Abstract
Objective
As dopamine is closely linked to locomotor activities, animal studies on locomotor activities using dopaminergic agents were widely done. However, most of animal studies were performed for a short period that there is a lack of longitudinal study on the effects of dopaminergic agents on locomotor activities. This study aimed to examine the long- term effect of a dopamine D2, D3 agonist quinpirole on locomotor activities in mice using a home-cage monitoring system.
Methods
The locomotor activities of Institute Cancer Research mice were measured by infrared motion detectors in home-cages under the 12-hour dark and 12-hour light condition for three days after the quinpirole injection. Quinpirole was injected at a concentration of 0.5 mg/kg intraperitoneally in the beginning of the dark phase. The locomotor activities before and after the quinpirole administration were compared by the Wilcoxon signed-rank test and one-way repeated measures ANOVA.
Results
After the quinpirole administration, the 24-hour total locomotor activity did not change (p = 0.169), but activities were significantly increased in the 12-hour dark phase sum (p = 0.013) and decreased in the 12-hour light phase sum (p = 0.009). Significant increases in the activities were observed in the dark-light difference (p = 0.005) and dark-light ratio (p = 0.005) as well.
Conclusion
This study suggests that quinpirole injection entrains the circadian rest-activity rhythm of locomotor activities. Therefore, quinpirole can be a drug that mediates locomotor activity as a dopamine agonist as well as a modulator of the circadian rhythms.
Keywords: Quinpirole, Dopamine, Infrared detector, Locomotion, Circadian rhythms
INTRODUCTION
Dopamine is one of the neurotransmitters that has crucial role in brain functions [1-3]. In particular, locomotor activity can be differentially regulated by biological conditions contributing to dopamine status in brain [4]. A study showed that disruptions in the dopamine transporter gene increased locomotor activity because lack of the transporter made dopamine to stay in the extracellular space 100 times longer than the wild type [5]. Another study showed that dopamine depletion due to lack of tyrosine hydroxylase expression caused a reduction of spontaneous locomotor activity in mice [6]. As such, alterations in the dopamine level could lead to a change in locomotor activities, and it is important to maintain a proper level of dopamine in the body.
Since dopamine is closely linked to locomotor activities, studies using dopaminergic agents on locomotor activities are widely done. Haloperidol, a dopamine receptor antagonist attenuated picrotoxin-induced locomotor hyperactivity in the open field test [7]. This result implies that locomotor hyperactivity induced by picrotoxin as GABA antagonist is mediated through dopamine re-ceptor. Dopamine D2, D3 receptors are known to modulate locomotor activity, the receptors are located in the presynaptic a postsynaptic neurons. Sophisticated tunings of locomotor activities are regulated by these receptors. Thus, their agonists and antagonists could have crucial roles on the modulation of locomotor activity, and these agents could be utilized as chronotheraputics by modulating the rest-activity rhythm [8,9]. A dopamine D2, D3 receptor agonist quinpirole and dopamine D2 receptor antagonist sulpiride were administrated to Long-Evans rats to manipulate D2 receptor [10]. The result showed that quinpirole had a had an increase of locomotion while sulpiride caused a decrease in locomotor activity [10]. A study that investigated the relationship between dorsal striatum or nucleus accumbens and locomotor activities in rats revealed that the administration of quinpirole has a locomotor activating effect in both brain sites [11]. An-other study supported this idea. The locomotor activity of Wistar rats were observed using a photocell arena, in this research as well, the quinpirole administrated group show-ed higher locomotor activity then the control group [12].
