Abstract
Animal studies have pointed at the liver as a hotspot for per- and polyfluoroalkyl substances (PFAS) accumulation and toxicity, however, these findings have not been replicated in human populations. We measured concentrations of seven PFAS in matched liver and plasma samples collected at the time of bariatric surgery from 64 adolescents in the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. Liver:plasma concentrations ratios were perfectly explained (r2 > 0.99) in a multi-linear regression (MLR) model based on toxicokinetic (TK) descriptors consisting of binding to tissue constituents and membrane permeabilities. Of the seven matched plasma and liver PFAS concentrations compared in this study, the liver:plasma concentration ratio of perfluoroheptanoic acid (PFHpA) was considerably higher than the liver:plasma concentration ratio of other PFAS congeners. Comparing the MLR model with an equilibrium mass balance model (MBM) suggested that complex kinetic transport processes are driving the unexpectedly high liver:plasma concentration ratio of PFHpA. Intra-tissue MBM modeling pointed to membrane lipids as the tissue constituents that drive the liver accumulation of long-chain, hydrophobic PFAS, whereas albumin binding of hydrophobic PFAS dominated PFAS distribution in plasma. The liver:plasma concentration dataset, empirical MLR model, and mechanistic MBM modeling allow the prediction of liver from plasma concentrations measured in human cohort studies. Our study demonstrates that combining biomonitoring data with mechanistic modeling can identify underlying mechanisms of internal distribution and specific target organ toxicity of PFAS in humans.
Keywords: PFAS, human exposure, biopsy samples, liver accumulation, correlation, toxicokinetics
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a group of more than 9,000 chemicals1 that have widespread use in industrial and consumer products as stain-, grease-, and water-repellants, surfactants, and lubricants.2 Anionic perfluoroalkyl acids are a class of PFAS that are hydrophobic, thermally stable, and resistant to degradation3 and are the focus of this work. These PFAS are ubiquitously found in the environment and detected in virtually all humans,4 and have been linked to multiple adverse health effects, including increased risk of non-alcoholic fatty liver disease (NAFLD) and cancer.5–7 The chronic PFAS exposure humans face and the increasing evidence of multifaceted adverse health effects require more knowledge about the mechanisms underlying the accumulation and distribution of PFAS in humans.
Most human biomonitoring studies have detected PFAS in plasma as these matrices are easily obtained. Depending on their hydrophobicity (C-chain length) and functional groups (carboxylic acids, sulfonic acids), PFAS have shown to be highly bioaccumulative in humans, with estimated elimination half-lives of 3.5 and 4.8 years for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), respectively.8 In contrast, shorter half-lives in humans have been found for short-chain PFAS such as perfluorobutanesulfonate (PFBS) and perfluorobutanoic acid (PFBA),9 supported by animal studies.10,11 The physiological and chemical binding mechanisms underlying tissue accumulation and retention of PFAS in humans are still poorly understood. Enterohepatic circulation and binding to liver proteins like fatty-acid binding proteins (FABPs) have been suggested to contribute to delayed elimination of long-chained PFAS,12 suggesting the liver as a primary repository for PFAS accumulation. However, concentrations in human livers have rarely been reported and never paired with simultaneously measured blood concentrations, limiting the establishment of a robust direct link between PFAS accumulation and liver-associated adverse health effects.
Epidemiological studies have linked PFAS exposure to dysregulated lipid metabolism and sterol homeostasis manifested in NAFLD and nonalcoholic steatohepatitis (NASH).5,6,13 Additionally, previous studies have demonstrated that PFAS preferentially accumulate in the liver;6,14,15 however, the mechanisms linking PFAS with these metabolic health risks remains unclear.2 While laboratory animal studies have measured liver-specific concentrations of PFAS, no previous human study has measured both liver and plasma specific concentrations of PFAS. Measuring concentrations of individual PFAS in the key target tissue (i.e., liver) and in plasma can help determine whether plasma alone is adequate for assessing health effects of PFAS in liver. Furthermore, laboratory based experimental studies have been used to elucidate potential mechanisms. Toxicological studies with cell-based bioassays (in vitro) and mammals (in vivo) suggest that PFAS interact with peroxisome proliferator-activated receptors (PPARs),16 upregulate bile acid synthesis,17 and altered lipogenesis,18 leading to disrupted lipid metabolism and excessive storage of lipids in the liver.19 However, PFAS accumulation in the human liver remains unknown, demanding for tools to predict liver from blood concentrations that is commonly sampled in human biomonitoring studies.
As an alternative to human cohort studies or biomonitoring studies, plasma-PFAS concentrations in humans have been predicted by one-compartment toxicokinetic (TK) models.20 However, these models neglect the distribution of chemicals between organs, and hence are uninformative of PFAS concentrations in specific organs like the liver. Scarcity on physiological parameters and physicochemical properties is a limitation to addressing organ distribution of PFAS in multi-compartment physiologically-based toxicokinetic models (PBTK).21 Simultaneous measurements of liver and blood concentrations could pave the way to mechanistically analyze the relative importance of sorption to tissue components (e.g., proteins, lipids), permeability (i.e., membrane diffusion), and physiological processes (e.g., enterohepatic circulation) to liver-blood partitioning of PFAS. TK descriptors like partition coefficients to proteins and lipids and permeabilities through biological membranes have been measured for the most common PFAS,22–24 and could be used to explain liver:plasma concentration ratios and derive empirical models to predict liver from blood concentrations measured in population studies.
For the first time, we report a matched dataset of PFAS concentrations measured simultaneously in the plasma and liver of 64 individuals as part of Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) Study. In this paper we aimed to assess how well liver concentrations can be predicted from plasma concentrations in human biomonitoring studies. First, we aimed to correlate plasma and liver-PFAS concentrations. As an alternative predictive method, multi-linear regression models (MLR) were derived to assess the suitability of TK descriptors to predict plasma:liver concentration ratios. The MLR models incorporated literature TK descriptors of PFAS, and physiological plasma and liver parameters measured in Teen-LABS and/or reported in the literature, including partition coefficients to serum albumin, phospholipids, FABPs, and storage lipids, as well as membrane permeabilities. The seven PFAS measured in our study covered a large chemical space in terms of carbon chain length (C6 – C11) and functional groups (carboxylic and sulfonic acids). For mechanistic analyses of the relative importance of protein and lipid binding to plasma:liver distribution of PFAS, experimental plasma:liver concentration ratios were compared to predicted values generated by a two-compartment physiologically-based mass balance model (MBM) that was parameterized with the TK descriptors and corresponding physiological data.
Materials and Methods
Study population.
