Abstract
We determined the inhibitory activities of gatifloxacin against Staphylococcus aureus topoisomerase IV, Escherichia coli DNA gyrase, and HeLa cell topoisomerase II and compared them with those of several quinolones. The inhibitory activities of quinolones against these type II topoisomerases significantly correlated with their antibacterial activities or cytotoxicities (correlation coefficient [r] = 0.926 for S. aureus, r = 0.972 for E. coli, and r = 0.648 for HeLa cells). Gatifloxacin possessed potent inhibitory activities against bacterial type II topoisomerases (50% inhibitory concentration [IC50] = 13.8 μg/ml for S. aureus topoisomerase IV; IC50 = 0.109 μg/ml for E. coli DNA gyrase) but the lowest activity against HeLa cell topoisomerase II (IC50 = 265 μg/ml) among the quinolones tested. There was also a significant correlation between the inhibitory activities of quinolones against S. aureus topoisomerase IV and those against E. coli DNA gyrase (r = 0.969). However, the inhibitory activity against HeLa cell topoisomerase II did not correlate with that against either bacterial enzyme. The IC50 of gatifloxacin for HeLa cell topoisomerase II was 19 and was more than 2,400 times higher than that for S. aureus topoisomerase IV and that for E. coli DNA gyrase. These ratios were higher than those for other quinolones, indicating that gatifloxacin possesses a higher selectivity for bacterial type II topoisomerases.
Quinolone antibacterial agents have potent activity against gram-positive and -negative bacteria and are currently used as therapy for various bacterial infections. The antibacterial activities of quinolones are related in their inhibitory activities against DNA gyrase and topoisomerase IV (3, 13, 28). Both enzymes are members of the type II topoisomerase family that controls bacterial DNA topology by passing a DNA double helix through another, by using a transient double-strand break (18).
It has recently been reported that the primary target of several quinolones in Escherichia coli is DNA gyrase (8, 13, 16, 17) and that in Staphylococcus aureus is topoisomerase IV (2, 4, 6, 22, 31). Quinolones also inhibit mammalian type II topoisomerases such as topoisomerase II (11, 12, 14, 21, 25), and their inhibitory potencies for topoisomerase II have been correlated with their cytotoxicity (25). Therefore, it is important to determine the inhibitory activities of quinolones against bacterial and mammalian type II topoisomerases to clarify the selective toxicities.
Gatifloxacin (AM-1155), a newly developed quinolone, has shown potent activity against gram-positive and -negative bacteria, and it has been reported that gatifloxacin inhibits DNA gyrase of E. coli, Pseudomonas aeruginosa, Micrococcus luteus, and S. aureus, like other quinolones (10, 30). However, little is known about the inhibitory activities of gatifloxacin against S. aureus topoisomerase IV and HeLa cell topoisomerase II. In this study, we determined the inhibitory activity of gatifloxacin against bacterial primary target enzymes and mammalian topoisomerase II, and herein we discuss its selectivity.
(A part of this work was presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October 1997.)
MATERIALS AND METHODS
Quinolones.
Gatifloxacin, ciprofloxacin, clinafloxacin, fleroxacin, levofloxacin, lomefloxacin, norfloxacin, ofloxacin, sparfloxacin, tosufloxacin, and pipemidic acid were synthesized by our company. Enoxacin and nalidixic acid were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Bacterial strains, cell line, and plasmids.
S. aureus MS5935 is a quinolone-susceptible clinical isolate, as reported previously (9). E. coli NIHJ JC-2 and HeLa cells were obtained from stocks in our laboratory. E. coli BL21(DE3) and pET11a were purchased from Stratagene (La Jolla, Calif.). pRSET A and kinetoplast DNA were purchased from Invitrogen (San Diego, Calif.) and TopoGEN Inc. (Columbus, Ohio), respectively. Supercoiled pBR322 was prepared by the cesium chloride density gradient method and was relaxed by topoisomerase I (prepared from HeLa cells in our laboratory) (19).
