Abstract
BACKGROUND:
This study evaluated the current clinical trends, risk factors, and temporal effects of post-transplant dialysis on outcomes following orthotopic heart transplantation after the 2018 United States adult heart allocation policy change.
METHODS:
The United Network for Organ Sharing (UNOS) registry was queried to analyze adult orthotopic heart transplant recipients after the October 18, 2018 heart allocation policy change. The cohort was stratified according to the need for post-transplant de novo dialysis. The primary outcome was survival. Propensity score-matching was performed to compare the outcomes between 2 similar cohorts with and without post-transplant de novo dialysis. The impact of post-transplant dialysis chronicity was evaluated. Multivariable logistic regression was performed to identify risk factors for post-transplant dialysis.
RESULTS:
A total of 7,223 patients were included in this study. Out of these, 968 patients (13.4%) developed post-transplant renal failure requiring de novo dialysis. Both 1-year (73.2% vs 94.8%) and 2-year (66.3% vs 90.6%) survival rates were lower in the dialysis cohort (p < 0.001), and the lower survival rates persisted in a propensity-matched comparison. Recipients requiring only temporary post-transplant dialysis had significantly improved 1-year (92.5% vs 71.6%) and 2-year (86.6 % vs 52.2%) survival rates compared to the chronic post-transplant dialysis group (p < 0.001). Multivariable analysis demonstrated low pretransplant estimated glomerular filtration (eGFR) and bridge with extracorporeal membrane oxygenation (ECMO) were strong predictors of post-transplant dialysis.
CONCLUSIONS:
This study demonstrates that post-transplant dialysis is associated with significantly increased morbidity and mortality in the new allocation system. Post-transplant survival is affected by the chronicity of post-transplant dialysis. Low pretransplant eGFR and ECMO are strong risk factors for post-transplant dialysis.
Keywords: orthotopic heart, transplantation, renal failure, dialysis, adverse events, survival
Heart failure (HF) is a major public health concern with the prevalence nearing 3% in the United States.1 Orthotopic heart transplantation (OHT) continues to be the gold standard treatment for end-stage HF after medical therapy failure.2,3 The number of OHTs performed annually has steadily increased with more than 3,800 cases in 2021.4 A new 6-tier heart allocation system was introduced on October 18, 2018 through the United Network for Organ Sharing (UNOS).2,5 The new allocation system was designed to address the limitations of the prior 3-tier allocation system by improving candidate risk stratification. Such efforts are focused on improving the need-based prioritization and more equitable geographic access to donor organs. There has been a nationwide trend of increasing transplantations performed on patients with higher-risk features following the recent allocation policy change, including those who are bridged to transplantation with temporary mechanical circulatory support (MCS) devices over conventional durable left ventricular assist therapy.6
Recent studies have demonstrated a positive impact of the new system on waitlist times and waitlist mortality with comparable survival rates to the prior systems.7-10 However, post-transplant complications continue to be problematic, including post-transplant renal disease.2,3 Renal insufficiency is relatively common in end-stage HF and can be exacerbated in the setting of heart transplantation operation and immunosuppression, and it is associated with increased morbidity and mortality following heart transplantation.11-13 Previously reported rates of renal failure requiring dialysis following heart transplantation vary widely, ranging from 4% to 28%.12-15 There is a paucity of literature evaluating the clinical trend and impact of post-transplant dialysis on outcomes under the new allocation system. In this study, we aimed to evaluate the incidence, predictors, and temporal effects of post-transplant dialysis on outcomes following OHT under the new allocation system.
Materials and methods
Data source
The UNOS database was utilized for this study. This is a prospectively collected registry of all solid organ transplantations performed in the United States. Patient and medical center identifiers were excluded from the analysis. This study was approved by the institutional review board at the University of Pittsburgh, and the need for informed consent for the study was waived.
Study population
This study included all adult patients (age ≥18 years) who underwent isolated OHT between October 18, 2018 and June 30, 2021 after the 2018 heart allocation policy change. Follow-up for these recipients was extended to June 30, 2022. Multivisceral transplant, heterotopic heart transplant, and/or redo OHT recipients were excluded. Additionally, patients that underwent dialysis prior to transplantation were excluded. Patients were stratified into 2 cohorts based on whether post-transplant renal failure requiring de novo dialysis occurred during the index hospitalization.
Baseline characteristics and outcomes
Recipient, donor, and transplant characteristics and outcomes data were collected from the UNOS database and were compared between the 2 groups. These demographics included age, gender, race, body mass index (BMI), HF etiology, transplant-related variables, comorbidities, most recent preoperative laboratory values, preoperative interventions, and immunosuppressants. The incidence of post-transplant dialysis in the new system (November 1, 2018-June 30, 2021) was compared to a seasonally matched, equal-length time period prior to the allocation system change (November 1, 2015-June 30, 2018). Seasonally matching was defined as time periods corresponding to the same months of the year.
The primary outcome was survival at 1 and 2 years following OHT. The secondary outcomes included postoperative complications, permanent pacemaker, hospital length of stay, rates of acute rejection requiring medical therapy, hospitalization for infection after discharge, functional status at last follow-up, and graft survival. Furthermore, a propensity score-matched comparison was made between the 2 cohorts of patients with similar baseline characteristics.
Subanalyses were performed to assess the temporal effects of dialysis on survival by further stratifying the post-transplant dialysis cohort into temporary and chronic dialysis groups. Chronic dialysis was defined as patients who developed a nonrecoverable, end-stage renal disease (ESRD) and are expected to remain on dialysis. A 90-day conditional survival analysis was also performed given the immortal time bias in the temporary dialysis group. Lastly, the impact of dialysis timing was evaluated by comparing 1- and 2-year survival between recipients who underwent pretransplant dialysis vs those who underwent de novo post-transplant dialysis.
Data analysis
Baseline characteristics, including demographic and clinical data, are presented as frequency (percentage) for categorical variables and mean (±standard deviation) or median (interquartile range) for continuous variables. Pearson’s chi-square test or Fisher’s exact test were utilized for categorical comparisons. Either Student’s t-test or analysis of variance was employed for parametric continuous variables, and Wilcoxon rank-sum test was utilized for nonparametric data. Kaplan-Meier analysis was utilized to compare overall survival between the groups.
Propensity score-matching was performed to match 2 groups of patients with similar baseline recipient, donor, and transplant characteristics. Matching was done on a 1:1 basis using nearest neighbor matching without replacement and caliper setting of 0.2 of the standard deviation of the logit propensity score. A standardized mean difference of <15% was considered adequately matched, and a standardized mean difference <10% was considered well-matched.
Multivariable logistic regression was performed to identify risk-adjusted predictors for post-transplant dialysis among transplant recipients in this study. Additionally, a separate multivariable model was constructed to identify predictors of chronic dialysis needs among those requiring dialysis in the acute post-transplant period. In these models, potential covariates were selected by performing univariable logistic regression on all baseline recipient, donor, and transplant-related variables, and all variables with significant associations (p < 0.05) with post-transplant dialysis were selected. Stepwise, backwards selection of potential covariables with a threshold of p < 0.2 was then implemented to build the final model. The multivariable models were tested for significant interactions, as well as multicollinearity. Patients with missing data were removed from multivariable modeling. The statistical analyses were performed using Stata (StataCorp LP, College Station, TX) version 16 statistical software.
