Abstract
Objective:
Epigenetic modifications such as DNA methylation play a key role in male infertility etiology. This study aimed to explore the global DNA methylation status in testicular spermatogenic cells of varicocele-induced rats and consider their semen quality, with a focus on key epigenetic marks, namely 5-methylcytosine (5-mC) and 5-hydroxymethylcytosine (5-hmC), as well as the mRNA and proteins of ten-eleven translocation (TET) methylcytosine dioxygenases 1-3.
Materials and Methods:
In this experimental study, 24 mature male Wistar rats (8 in each group) were assigned amongst the control, sham, and varicocele groups. Sperm quality was assessed, and DNA methylation patterns of testicular spermatogenic cells were investigated using reverse transcription-polymerase chain reaction (RT-PCR), western blot, and immunofluorescence techniques.
Results:
Sperm parameters, chromatin and DNA integrity were significantly lower, and sperm lipid peroxidation significantly increased in varicocele-induced rats in comparison with control rats. During spermatogenesis in rat testis, 5-mC and 5-hmC epigenetic marks, and TET1-3 mRNA and proteins were expressed. In contrast to the 5-mC fluorescent signal which was presented in all testicular cells, the 5-hmC fluorescent signal was presented exclusively in spermatogonia and a few spermatids. In varicocele-induced rats, the 5-mC signal decreased in all cells within the tubules, whereas a strong signal of 5-hmC was detected in seminiferous tubules compared to the control group. As well, the levels of TET2 mRNA and protein expression were significantly upregulated in varicocele-induced rats in comparison with the control group. Also, our results showed that the varicocele-induced animals exhibited strong fluorescent signals of TET1-3 in testicular cells, whereas weak fluorescent signals were identified in the seminiferous tubules of the control animals.
Conclusion:
Consequently, we showed TET2 upregulation and the 5-hmC gain at testicular levels are associated with varicocele and sperm quality decline, and therefore they can be exploited as potential biomarkers of spermatogenesis.
Keywords: DNA Methylation, Male Infertility, Sperm, Varicocele, 5-Methylcytosine
Introduction
Varicocele as a public and remediable risk factor of male infertility, affects 15% of the general male population, within the infertile men cohort, the varicocele frequency is higher, being recognized in 35 to 50% of males with primary infertility, while in men with secondary infertility, it is 80% (1). Varicocele is the expansion and torsion of the scrotal venous pampiniform plexus which leads to pathological problems influencing particularly the left testicle (2). It is associated with a reduction of sperm count, motility, and normal morphology (3). Although various studies have recently been carried out on different aspects of varicocele, the varicocele etiology is multifactorial, and its physio-pathological mechanisms are uncertain (4). Overall, numerous factors such as oxidative stress, hypoxia, hyperthermia, apoptosis, and inflammation may be implicated (5, 6).
The alterability of clinical phenotypes related to varicocele proposes the attendance of indefinite related genetic factors and epigenetic alterations that may be significant in the varicocele etiology (3). DNA methylation is the most abundant epigenetic modification that directly influences the DNA, and it is necessitated for genomic imprinting, regulation of gene expression, sperm chromatin structure, and embryonic development (7). DNA methylation is regulated by the functional interaction between two enzyme families: DNA methyltransferases (DNMTs) and ten-eleven translocation (TET) methylcytosine dioxygenases, which regulate DNA methylation and demethylation, respectively. DNMTs catalyze the transfer of a methyl group from S-adenosylmethionine (SAM) to the C5 position of cytosine to produce 5-methylcytosine (5-mC), while TET proteins are Fe (II)/2-oxoglutarate-dependent dioxygenases that oxidize 5-mC to 5-hydroxymethylcytosine (5-hmC), 5-formylcytosine (5-fC), and 5-carboxylcytosine (5-caC) in DNA (8).
Abnormal DNA methylation can cause male infertility by leading to abnormal sperm parameters (9), this has been observed in men with varicocele too (1, 10, 11). Previous studies have shown that the severity of varicocele can affect the extent of sperm DNA methylation alteration (1, 12), which could be related to increased sperm DNA damage. This suggests that men with high-grade varicocele may be more likely to experience infertility (13).
Therefore, perception of pathophysiological mechanisms and cellular and molecular alterations during spermatogenesis in varicocele disorder is crucial in developing a remedy and for proper guidance and intervention. This study was designed to evaluate sperm parameters, sperm oxidative status, and the level of sperm DNA damage as well as testicular DNA methylation patterns in the rat model with varicocele induction.
Materials and Methods
Experimental design and animals
In this experimental study, 24 male Wistar rats weighing 150-200 g (two months of age) were purchased from the animal house belonging to the Royan Institute of Animal Biotechnology (Isfahan, Iran). Rats were held in the animal house in the standard environment, with a temperature of 22 ± 2°C, a humidity of 45-65%, and a 12-hr light/12- hr dark cycle, with access to water and food ad libitum. This experiment was ratified by the Ethics Committee of Royan Institute (IR.ACECR.ROYAN.REC.1400.049). After the acclimatization period (1 week), Wistar rats were randomly allocated into three experimental groups (eight rats per group): i. Control (with no intervention), ii. Sham (undergone a simple laparotomy), and iii. Varicocele (varicocele-induced).
Varicocele induction procedure
For the varicocele induction, rats were anesthetized via intraperitoneal injection of Ketamine (100 mg/kg) and Xylazine (10 mg/kg) using Guide for the Care and Use of Laboratory Animals (14). After the abdominal midline slice, the left renal vein, left adrenal vein, and left spermatic vein were then recognized. The undersurface of the left renal vein was attentively cleaned from adipose and connective tissues to make a channel and exposed in a medial site from the entry point of the left spermatic vein and left adrenal vein. Then, a 4-0 silk suture was elapsed through underneath the left renal vein and tied over a needle which had a 1 mm diameter and was set parallel to the left renal vein, next, the deletion of needles induces varicocele by enhancing the intravenous pressure lateral to the obstruction site, which was transferred to the pampiniform plexus. Lastly, the abdomen contents were rebounded, and the slice (in two layers) was closed by a 4-0 silk suture. In the sham group, partial ligation of the left renal vein was not carried out. The surgery was performed according to Turner’s protocol (15).
