Abstract
Fluoroquinolone resistance (FQ-R) in clinical isolates of Enterobacteriaceae species has been reported with increasing frequency in recent years. Two mechanisms of FQ-R have been identified in gram-negative organisms: mutations in DNA gyrase and reduced intracellular drug accumulation. A single point mutation in gyrA has been shown to reduce susceptibility to fluoroquinolones. To determine the extent of gyrA mutations associated with FQ-R in enteric bacteria, one set of oligonucleotide primers was selected from conserved sequences in the flanking regions of the quinolone resistance-determining regions (QRDR) of Escherichia coli and Klebsiella pneumoniae. This set of primers was used to amplify and sequence the QRDRs from 8 Enterobacteriaceae type strains and 60 fluoroquinolone-resistant clinical isolates of Citrobacter freundii, Enterobacter aerogenes, Enterobacter cloacae, E. coli, K. pneumoniae, Klebsiella oxytoca, Providencia stuartii, and Serratia marcescens. Although similarity of the nucleotide sequences of seven species ranged from 80.8 to 93.3%, when compared with that of E. coli, the amino acid sequences of the gyrA QRDR were highly conserved. Conservative amino acid substitutions were detected in the QRDRs of the susceptible type strains of C. freundii, E. aerogenes, K. oxytoca (Ser-83 to Thr), and P. stuartii (Asp-87 to Glu). Strains with ciprofloxacin MICs of >2 μg/ml expressed amino acid substitutions primarily at the Gly-81, Ser-83, or Asp-87 position. Fluoroquinolone MICs varied significantly for strains exhibiting identical gyrA mutations, indicating that alterations outside gyrA contribute to resistance. The type and position of amino acid alterations also differed among these six genera. High-level FQ-R frequently was associated with single gyrA mutations in all species of Enterobacteriaceae in this study except E. coli.
Fluoroquinolones are broad-spectrum antimicrobial agents effective in the treatment of a wide range of infections. However, widespread use of this class of agents has resulted in an increasing incidence of fluoroquinolone resistance (13). Mechanisms of resistance to quinolones include alterations in DNA gyrase and topoisomerase IV and decreased intracellular accumulation of the antimicrobial agent due to modifications of membrane proteins (3, 12).
The primary target of fluoroquinolones in gram-negative bacteria is DNA gyrase, a type II topoisomerase required for DNA replication and transcription (3). DNA gyrase, which is composed of two A subunits and two B subunits, is encoded by the gyrA and gyrB genes. In these organisms, resistance to fluoroquinolones has been shown to be associated most frequently with alterations in gyrA (7, 9, 29). The mutations are localized at the 5′ end of the gene (nucleotides 199 to 318 in the Escherichia coli gene sequence) in an area designated as the quinolone resistance-determining region, or QRDR, near Tyr-122, which binds the transiently cleaved DNA (14, 28). Genetic characterization of gyrA mutations associated with fluoroquinolone resistance in E. coli has been well defined by DNA sequence analysis of resistant strains of both clinical isolates and mutants selected in vitro (4, 11, 17, 24, 28). These mutations are found most frequently in the Ser-83 and Asp-87 codons and in the corresponding codon positions of the gyrA genes from several other organisms (6, 7, 9, 22, 25). However, the mechanisms of quinolone resistance for many of the enteric species associated with opportunistic infections have not been well defined.
In this study, the DNA sequences of the gyrA QRDRs of fluoroquinolone-resistant clinical isolates representing eight species of Enterobacteriaceae were determined. Oligonucleotide primers selected from conserved gyrA gene sequences flanking the QRDR were used to amplify and sequence the 5′ region of gyrA from the American Type Culture Collection (ATCC) type strain for each species and from 60 fluoroquinolone-resistant strains. DNA and deduced amino acid sequences were aligned, and the similarities and differences were characterized. Within each species, the amino acid sequences of the QRDRs from isolates with decreased fluoroquinolone susceptibilities were compared with those of the type strain, and amino acid substitution profiles were analyzed for association with fluoroquinolone resistance and for correlation with the ciprofloxacin (CIP) MIC.
(This study was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October 1997.)
MATERIALS AND METHODS
Bacterial strains and determination of antimicrobial susceptibility profiles.
Type strains of Enterobacteriaceae were those designated by the ATCC. Fluoroquinolone-resistant and -susceptible clinical isolates were obtained during the Intensive Care Antimicrobial Resistance Epidemiology (ICARE) study, in which isolates were collected from 39 hospitals across the United States between June 1994 and April 1997 (1). Strains were selected from among the ICARE isolates to represent a range of CIP MICs and geographical locations for each species group. Duplicate isolates from the same patient were excluded.
MICs were determined by the broth microdilution method with cation-adjusted Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) according to the methods of the National Committee for Clinical Laboratory Standards (18). CIP was obtained from Bayer Corporation (West Haven, Conn.), ofloxacin (OFLX) was purchased from Sigma Chemical Co. (St. Louis, Mo.), and sparfloxacin (SPAR) was obtained from Rhône-Poulenc Rorer (Collegeville, Pa.). Antibiotic concentrations in susceptibility testing were as follows: CIP, 0.06 to 8 μg/ml; OFLX, 0.25 to 8 or 0.06 to 32 μg/ml; and SPAR, 0.008 to 2 μg/ml. Quality control organisms for fluoroquinolone MIC determinations were Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 29213, E. coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853.
Amplification of the 5′ region of gyrA.