However, until now, most studies that investigated the relationship between dopaminergic agents and locomotor activities have seen a short period time [13]. For instance, in the open field test, which is a method to observe the locomotor activities of animals, an instruction suggested five-minute sessions for novel environments and thirty-minute sessions for familiar environments [14], and traditional animal behavior studies only observed for 2 hours at maximum [15]. This might be because the researchers in previous studies believed that making short term observations was meaningful enough for monitoring the drug effects. Also, in case of the open field test, its protocol hindered long term observations as researchers need to monitor and analysis data manually. Therefore, the short term effects of drugs were observed in most previous studies. Knowing that the animals have circadian rhythms, monitoring the locomotor activities for a short term may not be sufficient to understand the effect of the drug that is being used. Also, the effect of medication can be affected by various conditions. For example, the circadian oscillations in locomotor activities and the environment of experiment would give different outcomes depending on the time when the experiment is taken [16, 17]. Therefore, it is necessary to observe the animal on a long-term base. A system called ‘home-cage monitor’ has been used to monitor the locomotor activities of animals longitudinally [18]. Home-cage monitoring system is used for measuring the locomotor activities of animals. Digitized signals are collected by the detectors and this information can be translated into some variables such as sleep-wake states and locomotor activities [19,20]. For instance, an infrared detector is attached on each cage and detects the infrared signal of animals due to their body temperature, then the signal is converted to numerical values for further analysis [21]. This system allows the researchers to obtain the longitudinal data of locomotor activities of animals conveniently that many studies are using it recently. Some genetic studies to validate neuropsychiatric disorder models used a home-cage system to observe the locomotor activity of the tested animal for one week with a 12 h:12 h light-dark cycle [22,23]. Despite the importance of a longitudinal study, there is a lack of studies that explored the effect of dopaminergic agents on the locomotor activities of mice using a home-cage system. Therefore, this study aimed to observe the longitudinal effect of a dopamine D2, D3 agonist quinpirole on locomotor activity using a home-cage monitoring system. When observing the effects of quinpirole longitudinally, the effects of quinpirole could be different according to dark and light phases.
METHODS
Subjects
A total of 10 male Institution Cancer Research male mice were used in the experiment (weight 34−38 g, age 5−6 weeks). The animals were placed individually in cages under constant conditions with constant temperature (20−25°C) and humidity (40−60%). Food and water were provided ad libitum. The light-dark cycle was controlled with a 12 h:12 h light-dark cycle (light phases: lights on from 5 PM to 5 AM, dark phases: lights off from 5 AM to 5 PM) by Light Control System (iW Blast Powercore/ Colorplay 3/Data Enabler Pro; Philips). This study was approved by Institutional Animal Care and Use Committee at Pusan National University Hospital (PNUH-2017-018).
Experimental Procedure and Measurements
Infrared motion detector Mlog system (Biobserve Inc.) was used to measure the locomotor activities of the animals. Mlog system comprises infrared detectors that each detector is placed on each home-cage and detects the movements of the animal. If the animal moves, its motion is detected and measured every second. This observed data is transformed into numerical values and recorded. This system allowed to observe the locomotor activities of the animals 24 hours continuously.
The mice were individually placed in cages for one week as an accommodation period to adapt to the new environment. After this accommodation, the locomotor activities of each animal were measured by the infrared motion detectors. During the dark phase, at 5 PM, quinpirole 0.5 mg/kg was administrated intraperitoneally (mice weight: 38−43 g, total doses: 0.019−0.022 mg). After the injection, the animals were placed back into the cages and their locomotor activity data were collected for three days.
Data Analysis
The raw locomotor activities data was collected in every second. To handle data effectively, the data was aggregated into 12 hours and 24 hours, then visualization and possible trends were searched using following five factors. The 24-hour sum, which is the summation of the locomotor activities during 24 hours, the dark phase sum, which is the summation of the locomotor activities during the dark phase, the light phase sum, which is the summation of the locomotor activities during the light phase, the dark-light difference, which is the difference in the locomotor activities between the dark and light phases that was calculated by subtracting the locomotor activities in the light phase from that of the dark phase and the dark-light ratio, which is the ratio in the locomotor activities between the dark and light phases that was calculated by dividing the locomotor activities in the light phase from that of the dark phase. Manipulations of the numerical Mlog data were done by MATLAB R2021b (MathWorks). To find statistical significance, Wilcoxon signed-ranked test and one-way repeated measures ANOVA Statistics was performed using IBM SPSS version 22.0 (IBM Co.).
