Abstract
Background:
Mucinous rectal cancer is associated with a higher incidence of microsatellite instability, and a poorer response to neoadjuvant chemoradiotherapy compared to other subtypes of rectal adenocarcinoma. Immune checkpoint inhibitors are an emerging family of anti-cancer therapeutics associated with highly variable outcomes in colorectal cancer. Though the immune landscape of mucinous rectal cancer has not been fully explored, the presence of mucin is thought to act as a barrier preventing immune cell infiltration.
Objective:
The aim of this study was to determine the immune properties of mucinous rectal cancer and investigate the degree of lymphocyte infiltration in this cohort.
Design:
This is a retrospective cohort study which involved, multiplexed immunofluorescence staining of tumor micro-arrays.
Settings:
Samples originated from a single university teaching hospital.
Patients:
Our cohort included 15 cases of mucinous and 43 cases of non-mucinous rectal cancer
Main Outcome Measures:
Immune cells were classified and quantified. Immune cell counts were compared between mucinous and non-mucinous cohorts. Immune marker expression within tumor epithelial tissue was evaluated to determine degree of lymphocyte infiltration.
Results:
Cytotoxic (p=0.022), and regulatory T-cells (p=0.010) were found to be overrepresented in the mucinous cohort compared to the non-mucinous group. PD-1 expression was also found to be significantly greater in the mucinous group (p=0.001). CD3 (p=0.001) and CD8 (p=0.054) expression within tumor epithelium was also higher in the mucinous group, suggesting adequate immune infiltration despite the presence of mucin. Microsatellite instability status was not found to be a predictor of immune marker expression in our analysis.
Limitations:
The relatively small size of the cohort.
Conclusion:
Mucinous rectal cancer is associated with an immune rich tumour microenvironment, which was not associated with microsatellite instability status.
Keywords: Mucinous, Rectal Cancer, Multiplex Imaging, Microsatellite Instability, Immunotherapy, Tumor Microenvironment
Introduction:
Up to 5-15% of all rectal adenocarcinomas are of the mucinous subtype 1. These tumors are characterised by an abundance of extracellular mucin that constitutes more than 50 % of tumor volume 2. Mucinous colon and rectal adenocarcinomas have a high incidence of Microsatellite instability (MSI) and are more likely to demonstrate BRAF mutation compared to non-mucinous colorectal adenocarcinomas 3, 4. Mucinous rectal cancer is also associated with reduced rates of pathological complete response (pCR) and tumour down-staging following neoadjuvant chemoradiotherapy 5. As a result, patients treated for this disease have an increased likelihood of having a positive resection margin and are more likely to have a poor definitive outcome 5. Evidently, there is a need to identify alternative treatment strategies within this cohort.
The immune system is recognised as having a key role in preventing cancer progression 6. Treatment strategies aimed at promoting anti-cancer immune activity have emerged, and form the basis of anti-tumor immunotherapy 7. Critical to the anti-tumor immune response, is the activation, dissemination and cytotoxic effect of T-cells in response to the identification of tumor neoantigens 8. Tumor cells promote immunosuppression via inhibitory ligand-receptor interactions with various immune cells within the tumor microenvironment, these interactions are known as immune checkpoints 9, 10. Response to immune checkpoint inhibition (ICI) is highly variable in colorectal cancer (CRC) and is heavily dependent upon the degree of pre-treatment T-cell infiltration 11. At present, usage is limited to the treatment of patients with MSI-high, metastatic disease 11, 12. However, clinical trials exploring the utility of ICI to treat MSI-high rectal tumors in the neoadjuvant setting are underway, with early results demonstrating great promise 13.
As mentioned, mucinous CRCs have an increased incidence of MSI and are more likely to demonstrate a mismatch repair deficiency 4, 14. These characteristics are associated with a high neoantigen burden and increased immunogenicity 15. However, mucinous tumors also feature abundant extracellular MUC2 protein. MUC2 is thought to behave as a physical barrier in mucin, preventing immune cell infiltration 16. Additionally, mucin contains adhesion ligands and suppressive cytokines which may prevent infiltration of antigen-presenting cells or effector cells involved in the T-cell mediated host immune response 17. A previous study by Tozawa et al. found mucinous colorectal tumors to be associated with reduced peritumoral lymphocyte infiltration compared to non-mucinous CRCs 18. A further analysis by Nazemalhosseini-Mojarad et al. found no difference in the distribution of stromal or infiltrative CD8+ lymphocytes in CRCs with mucinous characteristics compared to non-mucinous CRCs 19. However a comprehensive analysis of the immune landscape of mucinous CRC has to date not been performed.