Oligonucleotide primers were designed on the basis of homologous regions of gyrA from E. coli (21) and the gyrA sequence reported for Klebsiella pneumoniae (8). Primer gyrA6 (5′-CGACCTTGCGAGAGAAAT-3′) corresponds to nucleotides 6 to 23 and gyrA631R (5′-GTTCCATCAGCCCTTCAA-3′) is complementary to nucleotides 631 to 614 of the E. coli gyrA sequence.
The gyrA gene fragments were amplified from chromosomal DNA present in crude cell lysates prepared by the method of Conrad et al. (4). Using a GeneAmp 9600 PCR System (Perkin-Elmer, Applied Biosystems Division [PE-ABI], Foster City, Calif.), amplifications were carried out in 50-μl volumes containing 50 pmol of each primer, 200 μM deoxynucleoside triphosphates, 1× reaction buffer with 1.5 mM MgCl2 (PE-ABI), 1 U of native Taq polymerase (PE-ABI), and 10 μl of cell lysate, containing approximately 100 ng of chromosomal DNA. An initial 4-min period of denaturation at 94°C was followed by 30 cycles of denaturation (1 min at 94°C), annealing (30 s at 55°C), and extension (45 s at 72°C) and then a final cycle of 72°C for 10 min. Amplification products were visualized by agarose gel electrophoresis and ethidium bromide staining to confirm the sizes of the gene fragments. PCR products were purified on QIAquick spin columns (QIAGEN, Chatsworth, Calif.).
DNA sequencing and analysis.
Oligonucleotides gyrA6 and gyrA631R were also used as primers for direct sequencing of the amplified gyrA gene fragments. The DNA sequences were determined with ABI Prism Dye Terminator or dRhodomine Terminator Cycle Sequencing reactions, using an ABI 377 automated sequencer (PE-ABI). Products from sequencing reactions were purified on Centri-Sep spin columns (Princeton Separations, Adelphia, N.J.). To eliminate errors caused by amplification artifacts, the forward and reverse sequences of each QRDR were determined for products from at least two independent PCRs. The GCG analysis programs (Genetics Computer Group, Madison, Wis.) were used for DNA and amino acid sequence alignments.
Nucleotide sequence accession numbers.
The partial sequences of the gyrA genes reported in this study were assigned the following GenBank accession numbers: Citrobacter freundii (ATCC 8090), AF052253; E. coli (ATCC 11775), AF052254; Enterobacter aerogenes (ATCC 13048), AF052255; Enterobacter cloacae (ATCC 13047), AF052256; Klebsiella oxytoca (ATCC 13182), AF052257; K. pneumoniae (ATCC 13883), AF052258; Providencia stuartii (ATCC 29914), AF052259; and Serratia marcescens (ATCC 13880), AF052260.
RESULTS
Amplification of the gyrA genes from eight species of Enterobacteriaceae.
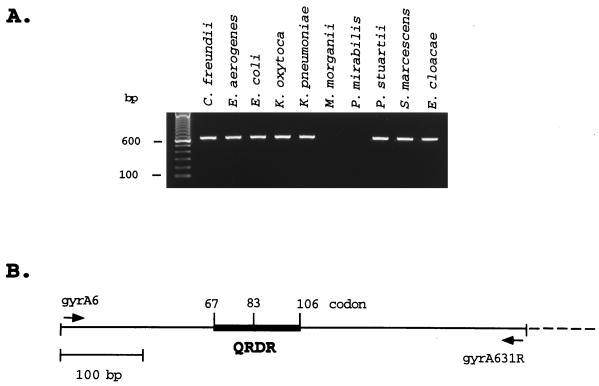
PCRs with oligonucleotide primers gyrA6 and gyrA631R amplified the expected 626-bp DNA fragments from the type strains of C. freundii, E. aerogenes, E. cloacae, E. coli, K. oxytoca, K. pneumoniae, P. stuartii, and S. marcescens (Fig. 1) and from 60 fluoroquinolone-resistant clinical isolates of these eight species. No amplification products were detected for Proteus mirabilis or Morganella morganii, indicating that the sequences of the 5′ region of gyrA in these two organisms diverge from the conserved sequences shared by the other species listed.
FIG. 1.
PCR amplification of the gyrA QRDR sequences from type strains of Enterobacteriaceae species. (A) Amplification of the predicted 626-bp fragment (including primers) from the 5′ end of the gyrA gene from various species of the Enterobacteriaceae. In the left-most lane are molecular size markers (100-bp DNA ladder). (B) Schematic diagram of the gyrA region amplified by synthetic oligonucleotide primers gyrA6 and gyrA631R (arrows), including the 120-bp QRDR (heavy line) encoding amino acids 67 to 106 of the E. coli GyrA protein (21).
Genetic analysis of the gyrA QRDR.