RESULTS
The Changes of the Locomotor Activities Before and After the Quinpirole Administration
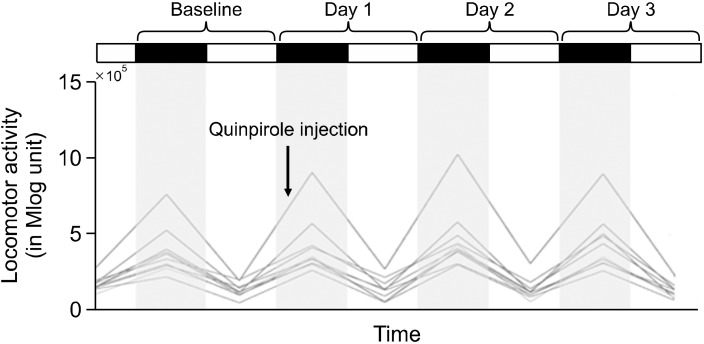
The locomotor activities observed for the baseline and three days after the quinpirole administration are shown in Figure 1. When comparing the locomotor activities before and after quinpirole administration, which is presented in Table 1, the 24-hour sum, which is the parameter for the sum of the locomotor activities from each day did not show significant change after the quinpirole injection (Z= −1.376, p = 0.169). The 12-hour dark phase sum which is the sum of locomotor activities during dark phase, was significantly increased after quinpirole administration (Z= −2.497, p = 0.013). The 12-hour light phase sum, which is the sum of locomotor activities during light phase, was significantly decreased (Z= −2.599, p = 0.009). The dark-light phase difference, the difference of the locomotor activities between dark and light phase, and ratio, which is the parameter that represents the ratio of the locomotor activities between dark and light phases, were significantly increased after quinpirole injection (dark-light difference: Z= −2.803, p = 0.005; ratio: Z= −2.803, p = 0.005).
Fig. 1.
The locomotor activities of animals. The locomotor activities of the animals at baseline before the quinpirole administration and for three days (day 1−3) after the quinpirole administration are pre-sented by using infrared detectors on home-cages. The timing of quinpirole administration is indicated with an arrow.
Table 1.
Comparison of the locomotor activities between before and after the quinpirole administration
| Parameters | Before injection | After injection | Z-score | p value |
|---|---|---|---|---|
| 24 hr sum | 5.406 × 105 ± 1.922 × 105 | 5.853 × 105 ± 2.480 × 105 | −1.376 | 0.169 |
| Dark phase sum | 3.561 × 105 ± 1.536 × 105 | 4.530 × 105 ± 1.935 × 105 | −2.497 | 0.013 |
| Light phase sum | 1.682 × 105 ± 4.367 × 104 | 1.362 × 105 ± 5.824 × 104 | −2.599 | 0.009 |
| Dark-light difference | 1.879 × 105 ± 1.211 × 105 | 3.088 × 105 ± 1.340 × 105 | −2.803 | 0.005 |
| Dark-light ratio | 2.113 ± 0.566 | 3.579 ± 0.898 | −2.803 | 0.005 |
Locomotor activity values of before and after injections are presented as mean ± standard deviation.
The Changes of the Locomotor Activities Induced by Quinpirole According to Time
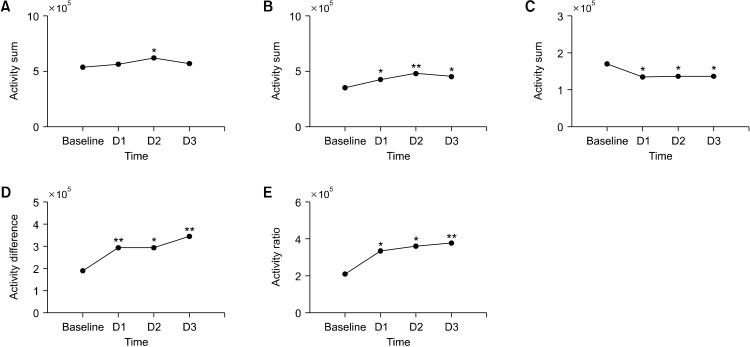
The changes of the locomotor activities induced by quinpirole according to time are analyzed by one-way repeated measures analysis of variance as shown in Figure 2. There was no significant difference in the 24-hour total locomotor activities, however, all other parameters showed statistical significance. The 12-hour dark phase sum increased significantly after quinpirole administration (F = 14.618, p = 0.004). The 12-hour light phase sum significantly decreased after the injection (F = 2.980, p = 0.049). The dark-light phase difference increased after the injection (F = 12.379, p = 0.007). Also, the dark-light phase ratio showed significant increase in the locomotor activities according to the quinpirole administration time (F = 9.772, p = 0.012).
