Abstract
Purpose
To compare amblyopia treatment outcomes between patients with neurodevelopmental disorders and their typically developing peers.
Methods
Of 2,311 patients diagnosed with amblyopia between 2010 and 2014 at Boston Children’s Hospital, 460 met inclusion criteria (age 2–12 with anisometropic, strabismic, or mixed amblyopia [interocular difference (IOD) ≥2 lines]). Treatment and visual outcomes were analyzed according to neurodevelopmental status: neurodevelopmental delay (DD) versus typical development (TD).
Results
The DD group (n = 54) and TD group (n = 406) were similar in demographics, amblyogenic risk factors, baseline visual measures, prescribed therapy, and adherence (P ≥ 0.10). Between-visit follow-up time was longer for the DD group (0.65 [0.42– 0.97] years) than for the TD group (0.5 [0.36–0.82] years; P = 0.023). IOD improved similarly in each group by the last visit (DD, −0.15 logMAR [−0.31 to −0.02]; TD, −0.2 logMAR [−0.38 to −0.1]; P = 0.09). Each group reached amblyopia resolution by the last visit at similar frequencies (DD, 23/54 [43%]; TD, 211/406 [52%]; P > 0.2). DD diagnosis did not independently influence amblyopia resolution (HR, 0.77; 95% CI, 0.53–1.12; P = 0.17), but each additional month of interval time between follow-up visits reduced the likelihood of resolution by 2.7% (HR, 0.67; 95% CI, 0.51–0.87; P = 0.003).
Conclusions
Patients with DD and those with TD responded similarly to amblyopia therapy; however, follow-up intervals were longer in patients with DD and correlated with the likelihood of persistent amblyopia, suggesting that greater efforts at assuring follow-up may benefit patients with DD.
Ophthalmic disorders occur more frequently in children with neurodevelopmental disorders, including those along the autism spectrum.1,2 Population-based data indicate that 10%−13% of children with neurodevelopmental disorders harbor an ophthalmologic diagnosis, compared with 3.5% of typically developing children.3 The prevalence of strabismus and clinically significant refractive errors are higher among these patients than in typically developing children.1,2,4,5 Thus, children with neurodevelopmental disorders are also at increased risk for amblyopia,6,7 a common form of visual impairment with a limited window for effective treatment.8 How neurodevelopmental delay conveys this increased risk for amblyopia is unclear, but the fact that amblyopia is also considered a neurodevelopmental disorder of vision may suggest a shared mechanism of dysfunctional synaptic plasticity.
We sought to determine how amblyopia treatments and outcomes for patients with neurodevelopmental disorders compare with those of neurotypical children. To our knowledge, no study to date has directly addressed these questions.1,3,7 We hypothesized that patients with neurodevelopmental disorders would have relatively worse outcomes compared with their neurotypical counterparts. Applying a large, established, institutional retrospective database of patients with amblyopia,9–13 we compared amblyopia treatment and outcomes between patients with and without a neurodevelopmental disorder diagnosis in an effort to guide a tailored approach to ophthalmic care in this vulnerable population.
Subjects and Methods
This retrospective, comparative cohort study was conducted at Boston Children’s Hospital, was approved by the Boston Children’s Hospital institutional review board, adhered to the tenets of the Declaration of Helsinki, and complied with the US Health Insurance Portability and Accountability Act of 1996.
A database of all patients billed for a diagnosis of amblyopia (ICD 9 codes 368.00–368.03) between 2010 and 2014 was created. Details of this database, described as the Boston Amblyopia Study, are provided by Shoshany and colleagues.10,11 In brief, inclusion criteria were patients ages 2 through 12 years at the time of presentation with amblyopia, which was defined as an interocular difference (IOD) of ≥2 logarithm of the minimum angle of resolution (logMAR) lines. Amblyopia severity was categorized as “moderate” (20/40–20/80 best-corrected visual acuity [BCVA]) of the amblyopic eye) and “severe” (>20/80 BCVA of the amblyopic eye). Patients with “mild” (<20/40 BCVA of the amblyopic eye) amblyopia were excluded.14,15,16 Three amblyopia subtypes were defined: (1) “anisometropic,” ≥1.00 D difference between eyes in spherical equivalent (SE) or ≥1.50 D difference in astigmatism between corresponding meridians in the two eyes (using cycloplegic refraction); (2) “strabismic,” presence of a heterotropia on examination at distance or near fixation (with or without optical correction); or (3) “mixed,” met criteria for strabismic and anisometropic amblyopia. Patients with amblyopia resulting from visual deprivation (ptosis, aphakia, etc) or not meeting the inclusion criteria above were excluded.
Clinical data collected from each visit included patient demographics, ocular history, past amblyopia treatment, best corrected visual acuity, sensorimotor evaluation, stereopsis measurement, cycloplegic refraction, treatment recommendation and adherence, treatment length, requested visit follow-up time, and actual visit follow-up time. BCVA measures were obtained using Snellen, HOTV, or LEA optotypes (linear, crowding bars, or single letters) based on the patient’s age and literacy.