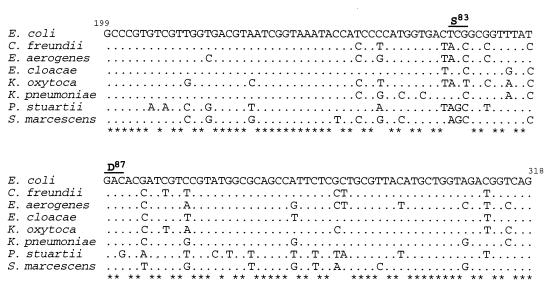
The DNA sequences of the gyrA gene fragments for the type strains and clinical isolates extended from nucleotides 24 to 613 (590 bp), including the QRDR (nucleotides 199 to 319 of the corresponding E. coli sequence) and excluding the primer sequences. Alignment of DNA sequences for the QRDRs from the type strains of eight enterobacterial species with the E. coli sequence revealed numerous nucleotide substitutions in the former (Fig. 2). However, 87 of the 120 nucleotides comprising the QRDR (72.5%) were conserved. The greatest degree of similarity to the gyrA QRDR sequence from E. coli was found in the sequence of the E. cloacae type strain (93.3% identity) (Table 1), while P. stuartii (80.8% identity) displayed the lowest degree of similarity. Comparisons of all eight species revealed that the gyrA QRDRs of K. pneumoniae and P. stuartii, with 75.8% identical nucleotides, were the least similar. Significant diversity of gyrA QRDR sequences was also noted among species of the same genus. The E. aerogenes and E. cloacae sequences exhibited 88.3% identity, while K. pneumoniae and K. oxytoca showed 87.5% identity in this region, which is a lower degree of similarity than was noted among several species from different genera.
FIG. 2.
DNA sequence similarities of the gyrA QRDRs from eight species of Enterobacteriaceae. Dots indicate nucleotide positions identical to the corresponding E. coli gyrA sequence. Nucleotide positions conserved in all sequences are designated by asterisks. The Ser-83 and Asp-87 codons, in which mutations frequently associated with fluoroquinolone resistance are found, are indicated by the solid bars above the sequence. Numbers refer to the nucleotide positions in the E. coli gyrA sequence (21).
TABLE 1.
Similarity of gyrA QRDR sequences among Enterobacteriaceae speciesa
| Species | % Identity
|
|||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | C. freundii | E. aerogenes | E. cloacae | K. oxytoca | K. pneumoniae | P. stuartii | S. marcescens | |
| E. coli | 100 | 89.2 | 86.7 | 93.3 | 86.7 | 90.0 | 80.8 | 84.2 |
| C. freundii | 100 | 92.5 | 92.5 | 95.0 | 87.5 | 82.5 | 83.3 | |
| E. aerogenes | 100 | 88.3 | 90.0 | 88.3 | 82.5 | 84.2 | ||
| E. cloacae | 100 | 89.2 | 90.8 | 83.4 | 83.4 | |||
| K. oxytoca | 100 | 87.5 | 79.2 | 83.4 | ||||
| K. pneumoniae | 100 | 75.8 | 86.7 | |||||
| P. stuartii | 100 | 81.7 | ||||||
| S. marcescens | 100 | |||||||
DNA sequences from ATCC type strains.
The gyrA QRDR sequence of the E. coli type strain (ATCC 11775) was also compared with the E. coli K12 gyrA sequence reported by Swanberg and Wang (21). Alignment of the two sequences revealed four nucleotide differences at the following positions (ATCC 11775 versus K12): 255, C to T; 267, T to C; 273, C to T; and 300, T to C. All of these substitutions were silent.
When the DNA sequence of the QRDR from the K. pneumoniae type strain was compared with the gyrA sequence from K. pneumoniae M5a1 reported by Dimri and Das (8), differences were detected in 15 of 120 nucleotides, and one nucleotide substitution resulted in an amino acid change. The substitution of T for A at nucleotide position 247 in the M5a1 strain altered the deduced amino acid codon for Ser-83 to Thr. Alignment of the complete 590-bp gyrA fragment from the K. pneumoniae type strain and the sequence of the corresponding region from gyrA of strain M5a1 revealed 76 mismatches within the 590 bp (data not shown). However, when the QRDR sequence from the K. oxytoca type strain was compared with the analogous region of the M5a1 gyrA sequence, only four nucleotide mismatches were detected, all of which were silent, and only 19 of 590 bp were mismatched in the alignment with the total K. oxytoca gene fragment. When the amino acid sequences were compared, Thr-83 was detected in the M5a1 strain and in the type strain and all fluoroquinolone-susceptible clinical isolates of K. oxytoca, while Ser-83 was detected in the type strain and all susceptible isolates of K. pneumoniae (Table 2). These data suggest that the sequence reported for the M5a1 strain may actually be from a strain of K. oxytoca and not from K. pneumoniae.
TABLE 2.
