Abstract
Epilepsy is a disease characterized by the periodic occurrence of seizures. Seizures can be controlled by antiseizure medications, which can improve the lives of individuals with epilepsy when given proper treatment. Therefore, this study aimed to review the scientific literature on brain neuroplasticity after treatment with antiseizure drugs in different regions of the brain. According to the findings, that several antiseizure, such as lamotrigine, diazepam, levetiracetam, and valproic acid, in addition to controlling seizures, can also act on neuroplasticity in different brain regions. The study of this topic becomes important, as it will help to understand the neuroplastic mechanisms of these drugs, in addition to helping to improve the effectiveness of these drugs in controlling the disease.
Keywords: Neuroplasticity, Neuronal plasticity, Anticonvulsant drugs, Antiepileptic, Seizures, Epilepsy.
INTRODUCTION
Epilepsy affects about 70 million people worldwide, it is a chronic neurological disease. The prevalence of this pathology is observed worldwide, with underdeveloped countries being the most susceptible [1]. It is a pathology that is related to recurrent seizures that, in some cases, require lifelong treatment [2].
Epilepsy affects people of both sexes of all ages; however, it occurs more frequently in men. People with epilepsy tend to have more physical problems due to trauma linked to seizures and higher rates of psychosocial disorders such as anxiety and depression. The risk of dying prematurely is also notable, being up to three times higher when compared to individuals in the general population [3]. Seizures can be triggered by an imbalance of neurotransmitters, in addition to changes in the expression of receptors and ion channels [4]. Such seizures encompass abnormal alterations in the neuronal electrical mechanism and are associated with an imbalance of excitatory and inhibitory actions in brain networks. Glial cells also play a role in the generation of seizures, as they act in the modulation of neural functions [5].
Medications that treat seizures are often called antiseizure medications. The mainstay of epilepsy treatment is antiseizure drugs, which may also be called antiepileptic drugs. Studies show that antiseizure has provided an improvement in the lives of epileptic individuals. Most patients can live a seizure-free life when they receive proper treatment. However, antiseizure work to control seizures, but they do not cure epilepsy. The goal of antiseizure drug therapy is to prevent seizures and reduce side effects. These drugs can provide some morphological, biochemical, and functional changes and, therefore, can promote or decrease neuroplasticity in different regions of the brain [6].
Neuroplasticity can be conceptualized as the brain’s ability to form new connections, due to intrinsic or extrinsic stimuli or in response to sensory stimuli, damage, or dysfunction. It acts in the reorganization of neuronal functions, aiming at rapid adaptation and self-repair. Neuroplasticity can be adaptive when it is related to the gain of function, and maladaptive when it is related to the loss of function or increase in injuries. The neuroplasticity mechanism can be observed in the clinic, presenting an important function, and may be associated with the mechanism of structural and functional recovery in different clinical contexts [7,8]. Therefore, this study aimed to review the scientific literature on brain neuroplasticity after treatment with antiseizure drugs in different regions of the brain.
To carry out this study, the search bases PubMed, Portal Capes, Google Scholar, and Scielo were used. Studies were selected only in English and without the restriction of dates. Only those that were related to the action of antiseizure in the process of neuroplasticity were considered valid for discussion in this work.
EPILEPSY
Epilepsy is a neurological pathology that affects millions of individuals worldwide. This disease has as its main feature, the recurrence of spontaneous seizures due to brain hyperactivity. Such crises can impair the physical, mental, or behavioral functioning of individuals with this disease [9-11].
Convulsive seizures can be generated by the synchronous firing of a situated group of neuronal cells, which are called epileptic foci [12]. Epilepsy can be conceptualized as the occurrence of two or more unprovoked seizures occurring more than 24 hours apart. Most seizures end after 2 to 3 minutes, however, when the brain’s inhibition mechanisms fail, seizures can be prolonged. After 5 minutes of seizure, the status epilepticus term is applied [3,5,11].
Status epilepticus can be defined as a condition that results from the failure of mechanisms responsible for ending seizures or for initiating mechanisms that stimulate abnormally prolonged seizures. This condition can promote long-term consequences, including injury and death of neurons, as well as changes in neuronal networks depending on the type and time of seizures [13]. Epilepsies can be generalized or focal. Generalized seizures affect both cerebral hemispheres, while focal seizures occur in a small region of the cerebral cortex or a deep region of the brain. There are about 1,000 genes related to this patho-logy. The inhibitory action mediated by g-aminobutyric acid (GABA) and the excitation mediated by glutamate constitutes the basis of the disease [14].
ANTISEIZURE IN THE TREATMENT OF EPILEPSY
The use of antiseizure in the treatment of epilepsy is widespread. These drugs were designed to suppress neuronal hyperexcitability and thus suppress epileptic seizures [15]. These drugs consist of “symptomatic” agents, inhibit the symptoms of the disease, and are considered a key factor in the treatment of this pathology. Antiseizure are classified according to the Anatomical Therapeutic Chemical Classification System (ATC), in this system, drugs are divided according to their therapeutic, chemical characteristics and sites of action and are classified based on their most important therapeutic use, in principle basics of the ATC code, however, a medicine can present more than one ATC code. The ATC codes of the antiseizure listed in this study are described in Table 1 [16].
Table 1.
Antiseizure drugs and ATC code
| Anticonvulsants | ATC code |
|---|---|
| Carbamazepine | N03AF01 |
| Diazepam | N05BA01 |
| Lamotrigine | N03AX09 |
| Levetiracetam | N03AX14 |
| Phenobarbital | N03AA02 |
| Phenytoin | N03AB02 |
| Primidone | N03AA03 |
| Topiramate | N03AX11 |
| VPA | N03AG01 |
ATC, Anatomical Therapeutic Chemical Classification System; VPA, valproic acid.