This study represents the first analysis specifically looking at the immune landscape of mucinous rectal cancer; we herein employed an innovative multiplexed immunofluorescence imaging technique, which is comprised of a repeated stain-image-dye-inactivation sequence to evaluate a panel of important immune antibodies in a cohort of rectal tumors 20, 21. The aim of this study was to combine quantitative analytics with spatial tissue profiling to characterise the immune landscape of mucinous rectal cancer and determine the infiltrative capacity of lymphocytes in mucinous rectal cancer.
Methods:
Patient Cohort
Formalin-fixed, paraffin-embedded (FFPE) primary tumor tissue sections were obtained from 53 patients with stage I-III mucinous or non-mucinous rectal cancer. Mucinous tumors were defined by a consultant pathologist (TO’G, Beaumont Hospital) as those with greater than 50% of the tumor composed of extracellular mucin according to WHO classification 22. Clinical and pathological variables collected for analysis included; patient age, gender, disease stage, exposure to neoadjuvant chemoradiotherapy, microsatellite stability status, KRAS mutation status, BRAF mutation status, lymphovascular invasion status, perineural invasion status and extramural venous invasion status. Tissue was provided from the Beaumont Hospital Colorectal Biobank with written consent provided by all patients. Institutional ethical approval was granted by the Beaumont Research and Ethics Committee.
Immunofluorescence staining of tumor microarrays
Three tumor cores per patient were prepared on a microarray slide, each core was 1mm in diameter. All cores were taken from resected surgical specimens. Punches were taken from different regions within the centre of the tumor, based on identification by pathology (TO’G, Beaumont Hospital). Multiplexed immunofluorescence staining of the tumor micro-array were performed using the Cell DIVE™ technology (Leica Microsystems). This involves multiple rounds of antibody staining performed on the same tissue section with mild dye oxidation between successive rounds of staining and imaging 23. Epithelial cells and stromal cells were segmented using antibody stains against DAPI, pan-cytokeratin (CK-26), ribosomal S6 and Na+K+ATPase. For the purpose of this study we focused on expression of immune markers; CD3, CD4, CD8, CD20, programmed cell death protein 1 (PD-1), forkhead box P3 (FOXP3) and human leukocyte antigen class 1 (HLA1).
Antibodies were acquired commercially and underwent a multi-step process of validation and conjugation (as previously described by Gerdes et al. 23). Sources of antibodies are outlined in supplementary Table 1. Immune cells were classified according to expression profiles and were quantified at a patient level, using methods previously published by our group (See Table 1) 21. The cell type composition in CRC core tissues varied significantly, with some cores showing predominantly cancerous/epithelial cells in the absence of immune cell infiltration, and others showing very high levels (up to 55%) of immune cells. A bootstrap analysis using randomly sampled pairings; found cell type composition in cores from the same patient, to be more similar to each other compared to random pairings, suggesting that cell type composition was a biological feature of individual tumors.
Table 1.
Expression patterns utilised to classify immune cells.
| Immune Cell | Expression Classification |
|---|---|
| Cytotoxic T Cell | (CD3+, CD4+, CD8+, FOXP3−) |
| Regulatory T Cell | (CD3+, CD4+, CD8−, FOXP3+) |
| Helper T Cell | (CD3+, CD4+ CD8−, FOXP3−) |
| B Cell | (CD3−, CD4−, CD8−, CD20+) |
| Other Immune Cell | (CD3−, CD4+, CD8−, CD20−) |
Immunofluorescent images were processed and cells were segmented and quantified as described previously 23–25. For quality control (QC) analysis, we extended the method presented in Bello et al. We generated automated QC scores (0–1) for every cell in each imaging round by correlating baseline DAPI images with all corresponding DAPI images from other multiplexing rounds 26. Following quantification, slides were normalized for batch effects and exposure time for each channel/marker analyzed. To correct for a possible batch effects between slides, cells’ mean intensity were normalized using upper-quantile normalization, grouped by protein marker and slide. Secondly, quantiles of the normalized intensities were plotted against their rankits, and an affine transformation matrix to rotate the function to the main diagonal were calculated in regulator and helper T cells. Obtained transformation matrices were applied on the intensities, and pixel intensity values were restored using linear regression and upper-quantile normalized values. Solely for the batch correction, cells within 5% of the images’ margins were excluded for the calculation of the reference values. The batch correction was quality controlled with cell lines spotted in parallel to tissue samples.
Statistical analysis
Variable frequencies were reported as means with standard deviation, medians with inter-quartile range or as percentages. The distribution of continuous variables was compared between groups using a Mann-Whitney U test for unpaired non-parametric variables and an unpaired t-test for parametric variables. Categorical variable frequency was compared between groups using the Chi-square test. A p value of 0.05 was defined as the cut-off for statistical significance. Comparisons of cell types and expressions between non-mucinous and mucinous groups was performed using f-test.
