarians. Frailty in nonagenarians remains a concern because TAVR complications can have a devastating impact on the ability of these patients to perform daily living activities. Thus, careful consideration prior to TAVR referral needs to address the potential futility of the procedure in a given patient. Even though, in the present study, we demonstrate survival benefit and improvement in QoL indices, in some other cases, the preoperative cognitive impairments, comorbidities, and frailty status may outweigh the potential benefit of TAVR, in terms of symptoms improvement. Moreover, potential new-onset arrhythmias/conduction deficits or other complications (eg, stroke, acute kidney injury) related to TAVR could lead to a rapid decline in functional and QoL status in a nonagenarians patient treated with TAVR. Nonetheless, our study suggests that TAVR can be performed safely in well-selected and mildly frail nonagenarians, with a low incidence of procedural complications.
Limitations
Due to the retrospective nature of the study, a selection bias for nonagenarian patients undergoing TAVR cannot be entirely ruled out, as the patients who were more likely to survive were probably directed to TAVR after referral to the heart team, compared to patients directed to MT. However, a near-60% survival rate at 5 years among nonagenarian TAVR patients, and improved survival compared to an age- and gender-matched general population of nonagenarians, suggests that the selection process of an expert heart team is associated with excellent outcomes in this specific patient subgroup. Frailty assessment was available only for TAVR patients and was accomplished mostly by the “eyeball” test, which limits our assessment of the differences in risk profile between MT and TAVR patients in this specific population, but further studies should make a direct comparison of frailty between these 2 groups and observe the evolution of frailty status during follow-up. Although the Rockwood Clinical Frailty Scale was also available in ∼50% of our patients, future studies should use more accurate and reliable quantitative frailty measures, given the known limitations of the “eyeball” assessment.35 Measures of QoL significantly improved post-TAVR, but unfortunately, these were not available for all patients. Nonetheless, paired analyses were used to assess evolution between follow-ups. However, the completeness of the follow-up varies between questionnaires. Patients whose QoL improved the most have a higher probability of participating in such evaluations, which could have artificially enhanced the observed benefit with TAVR. Also, the provincial life-expectancy curves were able to be adjusted for only age and gender. Thus, some important comorbidities, frailty, and potential terminal diseases in the general population were not accounted for in the latter analysis.
Conclusion
Among mildly frail nonagenarians, TAVR is associated with a significant survival benefit, compared with MT, as well as significant improvement in several QoL indices and function (ie, 6MWT score). These patients experience a slightly better survival rate, compared to that of an age- and sex-matched general population. On the basis of these data and previous reports, nonagenarians should not be denied TAVR based solely on age.
Acknowledgements
The authors thank Serge Simard, Yasmine Babaki, and Mélanie Côté for administrative and statistical analysis support.
Ethics Statement
The study was approved by our institutional research ethics board who waived individual patient consent due to the retrospective nature of the study (approval number 2021–3627, 22050).
Patient Consent
Waived due to retrospective nature of this study.
Funding Sources
J.B. is supported by a doctoral scholarship from the Canadian Institutes of Health Research (CIHR, Ottawa, Canada). J.R.-C. holds the Famille Jacques Larivière Chair in Structural Heart Diseases.
Disclosures
J.R.-C. has received institutional research grants from Medtronic, Edwards Lifesciences, and Boston Scientific. S.M. has received institutional research grants from Edwards Lifesciences and Abbott, and has served as clinical proctor for Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest to disclose.
Footnotes
See page 790 for disclosure information.