Alterations in GyrA and susceptibilities of fluoroquinolone-resistant clinical isolates of Enterobacteriaceae species
| Strain | MIC (μg/ml) of:
|
Amino acid (codon) change at positiona:
|
||||
|---|---|---|---|---|---|---|
| CIP | OFLX | SPAR | 81 | 83 | 87 | |
| C. freundii | ||||||
| ATCC 8090 | ≤0.12 | ≤0.25 | ≤0.008 | Gly (GGT) | Thr (ACC) | Asp (GAC) |
| 7377 | 2 | 8 | >2 | — | Ile (ATC) | — |
| 0759 | 2 | 8 | >2 | — | Ile (ATC) | — |
| 9085 | 4 | 8 | 2 | — | Ile (ATC) | — |
| 9417 | 8 | 8 | >2 | — | Ile (ATC) | — |
| 1958 | ≥16 | ≥16 | >2 | — | Ile (ATC) | Gly (GGC) |
| 5757 | ≥16 | ≥16 | >2 | — | Ile (ATC) | Gly (GGC) |
| 9023 | ≥16 | ≥16 | >2 | — | Ile (ATC) | — |
| E. aerogenes | ||||||
| ATCC 13048 | 0.5 | 1 | 0.25 | Gly (GGT) | Thr (ACC) | Asp (GAC) |
| 1747 | ≤0.12 | <0.25 | 0.06 | — | — | — |
| 2786 | 2 | 4 | 2 | — | Ile (ATC) | — |
| 9032 | 4 | 4 | 2 | — | Ile (ATC) | — |
| 5593 | 8 | 8 | >2 | — | Ile (ATC) | — |
| 9433 | 8 | ≥16 | >2 | — | Ile (ATC) | — |
| 3521 | 8 | ≥16 | >2 | — | Ile (ATC) | — |
| 5590 | 8 | ≥16 | >2 | — | Ile (ATC) | — |
| 2775 | ≥16 | 32 | >2 | — | Ile (ATC) | — |
| E. cloacae | ||||||
| ATCC 13047 | ≤0.12 | ≤0.25 | 0.03 | Gly (GGT) | Ser (TCC) | Asp (GAC) |
| 1700 | ≤0.12 | <0.25 | 0.06 | — | — | — |
| 1524 | 0.25 | 0.5 | 0.12 | — | — | Asn (AAC) |
| 1963 | 2 | 4 | 2 | — | Phe (TTC) | — |
| 1286 | 2 | 4 | 1 | — | Tyr (TAC) | — |
| 3529 | 4 | 8 | >2 | — | Thr (ACC) | — |
| 1544 | 8 | 8 | >2 | — | Tyr (TAC) | — |
| 1627 | 8 | ≥16 | >2 | — | Tyr (TAC) | — |
| 9028 | ≥16 | 8 | >2 | — | Ile (ATC) | — |
| 1224 | ≥16 | 8 | >2 | — | Phe (TTC) | Asn (AAC) |
| 1251 | ≥16 | ≥16 | >2 | — | Ile (ATC) | — |
| 63 | ≥16 | ≥16 | >2 | — | Phe (TTC) | — |
| 105 | ≥16 | ≥16 | >2 | — | Phe (TTC) | — |
| 1783 | ≥16 | ≥16 | >2 | — | Tyr (TAC) | — |
| 9030 | ≥16 | ≥16 | >2 | — | Tyr (TAC) | — |
| 9031 | ≥16 | >32 | >2 | — | Ile (ATC) | — |
| E. coli | ||||||
| ATCC 11775 | ≤0.12 | ≤0.25 | 0.03 | Gly (GGT) | Ser (TCG) | Asp (GAC) |
| 748 | ≤0.12 | ≤0.25 | 0.03 | — | — | — |
| 3535 | 1 | 2 | 2 | — | Leu (TTG) | — |
| 5524 | 2 | 8 | 2 | — | Leu (TTG) | — |
| 9419 | ≥16 | 32 | >2 | — | Leu (TTG) | Gly (GGA) |
| 9421 | ≥16 | 32 | >2 | — | Leu (TTG) | Tyr (TAC) |
| 9425 | ≥16 | >32 | >2 | — | Leu (TTG) | Asn (AAC) |
| K. pneumoniae | ||||||
| ATCC 13883 | 0.5 | ≤0.25 | 0.06 | Gly (GGC) | Ser (TCC) | Asp (GAC) |
| 570 | ≤0.12 | ≤0.25 | 0.06 | — | — | — |
| 1961 | ≤0.12 | ≤0.25 | 0.03 | — | — | — |
| 1361 | 1 | 2 | 0.5 | — | Phe (TTC) | — |
| 1362 | 1 | 2 | 1 | — | Phe (TTC) | — |
| 1177 | 4 | ≥16 | >2 | — | Phe (TTC) | — |
| 682 | ≥16 | ≥16 | >2 | — | Phe (TTC) | — |
| 1768 | ≥16 | 32 | >2 | — | Tyr (TAC) | — |
| 1775 | ≥16 | >32 | >2 | — | Phe (TTC) | Gly (GGC) |
| 1565 | ≥16 | >32 | >2 | — | Tyr (TAC) | Asn (AAC) |
| K. oxytoca | ||||||
| ATCC 13182 | ≤0.12 | ≤0.25 | 0.12 | Gly (GGT) | Thr (ACT) | Asp (GAC) |
| 702 | ≤0.12 | ≤0.25 | 0.03 | — | — | — |
| 2538 | ≤0.12 | ≤0.25 | 0.06 | — | — | — |
| 57 | 0.5 | 0.05 | 0.25 | — | Ile (ATT) | — |
| 1577 | 4 | 4 | 2 | — | Ile (ATT) | — |
| 1817 | 8 | ≥16 | >2 | — | Ile (ATT) | — |
| 466 | ≥16 | 8 | >2 | — | Ile (ATT) | — |
| 32 | ≥16 | ≥16 | >2 | — | Ile (ATT) | — |
| 1578 | ≥16 | ≥16 | >2 | — | Ile (ATT) | — |
| 1612 | ≥16 | ≥16 | >2 | — | Ile (ATT) | — |
| 2777 | ≥16 | >32 | >2 | — | Ile (ATT) | — |
| P. stuartii | ||||||
| ATCC 29914 | ≤0.12 | ≤0.25 | ≤0.12 | Gly (GGT) | Ser (AGC) | Glu (GAG) |
| 1571 | 0.25 | 1 | 0.5 | — | — | — |
| 1284 | 2 | 4 | 2 | — | Arg (CGC) | — |
| 2469 | 8 | ≥16 | >2 | — | Ile (ATC) | — |
| 2783 | ≥16 | ≥16 | 2 | — | Arg (AGG) | — |
| 9428 | ≥16 | ≥16 | >2 | — | Ile (ATC) | — |
| 7375 | ≥16 | 32 | >2 | — | Ile (ATC) | — |
| 2468 | ≥16 | 32 | >2 | — | Ile (ATC) | — |
| 1773 | ≥16 | >32 | >2 | — | Arg (AGG) | — |
| S. marcescens | ||||||
| ATCC 13880 | 0.5 | 1 | 1 | Gly (GGT) | Ser (AGC) | Asp (GAC) |
| 1714 | 0.25 | 1 | 1 | — | — | — |
| 9745 | 2 | 4 | 2 | — | Ile (ATC) | — |
| 1221 | 4 | 8 | >2 | Cys (TGT) | — | — |
| 1969 | 4 | ≥16 | >2 | — | — | Asn (AAC) |
| 1570 | 8 | 8 | >2 | Cys (TGT) | — | — |
| 5591 | 8 | ≥16 | >2 | — | Arg (AGA) | — |
| 1568 | ≥16 | ≥16 | >2 | — | Arg (CGC) | — |
Versus the ATCC type strain sequence. Numbers correspond to the amino acid positions of E. coli GyrA (21). —, no change.