Billing codes relevant to neurodevelopmental diagnoses were queried and collected for all patients from the hospital’s billing database, using ICD-10 codes or ICD-9 equivalents as required. Patients were divided into two groups: (1) neurodevelopmental disorder (DD), and (2) typical development (TD). DD patients were preliminarily identified if their record contained any of the following billing codes: autism spectrum disorders (ICD-10: F84); language/speech delays (ICD-10: F80); motor delays (ICD-10: F82); behavioral disorders (ICD-10: F90–98); intellectual disabilities (ICD-10: F70–79); in utero–, neonatal-. or infancy-related diagnoses associated with neurodevelopmental delays (ICD-10: O60–77, O85–92, P00-P96); and chromosomal or genetic syndromes known to be associated with neurodevelopmental disorders (ICD-10: Q90–99) and confirmed on medical record review. Patient records were also categorized into subgroups based on the ICD code category. Patient records that contained billing codes from multiple ICD billing categories were included in each ICD billing category in which they possessed a billing code. After DD patients were identified by billing codes, their records were manually reviewed to confirm the diagnosis. TD patients were defined as amblyopia patients who did not possess a neurodevelopmental diagnostic billing code. Any patient records with diagnostic ambiguity regarding the nature or origin of the neurodevelopmental delay was reviewed and discussed by three coauthors (RNC, CLW, and EDG) and resolved.
Statistical Analysis
To avert potential confounding effects of neurodevelopmental status on visual acuity testing, we chose IOD as our primary outcome measure. Amblyopia resolution was defined as the first visit where the patient’s IOD was measured as <2 logMAR lines. Changes in visual acuity, IOD, and stereopsis were assessed at an interval of 6 ± 3 months (for those who had a visit in that time frame) and at the final follow-up visit on treatment.
Patient demographics, treatment characteristics, visual measures (visual acuity and IOD), and stereopsis were recorded as frequency and percentage for categorical data and median and interquartile range for continuous data and analyzed using nonparametric methods. Comparisons between patient groups were performed using the χ2 test, the Fisher exact test, or the Wilcoxon rank-sum test, as indicated. Analysis of time to amblyopia resolution was performed using Kaplan-Meier curve analysis and log-rank testing. Adjusted hazard ratios were computed using a multivariable Cox proportional hazards regression model including the following variables: patient group, amblyopia severity, amblyopia mechanism, glasses adherence, patching adherence, atropine adherence, previous amblyopia treatment, and average follow-up interval. A post hoc power calculation revealed that the achieved sample size of 460 patients provides 80% power for detecting a hazard ratio of 0.77 (conversely 1.30) based on Cox proportional hazards regression modeling (two-tailed, 0.05 alpha level). Statistical analyses were performed using R (version 4.02, R Foundation for Statistical Computing, Vienna, Austria) and RStudio (version 1.3.1073, RStudio PBC, Boston, MA) as described previously.10 Power calculations were performed using Stata (version 16.0; StataCorp LLC, College Station, TX).
Results
Of the 2,311 patients collected in the database, 460 met study inclusion/exclusion criteria. Median (interquartile range) presenting age was 5.4 (4.2, 6.8) years, with 222 female patients (48.3%) and 177 patients (38.5%) having received amblyopia treatment (glasses or occlusion) prior to presentation. Median presenting amblyopic eye BCVA was 0.52 (0.40, 0.70) logMAR lines, equivalent to 20/66 (20/50, 20/100) Snellen acuity; median IOD was 0.4 (0.3, 0.6) logMAR lines, and median stereopsis was 5.99 (4.61, 9.21) log arcsec, equivalent to 400 (100, 10,000) arcsec.
Of the 460 patients in our sample, 54 (11.7%) carried a DD diagnosis. Of note, 18 (33%) of the DD patients’ diagnoses were of a genetic etiology (chromosomal or monogenetic), and 14 (26%) were along the autism spectrum (Table 1). There were no significant differences between TD and DD groups for presenting age (eSupplement 1, available at jaapos.org), sex, race, ethnicity, family history of amblyopia, mechanism of amblyopia, severity of amblyopia, prior treatment history, first visit BCVA in the amblyopic eye, IOD, log stereopsis, amblyopia treatment prescription rates and compliance, length of treatment, number of visits, or provider-requested follow-up times (Tables 2 and 3).
Table 1.
Frequency of neurodevelopmental diagnoses
| Diagnoses | Frequency, no. (%)a N = 54 |
|---|---|
| Developmental delay not otherwise specified | 19 (35) |
| Chromosomal/genetic abnormalities | 18 (33) |
| Speech and language delay | 18 (24) |
| Autism spectrum disorders | 14 (26) |
| In utero–, neonatal-, infancy incident–related delays | 11 (20) |
| Motor delay | 6 (11) |
Patients may possess multiple neurodevelopmental diagnoses.
Table 2.