The Kruskal-Wallis H-test was used for variance analysis between categorical clinical features and CD3, CD8, CD20, FOXP3 and PD-1 expression. For the continuous clinical features (age), Spearman correlation was measured. Data were analysed using IBM SPSS Statistics Version 25.0 (IBM Corp, NY, USA), Python Version 3.8 (Python Software Foundation, Wilmington, DE, USA) and R Version 4.0.5 (R Foundation, Vienna, Austria).
Results:
Characteristics of included Mucinous and Non-Mucinous Rectal Cancers
Our cohort included 15 cases (26%) of mucinous and 43 cases (74%) of non-mucinous rectal cancer. 56.8% of the cohort were male, the median age was 70.5 years. 47% (n=27) of the cohort were American Joint Committee on Cancer (AJCC) stage 3.
The clinical and pathologic characteristics of the included patients are summarised in Table 2. 66% of the cohort underwent neoadjuvant chemoradiotherapy. 14% (n=2) of the mucinous cohort were found to have MSI compared to 2% (n=1) of the non-mucinous group. No statistically significant differences were observed between the groups with respect to patient clinical or pathological characteristics.
Table 2.
Clinical and pathological profile of mucinous and non-mucinous rectal cancers.
| Variable | Non-Mucinous RC (N=43) | Mucinous RC (N=15) | p Value | |
|---|---|---|---|---|
| Male | 58.1% (25) | 53.3 % (8) | 0.75 | |
|
| ||||
| Age | Median (IQR) | 70(42-89) | 71 (29-81) | 0.10 |
|
| ||||
| Stage | AJCC 1 | 11.6% (5) | 13.3% (2) | 0.17 |
| AJCC 2 | 34.8% (15) | 60.0% (9) | ||
| AJCC 3 | 53.4%(23) | 26.0% (4) | ||
| AJCC4 | 0.0%(0) | 0.0% (0) | ||
|
| ||||
| T Stage | Tis-T1-T-2 | 25.5% (11) | 20.0% (3) | 0.28 |
| T3 | 65.1% (28) | 60.0% (9) | ||
| T4 | 9.3% (4) | 20.0% (3) | ||
|
| ||||
| N Stage | N0 | 48.8% (21) | 73.3% (11) | 0.52 |
| N1 | 41.8%(18) | 6.7% (1) | ||
| N2 | 9.3%(4) | 20.0% (3) | ||
|
| ||||
| M Stage | M0 | 100% (43) | 100% (15) | NA |
| M1 | 0.0% (0) | 0.0% (0) | ||
|
| ||||
| Neoadjuvant CRT(n=56) a | 69.8% (30) | 53.3% (8) | 0.32 | |
|
| ||||
| Adjuvant CRT (n=52) a | 39.5% (17) | 53.3% (8) | 0.26 | |
|
| ||||
| MSI (n=55) a | 2.4% (1) | 14.3%(2) | 0.09 | |
|
| ||||
| KRAS (n=55) a | Mutant | 17.1%(7) | 35.7%(5) | 0.15 |
|
| ||||
| BRAF (n=54) a | Mutant | 2.4% (1) | 7.7%(1) | 0.38 |
|
| ||||
| LVI (n=57) a | 19%(8) | 6.7%(1) | 0.26 | |
|
| ||||
| Perineural Invasion(n=56) a | 11.9%(5) | 14.3%(2) | 0.82 | |
|
| ||||
| Extramural Invasion(n=56) a | 19.0%(8) | 7.1%(1) | 0.29 | |
|
| ||||
| DFS (Months) | Mean(SD) | 47(26.1) | 46(30.2) | 0.97 |
|
| ||||
| DSS(Months) | Mean(SD) | 52(24.1) | 55(33.5) | 0.63 |
AJCC American Joint Committee on Cancer, CRT Chemoradiotherapy, DFS Disease Free Survival, DSS Disease Specific Survival, LVI Lymphovascular Invasion, SD Standard Deviation. Categorical data reported as % (n).
Data not available in full cohort: n in parentheses = number with data available.
Immune Profile of Mucinous Rectal Cancer
Immune cells were classified as shown in Table 1. Total cell counts per core were quantified and a mean count per patient was calculated. Normalised cell frequencies were compared between mucinous and non-mucinous groups. Cytotoxic T-cells (p=0.022), regulatory T-cells (p=0.010), and those cells categorised as other immune cells (p=0.036), were found to be present in significantly greater abundance in the mucinous cohort (Fig. 1). Total PD-1 and HLA1 expression per core was quantified, and again a mean count per patient was calculated. Significantly higher expression of PD-1 (p=0.001) was observed in the mucinous cohort. HLA1 expression was not statistically different between the two groups (p=0.443) (Fig. 2).