The DNA sequence of the QRDR from the S. marcescens type strain (ATCC 13880) was identical to the sequence published by Kim et al. (ATCC 14756) (15). In the sequence flanking the QRDR, one nucleotide difference was found (nucleotide position 321, T to C), with no change in amino acid sequence. The C. freundii QRDR sequence was identical to that determined by Nishino et al. (19).
Amino acid sequence analysis.
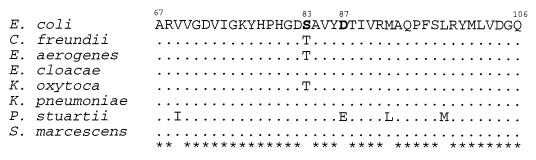
The deduced amino acid sequences of the QRDRs for the eight enterobacterial species were highly conserved (Fig. 3). The QRDR amino acid sequences of E. cloacae, K. pneumoniae, and S. marcescens were identical to the E. coli QRDR. Sequence analysis of C. freundii, E. aerogenes, and K. oxytoca strain QRDRs revealed one conservative substitution, Ser-83 to Thr, which was identified in all fluoroquinolone-susceptible isolates of these species as well as the type strains. Only P. stuartii exhibited more than one amino acid substitution in this region. In this organism, two conservative changes were detected: Val-69 to Ile and Asp-87 to Glu. In addition, all P. stuartii strains exhibited Leu-92-to-Met and Met-98-to-Leu substitutions when compared with the other members of the Enterobacteriaceae family included in this study.
FIG. 3.
Alignment of deduced amino acid sequences of the QRDRs of Enterobacteriaceae type strains. Amino acid differences are noted, and dots indicate amino acids identical to the corresponding E. coli sequence. Amino acid positions conserved in all sequences are designated by asterisks. Numbers refer to the amino acid positions in the E. coli GyrA sequence (21).
Detection of mutations in gyrA sequences amplified from clinical isolates.
After the DNA and amino acid sequences of the QRDRs from the quinolone-susceptible type strains were determined, the 5′ regions of gyrA from ciprofloxacin-resistant and -susceptible clinical isolates were amplified, sequenced, and analyzed for mutations leading to amino acid changes associated with fluoroquinolone resistance. Clinical isolates from each species were selected to represent a range of CIP MICs. The codon and amino acid alterations detected in fluoroquinolone-resistant and -susceptible clinical isolates and the CIP, OFLX, and SPAR MICs are summarized in Table 2 (amino acid positions refer to the corresponding positions in the E. coli GyrA protein).
In E. coli, a C-to-T substitution at the second position of the codon, resulting in a Ser-83-to-Leu mutation, was consistent for all fluoroquinolone-resistant isolates. In strains with double mutations, the second alteration involved the codon for Asp-87 and the amino acid substitution was Gly, Tyr, or Asn. A single mutation in codon 83 of gyrA was associated with decreased susceptibility or low levels of resistance to fluoroquinolones, and double mutations (codons 83 and 87) were associated with high levels of resistance.
All clinical isolates of C. freundii with reduced susceptibility to fluoroquinolones exhibited Thr-83-to-Ile mutations resulting from C-to-T substitutions at nucleotide position 248. Two isolates, C. freundii 1958 and 5757, had double mutations of Thr-83 to Ile and Asp-87 to Gly. CIP MICs of ≥16 μg/ml in C. freundii were associated with both single and double mutations.
Also, in all E. aerogenes isolates examined, mutations in codon 83 resulted in Thr-83-to-Ile substitutions. No double mutations were detected in gyrA genes from seven strains of E. aerogenes with reduced susceptibility to fluoroquinolones. However, CIP MICs for isolates with the single mutation ranged from 2 to ≥16 μg/ml.
The gyrA gene fragments from clinical isolates of E. cloacae exhibited numerous nucleotide substitutions, resulting in changes of Ser-83 to Phe, Ile, or Tyr. The only substitution detected in E. cloacae 3529 (CIP MIC = 4) was Ser-83 to Thr, which would not be expected to result in decreased susceptibility since Thr-83 is found in susceptible strains of K. oxytoca, C. freundii, and E. aerogenes. In E. cloacae 1524, the only GyrA alteration was a change of Asp-87 to Asn. There was no alteration of Ser-83, and only a marginal decrease in susceptibility to fluoroquinolones (CIP MIC = 0.25 μg/ml) was detected for this strain.
