Abstract
The mechanisms of fluconazole resistance in three clinical isolates of Candida krusei were investigated. Analysis of sterols of organisms grown in the absence and presence of fluconazole demonstrated that the predominant sterol of C. krusei is ergosterol and that fluconazole inhibits 14α-demethylase in this organism. The 14α-demethylase activity in cell extracts of C. krusei was 16- to 46-fold more resistant to inhibition by fluconazole than was 14α-demethylase activity in cell extracts of two fluconazole-susceptible strains of Candida albicans. Comparing the carbon monoxide difference spectra of microsomes from C. krusei with those of microsomes from C. albicans indicated that the total cytochrome P-450 content of C. krusei is similar to that of C. albicans. The Soret absorption maximum in these spectra was located at 448 nm for C. krusei and at 450 nm for C. albicans. Finally, the fluconazole accumulation of two of the C. krusei isolates was similar to if not greater than that of C. albicans. Thus, there are significant qualitative differences between the 14α-demethylase of C. albicans and C. krusei. In addition, fluconazole resistance in these strains of C. krusei appears to be mediated predominantly by a reduced susceptibility of 14α-demethylase to inhibition by this drug.
As the use of azole antifungal agents has risen dramatically, there has been an increase in the number of reports of azole-resistant isolates of Candida, especially in patients with advanced AIDS. In addition to the development of resistance by Candida albicans and Candida glabrata, it has been reported that the frequent use of fluconazole can select for the emergence of Candida krusei as a commonly isolated opportunistic pathogen in some medical centers (1, 33). This finding is clinically significant because C. krusei can cause serious infections in susceptible patients (8, 19). Furthermore, this organism is usually intrinsically resistant to fluconazole, both in vitro (3) and in vivo (4).
Three general mechanisms of azole resistance have been described for species of Candida. The first is an alteration in the target enzyme, 14α-demethylase. Inhibition of this enzyme by azoles causes an accumulation of C14 methylated sterols which likely disrupt membrane structure (9). In some resistant organisms, there is overexpression of the 14α-demethylase gene and/or the enzyme is less susceptible to azole inhibition (15, 24, 32). The second mechanism is decreased drug accumulation, mediated by either diminished uptake or increased efflux of the drug (22, 26). The third mechanism of resistance is the presence of a deficiency in C5(6) desaturase. Organisms deficient in this enzyme produce 14-methylfecosterol and remain viable when 14α-demethylase activity is inhibited (5, 14).
To determine if fluconazole resistance in C. krusei is mediated by one or more of these mechanisms, we analyzed the effects of this antifungal agent on sterol synthesis by three strains of C. krusei. In addition, the fluconazole uptake and cytochrome P-450 content of these organisms were measured. Our results indicate that the predominant mechanism of fluconazole resistance in these organisms is a 14α-demethylase with reduced susceptibility to the inhibitory effects of fluconazole.
MATERIALS AND METHODS
Fungal strains and susceptibility testing.
Three clinical isolates of C. krusei, strains 91-1158, 91-1159, and 91-1161, were generously provided by Michael Rinaldi (San Antonio, Tex.). A fourth isolate of C. krusei, ATCC 6258, was obtained from the American Type Culture Collection (Rockville, Md.). Two clinical isolates of C. albicans, Y01.345 and SC5314, were supplied by Christopher Hitchcock and William Fonzi (Georgetown University School of Medicine, Washington, D.C.), respectively. The susceptibilities of the organisms to fluconazole and itraconazole were determined at 24 and 48 h by the National Committee for Clinical Laboratory Standards M27-A broth microdilution method at an inoculum of 103 organisms per well (20). The medium was RPMI 1640 (Irvine Scientific, Santa Ana, Calif.) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS). The MICs were defined as the concentrations of drug that reduced growth by 80% compared to that of organisms grown in the absence of drug.
Sterol analysis.