Preparation of type II topoisomerases.
The grlA and grlB genes of S. aureus encoding both subunits of topoisomerase IV were amplified by PCR. PCR was performed with genomic DNA from S. aureus MS5935 by using eLONGase enzyme mix (GIBCO BRL, Rockville, Md.) with the oligonucleotides 5′-GGAATTCCATATGAGTGAAATAATTCAAGATT-3′ and 5′-CGGGATCCATTATGTGGTGGTATATCTGTCGC-3′ as primers for the first half of grlA, 5′-CCTAACTTACTAGTGAATGGTTCTACAGG-3′ and 5′-CGGGATCCTAGTTTTTAGCTAATATACATGTCTAT-3′ for the second half of grlA, and 5′-GGCGAAATCATTGCATATGAATAAACAAAATAATTATTCAGATGAT TCAATACAGG-3′ and 5′-CTTGAATTAGGATCCTCACTAGATTTCCTC-3′ for grlB. Based on the sequence published by Ferrero et al. (4), the primers were designed to introduce a NdeI site at the initiation codon and a BamHI site downstream from the termination codon. DNA was amplified for 30 cycles of 1 min at 95°C for denaturation, 1 min at 55°C for annealing, and 2 min at 70°C for polymerization. The first half and the second half of the grlA fragment were digested with NdeI and PstI and with BamHI and PstI, respectively. The grlB fragment and vector plasmids pET11a and pRSET A were digested with both NdeI and BamHI. Ligation was performed to construct grlA-pET11a (pKY3403) and grlB-pRSET A (pKY3405). GrlA and GrlB were individually induced by the addition of IPTG (isopropyl-1-thio-β-d-galactopyranoside) to cultures of E. coli BL21(DE3) transformed by pKY3403 and pKY3405, respectively. Cell extracts of BL21(DE3)/pKY3403 and BL21(DE3)/pKY3405 were prepared by the procedure of Peng and Marians (26). Fully active topoisomerase IV was reconstituted by preincubating GrlA and GrlB on ice for at least 30 min.
DNA gyrase from E. coli NIHJ JC-2 was prepared by affinity chromatography on novobiocin-Sepharose by the method of Sato et al. (27).
Topoisomerase II from HeLa cells was obtained by the method of Miller et al. (19).
Enzyme assay.
The decatenation activity of the reconstituted topoisomerase IV was determined by the method of Peng and Marians (26) with minor modifications. The reactions were analyzed by electrophoresis, and DNA quantification in agarose gels was carried out after ethidium bromide staining. The brightness of the bands corresponding to decatenated monomers of kinetoplast DNA was determined by densitometric analysis with FMBIO II Multi-View (Hitachi Software Engineering Co., Ltd., Yokohama, Japan).
The supercoiling activity of DNA gyrase was determined by the method of Gellert et al. (7) with minor modifications. Analysis was performed as described for the topoisomerase IV assay.
The relaxation activity of topoisomerase II was determined by the method of Oomori et al. (25).
The inhibitory effect of each quinolone on type II topoisomerase was assayed by determining the concentration required to inhibit 50% of the enzyme reaction (IC50). Selectivity was determined by dividing the IC50 for HeLa cell topoisomerase II by the IC50 for bacterial type II topoisomerase.
Determination of MICs and cytotoxicities.
MICs were measured by an agar dilution method (10) with Mueller-Hinton medium (Difco Laboratories, Detroit, Mich.).
Growth inhibition of HeLa cells was determined by the procedure of Aggarwal et al. (1). Cytotoxicity was expressed as the concentration required to inhibit 50% of HeLa cell growth.
Statistical analysis.
Correlation was determined by a linear regression analysis. A P value of <0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
Inhibitory activities of quinolones for bacterial type II topoisomerases.