Modern treatment of epilepsy began with the use of potassium bromide [17]. Since the discovery of the first antiseizure bromide in 1857, several antiseizure have been developed, such as phenobarbital, phenytoin, primidone, and many others. In recent years, a range of new antiseizure drugs and non-pharmacological remedies have been introduced in the treatment of epilepsy. Such drugs are designed to treat specific pathophysiological deficiencies, such as; the dissemination or generation of convulsive crises, in which the old drugs are no longer useful. However, even with the availability of all antiseizure drugs, whether new or old, coupled with the arrival of new techniques, seizures are specifically difficult to treat [1].
The development of antiseizure drugs was based on screening activities against seizures in acute seizure types, more specifically maximal electroshock and Pentylen-etetrazol [18]. Initially, drugs such as phenobarbital, carbamazepine, benzodiazepines, and valproic acid (VPA) were introduced to treat people with epilepsy. After the 1990s, new generation drugs were developed, such as lamotrigine, topiramate, levetiracetam, and others. These new drugs have some advantages, such as fewer side effects, in addition to improved pharmacokinetic properties [19]. However, old generation antiseizure drugs, such as phenobarbital, primidone, phenytoin carbamazepine, and benzoadiazepine, are efficient and widely used drugs, however, they have significant adverse effects, in addition to failing to control seizures [1].
The antiseizure effect of these drugs can be achieved by modifying neuronal burst properties and reducing synchronization in neuronal assemblies. The antiseizure activity of some drugs may also be associated with neuronal or GABA receptor modulation, considered the main inhibitory neurotransmitter. Antiseizure acts on several molecular targets to selectively alter neuronal excitability so that the trigger associated with the seizure is blocked [20]. Targets for antiseizure include ion channels, neurotransmitters, as well as enzymes that metabolize neuro-transmitters. Voltage-gated ion channels are considered molecular targets of numerous antiseizure drugs. Such ion channels include sodium, calcium, and potassium chan-nels. Drugs with antiseizure activities inhibit or increase ionic currents through the channels [21]. For this purpose, antiseizure are usually prescribed according to the following categories: (I) drugs that block the sodium channel; (II) drugs that activate GABA; (III) drugs that block the calcium channel; (IV) drugs that inhibit glutamate receptors [1].
Phenobarbital, one of the first barbiturates introduced, works by affecting the duration and intensity of seizures and causes a sedative effect. This drug causes an increase in GABA neurotransmission. Phenytoin, another drug used, is considered the first non-sedative drug. This drug affects membrane excitability in voltage-gated sodium channels [22]. However, one of the most used drugs in seizures is carbamazepine. Its action occurs through the inhibition of calcium and sodium channels. Another drug is VPA, which acts by increasing the action of GABA and inhibiting sodium channels. VPA, among several functions, acts by potentiating GABAergic function, increasing GABA release and reducing its catabolization, and increasing the density of GABA type B receptors. Another drug used is Lamotrigine, which is an antiseizure derived from phenyltriazine and is used in the treatment associated with seizures. Its action occurs by blocking voltage-sensitive sodium and calcium channels [23].
NEUROPLASTICITY
In the past, it was believed that the mammalian central nervous system (CNS) was a completely stable structure, from birth, implying that it was a rigid structure and incapable of being modified. However, over the years, it has been proven that the brain is modifiable from the moment it is stimulated. The first to speak of neuroplasticity was researcher William James, about 120 years ago. He mentioned that the human brain can change continuously [24]. However, the first to define the term “neuro-plasticity” was the neuroscientist Jerzy Konorski, in 1948, and the first to demonstrate neuroplasticity in real cases was the researcher Paul Bach-y-Rita, who stated that healthy regions of the brain can assume functions of injured parts [25].
The brain is a structure with some flexibility that can be adjusted throughout life, through new experiences [26]. Neuroplasticity is a property of the nervous system (SN) that can be defined as the ability to change in response to a lived experience, that is, it is an adaptive capacity of the SN to various changes in environmental conditions. Some research indicates that the production, differentiation, and survival of new neuronal cells consist of neuroplastic mechanisms, regulated by experience [27]. Every time some mechanism of energy or information from the environment, both external and internal, affects the SN, some marks remain, providing it with a certain alteration. As this mechanism happens daily, neuroplasticity becomes a constant feature of neurological actions. The concept of neuroplasticity is based on permanent changes in SN cells, and such changes occur due to stimuli that may be environmental or even some damage to nerve cells [28]. The purpose of neuroplasticity is to remodel, change and reorganize [25]. Neuroplasticity occurs with greater intensity in the early stages of life. In adults, neuroplasticity is slower and limited; however, when stimuli such as, for example, a learning task or even a physical exercise occur, neuroplasticity can increase in intensity. Throughout life, neural pathways and circuits remain plastic and undergo modifications over the years, involving the challenge of repairing the damage that can be caused to the NS [6,28].
Neuroplasticity allows the brain to form new connections, due to intrinsic or extrinsic stimuli or in response to sensory stimulation, damage, or dysfunction. Neuroplasticity acts in the reorganization of neuronal functions, aiming at rapid adaptation and self-repair [29]. Neuroplastic alterations in the NS are consequences arising from the natural development of an individual, but they can also be the result of many types of damages and injuries, lived experiences resulting from environmental stimuli, in addition to learning and memory processes. The neuroplasticity process can positively or negatively influence individuals of all ages and throughout life [30]. Neuroplasticity is of real importance in the process of brain adaptation, and this, when considered maladaptive, contributes to disorders such as depression, and post-traumatic stress disorder. It is a very complex process and its underlying mechanism still needs to be better understood. However, it is understood that neuroplasticity encompasses functional and morphological adaptations [31]. Therefore, neuroplasticity is multiple physiological mechanisms that are general to the biology of the brain, but which are also particular to each network of neurons or microenvironments. However, such a mechanism represents a complex subject that requires the involvement of processes and basic components of biochemistry, since this process is not only due to structural alterations of a set of dendrites but to intra and extracellular adaptive processes which fulfill more of a signaling pathway at the molecular level [27].