For sterol extraction, each strain of C. krusei was grown for 24 h at 37°C on a rotary shaker. The medium was Sabouraud dextrose broth (Difco, Detroit, Mich.), with and without fluconazole. The concentration of fluconazole was 16 μg/ml, the highest concentration at which these organisms would grow in this medium. The organisms were harvested by centrifugation and washed twice in 0.85% saline, and their total sterols were extracted by ethanolic KOH, as described previously (6, 7). The resultant sterols were further purified by thin-layer chromatography on PK6F silica gel 60-Å plates (Whatman, Clifton, N.J.) with a solvent system of petroleum ether-diethyl ether (3:1, vol/vol) (27). The sterols were eluted from the silica in chloroform-diethyl ether-ethanol (1:1:1). After being derivatized with hexamethyldisilazane and trimethylchlorosilane (28), the sterols were redissolved in hexane and analyzed by gas chromatography-mass spectrometry. The sterols were identified by comparison to known standards and published data (16, 17, 23).
Fluconazole accumulation.
A filter-based assay was used to measure the accumulation of fluconazole by the organisms (22). These organisms were grown to exponential phase in Sabouraud dextrose broth and suspended in phosphate-buffered saline (pH 7.5) containing 5% glucose (wt/vol) at 108 organisms per ml. Next, a mixture of [3H]fluconazole (specific activity, 715 GBq/mmol) and unlabeled fluconazole was added to the cells so that the final concentration of fluconazole was 100 nM (0.2 μCi/ml). At selected intervals, aliquots were removed and the organisms were collected by filtration. Next, the organisms were washed four times in phosphate-buffered saline containing 100 μM unlabeled fluconazole. The amount of cell-associated radioactivity was determined by scintillation counting. All experiments were performed in triplicate.
Carbon monoxide difference spectra of microsomes.
The cytochrome P-450 content of the organisms was analyzed by measuring their carbon monoxide difference spectra. Organisms were grown to exponential phase in Sabouraud dextrose broth at 37°C and harvested by centrifugation. They were spheroplasted with lyticase (Sigma, St. Louis, Mo.) in sorbitol buffer (1.5 M sorbitol in 10 mM Tris buffer, pH 7.4) and resuspended in 10 mM Tris buffer, pH 7.4, containing 0.65 M sorbitol, 0.1 mM EDTA, 0.1 mM glutathione, and protease inhibitors (Boehringer Mannheim, Indianapolis, Ind.) (10). All subsequent steps were carried out at 4°C. The spheroplasts were broken into microsomes by sonication, after which debris and unbroken cells were removed by centrifugation at 1,500 × g for 10 min followed by 25,000 × g for 25 min. The microsomes were harvested with calcium chloride by the method of Käppeli et al. (13), washed once in 10 mM Tris buffer, pH 7.4, containing 150 mM potassium chloride, and resuspended in 100 mM Tris containing 0.65 M glycerol, 0.1 mM EDTA, and 0.1 mM glutathione. The carbon monoxide difference spectra of the microsomes were measured, and their cytochrome P-450 content was calculated by using an extinction coefficient of 91 liters/mmol/cm (21). The protein concentration of each microsome suspension was determined by the Bio-Rad protein assay (Bio-Rad, Hercules, Calif.). For each organism, the cytochrome P-450 contents of at least three different preparations of microsomes were measured.
Sterol biosynthesis by cell extracts.
To determine the effects of fluconazole on the synthesis of ergosterol from [14C]mevalonic acid, the organisms were grown in Sabouraud dextrose broth until late exponential phase. The organisms were broken by vortexing with glass beads in 0.1 M potassium phosphate, pH 7.5. Debris and unbroken cells were removed by centrifugation at 2,000 × g for 5 min followed by 10,000 × g for 10 min. To measure sterol biosynthesis, 925 μl of the resultant supernatant was added to 75 μl of cofactor buffer to achieve the following final concentrations: 1 μM NADP, 1 μM NADPH, 1 μM NAD, 7 μM glucose-6-phosphate, 5 μM ATP, 3 μM reduced glutathione, 2 μM MnCl2, and 0.25 μCi [14C]mevalonic acid (10, 12). Selected concentrations of fluconazole were added to the reaction mixtures before the addition of the cell extracts. After incubation at 37°C for 2 h, the reaction was stopped with ethanolic KOH. The samples were saponified at 80°C for 45 min, after which the nonsaponifiable lipids were extracted with petroleum ether (bp 40 to 60°C). The samples were dried under nitrogen and redissolved in chloroform. The sterols were separated by thin-layer chromatography on silica gel LK6D (Whatman) with a solvent system of petroleum ether-diethyl ether (3:1, vol/vol) (27). The sterols were visualized by iodine staining and identified by comparison with commercially available standards which were run in parallel. Next, the sterol-containing bands were scraped from the plates and 14C incorporation was determined by liquid scintillation counting. All experiments were repeated at least three times with different cell extracts.
