Abstract
Objective
Antipsychotic drugs are known as the major cause of non-neoplastic hyperprolactinemia. This study aimed to investigate the levels of serum prolactin depending on the use of antipsychotic drugs in patients through the Clinical Data Warehouse (CDW).
Methods
We conducted a cohort search in the CDW application and got 260 patients’ medical records diagnosed with schizophrenia, schizotypal and delusional disorders, manic episodes, and bipolar affective disorders who were taking one of risperidone, blonanserin, amisulpride, and olanzapine. After that, we reviewed the medical data and used the ANCOVA analysis and the post hoc test to compare serum prolactin levels among four antipsychotic drug groups.
Results
Among the 117 subjects included in the analysis, the mean serum prolactin level was 64.6 ± 54.6 ng/ml. Serum prolactin levels were significantly higher in subjects taking risperidone or amisulpride compared to blonanserin and olanzapine. The female subjects who took blonanserin, olanzapine, and risperidone had significantly higher prolactin levels, but there was no difference in serum prolactin levels between sex in the subjects who took amisulpride.
Conclusion
This study suggests the need for regular monitoring of serum prolactin levels in patients who are taking antipsychotics, especially in female patients. And we showed that there is a possibility to conduct more effective and simpler big data research using the CDW. Further studies on the subjects with controlled confounding variables and larger sample groups are needed.
Keywords: Antipsychotic agents; Prolactin; Hyperprolactinemia; Affective disorder, psychotic; Big data
INTRODUCTION
Antipsychotic drugs are the first treatment of choice for psychosis, such as schizophrenia, schizoaffective disorder, and bipolar disorder. Recently, these drugs have also been prescribed for various psychiatric disorders (Attention/Deficit Hyperactivity Disorder, anxiety disorder, depression eating disorder, etc.) as well as psychosis [1]. These antipsychotic drugs are known to be the main cause of non-neoplastic hyperprolactinemia [2], because they block the D2 dopamine receptor thereby causing hyperprolactinemia by interfering with the inhibition of dopamine-induced prolactin secretion [3]. A few studies have reported the occurrence of hyperprolactinemia in up to 70% of patients taking antipsychotic drugs [4]. Recently, atypical antipsychotics, which have fewer extrapyramidal side effects than typical antipsychotics, have been widely prescribed as a treatment option for psychosis [5]. But atypical antipsychotic drugs with high D2 receptor occupancy, such as risperidone and paliperidone, have been known to cause hyperprolactinemia more frequently than haloperidol which is a typical antipsychotic drug. Amisul-pride is also known as a antipsychotic drug that causes prolactin level elevation [6,7]. On the other hand, aripiprazole is known to have an effect on the decrease of prolactin secretion compared to typical antipsychotics [8]. Quetiapine has lower affinity for dopamine D2 receptors and is also known to have lower levels of prolactin compared to risperidone in children [9]. In comparison, olanzapine is known as associated with mild and transient hyperprolactinemia [6]. But there have been various findings about olanzapine. One categorical data of olanzapine shows a prevalence of hyperprolactinemia between 6−40% [10,11] and there was a doubling of the basal values from 9 to 18 ng/ml in a small cohort study of male schizophrenia subjects using olanzapine [12]. Meanwhile, in the case of blonanserin, there have been several studies compared to risperidone [13,14] but studies with other antipsychotics are insufficient until now.
Although there are inconsistencies about serum prolactin normal ranges in many studies, the upper limit of 19 ng/ml for males and 24 ng/ml for non-pregnant and non- feeding females are used in recent studies [8,15]. Hyper-prolactinemia inhibits the secretion of luteinizing hormone (LH) and follicle-stimulating hormone from the pituitary gland, thereby resulting in low levels of gonadal steroids and hypogonadism [3,16]. Hyperprolactinemia can cause sexual dysfunction, galactorrhea, gynecomastia, amenorrhea, infertility, etc. in both males and females. Long-term hyperprolactinemia causes bone density decline, osteoporosis, and fractures, and even increases the risk of some types of cancer such as breast cancer and cardiovascular disease [3]. Therefore, clinicians should not only monitor and manage the risk of hyperprolactinemia side effects in patients using antipsychotic drugs but also carry early intervention in case of the occurrence of hy-perprolactinemia. However, studies on the frequency of hyperprolactinemia among patients taking antipsychotic drugs have not been actively conducted in South Korea, and the prevalence is between 30−60%, with a large gap between studies [17]. Clinical interest in antipsychotic drug-induced hyperprolactinemia has been constantly increasing with the recent publication of the Korean Psy-chosomatic Society for Treatment Guidelines for Antipsy-chotics Drug-Induced Hyperprolactinemia. However, many patients still do not receive appropriate education and adequate monitoring form clinicians about hyperprolactine-mia. In addition, because of hyperpro-lactinemia, various departments such as obstetrics and gynecology, and endocrinology are wasting immense medical costs and medical resources due to overlapping treatment and examination without resolving the fundamental cause, thereby making patients suffer [18].