Sample collection
Eight weeks after the progress of varicocele induction, all rats were sacrificed, and their left testis was dissected and washed, and then a portion of each testicular tissue was fixed with formalin 10% fixative for immunohistochemical evaluation, and the residuary testicular tissues were utilized for the western blot analysis. The left epididymides were dissected, and for sperm retrieval, the caudal epididymides were put in a petri dish containing 5 ml of sperm washing medium (VitaSperm™ Innovative Biotech, Tehran, Iran) at 37˚C with 5% CO2 for 30 minutes (2).
Sperm parameters assessment
For sperm motility, ten microliters of sperm suspension were placed on pre-warmed clean slides, and then the motility of two hundred spermatozoa at random 10 microscopic fields was evaluated (CX31 OLYMPUS, Japan, 400× magnification). The percentages of motile (progressive and nonprogressive) sperm were recorded. For sperm concentration, ten microliters of diluted sperm suspension were assessed using a Makler chamber. Heads of sperm were considered in 5 rows by light microscopy (CX31 OLYMPUS, Japan, 400× magnification). The sperm concentration was shown as millions of sperm/mL. Sperm morphology was quantified by Eosin (1%, Merck, Germany) -Nigrosine (10%, Merck, Germany) staining. Forty microliters of Eosin were mixed with twenty microliters of sperm suspension. After 5 minutes, sixty microliters of Nigrosine were added. Thin smears were prepared with twenty microliters of sperm preparation on the slides. The morphology of two hundred spermatozoa was randomly assessed on the light microscope (CX31 OLYMPUS, Japan, 1000× magnification), afterward, the percentage of sperm abnormal morphology was reported (2).
Sperm lipid peroxidation evaluation
Sperm lipid peroxidation was evaluated by BODIPY 581/591 C11 dye. In brief, two million washed spermatozoa were exposed to a final concentration of 5 µM of BODIPY C11 dye for 30 minutes at 37˚C. Positive control was conducted for each sample by adding 2 mM H2 O2 to the washed sample. The sperm lipid peroxidation percentage was quantified using a FACSCalibur (Becton Dickinson, San Jose, CA, USA) Flow Cytometer (16).
Sperm chromatin maturity and DNA integrity assessment
Aniline blue (AB), an acidic dye that interrelates with lysine-rich histones, was exploited to evaluate the spermatic nucleus maturation level. In brief, after the smear preparation, the smears were fixed with 3% glutaraldehyde for 2 hr. Then, slides were dyed with AB (aqueous 5%) in 4% acetic acid (pH=3.5, 120 minutes), and then washed with phosphate-buffered saline (PBS 1X). A light microscope (CX31 OLYMPUS, Japan, 1000× magnification) was exhausted to assess the percentage of immature sperms with abnormal persistent histone content (AB-positive sperm). For each sample, at least two hundred spermatozoa were randomly considered. To improve our assessment of the ideal sperm nucleus structure, we also evaluated sperm nuclear chromatin protamination using chromomycin A3 (CMA3) staining. In brief, 20 μl of Carnoy solution (methanol/acetic acid, 3: 1) was added to 20 μl of sperm sample for 5 minutes at 4°C. After the smear provision, thereafter, the smears were treated with 150 μl CMA3 solution (0.25 mg/mL CMA3 in McIlvaine buffer [7 mL 0.1 M citric acid, 32.9 mL 0.2 M Na2 HPO4 .7H2 O, pH=7.0, containing 10 mM MgCl2) for 2 hours. After washing with PBS 1X, microscopic investigations of the slides were completed by a fluorescent microscope (BX51 OLYMPUS, Japan, 1000× magnification) with suitable filters (460-470 nm). For each sample, a minimum of two hundred spermatozoa were randomly counted based on sperm with light yellow fluorescence (CMA3+) that were considered spermatozoa with protamine deficiency (17). The Aridine orange (AO) staining was carried out to identify sperm DNA integrity. In this staining, after fixation of prepared smears with Carnoy’s solution at 4°C, smears were then dyed with AO in citrate-phosphate buffer (80 ml 0.1 M citric acid+5 ml 0.3 M NaH2 PO4, pH=2.5). Next step, the smears were washed with PBS 1X, and for each slide, two hundred sperm atozoa were randomly counted by a fluorescent microscope (BX51 OLYMPUS, Japan, 1000× magnification). The percentage of sperms with a red or orange nucleus was considered to display DNA damage (2).
Quantitative real time-polymerase chain reaction method
To isolate total RNA, 50 mg of rat testicular tissue, stored at -80°C, was homogenized with 1 ml of TRIzol reagent (Yekta Tajhiz Azma, Iran). The RNA quality and concentration were evaluated by a Thermo Scientific NanoDrop® ND-1000 spectrophotometer. The cDNA synthesis was performed by cDNA synthesis kit according to the manufacturer’s instruction (Biotechrabbit™ cDNA Synthesis Kit), subsequent Real-time PCR was carried out by SYBR green (Yekta Tajhiz Azma, Iran) fluorescent dye by ABI (Applied Biosystems StepOnePlus™). All data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and expressed as 2-ΔΔCt (17). The list of primers is summarised in Table 1.
Western blot technique
In brief, after protein extraction of rat testicular tissues with the TRIzol reagent (Yekta Tajhiz Azma, Iran), the concentration of protein was identified by Bradford’s assay. Protein samples were exposed to 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and then transported to polyvinylidene difluoride (PVDF) membranes. The samples were probed with GAPDH (1:5000, MAB374), and antibodies against TET1 (1:6000, GTX124207), TET2 (1:50, ab94580), and TET3 (1:100, NBP2-20602) as formerly explained (18). Protein expression was detected via goat anti-rabbit IgG-horseradish peroxidase (HRP) secondary antibody (1: 5000, sc-2301), and identified by an enhanced chemiluminescence system (ECL, Santa Cruz, USA) following the manufacturer’s instructions. Proteins expression was normalized for GAPDH expression as an internal control, and the signal quantification was attained via Image J software.