RESULTS
Growth in fluconazole reduced the ergosterol content of C. krusei.
Fluconazole MICs for C. krusei 91-1158, 91-1159, and 91-1161 were all high, whereas both strains of C. albicans were susceptible to this drug (Table 1). C. albicans Y01.345 was slightly more susceptible to itraconazole than C. albicans SC5314 or the three strains of C. krusei. Analysis of the sterols of the organisms by gas chromatography-mass spectrometry revealed that the predominant sterol in C. krusei was ergosterol (Table 2). Growing all three strains of C. krusei in the presence of fluconazole caused a decrease in their ergosterol content and a marked increase in lanosterol. Other 14-methyl sterols that were increased in organisms exposed to fluconazole were 14-methylfecosterol and eburicol. These results suggest that fluconazole inhibits 14α-demethylase in C. krusei. No 14-methyl-ergosta-8,24(28)-dien-3,6-diol was detected, although this sterol has been reported to accumulate when C. krusei is exposed to azoles (18, 31).
TABLE 1.
Antifungal susceptibilities of C. albicans and C. krusei
| Organism | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Fluconazole
|
Itraconazole
|
|||
| 24 h | 48 h | 24 h | 48 h | |
| C. albicans Y01.345 | 0.25 | <0.25 | 0.125 | 0.125 |
| C. albicans SC5314 | 0.5 | 1 | 0.5 | 0.5 |
| C. krusei 91-1158 | 32 | 64 | 0.5 | 0.5 |
| C. krusei 91-1159 | 16 | 32 | 0.5 | 0.5 |
| C. krusei 91-1161 | 16 | 32 | 0.5 | 0.5 |
TABLE 2.
Effect of fluconazole on the sterols of C. krusei
| Sterol | Proportion of total sterols (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Control (no drug)
|
Fluconazolea
|
|||||||
| 91-1158 | 91-1159 | 91-1161 | ATCC 6258 | 91-1158 | 91-1159 | 91-1161 | ATCC 6258 | |
| Cholesta-7,24-dienol | 1.3 | 1.8 | 1.3 | 1.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ergosterol | 88.9 | 86.5 | 91.1 | 93.0 | 82.1 | 83.4 | 83.7 | 72.9 |
| Ergosta-7,22-dienol | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 |
| 14-Methylfecosterol | 0.0 | 0.0 | 0.0 | 0.0 | 2.1 | 1.2 | 1.8 | 1.5 |
| Ergosta-8,24(28)-dienol | 0.0 | 3.2 | 2.3 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 |
| Fecosterol | 2.9 | 0.0 | 0.0 | 1.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ergosta-5,7-dienol | 2.6 | 3.7 | 2.3 | 1.4 | 0.5 | 1.5 | 0.6 | 1.1 |
| Episterol | 1.6 | 2.4 | 1.8 | 1.1 | 0.5 | 0.0 | 0.2 | 0.3 |
| Lanosterol | 0.4 | 0.4 | 0.3 | 0.5 | 5.9 | 9.0 | 10.9 | 19.5 |
| 4-Methylfecosterol | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 |
| 4,14-Dimethyl-cholesta-8,24-dienol | 2.3 | 2.0 | 1.0 | 0.6 | 5.9 | 0.0 | 0.0 | 0.0 |
| Eburicol | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 1.8 | 0.5 | 2.0 |
| Unidentified sterols | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 2.2 | 2.5 |
Organisms were grown with fluconazole (16 μg/ml) for 24 h.
The 14α-demethylase from cell extracts of C. krusei showed reduced susceptibility to inhibition by fluconazole.
Although fluconazole appeared to inhibit 14α-demethylase in C. krusei, exposure to a relatively high concentration of drug was required to produce this inhibition. Therefore, we examined whether the enzyme itself was resistant to the inhibitory effects of fluconazole. We determined the effects of this drug on the synthesis of ergosterol from [14C]mevalonic acid in cell extracts of three strains of C. krusei and two isolates of C. albicans. We found that the concentration of fluconazole required to inhibit the synthesis of ergosterol by 50% (IC50) was 16- to 46-fold higher in cell extracts from C. krusei than in extracts from either strain of C. albicans (Table 3). This difference indicates that reduced fluconazole susceptibility of the 14α-demethylase from C. krusei is likely the predominant mechanism of fluconazole resistance in these isolates.