The study is based on data from the Teen-LABS study (ClinicalTrials.gov number, NCT00465829), a prospective, multicenter, observational study of adolescents (≤19 years of age) who underwent bariatric surgery from 2007 through 2012 and enrolled at participating clinical centers in the United States: Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio), Nationwide Children’s Hospital (Columbus, Ohio), the University of Pittsburgh Medical Center (Pittsburgh, Ohio), Texas Children’s Hospital (Houston, Texas) and the Children’s Hospital of Alabama (Birmingham, Alabama).25,26 Study inclusion criteria included (1) adolescents up to age 19, (2) adolescents approved for bariatric surgery, (3) agreement to participate in the Teen-LABS study and understanding including signing of Informed Consent/Assent.25,26 Exclusion criteria included (1) age 19 or greater, (2) unable to sign Informed Consent/Assent.25,26 The present study includes 64 participants with plasma and liver biopsies collected at the time of surgery. The Teen-LABS steering committee, which included a site principal investigator from each participating center, worked in collaboration with the data coordinating center and project scientists from the National Institute of Diabetes and Kidney Disease (NIH-NIDDK) to design and implement the study.25–27 All bariatric procedures were performed by surgeons who were specifically trained for study data collection (Teen-LABS–certified surgeons).25,26 The study protocol, assent/consent forms, and data and safety monitoring plans were approved by the Institutional Review Boards of each institution and by the independent data and safety monitoring board prior to study initiation.25,27 Written informed consent or assent, as appropriate for age, was obtained from all parents/guardians and adolescents.25–28 Additionally, the study was approved by the University of Southern California review board.
Data collection.
Standardized methods for data collection have been described previously.25,26,28 Fasting blood specimens were drawn at the preoperative visit. All laboratory assays were performed by the Northwest Lipid Metabolism and Diabetes Research Laboratories (Seattle, WA). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation except for participants whose triglycerides were ≥400 mg/dl, for whom LDL cholesterol was measured directly by beta-quantification. Liver histology and liver biopsy methodology has been previously detailed,28 however, briefly, liver biopsies were obtained by core needle technique after induction of anesthesia and before performing the bariatric surgery procedure. Due to the observational study design and lack of published consensus on whether intra-operative liver biopsies should be standard of care at time of bariatric surgery, the decision to perform a liver biopsy was deferred to the surgical teams at each site. Accordingly, 99% of all biopsies were performed at the sites where intraoperative biopsy was standard of care. Participants with insufficient liver tissue or taking medications that may cause or treat NASH were excluded from analysis. Liver biopsy specimens were stained with hematoxylin-eosin and Masson’s trichrome stains and reviewed and scored centrally by an experienced hepatopathologist using the validated NASH Clinical Research Network scoring system.29 NAFLD was defined by the histopathological diagnosis. Detailed descriptions of study methods, comorbidity and other data definitions, case report forms, and laboratory testing are included in previous publications.26,27 For this analysis covariates were obtained at the time of surgery (baseline visit) at an in-person visit with trained study personnel.27 Data collected were maintained in a central database by the data coordinating center.
Liver-PFAS Laboratory Analysis.
Liver biopsy samples, collected at the time of bariatric surgery, were analyzed for PFAS concentrations at the environmental medicine laboratory at the University of Southern Denmark. The samples were transported on dry ice with temperature logging by World Courier (AmerisourceBergen Corporation, Conshohocken, PA), and stored at −80°C until analysis. The liver samples were thawed overnight at 5°C. The biopsies were drip dried on a lint free paper tissue and accurately weighted into a 2 mL polypropylene microtube (SARSTEDT, Nümbrecht, Germany). The liver biopsies ranged in weight from 17 to 124 mg, with an average weight of 82 mg. The liver samples were homogenized with three 5 mm steel balls on a Retsch Mixer Mill 200 at 25 Hz for 5 minutes after addition of 30 μL 20 ng/mL isotope labeled PFAS analogues (as internal standards) and 200 μL 0.06 M zinc sulphate in methanol to promote precipitation of proteins and blood cells. The samples were then centrifuged at 23,000 g for 20 minutes after which 160 μL of the supernatant were taken out and mixed with 400 uL of methanol. The subsequent analytical procedure was identical to the method the laboratory utilizes for serum and whole blood samples with on-line solid-phase extraction performed by a Thermo Scientific EQuan MAX liquid chromatographic system, directly coupled to a TSQ Quantiva triple quadrupole mass spectrometer.30,31 Analytical standards (PFAC-24PAR) and mass-labelled internal standards (MPFAC-24ES) were purchased from Wellington Laboratories Inc, Guelph, Ontario, Canada. The precision of the method was controlled in spiked pig liver samples and ranged for the identified compounds from 92–104.5%. The limit of detection (LOD) for the identified PFAS compounds was 0.03 ng/g liver tissue. Values below the LOD were replaced with ½ LOD. The batch of samples also included serum quality control samples from the German External Quality Assessment Program (G-EQUAS) as well as NIST 1957, that were extracted the same way as the liver biopsies, and the results of these were well within the acceptance range.
Plasma-PFAS Laboratory Analysis.
The samples were transported on dry ice with temperature logging by World Courier (AmerisourceBergen Corporation, Conshohocken, PA), and stored at −80°C until analysis. The samples were analyzed by on-line solid phase extraction followed by LC-MS/MS as previously described.32,33 The LOD was 0.03 ng/mL for all the reported compounds. The batch imprecision for the quality controls was better than 6%.
Toxicokinetic (TK) descriptors.
Toxicokinetic (TK) descriptors were derived from the literature as input for multi-linear regression and mass balance models. Partition coefficients (K, concentration ratio between two phases) of the study PFAS to bovine serum albumin (BSA, KBSA/w, Lw/LBSA), membrane lipids (phospholipids, Kmem/w, Lw/Lmem), and structural proteins (SP, KSP/w, Lw/LSP) were obtained from the literature22–24,34,35 as reported in Table S2. BSA was used as a surrogate for human serum albumin (HSA) as a consistent data set of KBSA/w was available for the study PFAS and it has been shown that chemical binding to BSA compares to HSA.36 Association constants with FABPs were derived from multiple studies37–40 and transformed into partition coefficients as described in Endo and Goss41; apparent permeabilities (Papp, cm/s) of the study PFAS were reported in Ebert, et al.23. All TK descriptors and their adaptations from the literature are reported in Table S2 of the Supporting Information.
Descriptive statistics.
Descriptive statistics were determined for PFAS measured in liver and plasma—geometric mean, 95% confidence intervals, and percentage below the limit of detection. For liver and plasma-PFAS values less than LOD, values were substituted by the half of the LOD value. Spearman’s rank correlation (r) was used to examine the correlations between plasma and liver-PFAS concentrations. R version 4.4.2 was used for the analysis of descriptive statistics.