K. pneumoniae isolates exhibited either single or double mutations involving Ser-83 and Asp-87. All single mutations involved either a C-to-T or C-to-A change at the second position in codon 83, resulting in alteration of Ser to either Phe or Tyr, respectively. In two double mutants, K. pneumoniae 1775 and 1565, a nucleotide substitution at the first or second position of codon 87 resulted in a change of Asp to Gly or Asn. CIP MICs ranged from 1 to ≥16 μg/ml, and double mutations were not required for high-level resistance. In the group of K. pneumoniae strains with single mutations, no specific mutation (Ser-83 to Phe or Tyr) was associated with low or high levels of fluoroquinolone resistance.
K. oxytoca mutations were confined to the Thr-83 codon and were consistently C-to-T substitutions in the second position, resulting in Thr-to-Ile alterations, similar to C. freundii and E. aerogenes. CIP MICs associated with this alteration ranged from 0.5 to ≥16 μg/ml.
Changes in the QRDR of P. stuartii gyrA were also confined to codon 83; however, the nucleotide substitutions differed. Single nucleotide substitutions included A to C at the first position and C to G at the third position, both resulting in Ser-to-Arg mutations, and G to T in the second position, resulting in a Ser-to-Ile mutation. CIP MICs ranged from 2 to ≥16 μg/ml for P. stuartii isolates with alterations of Ser-83.
The fluoroquinolone-resistant clinical isolates of S. marcescens displayed the greatest diversity in mutations, including Gly-81 to Cys, Ser-83 to Ile or Arg, and Asp-87 to Asn. No double mutations were detected in the gyrA QRDRs from the six isolates examined. The GyrA sequences from clinical isolates S. marcescens 1221 (CIP MIC = 4) and 1570 (CIP MIC = 8) revealed only Gly-81 to Cys substitutions, and Asp-87 to Asn was the only alteration detected in strain 1969.
DISCUSSION
The association of DNA gyrase A mutations with fluoroquinolone resistance has been established for both gram-negative and gram-positive organisms (3). In the family Enterobacteriaceae, E. coli is the only species for which mutations in gyrA leading to fluoroquinolone resistance have been well characterized (4, 10, 23, 28). In E. coli, a single point mutation in gyrA results in decreased susceptibility to fluoroquinolones (27), and high-level resistance is associated with double amino acid substitutions in the GyrA protein (4, 10, 24). Although additional factors, such as mutations in the ParC subunit of topoisomerase IV and decreased intracellular drug accumulation, have been shown to play a complementary role by increasing the level of resistance (17, 26), in vitro studies with E. coli suggest that the first step in selection for decreased susceptibility to fluoroquinolones is an alteration of Ser-83 (11).
The similarity of the N-terminal region of the gyrA sequence of E. coli (21) to that reported for K. pneumoniae (8) was exploited to design oligonucleotide primers that amplified 590-bp gene fragments, including the QRDRs, from type strains of 8 of the 10 Enterobacteriaceae species tested. No amplification products were detected for M. morganii or Proteus mirabilis. Based on DNA relatedness studies, species of Proteus, Providencia, and Morganella are less than 25% related to other genera of Enterobacteriaceae (2). Therefore, the failure of the primers to amplify the gene fragments from Proteus mirabilis and M. morganii was less remarkable than the successful amplification of the fragment from P. stuartii and served to emphasize the highly conserved nature of the 5′ region of gyrA sequences among the Enterobacteriaceae.
Although the G+C content of the Enterobacteriaceae genomes ranges from 38 to 60 mol% (2) and the diversity of the DNA sequences for the gyrA QRDRs from the type strains, when compared with the corresponding region in the E. coli gyrase gene, ranged from 6.7% for E. cloacae to 19.2% for P. stuartii, the amino acid sequence of GyrA was highly conserved among the eight species in this study. Nucleotide substitutions, which were found primarily at the third codon position, may reflect the preferential codon usage required to preserve both the amino acid sequence of the gyrase A subunit and the characteristic moles percent G+C of each species. When compared with the corresponding region of the E. coli parC gene, nucleotide sequence similarities of the amplified gene fragments decreased to 60 to 62% for all species examined. Based on these findings, it was concluded that the gene fragments represented sequences from the gyrA gene.
The detection of Ser-83 in susceptible strains of K. pneumoniae is consistent with the gyrA gene fragment sequence reported by Deguchi et al. (6). The presence of Thr instead of Ser at position 83, evident in C. freundii, E. aerogenes, and K. oxytoca, is also noted in gyrA genes from susceptible strains of Pseudomonas aeruginosa (16) and Campylobacter jejuni (25). Previous studies of Pseudomonas aeruginosa and Campylobacter jejuni led to the suggestion that the intrinsically lower quinolone susceptibilities of these two organisms might be due to the presence of Thr at position 83 (25). The susceptibility profiles of fluoroquinolone-susceptible strains of C. freundii and K. oxytoca (CIP MICs, ≤0.12 μg/ml) do not support this premise. However, the gyrA sequence from one strain of E. cloacae, 3529, revealed a deduced Ser-83-to-Thr substitution associated with fluoroquinolone resistance (CIP MIC = 4), a conservative mutation that would not be expected to lead to a significant decrease in susceptibility. Similarly, Nishino et al. (19) have analyzed several clinical isolates of C. freundii, exhibiting decreased susceptibility to fluoroquinolones (MICs, 1.56 μg/ml), in which no mutations were found in gyrA or parC. As with these strains, further genetic analysis will be required to determine the mechanism(s) of resistance in E. cloacae 3529.