References
- 1.Statistics Canada . Statistics Canada; 2017. A portrait of the population aged 85 and older in 2016 in Canada. Census in Brief.https://www12.statcan.gc.ca/census-recensement/2016/as-sa/98-200-x/2016004/98-200-x2016004-eng.cfm Available at: [Google Scholar]
- 2.Kapadia S.R., Leon M.B., Makkar R.R., et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–2491. doi: 10.1016/S0140-6736(15)60290-2. [DOI] [PubMed] [Google Scholar]
- 3.Leon M.B., Smith C.R., Mack M.J., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 4.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 5.Mentias A., Saad M., Desai M.Y., et al. Temporal trends and clinical outcomes of transcatheter aortic valve replacement in nonagenarians. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thourani V.H., Jensen H.A., Babaliaros V., et al. Outcomes in nonagenarians undergoing transcatheter aortic valve replacement in the PARTNER-I trial. Ann Thorac Surg. 2015;100:785–792. doi: 10.1016/j.athoracsur.2015.05.021. discussion 793. [DOI] [PubMed] [Google Scholar]
- 7.Deharo P., Bisson A., Herbert J., et al. Outcomes in nonagenarians undergoing transcatheter aortic valve implantation: a nationwide analysis. EuroIntervention. 2020;15:1489–1496. doi: 10.4244/EIJ-D-19-00647. [DOI] [PubMed] [Google Scholar]
- 8.Elgendy I.Y., Mahmoud A.N., Elbadawi A., et al. In-hospital outcomes of transcatheter versus surgical aortic valve replacement for nonagenarians. Catheter Cardiovasc Interv. 2019;93:989–995. doi: 10.1002/ccd.28050. [DOI] [PubMed] [Google Scholar]
- 9.Crum R.M., Anthony J.C., Bassett S.S., Folstein M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 10.Church S., Rogers E., Rockwood K., Theou O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20:393. doi: 10.1186/s12877-020-01801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afilalo J. The Clinical Frailty Scale: upgrade your eyeball test. Circulation. 2017;135:2025–2027. doi: 10.1161/CIRCULATIONAHA.116.025958. [DOI] [PubMed] [Google Scholar]
- 12.Mishra R.K., Yang W., Roy J., et al. Kansas City Cardiomyopathy Questionnaire score is associated with incident heart failure hospitalization in patients with chronic kidney disease without previously diagnosed heart failure: Chronic Renal Insufficiency Cohort Study. Circ Heart Fail. 2015;8:702–708. doi: 10.1161/CIRCHEARTFAILURE.115.002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diez C., Koch D., Kuss O., et al. Risk factors for mediastinitis after cardiac surgery - a retrospective analysis of 1700 patients. J Cardiothorac Surg. 2007;2:23. doi: 10.1186/1749-8090-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer M.T.D., Goldsmith K.A., Sharples L.S., Buxton M.J. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13. doi: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics Canada. Table 13-10-0114-01 Life expectancy and other elements of the complete life table, three-year estimates, Canada, all provinces except Prince Edward Island. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011401&request_locale=en Available at:
- 16.Kappetein A.P., Head S.J., Généreux P., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 17.Vlastra W., Chandrasekhar J., Vendrik J., et al. Transfemoral TAVR in nonagenarians. JACC Cardiovasc Interv. 2019;12:911–920. doi: 10.1016/j.jcin.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:35–71. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 19.Baron S.J., Arnold S.V., Wang K., et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837–845. doi: 10.1001/jamacardio.2017.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold S.V., Spertus J.A., Vemulapalli S., et al. Quality-of-life outcomes after transcatheter aortic valve replacement in an unselected population: a report from the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017;2:409–416. doi: 10.1001/jamacardio.2016.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalili H., Bansal P., Al Taii H., et al. Quality of life outcomes after transcatheter aortic valve replacement in nonagenarians. J Invasive Cardiol. 2020;32:375–379. doi: 10.25270/jic/20.00027. [DOI] [PubMed] [Google Scholar]
