Abstract
Dietary calcium (Ca) intake is needed to attain peak bone mineral density (BMD). Habitual low Ca intake increases intestinal Ca absorption efficiency to protect bone mass but the mechanism controlling, and the impact of genetics on, this adaptive response is not clear. We fed 11 genetically diverse inbred mouse lines a normal (0.5%) or low (0.25%) Ca diet from 4-12 wks of age (n=8 per diet per line) and studied the independent and interacting effects of diet and genetics on Ca and bone metabolism. Significant genetic variation was observed in all bone, renal, and intestinal phenotypes measured including Ca absorption. Also, adaptation of Ca absorption and bone parameters to low dietary Ca was significantly different among the lines. Ca absorption was positively correlated to femur BMD (r=0.17, p=0.02), and distal femur BV/TV (r=0.34, p<0.0001). While Ca absorption was correlated to 1,25 dihydroxyvitamin D (1,25(OH)2D) (r=0.35, P<0.0001), the adaptation of Ca absorption to low Ca intake did not correlate to diet-induced adaptation of 1,25(OH)2D across the 11 lines. Several intestinal proteins have been proposed to mediate Ca absorption; claudins 2 and 12, voltage gated Ca channel v1.3 (Cav1.3), plasma membrane Ca ATPase 1b (PMCA1b), transient receptor potential vanilloid member 6 (TRPV6) and calbindin D9k (CaBPD9k). Only the mRNA levels for TRPV6, CaBPD9k, and PMCA1b were related to Ca absorption (r= 0.42, 0.43, and 0.21, respectively). However, a significant amount of the variation in Ca absorption is not explained by the current model and suggests that novel mechanisms remain to be determined. These observations lay the groundwork for discovery-focused initiatives to identify novel genetic factors controlling gene-by-diet interactions affecting Ca/bone metabolism.
Keywords: calcium, intestinal absorption, genetics, diet, bone
Introduction:
Whole body calcium (Ca) homeostasis is maintained by the coordination of a three-tissue axis of intestine, kidney, and bone(1) and this coordination is crucial for developing peak bone mass and minimizing adult bone loss(2,3). In humans, fractional Ca absorption is positively correlated to bone mass (4-6) while low Ca absorption efficiency is associated with increased fracture risk (7) and can reduce the efficacy of Ca treatment in the prevention of osteoporosis (4). Despite these relationships, we still lack a clear understanding for how Ca absorption occurs.
Ca absorption follows both a saturable, transcellular pathway and a passive, paracellular pathway that is directly proportional to dietary Ca intake.(8) Active intestinal Ca absorption is especially crucial to the development of optimal bone density when dietary Ca intake is low. During these periods, renal conversion of serum 25 hydroxyvitamin D (25OHD) to the active hormone 1,25 dihydroxyvitamin D (1,25(OH)2D) is increased. Molecular events regulated by binding of 1,25(OH)2D to the vitamin D receptor (VDR) subsequently increase Ca absorption efficiency as well as reduce urinary Ca loss.(9-11) Deletion of intestinal VDR in mice reduces active intestinal Ca absorption efficiency by 70% and this is directly responsible for the hypocalcemia and osteomalacia seen in VDR knockout mice.(11)
The most studied mechanism for vitamin D-regulated intestinal Ca absorption is the facilitated diffusion model.(8) In this model, 1,25(OH)2D increases the expression of target genes whose protein products mediate Ca entry into the enterocyte through the apical membrane channel transient receptor potential vanilloid member 6 (TRPV6), movement through the cell by binding to calbindin D9k (CaBPD9k), and extrusion from the cell through the ATP dependent-plasma membrane Ca pump, PMCA1b.(8) While close relationships exist between components of the facilitated diffusion model in CaCo-2 cells(12) and C57BL/6J mice(13,14) recent studies in knockout mice indicate that the facilitated diffusion model may not be accurate (15).
In human studies, the efficiency of intestinal Ca absorption varies from 7-75%.(16-18) The variation in intestinal Ca absorption efficiency in humans is likely due to the influence of multiple physiologic (e.g. growth, pregnancy, lactation, aging), environmental variables (e.g. dietary Ca intake, vitamin D status), and genetic factors. Twin studies, genetic mapping studies in mice, and GWAS in humans reveal that several aspects of whole-body Ca and bone homeostasis are influenced by genetics, e.g. multiple bone endpoints(19,20) (human findings reviewed in(21)), serum 25OHD (22). However, less information is available for the impact of genetics on the efficiency of Ca absorption and the adaptive upregulation of Ca absorption to low dietary Ca intake. Two groups have reported that the efficiency of intestinal Ca absorption is higher in C3H/HeJ mice compared to C57BL/6J mice, suggesting genetic background may influence this trait.(23,24) In addition, racial differences in the ability of adolescent girls to increase Ca absorption efficiency during periods of low dietary Ca intake indicate that this adaptive response also has a genetic component.(25,26)
To determine the genetic contribution to the efficiency of Ca absorption, we examined 11 inbred lines of mice fed defined diets containing either high or low Ca content from weaning to 12 wks of age. Using this population, we have identified genetic variation in intestinal Ca absorption efficiency as well as a number of other parameters relevant to whole body Ca homeostasis. There were independent genetic effects controlling the adaptive response of Ca absorption and other parameters to low dietary Ca intake. Collectively, our findings suggest that Ca absorption physiology is more complex than suggested by the facilitated diffusion model and that novel genetic factors affecting Ca absorption as well as diet-induced adaptation of Ca/bone metabolism have yet to be identified.
Materials and Methods
Experimental Design
Four week old, male mice from 11 common laboratory inbred strains were obtained from The Jackson Labs (Bar Harbor, ME): 129S1/SV1mJ (129S), A/J, AKR/J (AKR), C3H/HeJ (C3H), C57BL/6J (B6), CAST/EiJ (CAST), CBA/J (CBA), DBA/2J (DBA), PWK/PhJ (PWK), SWR/J (SWR), and WSB/EiJ (WSB). This panel of lines was chosen to encompass three mouse subspecies (Mus musculus domesticus, M.m.musculus, and M.m. castaneus), to include classical inbred strains as well as more genetically divergent wild-derived inbred lines, and to represent parental strains of available genetic mapping resources.(27) To minimize differences in availability and breeding efficiency among the lines, lines were shipped in groups of 4-8 mice within each of two shipment periods. At arrival, an equal number of mice from each line were randomly assigned to either a 0.5% Ca (adequate) or 0.25% Ca (low) diet (AIN93G base with 200 IU vitamin D3/kg diet, Research Diets, New Brunswick, NJ) (n=8/diet/line). Dietary Ca levels were chosen to maintain Ca homeostasis (0.5% Ca) or elicit an adaptive response in serum 1,25(OH)2D (0.25% Ca). Mice were maintained in rooms with UV blocking filters over lights (Pegasus Lighting, Beaver Falls, PA) and a 12 h light/dark cycle; they were given food and water ad libitum. At 12 wks of age mice were fasted overnight, anesthetized with a cocktail of ketamine and xylazine, and Ca absorption was measured by Ca45 appearance in the serum 10 min after an oral gavage test as previously described (full details in Supplemental Methods).(10) Blood was drawn and serum was prepared for the analysis of intestinal Ca absorption by liquid scintillation and 1,25(OH)2D levels by radioimmunoassay as previously described.(11,28) Duodenum and kidney were prepared for mRNA analysis are described in the Supplemental Methods. Data from a pilot study characterizing the response of B6, DBA, and PWK mice to dietary Ca restriction were included in the final analysis of available phenotypes (total sample size for these lines=16-24/diet). All animal experiments were approved by the Purdue University Animal Care and Use Committee.