Bacterial and mammalian type II topoisomerases are known to be essential enzymes for cell growth (24). Based on genetic analysis, it has been reported that the primary targets of the quinolones tested so far in E. coli and S. aureus are DNA gyrase and topoisomerase IV, respectively (2, 4–6, 16, 17, 22, 31). In our study, gatifloxacin showed a higher inhibitory activity against S. aureus topoisomerase IV (IC50 = 13.8 μg/ml) and E. coli DNA gyrase (IC50 = 0.109 μg/ml) than did the other quinolones tested, except for clinafloxacin and ciprofloxacin (Table 1).
TABLE 1.
Inhibitory activities of quinolones against type II topoisomerases
| Quinolone | Activity (μg/ml) against:
|
|||||
|---|---|---|---|---|---|---|
|
S. aureus MS5935 topoisomerase IV
|
E. coli NIHJ JC-2 DNA gyrase
|
HeLa cell topoisomerase II
|
||||
| IC50 | MIC | IC50 | MIC | IC50 | Cytotoxicity | |
| Gatifloxacin | 13.8 | 0.05 | 0.109 | 0.0063 | 265 | 122 |
| Clinafloxacin | 3.23 | 0.025 | 0.045 | 0.0032 | 29.7 | 60.1 |
| Ciprofloxacin | 11.8 | 0.39 | 0.072 | 0.0032 | 34.4a | 105 |
| Sparfloxacin | 22.3 | 0.05 | 0.130 | 0.0125 | 102 | 72.5 |
| Ofloxacin | 35.7 | 0.39 | 0.211 | 0.0125 | 132a | 301 |
| Tosufloxacin | NDb | ND | 0.165 | 0.0125 | ND | ND |
| Levofloxacin | 19.9 | 0.20 | 0.186 | 0.0063 | ND | ND |
| Lomefloxacin | 35.2 | 0.39 | 0.171 | 0.025 | 238 | 216 |
| Norfloxacin | 26.0 | 0.78 | 0.309 | 0.025 | 87.3a | 139 |
| Fleroxacin | 45.7 | 0.39 | 0.378 | 0.025 | 193a | 349 |
| Enoxacin | 55.4 | 0.78 | 0.482 | 0.05 | 81.8 | 67.9 |
| Pipemidic acid | 350 | 25 | 3.18 | 0.78 | ND | ND |
| Nalidixic acid | 435 | 25 | 7.25 | 0.78 | 169a | 277 |
Data from previous study (25).
ND, not determined.
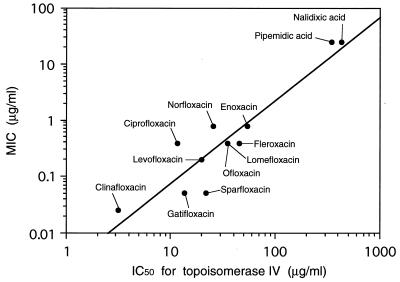
The inhibitory activities of quinolones against E. coli DNA gyrase have been shown to correlate with their antimicrobial activities (12). However, little is known about the relationship between the antimicrobial activities of quinolones and their inhibitory activity against S. aureus topoisomerase IV (29). In this study, we statistically determined the correlation between the inhibitory activity of many quinolones against S. aureus topoisomerase IV and their antibacterial activity and observed a significant correlation between not only the IC50s of the quinolones for E. coli DNA gyrase and their MICs (correlation coefficient [r] = 0.972, P < 0.01) but also the IC50s of the quinolones for S. aureus topoisomerase IV and their MICs (r = 0.926, P < 0.01) (Fig. 1). These results suggest that the antibacterial activities of the quinolones tested against E. coli and S. aureus are basically determined by the inhibition of DNA gyrase and topoisomerase IV, respectively.
FIG. 1.
Correlation between the IC50 for topoisomerase IV and the MIC for S. aureus (r = 0.926; P < 0.01).
The IC50 of gatifloxacin for S. aureus topoisomerase IV was almost equal to that of ciprofloxacin. However, the antibacterial activity of gatifloxacin against S. aureus was eight times higher than that of ciprofloxacin. A similar discrepancy was also observed between sparfloxacin and norfloxacin. These phenomena suggest that some factors besides the inhibition of topoisomerase IV influence the antibacterial activity of quinolones against S. aureus. These factors might include inhibition of S. aureus DNA gyrase and excretion mechanisms of quinolones in S. aureus, such as the quinolone efflux protein NorA (6, 15, 23).