Multiple-linear Regression.
For regression models, the log2 of PFAS concentrations was used to normalize the right-skewed distributions. Plasma-PFAS values less than the level of detection (LOD), were substituted by the half of the LOD value. Multiple-linear regression models and partial r2 analyses were run in R using the package rsq for generalized linear models. We tested experimental liver:plasma concentration ratios as response variable and TK descriptors as independent variables to evaluate the predictive power of all combinations of the five TK descriptors described above and summarized in Table S2. We also investigated the predictive power of physiological parameters measured for individual patients in Teen-LABS (albumin, total cholesterols, triglyceride levels).
Mass Balance Model (MBM).
A physiologically-based MBM was adapted from previous studies to predict and mechanistically describe liver:plasma concentration ratios.34,42 The applied MBM is a two-compartment model (i.e., plasma and liver) that assumes equilibrium between the compartments at all times (i.e., no flux) and neglects complex toxicokinetic processes (e.g., membrane permeability). We used the MBM to evaluate to what extent experimental plasma:liver concentrations can be explained by binding to proteins and lipids (i.e., tissue retention), or if the inclusion of more complex kinetic processes is necessary. The plasma and liver compartments are composed of different kinds of proteins and lipids as well as non-sorptive phases (water, Fig. 1). Partition coefficients were used as quantitative descriptors of the binding affinities of PFAS to tissue proteins and lipids. Protein and lipids of plasma samples were measured and used as input parameters for the model. Missing physiological parameters were derived from the literature34 or estimated from plasma biomarkers (see Table S3 for details). The plasma was assumed to be composed of albumin (~4.2% as measured in this study), phospholipids (~0.15%, as predicted from cholesterol levels), structural proteins (2.2%), non-sorptive molecules (storage lipids, cholesterol, sugars), and water (~93%).34 Note that by accounting for the composition of plasma, the MBM accounts for the differences in the binding affinity between plasma and whole blood. As compositions of the liver samples were not measured, water, protein, and lipid volume fractions were assumed to be 73%, 18.1%, and 4.9%, respectively.34 Of these proteins, 1.7% and 0.66% were assumed to be FABPs and albumin.34,43 The remaining proteins were assumed structural proteins. Of the liver lipids, 24% were assumed to be membrane lipids and 76% were storage lipids.34,44 The MBM was parameterized with experimental partition coefficients from the literature that were converted to units of L/L (see Table S2 for details). Liver-blood partition coefficients (Kliver/plasma, Lplasma/Lliver) were derived as
| (1) |
where Kliver/w is the liver-water (Lw/Lliver) and Kplasma/w is the plasma-water partition coefficient (Kplasma/w, Lw/Lplasma). By combining structural tissue composition and corresponding partition coefficients, Kliver/w and Kplasma/w were calculated as
| (2) |
| (3) |
Figure 1.
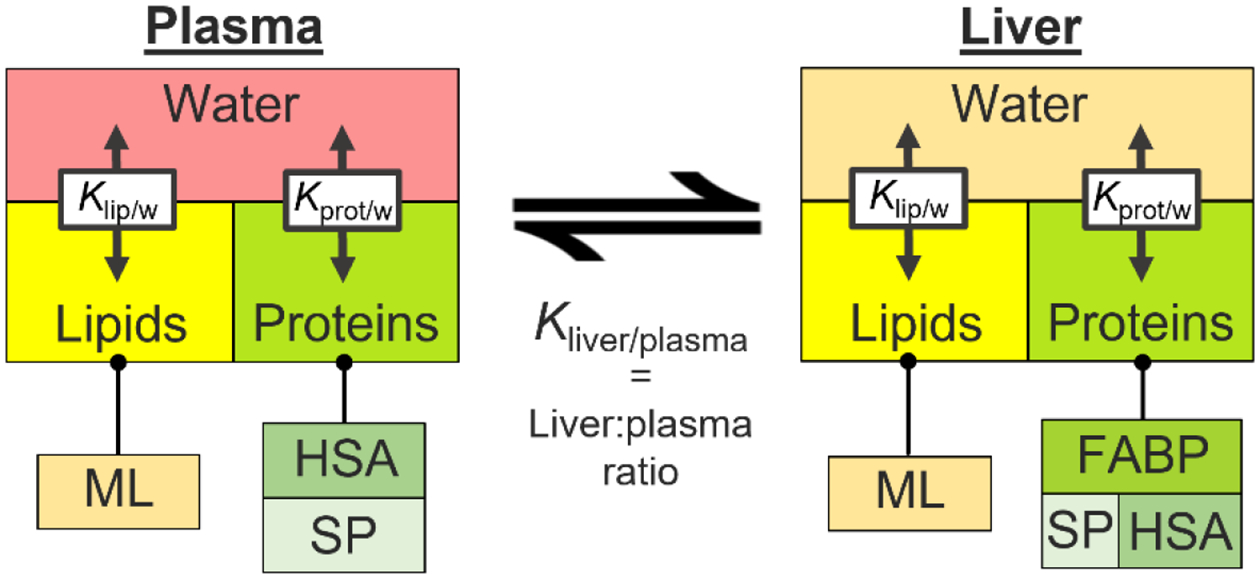
Structure of the steady-state mass balance model used to predict plasma-liver ratios from protein, lipid, and water compositions of the tissues and respective partition coefficients. ML = membrane lipids; HSA = human serum albumin; SP = structural proteins; FABP = fatty-acid binding proteins; Klip/w = lipid-water partition coefficient; Kprot/w = protein-water partition coefficient.
Results and Discussion
Cohort characteristics.
There are 64 Teen-LABS participants included in this study. These participants had both liver and plasma samples collected at the time of bariatric surgery. Descriptive characteristics of study participants are presented in Table 1—the mean age of participants at the time of bariatric surgery was 16.7 years (SD: 1.7 years). Most study participants were female (73.4%). Participants have severe obesity; the mean BMI at baseline was 51.3 kg/m2 (min to max, 34.0 to 87.7 kg/m2), and 42.2% of participants have histopathological determined NAFLD.
Table 1.
Teen-LABS Study Participant Characteristics (N=64)
| n (%)/mean (SD) | |
|---|---|
| Female | 47 (73.4) |
| Age (years) | 16.7 (1.7) |
| eGFRa (mL/min/1.73m2) | 111.0 (27.5) |
| Albumin (g/dl) | 4.2 (0.27) |
| BMIb (kg/m2) | 51.3 (8.6) |
| NAFLDc (y/n) | 27 (42.2) |
Participant characteristics were determined at the time of bariatric surgery. NAFLD (yes/no) was determined via histopathology diagnosis.
eGFR = estimated glomerular filtration rate;
BMI = body mass index;
NAFLD = non-alcoholic fatty liver disease
Experimental blood and liver concentration in the Teen-LABS study.