Recently, clinical analysis using Clinical Data Warehouse (CDW) was actively conducted, which required using vast clinical data instead of directly checking and entering patient’s written or electronic medical records. It means that big medical data can be obtained without spending a lot of manpower and time through CDW [19].
Therefore, this study aimed to investigate whether hyperprolactinemia occurs due to the administration of atypical antipsychotic drugs, risperidone, and amisulpride, known to cause hyperprolactinemia frequently as well as olanzapine which has various results and blonanserin relatively lacking compared with other antipsychotic drugs through the CDW.
METHODS
Clinical Data Warehouse
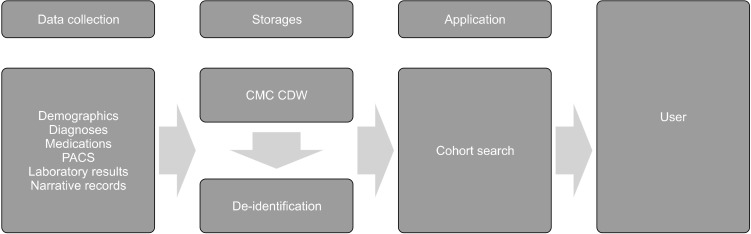
The Catholic Medical Center (CMC) has been operating the CDW that integrates all eight hospitals under the CMC since November 15, 2019. Currently, the CMC CDW doesn’t not only integrate and collect demographic data such as age, gender, and death of about 15 million patients in eight affiliated hospitals under the CMC, but also diagnoses based on International Classification of Diseases- 10 (ICD-10), medical information in the narrative reports, medications, laboratory medical tests, and radiological records. These stored data can be searched under the conditions desired by the researchers, and the extracted data can be obtained as an Excel file in which patient personal information is de-identified after approval from the Institutional Review Board (IRB) of the Catholic University of Korea (Fig. 1).
Fig. 1.
Overview of the Clinical Data Warehouse (CDW).
PACS, picture archiving and communication system; CMC, Catholic Medical Center.
Subjects
In this study, we used data from patients who visited St. Vincent’s Hospital among eight hospitals through CDW and conducted a cohort search in the CDW application following conditions.
1) In-patients and outpatients aged more than 18 years from March 1, 2017, to May 31, 2022, at the Depart-ment of Psychiatry at St. Vincent’s Hospital, The Catholic Uni-versity of Korea.
2)Patients who were diagnosed in the database according to the ICD-10 for schizophrenia, schizotypal and delusional disorders, manic episodes, and bipolar affective disorder (F20, F22, F23, F25, F29, F30, F31).
3) Patients who were taking one of risperidone, blonanserin, amisulpride, and olanzapine.
4) Patients who had an assessment of serum prolactin levels at least once by venous sampling.
As a result, we received about 260 patients’ de-identified data.
Among 260 patients, the patients with the applicable following criteria were excluded from the present study through data review.
1) If two or more antipsychotic drugs were taken at the same time
2) If the serum prolactin level test was not measured after taking the antipsychotic drug
3) If an antipsychotic long-acting injection such as Abilify maintena, Invega sustenna, Invega trinza were injected
4) If there was a pituitary disease known to affect prolactin levels: prolactinoma, pituitary adenoma, hypophysitis, etc. in the Brain magnetic resonance imaging (MRI) [2]
Although there is some inconsistency in the literature regarding the normal range of serum prolactin, in general, drug-induced hyperprolactinemia ranges between 25−100 ng/ml [8,20]. So we divided the subjects into four groups according to the prolactin level: severe hyperprolactinemia (> 100 ng/ml), moderate hyperprolac-tinemia (51−100 ng/ml), mild hyperprolactinemia (25−50 ng/ml), and normal (< 25 ng/ml) [21].
In addition, this study was conducted in accordance with the approval from the IRB of the Catholic University of Korea for the protection of patient clinical data infor-mation.
Statistical Analysis
Descriptive statistics (mean, standard deviation, frequency, and percentage) were used for the general characteristics of the four groups: amisulpride, blonanserin, olanzapine, and risperidone. We used logistic regression analysis, including the equivalent dose of chlorpromazine of the four antipsychotics, to evaluate the factors affecting the occurrence of hyperprolactinemia. Depending on the characteristics of the variables, the analysis of covariance (ANCOVA) was performed to know the effect of serum prolactin levels according to the antipsychotic drugs. Chlorpromazine equivalent dose and sex were used as covariates. The Bonferroni test was conducted also for post hoc analysis of the ANCOVA test. Additionally, we conducted the ranked ANCOVA test to find the effect of serum prolactin levels according to sex in the four antipsychotic groups. We used chlorpromazine equivalent dose as a covariate in this test. The extracted data were analyzed using SPSS version 21.0 (IBM Co.), and differences between groups were considered to be significant when p < 0.05.
RESULTS
Among the 260 patients, 143 patients were excluded based on the exclusion criteria, and a total of 117 subjects were selected for this study.