Bone Phenotyping
Formalin-fixed femora were scanned using a PIXImus densitometer (Lunar; GE-Healthcare, Madison, WI) to yield bone mineral content (BMC, g) and bone mineral density (BMD, g/cm2) or by microcomputed tomography (μCt 40, Scanco Medical, AG, Bassersdorf, Switzerland) at the midshaft and distal metaphysis.(29) Samples were scanned for μCt while immersed in 70% ethanol. Images were obtained using a cubic voxel size of 16 μm, X-ray tube potential of 55 kVp, an X ray intensity of 145 μA, and 300 ms integration time (Supplemental Methods). The reproducibility of this method for mouse femur is reported elsewhere.(29,30)
Statistical Analysis
Data points with a z score in the extreme 2.5% of either end of a line/diet group distribution were removed as outliers. Adherence to a normal distribution was determined by Anderson-Darling tests. Data not normally distributed were transformed as follows: Ca absorption (log 10); VDR (y0.5); TRPV6 and 1,25(OH)2D (y0.25); duodenal and renal CaBPD9k, PMCA1b, CLDN2, CLDN12, Cav1.3, Tb.Sp, Tb.Th, BV/TV, TRPV5, and CaBPD28k (natural log). Adherence to a normal distribution was confirmed after transformation. Each phenotype was assessed for the presence of main effects (line, diet) and a line-by-diet interaction using ANCOVA with body weight (BW) and femur length (FL) as body size covariates.(31) When a significant F statistic was detected, specific a priori post-hoc comparisons were made using a permutation-based t-test procedure (Supplemental Table 3). Relationships between phenotypes were determined after, significant, independent confounding effects of BW and/or FL were removed by linear regression (31). Phenotypes affected by BW and FL were: BMD, BMC, Tb.N, Tb.Sp, and renal CaBPD9k; while Ca absorption, TRPV6, duodenal CaBPD9k, PMCA1b, VDR, CLDN2, CLDN12, 1,25(OH)2D, Tb.Th, Ct.Th, and Ct.Ar/Tt.Ar were affected by BW only and BV/TV was affected by FL only. The resulting residual values were used in Pearson’s correlations, full model linear regression, and principal components analysis (PCA). The number of factors extracted in PCA was based on the Kaiser criterion (eigenvalue >1) and scree plot examination.(32) Factors were next rotated using the orthogonal Varimax rotation. Factor loadings >0.4 or <−0.4 were used for interpretation of each principal component.
For several phenotypes, a unique adaptation parameter reflecting the response to low dietary Ca intake was generated for each mouse on the 0.25% Ca diet. This was calculated as the percent difference between the phenotype value for an individual (i) fed the 0.25% Ca diet (x) and the line (j) mean for the phenotype value from the 0.5% Ca diet (y), standardized to the line mean for the phenotype value from the 0.5% Ca diet and multiplied by 100, i.e.. The adaptation parameter was normally distributed and not affected by body-size covariates for any phenotype. Summary statistics of adaptation parameters are given in Supplemental Table 4. The effect of genetic variation on the adaptation parameter was tested in a one-way ANOVA. The impact of low dietary Ca intake on phenotypes within individual lines was determined using a one-sample t-test (H0=0). All analyses were conducted using SAS Enterprise 4.2 (SAS Institute, Inc., Cary, NC) and significance determined at p<0.05.
Our primary research goal was to determine the effect of genetics on Ca absorption and so the study was powered based on variance estimates from B6 mice for this phenotype (n=8, 50% difference between dietary groups, SD = 30% of mean, α = 0.05, power = 0.872). Using this sample size we had sufficient power to detect significant differences in mRNA endpoints (100% difference, SD = 50%, power = 0.96) and distal femur μCT parameters (30% difference, SD = 20%, power = 0.797) but femur midshaft, BMD, and BMC had reduced power 0.461). Our linear associations with n=123 have the power (0.8) to see a significant correlation of r = 0.25 (p<0.05).
Results
Ca absorption and its adaptation to low dietary Ca intake
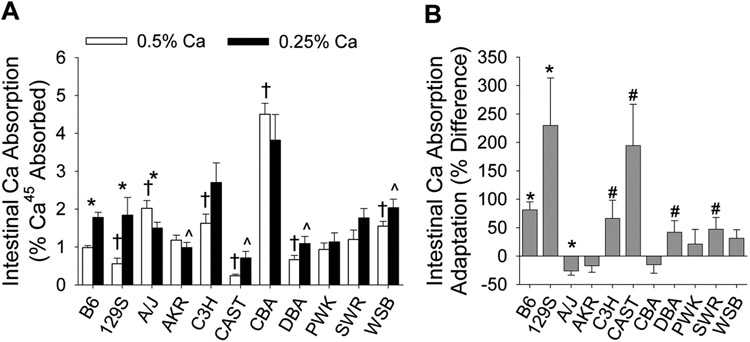
Unadjusted values and least squares means from ANCOVA are provided for all parameters in Supplementary Tables 1 and 2, respectively. Intestinal Ca absorption was significantly affected by genetic background (line) regardless of the level of dietary Ca fed (Fig. 1A, p<0.0001) with CBA, A/J, and WSB lines having the highest absorption efficiency on the 0.5% Ca diet and 129S, CAST, and DBA lines having the lowest.
Fig. 1.