All seven PFAS included in this analysis were detected in 100% of liver samples. With the exception of PFHpA (detected in 89% of plasma samples) and PFUnDA (detected in 88% of plasma samples) the additional five PFAS were detected in 100% of plasma samples (Table S1). Figure 2 reports the concentrations of the seven PFAS in plasma, liver, and corresponding liver:plasma concentrations ratios. The concentrations of PFOA and PFOS were generally highest in plasma (Fig. 2A), revealing that these phased-out PFAS (PFOS was phased out of production in 2002, and U.S. manufacturers eliminated PFOA emissions and product content at the end of 2015)45 were still major chemical contaminants in humans at the time of the bariatric surgery. Among the measured PFAS, PFOS had the highest liver concentration, whereas median PFOA and shorter chain carboxylic and sulfonic acids (PFHpA and PFHxS) concentrations in livers were sub- ng/g concentrations (Fig. 2B). Hydrophobic PFAS with longer C-chain length accumulated in livers to a higher extent than their hydrophilic alternatives, with liver:plasma concentration ratios of 1.52 (PFOS, C8), 1.37 (PFNA, C9), 1.83 (PFDA, C10), and 3.64 (PFUnDA, C11), as compared to 0.12 (PFHxS, C6) and 0.26 (PFOA, C8) (Fig. 2C). Notably, plasma concentrations of the shorter-chain PFHxS (C6) reach levels comparable to PFOS (C8) and PFOA (C8), however, liver concentrations were low and liver:plasma concentrations ratios were the lowest amongst the tested PFAS, indicating low accumulation tendency of PFHxS in human liver. While found in low concentrations in plasma (geometric mean [GM] = 0.08 ng/mL) and liver (GM = 0.17 ng/g liver), the highest liver:plasma ratios were found for PFHpA (C7), which was found in concentration ratios 26x higher than PFOA (C8). Large variabilities in liver:plasma concentration ratios of PFHpA were observed between individual participants (6.8±14.5). Despite low concentrations in liver and plasma, the high accumulation of PFHpA in the liver relative to plasma demonstrates that short-chain PFAS can be similarly or even more bioaccumulative in target organs like the liver than longer-chain, hydrophobic PFAS. This observation questions the increased use in short-chain PFAS as less bioaccumulative alternatives to legacy PFAS.
Figure 2.

(A) Concentrations of PFAS measured in plasma (ng/mL); (B) Concentrations of PFAS measured in liver (ng/g liver); (C) The ratio of liver to plasma PFAS. PFAS are organized in order of C-chain length and function group: PFHxS (C6), PFHpA (C7), PFOA (C8), PFOS (C8), PFNA (C9), PFUnDA (C11).
Liver:plasma correlation.
Spearman correlations indicate moderate to strong correlations between liver and plasma concentrations of six PFAS (Fig. 3), with coefficients ranging from 0.65 (PFUnDA) to 0.93 (PFHxS). Correlations between the chemicals were generally increasing with structural similarity (C-chain length and functional group), indicating that liver:plasma partitioning of PFAS is dependent on the physicochemical properties of the chemical. The strong correlations between plasma and liver concentrations suggest that liver accumulation can be predicted well from plasma concentrations for PFHxS (r = 0.93) and PFOA (r = 0.88); whereas prediction of liver concentrations from experimental plasma concentrations is not straightforward for PFOS (r = 0.77), PFNA (r = 0.72), PFDA (r = 0.73), and PFUnDA (r = 0.65). For PFHpA, no correlation has been found (r = 0.19), indicating a lacking relationship between liver and plasma concentrations and/or that complex mechanisms are underlying the high accumulation of PFHpA in the liver (Fig. 2C). Interestingly, liver:plasma concentration ratios were not significantly different between NAFLD-positive and NAFLD-negative patients (Fig. S1), indicating that plasma:liver distribution of PFAS is not affected by NAFLD. Based on these observations, we aimed to assess the ability of TK descriptors to predict liver concentrations from experimental plasma concentrations. These descriptors are commonly used to assess the toxicokinetics/pharmacokinetics (i.e., absorption, distribution, metabolism, and elimination, ADME) of chemical agents or drugs. Here, we used partition coefficients to biomaterials abundant in both plasma and liver and diffusion rates over biological membranes (permeability), as described in the Materials & Methods section.
Figure 3.

Spearman correlation coefficients (r), smoothed histogram, and scatter plot for seven PFAS congeners measured in plasma (ng/mL) and liver (ng/g liver) samples from 64 participants from the Teen-LABS study.
Multilinear regression models to predict liver:plasma concentration ratios.
As liver:plasma ratios did not correlate with single TK descriptors (Fig. S2, r2 ≤ 0.29), all combinations of TK descriptors were tested as independent variables in MLR models. Combinations of two TK descriptors resulted in poor correlations (r2 ≤ 0.37, Table S6). MLR models with average predictive power (r2 = 0.33 – 0.73) were derived from combinations of three or four partition coefficients as indicators for binding to tissue constituents, with combining KBSA/w, KFABP/w, KSP/w, and Kmem/w to result in the most promising MLR model (r2 = 0.73). The addition of permeability (Papp) as a more complex, kinetic parameter for the capacity of PFAS to diffuse through biological membranes improved the MLR models considerably, achieving excellent predictive power across all seven PFAS when combining all partitioning coefficients with permeability (r2 = 0.991, Fig. 4), which points at permeability as a key mechanism for plasma:liver distribution of PFAS. Partial r2 analyses point at protein binding (albumin, FABPs, and structural proteins) and membrane permeability as most influential parameters in the MLR model (Fig. 4, partial r2 ≥ 0.97), whereas binding to membrane lipids was less important (partial r2 = 0.87) but still increased the predictive power as compared to a protein binding- and permeability-based model (r2 = 0.99 as compared to r2 = 0.97). The necessity to include all TK descriptors to achieve excellent predictive power confirms that blood:liver distribution of PFAS is mainly driven by both competitive binding between plasma and liver proteins and lipids as well as the permeability of the chemical through biological membranes.24 In contrast to TK descriptor correlations, blood levels of albumin, cholesterol, and triglycerides did not explain individual plasma:liver concentration ratios (Fig. S9). These findings emphasize the necessity to extend the currently limited experimental database on these TK descriptors and their inclusion in multi-compartment PBTK models that simulate PFAS disposition amongst different tissues. Given the strong correlation with TK descriptors, liver:plasma concentration ratios and liver PFAS concentrations (ng/g liver) from plasma concentrations (ng/mL) can be accurately predicted for all seven PFAS from:
| (4) |
Note that the partition coefficients (K) and apparent membrane permeabilities (Papp) must be entered in units of L/kg and cm/s, respectively. Standard errors and 95% confidence intervals of model parameters are reported in Table S2 of the Supplementary Material. Note that the MLR model has been derived from a cohort of adolescents that underwent bariatric surgery. Its ability to accurately predict liver:plasma concentration ratios has to be validated based on another independent dataset that concurrently measured liver:plasma concentrations, which is currently not available in the literature. Multi-linear regression modeling was performed with liver:plasma concentration ratios of NAFLD-positive (n = 27) and NAFLD-negative patients (n = 37). Similar to the MLR model of the whole data set, the models achieved excellent predictive power (Fig. S4). The resulting model parameters were comparable (78%−186% variation between the models), suggesting that NAFLD does not influence the prediction of liver:plasma concentration ratios by our MLR model.