Demographic Data
The demographic and clinical data of 117 subjects are presented in Table 1.
Table 1.
Characteristics of subjects
| Variable | Total patients (n = 117) |
|---|---|
| Age | 47.4 ± 18.5 |
| Female | 69 (59.0) |
| Serum prolactin level (ng/ml) | 64.6 ± 54.6 |
| Hyperprolactinemia incidence | 82 (70.1) |
| ICD-10 Diagnosis (na) | |
| F20 (Schizophrenia) | 55 |
| F22 (Delusional disorder) | 4 |
| F23 (Acute and transient psychotic disorder) | 4 |
| F24 (Induced delusional disorder) | 0 |
| F25 (Schizoaffective disorder) | 6 |
| F29 (Unspecified nonorganic psychosis) | 2 |
| F30 (Mania) | 6 |
| F31 (Bipolar affective disorder) | 73 |
Values are presented as mean ± standard deviation or number (%).
ICD-10, International Classification of Diseases-10.
aDuplicate patients: total 30 subjects.
There were more female subjects (48 males, 69 females), and the average age was 47.4 ± 18.5 years (male: 46.4 ± 18.3 years, female: 48.1 ± 18.8 years).
A total of 46 subjects used olanzapine, 43 subjects used risperidone, 18 subjects used blonanserin, and 10 subjects used amisulpride (Table 2).
Table 2.
Characteristics among patients receiving each atypical antipsychotic drugs
| Variable | AMSP | BLON | OZP | RPR |
|---|---|---|---|---|
| Patients | 10 | 18 | 46 | 43 |
| Female | 8 (80.0) | 11 (61.1) | 25 (54.3) | 25 (58.1) |
| Age (yr) | 47.1 ± 13.3 | 37.7 ± 15.4 | 53.7 ± 19.5 | 44.7 ± 17.7 |
| Mean dose (mg) | 860 | 15.7 | 10.1 | 4.0 |
| CPZ equivalent dose (mg) | 430 | 391.7 | 404.3 | 402.3 |
| Serum prolactin level (ng/ml) | 122.4 ± 56.0 | 49.8 ± 45.5 | 43.3 ± 47.2 | 80.2 ± 52.0 |
Values are presented as number only, number (%), or mean ± standard deviation.
AMSP, amisulpride; BLON, blonanserin; OZP, olanzapine; RPR, risperidone; CPZ, chlorpromazine.
According to the ICD-10, 55 subjects were diagnosed with schizophrenia, 4 subjects were diagnosed with delusional disorder, 4 subjects were diagnosed with acute and transient psychotic disorder, 6 cases were diagnosed with schizoaffective disorder, and 2 subjects were diagnosed with unspecified nonorganic psychosis. It was found that a total of 73 subjects were diagnosed with bipolar affective disorder and 6 with mania. There was a total of 30 subjects with duplicate diagnoses (Table 1).
Clinical Data
The average serum prolactin level after taking four antipsychotic drugs was 64.6 ± 54.6 ng/ml (Table 1). The average serum prolactin level was 32.8 ± 20.9 ng/ml in males and 86.8 ± 59.8 ng/ml in females.
Among the total subjects, the prevalence of hyperpro-lactinemia: serum prolactin level of more than 25 ng/ml was 70.1% (n = 82), of which 54.2% (n = 26) were male and 81.6% (n = 56) were female (Table 1).
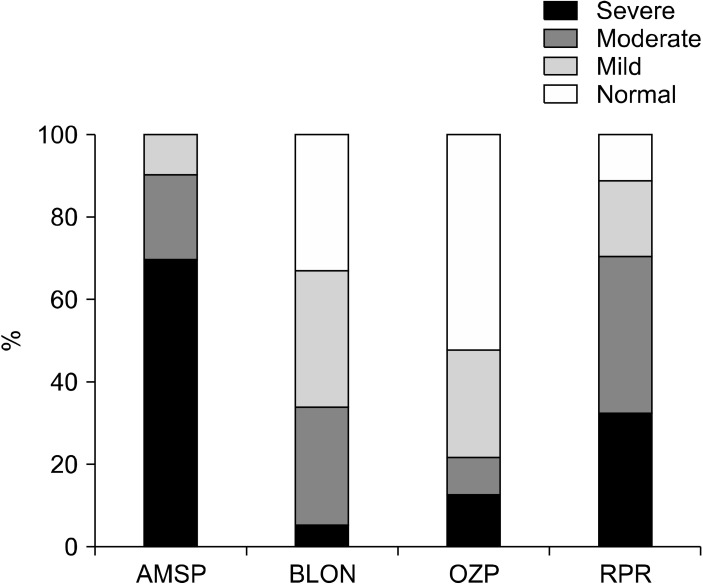
Hyperprolactinemia was observed in 100% of subjects taking amisulpride, 88.4% of subjects taking risperidone, 66.7% of patients taking blonanserin, and about 47.8% of subjects taking olanzapine (Fig. 2).