Fig 1.
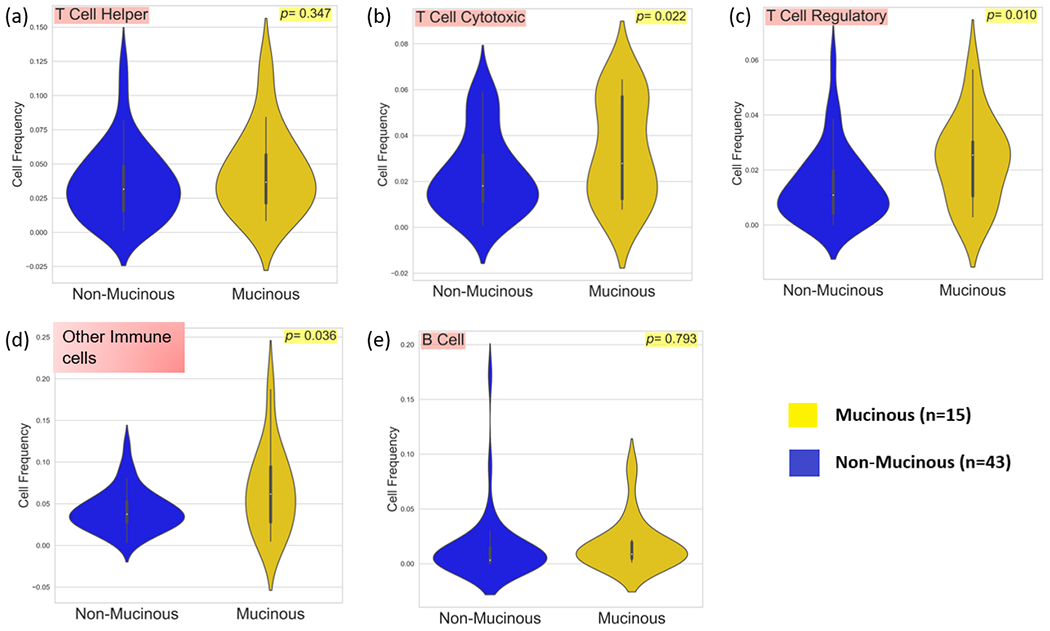
Violin plots comparing a, Cytotoxic T-Cell b, Regulatory T-Cell c, Helper T-Cell d, Other immune cells and e, B-Cell frequencies between 15 mucinous and 43 non-mucinous rectal cancers.
Fig. 2.
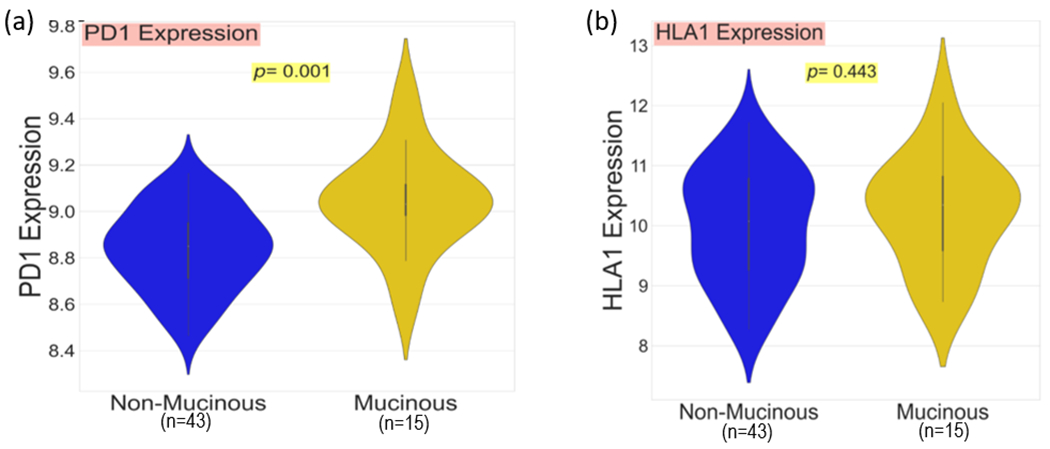
Violin plot comparing a, PD-1 expression and b, HLA1 expression between 15 mucinous and 43 non-mucinous rectal cancers.
Tumor Infiltration
It has been suggested the combined presence of MUC2 proteins, suppressive cytokines and adhesion ligands within the pools of mucin found in mucinous rectal cancer, may act to prevent immune cell infiltration 16, 27. To investigate this hypothesis, following segmentation we quantified CD3, CD4 and CD8 expression within tumour epithelial tissue. Expression values per core were quantified and normalised, a mean expression value per patient was calculated. Following segmentation, immune cell classifications were no longer viable and single marker expression values were used to identify infiltration. Expression values were compared between the mucinous and non-mucinous groups. The mucinous cohort were associated with elevated CD3 (p=0.001), CD4 (p=0.746) and CD8 (p=0.054) mean expression within tumor epithelial tissue (Fig. 3). This confirms the anti-immune properties of mucin do not prevent T-cell infiltration in mucinous rectal cancer (See Fig. 4).