Demographics, patient characteristics, and treatment data in all patients 2–12 years of age
| Variable | Typically developinga | Neurodevelopmental disordersa | P valueb |
|---|---|---|---|
| n = 406 | n = 54 | ||
| Age, years | 5.36 (4.57, 6.69) | 5.37 (4.15, 6.18) | 0.505 |
| Younger group (2–6) | 316 (77.8%) | 44 (81.5%) | 0.664 |
| Older group (7–12) | 90 (22.2%) | 10 (18.5%) | 0.664 |
| Sex | 0.107 | ||
| Female | 202 (49.8%) | 20 (37.0%) | |
| Male | 204 (50.2%) | 34 (63.0%) | |
| Race (n = 374) | (n = 321) | (n = 51) | 0.372 |
| White | 241 (74.6%) | 41 (80.4%) | |
| Non-White | 80 (24.8%) | 10 (19.6%) | |
| Ethnicity (n = 347) | (n = 301) | (n = 44) | 0.984 |
| Hispanic or Latino | 34 (11.2%) | 5 (11.4%) | |
| Not Hispanic or Latino | 267 (88.7%) | 39 (88.6%) | |
| Family history of amblyopia | 130 (31.9%) | 22 (40.7%) | 0.197 |
| Type of amblyopiac | 0.634 | ||
| Anisometropic | 243 (59.9%) | 29 (53.7%) | |
| Strabismic | 85 (20.9%) | 12 (22.2%) | |
| Mixed | 78 (19.2%) | 13 (24.1%) | |
| Severity of amblyopia | 0.273 | ||
| Moderate: 20/40 ≤ 20/80 BCVA | 263 (66.0%) | 31 (57.4%) | |
| Severe: >20/80 BCVA | 138 (34.0%) | 23 (42.6%) | |
| Prior amblyopia treatment | 153 (37.7%) | 24 (44.4%) | 0.418 |
| Treatment compliance | |||
| Treated with glasses | 380 (93.6%) | 48 (88.9%) | 0.321 |
| Median glasses compliance | 100% (100%, 100%) | 100% (75%, 100%) | 0.356 |
| Treated with patching | 328 (80.8%) | 40 (74.1%) | 0.328 |
| Median patching compliance | 60% (25%, 100%) | 50% (20%, 100%) | 0.515 |
| Treated with atropine | 98 (24.1%) | 12 (22.2%) | 0.889 |
| Median atropine compliance | 75% (0%, 100%) | 75% (20%, 90%) | 0.718 |
BCVA, best-corrected visual acuity.
Data are presented as frequency (percentage) for categorical data and median (25th, 75th percentile) for continuous data.
Obtained using the χ2 test, Fisher exact test, or Wilcoxon rank-sum test, as appropriate.
Anisometropic: ≥1.00 D of spherical equivalent or ≥1.5 D of astigmatism between eyes. Strabismic: heterotropia upon exam. Mixed: meet criteria for anisometropic and strabismic.
Table 3.
Comparison of visual acuity outcomes at first, 6-month, and last visits
| Variable | Typically developinga | Neurodevelopmental disordersa | P valueb |
|---|---|---|---|
| First visit comparison | n = 406 | n = 54 | |
| Amblyopic eye VA, logMAR | 0.48 (0.4, 0.7) | 0.60 (0.4, 0.8) | 0.100 |
| Fellow eye VA, logMAR | 0.1 (0.0, 0.18) | 0.1 (0.0, 0.30) | 0.034c |
| IOD, logMAR | 0.4 (0.3, 0.6) | 0.4 (0.3, 0.6) | 0.604 |
| n = 350 | n = 38 | ||
| Log stereopsis | 5.99 (4.61, 9.21) | 8.01 (5.30, 9.21) | 0.101 |
| Analysis of improvement from first to 6-month visit | n = 276 | n = 35 | |
| Amblyopic eye VA, logMAR | 0.3 (0.18, 0.48) | 0.4 (0.3, 0.57) | 0.032c |
| Change in amblyopic eye VA, logMAR | −0.22 (−0.4, −0.12) | −0.18 (−0.37, −0.08) | 0.168 |
| Fellow eye VA, logMAR | 0.0 (0.0, 0.1) | 0.1 (0.0, 0.18) | 0.044c |
| Change in fellow eye VA, logMAR | 0.0 (−0.1, 0.0) | 0.0 (−0.09, 0.04) | 0.886 |
| IOD, logMAR | 0.2 (0.10, 0.38) | 0.22 (0.09, 0.41) | 0.678 |
| Change in IOD, logMAR | −0.20 (−0.33, −0.10) | −0.18 (−0.34, −0.05) | 0.200 |
| Percent with <0.20 (logMAR) of IOD | 130 (47.1%) | 14 (40.0%) | 0.539 |
| n = 241 | n = 27 | ||
| Log stereopsis | 4.61 (4.25, 8.01) | 5.99 (4.5, 9.21) | 0.123 |
| Change in log stereopsis | 0.0 (−1.2, 0.0) | 0.0 (0.0, 0.0) | 0.005c |
| Analysis of improvement at last visit | n = 406 | n = 54 | |
| Amblyopic eye VA, logMAR | 0.18 (0.1, 0.4) | 0.39 (0.18, 0.48) | 0.002c |
| Change in amblyopic eye VA, logMAR from first visit | −0.3 (−0.44,−0.12) | −0.23 (−0.4, −0.09) | 0.244 |
| Fellow eye VA, logMAR | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.18) | <0.001c |
| Change in fellow eye VA, logMAR | −0.08 (−0.12, 0.0) | 0.0 (−0.17, 0.0) | 0.510 |
| IOD, logMAR | 0.18 (0.08, 0.4) | 0.26 (0.1, 0.4) | 0.318 |
| Change in IOD (logMAR) from first visit | −0.2 (−0.38, −0.1) | −0.15 (−0.31, −0.02) | 0.087 |
| Percent with <0.20 (logMAR) of IOD | 211 (52.0%) | 23 (42.6%) | 0.250 |
| n = 367 | n = 42 | ||
| Log stereopsis | 4.61 (3.91, 8.01) | 5.30 (4.61, 8.91) | 0.028c |
| Change in log stereopsis from first visit | −0.32 (−1.38, 0.0) | 0.0 (−0.67, 0.0) | 0.042c |
| Treatment information | |||
| Treatment length, years | 2.27 (1.2, 4.36) | 2.56 (1.47, 4.75) | 0.160 |
| Number of visits | 6 (3, 9) | 6 (3, 10) | 0.868 |
| Average time requested between visits, years | 0.31 (0.25, 0.39) | 0.33 (0.25, 0.45) | 0.104 |
| Average follow-up time between visits, years | 0.5 (0.36, 0.82) | 0.65 (0.42, 0.97) | 0.023c |
IOD, interocular difference; LogMAR, logarithmic minimal angle of resolution; VA, visual acuity.