Results
Baseline recipient, donor, and transplant characteristics
A total of 8,801 patients underwent isolated OHT during the study period. After excluding pediatric, pretransplant dialysis, and retransplant recipients, a total of 7,223 patients were included and analyzed. Out of these, 968 patients (13.4%) required post-transplant de novo dialysis. When comparing the rate of post-transplant dialysis to a seasonally matched, equal-length time period preceding the allocation system change, there was a higher number of recipients with post-transplant renal failure requiring dialysis under the new allocation system (13.4% vs 11.0%, p < 0.001) (Figure S1A and B).
The recipients requiring post-transplant dialysis had a higher proportion of White race and blood type A and a lower proportion of nonischemic HF etiology. Furthermore, dialysis recipients had higher BMI, higher serum total bilirubin, lower pretransplant estimated glomerular filtration rate (eGFR), and higher mean pulmonary capillary wedge pressure. Furthermore, the patients requiring dialysis had higher rates of pretransplant infection, transfusion, mechanical ventilation (MV), extracorporeal membrane oxygenation (ECMO), and prior sternotomy. Recipients in the dialysis group were more likely to receive both sex- and race-matched grafts with a longer cold ischemia time (Table 1).
Table 1.
Baseline Recipient, Donor, and Transplant Characteristics Stratified by Post-Transplant Dialysis Requirement
| No dialysis (n = 6,255) | Post-transplant dialysis (n = 968) | p-value | |
|---|---|---|---|
| Recipient characteristics | |||
| Age (years) | 57 (46-63) | 57 (48-64) | 0.095 |
| Female | 1,729 (27.6%) | 258 (26.7%) | 0.52 |
| Race | 0.003 | ||
| White | 3,893 (62.6%) | 659 (68.4%) | |
| Black | 1,466 (23.6%) | 196 (20.3%) | |
| Hispanic | 608 (9.8%) | 75 (7.8%) | |
| Asian | 225 (3.6%) | 26 (2.7%) | |
| Other | 28 (0.5%) | 8 (0.8%) | |
| Missing | 35 (0.6%) | 4 (0.4%) | |
| Body mass index (kg/m2) | 27.58 ± 4.94 | 28.91 ± 4.84 | <0.001 |
| Recipient blood type | 0.017 | ||
| A | 2,469 (39.5%) | 403 (41.6%) | |
| AB | 306 (4.9%) | 60 (6.2%) | |
| B | 997 (15.9%) | 121 (12.5%) | |
| O | 2,483 (39.7%) | 384 (39.7%) | |
| Heart failure etiology | <0.001 | ||
| Non-Ischemic | 3,530 (56.4%) | 487 (50.3%) | |
| Ischemic | 1,760 (28.1%) | 272 (28.1%) | |
| Congenital | 204 (3.3%) | 67 (6.9%) | |
| Restrictive | 278 (4.4%) | 48 (5.0%) | |
| Valvular | 54 (0.9%) | 18 (1.9%) | |
| Hypertrophic | 8 (0.1%) | 1 (0.1%) | |
| Other | 199 (3.2%) | 43 (4.4%) | |
| Missing | 222 (3.5%) | 32 (3.3%) | |
| Prior sternotomy and/or cardiac surgery | 2,606 (41.7%) | 496 (51.2%) | <0.001 |
| Diabetes mellitus | 1,668 (26.7%) | 287 (29.6%) | 0.053 |
| Total bilirubin (mg/dL) | 0.94 ± 1.52 | 1.30 ± 2.61 | <0.001 |
| eGFR (mL/min/1.73 m2) | 72.32 ± 34.83 | 65.27 ± 78.84 | <0.001 |
| Positive CMV serology | 3,390 (54.2%) | 519 (53.6%) | 0.74 |
| Pretransplant infection | 622 (10.0%) | 122 (12.7%) | 0.010 |
| Blood transfusion while on waitlist | 766 (12.3%) | 172 (17.9%) | <0.001 |
| Pretransplant mechanical ventilation | 101 (1.6%) | 40 (4.1%) | <0.001 |
| Intravenous inotropes | 2,433 (38.9%) | 355 (36.7%) | 0.19 |
| Intra-aortic balloon pump | 1,797 (28.7%) | 257 (26.5%) | 0.16 |
| ECMO | 265 (4.2%) | 80 (8.3%) | <0.001 |
| VAD support | 0.44 | ||
| None | 3,983 (63.7%) | 595 (61.5%) | |
| LVAD | 2,135 (34.1%) | 349 (36.1%) | |
| RVAD | 22 (0.4%) | 3 (0.3%) | |
| TAH | 24 (0.4%) | 2 (0.2%) | |
| LVAD + RVAD | 91 (1.5%) | 19 (2.0%) | |
| Mean pulmonary artery pressure (mm Hg) | 26.91 ± 10.11 | 27.57 ± 10.05 | 0.067 |
| Mean capillary wedge pressure (mm Hg) | 17.70 ± 8.76 | 18.57 ± 8.64 | 0.006 |
| Pulmonary vascular resistance (Woods units) | 2.39 ± 4.50 | 2.23 ± 1.71 | 0.29 |
| Trans-pulmonary gradient (mm Hg) | 9.14 ± 5.63 | 9.02 ± 5.84 | 0.55 |
| Donor characteristics | |||
| Age (years) | 32 (24-40) | 32 (24-40) | 0.95 |
| Female | 1,777 (28.4%) | 284 (29.3%) | 0.55 |
| Race | 0.90 | ||
| White | 3,956 (63.2%) | 628 (64.9%) | |
| Black | 1,026 (16.4%) | 152 (15.7%) | |
| Asian | 1,079 (17.3%) | 159 (16.4%) | |
| Hispanic | 101 (1.6%) | 16 (1.7%) | |
| Other | 93 (1.5%) | 13 (1.3%) | |
| Body mass index (kg/m2) | 27.93 ± 6.25 | 28.22 ± 6.37 | 0.18 |
| Blood type | 0.16 | ||
| A | 2,262 (36.2%) | 380 (39.3%) | |
| AB | 64 (1.0%) | 7 (0.7%) | |
| B | 707 (11.3%) | 94 (9.7%) | |
| O | 3,222 (51.5%) | 487 (50.3%) | |
| Diabetes mellitus | 230 (3.7%) | 47 (4.9%) | 0.077 |
| Hypertension | 2,179 (34.9%) | 325 (33.6%) | 0.44 |
| Serum creatinine (mg/dL) | 1.67 ± 1.77 | 1.65 ± 1.77 | 0.75 |