Figure 4.

Experimental liver:plasma concentration ratios predicted by the multilinear regression model that includes serum albumin-, fatty-acid binding protein-, phospholipid, and structural protein--water partition coefficients (KBSA/w, KFABP/w, Kmem/w, KSP/w), and apparent membrane permeabilities (Papp) of the PFAS. The dotted red line represents a 1:1 agreement.
Mechanistic discussion based on mass balance model predictions.
Following the excellent multi-linear correlation between TK descriptors and liver:plasma concentration ratios, we aimed to further investigate the partitioning between plasma and liver using a two-compartment MBM. The MBM accounts for the protein, lipid, and water composition of plasma and liver and the corresponding partition coefficients of the PFAS to these individual phases to derive liver-blood partition coefficients (Kliver/blood),34 which correspond to liver:plasma concentration ratios (Fig. 1). The MBM was able to explain liver:plasma concentration ratios of PFHxS, PFOA, PFDA, and PFOS, within a factor of 2 (Fig. 5). PFHpA liver:plasma concentration ratio was largely underestimated by a factor of 76, again revealing that transport of PFHpA from the blood stream into the liver underlies mechanisms of distribution not sufficiently explained by partition coefficients alone. The MBM does not account for kinetic processes like permeability, which was shown to be an important parameter in the empirical MLR model (Fig. 4).
Figure 5.

Experimental against liver:plasma concentration ratios predicted by the mass balance model accounting for the protein and lipid composition of both tissues.
Intra-tissue distribution modeling indicated that membrane lipids are driving liver accumulation of the hydrophobic, long-chain PFOS (C8), PFNA (C9), and PFDA (C10) (Fig. S5A), agreeing with the PFAS-phospholipid hypothesis supported by evidence from diverse species.46–48 In contrast, protein sorption is predicted to dominate the liver accumulation of PFBS (C3), PFHxA (C6), PFHpA (C7), and PFOA (C8). PFAS partitioning in plasma is strongly dominated by proteins, especially albumin (Fig. S6), as shown in previous studies.49 Liver:plasma concentration ratios in this study were derived from adolescents with severe obesity, potentially limiting the applicability of the derived MLR and MBM models to predict liver concentrations of adults with normal weight and liver physiology. As the majority of lipids enriched in NAFLD are triglycerides (storage fats) with very weak binding capacity for PFAS,50 the overall liver:blood partitioning is not expected to change substantially (Fig. S5B). However, physiological transport processes such as enterohepatic recirculation can be affected during fatty liver disease,51 which may influence PFAS accumulation.12 Binding to human FABPs was suggested to be an important driver of PFAS accumulation in human liver based on computed structural models of the interactions between PFAS and FABPs.40,52 Further compartmentalization of liver proteins indicated that most of the long chain and short chain PFAS are predominantly bound to structural proteins and albumins, whereas binding to FABPs was more relevant for PFOA and PFNA (Fig. S7). Comparing the outcome of the MLR and MBM models indicated that permeability is an important TK descriptor to predict liver-plasma distribution of certain PFAS (e.g., PFHpA, PFNA, PFUnDA). The inclusion of permeability to predict plasma-liver distribution in a mechanistic model would require time-dependent PBTK modeling. However, complex PBTK modeling of PFAS distribution in humans is currently limited to the availability of TK and physiological descriptors and will require parameterization to move forward complex PBTK modeling of PFAS in humans.
Implications, Limitations, and Outlook.
We present a unique dataset of matched plasma and liver concentrations of seven common PFAS that were simultaneously measured for 64 study participants in adolescents with severe obesity. As previously stated, liver:plasma concentration ratios in this study were derived from adolescents with severe obesity and have not been validated in an independent dataset, which potentially limits the applicability of the derived MLR and MBM models to predict liver concentrations of adults with normal weight and liver physiology. Until our research is replicated among or contrasted with healthy humans of normal weight, this dataset can serve as reference for studies investigating liver accumulation of PFAS to assess the contribution of PFAS exposure to the increase incidence of liver pathology and diseases.6 Still, both the MLR and MBM models need to be evaluated with independent liver:plasma concentration data sets before it can be used to predict liver from plasma concentrations in a broader context. Our MLR analysis emphasized that plasma:liver distribution of PFAS can be explained using literature TK descriptors. It must be clarified to what extent the MLR model can be applied to PFAS with other physicochemical properties, which again requires further datasets. The excellent predictive power (r2 > 0.99) of the liver:plasma MLR model is promising and suggests deriving further empirical models to predict the accumulation of PFAS in other tissues suspected to be hotspots of toxicity, such as the brain, lung, and kidneys.15,53,54 Currently, TK descriptors relevant to the accumulation of PFAS in tissues are limited to ~10 PFAS and a handful of reference proteins and lipids. The measurement of TK descriptors for a larger chemical space and further biomaterials will pave the way for a comprehensive analysis of inter- and intra-tissue accumulation of PFAS and the derivation of predictive models. Such analyses and predictive models will further identify PFAS with particularly high accumulation and persistence in tissues, as shown for PFHpA in our study. It should be emphasized, however, that the predicting liver:blood ratios of PFHpA using the MBM, MLR, or just plasma is not recommended because of the large variability between individuals in our study. The prediction model therefore lacks precision for PFHpA. Future work focused on additional data on PFHpA and driving biological processes will perhaps allow us to better explain this variability using models and then provide reliable predictions. A possible explanation for PFHpA elimination could be related to the short half-life— PFHpA has a half-life of 2 months instead of years for many of the other PFAS congeners.9 Recent change in exposure patterns of PFHpA have a greater impact to the exposure measured at the time of biopsy. Additionally, measuring biomarkers that are suspected to be particularly relevant for PFAS distribution simultaneously to chemical concentrations might explain variability in PFAS body burdens between individuals. For example, given the indication of active transport of PFHpA into the liver, relevant biomarkers could include active transporters that facilitate the influx and outflux of PFAS, such as organic acid transporter proteins (OATP) and Na+/taurocholate co-transporting polypeptide (NTCP) in liver tissue.55–57 Methodological advances are required to reduce the amount of tissue necessary to detect PFAS and various biomarkers as well as to derive TK descriptors for a large number of legacy and novel emerging PFAS. In this context, our study demonstrates the suitability of empirical MLR and mechanistic MBM models to analyze and predict inter- and intra-tissue distribution of PFAS. Combining high throughput biomonitoring techniques with empirical and mechanistic analyses and models will greatly contribute to identify the mechanisms underlying internal distribution and specific target organ toxicity of PFAS in humans, eventually contributing to phase-out bioaccumulative and toxic PFAS.