Data are presented as median (25th, 75th percentile) for continuous data and frequency (percentage) for categorical data.
Obtained using the Fisher exact test or Wilcoxon rank-sum test, as appropriate.
Denotes statistical significance.
Although there was no difference in average provider-requested follow-up times, dichotomization of follow-up times revealed patients with DDs were significantly more likely to have provider-requested follow-up intervals of ≥180 days (DD, 13/54 [24%]; TD, 49/406 [12%]; P = 0.027) and significantly less likely to have provider-requested follow-up intervals of <90 days (DD, 3/54 [6%]; TD, 72/406 [18%]; P = 0.038). Additionally, it took patients with DDs significantly longer time to return for their follow-up appointment compared to TD patients (DD: 0.65 [0.42, 0.97] years, TD: 0.5 [0.36, 0.82] years; P = 0.023). See Table 3.
Interim visits 6 ± 3 months after presentation were available for 311 of 462 patients: 276 of 406 TD (68%) and 35 of 54 DD (65%) (P > 0.6). The proportion of each group included at the 6-month visit timepoint was not different between groups (TD, 276/406 [68%]; DD, 35/54 [65%]; P = 0.641, χ2 test). Amblyopic eye visual acuity improved similarly between TD and DD groups (TD, −0.22 [−0.4, −0.12] logMAR lines; DD, −0.18 [−0.37, −0.08] logMAR lines; P = 0.17). See Figure 1A. IOD improved similarly between groups (TD, −0.20 [−0.33, −0.10] logMAR lines; DD, −0.18 [−0.3, −0.05] logMAR lines; P = 0.20). See Figure 1B. By the 6-month visit, 47% of TD patients and 40% of patients with DDs reached amblyopia resolution criteria (P > 0.54). See Table 3. TD patients had greater improvement in stereoacuity compared to patients with DDs (TD, 0.0 [−1.2, 0.0] log arcsec; DD, 0.0 [0.0, 0.0] log arcsec; P = 0.005).
FIG 1.

Comparison of change in amblyopic eye best-corrected visual acuity (A) and change in interocular difference in typically developing patients versus patients with neurodevelopmental disorder at the 6-month visit and last visit (B). Box plots describe the median, 25th and 75th percentiles, with tails representing all other data and outliers are displayed as individual data points. Improvement in amblyopic eye visual acuity did not differ between groups at either visit.
At the last visit, both groups had similar improvements in IOD and similar proportions of patients who reached amblyopia resolution (P values ≥ 0.08). See Table 3 and Figure 1B. Although improvements in amblyopic and fellow eye BCVA did not differ between groups (P values > 0.2), TD patients had significantly better final BCVAs compared with patients with DDs for both amblyopic (TD, 0.18 [0.1, 0.4] logMAR lines; equivalent to 20/30 [20/25, 20/50] Snellen acuity; DD: 0.39 [0.18, 0.48] logMAR lines; P = 0.002; equivalent to 20/50 [20/30, 20/60] Snellen acuity) and fellow eyes (TD, 0.0 [0.0, 0.0] logMAR lines; equivalent to 20/20 [20/20, 20/20]; DD, 0.0 [0.0, 0.18] logMAR lines; equivalent to 20/20 [20/20, 20/30]; P < 0.001). See Table 3. TD patients had significantly greater improvement in stereoacuity compared with patients with DDs (TD, −0.32 [−1.38, 0.0] log arcsec; DD, 0.0 [−0.67, 0.0] log arcsec; P = 0.042). Consequently, TD patients had better stereoacuity measures at their last visit compared to patients with DDs (TD: 4.61 [3.91, 8.01] log arcsec, equivalent to 100 [50, 3000] arcsec; DD: 5.30 [4.61, 8.91] log arcsec, equivalent to 200 [100, 7400]; P = 0.028). Median time to resolution of amblyopia was similar between groups (TD, 0.60 [0.3, 1.15] years; DD, 0.60 [0.26, 1.18] years; P > 0.7). Modified survival analysis controlling for the potential confounding factor of disparate follow-up intervals between groups revealed that differences in the probability of amblyopia resolution did not reach the threshold for statistical significance (P = 0.07). See Figure 2.
FIG 2.