Fig. 3.
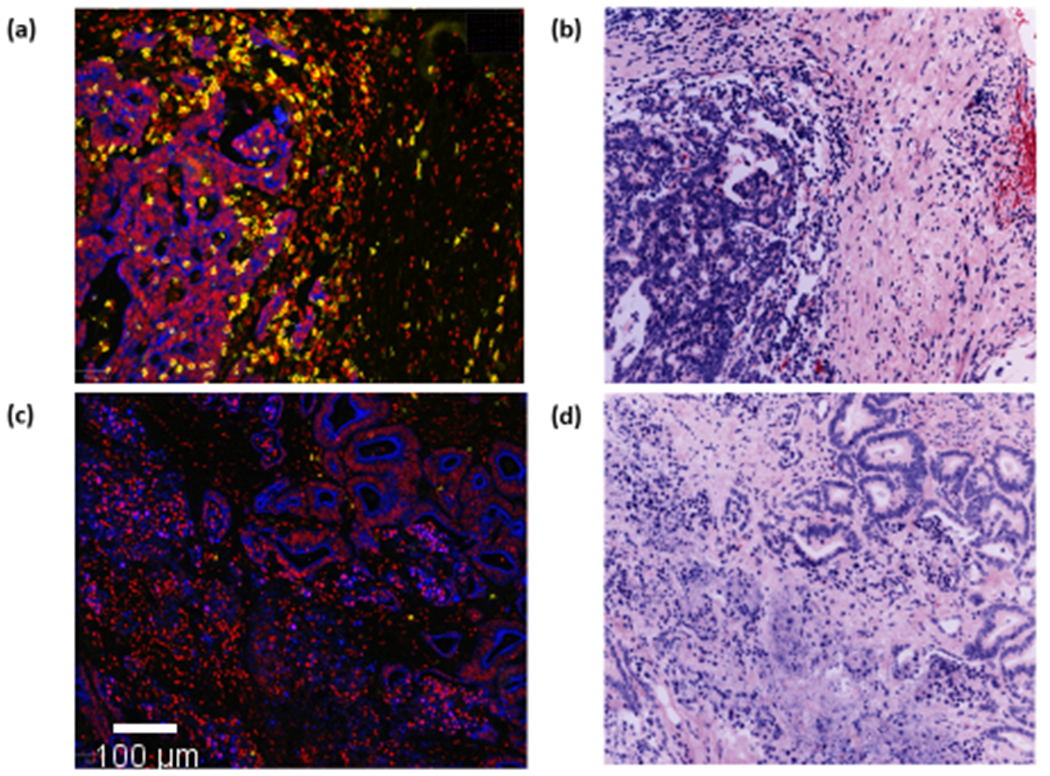
Violin plots comparing mean expression of a, CD3 b, CD4 and c, CD8 within epithelial (cancer) tissue between 15 mucinous and 43 non-mucinous rectal cancers.
Fig. 4.
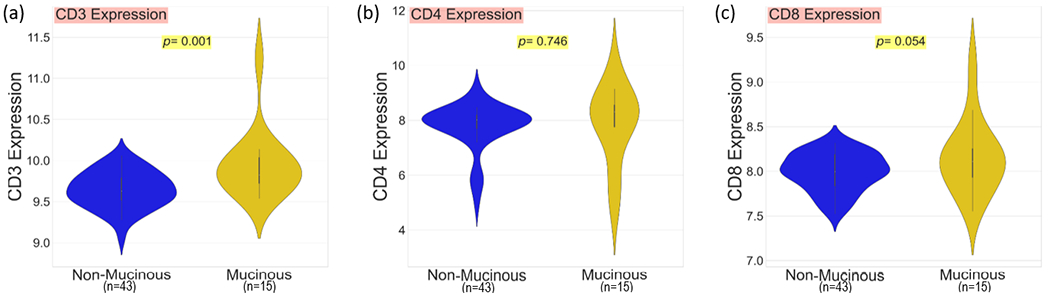
QuPath images with DAPI (red), Pancytokeratin (blue), and CD8 (yellow) staining; (a) A single core from a patient in the mucinous cohort demonstrating excellent CD8 expression within epithelial (cancer) tissue. (b) The corresponding core to image (a) with haemotoxylin and easin staining. (c) A single core from a patient from the NOS with poor CD8 expression within epithelial (cancer) tissue. (d) The corresponding core to image (c) with haemotoxylin and easin staining. (1 pixel ~ 0.325 micrometer)
Correlating Immune Marker Expression with Clinical and Pathologic Characteristics
Clinical and pathological variables including the effect of neoadjuvant chemoradiation, MSI status, age, sex and mucinous status were included in a correlative model to analyse immune marker expression. Correlative coefficients are reported in Table 3. Mucinous status correlated significantly with CD3 (p < 0.05), CD20 (p < 0.05), FOXP3 (p < 0.05) and PD-1 (p < 0.05) expression. Receipt of neoadjuvant chemoradiotherapy was associated with increased CD3 (p < 0.05) and PD-1 (B = 4.511; p < 0.05) expression. MSI status correlated significantly with CD4 expression (p < 0.05).