Supplementary Material
Synopsis:
Paired liver:plasma concentrations of perfluoroalkyl substances (PFAS) in humans were mechanistically analyzed using multilinear regression and physiologically-based mass balance modeling.
Source of Funding
The results reported herein correspond to specific aims of grant R01ES030691 to Dr. Chatzi from the National Institute of Environmental Health Science (NIEHS). Additional funding from NIEHS supported Dr. Chatzi (R01ES030364, R01ES029944, R21ES029681, R21ES028903, and P30ES007048), Dr. Baumert (R01ES030691), Dr. Goodrich (T32-ES013678), Dr. Valvi (R01ES033688, R21ES029328, P30ES023515), Dr. Walker (R01ES030691, U2CES030859, R01ES032831) and Dr. La Merrill (R01ES030364). Dr. Conti (R21ES029681, R01ES030691, R01ES030364, R01ES029944, P01CA196569, and P30ES007048), Dr. McConnell (P30ES007048, P2CES033433), Dr. Grandjean (P42ES027706), Dr. Stratakis has received funding from the Ministry of Science and Innovation and State Research Agency through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018-000806-S) and from IJC2020-043630-I financed by MCIN/AEI/10.13039/501100011033 and the European Union “NextGenerationEU/PRTR”). Dr. La Merrill was additionally supported by the California Environmental Protection Agency (20-E0017). The Teen-LABS consortium is supported by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through grants for a clinical coordinating center (UM1DK072493; Inge) and the Data Coordinating Center (UM1DK095710; Xie). Dr. Sisley is supported by grants from the USDA (6250-51000-053) and the NIH (R01DK128117-01A1).
Footnotes
Conflict of interest disclosures.
The authors declare that they have no conflicts of interest apart from Drs. Grandjean and Bartell who have provided paid expert assistance in legal cases involving PFAS exposed populations.
Declaration of competing financial interests.
All other authors declare they have no actual or potential competing financial interests.
Human Subjects
Ethics approval for this study was provided by the University of Southern California Institutional Review Board (IRB protocols HS-19-00057). Prior to participation, written informed assent/consent were obtained from participants and their guardians. The Teen–Longitudinal Assessment of Bariatric Surgery (Teen–LABS) study (ClinicalTrials.gov number, NCT00474318) was designed as prospective, multicenter, observational study of consecutive cases of bariatric surgery offered to adolescents. The study methodology has been previously described.
Supplemental Information
Descriptive statistics for liver and plasma PFAS; MLR model parameters; physicochemical properties; predicted liver:plasma PFAS ratio; comparison of PFAS distribution in healthy and diseased liver; MBM parameters
References
- 1.U.S. Environmental Protection Agency. Comptox Chemicals Dashboard: Master List of PFAS Substances (Version2), < https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster> (
- 2.Fenton SE; Ducatman A; Boobis A; DeWitt JC; Lau C; Ng C; Smith JS; Roberts SM Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem 40, 606–630 (2021). 10.1002/etc.4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen A; Kannan K; Mabury SA; van Leeuwen SP Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7, 513–541 (2011). 10.1002/ieam.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA & Needham LL Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115, 1596–1602 (2007). 10.1289/ehp.10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassler J; Ducatman A; Elliott M; Wen S; Wahlang B; Barnett J; Cave MC Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut 247, 1055–1063 (2019). 10.1016/j.envpol.2019.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costello E, Rock S, Stratakis N, Eckel SP, Walker DI, Valvi D, Cserbik D, Jenkins T, Xanthakos SA, Kohli R, Sisley S, Vasiliou V, La Merrill MA, Rosen H, Conti DV, McConnell R, Chatzi L. Exposure to per- and Polyfluoroalkyl Substances and Markers of Liver Injury: A Systematic Review and Meta-Analysis. Environ Health Perspect 130, 46001 (2022). 10.1289/ehp10092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y; Lin N; Dai C; Xu J; Zhang Y; Xu M; Wang F; Li Y; Chen D Occurrence and distribution of per- and polyfluoroalkyl substances (PFASs) in human livers with liver cancer. Environ Res 202, 111775 (2021). 10.1016/j.envres.2021.111775 [DOI] [PubMed] [Google Scholar]
- 8.Olsen GW; Burris JM; Ehresman DJ; Froehlich JW; Seacat AM; Butenhoff JL; Zobel LR Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115, 1298–1305 (2007). 10.1289/ehp.10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Fletcher T, Pineda D, Lindh CH, Nilsson C, Glynn A, Vogs C, Norström K, Lilja K, Jakobsson K, Li Y. Serum Half-Lives for Short- and Long-Chain Perfluoroalkyl Acids after Ceasing Exposure from Drinking Water Contaminated by Firefighting Foam. Environ Health Perspect 128, 077004 (2020). 10.1289/EHP6785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang S-C; Noker PE; Gorman GS; Gibson SJ; Hart JA; Ehresman DJ; Butenhoff JL Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reproductive Toxicology 33, 428–440 (2012). 10.1016/j.reprotox.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 11.Olsen GW; Chang S-C; Noker PE; Gorman GS; Ehresman DJ; Lieder PH; Butenhoff JL A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 256, 65–74 (2009). 10.1016/j.tox.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 12.Cao H, Zhou Z, Hu Z, Wei C, Li J, Wang L, Liu G, Zhang J, Wang Y, Wang T, Liang Y. Effect of Enterohepatic Circulation on the Accumulation of Per- and Polyfluoroalkyl Substances: Evidence from Experimental and Computational Studies. Environmental Science & Technology 56, 3214–3224 (2022). 10.1021/acs.est.1c07176 [DOI] [PubMed] [Google Scholar]