Fig. 2.
The severity of hyperprolactinemia according to antipsychotic drugs (%).
AMSP, amisulpride; BLON, blonanserin; OZP, olanzapine; RPR, risperidone.
Severe hyperprolactinemia, defined as a serum prolactin level higher than 100 ng/ml, was reported in only 1 subject (5.6%) who took blonanserin, in 6 subjects (13%) who took olanzapine, in 14 subjects (32.6%) who took risperidone, and in 7 subjects (70%) who took amisulpride (Fig. 2).
The occurrence of hyperprolactinemia were found to have statistical significance according to sex, with female subjects reporting hyperprolactinemia after taking antipsychotics more than male subjects (p = 0.001). In addition, the equivalent dose of chlorpromazine was found to have an effect on the occurrence of hyperprolactinemia also (p < 0.001). On the other hand, the subject’s age was found to have no effect on the occurrence of hyperprolactinemia (p = 0.253) (Table 3).
Table 3.
The logistic regression analyses on risk factors for hyperprolactinemia
| Predictor variables | B | Standard error | pvalue | Odds ratio | 95% Cl for odds ratio | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | −0.017 | 0.015 | 0.253 | 0.983 | 0.956 | 1.012 |
| Sex | −2.160 | 0.628 | 0.001 | 0.115 | 0.034 | 0.395 |
| CPZ equivalent dose | 0.005 | 0.001 | < 0.001 | 1.005 | 1.002 | 1.008 |
CPZ, chlorpromazine; CI, confidence interval.
Considering each antipsychotic drug, in the case of amisulpride, the average serum prolactin level was the highest at 122.4 ± 56.0 ng/ml, followed by risperidone 80.2 ± 52.0 ng/ml, blonanserin 49.8 ± 45.5 ng/ml, and olanzapine 43.3 ± 47.2 ng/ml. The average dosage of each drug was amisulpride 860 mg, blonanserin 15.7 mg, olanzapine 10.1 mg, and risperidone 4.0 mg. When converted to an equivalent dose of chlorpromazine, amisulpride was administered at the highest dose of 430 mg, followed by olanzapine 404.3 mg, risperidone, and blonanserin at equivalent doses of 402.3 mg and 391.7 mg, respectively (Table 2) [22].
The ANCOVA test was performed because there was significant interaction among sex, chlorpromazine equivalent dose, and hyperprolactinemia. The results of the ANCOVA test found that significant difference between antipsychotics and serum prolactin levels even though considering sex and chlorpromazine equivalent dose (p < 0.001) (Table 4). According to the post hoc test, amisulpride and risperidone showed higher serum prolactin levels than blonanserin and olanzapine. But, there was no significant difference between amisulpride and risperidone (p = 0.265). Blonanserin and olanzapine showed relatively low serum prolactin levels compared to amisulpride and risperidone, but could not find statistical significance between blonanserin and olanzapine (Table 5). Sex was found to affect the occurrence of hyperprolac-tinemia in this study, we did a comparison of serum prolactin levels between sex in each antipsychotic drug through the ranked ANCOVA using chlorpromazine equi-valent dose as a covariate. Blonanserin, olanzapine, and risperidone were found higher serum prolactin levels in females, but no statistical significance between sex was found in the subject who took amisulpride (pvalue = 0.123) (Table 6).
Table 4.
Results of the ANCOVA analysis between serum prolactin levels and antipsychotic drugs
| Covariates | ANCOVA | Eta coefficient |
|---|---|---|
| CPZ equivalent dose | F = 10.702, pvalue < 0.001 | 0.224 |
| Sex |
ANCOVA, analysis of covariance; CPZ, chlorpromazine.
Table 5.
The Bonferroni post-hoc analysis according toantipsychotic drugs
| Comparison | Mean difference | 95% Cl for mean difference | pvalue | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| AMSP | BLON | 60.2 | 17.5 | 102.9 | 0.001 |
| OZP | 64.0 | 26.0 | 102.0 | < 0.001 | |
| RPR | 28.9 | −9.2 | 67.0 | 0.265 | |
| BLON | AMSP | −60.2 | −102.9 | −17.5 | 0.001 |
| OZP | 3.8 | −26.1 | 33.8 | 1.000 | |
| RPR | −31.3 | −61.6 | −1.0 | 0.038 | |
| OZP | AMSP | −64.0 | −102.0 | −26.0 | < 0.001 |
| BLON | −3.8 | −33.8 | 26.1 | 1.000 | |
| RPR | −35.1 | −58.0 | −12.3 | < 0.001 | |
| RPR | AMSP | −28.9 | −67.0 | 9.2 | 0.265 |
| BLON | 31.3 | 1.0 | 61.6 | 0.038 | |
| OZP | 35.1 | 12.3 | 58.0 | < 0.001 | |
AMSP, amisulpride; BLON, blonanserin; OZP, olanzapine; RPR, risperidone; CI, confidence interval.
Table 6.