Immunofluorescence procedure
Immunofluorescence analysis was performed on fixed seminiferous tubules of rats using specific antibodies against 5-mC, 5-hmC, and TET1-3. This technique enabled the localization and evaluation of cytosine chemical modifications and the expression patterns of TET1-3 proteins. The rat testicular tissues were dissected and then fixed in 10% formalin buffer, embedded in paraffin wax, and then sections were cut (5 μm), deparaffinized, and rehydrated. Before the staining procedure, the slides were pre-heated for 25 minutes in an oven at 60°C (Samsung, Model CE117AE, South Korean) and then deparaffinized in Xylol (3x, each10 minutes), two times in 100% ethanol (each 5 minutes), in 96% ethanol (5 minutes) and 70% ethanol (5 minutes), and subsequently rehydrated. Next step, the slides were plunged in boiling citrate buffer (10% citrate buffer, pH=6) for 30 minutes, finally resumed to room temperature, and then rinsed in cold PBS (3x, each 5 minutes). After, all slides were immersed in 2% Triton X-100-PBS (5 minutes) and blocked by 3% bovine serum albumin (BSA) in 2% Triton X-100-PBS for 1 hour, and then subjected to the primary antibody against 5-mC (1:500, BI-MECY-0100), 5-hmC (1:500, NBP2-50099), TET1 (1:400, GTX124207), TET2 (1:400, ab94580), and TET3 (1:400, NBP2-20602) overnight at 4°C. Then, to be rinsed in PBS (3x, each 5 minutes) and subjected to secondary fluorescein isothiocyanate (FITC)-conjugated antibodies (AP124F, 1:200 for 5-mC, F1763; 1:300 for 5-hmC, and sc-2012; 1:80 for TET1-3) at room temperature for 1 hour. After washing with PBS, finally, the nuclei of testicular cells were stained using propidium iodide (PI). The testicular cells were imaged under a fluorescence microscope (BX51 OLYMPUS, Japan, 200× magnification) (18, 19).
Statistical analysis
The statistical analyses were carried out with SPSS software (SPSS Science, Chicago, IL, USA). Before statistical analysis, the normality of data was executed with the Shapiro-Wilk test. All data were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with post hoc Tukey HSD was employed for sperm parameters data, while independentsample t tests were used to identify differences between groups for reverse transcription-polymerase chain reaction (RT-PCR) and western blotting data. Graphs were shaped with GraphPad Prism statistical software package version 8 (San Diego, California, USA). P<0.05 were considered statistically significant. In this study, considering that there was no significant difference between the control and sham groups in sperm parameters, only the control group was used to evaluate gene and protein expression and immunofluorescence.
Results
Animal weight and testicular volume
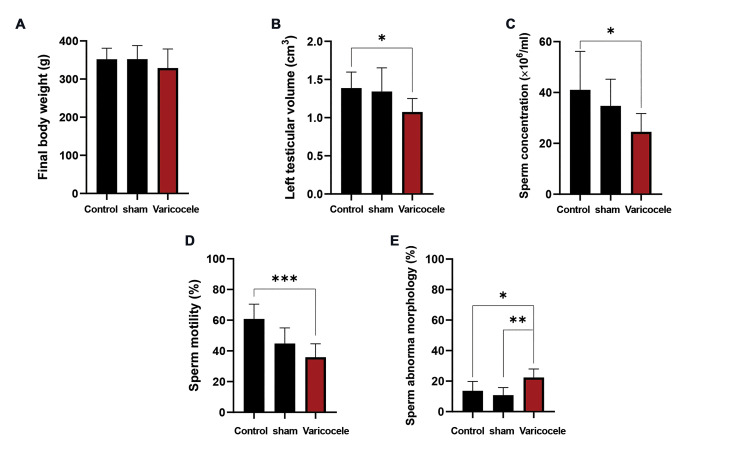
As indicated in Figure 1A, the final body weight was reduced in the varicocele group (328.75 ± 50.18 g) in comparison with the control (351.87 ± 28.94 g) and sham (352.00 ± 35.74 g) groups, but the difference was not significant. While varicocele induction resulted in a significant reduction (1.07 ± 0.17 cm3) in left testis volume compared to the control (1.38 ± 0.21 cm3 ; P=0.04, Fig .1B), no significant difference was detected between the varicocele group and the sham group (1.34 ± 0.31 cm3, P>0.05).
Fig.1.
The effect of varicocele induction on the rat body weight, testicular volume, and sperm parameters. Comparison of mean A. Final body weight (g), B. Left testicular volume (cm3), C. Sperm concentration (million /ml), D. Sperm motility (%), and E. Sperm abnormal morphology (%) of varicocele induction group with control and sham groups. Data are mean ± standard deviation (SD) (n=8). * ; P<0.05, **; P<0.01, and ***; P<0.001.
Sperm parameters
The results demonstrated that varicocele induction led to a significant decrease in the mean sperm concentration (24.50 ±7.24, Fig .1C) and motility (35.87 ± 8.72, Fig .1D) compared to the control (41.05 ± 15.13; P=0.02 and 60.75 ± 9.71; P=0.001, respectively) group. Also, in the varicocele condition, the mean percentage of sperm abnormal morphology (22.37 ± 5.57, Fig .1E) significantly increased in comparison with the control (13.64 ± 6.18, P=0.02) and sham (10.83 ± 4.98, P=0.004) groups.
Sperm lipid peroxidation, chromatin maturity, and DNA damage
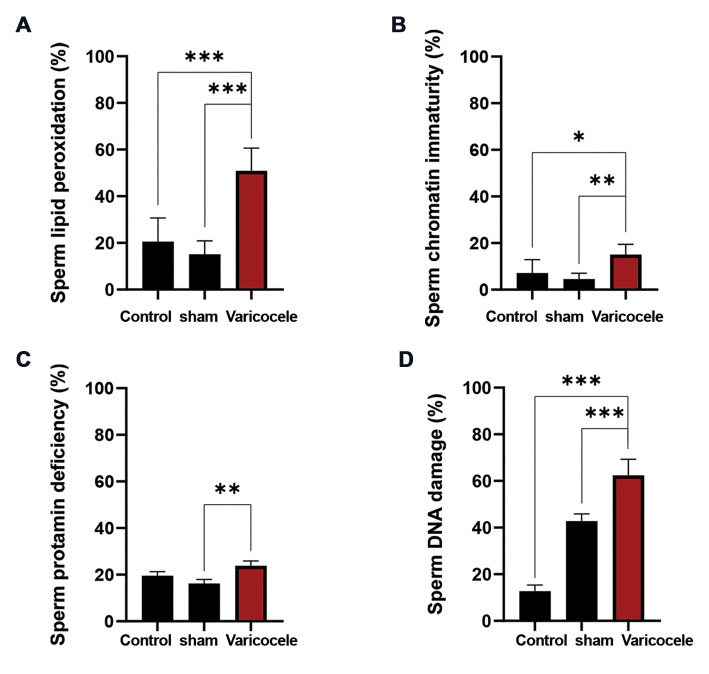
As shown in Figure 2A, the varicocele induction resulted in a significant increase (50.85 ± 9.75) in the percentages of sperm lipid peroxidation in comparison with the control (20.50 ±10.18, P<0.001) and sham (15.14 ± 5.72, P<0.001) groups. The mean percentages of spermatozoa with abnormal persistence of histones in varicocele animals (15.00 ± 4.47, Fig .2B) were significantly higher than in control (7.14 ± 5.72, P=0.02) and sham (4.50 ± 2.50, P=0.003) animals. Besides, varicocele induction in rats led to a significant increase (23.83 ± 2.08, Fig .2C) in sperm protamine deficiency in comparison with sham (16.16 ± 1.75, P=0.006), while there was no significant difference between varicocele and control (19.50 ± 1.80) groups. Also, the mean percentages of spermatozoa with DNA damage were found to be remarkably higher in the varicocele (62.35 ± 6.96, Fig .2D) group compared to the control (12.68 ± 2.69, P<0.001) and sham (42.78 ± 3.12, P<0.001) groups.