MOLECULAR MECHANISMS OF NEUROPLASTICITY
Regarding the molecular mechanism of neuroplasticity, evidence indicates that neuroplasticity arises from a series of molecular events, which are interrelated to specific neuroplastic events [32]. The basis of neuroplasticity is constituted by molecules and their interactions, which are behind the subcellular, synaptic, etc. Molecular events related to the neuroplasticity process are organized into structural, which consists of neurogenesis and formation of the dendritic column, and functional, which encompasses changes in the release of chemical mediators, receptor sensitivity, and activation of postsynaptic neuronal mechanisms [33].
Neuronal functions, including synaptic neuroplasticity, require proper regulation of proteins, which are primarily controlled by phosphorylation and dephosphorylation mechanisms. Therefore, broadly specific enzymes and proteins play an important role in brain neuroplasticity [32]. Brain-derived neurotrophic factor (BDNF), belonging to the neurotrophin family, is a key factor in the neuroplastic mechanism, as it acts on neuroplasticity, and structural changes and acts on synapses, as it is responsible for positively regulating the synthesis of proteins related to brain changes synaptic [34].
According to the molecular view, one of the essential points for carrying out the mechanism of neuroplasticity by Long-Term Potentiation (LTP) or Long-Term Depres-sion (LTD) is the concentration and cellular handling of Ca2+. LTP normally requires the presence of N-methyl D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-iso-xazolepropionic acid, and kainate receptors. LTD, in turn, requires the presence of Ca2+ channels, and NMDA receptors, in addition to metabotropic glutamate-type channels [27]. LTP is dependent on Ca2+ entry through NMDA receptors of the postsynaptic neuron, as well as protein kinase II autophosphorylation processes, which are Ca2+ dependent. The increase in Ca2+ levels within astrocytes is dependent on the action of the neuron and provides for the release of several gliotransmitters in the synapse, enabling mechanisms to control synaptic activity [33].
Astrocytes express metabotropic and ionotropic receptors, which are activated through the release of neurotransmitters such as norepinephrine, acetylcholine, and glutamate [6]. Neuroplasticity processes do not only occur at the intraneuronal and intersynaptic level but can also occur in the extracellular environment, such as the induction of cell adhesion molecules and neuroplastic mechanisms linked to astrocytes. Neuroplasticity, which is more in line with biology at the neuronal and interneuronal level, needs the participation of neuroglia that perform actions of neovascularization, energy level regulation, metabolic modulation, astrocytic regulation of calcium current for synaptogenesis, in addition to neuron signaling. Thus, it is observed that there are neuroplastic mechanisms of extraneuronal origin [27].
ANTISEIZURE AND NEUROPLASTICITY
Some chemical substances, when in contact with brain regions, can promote morphological and/or biochemical changes. These alterations can be beneficial or harmful to the CNS [35]. Antiseizure acts on the CNS by several specific mechanisms, such mechanisms provoked by these drugs, can affect neuroplasticity, increasing or decreasing the processes of neurogenesis, synaptogenesis, and synaptic neuroplasticity, that is, antiseizure re capable of promoting increased neuroplasticity or even reduce neuroplasticity in certain areas of the brain [36].
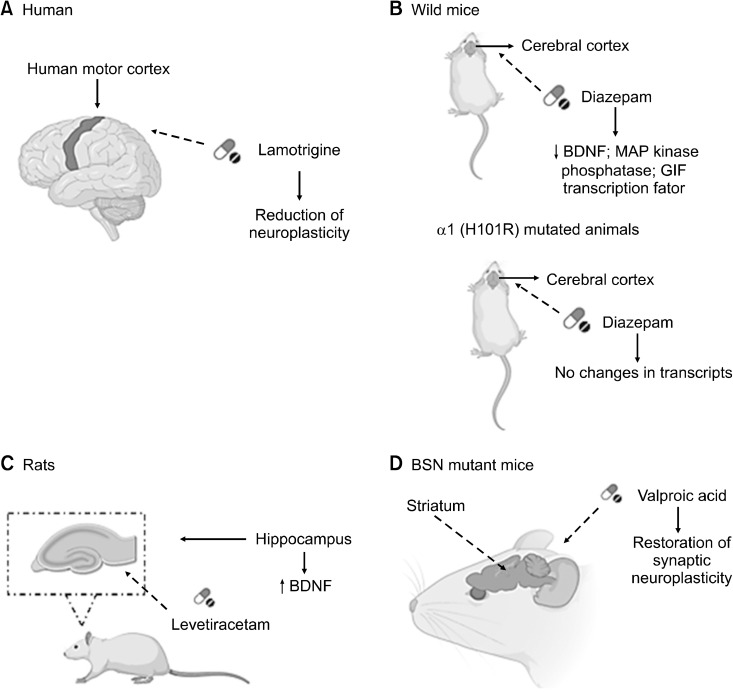
A study was conducted on the effects of Lamotrigine (LTG) on human motor cortex neuroplasticity induced by PAS25 (Paired Associative Stimulation), which is a protocol used to study synaptic neuroplasticity in the form of LTP and LTD. In this study, a single dose of LTG (300 mg) was used to test the motor cortical neuroplasticity induced by PAS25. For this research, volunteers who had no history of neurological diseases and were not using any active medication for the CNS at the time of the study were used. In this study, the following tests were performed: electromyographic recording to record the motor evocations of the abductor pollicis brevis muscle; magnetic stimulation, and paired associative stimulation. The authors mention that in people with an LTP-like response to PAS25, administration with LTG reduced the LTP-like neuroplasticity induced by PAS25. According to the authors of this study, a possible explanation for these effects of LTG on neuroplasticity similar to LTP is the depressant action of this antiseizure drug on corticospinal excita-bility. The reduction in the baseline excitability of the motor cortex is possibly due to the blockade of voltage-gated sodium and calcium channels by LTG. Thus, it was observed that the individual Resting Motor Threshold (MT) is inversely associated with the magnitude of LTP-like neuroplasticity induced by PAS. In this way, the reduction in cortical excitability induced by this drug, indexed by an elevation in MT, may have decreased the likelihood of inducing LTP-like neuroplasticity. In general, LTG differentially modulated cortical neuroplasticity induced by non-invasive brain stimulation in healthy humans, depending on their individual intrinsic disposition to manifest neuroplasticity in the form of LTP or LTD after paired associative stimulation. In individuals who showed a similar response to LTP induced by PAS25, LTG reduced neuroplasticity, the same occurring with individuals who showed a similar response to LTD. The authors also mention that LTG reduced glutamate release (Fig. 1A) [37].