Ca absorption and adaptation of Ca absorption to low Ca intake is variable among 11 inbred mouse lines. Bars reflect the mean ±SEM (n=4-14 per diet for each line). (A) Unadjusted Ca absorption values, * dietary groups within a line differ significantly (p<0.05); line mean differs significantly relative to the B6 reference line (p<0.05), † for the 0.5% Ca group, ^ for the 0.25% Ca group. (B) Adaptation of Ca absorption to low dietary Ca intake; adaptation significantly differs from 0 (*, p<0.05; #, p<0.1).
As expected, Ca absorption efficiency was significantly increased by low dietary Ca intake in the B6 reference line (+82%, p<0.0001). However, the adaptation of Ca absorption changed to low dietary Ca stress varied significantly among the inbred lines (line-by-diet interaction, p=0.009). Low Ca intake significantly increased Ca absorption only in the B6 and 129S lines (Fig. 1A). Consistent with this observation, analysis of the Ca absorption adaptation parameter shows that the B6 and 129S lines were significantly up-regulated (p<0.05) while a trend towards increased Ca absorption was also seen for CAST, DBA, C3H, and SWR (p<0.1, Fig. 1B). Lines with no diet-induced increase in Ca absorption were: A/J, AKR, CBA, PWK, and WSB.
Bone parameters, and their adaptation to low dietary Ca intake
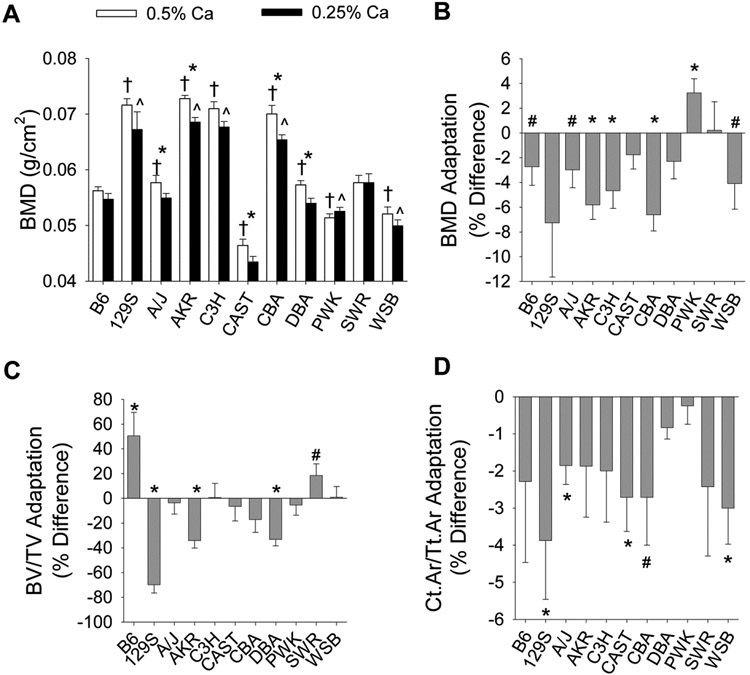
BMD was affected by significant line and diet main effects, as well as a significant line-by-diet interaction (p=0.038). Variability in BMD across the population for each diet can be seen in Fig. 2A. Similar levels of variation were also seen for Ct.Ar/Tt.Ar, distal femur BV/TV, Tb.Th, Tb.N, and Tb.Sp (Supplemental Table 3). However, the impact of line on bone loss due to dietary Ca restriction was different among BMD, Ct.Ar/Tt.Ar, and BV/TV (line effect p=0.005, p=0.8, p<0.0001, respectively; Fig. 2B-D). Only PWK mice were resistant to diet-induced bone loss in all three measures. The adaptive response of distal femur BV/TV to low Ca diets was most heterogeneous with significant loss of BV/TV observed in 129S, AKR, and DBA lines, no change in A/J, C3H, CAST, CBA, PWK, and WSB lines, and an increase in B6 (p=0.04) and SWR lines (p=0.1) (Fig. 2D).
Fig. 2.
Bone parameters and adaptation of bone parameters to low Ca diets are variable among 11 inbred mouse lines. Bars reflect the mean ±SEM (n=7-20 per diet for each line). (A) Unadjusted BMD values, * dietary groups within a line differ significantly (p<0.05); line mean differs significantly relative to the B6 reference line (p<0.05), † for the 0.5% Ca group, ^ for the 0.25% Ca group. (B-D) Adaptation to low dietary Ca intake for (B) BMD, (C) BV/TV, and (D) Ct.Ar/Tt.Ar; adaptation significantly differs from 0 (*, p<0.05; #, p<0.1).
Ca absorption efficiency is correlated to bone mass
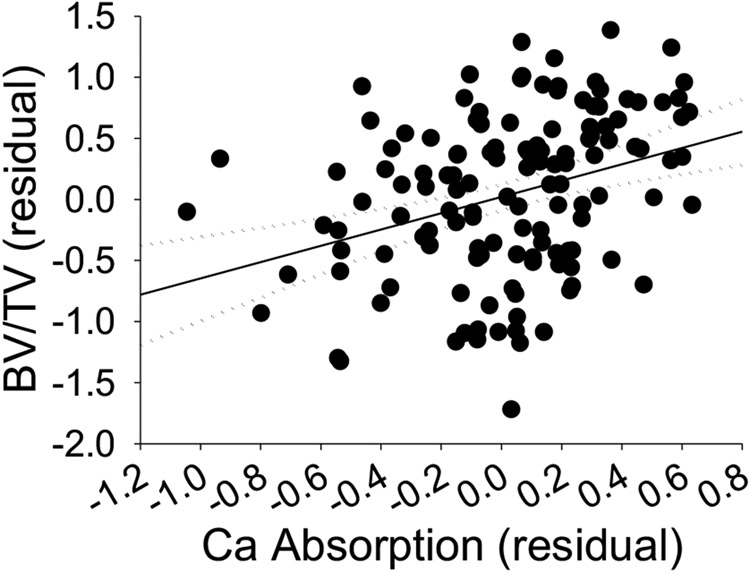
Ca absorption was positively correlated to BMD (r=0.17, p=0.02, Table 1). This effect was due to the beneficial impact of Ca absorption on trabecular bone (BV/TV, Fig. 3, Tb. Th, Table 1). In contrast, while Ct.Ar/Tt.Ar correlated to Ca absorption in mice on the 0.5% Ca diet (r = 0.25, p for trend = 0.052), the relationship was not significant for mice fed the 0.25% Ca diet or for the combined population.
Table 1.