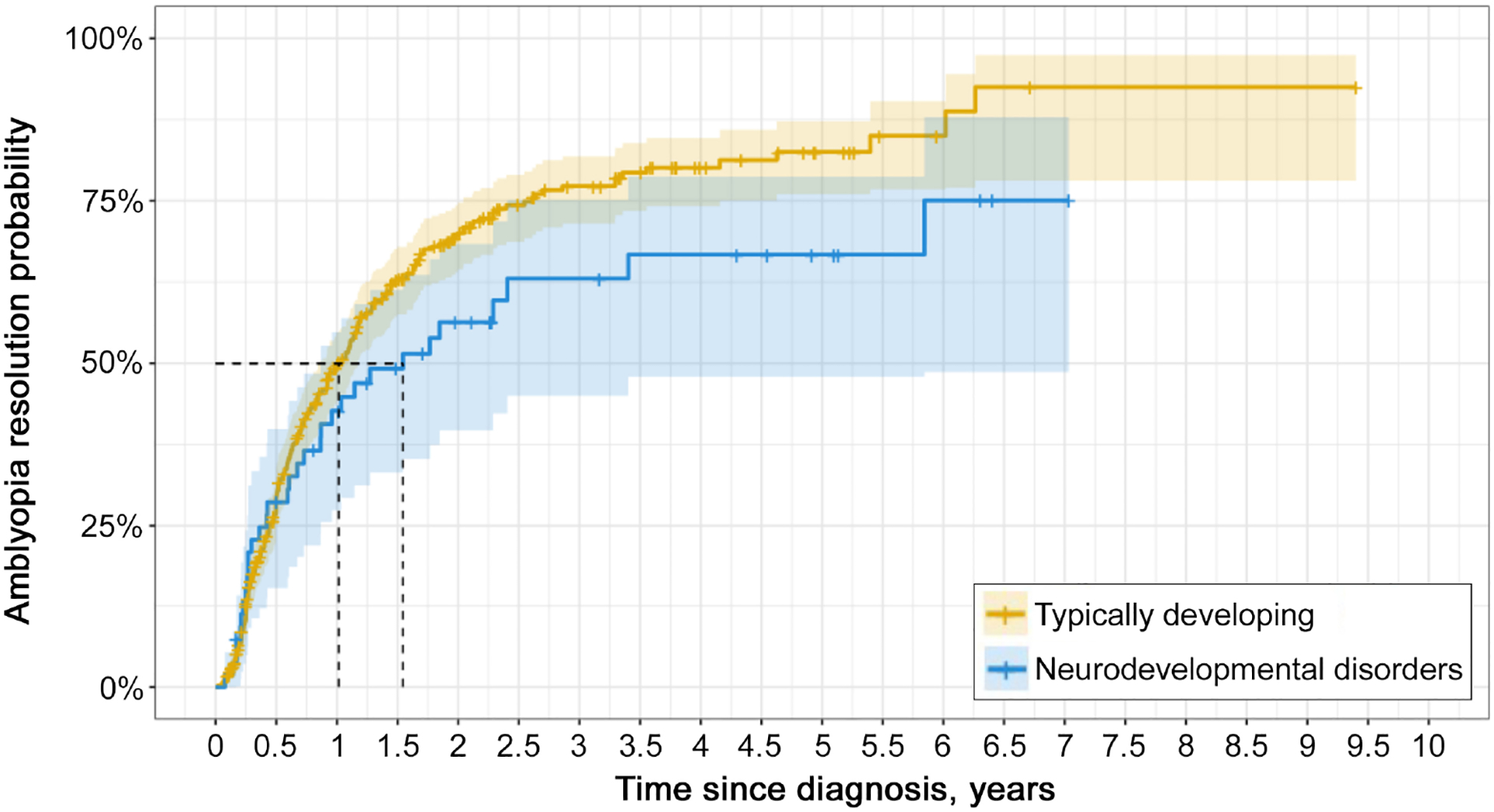
Kaplan-Meier curve of time to amblyopia resolution (IOD <2 logMAR lines) stratified by patient group. Solid lines represent the probability function of patients achieving amblyopia resolution over time, vertical ticks on the line represent the time point where a patient completed treatment without achieving resolution, and the shaded regions represent the 95% confidence intervals. Dashed black lines represent the median (50%) survival probability time points for each patient group. Log-ranked test comparing the two-survival function was not significant (P = 0.07).
Likelihood of amblyopia resolution was not associated with patient diagnostic group (DD vs TD) when evaluated using cox-proportional hazard modeling (HR, 0.77; 95% CI, 0.53–1.12; P = 0.17). Regardless of their diagnostic group membership, patients with >50% adherence to glasses treatment (HR, 1.50; 95% CI, 1.04–2.18; P = 0.031) and >50% adherence to patching treatment (HR, 1.39; 95% CI, 1.09–1.78; P = 0.008) were significantly more likely to have resolution of amblyopia by the final visit (Figure 3, eSupplement 2, available at jaapos.org). Patients with severe amblyopia (HR, 0.46; 95% CI, 0.35–0.60; P < 0.001), regardless of diagnostic group, were less likely to have resolution of amblyopia. Across both groups, every additional year of between-visit follow-up interval time contributed 33% lower likelihood of amblyopia resolution by the final visit, or 2.7% lower likelihood for each additional month of between-visit follow-up interval time (HR, 0.67; 95% CI, 0.51–0.87; P = 0.003). See Figure 3 and eSupplement 2. Amblyopic risk factor (strabismus and mixed mechanism vs. anisometropia), atropine adherence, and prior amblyopia treatment were not associated with the likelihood of amblyopia resolution (P values > 0.056).
FIG 3.