Fig. 1.
(A) Administration with Lamotrigine (300 mg) provided a reduction in neuroplasticity in the human motor cortex. (B) In wild- type mice, administration with dia-zepam (30 mg/kg) provided a reduc-tion in transcript expression levels related to the neuroplastic effect, however, in α1 (H101R) mutated animals, these changes were not observed. (C) Levetiracetam (40 mg/kg) administration enabled an increase in BDNF expression in the rat hippo-campus. (D) Valproic acid (400 mg/kg) treatment in BSN mutant mice made it possible to restore synaptic neuro-plasticity in the striatum.
BDNF, brain-derived neurotrophic factor; MAP, mitogen activated protein; BSN, bassoon.
One study evaluated the possible alterations in the levels of transcription, in the cerebral cortex, induced by the acute administration of diazepam in wild-type and point- mutated α1 mice (H101R). The animals received diazepam intraperitoneally (30 mg/kg). Several cortical areas were analyzed by microarrays and the RNA by real-time PCR. In their findings, it was observed that in wild animals, diazepam reduced the expression levels of calmodulin and BDNF, mitogen activated protein kinase phos-phatase, GIF transcription factor, c-fos, and gene-A, but none of these transcripts was altered in α1 (H101R) mutant animals after diazepam administration. Therefore, the sedative effect promoted by this drug is related to the selective negative regulation of these transcripts, which are involved in neuroplasticity and neurotrophic responses (Fig. 1B) [38].
A study whose objective was to analyze gene expression in the hippocampus of rats, which suffered seizures induced by the kindling model, an animal model used for the development of seizures and epilepsy, and to examine the effect of levetiracetam (40 mg/kg) in these animals. Mice underwent Kindling and received levetiracetam and then gene expression assays were performed. Research has shown that there was an expression of many genes, which are related to synaptic neuroplasticity, including BDNF. The authors of this study suggest that levetiracetam may promote attenuation of gene expression, which is known to provide synaptic regulation and remodeling (Fig. 1C) [39].
As for the striatum, one study aimed to analyze the TrkB/BDNF system and its involvement in striated modifications, which would be associated with the mutation of the bassoon (BSN) gene. Behavioral and neuroplastic observations were made in BSN mutant mice and to find out whether such changes would be dependent on seizure activity, TrKB levels were measured, and BDNF distribution was evaluated in the striatum of epileptic mice treated with VPA (400 mg/kg). The results showed that treatment with VPA caused a reduction in epileptic seizures, inhibited the appearance of pathological forms in fast-spiking interneurons, and provided the restoration of synaptic neuroplasticity of the corpus striatum (Fig. 1D) [40].
In addition to its antiseizure effect, VPA can also be considered an epigenetic drug, as it directly inhibits histone deacetylases (HDACs), belonging to a family of enzymes that act in the modification of the N-terminal tails of histones, modifying the interaction between histones and DNA, acting as an epigenetic regulator of gene expression [41]. In addition to inhibiting HDAC, VPA also acts on chromatin structural modification and gene ex-pression. Such mechanisms of epigenetic modifications can affect neurogenesis and reduce BDNF expression, which is closely related to the neuroplasticity process [42-44].
Bearing in mind that VPA is an antiseizure, in the clinic it is also used by women with epilepsy during pregnancy, however, the use of VPA during pregnancy is capable of promoting changes in the proliferation/differentiation of progenitor cells’ neural networks, in addition to inhibiting HDACs, causing neocortical dysgenesis [45]. Evidence indicates that transient exposure to VPA in the offspring’s brain in mouse models in the embryonic phase, suppresses neurogenesis after growth, negatively interfering with learning and memory. In this way, it is understood that the inhibition of histone deacetylation in the embryonic stage by VPA affects brain development, specifically in neural stem cells, impairing quantitative and qualitative neurogenesis [46].