Fig.2.
The effect of varicocele induction on the sperm lipid peroxidation and DNA integrity. Comparison of mean A. Sperm lipid peroxidationBODIPY staining (%), B. Chromatin immaturity-Aniline blue staining (%), C. Protamine deficiency-Chromomycine A3 staining (%), and D. DNA damage-Acridine orange staining (%) of varicocele induction group with control and sham groups. Data are mean ± standard deviation (SD) (n=8). *; P<0.05, **; P<0.01, and ***; P<0.001
The localization and expression levels of 5-mC and 5-hmC in the testicular tissue
Next, immunofluorescence staining of rat testicular tissue sections was performed to detect 5-mC and 5-hmC. A strong signal was detected for 5-mC from developing spermatogonia to spermatozoa in control animals (Fig .3A). However, the findings of this study demonstrated a decrease in the 5-mC signal from the cells at the basal membrane to the cells located in the lumen of the tubules in varicoceleinduced rats (Fig .3B). The study's findings indicated that in control animals, the 5-hmC signal was present mostly in spermatogonia and a few spermatids during their developing stages (Fig .3C), while, in rats with varicocele induction (Fig. 3D), a strong immunofluorescence signal of 5-hmC was detected in the seminiferous tubules.
Fig.3.
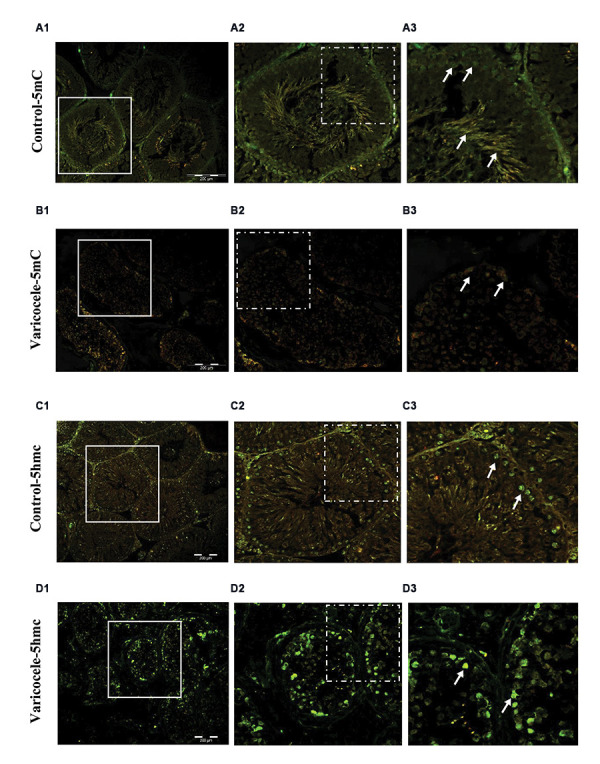
Varicocele increased hypomethylation. Immunofluorescent staining for 5-mC and 5-hmC. A. Note the high fluorescent signal (arrows) in the control group for 5-mC, B. Which is considerably decreased in the varicocele group. C. See the low fluorescent signal (arrows) in the control group for 5-mhC, D. Which is enhanced in the varicocele group (scale bar: 200 µm, magnification: 200×).
Table 1.
Primer sequences of genes analyzed by real-time polymerase chain reaction
|
| ||
|---|---|---|
| Name | Primer sequence (5´-3´) | Tm (°C) |
|
| ||
| Ten-eleven translocation (TET)1 | F: GAAGCCCACAACTACCAC | 56 |
| R: TTCCTAAATCTGCCTCGGTCA | ||
| Ten-eleven translocation (TET)2 | F: CCTTATTATACCCATCTAGGAGC | 62 |
| R: TCCGATACACCCATTTAGCAA | ||
| Ten-eleven translocation (TET)3 | F: GGTCACAGCCTGCATGGACT | 65 |
| R: AGCGATTGTCTTCCTTGGTCAG | ||
| Glyceraldehyde-3-Phosphate | F: TATGACTCTACCCACGGCAAG | 54 |
| Dehydrogenase (GAPDH) | R: ATACTCAGCACCAGCATCACC | |
|
| ||
Tm; primer melting temperature.