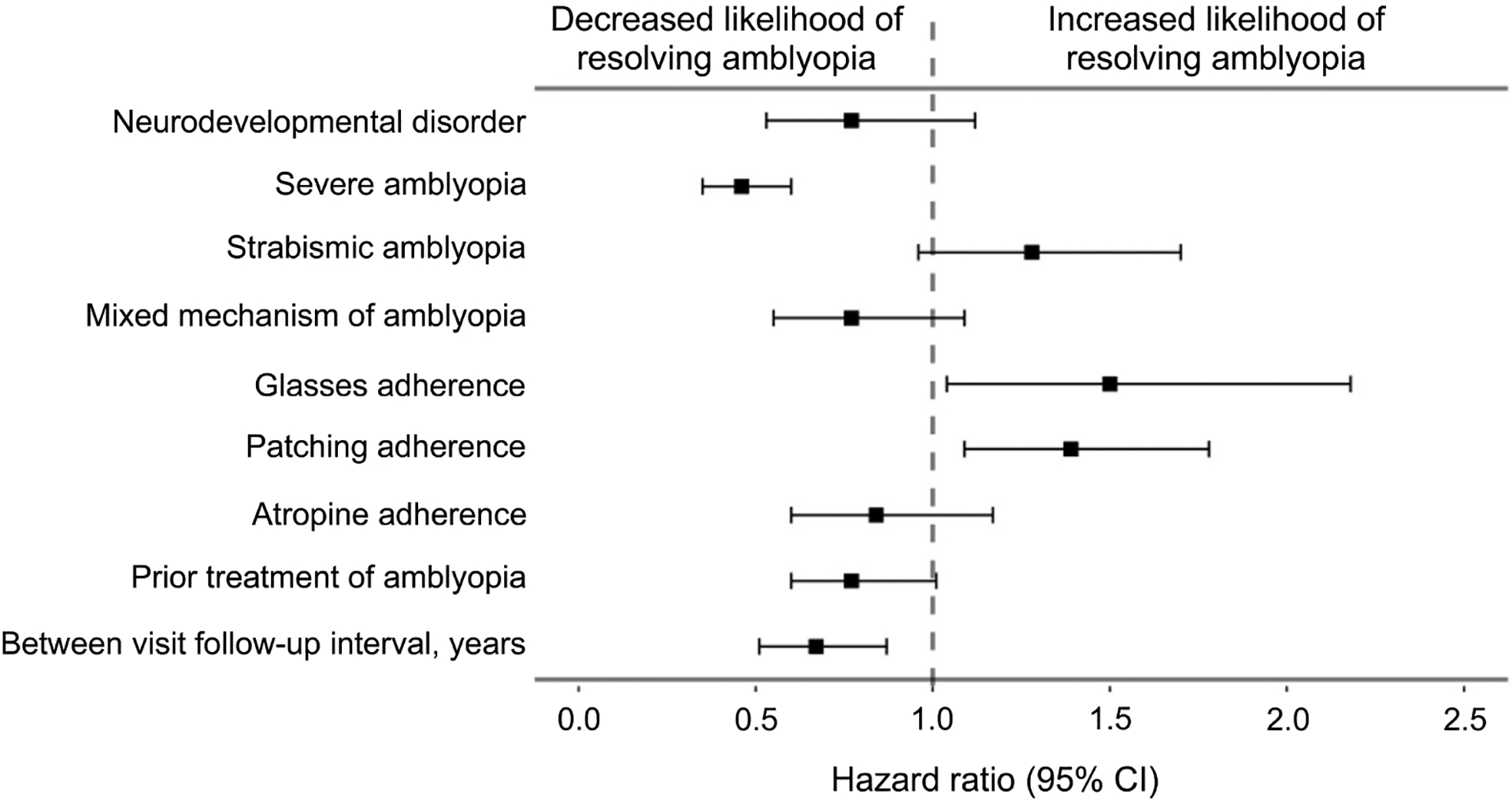
Forest plot of hazard ratios for amblyopia resolution in TD and DD patients. Boxes represent the calculated hazard ratios determined from Cox regression, and the left and right arms represent the 95% confidence interval of the hazard ratios.
Subgroup Analysis
We evaluated how outcomes of primary subgroups of the DD group, defined as (1) those with a genetic etiology (N = 18), and (2) those along the autism spectrum (N = 14), compared to outcomes of TD patients. Patients in the genetic DD subgroup were prescribed glasses significantly less often compared to their TD cohorts (genetic DD, 13 [72.2%]; P = 0.007), despite similar self-reported treatment adherence (genetic DD, 100% [100%,100%]; P = 0.914). Patients with genetic DDs had significantly less improvement in IOD from the first to last visit compared to the TD patients (genetic DD, −0.13 logMAR lines [−0.22, −0.01]; P = 0.016). The genetic DD group also had longer treatment duration compared to the TD group (genetic DD, 3.25 years [2.04, 5.13]; P = 0.034) and longer mean between-visit follow-up intervals (genetic DD: 0.68 years [0.46, 1.51]; P = 0.045). Modified univariate survival analysis revealed that amblyopia was significantly less likely to resolve in the genetic DD subgroup compared to the TD group (P = 0.022), though this association did not meet the statistical significance threshold on multivariable analysis (HR, 0.47; 95% CI, 0.22–1.01; P = 0.052).
Patients in the autism spectrum DD subgroup did not differ from the TD group in any demographics, visual measures, or treatment factors. Improvement in IOD (autism spectrum DD, −0.14 logMAR lines [−0.41, −0.07]; P = 0.495) and amblyopic eye BCVA (autism spectrum DD, −0.25 logMAR lines [−0.40, −0.07]; P = 0.387) from first to last visit were similar between the two groups. Stereoacuity improvement from first to last visit for patients in the autism spectrum DD subgroup did not significantly differ from the TD group (autism spectrum DD, 0.0 log arcsec [−0.51, 0.00]; P = 0.483). The autism spectrum DD group also had similar treatment duration (autism spectrum DD: 1.85 years [1.04, 5.01]; P = 0.905) and average between-visit follow-up intervals (autism spectrum DD, 0.70 years [0.42, 0.81]; P = 0.349) compared to the TD group.