| Graft LVEF <50% | 82 (1.3%) | 19 (2.0%) | 0.11 |
| Positive CMV serology | 3,870 (62.1%) | 577 (60.0%) | 0.20 |
| Hepatitis C | 757 (12.1%) | 98 (10.1%) | 0.076 |
| Matching and transplant characteristics | |||
| Sex matched | 4,899 (78.3%) | 788 (81.4%) | 0.029 |
| Race matched | 3,016 (48.2%) | 525 (54.2%) | <0.001 |
| HLA matched (≤3 loci mismatch) | 819 (13.1%) | 114 (11.8%) | 0.26 |
| ABO matched | 5,300 (84.7%) | 815 (84.2%) | 0.67 |
| CMV matched | 3,296 (52.9%) | 494 (51.4%) | 0.36 |
| Waitlist time (days) | 34 (9-186) | 35 (8-221.5) | 0.97 |
| Donor distance to transplanting center (nautical miles) | 222 (84-402) | 243.5 (97.5-406) | 0.078 |
| Graft cold ischemic time (hours) | 3.4 (2.8-4.0) | 3.5 (3.0-4.1) | <0.001 |
| Immunosuppression | |||
| Induction regimen | <0.001 | ||
| ATGAM | 94 (2.0%) | 25 (3.3%) | |
| Thymoglobulin | 1,054 (22.0%) | 185 (24.5%) | |
| Basiliximab | 1,517 (31.6%) | 294 (38.9%) | |
| Steroids only | 2,007 (41.8%) | 229 (30.3%) | |
| Other | 128 (2.7%) | 23 (3.0%) | |
| Missing | 1,455 (23.3%) | 212 (21.9%) | |
| Components of initial maintenance regimen | |||
| Steroids | 5,724 (91.5%) | 871 (90.0%) | 0.12 |
| Tacrolimus | 6,012 (96.1%) | 853 (88.1%) | <0.001 |
| Mycophenolate mofetil | 5,515 (88.2%) | 767 (79.2%) | <0.001 |
| Mycophenolic acid | 574 (9.2%) | 83 (8.6%) | 0.54 |
| Cyclosporin | 40 (0.6%) | 20 (2.1%) | <0.001 |
Abbreviations: CMV, cytomegalovirus; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; HLA, human leukocyte antigen; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; RVAD, right ventricular assist device; TAH, total artificial heart; VAD, ventricular assist device.
As for transplant immunosuppression, induction with basiliximab was most commonly used in the dialysis group whereas induction with steroid only was most commonly used in the nondialysis group. Maintenance immunosuppression therapy with tacrolimus or mycophenolate mofetil was lower in the dialysis group. However, the rate of cyclosporin utilization was higher in the dialysis group (Table 1).
Impact of post-transplant dialysis on outcomes
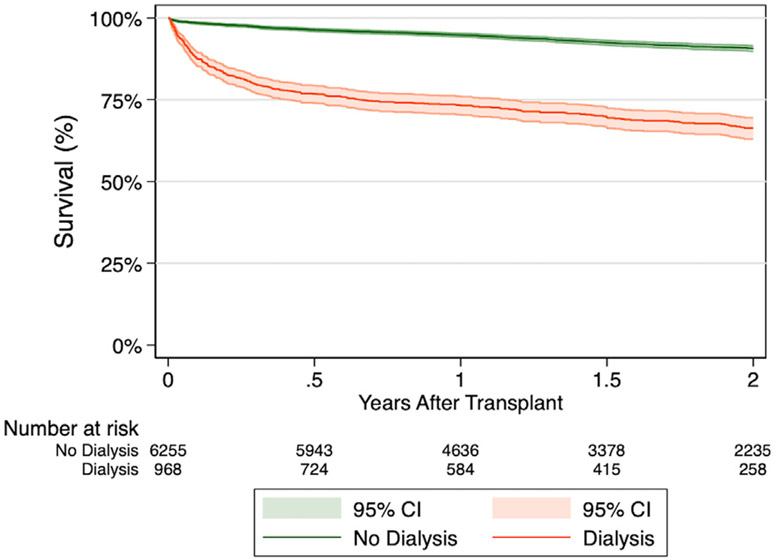
The recipients requiring post-transplant dialysis experienced significantly lower 1-year (73.2% vs 94.8%, p < 0.001) and 2-year (66.3% vs 90.6%, p < 0.001) survival compared to recipients without post-transplant dialysis (Figure 1). Recipients in the dialysis group had significantly higher rates of stroke, permanent pacemaker, longer hospital length of stay, and hospitalization for infection after discharge. Furthermore, recipients in the dialysis group were less likely to be functionally independent with higher rates of hospitalization and assistance requirement during the last follow-up visit. The rate of acute rejection requiring medical therapy was comparable (Table 2).
Figure 1.
Unadjusted Kaplan-Meier estimates for survival following heart transplantation stratified by post-transplant dialysis requirements in unmatched cohorts.
Table 2.
Post-Transplant Outcomes Stratified by Post-Transplant Dialysis Requirement
| No dialysis (n = 6,255) | Post-transplant dialysis (n = 968) | p-value | |
|---|---|---|---|
| Stroke | 182 (2.9%) | 87 (9.1%) | <0.001 |
| Permanent pacemaker | 104 (1.7%) | 27 (2.8%) | 0.014 |
| Hospital LOS (days) | 16 (11-22) | 33 (21-57) | <0.001 |
| Treated acute rejection | 671 (10.7%) | 118 (12.2%) | 0.17 |
| Hospitalization for infection after discharge | 811 (33.5%) | 160 (45.1%) | <0.001 |
| Functional status at last follow-up | <0.001 | ||
| Hospitalized | 261 (5.0%) | 174 (21.1%) | |
| Requires assistance | 658 (12.7%) | 178 (21.6%) | |
| Functionally independent | 4,274 (82.3%) | 471 (57.2%) |
Abbreviation: LOS, length of stay.
Propensity score-matched comparisons
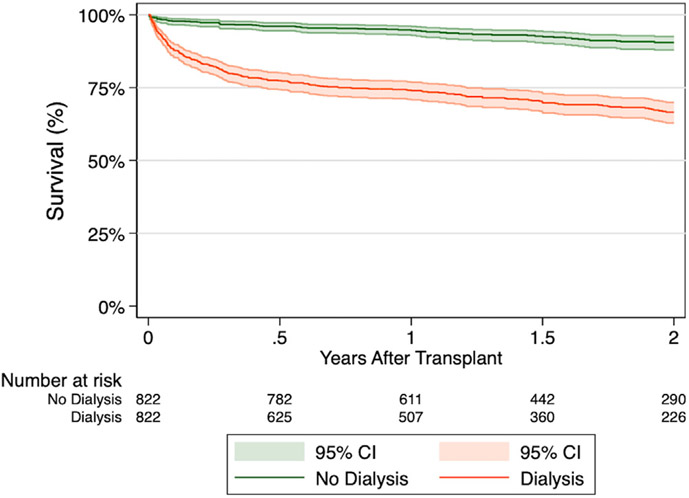
Propensity score-matching resulted in 1,644 patients with 822 patients requiring post-transplant dialysis and 822 patients not requiring post-transplant dialysis. Both groups were well-matched with respect to baseline recipient, donor, and transplant-related characteristics, except for the rate of ABO-matched graft and initial maintenance immunosuppression regimen (Table 3). Kaplan–Meier survival estimates remained lower in the dialysis group at 1-year (74.0% vs 94.7%, p < 0.001) and 2-year (66.6% vs 90.5%, p < 0.001) (Figure 2). In an unadjusted analysis, post-transplant dialysis was associated with over 6-fold increased risk of 2-year mortality following OHT in this propensity score-matched population (hazard ratio [HR] 6.20, 95% confidence interval [CI] 5.58-6.89, p < 0.001).