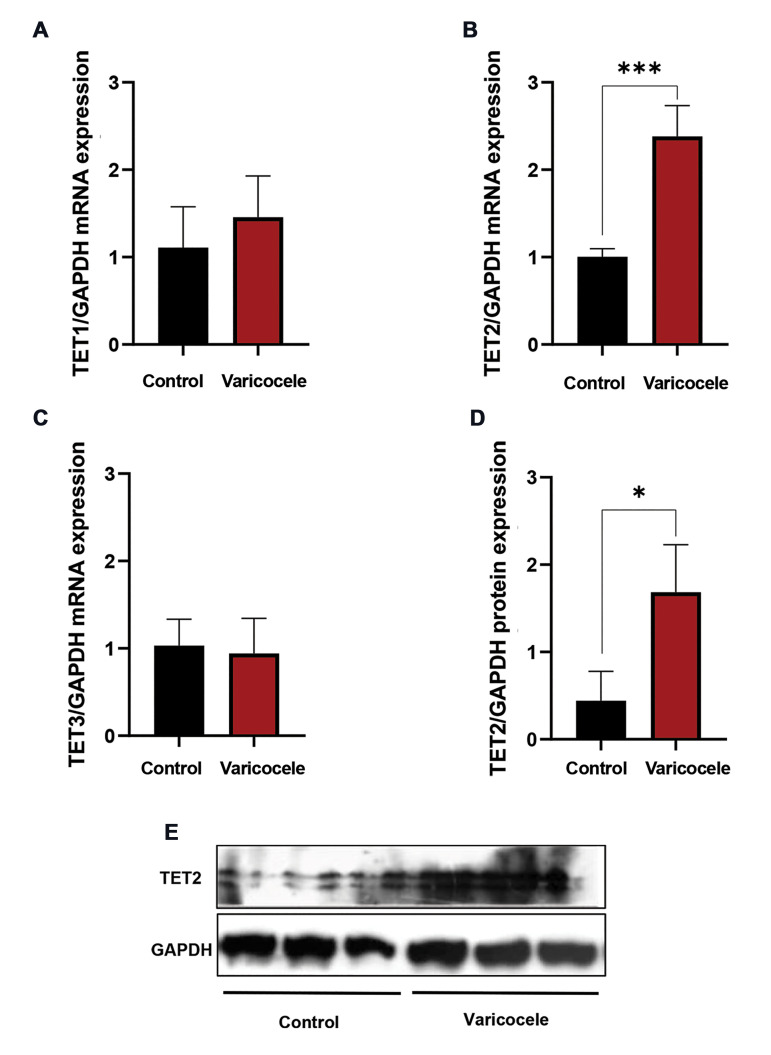
The levels of TET1-3 mRNA and TET2 protein expression
As represented in Figure 4A-C, the testicular tissue of rats with varicocele induction, expressed a significantly high level of TET2 mRNA (2.38 ± 0.35) in comparison with the control (1.00 ± 0.09, P<0.001) group, whereas no statistically significant differences were found in the expression levels of TET1 and TET3 mRNA between the varicocele and control group. Similarly, our findings presented that the TET2 expression at the protein level was significantly increased in varicocele-induced rats (1.68 ± 0.54) in comparison with the control (0.44 ± 0.33, P=0.03, Fig .4D, E) animals.
Fig.4.
The effect of varicocele induction on the ten-eleven translocation (TET) methylcytosine dioxygenases 1-3 expressions in rat testicular tissue. Comparison of mean mRNA levels of A. TET1, B. TET2, C. TET3, and D, E. Protein level of TET2 of varicocele induction group with the control group. Data are mean ± standard deviation (SD) (n=4 for RT-PCR and n=3 for western blotting). *; P<0.05 and ***; P<0.001.
The localization and expression level of TET1-3 in the testicular tissue
The localization and expression levels of TET1-3 in the testis of rats were identified by immunofluorescence staining (Fig .5A-F). These results displayed that TET1- 3 was present in different testicular cells, although with various distributions. Consistent with RT-PCR and western blot, the control animals exhibited weak fluorescent signals of TET1-3, especially TET2, in testicular cells (Fig.5A, C, E), whereas strong fluorescent signals were detected in seminiferous tubules of rats with varicocele induction (Fig.5B, D, E).
Fig.5.
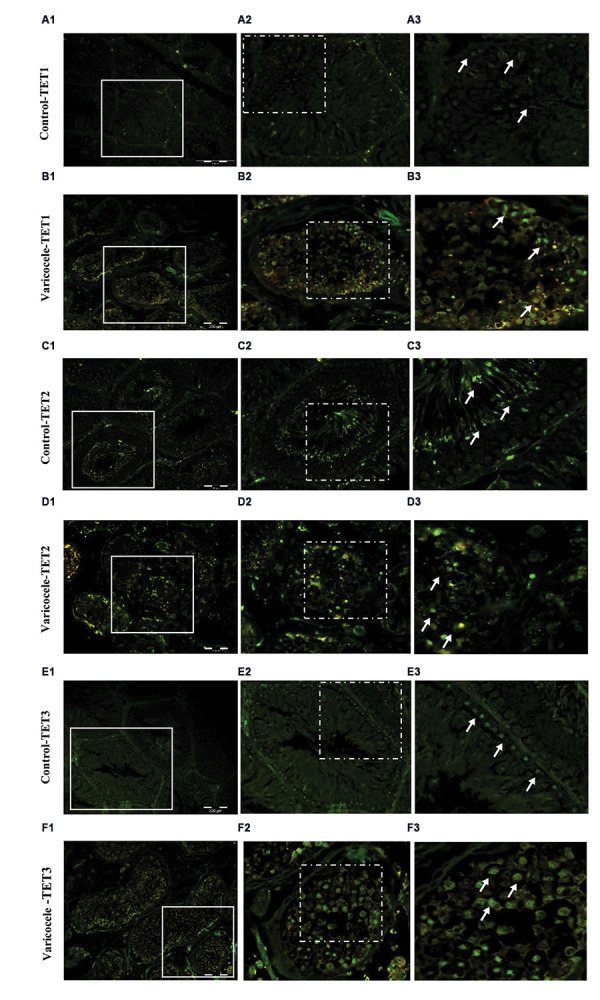
Varicocele induced ten-eleven translocation (TET) methylcytosine dioxygenases 1-3 enzyme activities in rat testicular tissue. A-F. Immunofluorescent staining for TET1-3. A, C, E. Note the weak fluorescent signal (arrows) of TET1-3 in the control group, B, D, F. Which is strong in the varicocele group (scale bar: 200 µm, magnification: 200×).
Discussion
One of the widespread risk factors for male infertility is varicocele as it negatively influences sperm parameters, sperm molecular and ultrastructural characteristics, and the testicular microenvironment (1). The varicocele etiology, the accurate effect of varicocele on male infertility, and the fundamental origins of the disturbance are still unclear; although literature has revealed that reactive oxygen species (ROS) and the subsequent oxidative stress perform a significant role in the pathogenesis of varicocelerelated male infertility (4, 20, 21). However, the variety of phenotypes associated with varicocele proposes an intricate multifactorial disorder in which genetic, epigenetic, and environmental factors act a significant role in varicocele etiology (1). In particular, epigenetic modifications including DNA methylation, histone modifications, and non-coding RNAs have been found to adjust the transcription process and spermatogenesis (22).
In line with the literature, the results of the current study showed sperm with significantly lower sperm quality such as low sperm concentration, motility, and higher sperm abnormal morphology, chromatin immaturity, lipid peroxidation, and DNA damage for rats with varicocele induction in comparison with control animals.These sperm pathologies are mainly related to varicocele-induced oxidative stress (2, 5, 23, 24). Local testicular heat stress, hypoxia, and inflammation instigated by varicocele can break the regional oxidative balance, and stimulate oxidative stress and excessive ROS production, which is associated with insufficient sperm histone-protamine replacement and/or improper DNA methylation. One should mention that insufficient sperm histoneprotamine replacement or poor sperm chromatin condensation performs an important role in DNA stability to ROS, which can influence the fertility ability in men and animals with varicocele (19, 20).