Correlation of Bone Density and Morphometry Parameters to Ca Absorption
| Phenotype | r (95% CL) | p | n |
|---|---|---|---|
| BMD | 0.172 (0.025-0.312) | p=0.02 | n=176 |
| BMC | 0.048 (−0.104-0.198) | p=0.54 | n=168 |
| Ct.Ar/Tt.Ar | 0.085 (−0.086-0.251) | p=0.33 | n=134 |
| Ct.Th | 0.102 (−0.066-0.265) | p=0.24 | n=138 |
| BV/TV | 0.338 (0.178-0.481) | p<0.0001 | n=133 |
| Tb.Th | 0.277 (0.134-0.438) | p=0.0004 | n=140 |
| Tb.Sp | −0.106 (−0.269-0.063) | p=0.22 | n=137 |
| Tb.N | 0.055 (−0.115-0.221) | p=0.53 | n=135 |
Pearson’s correlation coefficients are given: r (upper, lower 95% confidence limits). Body size corrected residuals were used.
Fig. 3.
Distal femur BV/TV is significantly, positively correlated with intestinal Ca absorption. Pearson’s correlation was calculated using individual residual values from mice representing all 11 inbred lines from both diet groups with data points present for both phenotypes. Solid line = regression (r=0.34, p<0.05), dotted line = 95% confidence interval, n=133.
Regulation of intestinal Ca absorption by 1,25(OH)2D
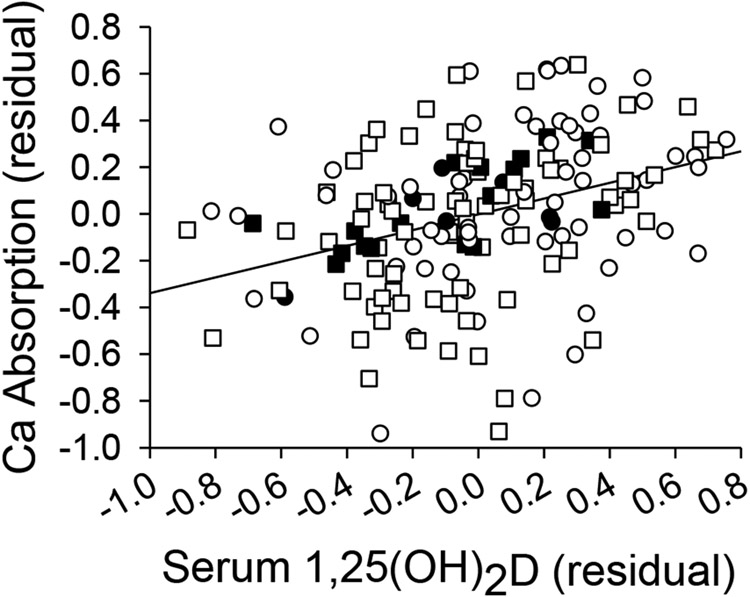
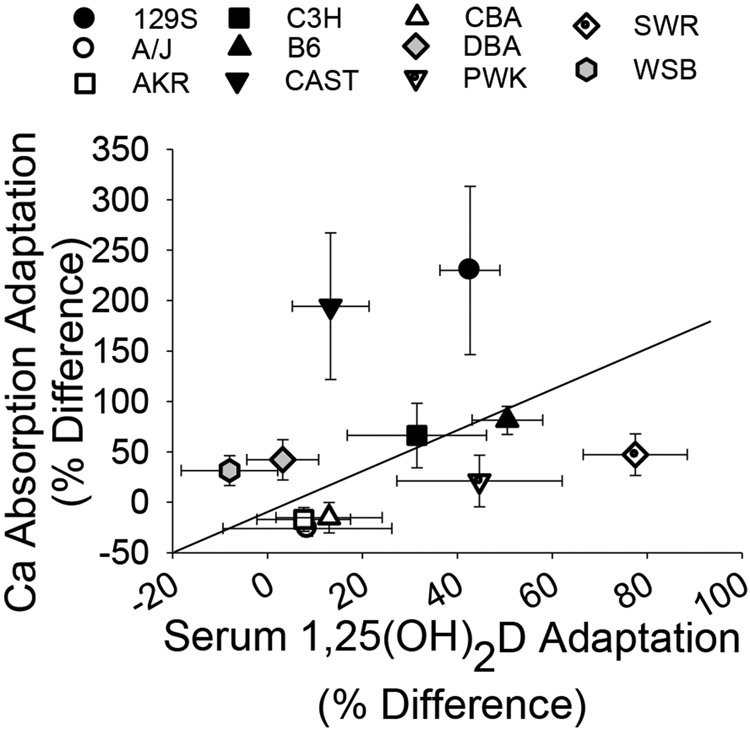
Using in situ ligated loops, we previously showed that Ca absorption efficiency is significantly correlated to serum 1,25(OH)2D in B6 mice (r = 0.92(14)). We confirmed this relationship in B6 mice using the oral gavage test (r=0.65, p=0.0006) (Fig. 4, black symbols). In the full panel of 11 inbred lines the relationship was still significant, but weakened (r=0.35, p<0.0001, Fig. 4; Supplemental Fig. 1) due to the high degree of diversity in the relationship between adaptation of Ca absorption and adaptation of serum 1,25(OH)2D to low Ca intake among the lines. Fig. 5 shows that only two lines had a proportional, diet-induced increase in Ca absorption and serum 1,25(OH)2D (B6, C3H). In contrast, other lines were hyper-responders (i.e. the diet-induced increase in Ca absorption was high relative to the diet-induced increase in serum 1,25(OH)2D in 129S and CAST), or hypo-responders (i.e. a blunted response in Ca absorption in relation to a large increase in serum 1,25(OH)2D in PWK and SWR). Two other lines increased Ca absorption with little to no corresponding increase in serum 1,25(OH)2D (i.e. vitamin D-independent in WSB, DBA), while three lines did not increase either Ca absorption or serum 1,25(OH)2D on a low Ca diet (i.e. non-responders were CBA, A/J, AKR) (Fig. 5, Supplemental Table 4).
Fig. 4.
Relationship between serum 1,25(OH)2D and intestinal Ca absorption across the 11 inbred lines (r=0.35, p<0.001, all symbols). Values for the B6 reference line alone are shown as filled symbols (r=0.65, p<0.01). 0.25% Ca diet (circles), 0.5% Ca diet (squares). Body size-corrected residual values are plotted, n=166.
Fig. 5.
Correlation between adaptation of serum 1,25(OH)2D and intestinal Ca absorption to low Ca diets in the 11 inbred lines. An adaptation parameter was calculated for each mouse on the 0.25% diet and the line mean values (± SEM) were plotted.