TABLE 3.
Comparison of total cytochrome P-450 content and fluconazole susceptibility of 14α-demethylases of C. albicans and C. kruseia
| Organism | Fluconazole IC50 (μM)b | Cytochrome P-450 content (nmol/mg of protein) |
|---|---|---|
| C. albicans Y01.345 | 0.017 ± 0.005 | 0.077 ± 0.037 |
| C. albicans SC5314 | 0.032 ± 0.006 | 0.058 ± 0.037 |
| C. krusei 91-1158 | 0.751 ± 0.384 | 0.052 ± 0.021 |
| C. krusei 91-1159 | 0.495 ± 0.208 | 0.065 ± 0.018 |
| C. krusei 91-1161 | 0.795 ± 0.436 | 0.077 ± 0.060 |
Results are the means ± standard deviations of at least three separate measurements.
Concentration of fluconazole required to inhibit the synthesis of ergosterol from [14C]mevalonic acid by 50%.
Carbon monoxide difference spectra also indicated that the cytochrome P-450 of C. krusei is different from that of C. albicans.
Next, we determined the carbon monoxide difference spectra of microsomes from these organisms. This procedure enabled us to evaluate further the possibility that fluconazole resistance was mediated by quantitative and/or qualitative changes in the 14α-demethylase of the organism. We found that the cytochrome P-450 content of the three strains of C. krusei was similar to that of the two strains of C. albicans (Table 3). Since 14α-demethylase accounts for the majority of cytochrome P-450 in yeasts, these findings suggest that the mechanism of fluconazole resistance in C. krusei is not mediated by overproduction of the target enzyme.
In these experiments, the Soret absorption maximum of microsomes from all three strains of C. krusei was located at 448 nm whereas the Soret peak from microsomes of C. albicans Y01.345 and SC5314 was located at 450 nm. This difference in absorption maxima also suggests that there are qualitative differences in the 14α-demethylase from the two different species of Candida.
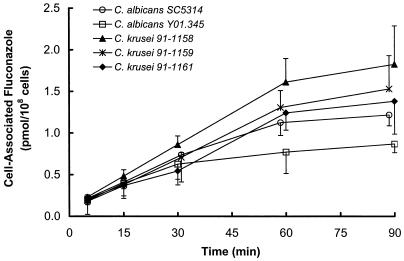
Fluconazole accumulation by C. krusei and C. albicans was similar.
To determine if reduced intracellular concentrations of the drug contributed to fluconazole resistance, the fluconazole accumulation of the three isolates of C. krusei was compared with that of the two strains of C. albicans. During the first 30 min, the accumulation of fluconazole by C. krusei was not consistently different from the accumulation of this drug by C. albicans (Fig. 1). However, at later time points, there was a trend towards greater accumulation of fluconazole by the strains of C. krusei. Of the organisms studied, C. albicans Y01.345 had the lowest fluconazole accumulation at 60 and 90 min. Therefore, the three isolates of C. krusei appear to be resistant to fluconazole by a mechanism that is independent of reduced fluconazole accumulation.
FIG. 1.
Accumulation of fluconazole by C. krusei and C. albicans. Results are the means of triplicate determinations. Error bars indicate standard deviations.
DISCUSSION
By comparing the effects of fluconazole on the synthesis of sterols by cell extracts of C. krusei and C. albicans, we determined that a major mechanism of fluconazole resistance in the three strains of C. krusei studied appears to be a reduced susceptibility of 14α-demethylase to fluconazole. The fluconazole IC50s for sterol synthesis by cell extracts of C. krusei ranged from 0.495 to 0.795 μM. These values were 16- to 46-fold greater than the corresponding IC50s for extracts of C. albicans. In the literature, there is substantial variability in the reported fluconazole IC50s for cell extracts of C. krusei. These values range from 0.080 to 1.38 μM (18, 31). Possible explanations for these differences include differences in methodology or strain-to-strain variations in the mechanism of fluconazole resistance in C. krusei. It is noteworthy that no previous investigators have reported the direct comparison of the fluconazole IC50 of C. krusei with that of a fluconazole-susceptible species of Candida. However, Venkateswarlu et al. (30) have reported that the fluconazole IC50 for cell extracts of different strains of C. albicans ranged from 0.042 to 0.055 μM, and we have determined previously that the fluconazole IC50 for 14α-demethylase purified from C. albicans is 0.074 μM (11). These values are only slightly higher than the ones reported here.