DNA methylation performs a key role in the regulation of gene expression and chromatin structure, which are important agents for spermatogenesis, sperm function, and embryonic development (1). Studies found a substantial negative correlation between global DNA methylation and DNA fragmentation in varicocele disorder (10) and damaged DNA is to a lesser extent prone to DNA methylation (25). While, a recent study revealed that prenatal exposure of male mice to a phytoestrogen-rich maternal diet in combination with a compromised redox system, resulted in global hypermethylation, and observed a positive correlation between DNA methylation and oxidative DNA damage in F1 generation testis and sperm, which these alterations may also influence F2 generation genetic integrity (26). It means that the persistent effects of maternal diets can be transferred later in life by DNA methylation changes. Therefore, one potential procedure that could serve as epigenetic cell memory, leading to differential gene expression, is DNA methylation (27).
The methylation of cytosine at the fifth carbon (5-mC) and the changed form of cytosine to 5-hmC are important epigenetic biomarkers that perform very critical roles in spermatogenesis (22). In this study, we performed immunofluorescence staining of testicular tissue sections to indicate 5-mC and 5-hmC localization and expression patterns. In accordance with previous results (28), 5-mC was present in all testicular cells, while 5-hmC was detectable almost exclusively in spermatogonia and only a few spermatids. The testicular histology analysis by Nettersheim et al. (29) revealed that human testicular tissue was marked by a high 5-hmC level in stem cell spermatogonia and a generally low 5-hmC level at the advanced differentiation stages. Besides, Gan et al. (30) showed elevated 5-hmC level was discovered in spermatogonia, which reduced to the round spermatid stage and enhanced anew in elongating spermatids and spermatozoa.
In our investigation, the fluorescent signal of 5-mC diminished in rats’ testicular tissue with varicocele induction, this is in agreement with the literature, which exhibits lower 5-mC values in the rat model of varicocele (19), and in men with varicocele (11) and oligoasthenozoospermia (31). In contrast to 5-mC, the immunofluorescent signal of 5-hmC was represented at a high level in testicular spermatogenic cells in varicocele-induced rats in comparison with control rats. In confirmation of the results of our present study, it was established that a high 5-hmC level was adversely correlated with normal sperm head morphology whereas positively correlated with sperm DNA damage (28), therefore seems 5-mC and 5-hmC levels are related to impaired spermatogenesis, sperm parameters, as well as, sperm chromatin protamination and DNA integrity in testicular pathological conditions (31, 32) like varicocele.
Based on the literature, DNA hypomethylation is primarily carried out by decreasing DNMT activity and increasing TETs (33). Therefore, an important question is by what process perform testicular spermatogenic cells in animals with varicocele acquire 5-hmC? There are two pathways of 5-hmC formation: planned TETs-mediated 5-mC oxidation (8) and ROS attack on 5-mC under oxidative stress conditions and convert the 5-mC to 5-hmC (34).
In our current study, at the mRNA level, TET1 and 3 did not change but TET2 level significantly increased in rats with varicocele induction, and also at the protein level, TET2 showed relatively strong expression. Interestingly, the TET2 protein structure differs from those of TET1 and TET3 and contains the IDAX CXXC4 domain that is crucial for CpG site detection, and downregulation of IDAX CXXC4 domain could result in TET2 overexpression and its improper recruitment to genomic regions where it is not normally found (35). Considering that the studies in this field are very limited, therefore, investigating the levels of TET2 and IDAX CXXC4 expression in normal and varicocele conditions will be important for future studies.
Another interesting point is that TET1 (36) and TET3 (37) are the maintenance DNA demethylases that do not deliberately decline the DNA methylation but especially avoid aberrant methylation extending into CpG islands in differentiated cells. Considering TET1 and 3 are recognized as maintenance demethylases, we did not see any significant difference in these gene expressions among the two groups. This might suggest that DNA methylation change in varicocele condition is not due to DNA maintenance aberration.
Also, our study in rats’ testicular tissue sections of seminiferous tubules in the varicocele and control conditions showed that TET1-3 have almost the same distribution as 5-hmC, and TET1-3 proteins showed relatively strong fluorescent signals in the spermatogenic cells in varicocele-induced rats in comparison with control rats. Our results displayed that active DNA demethylation (18) in the testicular spermatogenic cells in varicocele condition involves the alteration of 5-mC to 5-hmC mediated by TET1-3, especially TET2, but we could not rule out the ROS attack on the conversion of the 5-mC to 5-hmC.
In addition to the above-described mechanisms of active DNA demethylation, there are possible alternative pathways of DNA demethylation including demethylase activity imagined for DNMTs enzymes in oxidizing redox conditions, in the presence of high calcium and low SAM levels (38). In the varicocele state owing to delayed blood flow and the presence of excessive iron sedimentation, spermatozoa are subjected to oxidative stress and nutritional restriction (3). Moreover, increase intracellular calcium ions, leading to endoplasmic reticulum stress and apoptosis, have been considered one of the molecular mechanisms related to varicocele (39). Based on this background, Rashidi et al. (12) have reported that despite the sperm DNA hypomethylation status in men with varicocele, DNMTs enzymes expression was enhanced at both RNA and protein levels, which can reflect demethylase activity of DNMTs in individuals with varicocele. Considering the varicocele state has three prerequisite circumstances for DNMTs to act as demethylase, therefore it can physiologically perform significant roles in gene expression via epigenetic modification.
According to this issue, the incidence of high sperm DNA damage is mainly made happen by ROS, therefore in patients with a high percentage of 5-hmC-positive spermatozoa, sperm hydroxylation can also be caused by ROS attack (28), because in post-testicular sperm maturation, sperm have strongly declined cytoplasm volume which makes their genetic and epigenetic patterns very susceptible to interior and exterior agents, including ROS (40). Hence, there is this hypothesis that a high 5-hmC level in varicocele condition at the testicular level could result from active DNA demethylation, and at post-testicular level could cause by spontaneous hydroxymethylation by ROS attack following the reduction of cytoplasm and the differentiation of spermatogenic cells to spermatozoa, and our experimental approach can discern between the two approaches.