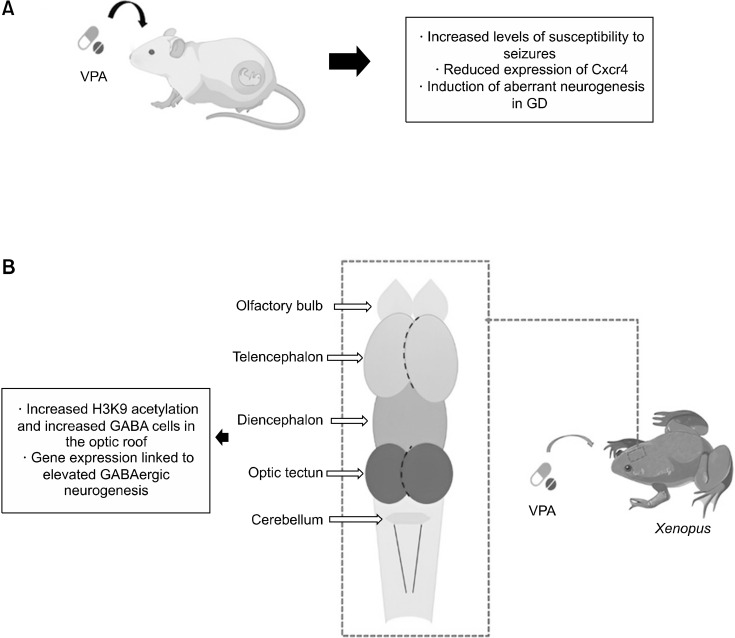
Using a mouse model, one study investigated the relationship between maternal use of VPA and increased susceptibility to seizures in the offspring. In this study, it was reported that prenatal exposure to VPA in mice increases levels of susceptibility to seizures in adult offspring, due to the incorrect location of newborn neurons in the hippocampal structure. The authors report that newly generated neurons by neural stem/progenitor cells (NS/PCs) integrate into the granule cell layer in the adult hippocampus, however, prenatal treatment with VPA promoted changes in the expression of genes related to cell migration in NS/PCs, thus increasing the abnormal location of newborn neurons in the hilum. The authors also report that VPA is capable of inducing changes in the expression of several genes, as an epigenetic drug, therefore, the prenatal exposure to VPA was able to induce aberrant neurogenesis in the DG of adult mice. It was therefore observed that VPA promoted changes in gene expression in NS/PCs in neurons. Therefore, the study showed that exposure to VPA in the prenatal period interferes with the migration of neurons in the DG of adult mice since there was a reduction in the expression of Cxcr4 in NS/PCs and, therefore, increased levels of susceptibility to seizures. However, the authors injected into the gyrus dentatus (GD) of mice that were exposed to VPA, retroviruses that expressed Cxcr4. With this, they observed that the susceptibility to seizures and aberrant neurogenesis were suppressed in these animals, that is, the replacement of Cxcr4 in NPCs enabled an adequate migration, thus neutralizing the high seizure sensitivity due to prenatal exposure to VPA (Fig. 2A) [47].
Fig. 2.
(A) Prenatal stage mice were treated with VPA. Prenatal exposure to this drug promoted increased levels of susceptibility to seizures, reduced expression of Cxcr4, and induction of aberrant neurogenesis in GD. (B) A study carried out in Xenopus treated with VPA, showed that the application of this drug promoted increased H3K9 acety-lation and increased GABA cells in the optic roof, and gene expression linked to elevated GABAergic neuro-genesis.
VPA, valproic acid; GD, gyrus dentatus; GABA, γ-aminobutyric acid.
One study investigated the process of epigenetic regulation of proliferation and differentiation of GABAergic neurons in the developing brain of Xenopus, treated with VPA. According to the authors, Xenopus is considered a reliable in vivo animal model, widely used for developmental research of the brain, neural neuroplasticity processes, and neurological diseases. In this study, it is possible to observe that numerous factors are able to affect the process of GABAergic neurogenesis. According to the researchers, the amount of GABAergic neurons undergoes dynamic modifications during the initial development in the optic tectum of these animals. Treatment with VPA, which inhibits HDAC, increases H3K9 acetylation and the amount of GABA cells in the optic tectum, in addition, the application of VPA reduces apoptotic cells and, according to electrophysiological records, VPA is capable of inducing an increase in the frequency of Minia-ture Inhibitory Postsynaptic Currents (mIPSCs) and no change in amplitude. With regard specifically to the neurogenesis process, the authors mention that VPA acts directly on HDACs to induce changes in the transcription machinery, resulting in gene expression linked to high GABAergic neurogenesis (Fig. 2B) [48].
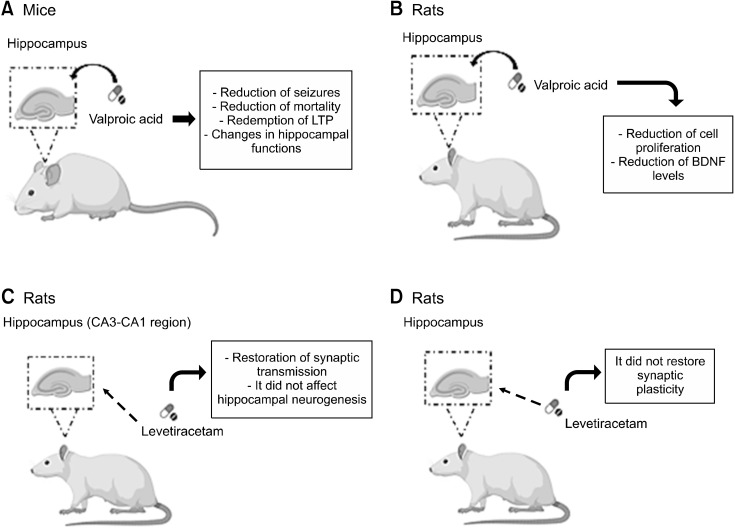
Still regarding VPA, a study evaluated the neuroplasticity and synaptic morphology of the hippocampus in a genetic model of epilepsy after treatment with VPA (400 mg/kg). For this, wild-type mice and mice lacking the Bassoon presynaptic protein were used as a model of epilepsy. The authors concluded in this study that treatment with VPA provides beneficial and deleterious actions on the functions of the hippocampus since early treatment with this drug can promote a reduction in seizures and mortality, in addition to rescuing LTP from the hippocampus in epileptic mice, for example. On the other hand, this drug is also capable of causing morphological abnormalities and memory dysfunction in normal animals. Thus, the authors of the study mention that chronic VPA, specifically when the treatment is carried out in the early stages of life, can promote changes in the functions of the hippocampus (Fig. 3A) [49].