Result of the ranked ANCOVA analysis between sex and each antipsychotic drug
| Antipsychotic drug | Serum prolactin level (ng/ml) | pvalue | |
|---|---|---|---|
| Male | Female | ||
| AMSP | 59.9 ± 9.7 | 138.0 ± 51.2 | 0.123 |
| BLON | 39.3 ± 20.3 | 56.5 ± 56.1 | 0.040 |
| OZP | 21.5 ± 8.3 | 61.6 ± 57.9 | 0.004 |
| RPR | 40.4 ± 25.4 | 108.9 ± 47.1 | <0.001 |
Values are presented as mean ± standard deviation.
AMSP, amisulpride; BLON, blonanserin; OZP, olanzapine; RPR, risperidone.
DISCUSSION
This study investigated the effects of antipsychotic drugs on serum prolactin levels by conducting clinical analysis of patients who were outpatients and inpatients at the Department of Psychiatry of a university hospital for about five years through CDW.
Hyperprolactinemia occurred in 70.1% (n = 82) of a total of 117 subjects. Among the four groups of our study, the amisulpride group, in particular, revealed the highest level of serum prolactin (122.4 ± 56.0 ng/ml), followed by risperidone (80.2 ± 52.0 ng/ml), blonanserin (49.8 ± 45.5 ng/ml), and olanzapine (43.3 ± 47.2 ng/ml). In an open-label study on prolactin elevation of atypical antipsychotic drugs, hyperprolactinemia occurred in 100% of subjects taking amisulpride and risperidone [23]. In a review study on the association between antipsychotic drugs and hyperprolactinemia, olanzapine showed a varied increase in prolactin levels ranging from 6% to 40% [6]. In the same study, risperidone showed a high level of hyperprolactinemia (about 89−100%), and in the study of 245 psychiatric patients receiving atypical antipsychotics in South Korea, about 79% of patients taking risperidone, demonstrated hyperprolactinemia. The incidence of hyperprolactinemia was higher compared to aripiprazole (10%), blonanserin (38%), olanzapine (29%), paliperidone (63%), and quetiapine (15%) [24]. In our study, hyperprolactinemia was observed in 100% of subjects taking amisulpride, 88.4% of subjects taking risperidone, 66.7% of patients taking blonanserin, about 47.8% of subjects taking olanzapine. The results are similar to those of the previous study; however, in the case of blonanserin, a higher rate of hyperprolactinemia, as noted though, the occurrence ranking was similar. It seems difficult to rule out the possibility that a higher rate of hyperprolactinemia occurred in the subjects because various doses were administered to subjects.
Severe hyperprolactinemia (> 100 ng/ml) has been reported to increase the risk of osteoporosis and, some cancers (breast cancer, endometrial cancer, etc.) and cardiovascular disease when it is persistent long-term hyperprolactinemia [25,26]. Especially in the case of hyperprolactinemia of more than 200 ng/ml, there might exist a possibility of adenoma development [20]. So additional organic cause evaluations, such as brain MRI and close observation, are recommended. Apparently, it is implied that additional evaluation and intervention, such as close observation and brain MRI are necessary for patients with moderate or severe hyperprolactinemia as defined in this study. Herein, about 90% of subjects taking amisulpride exhibited moderate-severe hyperprolactinemia thereby necessitating close observation and intervention, 69.8% in the risperidone group, 33.4% in the blonanserin group, and 21.7% in the olanzapine group.
Antipsychotic drugs are thought to induce hyperpro-lactinemia by binding with D2 dopamine receptors on the lactotroph cells and interfering with dopamine-induced inhibition of prolactin secretion in lactotroph cells with dependence on the strength of D2 blocking [3]. In addition, the lower the blood-brain barrier (BBB) penetration of each antipsychotic drug, the more it seems to affect the pituitary gland located outside the BBB, which causes a high rate of hyperprolactinemia [27]. Among antipsychotics, amisulpride has low lipophilicity than other antipsychotics, so it is assumed that amisulpride might be at a higher concentration in the pituitary gland, which is located outside the BBB, and leads to increased hyperprolactinemia than other antipsychotics [28]. However, the number of subjects who took amisulpride in this study was only 10, which is a small number of subjects compared to the cases with other drugs, so there might be a limitation on representativeness. In addition, amisulpride was admini-stered at the highest dose of chlorpromazine equivalent dose, so interpretation is likely to be limited. Meanwhile, risperidone is an atypical antipsychotic drug with a high D2 receptor occupancy index, which is presumed to cause higher and more frequent hyperprolactinemia [29].
Olanzapine has been reported to cause mild or transient hyperprolactinemia in several studies, similar to the case in this study. It is assumed that olanzapine has a lower D2 receptor occupancy than risperidone and paliperidone [8].