An additional finding was that the Soret maximum of microsomes of C. krusei was located at 448 nm, whereas it was located at 450 nm when microsomes of C. albicans were analyzed. This result also suggests that the 14α-demethylase of C. krusei is significantly different from that of C. albicans. Venkateswarlu et al. (29, 31) reported that the Soret peak of C. krusei microsomes was at 448 nm, which is similar to our findings. However, other investigators have found that the Soret peak of C. albicans microsomes is also located 448 nm, and we have determined that purified 14α-demethylase of C. albicans has a Soret peak at 447 (12, 15). It is possible that the difference between these results and our present findings is the result of differences in culture conditions and/or the methods used to purify the microsomes. For example, Sanglard et al. (25) found that the Soret maximum of microsomes of Candida tropicalis ranged from 447 to 450 nm, depending on the conditions under which the organisms were grown.
Burgener-Kairuz et al. (2) have determined the sequence of a 1.2-kb fragment of the C. krusei 14α-demethylase gene. They found that the deduced amino acid sequence of this fragment was only 80% similar to the corresponding portion of the 14α-demethylase of C. albicans. This finding also supports our conclusion that the 14α-demethylase of C. krusei differs significantly from that of C. albicans. It is not known which region(s) of the C. krusei 14α-demethylase is responsible for its reduced susceptibility to fluconazole, but this question is currently being investigated.
The reduced carbon monoxide difference spectra also indicated that the cytochrome P-450 content of C. krusei was similar to that of C. albicans. Other investigators have reported that the cytochrome P-450 contents of C. krusei and C. albicans are approximately 0.03 nmol/mg of protein and 0.02 to 0.05 nmol/mg of protein, respectively (12, 29–31). These results support the conclusion that C. krusei is not resistant to fluconazole because of overproduction of the target enzyme.
When the different strains of C. krusei were grown in the presence of fluconazole, we found a decrease in the amount of ergosterol and an increase in 14-methyl sterols, mainly lanosterol. Unlike other investigators, we did not find any evidence of 14-methyl-ergosta-8,24(28)-dien-3,6-diol in the organisms exposed to fluconazole (18, 31). This sterol was not detected in cell extracts from C. krusei ATCC 6258, even though Venkateswarlu et al. (31) reported its presence when this strain was grown in the presence of itraconazole. A likely explanation for this difference is that even though we grew the organisms in the maximal concentration of fluconazole that did not completely inhibit fungal growth, there was only partial inhibition of ergosterol synthesis. Others have observed that a strain of C. krusei grown in subinhibitory concentrations of itraconazole had a reduction in ergosterol content without the accumulation of 14-methyl-ergosta-8,24(28)-dien-3,6-diol (18). Thus, it is likely that, if it had been possible to grow the organisms in higher concentrations of fluconazole, we would have found the accumulation of this sterol. Nevertheless, because we found that fluconazole caused an accumulation of 14-methylfecosterol which was not converted to 14-methyl-ergosta-8,24(28)-dien-3,6-diol, we cannot completely rule out the possibility that C5(6) desaturase contributes to fluconazole resistance in some strains of C. krusei.
An additional finding was that the fluconazole accumulations of the three strains of C. krusei were similar to those of C. albicans. These results are different from those of Marichal et al. (18), who concluded that C. krusei is resistant to fluconazole on the basis of diminished drug accumulation. However, these investigators found that the fluconazole accumulation by exponential-phase organisms was actually fourfold greater than that observed with the strains of C. krusei used in this study. It is possible that differences in either methodology or the strains of C. krusei account for these differences in results.
Venkateswarlu et al. (31) also reported that itraconazole resistance in C. krusei is mediated by reduced drug accumulation. They found that a strain of C. krusei that was resistant to itraconazole accumulated less drug than did an itraconazole-susceptible isolate of C. krusei. While the two organisms had different susceptibilities to itraconazole, both exhibited high-level fluconazole resistance. These results demonstrate that resistance to fluconazole in C. krusei is mediated by a different mechanism than is itraconazole resistance.