Inhibitory activities of quinolones for mammalian topoisomerase II.
All the quinolones tested inhibited the relaxation activity of topoisomerase II from HeLa cells (Table 1). However, the IC50s of quinolones, except for nalidixic acid, for HeLa cell topoisomerase II were higher than those for both bacterial enzymes. The IC50s for topoisomerase II roughly correlated with the cytotoxicity (r = 0.648, P < 0.05). Therefore, it was suggested that the inhibitory activity of quinolones against topoisomerase II is one of the causes of the inhibition of HeLa cell growth. Gatifloxacin, with an IC50 of 265 μg/ml, showed the lowest inhibitory activity for topoisomerase II. In contrast, clinafloxacin and ciprofloxacin showed the highest inhibitory activities among the quinolones tested.
Selectivity of quinolones against bacterial and mammalian type II topoisomerases.
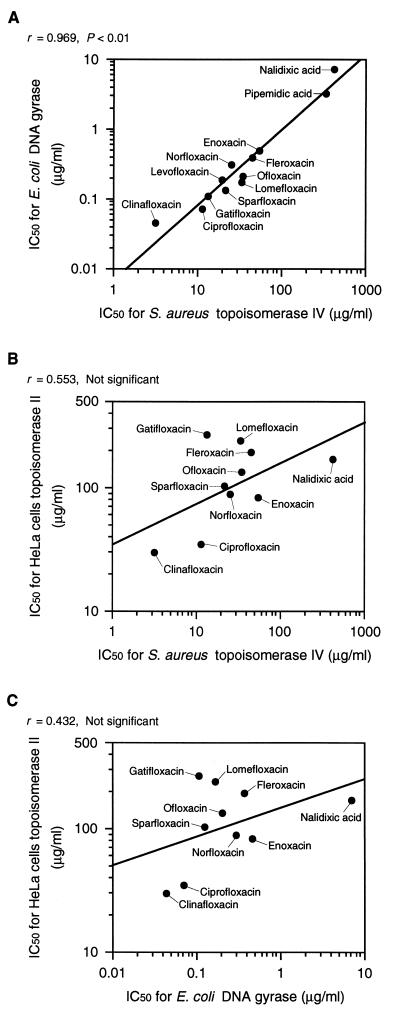
The correlations between the inhibitory activities for each type II topoisomerase are shown in Fig. 2.
FIG. 2.
Correlation between inhibitory activities against type II topoisomerases. (A) S. aureus topoisomerase IV vs. E. coli DNA gyrase; (B) S. aureus topoisomerase IV vs. HeLa cell topoisomerase II; (C) E. coli DNA gyrase vs. HeLa cell topoisomerase II. The correlation coefficient and significance are indicated at the top of each panel.
Ferrero et al. have reported that the S. aureus topoisomerase IV subunit GrlA and GrlB proteins have 32 and 49% identities with the E. coli DNA gyrase subunit GyrA and GyrB proteins, respectively (4). We showed that the inhibitory activities of quinolones against S. aureus topoisomerase IV closely correlate with those against E. coli DNA gyrase (r = 0.969, P < 0.01) (Fig. 2A). These data suggest that quinolones inhibit both enzymes in a similar manner. On the other hand, Hoshino et al. have reported that the inhibitory activities of various quinolones against E. coli DNA gyrase do not correlate with those against fetal calf thymus topoisomerase II (12). In this study, all the quinolones except nalidixic acid required concentrations (IC50s) to inhibit the topoisomerase II of HeLa cells that were higher than those needed to inhibit bacterial type II topoisomerases, and no significant correlation was observed between the inhibition of HeLa cell topoisomerase II and that of S. aureus topoisomerase IV as well as E. coli DNA gyrase (Fig. 2B and C). Morais Cabral et al. have reported differences in the rotation of the quaternary organization of the breakage-reunion domain, which includes the quinolone resistance-determining region, between prokaryotic (E. coli) DNA gyrase and eukaryotic (yeast) topoisomerase II (20). These differences might contribute to the only slight correlation between the inhibition of HeLa cell topoisomerase II and the inhibition of bacterial enzymes. Thus, there might be a large difference in the selectivities of the quinolones for bacterial and mammalian enzymes.