In the study about the analysis of therapeutic efficacy between blonanserin and risperidone, risperidone showed a 2.41-fold increase in serum prolactin level compared to blonanserin [13]. It has been reported that blonanserin led to a lower prolactin increase (34%) compared to risperidone (79%) and paliperidone (63%) in psychiatric patients receiving atypical antipsychotics in South Korea [24]. In our study, about 66.7% of the subjects who took blonanserin demonstrated hyperprolactinemia.
When considering the dose-dependent D2 receptor occupancy, there might be a limitation due to the use of various drug doses and the diversity of blood sampling conditions in each subject. However, only one case (5.6%) of severe hyperprolactinemia, was identified in the blonan-serin group, and it was the lowest rate among the four antipsychotic drugs. Blonanserin is known to block D2 dopamine receptors at a 20 times higher rate than risperidone, but it efficiently crosses the BBB than risperidone, amisulpride, or olanzapine. Hence, it seems to have a mild influence on the pituitary gland [30].
In addition, like previous studies [10], our study also found higher prolactin levels in female patients after taking antipsychotic drugs. The average serum prolactin level in female subjects was 86.8 ± 59.8 ng/ml and in male subjects, it was 32.8 ± 20.9 ng/ml. This reflects the sensitivity of females to prolactin levels and suggests that clinicians should be more sensitive in the assessment of prolactin levels in female patients. Notably, the subjects taking amisulpride showed an increase in serum prolactin levels regardless of sex, but the female subjects who were taking one of the antipsychotics among blonanserin, risperidone, and olanzapine had higher prolactin levels than the male subjects. Several studies have shown that prolactin increases according to the surge of progesterone, suggesting the possibility of fluctuation in prolactin levels during ovulation and follicular phases when the level of LH hormone increases rapidly [31].
However, in our study, the average age of women was 48.1 ± 18.8 years, which is only 1 year younger than the average age of menopause in South Korea, 49.7 years [32], so additional studies are needed on this topic. In the study on treatment-resistant schizophrenia patients with previous hyperprolactinemia, hyperprolactinemia was nor-malized in the female patients after taking blonanserin [14]. However, there exists a report that prolactin was rather elevated in the female patients who switched from olanzapine to blonanserin [33]. A study on prolactin levels and positive symptoms of schizophrenia following the use of olanzapine also reported higher prolactin levels in female patients as in our study. This seems to be due to differences in body fluid volume, body mass index, and hormonal changes that can affect the pharmacokinetics of antipsychotic drugs in female and male patients [34], but the effects of each drug require further follow-up studies.
This study has some limitations. First, the number of subjects is not equal between the different groups of drugs, and the representativeness of each group may be low because a small number of samples are included. Second, our study was a retrospective study because of the character of CDW. So we couldn’t control the condition of blood sampling for serum prolactin level. And we couldn’t control the dose and the duration of the drugs also, so it might influence serum prolactin levels. Also, various doses were administered for each drug at an equi-valent dose of chlorpromazine, so we performed an ANCOVA test to compensate for the dose of the administered antipsychotics. Nevertheless, it is difficult to completely rule out that there might be bias. Next, in order to exclude the administration of antidepressants that can increase serum prolactin levels, this study was conducted on the subject diagnosed with schizophrenia, schizotypal and delusional disorders, manic episodes, and bipolar affective disorder. And pituitary diseases that can cause hyperprolactinemia like prolactinoma, pituitary adenoma, and hypophysitis were excluded as criteria through reviewed Brain MRI. But due to the limitation of our CDW application, it was difficult to identify other drugs prescribed by other departments, and drugs prescribed by other hospitals were not known also. So, there might be a lack of consideration for drugs and diseases that could induce hyperprolactinemia. Fourth, our study selected the subjects according to the ICD-10 diagnosis entered on the Electronic Medical Record, however, it would be difficult to rule out the possibility of inaccurate diagnosis. Further studies involving control over the afore-mentioned confounding factors and a larger number of samples are necessitated.
Nevertheless, this study will be meaningful, as it is the first study in South Korea to use the CDW application and find the incidence of hyperprolactinemia according to antipsychotic drugs. The big data analysis through CDW enabled the evaluation of large amounts of data such as demographic characteristics, medical history, laboratory tests, and administration history in a relatively short time. Our study showed that characteristics of antipsychotic drugs could be evaluated using the CDW, and it suggested that it would help us to conduct more effective and simpler research in the future.
In conclusion, antipsychotic drugs are mostly used in psychosis patients, and it is the most common reason for non-neoplastic hyperprolactinemia. In our study, we found that amisulpride, risperidone, blonanserin, and olanzapine led to the highest prolactin levels in the specified order. Amisulpride and risperidone were found to induce hyperprolactinemia more extensively than blonanserin and olanzapine. Amisulpride showed elevated serum prolactin levels regardless of sex, but blonanserin, risperidone, and olanzapine led to higher prolactin levels in female subjects.
Consequently, it is proposed that clinicians should monitor serum prolactin levels carefully, especially in female patients taking antipsychotic drugs.