Correlations between duodenal mRNA levels and Ca absorption
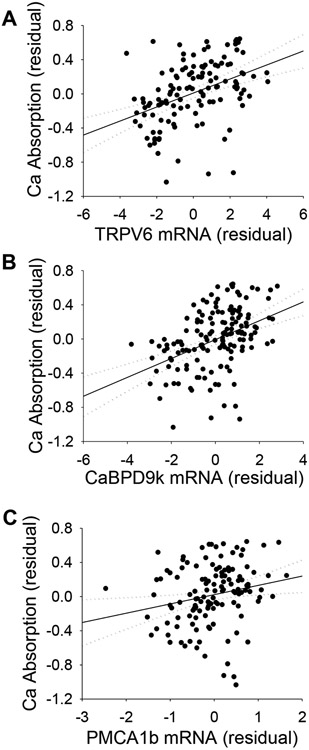
Several proteins have been proposed to contribute to basal and vitamin D-regulated intestinal Ca absorption, i.e. VDR, TRPV6, CaBPD9k, PMCA1b, CLDN2, CLDN12, and Cav1.3.(8) Line effects influenced the duodenal mRNA level for each of these genes (Supplemental Table 3). However, only TRPV6 and CaBPD9k mRNA levels were significantly increased by dietary Ca restriction. Consistent with their proposed roles in the facilitated diffusion model(8), TRPV6, CaBPD9k, and PMCA1b mRNA were each significantly, positively correlated with Ca absorption efficiency (Fig. 6A-C). Additionally, each gene target was positively correlated to serum 1,25(OH)2D (Table 2) and the three mRNA levels were closely correlated with one another (Table 2, Supplemental Fig. 2). When all three mRNAs were included in a multiple linear regression model for Ca absorption, only CaBPD9k mRNA level remained significant, indicating that these three factors are not independent determinants of Ca absorption. Duodenal CLDN2, CLDN12, and Cav1.3 mRNA levels were not significantly correlated to Ca absorption or to serum 1,25(OH)2 D levels (data not shown). Although VDR mRNA correlated with TRPV6, CaBPD9k, and PMCA1b mRNA, it did not correlate with Ca absorption (Table 2). Diet-induced changes in CaBPD9k mRNA, but not the other mRNAs, were correlated to the adaptation of Ca absorption to low Ca intake (r=0.38, p<0.0001, Supplemental Fig. 3). However, while VDR mRNA levels did not correlate to the adaptive increase in Ca absorption using mean values from the full panel of 11 inbred line (n = 11, r = 0.44, p = 0.17), a significant correlation was observed when the biological outlier line, A/J, was removed (n = 10, r = 0.81, p<0.01, data not shown).
Fig. 6.
Correlation between mRNA levels for the key members in the facilitated diffusion model and Ca absorption. (A) TRPV6 mRNA (r=0.42, p<0.001, n=127), (B) CaBPD9k mRNA (r=0.43, p<0.001, n=153), and (C) PMCA1b mRNA (r=0.21, p<0.05, n=129). Body size-corrected residuals with 95% confidence intervals are shown.
Table 2.
Pearson's Correlation Coefficients of Ca Absorption Regulators
| Ca Absorption | TRPV6 | CaBPD9k | PMCA1b | 1,25(OH)2D | |
|---|---|---|---|---|---|
| TRPV6 | 0.42 (0.26, 0.55) | ||||
| p<0.0001, n=127 | |||||
| CaBPD9k | 0.43 (0.27, 0.55) | 0.74 (0.65, 0.80) | |||
| p<0.0001, n=153 | p<0.0001, n=140 | ||||
| PMCA1b | 0.21 (0.04, 0.37) | 0.53, (0.40, 0.64) | 0.47, (0.33, 0.59) | ||
| p=0.02, n=129 | p<0.0001, n=142 | p<0.0001, n=141 | |||
| 1,25(OH)2D | 0.35 (0.21, 0.48) | 0.44, (0.30, 0.57) | 0.39, (0.27, 0.51) | 0.19, (0.02, 0.34) | |
| p<0.0001, n=166 | p<0.0001, n=139 | p<0.0001, n=189 | p=0.03, n=140 | ||
| VDR | −0.02 (−0.19, 0.15) | 0.45, (0.31, 0.54) | 0.23, (0.06, 0.37) | 0.53, (0.40, 0.63) | 0.09, (−0.07, 0.25) |
| p=0.82, n=132 | p<0.0001, n=147 | p=0.01, n=145 | p<0.0001, n=148 | p=0.29, n=146 |
Pearson’s correlation coefficients are given: r (upper, lower 95% confidence limits), p-value, n. Body size corrected residuals were used for analysis.
Renal mRNA Levels:
Renal levels of TRPV5, CaBPD28k, and CaBPD9k mRNA were significantly affected by genetic background (Supplemental Tables 1, 3) while only CaBPD28k and CaBPD9k were significantly influenced by diet and no significant line-by-diet interaction was detected for any of the three renal mRNAs. CaBPD28k and CaBPD9k mRNA were significantly, positively correlated with serum 1,25(OH)2D (r = 0.27 and 0.33, respectively, p<0.001) but TRPV5 mRNA was not (Supplemental Table 5). TRPV5 mRNA was negatively correlated with intestinal Ca absorption (r = −0.23, p=0.007) while TRPV5, CaBPD28k, CaBPD9k mRNA levels were negatively correlated with several bone parameters (e.g. r = −0.25, −0.43, −0.33 with Ct.Th., respectively, p ≤ 0.002, Supplemental Table 5).
Principal Components Analysis (PCA):
Two significant principal components (PC1, PC2) were extracted and they account for 30.8% and 25.9% of the total variance, respectively (Supplemental Table 6). PC1 contained intestinal Ca absorption, duodenal mRNA levels, and renal CaBPD9k mRNA. PC2 contained factors from each arm of the 3-tissue axis; Ct.Ar/Tt.Ar, BV/TV, Ca absorption, and renal mRNA levels.
Discussion
Identification of genetic diversity in inbred mouse lines has been the foundation for quantitative trait loci (QTL) mapping, candidate gene, and genome wide association studies to identify molecular determinants of phenotypes like BMD.(20) However, few studies have examined the effects of genetics on tissues controlling Ca metabolism other than bone, nor have interactions between genetics and diet been accounted for, resulting in inconsistencies in the association between dietary Ca intake and bone health.(33-37) Our study addresses this knowledge gap with a special focus on intestinal Ca absorption. We demonstrate that although feeding Ca restricted diets to growing mice initiates a physiological adaptation to protect bone, the robustness of this adaptive response is dependent on genetic background (Fig. 1 and 2, Supplemental Table 4). For most of the phenotypes we examined, there was no correlation between the basal genetic effect and adaptation of a phenotype to low Ca intake, demonstrating that these genetic effects are distinct.