Fig. 2.
The effect of the quinpirole injection over time. Each index of the locomotor activities was seen at baseline before the quinpirole administration, the first day (D1), the second day (D2), the third day (D3) after the quinpirole administration. (A) 24 hr sum. (B) Dark phsse sum. (C) Light phase sum. (D) Dark light difference. (E) Dark light ratio. The asterisks indicate the statistical significance when the data is compared to the baseline values by one-way repeated measures ANOVA.
*p < 0.05, **p < 0.01.
When the locomotor activities after the quinpirole administration was compared to that of the baseline using Wilcoxon signed-ranked test (Fig. 2), locomotor activities in the 24-hour sum showed an increasing trend and a significant increase in Day 2 compared to the baseline activity (Z= −1.988, p = 0.047). In the 12-hour dark phase sum, locomotor activities increased significantly in Day 1, 2, 3 (Day 1: Z= −1.988, p = 0.047; Day 2: Z= −2.599, p = 0.009; Day 3: Z= −2.497, p = 0.013) after the quinpirole administration. The locomotor activities of the 12-hour light phase sum decreased significantly by the quinpirole injection in all three days (Day 1: Z= −2.191, p = 0.028; Day 2: Z= −2.090, p = 0.037; Day 3: Z= −2.293, p = 0.022). The dark-light phase difference result showed that the locomotor activities were increased significantly in all three days compared to the baseline (Day 1: Z= −2.599, p = 0.009; Day 2: Z= −2.191, p = 0.028; Day 3: Z= −2.701, p = 0.007). Lastly, the dark-light phase ratio also showed a significant increase after the drug administration in all three days (Day 1: Z= −2.497, p = 0.013; Day 2: Z= −2.191, p = 0.028; Day 3: Z= −2.701, p = 0.007).
DISCUSSION
In this study, dopamine D2, D3 receptor agonist quinpirole showed an increase of locomotor activity during the dark phase in mice, which corresponds to the active daytime for humans. Previous studies indicated that quinpirole showed a biphasic pattern that signifies an initial suppression and late activation of locomotor activities after a quinpirole administration [24]. These studies observed locomotor activities for short-terms within 2 hours [25,26]. As there was a biphasic transition in the locomotor activities between 30 to 50 minutes after the quinpirole injection, the total locomotor activities during a short-term induced by quinpirole could be inconsistently increased or decreased [26]. Considering the biphasic transition and the increasing trend in the locomotor activity, there might be a possibility of an increase in the total locomotor activities after initial suppression if a long-term observation was made. In this study, the locomotor activities were longitudinally observed for three days after the entrainment using infrared motion detectors on home- cages. As expected, the locomotor activities of the mice were increased after an administration of quinpirole.