Footnotes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Jong-Hyun Jeong. Data acquisition: Jong-Hyun Jeong, Suhyung Kim. Formal analysis: Jong- Hyun Jeong, Suhyung Kim. Supervision: Jong-Hyun Jeong, Seung-Chul Hong, Ho-jun Seo, Tae-Won Kim, Yoo Hyun Um. Writing—original draft: Suhyung Kim. Writing—review & editing: Jong-Hyun Jeong, Seung-Chul Hong, Ho-jun Seo, Tae-Won Kim, Yoo Hyun Um.
References
- 1.Matei VP, Purnichi T, Mihailescu A, Grigoras R. Prolactin level in patients with first episode schizophrenia treated for one year with atypical antipsychotics. Acta Endocrinol (Buchar) 2018;14:483–490. doi: 10.4183/aeb.2018.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halperin Rabinovich I, Cámara Gómez R, García Mouriz M, Ollero García-Agulló D Grupo de Trabajo de Neuroendo-crinología de la SEEN, author. [Clinical guidelines for diagnosis and treatment of prolactinoma and hyperprolactinemia]. Endo-crinol Nutr. 2013;60:308–319. doi: 10.1016/j.endoen.2012.11.009. Spanish. [DOI] [PubMed] [Google Scholar]
- 3.Byerly M, Suppes T, Tran QV, Baker RA. Clinical implications of antipsychotic-induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorders: Recent developments and current perspectives. J Clin Psychopharmacol. 2007;27:639–661. doi: 10.1097/jcp.0b013e31815ac4e5. [DOI] [PubMed] [Google Scholar]
- 4.Inder WJ, Castle D. Antipsychotic-induced hyperprolactinaemia. Aust N Z J Psychiatry. 2011;45:830–837. doi: 10.3109/00048674.2011.589044. [DOI] [PubMed] [Google Scholar]
- 5.Poo SX, Agius M. Atypical antipsychotics for schizophrenia and/or bipolar disorder in pregnancy: Current recommendations and updates in the NICE guidelines. Psychiatr Danub. 2015;27 Suppl 1:S255–260. [PubMed] [Google Scholar]
- 6.Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psycho-pharmacol. 2008;22(2 Suppl):46–55. doi: 10.1177/0269881107088435. [DOI] [PubMed] [Google Scholar]
- 7.Chwieduk CM, Keating GM. Paliperidone extended release: A review of its use in the management of schizophrenia. Drugs. 2010;70:1295–1317. doi: 10.2165/11204840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics--a review. Hum Psychopharmacol. 2010;25:281–297. doi: 10.1002/hup.1116. [DOI] [PubMed] [Google Scholar]
- 9.Stevens JR, Kymissis PI, Baker AJ. Elevated prolactin levels in male youths treated with risperidone and quetiapine. J Child Adolesc Psychopharmacol. 2005;15:893–900. doi: 10.1089/cap.2005.15.893. [DOI] [PubMed] [Google Scholar]
- 10.Bushe C, Shaw M. Prevalence of hyperprolactinaemia in a naturalistic cohort of schizophrenia and bipolar outpatients during treatment with typical and atypical antipsychotics. J Psychopharmacol. 2007;21:768–773. doi: 10.1177/0269881107078281. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery J, Winterbottom E, Jessani M, Kohegyi E, Fulmer J, Seamonds B, et al. Prevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatment. J Clin Psychiatry. 2004;65:1491–1498. doi: 10.4088/JCP.v65n1108. [DOI] [PubMed] [Google Scholar]
- 12.Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry. 2002;159:133–135. doi: 10.1176/appi.ajp.159.1.133. [DOI] [PubMed] [Google Scholar]
- 13.Yu W, Lei L, Yu Y, Li Y, Shen Y, Li H. Model-based analysis of therapeutic efficacy of blonanserin and risperidone in schizophrenia patients and effects on prolactin: A randomized double-blind study. Hum Psychopharmacol. 2020;35:e2717. doi: 10.1002/hup.2717. [DOI] [PubMed] [Google Scholar]
- 14.Kawabe K, Horiuchi F, Ueno S. Blonanserin, a novel antipsychotic, is suitable for treating schizophrenia associated with hyperprolactinemia: a case series. Clin Neuropharmacol. 2013;36:239–241. doi: 10.1097/WNF.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 15.Kelly DL, Wehring HJ, Earl AK, Sullivan KM, Dickerson FB, Feldman S, et al. Treating symptomatic hyperprolactinemia in women with schizophrenia: Presentation of the ongoing DAAMSEL clinical trial (Dopamine partial Agonist, Aripiprazole, for the Management of Symptomatic ELevated prolactin) BMC Psychiatry. 2013;13:214. doi: 10.1186/1471-244X-13-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bushe CJ, Bradley A, Pendlebury J. A review of hyperpro-lactinaemia and severe mental illness: are there implications for clinical biochemistry? Ann Clin Biochem. 2010;47:292–300. doi: 10.1258/acb.2010.010025. [DOI] [PubMed] [Google Scholar]