Table 3.
A correlative model demonstrating association between clinical and pathological characteristics and immune marker expression. The age coefficient represents the correlation coefficient. For the other categorical variables, the coefficient represents the Kruskal-Wallis H statistic.
| Variable | CD3 | CD4 | CD8 | CD20 | FOXP3 | PD-1 |
|---|---|---|---|---|---|---|
| Male | 0.13 | 0.06 | 0.01 | 0.18 | 0.01 | 3.36 |
| Age | −0.15 | −0.15 | −0.14 | −0.09 | −0.05 | −0.22 |
| Stage | −0.17 | −0.01 | −0.15 | 0.01 | −0.15 | −0.07 |
| Neoadjuvant CRT | 3.84 * | 0.15 | 1.56 | 3.17 | 0.51 | 8.95 * |
| MSI-high | 0.07 | 5.51 * | 0.04 | 0.02 | 0.29 | 0.15 |
| Mucinous Status | 7.53 * | 1.53 | 2.63 | 6.41 * | 6.70 * | 8.40 * |
MSI microsatellite instability.
= p < 0.05.
Discussion and Conclusion:
The inclusion of neoadjuvant chemoradiotherapy as part of the standard treatment regimen for locally advanced rectal adenocarcinoma, has revolutionised management of this cohort, with pCR rates reportedly as high as 25% 28, 29. As a consequence of its inhibited response to neoadjuvant treatment, outcomes in mucinous rectal cancer have deteriorated comparatively 5. Investigation into the efficacy of alternative treatment strategies, such as immunotherapy in this group are warranted. 12. Our analysis has found mucinous rectal cancer to be associated with an immune rich tumor microenvironment. The concern that mucin may behave as a barrier preventing immune cell infiltration in mucinous adenocarcinoma was not borne out in our analysis 27. Our findings demonstrate that mucinous rectal cancer is associated with increased infiltration of CD3+, CD4+ and CD8+ lymphocytes compared to non-mucinous rectal cancer.
Previous data published by our group following interrogation of the cancer genome atlas found mucinous CRC to be associated with increased gene expression of immune checkpoints PD-L1 and TIM-3 30. PD-1 expression was found to be elevated significantly in the mucinous rectal cancer cohort in this current study. Elevated expression of PD-1 is indicative of T-cell exhaustion 31, 32. This is characterised by a dysfunctional state, associated with reduced T-cell proliferative capacity, cytokine production and cytotoxicity 33. PD-1 blockade with pembroluzimab causes reinvigoration of T-cell effector function 34. Interestingly, PD-1 postitive T-cells only, (as opposed to other T-cells present in the tumor microenvironment) are thought to become reinvigorated following immune checkpoint inhibition 34. Our findings that mucinous rectal cancers are associated with good T-cell infiltration as well as elevated PD-1 expression are pertinent and suggest mucinous tumors are likely to respond positively to ICI. This hypothesis is in keeping with findings from a clinical trial, which found a composite score of high extracellular mucin and increased PD-L1 expression to be predictive of a positive response to PD-1 blockade in the treatment of metastatic CRC 35.
Use of immune checkpoint inhibitors in CRC is reserved for patients with MSI-high, metastatic disease only 36. Interestingly only 2 of 15 (14%) of our mucinous cohort were found to be MSI-high, and MSI status was only found to be associated with increased CD4 expression, whilst mucinous status was strongly associated with CD3, CD20, FOXP3 and PD-1 expression. This suggests other factors, beyond MSI status may contribute towards immunogenicity in mucinous RC. One factor known to contribute significantly towards tumour neoantigen load and thus immunogenicity are polymerase (POLE) mutation rates 37. Analysis of POLE mutation rates in mucinous rectal cancer in the context of immune cell activity may further explain the immune activity evident in MSS mucinous rectal tumours in our cohort.