Discussion
We found that despite carrying a neurodevelopmental disorder, patients with DDs resolved their amblyopia and improved amblyopic eye visual acuity at similar levels to TD patients. DD patients had worse visual acuity at the last visit. This may reflect lower visual acuity as a manifestation of DDs or limitations in visual acuity testing methodology and its reliance on verbal responses to questions that may be difficult for patients with DDs. Testing limitations also extend to stereopsis and therefore contribute to the differences reported in stereopsis measurements and improvement in the DD group. Our post hoc analysis demonstrated that the study was sufficiently powered to detect clinically meaningful differences in amblyopia treatment outcomes between groups. Thus, we conclude that ultimately patients with DDs may be as responsive to standard amblyopia treatments as their neurotypical cohorts.
Patients with DDs returned at longer follow-up intervals on average (7.8 months) compared to TD patients (6.0 months), despite similar requested follow-up times from their eye care providers, similar treatment lengths, and number of eye care visits. Shoshany and colleagues9 recently identified requested follow-up time as a potential modifier for decreasing lost-to-follow-up rates in amblyopia treatment. Acknowledging that requested follow-up intervals were not different between groups or predictive of amblyopia resolution in our study, the requested time for follow-up is the only modifiable factor within the provider’s control. Our study design did not allow us to test why patients with DDs were seen at longer intervals. We speculate that there is a higher burden of care coordination for patients with DDs and their families, whether because of additional healthcare-related visits or otherwise. Providers should be aware of how follow-up delays may impact outcomes (decreasing likelihood of amblyopia resolution by 3% per month) and the vulnerability of patients with DDs in this regard.
Controlling for relevant factors of amblyopia treatment (amblyopia severity, etiology, treatment adherence, etc) and follow-up interval, testing of our hypothesis that DD patients are less likely to resolve amblyopia did not find differences that reached the threshold for statistical significance. However, the 6-month between-group difference in time to 50% probability of amblyopia resolution on survival analysis may be clinically meaningful. Overall, neurodevelopmental status does not contribute to the likelihood of eventual amblyopia resolution. Amblyopia resolution correlated as expected with amblyopia severity, follow-up interval, and adherence to therapy, further validating our conclusions regarding DD status and amblyopia treatment outcomes.17 Although we found no difference in amblyopia severity between groups in our sample, DD patients with more severe amblyopia may be more susceptible to suboptimal outcomes with prolonged follow-up intervals.
An important limitation of this study is the wide range of neurodevelopmental disorder causes and types, each of which were reflected by small samples that limited the power of subgroup analyses and outcome-relevant conclusions in specific DDs. We restricted our subgroup analysis to the most prevalent conditions (genetic and autism spectrum DDs), which revealed contrasting outcomes relative to TD patients. Patients in the autism spectrum DD subgroup did not differ significantly from the TD group in any demographics, visual measures, or treatment factors. However, the genetic DD subgroup was prescribed glasses less often and showed less than two lines of IOD improvement from first to last visit. They had longer between-visit follow-up intervals and lengths of treatment compared to the TD group. While univariate analysis revealed they were ultimately less likely to resolve amblyopia, in multivariate analysis the association did not meet the threshold for statistical significance. We attempted to control for additional potential confounders, including age at diagnosis, initial visual acuity, and length of follow-up, by using Kaplan-Meier analyses, log-rank testing, and a multivariable Cox proportional hazard regression where appropriate. Further investigations should consider the types of DDs and timing of DD diagnosis relative to amblyopia that deserve dedicated individual study.
Additional limitations of this retrospective study preclude specific treatment recommendations. Current methods of assessing visual acuity and stereopsis may hinder their accuracy in DD patients. Indeed, patients with DD had worse fellow eye visual acuity at baseline, 6-months, and final follow-up compared to TD patients (Table 3). To address this concern, we focused our outcome data on IOD rather than amblyopic eye visual acuity. We also separately examined outcomes as a function of severity.3 As with any retrospective amblyopia study, we could not objectively measure adherence to glasses or amblyopia treatment, which are known to vary from reports by families when measured objectively,18 and there is no data as to whether self-assessment of families of DD patients would be more or less accurate.
Available published literature on amblyopia in the setting of neurodevelopmental disorders focuses on the prevalence of amblyopia and its risk factors.3,6,7 Amblyopia (in conjunction with refractive and/or strabismic risk factors) is prevalent (10%−26%) among patients with DDs.1,4,19 In this study, we found the DD group comprised a subset (11.7%) of the total sample. While this study was powered to detect clinically meaningful differences in primary outcomes, our cohorts may not have been large enough to detect small increases in susceptibility of DD patients to suboptimal outcomes. On the other hand, the overall lack of a clear difference in amblyopia treatment outcome highlights that prescription of amblyopia treatment should not be withheld because of a concomitant DD. Indeed, the data suggest that greater effort to assure prompt follow-up may benefit these patients.
In conclusion, patients with DDs ultimately show similar responses to amblyopia therapy when compared to their TD peers. While care delivery for these patients may be more complex at several levels, it is important that patients with DDs are provided the clinical standard of care for amblyopia. Providers should anticipate and account for longer follow-up intervals in patients with DDs despite specifying the same timeframe as would be recommended for TD peers. Future studies focusing on larger numbers of patients in more narrowly defined cohorts could overcome the limitations of the present study and provide helpful information on treatment strategies and visual outcomes in this vulnerable population.