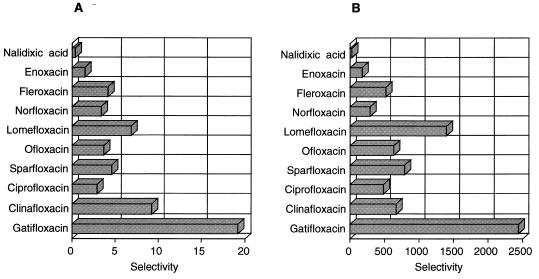
The selectivities of the quinolones are expressed in Fig. 3 as ratios of the IC50 for HeLa cell topoisomerase II to that for the bacterial enzyme. The IC50 of gatifloxacin for HeLa cell topoisomerase II was 19 and was more than 2,400 times higher than that for S. aureus topoisomerase IV and E. coli DNA gyrase. These ratios were higher than those for the other quinolones tested, indicating that gatifloxacin possesses a higher selectivity for bacterial type II topoisomerases.
FIG. 3.
Selectivity of quinolones against type II topoisomerases. Selectivity was calculated by dividing the IC50 for HeLa cell topoisomerase II by the IC50 for bacterial type II topoisomerase. (A) S. aureus topoisomerase IV vs. HeLa cell topoisomerase II; (B) E. coli DNA gyrase vs. HeLa cell topoisomerase II.
ACKNOWLEDGMENTS
We are grateful to Keiichi Hiramatsu (Department of Bacteriology, Juntendo University) for providing S. aureus MS5935. We also thank Eiji Wakabayashi for his technical support.
REFERENCES
- 1.Aggarwal B B, Moffat B, Harkins R N. Human lymphotoxin. J Biol Chem. 1984;259:686–691. [PubMed] [Google Scholar]
- 2.Blanche F, Cameron B, Bernard F-X, Maton L, Manse B, Ferrero L, Ratet N, Lecoq C, Goniot A, Bisch D, Crouzet J. Differential behaviors of Staphylococcus aureus and Escherichia coli type II DNA topoisomerases. Antimicrob Agents Chemother. 1996;40:2714–2720. doi: 10.1128/aac.40.12.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda H, Hori S, Hiramatsu K. Antibacterial activity of gatifloxacin (AM-1155, CG5501, BMS-206584), a newly developed fluoroquinolone, against sequentially acquired quinolone-resistant mutants and the norA transformant of Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:1917–1922. doi: 10.1128/aac.42.8.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gellert M, Mizuuchi K, O’Dea M H, Nash H A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci USA. 1976;73:3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper D C, Wolfson J S. Mechanisms of quinolone action and bacterial killing. In: Hooper D C, Wolfson J S, editors. Quinolone antibacterial agents. Washington, D.C: American Society for Microbiology; 1993. pp. 53–57. [Google Scholar]
- 9.Hori S, Ohshita Y, Utsui Y, Hiramatsu K. Sequential acquisition of norfloxacin and ofloxacin resistance by methicillin-resistant and -susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:2278–2284. doi: 10.1128/aac.37.11.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosaka M, Yasue T, Fukuda H, Tomizawa H, Aoyama H, Hirai K. In vitro and in vivo antibacterial activities of AM-1155, a new 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1992;36:2108–2117. doi: 10.1128/aac.36.10.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshino K, Sato K, Une T, Osada Y. Inhibitory effects of quinolones on DNA gyrase of Escherichia coli and topoisomerase II of fetal calf thymus. Antimicrob Agents Chemother. 1989;33:1816–1818. doi: 10.1128/aac.33.10.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoshino K, Sato K, Akahane K, Yoshida A, Hayakawa I, Sato M, Une T, Osada Y. Significance of the methyl group on the oxazine ring of ofloxacin derivatives in the inhibition of bacterial and mammalian type II topoisomerases. Antimicrob Agents Chemother. 1991;35:309–312. doi: 10.1128/aac.35.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino K, Kitamura A, Morrissey I, Sato K, Kato J, Ikeda H. Comparison of inhibition of Escherichia coli topoisomerase IV by quinolones with DNA gyrase inhibition. Antimicrob Agents Chemother. 