Conclusion
Our results demonstrated that sperm parameters, chromatin and DNA integrity, and also testicular spermatogenic cells DNA methylation pattern were impaired by varicocele induction. In varicocele conditions, high 5-hmC formation in testicular spermatogenic cells may be induced by active DNA demethylation through TETs enzyme activities, especially TET2. In addition, the high sperm DNA damage occurrence, which is mainly caused by ROS attack, can represent spontaneous high hydroxymethylation of 5-mC by ROS and hypomethylation at the post-testicular level. Therefore, we suggest that measuring the 5-mC/5- hmC at testicular and post-testicular levels can be a beneficial way to create prognostic epidata in male infertility cases, particularly varicocele, and knowing the mechanisms intricated in epigenetic modification unties the way for targeted remedy of varicocele pathological condition.
Acknowledgments
We would like to express our gratitude to the staff of Isfahan Fertility and Infertility Center as well as the staff of the Department of Animal Biotechnology, Reproductive Biomedicine Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran. This research received no specific grant from any funding agency in the public, commercial, or notfor-profit sectors. The authors declare no conflict of interest.
Author’s Contributions
H.T.D.; Performed varicocele induction, sperm functional analysis, western blot technique, data analysis, and manuscript write. N.N.; Analyzed Expression of TET2 and DNA Hypomethylation in Varicocele the data, performed literature research, drafted the manuscript, revised the manuscript, and the final scientific manuscript revision. F.R..; Performed western blot technique, and analyzed the data. M.H.N.-E., M.T.; Contributed to the conception, design, and coordination of the study, data analysis, revised the manuscript, and performed the final scientific manuscript revision. All authors read and approved the final manuscript.
References
- 1.Santana VP, James ER, Miranda-Furtado CL, Souza MF, Pompeu CP, Esteves SC, et al. Differential DNA methylation pattern and sperm quality in men with varicocele. Fertil Steril. 2020;114(4):770–778. doi: 10.1016/j.fertnstert.2020.04.045. [DOI] [PubMed] [Google Scholar]
- 2.Santana VP, Miranda-Furtado CL, de Oliveira-Gennaro FG, Dos Reis RM. Genetics and epigenetics of varicocele pathophysiology: an overview. J Assist Reprod Genet. 2017;34(7):839–847. doi: 10.1007/s10815-017-0931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadeghi N, Erfani-Majd N, Tavalaee M, Tabandeh MR, Drevet JR, Nasr-Esfahani MH. Signs of ROS-associated autophagy in testis and sperm in a rat model of varicocele. Oxid Med Cell Longev. 2020;2020:5140383–5140383. doi: 10.1155/2020/5140383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang QF, Wang S, Zhang H, Liu QL, Wei Y, Deng W, et al. Effects of alpha-lipoic acid on sperm quality in patients with varicocelerelated male infertility: study protocol for a randomized controlled clinical trial. Trials. 2022;23(1):1002–1002. doi: 10.1186/s13063-022-06951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorian K, Kadkhodaee M, Kianian F, Abdi A, Ranjbaran M, Ashabi G, et al. Long-term NaHS administration reduces oxidative stress and apoptosis in a rat model of left-side varicocele. Andrologia. 2020;52(2):e13496–e13496. doi: 10.1111/and.13496. [DOI] [PubMed] [Google Scholar]
- 6.Razi M, Tavalaee M, Sarrafzadeh-Rezaei F, Moazamian A, Gharagozloo P, Drevet JR, et al. Varicocoele and oxidative stress: New perspectives from animal and human studies. Andrology. 2021;9(2):546–558. doi: 10.1111/andr.12940. [DOI] [PubMed] [Google Scholar]
- 7.Rotondo JC, Lanzillotti C, Mazziotta C, Tognon M, Martini F. Epigenetics of male infertility: the role of DNA methylation. Front Cell Dev Biol. 2021;9:689624–689624. doi: 10.3389/fcell.2021.689624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onodera A, González-Avalos E, Lio CJ, Georges RO, Bellacosa A, Nakayama T, et al. Roles of TET and TDG in DNA demethylation in proliferating and non-proliferating immune cells. Genome Biol. 2021;22(1):186–186. doi: 10.1186/s13059-021-02384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Xu J, Yang T, Chen J, Li F, Shen B, et al. Genome-wide methylation analyses of human sperm unravel novel differentially methylated regions in asthenozoospermia. Epigenomics. 2022;14(16):951–964. doi: 10.2217/epi-2022-0122. [DOI] [PubMed] [Google Scholar]
- 10.Tavalaee M, Bahreinian M, Barekat F, Abbasi H, Nasr-Esfahani MH. Effect of varicocelectomy on sperm functional characteristics and DNA methylation. Andrologia. 2015;47(8):904–909. doi: 10.1111/and.12345. [DOI] [PubMed] [Google Scholar]
- 11.Bahreinian M, Tavalaee M, Abbasi H, Kiani-Esfahani A, Shiravi AH, Nasr-Esfahani MH. DNA hypomethylation predisposes sperm to DNA damage in individuals with varicocele. Syst Biol Reprod Med. 2015;61(4):179–186. doi: 10.3109/19396368.2015.1020116. [DOI] [PubMed] [Google Scholar]
- 12.Rashidi M, Tavalaee M, Abbasi H, Nomikos M, Nasr-Esfahani MH. Increased de novo DNA methylation enzymes in sperm of individuals with varicocele. Cell J. 2021;23(4):389–396. doi: 10.22074/cellj.2021.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santana VP, Miranda-Furtado CL, Pedroso DCC, Eiras MC, Vasconcelos MAC, Ramos ES, et al. The relationship among sperm global DNA methylation, telomere length, and DNA fragmentation in varicocele: a cross-sectional study of 20 cases. Syst Biol Reprod Med. 2019;65(2):95–104. doi: 10.1080/19396368.2018.1557762. [DOI] [PubMed] [Google Scholar]
- 14.NRC. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 15.Turner TT. The study of varicocele through the use of animal models. Hum Reprod Update. 2001;7(1):78–84. doi: 10.1093/humupd/7.1.78. [DOI] [PubMed] [Google Scholar]
- 16.Aitken RJ, Wingate JK, De Iuliis GN, McLaughlin EA. Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. Mol Hum Reprod. 2007;13(4):203–211. doi: 10.1093/molehr/gal119. [DOI] [PubMed] [Google Scholar]