Microbial influences may also contribute towards differences in immune cell activity between these cohorts. Previously, our group undertook a whole genome sequencing study; examining 10 mucinous rectal adenocarcinomas 38. A key finding from this study was the abundance of Fusobacterium nucleatum found within tumor tissue. This finding is interesting in the context of a growing body of evidence linking various members of the gut microbiome, including Fusobacterium with enhanced immunogenicity and responsivity to immunotherapy in cancer 39, 40. Further exploration into the relationship between members of the gut microbiome and immune activation in mucinous CRC are warranted.
Interestingly, receipt of neoadjuvant chemoradiotherapy was associated with increased expression of CD3 and PD-1. Chemoradiotherapy has previously been shown to enhance the immune response in CRC patients 41, 42. Though neoadjuvant therapy may not be effective with respect to inducing a tumoricidal effect in mucinous rectal cancer, it may act to prime the immune response. Combination therapies involving chemoradiotherapy and immunotherapy may be of clinical benefit in this group.
Though the number of patients included in this study are small, this is the first thorough investigation of the immune landscape of mucinous rectal cancer. Multiplexed imaging techniques enabled us to evaluate expression of multiple immune markers in unison. Mucin does not appear to behave as a barrier preventing immune infiltration and immune marker expression was not found to be overtly dependent on MSI status. Our findings suggest mucinous RC may be associated with increased immune activity. Given the inhibited response of this cohort to traditional adjuvant chemo- and radiotherapy, these findings should support future endeavours exploring the potential role of immunotherapy in mucinous CRC.
Acknowledgments and Funding:
This work was funded by a US-Northern Ireland-Ireland Tripartite grant from Science Foundation Ireland and the Health Research Board to JHMP (16/US/3301) and the National Cancer Institute under award number R01CA208179 (Systems Modelling of Tumor Heterogeneity and Therapy Response in Colorectal Cancer; to FG). WPD is supported by an RCSI Bon Secours Hospital MD StAR fellowship and the Beaumont Hospital Cancer Research and Development Trust. DL is supported by US-Ireland R01 award (NI Partner supported by HSCNI, STL/5715/15). BK is supported by Science Foundation Ireland through the SFI Centre for Research Training in Genomics Data Science under Grant number 18/CRT/6214 and EU’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant H2020-MSCA-COFUND-2019-945385.
Footnotes
Disclosures & Conflicts of Interest:
The Cell DIVE™ platform was developed by GE Research. EMcD and FG are current employees of GE Research. The other authors have no potential conflicts.
References:
- 1.Kang H, O’Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Diseases of the colon and rectum. 2005;48(6):1161–8. [DOI] [PubMed] [Google Scholar]
- 2.Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer. 1976;37(4):1891–900. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds IS, O’Connell E, Fichtner M, McNamara DA, Kay EW, Prehn JHM, et al. Mucinous adenocarcinoma is a pharmacogenomically distinct subtype of colorectal cancer. Pharmacogenomics J. 2020;20(3):524–32. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds IS, Furney SJ, Kay EW, McNamara DA, Prehn JHM, Burke JP. Meta-analysis of the molecular associations of mucinous colorectal cancer. The British journal of surgery. 2019;106(6):682–91. [DOI] [PubMed] [Google Scholar]
- 5.McCawley N, Clancy C, O’Neill BD, Deasy J, McNamara DA, Burke JP. Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Diseases of the colon and rectum. 2016;59(12):1200–8. [DOI] [PubMed] [Google Scholar]
- 6.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–11. [DOI] [PubMed] [Google Scholar]
- 7.Lee MY, Jeon JW, Sievers C, Allen CT. Antigen processing and presentation in cancer immunotherapy. Journal for immunotherapy of cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi V, Harari A, Coukos G. Neoantigen-Specific Adoptive Cell Therapies for Cancer: Making T-Cell Products More Personal. Frontiers in immunology. 2020;11:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petitprez F, Meylan M, de Reyniès A, Sautès-Fridman C, Fridman WH. The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies. Frontiers in immunology. 2020;11:784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer discovery. 2015;5(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahin IH, Akce M, Alese O, Shaib W, Lesinski GB, El-Rayes B, et al. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. British journal of cancer. 2019;121(10):809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen R, Rousseau B, Vidal J, Colle R, Diaz LA Jr., André T. Immune Checkpoint Inhibition in Colorectal Cancer: Microsatellite Instability and Beyond. Targeted oncology. 2020;15(1):11–24. [DOI] [PubMed] [Google Scholar]
- 13.Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. The New England journal of medicine. 2022;386(25):2363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrici J, Farzin M, Sioson L, Clarkson A, Watson N, Toon CW, et al. Mismatch repair deficiency as a prognostic factor in mucinous colorectal cancer. Modern Pathology. 2016;29(3):266–74. [DOI] [PubMed] [Google Scholar]