Bone is the most abundant store of Ca in the body and is influenced by regulatory mechanisms occurring at the bone, intestine, and kidney. Our data show that while significant gene-by-diet interactions control the adaptation of bone mass and intestinal Ca absorption to low dietary Ca intake, no interaction influenced the renal levels of transcripts related to Ca reabsorption. This suggests that while genetic variation affecting renal Ca handling may contribute to bone health, it may be less critical for the adaptive response to Ca restriction in growing mice. We expected that renal TRPV5, CaBPD28k, and CaBPD9k levels, as surrogate markers of renal Ca reabsorption, would be tightly correlated to each other and positively associated with both serum 1,25(OH)2D and bone parameters (i.e. improved Ca retention = improved bone).(9,38) However, while CaBPD9k and D28k mRNA levels were positively associated with serum 1,25(OH)2D levels (r = 0.33, 0.27, respectively, p<0.001), TRPV5 mRNA was not. The lack of correlation between renal TRPV5 and serum 1,25(OH)2D is consistent with previous reports showing modest changes in renal TRPV5 mRNA levels between wild-type and VDR knockout mice(10,13) and following injection with pharmacologic levels of 1,25(OH)2D(9). Previous studies report that urinary Ca excretion is higher in mice with TRPV5 gene deletion or low renal CaBPD9k and D28k levels(38,39) suggesting a positive role for these proteins in limiting bone loss and maintaining bone density. In contrast, we found that the three renal mRNA levels were individually, and in a principal components analysis, negatively associated with various bone endpoints. We hypothesize that this is an indirect effect that reflects a reduced need for renal Ca reabsorption when intestinal Ca absorption is high. This idea is supported in part by a negative association between renal TRPV5 mRNA and Ca absorption.
The primary goals for our study were to evaluate the genetic influences on intestinal Ca absorption as well as to use our genetically diverse population to learn more about mechanisms for Ca absorption and the contribution of Ca absorption to development of peak bone mass. Two previous studies showed that Ca absorption was greater in 8-12 week old female C3H than B6 mice fed a Ca-sufficient diet (0.4% or 1.2% Ca).(23,24) We have extended these observations to 11 inbred strains and our data reveal a large amount of variation in Ca absorption efficiency in mice fed an adequate or low Ca diet, as well as in the ability of mice to adapt to low dietary Ca intake (Fig. 1).
Armbrecht et al.(24) previously showed that the maximal response of Ca absorption to 1,25(OH)2D injection was not different between C3H and B6 mice. In contrast, our study examined a nutritionally and physiologically relevant condition – restriction of dietary Ca by 50%, i.e. similar to the relationship between Ca intake and Ca requirements seen in adult women in the U.S.(40) Previously, we reported that B6 mice follow the traditional model of adaptation to low dietary Ca intake(9); increases in serum 1,25(OH)2D induce active intestinal Ca absorption that protect mice from bone loss through a VDR dependent mechanism(8,9,13). However, while we see variation in the Ca absorption response to low dietary Ca intake across the 11 lines, this variation was not strongly associated with diet-induced increases in serum 1,25(OH)2D levels (Fig. 5). Subpopulations were identified in the panel of 11 lines that reflected “normal” and “hyper” adapters, vitamin D-independent adapters, vitamin D-resistant adapters, and non-adapters. The lack of a strong relationship between adaptation of Ca absorption and diet-induced changes in serum 1,25(OH)2D were not due to obvious line-specific differences in duodenal VDR mRNA level (Supplemental Tables 2 and 4). We previously reported that growth hormone or IGF-1 contributes to the residual Ca absorption efficiency that exists in growing VDR knockout mice.(13) However, we measured Ca absorption in mice that were past their rapid growth phase and our data was adjusted for body size to minimize growth-related effects on our phenotypes. Taken together, these observations indicate the existence of a vitamin D-independent, enhancing effect of low Ca intake on Ca absorption but the mechanism for this is not clear.
In B6 mice there is a close relationship between serum 1,25(OH)2D levels and Ca absorption efficiency (Fig. 3, r = 0.65; r = 0.9(14)). However, when the genetic diversity available in our 11 line panel is considered, the relationship of Ca absorption to serum 1,25(OH)2D is more similar to that reported in humans (r = 0.23-0.35)(41-43)(Fig. 4). Known environmental factors such as diet, age, and circulating hormones account for, at most, one quarter of the variation in true fractional Ca absorption seen in human populations.(41,44) Our data suggest that the remainder of the variation in Ca absorption is due to genetic factors and gene-by-diet interactions. Consistent with this concept, adolescent black girls have higher Ca absorption compared to white girls(45) and this may contribute to the higher bone deposition seen in black girls(46). Also, serum 1,25(OH)2D is a significant predictor of Ca absorption in black but not white women, suggesting an impact of genetics on this relationship.(16) Here we found that adaptive increases in Ca absorption were not strongly correlated to diet-induced changes in serum 1,25(OH)2D. This suggests that resistance or hyper-responsiveness to the action of 1,25(OH)2D may reflect defects in the VDR-dependent regulatory system.(12,14) However, there were also no obvious polymorphisms in the VDR gene that segregate with the responses of the mouse lines studied here. Identification of the genetic factors controlling Ca absorption has been difficult because studies on polymorphisms in candidate genes have been limited and inconsistent.(8) In addition, the environmental and genetic complexity of free-living human populations makes identifying gene-by-diet interactions difficult, especially for a hard to measure physiologic trait like Ca absorption. In contrast, our study in genetically well-characterized mouse models raised in a controlled environment provides a strong foundation for future gene mapping studies to identify the genetic variants that control intestinal Ca absorption efficiency as well as its adaptation to low dietary Ca intake.(27)
Our study has also allowed us to examine three models proposed to describe intestinal Ca absorption(8), i.e. the facilitated diffusion model, passive diffusion through the tight junction proteins CLDN2 and CLDN12 (47), and transcellular Ca transport through the voltage gated Ca channel Cav1.3 (48). Although CLDN2, CLDN12, and Cav1.3 mRNA levels were each detected in duodenum and each was significantly affected by line, none of them were influenced by diet nor were they significantly associated with Ca absorption or serum 1,25(OH)2D. This observation indicates they play a minimal role in Ca absorption under our experimental conditions (i.e. a low Ca load in our absorption test designed to reveal transcellular, not paracellular, Ca transport).