Literature Review
Literature search was performed in PubMed, without date restriction, on October 22–29, 2022, using the terms amblyopia and neurodevelopmental disorders. We reviewed all citations for reports of amblyopia outcomes in patient cohorts with neurodevelopmental disorders identified by the literature search. Foreign literature for which an abstract was available in English was also reviewed.
Supplementary Material
Grant Support:
Children’s Hospital Ophthalmology Foundation (RNC, DGH, EDG); NIH K08 EY030164 (EDG).
Financial disclosures:
Dr. David G. Hunter is an advisor and equity owner of Luminopia Inc and Rebion Inc. Dr. Eric D Gaier is an advisor, patent holder, and equity owner of Luminopia Inc, and a consultant to Stoke Therapeutics Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as a poster at the 46th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, April 9–11, 2021.
References
- 1.Ikeda J, Davitt BV, Ultmann M, Maxim R, Cruz OA. Brief report: incidence of ophthalmologic disorders in children with autism. J Autism Dev Disord 2013;43:1447–51. [DOI] [PubMed] [Google Scholar]
- 2.Hegde V, Jain R, Bappal A, Shambhu R. Ocular manifestations in children with developmental delay at a tertiary center in South India. Saudi J Ophthalmol 2021;35:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang MY, Doppee D, Yu F, Perez C, Coleman AL, Pineles SL. Prevalence of ophthalmologic diagnoses in children with autism spectrum disorder using the Optum dataset: a population-based study. Am J Ophthalmol 2021;221:147–53. [DOI] [PubMed] [Google Scholar]
- 4.Black K, McCarus C, Collins MLZ, Jensen A. Ocular manifestations of autism in ophthalmology. Strabismus 2013;21:98–102. [DOI] [PubMed] [Google Scholar]
- 5.Kabatas EU, Ozer PA, Ertugrul GT, Kurtul BE, Bodur S, Alan BE. Initial ophthalmic findings in Turkish children with autism spectrum disorder. J Autism Dev Disord 2015; 45:2578–81. [DOI] [PubMed] [Google Scholar]
- 6.Chang MY, Gandhi N, O’Hara M. Ophthalmologic disorders and risk factors in children with autism spectrum disorder. J AAPOS 2019;23:337.e1–6. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen LS, Skov L, Jensen H. Visual dysfunctions and ocular disorders in children with developmental delay. I. Prevalence, diagnoses and aetiology of visual impairment. Acta Ophthalmol Scand 2007;85:149–56. [DOI] [PubMed] [Google Scholar]
- 8.Holmes JM, Levi DM. Treatment of amblyopia as a function of age. Vis Neurosci 2018;35:E015. [DOI] [PubMed] [Google Scholar]
- 9.Shoshany TN, Chinn RN, Staffa SJ, Bishop K, Michalak S, Hunter DG. Identifying characteristics predictive of lost-to-follow-up status in amblyopia. Am J Ophthalmol 2021;230:200–206. [DOI] [PubMed] [Google Scholar]
- 10.Shoshany TN, Michalak S, Staffa SJ, Chinn RN, Bishop K, Hunter DG. Effect of primary occlusion therapy in asymmetric, bilateral amblyopia. Am J Ophthalmol 2020;211:87–93. [DOI] [PubMed] [Google Scholar]
- 11.Shoshany T The Boston Amblyopia Project: analyzing real-world outcomes in amblyopia treatment, identifying major challenges in management, and characterizing unique patient subgroups through a large retrospective database. Published online June 24, 2020.
- 12.Shoshany TN, Michalak S, Chinn R, Staffa SJ, Hunter DG. Evaluating amblyopia treatment success using the AAO IRIS50 measures. Ophthalmology 2020;127:836–8. [DOI] [PubMed] [Google Scholar]
- 13.Chinn R, Michalak S, Shoshany T, Bishop K, Staffa S, Hunter D. Effect of sequential and simultaneous patching regimens in unilateral amblyopia. Am J Ophthalmol 2022;233:48–56. [DOI] [PubMed] [Google Scholar]
- 14.Michalak SM, Chinn RN, Shoshany TN, Bishop K, Staffa SJ, Hunter DG. Subthreshold amblyopia: characterization of a new cohort. Am J Ophthalmol 2023; 251:156–64. [DOI] [PubMed] [Google Scholar]
- 15.Repka MX, Kraker RT, Holmes JM, et al. Atropine vs patching for treatment of moderate amblyopia: Follow-up at 15 years of age of a randomized clinical trial. JAMA Ophthalmol 2014;132:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck RW. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology 2003;110:2075–87. [DOI] [PubMed] [Google Scholar]
- 17.Wallace DK, Repka MX, Lee KA, et al. Amblyopia Preferred Practice Pattern®. Ophthalmology 2018;125:P105–P142. [DOI] [PubMed] [Google Scholar]
- 18.Scheiman MM, Hertle RW, Kraker RT, et al. Patching vs atropine to treat amblyopia in children aged 7 to 12 years: a randomized trial. Arch Ophthalmol 2008;126:1634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sing Lok Lau C, Man Kit Tong J, Wai Ho Tang E, Kai Wang Li K. Ocular features and autism spectrum disorder: a 10-year retrospective review. Indian Pediatr 2022;59:581–2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