Table 3.
Baseline Recipient, Donor, and Transplant Characteristics Stratified by Post-Transplant Dialysis Requirement in Propensity Score-Matched Cohorts
| No dialysis (n = 822) | Post-transplant dialysis (n = 822) | SMD | |
|---|---|---|---|
| Recipient characteristics | |||
| Recipient age (years) | 57 (49-64) | 57 (48-64) | 0.003 |
| Female | 226 (27.5%) | 225 (27.4%) | 0.003 |
| Race | 0.002 | ||
| White | 569 (69.2%) | 572 (69.6%) | |
| Black | 166 (20.2%) | 164 (20.0%) | |
| Hispanic | 65 (7.9%) | 63 (7.7%) | |
| Asian | 22 (2.7%) | 19 (2.3%) | |
| Other | 0 (0.0%) | 4 (0.5%) | |
| Body mass index (kg/m2) | 28.81 ± 4.90 | 28.90 ± 4.77 | 0.019 |
| Recipient blood type | 0.002 | ||
| A | 340 (41.4%) | 339 (41.2%) | |
| AB | 38 (4.6%) | 48 (5.8%) | |
| B | 125 (15.2%) | 106 (12.9%) | |
| O | 319 (38.8%) | 329 (40.0%) | |
| Heart failure etiology | 0.001 | ||
| Nonischemic | 418 (50.9%) | 419 (51.0%) | |
| Ischemic | 255 (31.0%) | 226 (27.5%) | |
| Congenital | 27 (3.3%) | 52 (6.3%) | |
| Restrictive | 33 (4.0%) | 43 (5.2%) | |
| Valvular | 14 (1.7%) | 13 (1.6%) | |
| Hypertrophic | 1 (0.1%) | 1 (0.1%) | |
| Other | 35 (4.3%) | 39 (4.7%) | |
| Diabetes mellitus | 242 (29.4%) | 252 (30.7%) | 0.027 |
| Total bilirubin (mg/dL) | 1.07 ± 2.05 | 1.16 ± 2.15 | 0.040 |
| eGFR (mL/min/1.73 m2) | 62.81 ± 25.62 | 61.31 ± 31.97 | 0.052 |
| Prior sternotomy and/or cardiac surgery | 437 (53.2%) | 413 (50.2%) | 0.058 |
| Positive CMV serology | 451 (54.9%) | 436 (53.0%) | 0.037 |
| Pretransplant infection | 85 (10.3%) | 94 (11.4%) | 0.035 |
| Blood transfusion while on waitlist | 135 (16.4%) | 135 (16.4%) | <0.001 |
| Pretransplant mechanical ventilation | 25 (3.0%) | 21 (2.6%) | 0.029 |
| Intravenous inotropes | 298 (36.3%) | 293 (35.6%) | 0.013 |
| Intra-aortic balloon pump | 232 (28.2%) | 224 (27.3%) | 0.022 |
| ECMO | 56 (6.8%) | 46 (5.6%) | 0.050 |
| Ventricular assist device | 0.002 | ||
| None | 504 (61.3%) | 502 (61.1%) | |
| LVAD | 300 (36.5%) | 302 (36.7%) | |
| RVAD | 0 (0.0%) | 2 (0.2%) | |
| TAH | 2 (0.2%) | 1 (0.1%) | |
| LVAD + RVAD | 16 (1.9%) | 15 (1.8%) | |
| Mean pulmonary artery pressure (mm Hg) | 28.04 ± 10.31 | 27.64 ± 10.06 | 0.039 |
| Mean capillary wedge pressure (mm Hg) | 18.95 ± 8.75 | 18.51 ± 8.58 | 0.051 |
| Pulmonary vascular resistance (Woods units) | 2.25 ± 1.81 | 2.25 ± 1.71 | 0.005 |
| Trans-pulmonary gradient (mm Hg) | 9.09 ± 6.15 | 9.13 ± 5.78 | 0.006 |
| Donor characteristics | |||
| Age (years) | 32 (25-40) | 32 (24-40) | 0.013 |
| Female | 257 (31.3%) | 244 (29.7%) | 0.034 |
| Race | 0.001 | ||
| White | 539 (65.6%) | 541 (65.8%) | |
| Black | 129 (15.7%) | 126 (15.3%) | |
| Asian | 131 (15.9%) | 131 (15.9%) | |
| Hispanic | 10 (1.2%) | 12 (1.5%) | |
| Other | 13 (1.6%) | 12 (1.5%) | |
| Body mass index (kg/m2) | 28.03 ± 5.73 | 28.21 ± 6.29 | 0.029 |
| Blood type | 0.012 | ||
| A | 320 (38.9%) | 323 (39.3%) | |
| AB | 7 (0.9%) | 7 (0.9%) | |
| B | 75 (9.1%) | 80 (9.7%) | |
| O | 420 (51.1%) | 412 (50.1%) | |
| Diabetes mellitus | 47 (5.7%) | 39 (4.7%) | 0.044 |
| Hypertension | 286 (34.8%) | 284 (34.5%) | 0.005 |
| Graft LVEF <50% | 16 (1.9%) | 17 (2.1%) | 0.009 |
| Positive CMV serology | 495 (60.2%) | 492 (59.9%) | 0.007 |
| Hepatitis C | 93 (11.3%) | 88 (10.7%) | 0.019 |
| Matching and transplant characteristics | |||
| Sex matched | 663 (80.7%) | 673 (81.9%) | 0.031 |
| Race matched | 460 (56.0%) | 451 (54.9%) | 0.022 |
| HLA matched (≤3 loci mismatch) | 95 (11.6%) | 97 (11.8%) | 0.008 |
| ABO matched | 691 (84.1%) | 700 (85.2%) | 0.30 |
| CMV matched | 424 (51.6%) | 426 (51.8%) | 0.005 |
| Waitlist time (days) | 37 (10-236) | 38.5 (9-246) | 0.002 |
| Donor distance to transplanting center (nautical miles) | 231.5 (97-414) | 240 (98-406) | 0.008 |
| Graft cold ischemic time (hours) | 3.5 (3.0-4.1) | 3.5 (3.0-4.1) | 0.029 |
| Immunosuppression | |||
| Induction regimen | <0.001 | ||
| ATGAM | 7 (1.1%) | 23 (3.6%) | |
| Thymoglobulin | 140 (22.4%) | 144 (22.6%) | |
| Basiliximab | 196 (31.3%) | 259 (40.6%) | |
| Steroids only | 267 (42.7%) | 193 (30.3%) | |
| Other | 16 (2.6%) | 19 (3.0%) | |
| Missing | 196 (23.8%) | 184 (22.4%) | |
| Components of initial maintenance regimen | |||
| Steroids | 749 (91.1%) | 745 (90.6%) | 0.73 |
| Tacrolimus | 784 (95.4%) | 723 (88.0%) | <0.001 |
| Mycophenolate mofetil | 718 (87.3%) | 648 (78.8%) | <0.001 |
| Mycophenolic acid | 73 (8.9%) | 75 (9.1%) | 0.86 |
| Cyclosporin | 8 (1.0%) | 19 (2.3%) | 0.033 |
Abbreviations: CMV, cytomegalovirus; ECMO, Extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; HLA, human leukocyte antigen; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; RVAD, right ventricular assist device; SMD, standardized mean difference; TAH, total artificial heart; VAD, ventricular assist device.