Fig. 3.
(A) Wild-type mice and mice lacking the presynaptic protein Bassoon were treated with 400 mg/kg of Valproic Acid. The treatment provided a reduction in seizures, and ortality, rescued hippocampal LTP, and promoted changes in hippocampal functions. (B) Rats were treated with 300 mg/kg of Valproic Acid. There was a reduction in cell proliferation in the dentate gyrus, in addition to a reduction in the levels of BDNF and Notch1. (C) The effect of levetiracetam (54 mg/kg) was evaluated on hippocampal synaptic neuroplasticity in rat CA3-CA1 regions. Treatment with this drug promoted the reestablishment of basal synaptic transmission, furthermore, this drug did not affect hippocampal neurogenesis. (D) Rats were treated with 300 mg/kg Levetiracetam. Treatment with this drug was not able to restore synaptic neuroplasticity in the dentate gyrus region of the hippocampus of these animals.
LTP, Long-Term Potentiation; BDNF, brain-derived neurotrophic factor.
Another study regarding VPA investigated the association between cognition and changes in hippocampal cell proliferation. In their findings, the authors observed that treatment in rats with VPA (300 mg/kg) decreased cell proliferation in the subgranular zone of the dentate gyrus in addition to significantly reducing the levels of BDNF, which is closely related to the neuroplastic effect, and Notch1. Thus, the authors mention that this drug can lead to cognitive impairment by decreasing hippocampal neurogenesis (Fig. 3B) [50].
One study evaluated the effects of levetiracetam (54 mg/kg) on hippocampal synaptic neuroplasticity in the CA3-CA1 and mossy fiber sprouting regions, in addition to chronic epileptic anxiety-like behavior in a lithium-pilocarpine rat model. In their findings, it was observed that treatment with this drug was able to restore basal synaptic transmission and the facilitation relationship of paired pulses in the synapses of the CA3-CA1 regions. Furthermore, levetiracetam did not affect epilepsy-induced adult hippocampal neurogenesis, mossy fiber sprouting in dentate gyrus cells, and anxiety-like behavior. The results of this study showed that, in addition to decreasing seizures, this antiseizure drug showed promising effects on synaptic transmission and structural neuroplasticity in chronic epilepsy (Fig. 3C) [51].
A study carried out whose objective was to characterize characterizing changes in synaptic neuroplasticity in the dentate gyrus region of the hippocampus in rats with early chronic epilepsy and observing whether treatment with levetiracetam (300 mg/kg) would be able to restore changes in baseline excitability, the short-term (facili-tation/depression) and LTP. In their findings, the authors mention that levetiracetam can act as an efficient antiseizure that potentiates the inhibitory transmission, increasing GABA release, in addition to suppressing the firing of glutamatergic neuronal cells in the dentate gyrus region. However, the authors clarify that the results of this study do not support the action of this drug as capable of restoring synaptic neuroplasticity (Fig. 3D) [52].
CONCLUSION
The studies addressed in this review showed that several antiseizure, in addition to controlling seizures, can also act on neuroplasticity in different brain regions, such as lamotrigine, diazepam, levetiracetam, and valproic acid, which, according to the findings, can promote some alterations in cells different structures, such as the cerebral cortex, hippocampus, striatum, and others. The study of this topic becomes important, as it will help to understand the mechanisms of these drugs on neuroplasticity, in addition to helping to improve the effectiveness of antiseizure in controlling the disease.
Footnotes
Funding
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. The main author of this study received doctoral grants from CAPES.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Débora Lopes Silva de Souza, Hosana Mirelle Goes e Silva Costa, Francisca Idalina Neta. Supervision: Claudio Lopes de Vasconcelos, José Rodolfo Lopes de Paiva Cavalcanti, Cynthia Cavalcanti de Albuquerque. Original writing: Débora Lopes Silva de Souza. Writing and editing review: Débora Lopes Silva de Souza, Claudio Lopes de Vasconcelos, José Rodolfo Lopes de Paiva Cavalcanti, Paulo Leonardo Araujo de Gois Morais, Cynthia Cavalcanti de Albuquerque, Fausto Pierdoná Guzen, Lucídio Clebeson de Oliveira, Luís Marcos de Medeiros Guerra.