Each of the four amino acid substitutions found in the type strain of P. stuartii is present in the GyrA protein of at least one other organism. Alignment of 13 microbial GyrA QRDR sequences by Tankovic et al. (22) revealed that the Val-69-to-Ile substitution is common to both gram-positive and gram-negative species. Glu-87 is typical of gram-positive organisms such as Streptococcus pneumoniae, Enterococcus faecalis, Bacillus subtilis, and Staphylococcus aureus but has not been described previously for a gram-negative organism. The combined substitutions of Leu for Met-92 and Met for Leu-98 are present in the GyrA sequence of Aeromonas salmonicida (20).
When the amino acid alterations of the fluoroquinolone-resistant clinical isolates and the corresponding MICs for CIP, OFLX, and SPAR were evaluated, no specific amino acid alterations were associated with low- or high-level resistance profiles. Consistent with previous studies (10, 24, 27), low-level fluoroquinolone resistance in E. coli was associated with single mutations in the GyrA protein and high-level resistance required double mutations. However, for all other species in this study, there were strains for which high-level fluoroquinolone resistance was associated with a gyrA QRDR sequence exhibiting a single mutation. The association of low- and high-level resistance in clinical isolates of the same species with identical gyrA mutations is consistent with recent reports of gyrA mutations associated with fluoroquinolone resistance in clinical isolates of C. freundii (19), E. cloacae (5), and S. marcescens (15).
Unlike the consistent single mutation resulting in the alteration of Thr-83 to Ile in GyrA of C. freundii, E. aerogenes, and K. oxytoca, several different nucleotide substitutions resulting in diverse amino acid changes were associated with fluoroquinolone resistance in S. marcescens. For the six clinical isolates of S. marcescens having CIP MICs of >2 μg/ml, five different nucleotide substitutions resulted in four distinct amino acid alterations at three codon positions, Gly-81, Ser-83, and Asp-87. In contrast with other enterobacterial species, mutation of Ser-83 was not required for high-level fluoroquinolone resistance in S. marcescens.
In summary, comparison of the gyrA gene sequences and amino acid alterations associated with fluoroquinolone resistance in the Enterobacteriaceae revealed numerous differences as well as similarities among the eight species examined. The amino acid sequences of these Enterobacteriaceae species were highly conserved, although the nucleotide sequences differed by as much as 19.2% from that of E. coli gyrA. E. coli was the only species in which double mutations of gyrA were required for high-level fluoroquinolone resistance. With the exception of S. marcescens, high-level resistance was associated primarily with alterations of the Ser or Thr at position 83. These data extend our understanding of the molecular mechanisms of fluoroquinolone resistance associated with gyrA mutations to include E. aerogenes, K. oxytoca, and P. stuartii and provide additional data on both type strain gyrA sequences and mutations associated with fluoroquinolone resistance in clinical isolates of E. coli, E. cloacae, K. pneumoniae, S. marcescens, and C. freundii.
ACKNOWLEDGMENTS
We thank Bertha Hill for MIC susceptibility testing, Caroline O’Hara for species identification, and Kamile Rasheed and George Killgore for helpful discussions. We also thank John McGowan, Jr., Lennox Archibald, Robert Gaynes, Scott Fridkin, and all of the personnel and hospitals of Project ICARE for providing clinical isolates.
REFERENCES
- 1.Archibald L, Phillips L, Monnet D, McGowan J E, Jr, Tenover F C, Gaynes R. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: increasing importance of the intensive care unit. Clin Infect Dis. 1997;24:211–215. doi: 10.1093/clinids/24.2.211. [DOI] [PubMed] [Google Scholar]
- 2.Brenner D J. Facultatively anaerobic gram-negative rods, family I. Enterobacteriaceae. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Baltimore, Md: The Williams and Wilkins Co.; 1984. pp. 408–420. [Google Scholar]
- 3.Cambau, E., and L. Gutmann. 1993. Mechanisms of resistance to quinolones. Drugs 45(Suppl. 3):15–23. [DOI] [PubMed]
- 4.Conrad S, Oethinger M, Kaifel K, Klotz G, Marre R, Kern W V. gyrA mutations in high-level fluoroquinolone-resistant clinical isolates of Escherichia coli. J Antimicrob Chemother. 1996;38:443–455. doi: 10.1093/jac/38.3.443. [DOI] [PubMed] [Google Scholar]
- 5.Deguchi T, Yasuda M, Nakano M, Ozeki S, Kanematsu E, Nishino Y, Ishihara S, Kawada Y. Detection of mutations in the gyrA and parC genes in quinolone-resistant clinical isolates of Enterobacter cloacae. J Antimicrob Chemother. 1997;40:543–549. doi: 10.1093/jac/40.4.543. [DOI] [PubMed] [Google Scholar]