Figure 2.
Comparison of survival stratified by post-transplant dialysis in propensity score-matched cohorts.
The dialysis group experienced a higher rate of stroke and a longer hospital length of stay. Furthermore, the recipients in the dialysis group were less likely to be functionally independent with higher rates of hospitalization and assistance requirement during the last follow-up visit. The rates of acute rejection requiring medical treatment, permanent pacemaker, and hospitalization for infection after discharge were equivalent (Table 4).
Table 4.
Post-Transplant Outcomes Stratified by Post-Transplant Dialysis Requirement in Propensity Score-Matched Cohorts
| No dialysis (n = 822) | Post-transplant dialysis (n = 822) | p-value | |
|---|---|---|---|
| Stroke | 31 (3.8%) | 74 (9.1%) | <0.001 |
| Permanent pacemaker | 12 (1.5%) | 23 (2.8%) | 0.058 |
| Hospital LOS (days) | 17 (12-24) | 33 (21-56) | <0.001 |
| Treated acute rejection | 79 (9.6%) | 103 (12.5%) | 0.059 |
| Hospitalization for infection after discharge | 116 (37.1%) | 134 (44.8%) | 0.051 |
| Functional status at last follow-up | <0.001 | ||
| Hospitalized | 34 (4.9%) | 146 (20.7%) | |
| Requires assistance | 98 (14.1%) | 158 (22.4%) | |
| Functionally independent | 562 (81.0%) | 400 (56.8%) |
Abbreviation: LOS, length of stay.
Impact of temporal factors of dialysis on survival
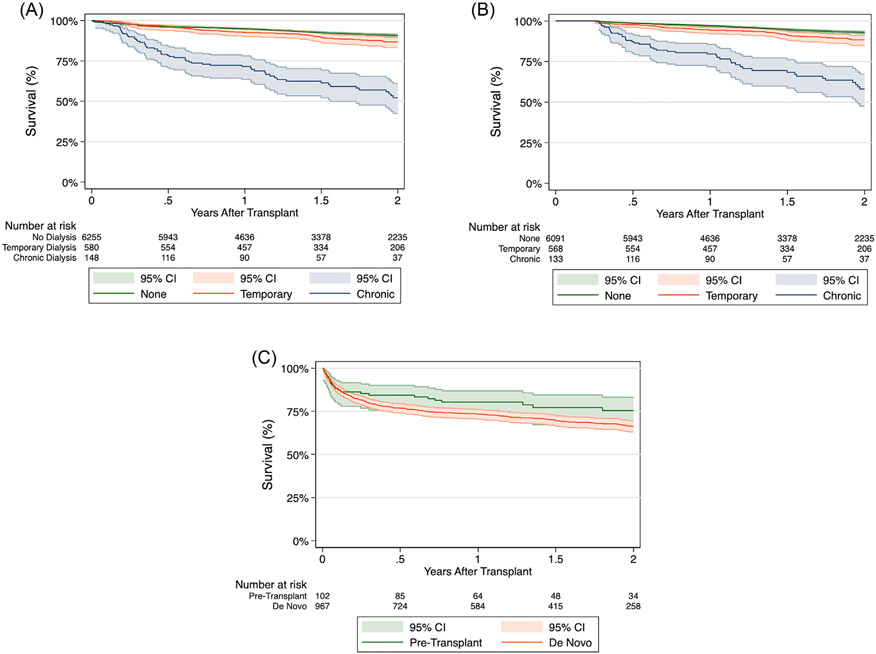
The impact of post-transplant dialysis chronicity was evaluated by further stratifying the post-transplant dialysis cohort into whether their dialysis requirement was temporary (n = 580) or chronic (n = 148). The temporary dialysis group had significantly improved 1-year (92.5% vs 71.6%, p < 0.001) and 2-year (86.6 % vs 52.2%, p < 0.001) survival compared to the chronic dialysis group (Figure 3A). Furthermore, 1-year (92.5 vs 71.6) and 2-year (86.5% vs 51.3%) graft survival rates were also significantly higher in the temporary dialysis group. However, the temporary dialysis group still had an increased risk of 2-year mortality (HR 1.43, 95% CI 1.10-1.86, p = 0.007) and 2-year graft failure (HR 1.44, 95% CI 1.11-1.87, p = 0.005) compared to the nondialysis group.
Figure 3.
Kaplan-Meier estimates for survival following heart transplantation. (A) Comparison of survival stratified by post-transplant dialysis requirements and dialysis chronicity. (B) Comparison of survival stratified by post-transplant dialysis requirements and dialysis chronicity with conditional 90-day survival. (C) Comparison of survival stratified by pretransplant dialysis and post-transplant de novo dialysis.
In the 90-day conditional survival analysis to account for immortal time bias in the temporary dialysis group, the temporary dialysis group continued to have higher 1-year (94.3% vs 79.7%, p < 0.001) and 2-year (88.4 % vs 58.1%, p < 0.001) survival compared to the chronic dialysis group (Figure 3B). Furthermore, the temporary dialysis group continued to have an increased risk of 2-year mortality compared to the nondialysis group (HR 1.70, 95% CI 1.27-2.27, p < 0.001).
When assessing the impact of dialysis timing, the post-transplant dialysis cohort was further divided into pretransplant (n = 102) and post-transplant de novo (n = 967) dialysis groups. The post-transplant dialysis group had comparable 1-year (80.4% vs 75.4%) and 2-year (73.3 % vs 66.4%) survival rates to the pretransplant dialysis group (p = 0.1068; Figure 3C).
Predictors of post-transplant dialysis and dialysis chronicity
Multivariable logistic regression with backwards stepwise selection was performed to identify risk factors for post-transplant dialysis. In the final model, higher BMI, HF etiologies (congenital, restrictive, and valvular), previous sternotomy and/or cardiac surgery, elevated total bilirubin, low pretransplant eGFR, MV, blood transfusion on the waitlist, ECMO, and increased graft cold ischemia time were associated with an increased odds of post-transplant dialysis (Table 5A). Low pretransplant eGFR, MV, and ECMO were strong risk factors for post-transplant dialysis, where eGFR <15 mL/min/1.73 m2 was associated with over 16-fold increased odds of post-transplant dialysis. Recipient blood type B, induction with steroids only, and maintenance immunosuppression with tacrolimus were associated with decreased odds of post-transplant dialysis (Table 5A). In this model, a total of 24% of patients were excluded due to missing data, of which 22.9% was from the induction regimen covariable. A model without this variable is displayed in Table S1, which demonstrated comparable results.