In conclusion, our data indicate that, in the strains of C. krusei studied, fluconazole resistance is largely the result of a decreased susceptibility of 14α-demethylase to the inhibitory effects of fluconazole. Diminished accumulation of fluconazole did not appear to contribute to fluconazole resistance in these isolates. However, it is possible that reduced fluconazole accumulation is the predominant mechanism of fluconazole resistance in other isolates of C. krusei. Future work will investigate whether different strains of C. krusei have different mechanisms of resistance to this drug and why the 14α-demethylase of this organism is less susceptible to inhibition by fluconazole.
ACKNOWLEDGMENTS
We thank Michael Mador and Trang Phan for technical assistance. We also appreciate the assistance of W.-N. Paul Lee and Anne Bergener at the Stable Isotope Facility at Harbor-UCLA Research and Education Institute.
This work was supported by a grant from Pfizer, Inc.
REFERENCES
- 1.Borg-von Zepelin M, Eiffert H, Kann M, Rüchel R. Changes in the spectrum of fungal isolates: results from clinical specimens gathered in 1987/88 compared with those in 1991/92 in the University Hospital Göttingen, Germany. Mycoses. 1993;36:247–253. doi: 10.1111/j.1439-0507.1993.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 2.Burgener-Kairuz P, Zuber J-P, Jaunin P, Buchman T G, Bille J, Rossier M. Rapid detection and identification of Candida albicans and Torulopsis (Candida) glabrata in clinical specimens by species-specific nested PCR amplification of a cytochrome P-450 lanosterol-α-demethylase (L1A1) gene fragment. J Clin Microbiol. 1994;32:1902–1907. doi: 10.1128/jcm.32.8.1902-1907.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dermoumi H. In vitro susceptibility of yeast isolates from the blood to fluconazole and amphotericin B. Chemotherapy. 1992;38:112–117. doi: 10.1159/000238950. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M A, Shen S-H, Haddad J, Tarry W F. Comparison of in vivo activity of fluconazole with that of amphotericin B against Candida tropicalis, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother. 1989;33:1443–1446. doi: 10.1128/aac.33.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geber A, Hitchcock C A, Swartz J E, Pullen F S, Marsden K E, Kwon-Chung K J, Bennett J E. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob Agents Chemother. 1995;39:2708–2717. doi: 10.1128/aac.39.12.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghannoum M A, Janini G, Khamis L, Radwan S S. Dimorphism-associated variations in the lipid composition of Candida albicans. J Gen Microbiol. 1986;132:2367–2375. doi: 10.1099/00221287-132-8-2367. [DOI] [PubMed] [Google Scholar]
- 7.Ghannoum M A, Swairjo I, Soll D R. Variation in lipid and sterol contents in Candida albicans white and opaque phenotypes. J Med Vet Mycol. 1990;28:103–115. [PubMed] [Google Scholar]
- 8.Goldman M, Pottage J C, Weaver D C. Candida krusei fungemia. Report of 4 cases and review of the literature. Medicine. 1993;72:143–150. [PubMed] [Google Scholar]
- 9.Hitchcock C A. Resistance of Candida albicans to azole antifungal agents. Biochem Soc Trans. 1993;21:1039–1047. doi: 10.1042/bst0211039. [DOI] [PubMed] [Google Scholar]
- 10.Hitchcock C A, Brown S B, Evans E G V, Adams D J. Cytochrome P-450-dependent 14α-demethylation of lanosterol in Candida albicans. Biochem J. 1989;260:549–556. doi: 10.1042/bj2600549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hitchcock C A, Dickinson K, Brown S B, Evans E G V, Adams D J. Interaction of azole antifungal antibiotics with cytochrome P-450-dependent 14α-sterol demethylase purified from Candida albicans. Biochem J. 1990;266:475–480. doi: 10.1042/bj2660475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hitchcock C A, Dickinson K, Brown S B, Evans E G V, Adams D J. Purification and properties of cytochrome P-450-dependent 14α-sterol demethylase from Candida albicans. Biochem J. 1989;263:573–579. doi: 10.1042/bj2630573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Käppeli O, Sauer M, Fiecther A. Convenient procedure for the isolation of highly enriched, cytochrome P-450-containing microsomal fraction from Candida tropicalis. Anal Biochem. 1982;126:179–182. doi: 10.1016/0003-2697(82)90126-9. [DOI] [PubMed] [Google Scholar]