In the facilitated diffusion model(49), TRPV6, CaBPD9k, and PMCA1b work in coordination to mediate Ca absorption, e.g. in B6 mice, TRPV6 and CaBPD9k levels are elevated by increased serum 1,25(OH)2D levels and they are associated with increased Ca absorption efficiency(9). However, the role of these proteins in Ca absorption has been questioned due to lack of a dramatic phenotype in TRPV6 and CaBPD9k knockout mice.(15) Our data indicate that TRPV6, CaBPD9k, and PMCA1b likely perform as a single functional unit; multiple linear regression and PCA indicated that TRPV6, CaBPD9k, and PMCA1b mRNA were not independent predictors of Ca absorption. However, the correlations of these messages with Ca absorption, while significant, are weak (r values ≤ 0.43 lead to r2 ≤ 0.18 or less), indicating that only a small portion of the variability in Ca absorption is dependent upon the facilitated diffusion model (Table 2). In addition, only the adaption of CaBPD9k mRNA to low Ca intake was significantly correlated to low dietary Ca-induced adaptation of Ca absorption. This observation supports our hypothesis that CaBPD9k expression is a response to the elevated intracellular Ca levels that accompany Ca absorption, but it does not strongly support an exclusive role for the facilitated diffusion model as the mediator of Ca absorption.(28)
In conclusion, we have shown that genetic variation and gene-by-diet interactions affect not only active intestinal Ca absorption, but also its relationship to bone. These interactions are partially accounted for by variation in the traditional cellular mediators (i.e. TRPV6, CaBPD9k, PMCA1b mRNA) and the hormonal regulator (i.e. 1,25(OH)2D) of Ca absorption. However, the characterization done here, on 11 inbred lines of mice in a carefully controlled environment, indicates that there are aspects of Ca homeostasis that remain to be discovered. Future studies using mouse genetic mapping populations, such as recombinant inbred line panels, are needed to map genetic loci responsible for our observation.(27) Further characterization of the gene-by-diet interactions identified here will provide insight into their impact on fracture risk and will provide scientific support for defining dietary requirements for individuals or genetically distinct subgroups.
Supplementary Material
Acknowledgements:
The authors thank Ms. Bernardine Frankel and Mr. John Replogle for their technical assistance. This work was supported by a National Institutes of Health grants DK540111 and ES019103 to JCF.
Footnotes
Supplemental data is included with this manuscript
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Norman AW. Intestinal calcium absorption: A vitamin D-hormone mediated adaptive response. Am J Clin Nutr. 1990;51:290–300. [DOI] [PubMed] [Google Scholar]
- 2.Matkovic V, Fontana D, Tominac C, Goel P, Chesnut CH. Factors that influence peak bone mass formation: a study of calcium balance and the inheritance of bone mass in adolescent females. Am J Clin Nutr. 1990;52:878–88. [DOI] [PubMed] [Google Scholar]
- 3.Nordin BE, O'Loughlin PD, Need AG, Horowitz M, Morris HA. Radiocalcium absorption is reduced in postmenopausal women with vertebral and most types of peripheral fractures. Osteoporos Int. 2004;15(1):27–31. [DOI] [PubMed] [Google Scholar]
- 4.Smith E, Need AG, Schultz CG, Horowitz M. Does the response of bone mass to calcium supplements depend on calcium absorption efficiency? Eur J Endocrinol. 2004;151(6):759–63. [DOI] [PubMed] [Google Scholar]
- 5.Hoover PA, Webber CE, Beaumont LF, Blake JM. Postmenopausal bone mineral density: relationship to calcium intake, calcium absorption, residual estrogen, body composition, and physical activity. Can J Physiol Pharmacol. 1996;74(8):911–7. [PubMed] [Google Scholar]
- 6.Nordin BE, Robertson A, Seamark RF, Bridges A, Philcox JC, Need AG, Horowitz M, Morris HA, Deam S. The relation between calcium absorption, serum dehydroepiandrosterone, and vertebral mineral density in postmenopausal women. J Clin Endocrinol Metab. 1985;60(4):651–7. [DOI] [PubMed] [Google Scholar]
- 7.Ensrud KE, Duong T, Cauley JA, Heaney RP, Wolf RL, Harris E, Cummings SR. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 2000;132(5):345–53. [DOI] [PubMed] [Google Scholar]
- 8.Fleet JC, Schoch RD. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci. 2010;47(4):181–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice Endocrinology. 2003;144(9):3885–94. [DOI] [PubMed] [Google Scholar]
- 10.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA. 2001;98(23):13324–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136(4):1317–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao A, Wood RJ, Fleet JC. Increased vitamin D receptor level enhances 1,25-dihydroxyvitamin D3- mediated gene expression and calcium transport in Caco-2 cells. J Bone Miner Res. 2001;16(4):615–24. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Kato S, Fleet JC. Vitamin D Receptor (VDR) Knockout Mice Reveal VDR-Independent Regulation of Intestinal Calcium Absorption and ECaC2 and Calbindin D9k mRNA J Nutr. 2003;133(2):374–80. [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Fleet JC. Intestinal Resistance to 1,25 Dihydroxyvitamin D in Mice Heterozygous for the Vitamin D Receptor Knockout Allele. Endocrinology. 2007;148:1396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149(6):3196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aloia JF, Chen DG, Yeh JK, Chen H. Serum vitamin D metabolites and intestinal calcium absorption efficiency in women. Am J Clin Nutr. 2010;92(4):835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alevizaki CC, Ikkos DG, Singhelakis P. Progressive decrease of true intestinal calcium absorption with age in normal man. J Nucl Med. 1973;14:760–62. [PubMed] [Google Scholar]
- 18.Heaney RP, Recker RR. Distribution of calcium absorption in middle-aged women. Am J Clin Nutr. 1986;43(2):299–305. [DOI] [PubMed] [Google Scholar]
- 19.Farber CR, Kelly SA, Baruch E, Yu D, Hua K, Nehrenberg DL, de Villena FP, Buus RJ, Garland T Jr., Pomp D. Identification of quantitative trait loci influencing skeletal architecture in mice: emergence of Cdh11 as a primary candidate gene regulating femoral morphology. J Bone Miner Res. 2011;26(9):2174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackert-Bicknell CL, Karasik D, Li Q, Smith RV, Hsu YH, Churchill GA, Paigen BJ, Tsaih SW. Mouse BMD quantitative trait loci show improved concordance with human genome-wide association loci when recalculated on a new, common mouse genetic map. J Bone Miner Res. 2010;25(8):1808–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards JB, Zheng HF, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet. 2012;13(8):576–88. [DOI] [PubMed] [Google Scholar]