References
- 1.Al-Otaibi F. An overview of structurally diversified anticonvulsant agents. Acta Pharm. 2019;69:321–344. doi: 10.2478/acph-2019-0023. [DOI] [PubMed] [Google Scholar]
- 2.Aksoy D, Güveli BT, Ak PD, Sarı H, Ataklı D, Arpacı B. Effects of oxcarbazepine and levetiracetam on calcium, ionized calcium, and 25-OH vitamin-D3 levels in patients with epilepsy. Clin Psychopharmacol Neurosci. 2016;14:74–78. doi: 10.9758/cpn.2016.14.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54:185–191. doi: 10.1159/000503831. [DOI] [PubMed] [Google Scholar]
- 4.Akyuz E, Polat AK, Eroglu E, Kullu I, Angelopoulou E, Paudel YN. Revisiting the role of neurotransmitters in epilepsy: An updated review. Life Sci. 2021;265:118826. doi: 10.1016/j.lfs.2020.118826. [DOI] [PubMed] [Google Scholar]
- 5.Dahlin M, Prast-Nielsen S. The gut microbiome and epilepsy. EBioMedicine. 2019;44:741–746. doi: 10.1016/j.ebiom.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kania BF, Wrońska D, Zięba D. Introduction to neural plasticity mechanism. J Behav Brain Sci. 2017;7:41–49. doi: 10.4236/jbbs.2017.72005. [DOI] [Google Scholar]
- 7.Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasmita AO, Kuruvilla J, Ling APK. Harnessing neuroplasticity: Modern approaches and clinical future. Int J Neurosci. 2018;128:1061–1077. doi: 10.1080/00207454.2018.1466781. [DOI] [PubMed] [Google Scholar]
- 9.Beyhan N, Kaymakcioglu BK, Gümrü S, Aricioglu F. Synthesis and anticonvulsant activity of some 2-pyrazolines derived from chalcone. Arab J Chem. 2017;10 Suppl 2:S2073–S2081. doi: 10.1016/j.arabjc.2013.07.037. [DOI] [Google Scholar]
- 10.Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 11.LaPenna P, Tormoehlen LM. The pharmacology and toxicology of third-generation anticonvulsant drugs. J Med Toxicol. 2017;13:329–342. doi: 10.1007/s13181-017-0626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizoguchi H, Yamada K. Roles of matrix metalloproteinases and their targets in epileptogenesis and seizures. Clin Psychopharmacol Neurosci. 2013;11:45–52. doi: 10.9758/cpn.2013.11.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manford M. Recent advances in epilepsy. J Neurol. 2017;264:1811–1824. doi: 10.1007/s00415-017-8394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anwar H, Khan QU, Nadeem N, Pervaiz I, Ali M, Cheema FF. Epileptic seizures. Discoveries (Craiova) 2020;8:e110. doi: 10.15190/d.2020.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boison D. The biochemistry and epigenetics of epilepsy: Focus on adenosine and glycine. Front Mol Neurosci. 2016;9:26. doi: 10.3389/fnmol.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch E, Johnell K, Kauppi K. Longitudinal effects of using and discontinuing central nervous system medications on cognitive functioning. Pharmacoepidemiol Drug Saf. 2023;32:446–454. doi: 10.1002/pds.5569. [DOI] [PubMed] [Google Scholar]
- 17.Brodie MJ, Sills GJ. Combining antiepileptic drugs--rational polytherapy? Seizure. 2011;20:369–375. doi: 10.1016/j.seizure.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins NA, Anderson LL, Gertler TS, Laux L, George AL, Jr, Kearney JA. Screening of conventional anticonvulsants in a genetic mouse model of epilepsy. Ann Clin Transl Neurol. 2017;4:326–339. doi: 10.1002/acn3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalkara S, Karakurt A. Recent progress in anticonvulsant drug research: Strategies for anticonvulsant drug development and applications of antiepileptic drugs for non-epileptic central nervous system disorders. Curr Top Med Chem. 2012;12:1033–1071. doi: 10.2174/156802612800229215. [DOI] [PubMed] [Google Scholar]
- 20.Palma E, Ruffolo G, Cifelli P, Roseti C, Vliet EAV, Aronica E. Modulation of GABAA receptors in the treatment of epilepsy. Curr Pharm Des. 2017;23:5563–5568. doi: 10.2174/1381612823666170809100230. [DOI] [PubMed] [Google Scholar]
- 21.Stefan H, Feuerstein TJ. Novel anticonvulsant drugs. Pharmacol Ther. 2007;113:165–183. doi: 10.1016/j.pharmthera.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Löscher W, Sills GJ, White HS. The ups and downs of alkyl-carbamates in epilepsy therapy: How does cenobamate differ? Epilepsia. 2021;62:596–614. doi: 10.1111/epi.16832. [DOI] [PubMed] [Google Scholar]
- 23.Zhu MM, Li HL, Shi LH, Chen XP, Luo J, Zhang ZL. The pharmacogenomics of valproic acid. J Hum Genet. 2017;62:1009–1014. doi: 10.1038/jhg.2017.91. [DOI] [PubMed] [Google Scholar]
- 24.Lamb S. Neuroplasticity: A century-old idea championed by Adolf Meyer. CMAJ. 2019;191:E1359–E1361. doi: 10.1503/cmaj.191099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demarin V, Morović S. Neuroplasticity. Period Biol. 2014;116:209–211. doi: 10.2307/j.ctv22jnnm9.22. [DOI] [Google Scholar]
- 26.Wenger E, Lövdén M. The learning hippocampus: Education and experience-dependent plasticity. Mind, Brain, and Edu-cation. 2016;10:171–183. doi: 10.1111/mbe.12112. [DOI] [Google Scholar]
- 27.Garcés-Vieira MV, Suárez-Escudero JC. Neuroplasticity: biochemical and neurophysiological aspects. CES Med. 2014;28:119–132. [Google Scholar]
- 28.Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Yan H, Yang Y, Xu M, Shi Y, Zeng W, et al. Occu-pational neuroplasticity in the human brain: A critical review and meta-analysis of neuroimaging studies. Front Hum Neurosci. 2020;14:215. doi: 10.3389/fnhum.2020.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaffer J. Neuroplasticity and clinical practice: Building brain power for health. Front Psychol. 2016;7:1118. doi: 10.3389/fpsyg.2016.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, Liu J, Wang M, Zhang Y, Li L. From serotonin to neuroplasticity: Evolvement of theories for major depressive disorder. Front Cell Neurosci. 2017;11:305. doi: 10.3389/fncel.2017.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulyaeva NV. Molecular mechanisms of neuroplasticity: An expanding universe. Biochemistry (Mosc) 2017;82:237–242. doi: 10.1134/S0006297917030014. [DOI] [PubMed] [Google Scholar]