- 17.Rahmani M, Tavalaee M, Hosseini M, Eskandari A, Shaygannia E, Sadeghi N, et al. Deferasirox, an iron-chelating agent, improves testicular morphometric and sperm functional parameters in a rat model of varicocele. Oxid Med Cell Longev. 2021;2021:6698482–6698482. doi: 10.1155/2021/6698482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni K, Dansranjavin T, Rogenhofer N, Oeztuerk N, Deuker J, Bergmann M, et al. TET enzymes are successively expressed during human spermatogenesis and their expression level is pivotal for male fertility. Hum Reprod. 2016;31(7):1411–1424. doi: 10.1093/humrep/dew096. [DOI] [PubMed] [Google Scholar]
- 19.Salmani S, Razi M, Sarrafzadeh-Rezaei F, Mahmoudian A. Testosterone amplifies HSP70-2a, HSP90 and PCNA expression in experimental varicocele condition: Implication for DNA fragmentation. Reprod Biol. 2020;20(3):384–395. doi: 10.1016/j.repbio.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Wang K, Gao Y, Wang C, Liang M, Liao Y, Hu K. Role of oxidative stress in varicocele. Front Genet. 2022;13:850114–850114. doi: 10.3389/fgene.2022.850114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majzoub A, Cho CL, Agarwal A, Esteves SC. In: Varicocele and male infertility: a complete guide. Esteves Sc, Cho CL, MajzoubA Agarwal A., editors. Cham: Springer International Publishing; 2019. Oxidative stress and varicocele pathophysiology series; pp. 55–71. [Google Scholar]
- 22.Gao Y, Zhao Y, Zhang H, Zhang P, Liu J, Feng Y, et al. Pubertal exposure to low doses of zearalenone disrupting spermatogenesis through ERα related genetic and epigenetic pathways. Toxicol Lett. 2019;315:31–38. doi: 10.1016/j.toxlet.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Shaygannia E, Nasr-Esfahani MH, Sotoodehnejadnematalahi F, Parivar K. Is ferroptosis involved in ROS-induced testicular lesions in a varicocele rat model? Basic Clin Androl. 2021;31(1):10–10. doi: 10.1186/s12610-021-00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassanin AM, Ahmed HH, Kaddah AN. A global view of the pathophysiology of varicocele. Andrology. 2018;6(5):654–661. doi: 10.1111/andr.12511. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Zhang F, Gao HH, Zhang JM. Effects of varicocele on DNA methylation pattern of H19 and Snrpn gene in spermatozoa and behavioural characteristics of adult rat offspring. Andrologia. 2017;49(1) doi: 10.1111/and.12591. [DOI] [PubMed] [Google Scholar]
- 26.Godschalk RWL, Janssen MCM, Vanhees K, van Doorn-Khosrovani SBVW, van Schooten FJ. Maternal exposure to genistein during pregnancy and oxidative DNA damage in testes of male mouse offspring. Front Nutr. 2022;9:904368–904368. doi: 10.3389/fnut.2022.904368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruno S, Williams RJ, Del Vecchio D. Epigenetic cell memory: the gene’s inner chromatin modification circuit. PLoS Comput Biol. 2022;18(4):e1009961–e1009961. doi: 10.1371/journal.pcbi.1009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efimova OA, Pendina AA, Tikhonov AV, Parfenyev SE, Mekina ID, Komarova EM, et al. Genome-wide 5-hydroxymethylcytosine patterns in human spermatogenesis are associated with semen quality. Oncotarget. 2017;8(51):88294–88307. doi: 10.18632/oncotarget.18331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nettersheim D, Heukamp LC, Fronhoffs F, Grewe MJ, Haas N, Waha A, et al. Analysis of TET expression/activity and 5mC oxidation during normal and malignant germ cell development. PLoS One. 2013;8(12):e82881–e82881. doi: 10.1371/journal.pone.0082881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gan H, Wen L, Liao S, Lin X, Ma T, Liu J, et al. Dynamics of 5-hydroxymethylcytosine during mouse spermatogenesis. Nat Commun. 2013;4:1995–1995. doi: 10.1038/ncomms2995. [DOI] [PubMed] [Google Scholar]
- 31.Olszewska M, Kordyl O, Kamieniczna M, Fraczek M, Jędrzejczak P, Kurpisz M. Global 5mC and 5hmC DNA levels in human sperm subpopulations with differentially protaminated chromatin in normo- and oligoasthenozoospermic males. Int J Mol Sci. 2022;23(9):4516–4516. doi: 10.3390/ijms23094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montjean D, Zini A, Ravel C, Belloc S, Dalleac A, Copin H, et al. Sperm global DNA methylation level: association with semen parameters and genome integrity. Andrology. 2015;3(2):235–240. doi: 10.1111/andr.12001. [DOI] [PubMed] [Google Scholar]
- 33.Hernández-Cruz EY, Arancibia-Hernández YL, Loyola-Mondragón DY, Pedraza-Chaverri J. Oxidative stress and its role in Cd-induced epigenetic modifications: use of antioxidants as a possible preventive strategy. Oxygen. 2022;2(2):177–210. [Google Scholar]
- 34.Madugundu GS, Cadet J, Wagner JR. Hydroxyl-radical-induced oxidation of 5-methylcytosine in isolated and cellular DNA. Nucleic Acids Res. 2014;42(11):7450–7460. doi: 10.1093/nar/gku334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko M, An J, Bandukwala HS, Chavez L, Aijö T, Pastor WA, et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature. 2013;497(7447):122–126. doi: 10.1038/nature12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin C, Lu Y, Jelinek J, Liang S, Estecio MR, Barton MC, et al. TET1 is a maintenance DNA demethylase that prevents methylation spreading in differentiated cells. Nucleic Acids Res. 2014;42(11):6956–6971. doi: 10.1093/nar/gku372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santiago M, Antunes C, Guedes M, Iacovino M, Kyba M, Reik W, et al. Tet3 regulates cellular identity and DNA methylation in neural progenitor cells. Cell Mol Life Sci. 2020;77(14):2871–2883. doi: 10.1007/s00018-019-03335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Wijst MG, Venkiteswaran M, Chen H, Xu GL, Plösch T, Rots MG. Local chromatin microenvironment determines DNMT activity: from DNA methyltransferase to DNA demethylase or DNA dehydroxymethylase. Epigenetics. 2015;10(8):671–676. doi: 10.1080/15592294.2015.1062204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Zhou T, Su YF, Hu ZY, Wei JJ, Wang W, et al. Prokineticin 2 overexpression induces spermatocyte apoptosis in varicocele in rats. Asian J Androl. 2020;22(5):500–506. doi: 10.4103/aja.aja_109_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016;28(1-2):1–10. doi: 10.1071/RD15325. [DOI] [PubMed] [Google Scholar]