- 17.Lee KH, Kang SH, Kang GY, Kim KH, Kim KK, Soh M, et al. The prevalence of hyperprolactinemia and amenorrhea and the association with sexual dysfunction in schizophrenic patients with antipsychotics. J Korean Neuropsychiatr Assoc. 2009;48:423–429. [Google Scholar]
- 18.Korean Psychosmatic Society, author. Treatment guidelines for antipsychotics drug-induced hyperprolactinemia. Korean Psychosmatic Society; 2022. [Google Scholar]
- 19.Kang E, Han SA, Kim S, Kim SM, Jang M, Lee HE, et al. Five-years of breast cancer management in a new hospital: Analysis using clinical data warehouse. J Breast Cancer. 2010;13:96–103. doi: 10.4048/jbc.2010.13.1.96. [DOI] [Google Scholar]
- 20.Serri O, Chik CL, Ur E, Ezzat S. Diagnosis and management of hyperprolactinemia. CMAJ. 2003;169:575–581. doi: 10.1097/01.pgo.0000873452.09562.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serri O, Li L, Mamputu JC, Beauchamp MC, Maingrette F, Renier G. The influences of hyperprolactinemia and obesity on cardiovascular risk markers: Effects of cabergoline therapy. Clin Endocrinol (Oxf) 2006;64:366–370. doi: 10.1111/j.1365-2265.2006.02469.x. [DOI] [PubMed] [Google Scholar]
- 22.Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69:440–447. doi: 10.1111/pcn.12275. [DOI] [PubMed] [Google Scholar]
- 23.Svestka J, Synek O, Tomanová J, Rodáková I, Cejpková A. Differences in the effect of second-generation antipsychotics on prolactinaemia: six weeks open-label trial in female in-patients. Neuro Endocrinol Lett. 2007;28:881–888. [PubMed] [Google Scholar]
- 24.Park YM, Lee SH, Lee BH, Lee KY, Lee KS, Kang SG, et al. Prolactin and macroprolactin levels in psychiatric patients receiving atypical antipsychotics: A preliminary study. Psychia-try Res. 2016;239:184–189. doi: 10.1016/j.psychres.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129–134. doi: 10.1192/bjp.bp.106.023671. [DOI] [PubMed] [Google Scholar]
- 26.Wang PS, Walker AM, Tsuang MT, Orav EJ, Glynn RJ, Levin R, et al. Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry. 2002;59:1147–1154. doi: 10.1001/archpsyc.59.12.1147. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Iglesias R, Mata I, Martínez-García O, Garcia-Unzueta MT, Amado JA, Valdizán EM, et al. Long-term effect of haloperidol, olanzapine, and risperidone on plasma prolactin levels in patients with first-episode psychosis. J Clin Psycho-pharmacol. 2012;32:804–808. doi: 10.1097/JCP.0b013e318272688b. [DOI] [PubMed] [Google Scholar]
- 28.Kopecek M, Bares M, Svarc J, Dockery C, Horácek J. Hyper-prolactinemia after low dose of amisulpride. Neuro Endocrinol Lett. 2004;25:419–422. [PubMed] [Google Scholar]
- 29.Besnard I, Auclair V, Callery G, Gabriel-Bordenave C, Roberge C. [Antipsychotic-drug-induced hyperprolactinemia: physiopathology, clinical features and guidance]. Encephale. 2014;40:86–94. doi: 10.1016/j.encep.2012.03.002. French. [DOI] [PubMed] [Google Scholar]
- 30.Tenjin T, Miyamoto S, Ninomiya Y, Kitajima R, Ogino S, Miyake N, et al. Profile of blonanserin for the treatment of schizophrenia. Neuropsychiatr Dis Treat. 2013;9:587–594. doi: 10.2147/NDT.S34433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford AM, Beasley CM, Jr, Tollefson GD. The acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentrations. Schizophr Res. 1997;26:41–54. doi: 10.1016/S0920-9964(97)00036-4. [DOI] [PubMed] [Google Scholar]
- 32.Korea Disease Control and Prevention Agency, author. Menopause [Internet] Korea Disease Control and Prevention Agency; Cheongju: 2021. [cited at 2022 Dec 19]. Available from: https://health.kdca.go.kr/healthinfo/biz/health/gnrlzHealthInfo/gnrlzHealthInfo/gnrlzHealthInfoView.do?cntnts_sn=421 . [Google Scholar]
- 33.Takahashi S, Suzuki M, Uchiyama M. One-year follow-up study of psychotic patients treated with blonanserin: a case series. Asia Pac Psychiatry. 2013;5:164–167. doi: 10.1111/j.1758-5872.2012.00232.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen YL, Cheng TS, Lung FW. Prolactin levels in olanzapine treatment correlate with positive symptoms of schizophrenia: Results from an open-label, flexible-dose study. Prim Care Companion J Clin Psychiatry. 2009;11:16–20. doi: 10.4088/PCC.08m00668. [DOI] [PMC free article] [PubMed] [Google Scholar]