Table 5.
Multivariable Logistic Regression Models. (A) Model for Dialysis Following Heart Transplant. (B) Model for Nonrecoverable, Chronic Dialysis Requirement Among Recipients With Post-Transplant Renal Failure Requiring Dialysis
| A. | |||
|---|---|---|---|
| Odds ratio | 95% confidence interval | p-value | |
| BMI, increasing per 1 kg/m2 | 1.04 | 1.03, 1.06 | <0.001 |
| Recipient blood type | |||
| A | Ref | Ref | Ref |
| AB | 1.21 | 0.85, 1.73 | 0.292 |
| B | 0.76 | 0.59. 0.98 | 0.037 |
| O | 0.91 | 0.76, 1.09 | 0.294 |
| Heart failure etiology | |||
| Nonischemic | Ref | Ref | Ref |
| Ischemic | 0.92 | 0.76, 1.12 | 0.426 |
| Congenital | 2.59 | 1.82, 3.68 | <0.001 |
| Restrictive | 1.52 | 1.05. 2.20 | 0.026 |
| Valvular | 2.02 | 1.04, 3.93 | 0.038 |
| Hypertrophic | 1.32 | 0.15, 11.74 | 0.806 |
| Previous sternotomy and/or cardiac surgery | 1.27 | 1.07, 1.51 | 0.007 |
| Total bilirubin, increasing, per 1 mg/dL | 1.09 | 1.04, 1.13 | <0.001 |
| eGFR at transplant, mL/min/1.73 m2 | |||
| ≥90 | Ref | Ref | Ref |
| 60-89 | 1.76 | 1.33, 2.32 | <0.001 |
| 30-59 | 2.82 | 2.15, 3.70 | <0.001 |
| 15-29 | 4.57 | 2.75, 7.61 | <0.001 |
| <15 | 16.51 | 3.51, 77.65 | <0.001 |
| Mechanically ventilated at time of transplant | 1.85 | 1.13, 3.06 | 0.015 |
| Blood transfusion on waitlist | 1.45 | 1.16, 1.80 | 0.001 |
| Pretransplant ECMO | 1.82 | 1.27, 2.60 | 0.001 |
| Sex-matched donor and recipient | 1.17 | 0.95, 1.43 | 0.131 |
| Race-matched donor and recipient | 1.12 | 0.95, 1.32 | 0.161 |
| Graft cold ischemia time, increasing, per hour | 1.11 | 1.03, 1.19 | 0.007 |
| Induction regimen | |||
| ATGAM | Ref | Ref | Ref |
| Thymoglobulin | 0.64 | 0.39, 1.05 | 0.077 |
| Basiliximab | 0.69 | 0.42, 1.12 | 0.132 |
| Steroids only | 0.47 | 0.29, 0.78 | 0.003 |
| Other | 0.65 | 0.34, 1.27 | 0.211 |
| Initial tacrolimus maintenance immunosuppression | 0.36 | 0.25, 0.47 | <0.001 |
| Initial mycophenolate mofetil maintenance immunosuppression | 0.83 | 0.65, 1.06 | 0.130 |
| 1,737 (24%) of study patients were excluded from the final model due to incomplete data. | |||
| B. | |||
| Odds ratio | 95% confidence interval | p-value | |
| BMI, increasing per 1 kg/m2 | 1.03 | 0.99, 1.07 | 0.179 |
| Previous sternotomy and/or cardiac surgery | 1.96 | 1.32, 2.90 | 0.001 |
| eGFR at transplant, mL/min/1.73 m2 | |||
| ≥90 | Ref | Ref | Ref |
| 60-89 | 1.75 | 0.77, 3.95 | 0.180 |
| 30-59 | 1.54 | 0.69, 3.42 | 0.293 |
| 15-29 | 2.69 | 0.95, 7.63 | 0.063 |
| <15 | 7.68 | 1.77, 33.28 | 0.006 |
| IABP at time of transplant | 0.69 | 0.43, 1.10 | 0.118 |
| Female donor | 1.48 | 0.99, 2.22 | 0.059 |
| ABO-matched donor and recipient | 0.49 | 0.31, 0.78 | 0.003 |
| Race-matched donor and recipient | 0.71 | 0.48, 1.03 | 0.071 |
| Mycophenolic acid as initial maintenance immunosuppression | 0.59 | 0.28, 1.26 | 0.173 |
| Cyclosporin as initial maintenance immunosuppression | 2.57 | 0.95, 6.92 | 0.062 |
Abbreviations: BMI, body mass index; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pump.
No study patients were excluded from the final model due to incomplete data.
Given the detrimental impact of chronic dialysis, a separate multivariable logistic regression was performed to identify risk factors for nonrecoverable, chronic post-transplant dialysis. The predictors of chronic post-transplant dialysis were previous sternotomy and low pretransplant eGFR (Table 5B), where eGFR <15 mL/min/1.73 m2 was associated with a 7-fold increased odds of chronic post-transplant dialysis. ABO-matched grafts were associated with decreased odds of chronic post-transplant dialysis (odds ratio 0.49, 95% CI 0.31-0.78, p = 0.003).
Discussion
This is the first study to evaluate the temporal effects of post-transplant dialysis on outcomes following OHT after the 2018 heart allocation policy change. Our results demonstrate that (1) there is a higher rate of post-transplant dialysis in the current allocation system compared to the prior system, (2) post-transplant dialysis is associated with increased morbidity and mortality, (3) post-transplant survival is affected by the chronicity of post-transplant dialysis, (4) timing of dialysis (before or after transplantation) does not influence outcomes, and (5) low pretransplant eGFR and ECMO are strong risk factors for post-transplant dialysis.
Heart transplantation is the gold-standard treatment for advanced HF patients. With improved survival, post-transplant renal disease is progressively becoming more prevalent.13 We observed that 13.4% of recipients experienced post-transplant renal failure requiring de novo dialysis, consistent with a previously reported incidence of 4% to 28%.14-18 Our results demonstrated that the rate of post-transplant dialysis was significantly higher in the new allocation system compared to the previous system. This finding is not surprising as the new allocation system was designed to improve the stratification of high-risk groups and prioritize candidates with high acuity, especially those bridged with ECMO or other forms of nondischargeable MCS.
In this study, our results confirmed that post-transplant dialysis is associated with higher morbidity and mortality. This is not surprising as these recipients requiring post-transplant dialysis had several well-known risk factors for worse outcomes including pretransplant infection, transfusion, MV, ECMO, and prior sternotomy. Additionally, higher rates of complications were associated with post-transplant dialysis, including stroke, longer hospitalization, and hospitalization for infection after discharge, and the recipients with post-transplant dialysis experienced increased physical frailty and decreased functional independence. As these factors are important prognostic markers, it raises a question regarding the impact of early post-transplant physical rehabilitation to mitigate the degree of physical deterioration to improve outcomes.