- 14.Kelly S L, Lamb D C, Kelly D E, Manning N J, Loeffler J, Hebart H, Schumacher U, Einsele H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5,6-desaturation. FEBS Lett. 1997;400:80–82. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 15.Lamb D C, Kelly D E, Schunck W H, Shyadehi A Z, Akhtar M, Lowe D J, Baldwin B C, Kelly S L. The mutation T315A in Candida albicans sterol 14α-demethylase causes reduced enzyme activity and fluconazole resistance through reduced affinity. J Biol Chem. 1997;272:5682–5688. doi: 10.1074/jbc.272.9.5682. [DOI] [PubMed] [Google Scholar]
- 16.Loeffler R S T, Hayes A L. Sterols of the plant pathogenic fungi Botrytis cinera and pyrenophora teres. Phytochemistry. 1990;29:3424–3425. [Google Scholar]
- 17.Loeffler R S T, Hayes A L. Effects of sterols biosynthesis inhibitor fungicides on growth and sterol composition of Ustilago maydis, Botrytis cinera and pyrenophora teres. Pestic Sci. 1992;35:7–17. [Google Scholar]
- 18.Marichal P, Gorrens J, Coene M-C, LeJeune L, Vanden Bossche H. Origin in differences in susceptibility of Candida krusei to azole antifungal agents. Mycoses. 1995;38:111–117. doi: 10.1111/j.1439-0507.1995.tb00032.x. [DOI] [PubMed] [Google Scholar]
- 19.McQuillen, D. P., B. S. Zingman, F. Meunier, and S. M. Levitz. Invasive infections due to Candida krusei: report of ten cases of fungemia that include three cases of endophthalmitis. Clin. Infect. Dis. 14:472–478. [DOI] [PubMed]
- 20.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast. Tentative standard. Document M27-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 21.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 22.Parkinson T, Falconer D J, Hitchcock C A. Fluconazole resistance due to energy-dependent drug efflux in Candida glabrata. Antimicrob Agents Chemother. 1995;39:1696–1699. doi: 10.1128/aac.39.8.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quail M A, Arnoldi A, Moore D J, Goosey M W, Kelly S L. Ketoconazole mediated growth inhibition in Botrytis cinera and Saccharomyces cerevisiae. Phytochemistry. 1993;32:273–280. [Google Scholar]
- 24.Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanglard D, Käppeli O, Fiecther A. The distinction of different types of cytochromes P-450 from the yeasts Candida tropicalis and Saccharomyces uvarum. Arch Biochem Biophys. 1986;251:276–286. doi: 10.1016/0003-9861(86)90075-5. [DOI] [PubMed] [Google Scholar]
- 26.Sanglard D, Kuchler K, Ischer F, Pagani J-L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorkhoh N A, Ghannoum M A, Ibrahim A S, Stretton R J, Radwan S S. Growth of Candida albicans in the presence of hydrocarbons: a correlation between sterol concentration and hydrocarbon uptake. Appl Microbiol Biotechnol. 1991;34:509–512. [Google Scholar]
- 28.Vandenheuvel F A, Court A S. Reference high-efficiency non-polar packed column for the gas-liquid chromatography of nanogram amounts of sterols. Part I. Retention time data. J Chromatogr. 1968;38:439–459. doi: 10.1016/0021-9673(68)85073-3. [DOI] [PubMed] [Google Scholar]
- 29.Venkateswarlu K, Denning D W, Kelly S L. Inhibition and interaction of cytochrome P450 of Candida krusei with azole antifungal drugs. J Med Vet Mycol. 1997;35:19–25. [PubMed] [Google Scholar]
- 30.Venkateswarlu K, Denning D W, Manning N J, Kelly S L. Comparison of D0870, a new triazole antifungal agent, to fluconazole for inhibition of Candida albicans cytochrome P-450 by using in vitro assays. Antimicrob Agents Chemother. 1996;40:1382–1386. doi: 10.1128/aac.40.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkateswarlu K, Denning D W, Manning N J, Kelly S L. Reduced accumulation of drug in Candida krusei accounts for itraconazole resistance. Antimicrob Agents Chemother. 1996;40:2443–2446. doi: 10.1128/aac.40.11.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White T C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wingard J R, Merz W G, Rinaldi M G, Johnson T R, Karp J E, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325:1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]