- 22.Bu FX, Armas L, Lappe J, Zhou Y, Gao G, Wang HW, Recker R, Zhao LJ. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum Genet. 2010;128(5):549–56. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Kalu DN. Strain differences in bone density and calcium metabolism between C3H/HeJ and C57BL/6J mice. Bone. 1999;25(4):413–20. [DOI] [PubMed] [Google Scholar]
- 24.Armbrecht HJ, Boltz MA, Hodam TL. Differences in intestinal calcium and phosphate transport between low and high bone density mice. Am J Physiol Gastrointest Liver Physiol. 2002;282(1):G130–G36. [DOI] [PubMed] [Google Scholar]
- 25.Wu L, Martin BR, Braun MM, Wastney ME, McCabe GP, McCabe LD, Dimeglio LA, Peacock M, Weaver CM. Calcium requirements and metabolism in Chinese-American boys and girls. J Bone Miner Res. 2010;25(8):1842–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver CM, McCabe LD, McCabe GP, Braun M, Martin BR, Dimeglio LA, Peacock M. Vitamin D status and calcium metabolism in adolescent black and white girls on a range of controlled calcium intakes. J Clin Endocrinol Metab. 2008;93(10):3907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts A, Pardo-Manuel DV, Wang W, McMillan L, Threadgill DW. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: implications for QTL discovery and systems genetics. Mamm Genome. 2007;18(6-7):473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui M, Li Q, Johnson R, Fleet JC. Villin promoter-mediated transgenic expression of transient receptor potential cation channel, subfamily V, member 6 (TRPV6) increases intestinal calcium absorption in wild-type and vitamin D receptor knockout mice. J Bone Miner Res. 2012;27(10):2097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, M++ller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Miner Res. 2010;25(7):1468–86. [DOI] [PubMed] [Google Scholar]
- 30.Kohler T, Beyeler M, Webster D, Muller R. Compartmental bone morphometry in the mouse femur: reproducibility and resolution dependence of microtomographic measurements. Calcif Tissue Int. 2005;77(5):281–90. [DOI] [PubMed] [Google Scholar]
- 31.Lang DH, Sharkey NA, Lionikas A, Mack HA, Larsson L, Vogler GP, Vandenbergh DJ, Blizard DA, Stout JT, Stitt JP, McClearn GE. Adjusting data to body size: a comparison of methods as applied to quantitative trait loci analysis of musculoskeletal phenotypes. J Bone Miner Res. 2005;20(5):748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23(3):392–9. [DOI] [PubMed] [Google Scholar]
- 33.MacInnis RJ, Cassar C, Nowson CA, Paton LM, Flicker L, Hopper JL, Larkins RG, Wark JD. Determinants of bone density in 30- to 65-year-old women: a co-twin study. J Bone Miner Res. 2003;18(9):1650–6. [DOI] [PubMed] [Google Scholar]
- 34.Ulrich CM, Georiou CC, Snow-Harter CM, Gillis DE. bone mineral density in mother-daughter pairs: relations to lifetime exercise, lifetime milk consumption, and calcium supplements. Am J Clin Nutr. 1996;63:72–79. [DOI] [PubMed] [Google Scholar]
- 35.Runyan SM, Stadler DD, Bainbridge CN, Miller SC, Moyer-Mileur LJ. Familial resemblance of bone mineralization, calcium intake, and physical activity in early-adolescent daughters, their mothers, and maternal grandmothers. J Am Diet Assoc. 2003;103(10):1320–5. [DOI] [PubMed] [Google Scholar]
- 36.Henderson NK, Price RI, Cole JH, Gutteridge DH, Bhagat CI. Bone density in young women is associated with body weight and muscle strength but not dietary intakes. J Bone Miner Res. 1995;10(3):384–93. [DOI] [PubMed] [Google Scholar]
- 37.Daniels ED, Pettifor JM, Schnitzler CM, Moodley GP, Zachen D. Differences in mineral homeostasis, volumetric bone mass and femoral neck axis length in black and white South African women. Osteoporos Int. 1997;7(2):105–12. [DOI] [PubMed] [Google Scholar]
- 38.Gkika D, Hsu YJ, van der Kemp AW, Christakos S, Bindels RJ, Hoenderop JG. Critical role of the epithelial Ca2+ channel TRPV5 in active Ca2+ reabsorption as revealed by TRPV5/calbindin-D28K knockout mice. J Am Soc Nephrol. 2006;17(11):3020–7. [DOI] [PubMed] [Google Scholar]
- 39.Zheng W, Xie Y, Li G, Kong J, Feng JQ, Li YC. Critical role of calbindin-D28k in calcium homeostasis revealed by mice lacking both vitamin D receptor and calbindin-D28k. J Biol Chem. 2004. [DOI] [PubMed] [Google Scholar]
- 40.Heaney RP. Nutritional factors in osteoporosis. Ann Rev Nutr. 1993;13:287–316. [DOI] [PubMed] [Google Scholar]
- 41.Wolf RL, Cauley JA, Baker CE, Ferrell RE, Charron M, Caggiula AW, Salamone LM, Heaney RP, Kuller LH. Factors associated with calcium absorption efficiency in pre- and perimenopausal women. Am J Clin Nutr. 2000;72(2):466–71. [DOI] [PubMed] [Google Scholar]
- 42.Kinyamu HK, Gallagher JC, Prahl JM, DeLuca HF, Petranick KM, Lanspa SJ. Association between intestinal vitamin D receptor, calcium absorption, and serum 1,25 dihydroxyvitamin D in normal young and elderly women. J Bone Miner Res. 1997;12(6):922–28. [DOI] [PubMed] [Google Scholar]
- 43.Need AG, Kemp A, Giles N, Morris HA, Horowitz M, Nordin BE. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int. 2002;13(1):83–88. [DOI] [PubMed] [Google Scholar]
- 44.Shapses SA, Sukumar D, Schneider SH, Schlussel Y, Brolin RE, Taich L. Hormonal and dietary influences on true fractional calcium absorption in women: role of obesity. Osteoporos Int. 2012;23(11):2607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryant RJ, Wastney ME, Martin BR, Wood O, McCabe GP, Morshidi M, Smith DL, Peacock M, Weaver CM. Racial differences in bone turnover and calcium metabolism in adolescent females. J Clin Endocrinol Metab. 2003;88(3):1043–7. [DOI] [PubMed] [Google Scholar]
- 46.Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, Weaver CM. Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr. 2007;85(6):1657–63. [DOI] [PubMed] [Google Scholar]
- 47.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and −12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19(5):1912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kellett GL. Alternative perspective on intestinal calcium absorption: proposed complementary actions of Ca(v)1.3 and TRPV6. Nutr Rev. 2011;69(7):347–70. [DOI] [PubMed] [Google Scholar]
- 49.Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol. 1986;250(5 Pt 1):G561–G69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.