A plethora of literature supports the adverse impact of post-transplant chronic kidney disease and ESRD on outcomes following OHT.19-22 Recent evidence suggests that the prognostic implication of post-transplant acute kidney injury (AKI) may differ compared to chronic kidney disease and ESRD.23-25 However, only a few studies have evaluated the long-term effect of AKI and temporary dialysis on heart transplant recipients.17,18 Moreover, no studies have assessed the temporal effect of dialysis in the new allocation system. In this study, our results demonstrated that the temporary dialysis group had a significantly improved survival compared to the chronic dialysis group, with survival rates nearing the nondialysis cohort. Additionally, the pre- or post-transplant timing of dialysis did not affect survival. The exact mechanism of the adverse impact of post-transplant renal failure and dialysis on outcomes is still under further investigation, but the current evidence suggests that other comorbidities associated with renal disease and the intrinsic burden associated with dialysis may play an important role.26,27 Furthermore, uremia-related factors may also contribute to worse outcomes, including fluid overload, electrolyte derangement, oxidative stress, endothelial dysfunction, insulin resistance, and excess sympathetic tone.28,29
In this study, several risk factors for post-transplant dialysis have been identified to adversely impact post-transplant outcomes. These include increased BMI, elevated bilirubin, low eGFR, prior sternotomy, pretransplant blood transfusion, ECMO, MV, and increased graft cold ischemia time.30-32 These findings are important for candidate selection and counseling when OHT is being considered. Identification and risk stratification may help mitigate the adverse effects of these high-risk features and improve outcomes in this vulnerable population.
In our study, low pretransplant eGFR was the strongest predictor of post-transplant dialysis. In a prior study utilizing the UNOS database, a similar conclusion was drawn where a lower pretransplant eGFR was directly proportional to the increased mortality with an adjusted hazard ratio for mortality of 1.55 (95% CI 1.41-1.70) for eGFR <30 mL/ min/1.73 m2.33 Moreover, low pretransplant eGFR was an independent predictor for ESRD and subsequent kidney transplantation.33 This is also in accordance with our results, where low pretransplant eGFR was an independent risk factor for ESRD requiring chronic dialysis. There is growing evidence supporting improved outcomes with combined heart-kidney (CHK) transplantation compared to heart transplants alone in recipients with advanced renal disease or on dialysis.34-37 Therefore, early consideration of CHK transplantation may be warranted in candidates with low pretransplant eGFR as these patients are at heightened risk for post-transplant dialysis and subsequent ESRD.
Bridge to transplant with ECMO was another significant risk factor with >80% increased odds of requiring post-transplant dialysis. This has a significant clinical implication as the new allocation system was designed to improve the stratification of high-risk groups and prioritize candidates with temporary MCS given their acuity.7 Prior studies have demonstrated that there are 4-times more patients bridged with ECMO compared to the old system.19,20 As more acutely ill patients supported with temporary MCS undergo OHT under the new allocation system, the incidence of post-transplant dialysis may become increasingly more prevalent. Therefore, additional studies are necessary to evaluate optimal renal protection methods and candidate stratification for CHK transplantation given the shift in allocation paradigm and recipient acuity and characteristics.
Study limitations
There are several limitations to this study. Foremost, the study is retrospective and nonrandomized in nature. The study only included and analyzed recipients after the allocation policy change leading to selection bias, which limits the generalizability of the findings to prior eras. Similar to other multicenter databases, the UNOS registry is prone to inaccurate data entry and missing data. Furthermore, there is limited granular information on the recipient, donor, and transplant-related characteristics. These include practice patterns, individualized postoperative management, surgeon preference, institutional preferences, bridging modality, and recipient and donor selection. Furthermore, given the limited, static nature of the available data, the dynamicity of the pretransplant renal function could not be captured. This database does differentiate between temporary and chronic dialysis needs in patient follow-up data records. However, the exact duration and other temporal factors of dialysis are not collected, which could be useful in the quantification of the impacts of dialysis duration on post-transplant outcomes. Furthermore, we acknowledge the immortal time bias in the temporary dialysis group given the period to survive dialysis support, which could have contributed to higher early survival. Lastly, given the broad spectrum of renal diseases and individualized renal replacement therapy, the inconsistency and heterogenicity of the cohort may have affected the results.
Conclusion
This is the first study to evaluate the temporal effects of post-transplant dialysis on outcomes following OHT after the 2018 adult heart allocation policy change. The present study of 7,223 adult OHT recipients from the current allocation system demonstrates that post-transplant dialysis is associated with worse outcomes. The rate of post-transplant renal failure requiring dialysis is significantly higher in the current allocation system compared to the prior system. Survival appears to be affected by the chronicity of post-transplant dialysis requirements, where there was a significant survival improvement with temporary dialysis. Low pretransplant eGFR and ECMO are strong predictors of post-transplant dialysis.
Supplementary Material
Abbreviations:
- AKI
acute kidney injury
- BMI
body mass index
- CHK
combined heart-kidney transplantation
- CI
confidence interval
- ECMO
extracorporeal membrane oxygenation
- eGFR
estimated glomerular filtration
- ESRD
end-stage renal disease
- HF
heart failure
- HR
hazard ratio
- MCS
mechanical circulatory support
- MV
mechanical ventilation
- OHT
orthotopic heart transplantation
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure statement
Dr. Kaczorowski received consultant and speaking fees for Medtronic and Abiomed. There are no direct conflicts of interest as it relates to this manuscript.
Disclaimer
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2023.01.004.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association [published correction appears in Circulation. 2017 Mar 7;135(10): e646] [published correction appears in Circulation. 2017 Sep 5;136 (10):e196]. Circulation 2017;135:e146–603. 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult heart transplantation report—2019; focus theme: donor and recipient size match [published correction appears in J Heart Lung Transplant. 2020 Jan;39(1):91]. J Heart Lung Transplant 2019;38:1056–66. 10.1016/j.healun.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt SA, Haddad F. The changing face of heart transplantation. J Am Coll Cardiol 2008;52:587–98. 10.1016/j.jacc.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Organ Procurement and Transplantation Network (OPTN). https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ Accessed March 12, 2022.