Meanwhile, in this study, the locomotor activities were increased in the 12-hour dark phase after the quinpirole administration but decreased in the locomotor activities during the 12-hour light phase. To our knowledge, there was no evidence on long-term effects of quinpirole on the locomotor activities during the dark and light phase. A study examined the diurnal rhythms of the locomotor activities by quinpirole 1 mg in C3H/HeJ mice [27]. How-ever, this study only reported the patterns of a short-term locomotor activities for 90 minutes after daytime and nighttime injections [27], which was different from our study that we observed longitudinally during 12 hours and 24 hours. The results of our long-term study imply that quinpirole could enhance the circadian rest-activity patterns. Considering a single administration of quinpirole during the dark phase and the half-life of around 1.8-hour [28], the decrease of the locomotor activities during the light phase might be caused by a dampening effect the effect diminished the increased total locomotor activities during dark phase due to the decrease of locomotor activities during light phase. These findings in the light phase needs to be confirmed whether a single quinpirole administration can enhance the circadian rest-activity rhythms or activity decrease by the dampening effect in a future study using a repeated administration design. However, other indices such as the difference and ratio of the locomotor activities in the 12-hour dark and light phases also suggested that the entrainment of the circadian rest-activity rhythms were augmented. Given that the locomotor activities after the quinpirole administration was increased during the dark phase and decrease during the light phase, the 24-hour total locomotor activities were expected to be unchanged. Taken together, quinpirole might have a potential to be a modulator for enhancing the circadian rest-activity rhythms.
The circadian rest-activity rhythms can be influenced by dopaminergic neurotransmissions [16]. Dopamine shows high levels in dark phase which is related to active locomotor activities and in contrast, low levels in light phase [29]. This study showed an enhancement of the circadian rest-activity rhythms as well as an increase of the locomotor activities in the dark phase. Considering that the dopamine D2, D3 agonist quinpirole injection was done at the dark phase when the dopamine neurotransmission level is high, the intrinsic circadian dopaminergic oscillation might be augmented by the injection. A study using methylphenidate similarly reported that an increase of dopamine induced by methylphenidate in dark phase was related to an increase in locomotion [30]. Given that the results of previous study and our study, extrinsic dopamine agonists in dark phase can enhance intrinsic circadian rest-activity rhythms when the circadian timing was synchronized.
However, there are some limitations in this study. Firstly, this study was a single arm study without a control group to observe the effect of dopamine D2, D3 agonist quinpirole on locomotor activities and only compared the changes in locomotor activities before and after a quinpirole injection. In future studies, to confirm the accurate effect of quinpirole, it is necessary to use a placebo and conduct randomized controlled trials. Secondly, quinpirole was only injected once in this study. It might be possible that the result may change if multiple administrations were given. Thirdly, in this study, only one type of quinpirole dosage, 0.5 mg/kg was used. This concentration was selected because it is commonly used [31], but as the result might alter depending on the concentration of administrated quinpirole, it is necessary to investigate the effects of different quinpirole dosages in future studies. Fourthly, we only conducted experiments at dark phase when mice were active. Considering mice have circadian rhythms, it is possible that the results might change if quinpirole was injected at light phase, therefore it is recommended to examine the effect of quinpirole on locomotor activities from dark and light phase administrations.
In this study, the effect of dopamine D2, D3 agonist quinpirole on locomotor activities were observed longitudinally through the home-cage monitoring system. These longitudinal observations suggest that the effects of dopamine D2, D3 agonist during the dark and light phases of the circadian rhythm may be different. These results can be clinically important information on how dopamine D2, D3 agonist affect rest-activity rhythm. Further-more, it can be said that this agent may be used as modulators of rest-activity rhythm on subjects with disrupted rhythms. When considering frequent prescriptions on dopamine-related psychiatric agents, these agents should be considered to have an influence on rest-activity rhythm. In this respect, the results of this study provide important clinical information on the relationship between dopamine and rest-activity rhythm. In future studies, it is required to confirm these circadian effects of quinpirole on locomotor activities and examine the differential effects by various conditions, such as different dosages, repeated administration, and circadian timing. That is, the effect of an agonist could be different depending on its dosage. Therefore, it is necessary to investigate the moderating effect of dopamine agonists. Also, this study observed the effect of single administration that it is possible that the overall locomotor activity could change with repeated administrations. In particular, it is needed to research on the modulating effect for rest-activity by repeated administration using a disruption animal model as a chrono-therapeutic. Furthermore, a study on observing the circadian timing effect, that reflects the differential effects according to administration time based on individual circadian rhythm, are necessary.