- 15.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine. 2015;372(26):2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hugen N, Brown G, Glynne-Jones R, de Wilt JH, Nagtegaal ID. Advances in the care of patients with mucinous colorectal cancer. Nature reviews Clinical oncology. 2016;13(6):361–9. [DOI] [PubMed] [Google Scholar]
- 17.Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nature reviews Immunology. 2016;16(10):639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tozawa E, Ajioka Y, Watanabe H, Nishikura K, Mukai G, Suda T, et al. Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: is mucinous carcinoma a distinct histological entity? Pathology, research and practice. 2007;203(8):567–74. [DOI] [PubMed] [Google Scholar]
- 19.Nazemalhosseini-Mojarad E, Mohammadpour S, Torshizi Esafahani A, Gharib E, Larki P, Moradi A, et al. Intratumoral infiltrating lymphocytes correlate with improved survival in colorectal cancer patients: Independent of oncogenetic features. J Cell Physiol. 2019;234(4):4768–77. [DOI] [PubMed] [Google Scholar]
- 20.Rajan A, Heery CR, Thomas A, Mammen AL, Perry S, O’Sullivan Coyne G, et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (Avelumab) treatment in advanced thymoma. Journal for immunotherapy of cancer. 2019;7(1):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindner AU, Salvucci M, McDonough E, Cho S, Stachtea X, O’Connell EP, et al. An atlas of inter- and intra-tumor heterogeneity of apoptosis competency in colorectal cancer tissue at single-cell resolution. Cell death and differentiation. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system: World Health Organization; 2010. [Google Scholar]
- 23.Gerdes MJ, Sevinsky CJ, Sood A, Adak S, Bello MO, Bordwell A, et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(29):11982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berens ME, Sood A, Barnholtz-Sloan JS, Graf JF, Cho S, Kim S, et al. Multiscale, multimodal analysis of tumor heterogeneity in IDH1 mutant vs wild-type diffuse gliomas. PLoS One. 2019;14(12):e0219724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindner AU, Salvucci M, McDonough E, Cho S, Stachtea X, O’Connell EP, et al. An atlas of inter- and intra-tumor heterogeneity of apoptosis competency in colorectal cancer tissue at single-cell resolution. Cell Death & Differentiation. 2022;29(4):806–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bello M, Can A, Xiaodong T, editors. Accurate registration and failure detection in tissue micro array images. 2008 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro; 2008. 14-17 May 2008. [Google Scholar]
- 27.Johansson MEV, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nature Reviews Immunology. 2016;16(10):639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. The British journal of surgery. 2012;99(7):918–28. [DOI] [PubMed] [Google Scholar]
- 29.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2018;16(7):874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connell E, Salvucci M, Reynolds IS, McNamara DA, Burke JP, Prehn JHM. Mucinous Colorectal Cancer is Associated With Expression of the TIM-3 Immune Checkpoint Independently of Microsatellite Instability (MSI) Status. Annals of Surgical Oncology. 2021;28(12):7999–8006. [DOI] [PubMed] [Google Scholar]
- 31.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell. 2015;27(4):450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia A, Zhang Y, Xu J, Yin T, Lu XJ. T Cell Dysfunction in Cancer Immunity and Immunotherapy. Frontiers in immunology. 2019;10:1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nature medicine. 2019;25(8):1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llosa NJ, Luber B, Siegel N, Awan AH, Oke T, Zhu Q, et al. Immunopathologic Stratification of Colorectal Cancer for Checkpoint Blockade Immunotherapy. Cancer immunology research. 2019;7(10):1574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nature medicine. 2020;26(4):566–76. [DOI] [PubMed] [Google Scholar]
- 37.Gong J, Wang C, Lee PP, Chu P, Fakih M. Response to PD-1 Blockade in Microsatellite Stable Metastatic Colorectal Cancer Harboring a POLE Mutation % J Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw. 2017;15(2):142–7. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds IS, Thomas V, O’Connell E, Fichtner M, McNamara DA, Kay EW, et al. Mucinous Adenocarcinoma of the Rectum: A Whole Genome Sequencing Study. Front Oncol. 2020;10:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World journal of gastroenterology. 2016;22(2):557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun JY, Yin TL, Zhou J, Xu J, Lu XJ. Gut microbiome and cancer immunotherapy. J Cell Physiol. 2020;235(5):4082–8. [DOI] [PubMed] [Google Scholar]
- 41.Teng F, Mu D, Meng X, Kong L, Zhu H, Liu S, et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. American journal of cancer research. 2015;5(6):2064–74. [PMC free article] [PubMed] [Google Scholar]
- 42.Matsutani S, Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, et al. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer science. 2018;109(4):966–79. [DOI] [PMC free article] [PubMed] [Google Scholar]