- 6.Deguchi T, Fukuoka A, Yasuda M, Nakano M, Ozeki S, Kanematsu E, Nishino Y, Ishihara S, Ban Y, Kawada Y. Alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV in quinolone-resistant clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1997;41:699–701. doi: 10.1128/aac.41.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deguchi T, Yasuda M, Nakano M, Ozeki S, Ezaki T, Saito I, Kawada Y. Quinolone-resistant Neisseria gonorrhoeae: correlation of alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV with antimicrobial susceptibility profiles. Antimicrob Agents Chemother. 1996;40:1020–1023. doi: 10.1128/aac.40.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimri G P, Das H K. Cloning and sequence analysis of gyrA gene of Klebsiella pneumoniae. Nucleic Acids Res. 1990;18:151–156. doi: 10.1093/nar/18.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiou M, Muñoz R, Román F, Cantón R, Gómez-Lus R, Campos J, De La Campa A G. Ciprofloxacin-resistant Haemophilus influenzae strains possess mutations in analogous positions of GyrA and ParC. Antimicrob Agents Chemother. 1996;40:1741–1744. doi: 10.1128/aac.40.7.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heisig P, Schedletzky H, Falkenstein-Paul H. Mutations in the gyrA gene of a highly fluoroquinolone-resistant clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1993;37:696–701. doi: 10.1128/aac.37.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heisig P, Tschorny R. Characterization of fluoroquinolone-resistant mutants of Escherichia coli selected in vitro. Antimicrob Agents Chemother. 1994;38:1284–1291. doi: 10.1128/aac.38.6.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper D C, Wolfson J S, Bozza M A, Ng E Y. Genetics and regulation of outer membrane protein expression by quinolone resistance loci nfxB, nfxC, and cfxB. Antimicrob Agents Chemother. 1992;36:1151–1154. doi: 10.1128/aac.36.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper D C. Bacterial resistance to fluoroquinolones: mechanisms and patterns. Adv Exp Med Biol. 1995;390:49–57. doi: 10.1007/978-1-4757-9203-4_4. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz D S, Wang J C. Mapping the active site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987;262:5339–5344. [PubMed] [Google Scholar]
- 15.Kim J H, Cho E H, Kim K S, Kim H Y, Kim Y M. Cloning and nucleotide sequence of the DNA gyrase gyrA gene from Serratia marcescens and characterization of mutations in gyrA of quinolone-resistant clinical isolates. Antimicrob Agents Chemother. 1998;42:190–193. doi: 10.1128/aac.42.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kureishi A, Diver J M, Beckthold B, Schollaardt T, Bryan L E. Cloning and nucleotide sequence of Pseudomonas aeruginosa DNA gyrase gyrA gene from strain PAO1 and quinolone-resistant clinical isolates. Antimicrob Agents Chemother. 1994;38:1944–1952. doi: 10.1128/aac.38.9.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moniot-Ville N, Guibert J, Moreau N, Acar J F, Collatz E, Gutmann L. Mechanisms of quinolone resistance in a clinical isolate of Escherichia coli highly resistant to fluoroquinolones but susceptible to nalidixic acid. Antimicrob Agents Chemother. 1991;35:519–523. doi: 10.1128/aac.35.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.Nishino Y, Deguchi T, Yasuda M, Kawamura T, Nakano M, Kanematsu E, Ozeki S, Kawada Y. Mutations in the gyrA and parC genes associated with fluoroquinolone resistance in clinical isolates of Citrobacter freundii. FEMS Microbiol Lett. 1997;154:409–414. doi: 10.1111/j.1574-6968.1997.tb12675.x. [DOI] [PubMed] [Google Scholar]
- 20.Oppegaard H, Sørum H. gyrA mutations in quinolone-resistant isolates of the fish pathogen Aeromonas salmonicida. Antimicrob Agents Chemother. 1994;38:2460–2464. doi: 10.1128/aac.38.10.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanberg S L, Wang J C. Cloning and sequencing of the Escherichia coli gyrA gene coding for the A subunit of DNA gyrase. J Mol Biol. 1987;197:729–736. doi: 10.1016/0022-2836(87)90479-7. [DOI] [PubMed] [Google Scholar]
- 22.Tankovic J, Perichon B, Duval J, Courvalin P. Contribution of mutations in gyrA and parC genes to fluoroquinolone resistance of mutants of Streptococcus pneumoniae obtained in vivo and in vitro. Antimicrob Agents Chemother. 1996;40:2505–2510. doi: 10.1128/aac.40.11.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truong Q C, Quabdesselam S, Hooper D C, Moreau N J, Soussy C J. Sequential mutations of gyrA in Escherichia coli associated with quinolone therapy. J Antimicrob Chemother. 1995;36:1055–1059. doi: 10.1093/jac/36.6.1055. [DOI] [PubMed] [Google Scholar]
- 24.Vila J, Ruiz J, Marco F, Barcelo A, Gon̄i P, Giralt E, Jimenez De Anta T. Association between double mutation in gyrA gene of ciprofloxacin-resistant clinical isolates of Escherichia coli and MICs. Antimicrob Agents Chemother. 1994;38:2477–2479. doi: 10.1128/aac.38.10.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Huang W M, Taylor D E. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob Agents Chemother. 1993;37:457–463. doi: 10.1128/aac.37.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiedemann B, Heisig P. Mechanisms of quinolone resistance. Infection. 1994;22:s73–s79. doi: 10.1007/BF01793570. [DOI] [PubMed] [Google Scholar]
- 27.Willmott C J R, Maxwell A. A single point mutation in the DNA gyrase A protein greatly reduces binding of fluoroquinolones to the gyrase-DNA complex. Antimicrob Agents Chemother. 1993;37:126–127. doi: 10.1128/aac.37.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida H, Nakamura M, Bogaki M, Ito H, Kojima T, Hattori H, Nakamura S. Mechanism of action of quinolones against Escherichia coli DNA gyrase. Antimicrob Agents Chemother. 1993;37:839–845. doi: 10.1128/aac.37.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]