Footnotes
Funding
This study was supported by Biomedical Research Institute Grant (202100230001), Pusan National Univer-sity Hospital, Republic of Korea.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Jeonghyun Park, Eunsoo Moon, Yoo Rha Hong, Jung Hyun Lee. Data acquisition: Jeonghyun Park, Hyun Ju Lim. Formal analysis: Jeonghyun Park, Hyun Ju Lim, Kyungwon Kim. Funding: Eunsoo Moon. Supervision: Eunsoo Moon. Writing−original draft: Jeonghyun Park, Eunsoo Moon. Writing−review & editing: Hyun Ju Lim, Kyungwon Kim, Yoo Rha Hong, Jung Hyun Lee.
References
- 1.Verma V. Classic studies on the interaction of cocaine and the dopamine transporter. Clin Psychopharmacol Neurosci. 2015;13:227–238. doi: 10.9758/cpn.2015.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama K, Sutoo D. Music enhances drunkenness: A phenomenon related to increased dopaminergic function. Clin Psychopharmacol Neurosci. 2010;8:156–159. [Google Scholar]
- 3.Dalton VS, Zavitsanou K. Rapid changes in D1 and D2 dopamine receptor binding in striatal subregions after a single dose of phencyclidine. Clin Psychopharmacol Neurosci. 2011;9:67–72. doi: 10.9758/cpn.2011.9.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart- Kasch S, Zhang G, et al. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyper-locomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi K, Sano H. Dopamine deficiency in mice. Brain Dev. 2000;22 Suppl 1:S54–S60. doi: 10.1016/S0387-7604(00)00134-0. [DOI] [PubMed] [Google Scholar]
- 7.Chang CK, Wang NL, Lin MT. Inhibition of the dopamine system in rat amygdala attenuates the picrotoxin-induced locomoter hyperactivity and hypertension. Clin Exp Pharmacol Physiol. 2004;31:284–288. doi: 10.1111/j.1440-1681.2004.03994.x. [DOI] [PubMed] [Google Scholar]
- 8.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 10.Stuchlik A, Rehakova L, Rambousek L, Svoboda J, Vales K. Manipulation of D2 receptors with quinpirole and sulpiride affects locomotor activity before spatial behavior of rats in an active place avoidance task. Neurosci Res. 2007;58:133–139. doi: 10.1016/j.neures.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Van Hartesveldt C, Cottrell GA, Potter T, Meyer ME. Effects of intracerebral quinpirole on locomotion in rats. Eur J Pharmacol. 1992;214:27–32. doi: 10.1016/0014-2999(92)90091-H. [DOI] [PubMed] [Google Scholar]
- 12.Mattingly BA, Rowlett JK, Lovell G. Effects of daily SKF 38393, quinpirole, and SCH 23390 treatments on locomotor activity and subsequent sensitivity to apomorphine. Psychopharmacology (Berl) 1993;110:320–326. doi: 10.1007/BF02251287. [DOI] [PubMed] [Google Scholar]
- 13.McDougall SA, Apodaca MG, Park GI, Teran A, Baum TJ, Montejano NR. MK801-induced locomotor activity in preweanling and adolescent male and female rats: Role of the dopamine and serotonin systems. Psychopharmacology (Berl) 2020;237:2469–2483. doi: 10.1007/s00213-020-05547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himanshu, Dharmila, Sarkar D, Nutan A. A review of behavioral tests to evaluate different types of anxiety and anti-anxiety effects. Clin Psychopharmacol Neurosci. 2020;18:341–351. doi: 10.9758/cpn.2020.18.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shieh KR, Yang SC. Formosan wood mice (Apodemus semotus) exhibit more exploratory behaviors and central dopaminergic activities than C57BL/6 mice in the open field test. Chin J Physiol. 2020;63:27–34. doi: 10.4103/CJP.CJP_47_19. [DOI] [PubMed] [Google Scholar]