- 22.Bernal E., Ariza-Solé A., Bayés-Genís A., et al. Management of nonagenarian patients with severe aortic stenosis: the role of comorbidity. Heart Lung Circ. 2018;27:219–226. doi: 10.1016/j.hlc.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Elgendy I.Y., Mahmoud A.N., Elbadawi A., et al. Trends of uptake and in-hospital mortality for transcatheter aortic valve implantation versus surgical aortic valve replacement in nonagenarians. Am J Cardiol. 2019;123:703–705. doi: 10.1016/j.amjcard.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Stehli J., Koh J.Q.S., Duffy S.J., et al. Comparison of outcomes of transcatheter aortic valve implantation in patients aged > 90 years versus < 90 years. Am J Cardiol. 2019;124:1085–1090. doi: 10.1016/j.amjcard.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Du Y., Fu M., et al. Clinical outcomes of transcatheter aortic valve replacement in nonagenarians: a systematic review and meta-analysis. J Interv Cardiol. 2019;2019 doi: 10.1155/2019/5819232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vendrik J., van Mourik M.S., van Kesteren F., et al. Comparison of outcomes of transfemoral aortic valve implantation in patients < 90 with those > 90 years of age. Am J Cardiol. 2018;121:1581–1586. doi: 10.1016/j.amjcard.2018.02.056. [DOI] [PubMed] [Google Scholar]
- 27.Scholtz S., Dimitriadis Z., Vlachojannis M., et al. Transcatheter aortic valve implantation in nonagenarians: procedural outcome and mid-term results. Heart Lung Circ. 2018;27:725–730. doi: 10.1016/j.hlc.2017.05.137. [DOI] [PubMed] [Google Scholar]
- 28.Okoh A.K., Chauhan D., Kang N., et al. The impact of frailty status on clinical and functional outcomes after transcatheter aortic valve replacement in nonagenarians with severe aortic stenosis. Catheter Cardiovasc Interv. 2017;90:1000–1006. doi: 10.1002/ccd.27083. [DOI] [PubMed] [Google Scholar]
- 29.Arnold S.V., Zhao Y., Leon M.B., et al. Impact of frailty and prefrailty on outcomes of transcatheter or surgical aortic valve replacement. Circ Cardiovasc Interv. 2022;15 doi: 10.1161/CIRCINTERVENTIONS.121.011375. [DOI] [PubMed] [Google Scholar]
- 30.Rajabali N., Rolfson D., Bagshaw S.M. Assessment and utility of frailty measures in critical illness, cardiology, and cardiac surgery. Can J Cardiol. 2016;32:1157–1165. doi: 10.1016/j.cjca.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Shimura T., Yamamoto M., Kano S., et al. Impact of the Clinical Frailty Scale on outcomes after transcatheter aortic valve replacement. Circulation. 2017;135:2013–2024. doi: 10.1161/CIRCULATIONAHA.116.025630. [DOI] [PubMed] [Google Scholar]
- 32.Zadrozny M., Hainzer N., Mehilli J., et al. TAVR in nonagenarians: an analysis investigating safety, efficacy, symptomatic improvement, and long-term survival. J Cardiol. 2021;78:44–50. doi: 10.1016/j.jjcc.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Stańska A., Jagielak D., Brzeziński M., et al. Improvement of quality of life following transcatheter aortic valve implantation in the elderly: a multi-centre study based on the Polish national TAVI registry. Kardiol Pol. 2017;75:13–20. doi: 10.5603/KP.a2016.0164. [DOI] [PubMed] [Google Scholar]
- 34.Rickard J., Cheng A., Spragg D., et al. Survival in octogenarians undergoing cardiac resynchronization therapy compared to the general population. Pacing Clin Electrophysiol. 2014;37:740–744. doi: 10.1111/pace.12337. [DOI] [PubMed] [Google Scholar]
- 35.McDonagh J., Prichard R., Ferguson C., et al. Clinician estimates of frailty compared to formal frailty assessment in adults with heart failure: a cross-sectional analysis. Heart Lung Circ. 2022;31:1241–1246. doi: 10.1016/j.hlc.2022.04.003. [DOI] [PubMed] [Google Scholar]