- 13.Roth K; Yang Z; Agarwal M; Liu W; Peng Z; Long Z; Birbeck J; Westrick J; Liu W; Petriello MC Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environment International 157, 106843 (2021). 10.1016/j.envint.2021.106843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzurro DM, Seeley M, Kerper LE & Beck BD Interspecies differences in perfluoroalkyl substances (PFAS) toxicokinetics and application to health-based criteria. Regul Toxicol Pharmacol 106, 239–250 (2019). 10.1016/j.yrtph.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 15.Pérez F; Nadal M; Navarro-Ortega A; Fàbrega F; Domingo JL; Barceló D; Farré M Accumulation of perfluoroalkyl substances in human tissues. Environment International 59, 354–362 (2013). 10.1016/j.envint.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 16.Behr A-C, Plinsch C, Braeuning A & Buhrke T Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicology in Vitro 62, 104700 (2020). 10.1016/j.tiv.2019.104700 [DOI] [PubMed] [Google Scholar]
- 17.Salihović S; Dickens AM; Schoultz I; Fart F; Sinisalu L; Lindeman T; Halfvarson J; Orešič M; Hyötyläinen T Simultaneous determination of perfluoroalkyl substances and bile acids in human serum using ultra-high-performance liquid chromatography–tandem mass spectrometry. Analytical and Bioanalytical Chemistry 412, 2251–2259 (2020). 10.1007/s00216-019-02263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahlang B; Jin J; Beier JI; Hardesty JE; Daly EF; Schnegelberger RD; Falkner KC; Prough RA; Kirpich IA; Cave MC Mechanisms of Environmental Contributions to Fatty Liver Disease. Current Environmental Health Reports 6, 80–94 (2019). 10.1007/s40572-019-00232-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modaresi SMS, Wei W, Emily M, DaSilva NA & Slitt AL Per- and polyfluoroalkyl substances (PFAS) augment adipogenesis and shift the proteome in murine 3T3-L1 adipocytes. Toxicology 465, 153044 (2022). 10.1016/j.tox.2021.153044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu XC; Tokranov AK; Liddie J; Zhang X; Grandjean P; Hart JE; Laden F; Sun Q; Yeung LWY; Sunderland EM Tap Water Contributions to Plasma Concentrations of Poly- and Perfluoroalkyl Substances (PFAS) in a Nationwide Prospective Cohort of U.S. Women. Environ Health Perspect 127, 067006 (2019). 10.1289/EHP4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Silva AO, Armitage JM, Bruton TA, Dassuncao C, Heiger-Bernays W, Hu XC, Kärrman A, Kelly B, Ng C, Robuck A, Sun M, Webster TF, Sunderland EM. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environmental Toxicology and Chemistry 40, 631–657 (2021). 10.1002/etc.4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allendorf F, Berger U, Goss KU & Ulrich N Partition coefficients of four perfluoroalkyl acid alternatives between bovine serum albumin (BSA) and water in comparison to ten classical perfluoroalkyl acids. Environ Sci-Proc Imp 21, 1852–1863 (2019). 10.1039/c9em00290a [DOI] [PubMed] [Google Scholar]
- 23.Ebert A, Allendorf F, Berger U, Goss KU & Ulrich N Membrane/Water Partitioning and Permeabilities of Perfluoroalkyl Acids and Four of their Alternatives and the Effects on Toxicokinetic Behavior. Environmental Science & Technology 54, 5051–5061 (2020). 10.1021/acs.est.0c00175 [DOI] [PubMed] [Google Scholar]
- 24.Droge STJ Membrane-Water Partition Coefficients to Aid Risk Assessment of Perfluoroalkyl Anions and Alkyl Sulfates. Environmental Science & Technology 53, 760–770 (2019). 10.1021/acs.est.8b05052 [DOI] [PubMed] [Google Scholar]
- 25.Inge TH, Zeller M, Harmon C, Helmrath M, Bean J, Modi A, Horlick M, Kalra M, Xanthakos S, Miller R, Akers R, Courcoulas A. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg 42, 1969–1971 (2007). 10.1016/j.jpedsurg.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, Harmon CM, Courcoulas A, Horlick M, Xanthakos SA, Dolan L, Mitsnefes M, Barnett SJ, Buncher R. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr 168, 47–53 (2014). 10.1001/jamapediatrics.2013.4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, Harmon CM, Zeller MH, Chen MK, Xanthakos SA, Horlick M, Buncher CR. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med 374, 113–123 (2016). 10.1056/NEJMoa1506699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xanthakos SA, Jenkins TM, Kleiner DE, Boyce TW, Mourya R, Karns R, Brandt ML, Harmon CM, Helmrath MA, Michalsky MP, Courcoulas AP, Zeller MH, Inge TH. High Prevalence of Nonalcoholic Fatty Liver Disease in Adolescents Undergoing Bariatric Surgery. Gastroenterology 149, 623–634 e628 (2015). 10.1053/j.gastro.2015.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005). 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 30.Vorkamp K; Nielsen F; Kyhl HB; Husby S; Nielsen LB; Barington T; Andersson AM; Jensen TK Polybrominated diphenyl ethers and perfluoroalkyl substances in serum of pregnant women: levels, correlations, and potential health implications. Arch Environ Contam Toxicol 67, 9–20 (2014). 10.1007/s00244-013-9988-z [DOI] [PubMed] [Google Scholar]
- 31.Valvi D; Hojlund K; Coull BA; Nielsen F; Weihe P; Grandjean P Life-course Exposure to Perfluoroalkyl Substances in Relation to Markers of Glucose Homeostasis in Early Adulthood. J Clin Endocrinol Metab 106, 2495–2504 (2021). 10.1210/clinem/dgab267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haug LS, Thomsen C & Becher G A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A 1216, 385–393 (2009). 10.1016/j.chroma.2008.10.113 [DOI] [PubMed] [Google Scholar]
- 33.Eryasa B; Grandjean P; Nielsen F; Valvi D; Zmirou-Navier D; Sunderland E; Weihe P; Oulhote Y Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ Int 130, 104874 (2019). 10.1016/j.envint.2019.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allendorf F, Goss K-U & Ulrich N Estimating the Equilibrium Distribution of Perfluoroalkyl Acids and 4 of Their Alternatives in Mammals. Environmental Toxicology and Chemistry 40, 910–920 (2021). 10.1002/etc.4954 [DOI] [PubMed] [Google Scholar]
- 35.Bischel HN, MacManus-Spencer LA, Zhang C & Luthy RG Strong associations of short-chain perfluoroalkyl acids with serum albumin and investigation of binding mechanisms. Environ Toxicol Chem 30, 2423–2430 (2011). 10.1002/etc.647 [DOI] [PubMed] [Google Scholar]