- 16.Blum ID, Zhu L, Moquin L, Kokoeva MV, Gratton A, Giros B, et al. A highly tunable dopaminergic oscillator generates ultradian rhythms of behavioral arousal. Elife. 2014;3:e05105. doi: 10.7554/eLife.05105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourguignon C, Storch KF. Control of rest: Activity by a dopaminergic ultradian oscillator and the circadian clock. Front Neurol. 2017;8:614. doi: 10.3389/fneur.2017.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iannello F. Non-intrusive high throughput automated data collection from the home cage. Heliyon. 2019;5:e01454. doi: 10.1016/j.heliyon.2019.e01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J, Jung MS, Moon E, Lim HJ, Oh CE, Lee JH. Prediction of locomotor activity by infrared motion detector on sleep-wake state in mice. Clin Psychopharmacol Neurosci. 2021;19:303–312. doi: 10.9758/cpn.2021.19.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong YR, Kim K, Moon E, Park J, Oh CE, Lee JH, et al. Machine learning algorithms for the prediction of locomotor activity by an infrared motion detector on the sleep-wake states in mice. Clin Psychopharmacol Neurosci. 2023;21:279–287. doi: 10.9758/cpn.2023.21.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein CJMI, Budiman T, Homberg JR, Verma D, Keijer J, van Schothorst EM. Measuring locomotor activity and behavioral aspects of rodents living in the home-cage. Front Behav Neurosci. 2022;16:877323. doi: 10.3389/fnbeh.2022.877323. Erratum in: Front Behav Neurosci 2022;16:943307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima R, Takao K, Hattori S, Shoji H, Komiyama NH, Grant SGN, et al. Comprehensive behavioral analysis of heterozygous Syngap1 knockout mice. Neuropsychopharmacol Rep. 2019;39:223–237. doi: 10.1002/npr2.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima R, Hattori S, Funasaka T, Huang FL, Miyakawa T. Decreased nesting behavior, selective increases in locomotor activity in a novel environment, and paradoxically increased open arm exploration in Neurogranin knockout mice. Neuro-psychopharmacol Rep. 2021;41:111–116. doi: 10.1002/npr2.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eilam D, Golani I. The ontogeny of exploratory behavior in the house rat (Rattus rattus): The mobility gradient. Dev Psychobiol. 1988;21:679–710. doi: 10.1002/dev.420210707. [DOI] [PubMed] [Google Scholar]
- 25.Eilam D, Szechtman H. Biphasic effect of D-2 agonist quinpirole on locomotion and movements. Eur J Pharmacol. 1989;161:151–157. doi: 10.1016/0014-2999(89)90837-6. [DOI] [PubMed] [Google Scholar]
- 26.Van Hartesveldt C, Meyer ME, Potter TJ. Ontogeny of biphasic locomotor effects of quinpirole. Pharmacol Biochem Behav. 1994;48:781–786. doi: 10.1016/0091-3057(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 27.Akhisaroglu M, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in quinpirole-induced locomotor behaviors and striatal D2/D3 receptor levels in mice. Pharmacol Biochem Behav. 2005;80:371–377. doi: 10.1016/j.pbb.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Whitaker NG, Lindstrom TD. Disposition and biotransfor-mation of quinpirole, a new D-2 dopamine agonist antihypertensive agent, in mice, rats, dogs, and monkeys. Drug Metab Dispos. 1987;15:107–113. [PubMed] [Google Scholar]
- 29.Kim J, Jang S, Choe HK, Chung S, Son GH, Kim K. Implications of circadian rhythm in dopamine and mood regulation. Mol Cells. 2017;40:450–456. doi: 10.14348/molcells.2017.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaytan O, Yang P, Swann A, Dafny N. Diurnal differences in sensitization to methylphenidate. Brain Res. 2000;864:24–39. doi: 10.1016/S0006-8993(00)02117-X. [DOI] [PubMed] [Google Scholar]
- 31.Amato D, Milella MS, Badiani A, Nencini P. Compulsive-like effects of quinpirole on drinking behavior in rats are inhibited by substituting ethanol for water. Behav Brain Res. 2007;177:340–346. doi: 10.1016/j.bbr.2006.11.016. [DOI] [PubMed] [Google Scholar]