- 33.Toricelli M, Pereira AAR, Souza Abrao G, Malerba HN, Maia J, Buck HS, et al. Mechanisms of neuroplasticity and brain degeneration: Strategies for protection during the aging process. Neural Regen Res. 2021;16:58–67. doi: 10.4103/1673-5374.286952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cichon N, Saluk-Bijak J, Gorniak L, Przyslo L, Bijak M. Flavonoids as a natural enhancer of neuroplasticity-an overview of the mechanism of neurorestorative action. Antioxi-dants (Basel) 2020;9:1035. doi: 10.3390/antiox9111035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Oliveira RMW. Neuroplasticity. J Chem Neuroanat. 2020;108:101822. doi: 10.1016/j.jchemneu.2020.101822. [DOI] [PubMed] [Google Scholar]
- 36.El-shaer NH, Al-Gabri NA. Patho-immunohistochemical study on the neuro-protective effects of ginkobiloba against carbamazepine-induced neurotoxicity in experimental albino rats. J Chem Pharm Res. 2018;10:174–181. [Google Scholar]
- 37.Delvendahl I, Lindemann H, Heidegger T, Normann C, Ziemann U, Mall V. Effects of lamotrigine on human motor cortex plasticity. Clin Neurophysiol. 2013;124:148–153. doi: 10.1016/j.clinph.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Huopaniemi L, Keist R, Randolph A, Certa U, Rudolph U. Diazepam-induced adaptive plasticity revealed by alpha1 GABAA receptor-specific expression profiling. J Neurochem. 2004;88:1059–1067. doi: 10.1046/j.1471-4159.2003.02216.x. [DOI] [PubMed] [Google Scholar]
- 39.Christensen KV, Leffers H, Watson WP, Sánchez C, Kallunki P, Egebjerg J. Levetiracetam attenuates hippocampal expression of synaptic plasticity-related immediate early and late response genes in amygdala-kindled rats. BMC Neurosci. 2010;11:9. doi: 10.1186/1471-2202-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghiglieri V, Sgobio C, Patassini S, Bagetta V, Fejtova A, Giampà C, et al. TrkB/BDNF-dependent striatal plasticity and behavior in a genetic model of epilepsy: Modulation by valproic acid. Neuropsychopharmacology. 2010;35:1531–1540. doi: 10.1038/npp.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jessberger S, Nakashima K, Clemenson GD, Jr, Mejia E, Mathews E, Ure K, et al. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci. 2007;27:5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, He X, Li Q, Kong X, Ou Z, Zhang L, et al. PI3K/AKT/mTOR signaling mediates valproic acid-induced neuronal differentiation of neural stem cells through epigenetic modifications. Stem Cell Reports. 2017;8:1256–1269. doi: 10.1016/j.stemcr.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinjo T, Ito M, Seki T, Fukuhara T, Bolati K, Arai H, et al. Prenatal exposure to valproic acid is associated with altered neurocognitive function and neurogenesis in the dentate gyrus of male offspring rats. Brain Res. 2019;1723:146403. doi: 10.1016/j.brainres.2019.146403. [DOI] [PubMed] [Google Scholar]
- 44.Pannangrong W, Sirichoat A, Wongsiri T, Wigmore P, Welbat JU. Valproic acid withdrawal ameliorates impairments of hippocampal-spatial working memory and neurogenesis. J Zhejiang Univ Sci B. 2019;20:253–263. doi: 10.1631/jzus.B1800340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujimura K, Mitsuhashi T, Shibata S, Shimozato S, Takahashi T. In utero exposure to valproic acid induces neocortical dysgenesis via dysregulation of neural progenitor cell proliferation/differentiation. J Neurosci. 2016;36:10908–10919. doi: 10.1523/JNEUROSCI.0229-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ideta-Otsuka M, Igarashi K, Narita M, Hirabayashi Y. Epigenetic toxicity of environmental chemicals upon exposure during development - bisphenol A and valproic acid may have epigenetic effects. Food Chem Toxicol. 2017;109:812–816. doi: 10.1016/j.fct.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Sakai A, Matsuda T, Doi H, Nagaishi Y, Kato K, Nakashima K. Ectopic neurogenesis induced by prenatal antiepileptic drug exposure augments seizure susceptibility in adult mice. Proc Natl Acad Sci U S A. 2018;115:4270–4275. doi: 10.1073/pnas.1716479115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao J, Luo Y, Lu Y, Wu X, Chen P, Zhang X, et al. Epigenetic regulation of GABAergic differentiation in the developing brain. Front Cell Neurosci. 2022;16:988732. doi: 10.3389/fncel.2022.988732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sgobio C, Ghiglieri V, Costa C, Bagetta V, Siliquini S, Barone I, et al. Hippocampal synaptic plasticity, memory, and epilepsy: Effects of long-term valproic acid treatment. Biol Psy-chiatry. 2010;67:567–574. doi: 10.1016/j.biopsych.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Umka J, Mustafa S, ElBeltagy M, Thorpe A, Latif L, Bennett G, et al. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience. 2010;166:15–22. doi: 10.1016/j.neuroscience.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 51.Salaka RJ, Nair KP, Sasibhushana RB, Udayakumar D, Kutty BM, Srikumar BN, et al. Differential effects of levetiracetam on hippocampal CA1 synaptic plasticity and molecular changes in the dentate gyrus in epileptic rats. Neurochem Int. 2022;158:105378. doi: 10.1016/j.neuint.2022.105378. [DOI] [PubMed] [Google Scholar]
- 52.González-H G, Contreras-García IJ, Sánchez-Huerta K, Queiroz CMT, Gallardo Gudiño LR, Mendoza-Torreblanca JG, et al. Levetiracetam reduced the basal excitability of the dentate gyrus without restoring impaired synaptic plasticity in rats with temporal lobe epilepsy. Brain Sci. 2020;10:634. doi: 10.3390/brainsci10090634. [DOI] [PMC free article] [PubMed] [Google Scholar]