1994;38:2623–2627. doi: 10.1128/aac.38.11.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussy P, Maass G, Tümmler B, Grosse F, Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase α primase complex, topoisomerase I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986;29:1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khodursky A B, Zechiedrich E L, Cozzarelli N R. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc Natl Acad Sci USA. 1995;92:11801–11805. doi: 10.1073/pnas.92.25.11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumagai Y, Kato J-I, Hoshino K, Akasaka T, Sato K, Ikeda H. Quinolone-resistant mutants of Escherichia coli DNA topoisomerase IV parC gene. Antimicrob Agents Chemother. 1996;40:710–714. doi: 10.1128/aac.40.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luttinger A. The twisted life of DNA in the cell: bacterial topoisomerases. Mol Microbiol. 1995;15:601–606. doi: 10.1111/j.1365-2958.1995.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller K G, Liu L F, Englund P T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981;256:9334–9339. [PubMed] [Google Scholar]
- 20.Morais Cabral J H, Jackson A P, Smith C V, Shikotra N, Maxwell A, Liddington R C. Crystal structure of the breakage-reunion domain of DNA gyrase. Nature. 1997;388:903–906. doi: 10.1038/42294. [DOI] [PubMed] [Google Scholar]
- 21.Moreau N J, Robaux H, Baron L, Tabary X. Inhibitory effects of quinolones on pro- and eucaryotic DNA topoisomerases I and II. Antimicrob Agents Chemother. 1990;34:1955–1960. doi: 10.1128/aac.34.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng E Y W, Trucksis M, Hooper D C. Quinolone resistance mediated by norA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 1994;38:1345–1355. doi: 10.1128/aac.38.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitiss J L. Roles of DNA topoisomerases in chromosomal replication and segregation. In: Liu L F, editor. DNA topoisomerases: biochemistry and molecular biology. San Diego, Calif: Academic Press, Inc.; 1994. pp. 103–134. [DOI] [PubMed] [Google Scholar]
- 25.Oomori, Y., T. Yasue, H. Aoyama, K. Hirai, S. Suzue, and T. Yokota. 1988. Effects of fleroxacin on HeLa cell functions and topoisomerase II. J. Antimicrob. Chemother. 22(Suppl. D):91–97. [DOI] [PubMed]
- 26.Peng H, Marians K J. Escherichia coli topoisomerase IV. Purification, characterization, subunit structure, and subunit interaction. J Biol Chem. 1993;268:24481–24490. [PubMed] [Google Scholar]
- 27.Sato K, Inoue Y, Fujii T, Aoyama H, Inoue M, Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Chemother. 1986;30:777–780. doi: 10.1128/aac.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen L L, Chu D T W. Type II DNA topoisomerases as antibacterial targets. Curr Pharm Design. 1996;2:195–208. [Google Scholar]
- 29.Tanaka M, Onodera Y, Uchida Y, Sato K, Hayakawa I. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2362–2366. doi: 10.1128/aac.41.11.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakabayashi E, Mitsuhashi S. In vitro antibacterial activity of AM-1155, a novel 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1994;38:594–601. doi: 10.1128/aac.38.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamagishi J, Kojima T, Oyamada Y, Fujimoto K, Hattori H, Nakamura S, Inoue M. Alterations in the DNA topoisomerase IV grlA gene responsible for quinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1157–1163. doi: 10.1128/aac.40.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]