- 36.Gülden M, Dierickx P & Seibert H Validation of a prediction model for estimating serum concentrations of chemicals which are equivalent to toxic concentrations in vitro. Toxicol in Vitro 20, 1114–1124 (2006). 10.1016/j.tiv.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 37.Yang D, Han J, Hall DR, Sun J, Fu J, Kutarna S, Houck KA, LaLone CA, Doering JA, Ng CA, Peng H. Nontarget Screening of Per- and Polyfluoroalkyl Substances Binding to Human Liver Fatty Acid Binding Protein. Environmental Science & Technology 54, 5676–5686 (2020). 10.1021/acs.est.0c00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Y, Zhu Y, Xu D, Feng X, Yu X, Shan G, Zhu L. Insights into the Competitive Mechanisms of Per- and Polyfluoroalkyl Substances Partition in Liver and Blood. Environmental Science & Technology (2022). 10.1021/acs.est.1c08493 [DOI] [PubMed] [Google Scholar]
- 39.Khazaee M, Christie E, Cheng W, Michalsen M, Field J, Ng C. Perfluoroalkyl Acid Binding with Peroxisome Proliferator-Activated Receptors α, γ, and δ, and Fatty Acid Binding Proteins by Equilibrium Dialysis with a Comparison of Methods. Toxics 9, 45 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Ren X-M & Guo L-H Structure-Based Investigation on the Interaction of Perfluorinated Compounds with Human Liver Fatty Acid Binding Protein. Environmental Science & Technology 47, 11293–11301 (2013). 10.1021/es4026722 [DOI] [PubMed] [Google Scholar]
- 41.Endo S & Goss KU Serum Albumin Binding of Structurally Diverse Neutral Organic Compounds: Data and Models. Chemical Research in Toxicology 45, 2293–2301 (2011). 10.1021/es200855w [DOI] [PubMed] [Google Scholar]
- 42.Endo S, Brown TN & Goss K-U General Model for Estimating Partition Coefficients to Organisms and Their Tissues Using the Biological Compositions and Polyparameter Linear Free Energy Relationships. Environmental Science & Technology 47, 6630–6639 (2013). 10.1021/es401772m [DOI] [PubMed] [Google Scholar]
- 43.Wiśniewski JR, Vildhede A, Norén A & Artursson P In-depth quantitative analysis and comparison of the human hepatocyte and hepatoma cell line HepG2 proteomes. J Proteomics 136, 234–247 (2016). 10.1016/j.jprot.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 44.Ruark CD, Hack CE, Robinson PJ, Mahle DA & Gearhart JM Predicting Passive and Active Tissue:Plasma Partition Coefficients: Interindividual and Interspecies Variability. Journal of Pharmaceutical Sciences 103, 2189–2198 (2014). 10.1002/jps.24011 [DOI] [PubMed] [Google Scholar]
- 45.National Toxicology Program. Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid or Perfluorooctane Sulfonate. (Office of Health Assessment and Translation, Divison of Toxicology Program, National Institute of Envrionmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, 2016). [Google Scholar]
- 46.Armitage JM, Arnot JA & Wania F Potential Role of Phospholipids in Determining the Internal Tissue Distribution of Perfluoroalkyl Acids in Biota. Environmental Science & Technology 46, 12285–12286 (2012). 10.1021/es304430r [DOI] [PubMed] [Google Scholar]
- 47.Dassuncao C, Pickard H, Pfohl M, Tokranov AK, Li M, Mikkelsen B, Slitt A, Sunderland EM. Phospholipid Levels Predict the Tissue Distribution of Poly- and Perfluoroalkyl Substances in a Marine Mammal. Environ Sci Technol Lett 6, 119–125 (2019). 10.1021/acs.estlett.9b00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robuck AR, McCord JP, Strynar MJ, Cantwell MG, Wiley DN, Lohmann R. Tissue-specific distribution of legacy and novel per- and polyfluoroalkyl substances in juvenile seabirds. Environ Sci Technol Lett 8, 457–462 (2021). 10.1021/acs.estlett.1c00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forsthuber M, Kaiser AM, Granitzer S, Hassl I, Hengstschläger M, Stangl H, Gundacker C. Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environment International 137, 105324 (2020). 10.1016/j.envint.2019.105324 [DOI] [PubMed] [Google Scholar]
- 50.Browning JD & Horton JD Molecular mediators of hepatic steatosis and liver injury. The Journal of Clinical Investigation 114, 147–152 (2004). 10.1172/JCI22422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong M, Zhu L, Bai L, Zhang X, Chen Y, Liu S, Zheng S, Pandol SJ, Han YP, Duan Z. Vitamin D deficiency promotes nonalcoholic steatohepatitis through impaired enterohepatic circulation in animal model. American Journal of Physiology-Gastrointestinal and Liver Physiology 307, G883–G893 (2014). 10.1152/ajpgi.00427.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheng N, Li J, Liu H, Zhang A & Dai J Interaction of perfluoroalkyl acids with human liver fatty acid-binding protein. Archives of Toxicology 90, 217–227 (2016). 10.1007/s00204-014-1391-7 [DOI] [PubMed] [Google Scholar]
- 53.Starnes HM, Rock KD, Jackson TW & Belcher SM A Critical Review and Meta-Analysis of Impacts of Per- and Polyfluorinated Substances on the Brain and Behavior. Frontiers in Toxicology 4 (2022). 10.3389/ftox.2022.881584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health. A Scoping Review 13, 1479–1492 (2018). 10.2215/cjn.04670418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang C-H, Glover KP & Han X Characterization of Cellular Uptake of Perfluorooctanoate via Organic Anion-Transporting Polypeptide 1A2, Organic Anion Transporter 4, and Urate Transporter 1 for Their Potential Roles in Mediating Human Renal Reabsorption of Perfluorocarboxylates. Toxicological Sciences 117, 294–302 (2010). 10.1093/toxsci/kfq219 [DOI] [PubMed] [Google Scholar]
- 56.Zhao W, Zitzow JD, Ehresman DJ, Chang SC, Butenhoff JL, Forster J, Hagenbuch B. Na+/Taurocholate Cotransporting Polypeptide and Apical Sodium-Dependent Bile Acid Transporter Are Involved in the Disposition of Perfluoroalkyl Sulfonates in Humans and Rats. Toxicological Sciences 146, 363–373 (2015). 10.1093/toxsci/kfv102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao W, Zitzow JD, Weaver Y, Ehresman DJ, Chang SC, Butenhoff JL, Hagenbuch B. Organic Anion Transporting Polypeptides Contribute to the Disposition of Perfluoroalkyl Acids in Humans and Rats. Toxicol Sci 156, 84–95 (2017). 10.1093/toxsci/kfw236 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