- 5.Estep JD, Soltesz E, Cogswell R. The new heart transplant allocation system: early observations and mechanical circulatory support considerations. J Thorac Cardiovasc Surg 2020;161:1839–46. 10.1016/j.jtcvs.2020.08.113,S0022-5223(20)32638-6. [DOI] [PubMed] [Google Scholar]
- 6.Kilic A, Mathier MA, Hickey GW, et al. Evolving trends in adult heart transplant with the 2018 heart allocation policy change. JAMA Cardiol 2021;6:159–67. 10.1001/jamacardio.2020.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awad MA, Shah A, Griffith BP. Current status and outcomes in heart transplantation: a narrative review. Rev Cardiovasc Med 2022;23:11. 10.31083/j.rcm2301011. [DOI] [PubMed] [Google Scholar]
- 8.Ganapathi AM, Lampert BC, Mokadam NA, et al. Allocation changes in heart transplantation: what has really changed? J Thorac Cardiovasc Surg 2021;165:724–33.e7. 10.1016/j.jtcvs.2021.03.031,S0022-5223(21)00510-9. [DOI] [PubMed] [Google Scholar]
- 9.Goff RR, Uccellini K, Lindblad K, et al. A change of heart: preliminary results of the US 2018 adult heart allocation revision. Am J Transplant 2020;20:2781–90. 10.1111/ajt.16010. [DOI] [PubMed] [Google Scholar]
- 10.Lazenby KA, Narang N, Pelzer KM, Ran G, Parker WF. An updated estimate of posttransplant survival after implementation of the new donor heart allocation policy. Am J Transplant 2022;22:1683–90. 10.1111/ajt.16931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Zhao X, Hammill BG, et al. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: insights from the get with the guidelines-heart failure registry. Circ Heart Fail 2018;11:e004646. 10.1161/CIRCHEARTFAILURE.117.004646. [DOI] [PubMed] [Google Scholar]
- 12.Heywood JT, Fonarow GC, Costanzo MR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007;13:422–30. 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Nadkarni GN, Chauhan K, Patel A, et al. Temporal trends of dialysis requiring acute kidney injury after orthotopic cardiac and liver transplant hospitalizations. BMC Nephrol 2017;18:244. 10.1186/s12882-017-0657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi AP, Vella JP. Acute kidney disease after liver and heart transplantation. Transplantation 2016;100:506–14. 10.1097/TP.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 15.Jokinen JJ, Tikkanen J, Kukkonen S, et al. Natural course and risk factors for impaired renal function during the first year after heart transplantation. J Heart Lung Transplant 2010;29:633–40. 10.1016/j.healun.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Escoresca Ortega AM, Ruíz de Azúa López Z, Hinojosa Pérez R, et al. Kidney failure after heart transplantation. Transplant Proc 2010;42:3193–5. 10.1016/j.transproceed.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Odim J, Wheat J, Laks H, et al. Peri-operative renal function and outcome after orthotopic heart transplantation. J Heart Lung Transplant 2006;25:162–6. 10.1016/j.healun.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Fortrie G, Manintveld OC, Constantinescu AA, van de Woestijne PC, Betjes MGH. Renal function at 1 year after cardiac transplantation rather than acute kidney injury is highly associated with long-term patient survival and loss of renal function: a retrospective cohort study. Transpl Int 2017;30:788–98. 10.1111/tri.12940. [DOI] [PubMed] [Google Scholar]
- 19.Cogswell R, John R, Estep JD, et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant 2020;39:1–4. 10.1016/j.healun.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Yang BQ, Itoh A, Masood MF, Hartupee JC, Schilling JD. Impact of new UNOS allocation criteria on heart transplant practices and outcomes. Transplant Direct 2020;7:e642. 10.1097/TXD.0000000000001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora S, Andreassen A, Simonsen S, et al. Prognostic importance of renal function 1 year after heart transplantation for all-cause and cardiac mortality and development of allograft vasculopathy. Transplantation 2007;84:149–54. 10.1097/01.tp.0000268810.61393.2c. [DOI] [PubMed] [Google Scholar]
- 22.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003;349:931–40. 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 23.Villar E, Boissonnat P, Sebbag L, et al. Poor prognosis of heart transplant patients with end-stage renal failure. Nephrol Dial Transplant 2007;22:1383–9. 10.1093/ndt/gfl811. [DOI] [PubMed] [Google Scholar]
- 24.Roest S, Hesselink DA, Klimczak-Tomaniak D, et al. Incidence of end-stage renal disease after heart transplantation and effect of its treatment on survival. ESC Heart Fail 2020;7:533–41. 10.1002/ehf2.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang TJ, Lin CH, Wei HJ, Wu MJ. Long-term outcomes and risk factors of renal failure requiring dialysis after heart transplantation: a nationwide cohort study. J Clin Med. 2020;9:2455. 10.3390/jcm9082455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension 2003;42:1050–65. 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 27.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 2008;3:505–21. 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa E, Rocha S, Rocha-Pereira P, et al. Cross-talk between inflammation,coagulation/fibrinolysis and vascular access in hemodialysis patients. J Vasc Access 2008;9:248–53. [PubMed] [Google Scholar]
- 29.Kendrick J, Chonchol MB. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol 2008;4:672–81. 10.1038/ncpneph0954. [DOI] [PubMed] [Google Scholar]
- 30.Weiss ES, Allen JG, Kilic A, et al. Development of a quantitative donor risk index to predict short-term mortality in orthotopic heart transplantation. J Heart Lung Transplant 2012;31:266–73. 10.1016/j.healun.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Kilic A, Hickey G, Mathier MA, et al. Outcomes of the first 1300 adult heart transplants in the United States after the allocation policy change. Circulation 2020;141:1662–4. 10.1161/CIRCULATIONAHA.119.045354. [DOI] [PubMed] [Google Scholar]
- 32.Singh SSA, Dalzell JR, Berry C, Al-Attar N. Primary graft dysfunction after heart transplantation: a thorn amongst the roses. Heart Fail Rev 2019;24:805–20. 10.1007/s10741-019-09794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habib PJ, Patel PC, Hodge D, et al. Pre-orthotopic heart transplant estimated glomerular filtration rate predicts post-transplant mortality and renal outcomes: an analysis of the UNOS database. J Heart Lung Transplant 2016;35:1471–9. 10.1016/j.healun.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Gill J, Shah T, Hristea I, et al. Outcomes of simultaneous heart-kidney transplant in the US: a retrospective analysis using OPTN/UNOS data. Am J Transplant 2009;9:844–52. 10.1111/j.1600-6143.2009.02588.x. [DOI] [PubMed] [Google Scholar]
- 35.Schaffer JM, Chiu P, Singh SK, Oyer PE, Reitz BA, Mallidi HR. Heart and combined heart-kidney transplantation in patients with concomitant renal insufficiency and end-stage heart failure. Am J Transplant 2014;14:384–96. 10.1111/ajt.12522. [DOI] [PubMed] [Google Scholar]
- 36.Karamlou T, Welke KF, McMullan DM, et al. Combined heart-kidney transplant improves post-transplant survival compared with isolated heart transplant in recipients with reduced glomerular filtration rate: analysis of 593 combined heart-kidney transplants from the United Network Organ Sharing database. J Thorac Cardiovasc Surg 2014;147:456–61. 10.1016/j.jtcvs.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Kilic A, Grimm JC, Whitman GJ, et al. The survival benefit of simultaneous heart-kidney transplantation extends beyond dialysis-dependent patients. Ann Thorac Surg 2015;99:1321–7. 10.1016/j.athoracsur.2014.09.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.