Abstract
Neurotransmitter release requires assembly of the SNARE complex fusion machinery, with multiple SNARE-binding proteins regulating when and where synaptic vesicle fusion occurs. The presynaptic protein Complexin (Cpx) controls spontaneous and evoked neurotransmitter release by modulating SNARE complex zippering. Although the central SNARE-binding helix is essential, post-translational modifications to Cpx’s C-terminal membrane-binding amphipathic helix regulate its ability to control synaptic vesicle fusion. Here, we demonstrate that RNA editing of the Cpx C-terminus modifies its ability to clamp SNARE-mediated fusion and alters presynaptic output. RNA editing of Cpx across single neurons is stochastic, generating up to eight edit variants that fine tune neurotransmitter release by altering the subcellular localization and clamping properties of the protein. Similar stochastic editing rules for other synaptic genes were observed, indicating editing variability at single adenosines and across multiple mRNAs generates unique synaptic proteomes within the same population of neurons to fine tune presynaptic output.
Graphical Abstract

In brief
Brija et al. identify stochastic RNA editing of Complexin within individual neurons that generates multiple variants that alter SNARE-mediated fusion and synaptic growth. Similar RNA editing rules were found for other synaptic genes, indicating stochastic editing across multiple mRNAs generates unique synaptic proteomes within neurons that fine tune synaptic transmission.
INTRODUCTION
Neuronal communication is initiated by Ca2+-evoked fusion of synaptic vesicles (SVs) in response to action potentials. Single SVs also fuse spontaneously to generate mini events. Complexin (Cpx) and the Ca2+ sensor Synaptotagmin 1 (Syt1) bind and regulate SNARE complexes to control whether SVs fuse spontaneously or through the evoked pathway.1–3 Cpx arrests zippering of the SNARE complex at the SV/plasma membrane interface to maintain SVs in a fusion-ready state that allows Ca2+-bound Syt1 to rapidly trigger release.4–6 Indeed, invertebrate Cpxs act as “fusion clamps” to reduce spontaneous release in the absence of Ca2+.7–9 Dynamic changes to Cpx function can also regulate spontaneous release to gate structural and functional presynaptic plasticity.10–12 The Cpx C-terminus is a key site for such regulatory control, as it encodes a conserved amphipathic helix that functions to localize Cpx to SVs and concentrate its activity at release sites.13 In Drosophila, a single cpx gene produces two isoforms, Cpx7A and Cpx7B, with different C-termini due to alternative splicing of exon 7.14,15 Cpx7B is regulated by protein kinase A (PKA) phosphorylation of a serine residue (S126) within this alternatively spliced exon, leading to elevated spontaneous release that triggers activity-induced structural plasticity.10 The Cpx7A isoform lacks this serine residue and instead undergoes RNA editing by ADAR (adenosine deaminase acting on RNA) to generate multiple Cpx7A proteins with unique C-terminal sequences. A-to-I editing can recode mRNAs by deaminating target adenosines, causing the resulting inosine base to be read as guanosine by the translation machinery.16 RNA editing of Cpx7A occurs at multiple sites, including editing of two adjacent adenosines that can change an unedited asparagine (N130) residue to a glycine (N130G), aspartate (N130D), or serine (N130S) near the phosphorylated S126 residue in Cpx7B.14,17
Given Cpx7A is the dominant isoform in the Drosophila nervous system,14 RNA editing of Cpx represents an attractive mechanism for regulating neurotransmitter release and structural plasticity across a larger population of neurons. Here we assayed the role of Cpx RNA editing in controlling neurotransmitter release. Single-cell RNA sequencing (RNA-seq) revealed multiple Cpx7A RNA editing variants can be simultaneously co-expressed in individual motoneurons (MNs), indicating RNA editing of Cpx does not act in an “all-or-none” fashion. Transgenic rescue demonstrates editing of Cpx can alter the protein’s subcellular localization and ability to clamp spontaneous SV fusion, leading to synaptic overgrowth. Rescue with a combination of edited and unedited Cpx reveals edited variants act in a semi-dominant fashion, consistent with a model where multiple Cpxs engage assembling SNARE complexes during SV fusion.18 Such a mechanism allows edited and unedited Cpx proteins to assert independent effects in a combinatorial fashion to control fusion dynamics. Together, these data indicate stochastic RNA editing of the Cpx C-terminus can generate distinct presynaptic output across individual Drosophila neurons.
RESULTS
Alternative splicing and RNA editing of Drosophila Complexin generate divergent C-terminal sequences
In contrast to four Cpx homologs in mammals, a single cpx gene is present in Drosophila. Drosophila cpx undergoes alternative splicing of exon 7 to generate two unique isoforms, Cpx7A and Cpx7B, that differ in their last ~20 amino acids (Figures 1A and 1B). The C-terminus of both isoforms encodes a membrane-binding amphipathic helix (Figures 1C and 1D) that is conserved across invertebrate and vertebrate Cpx homologs.13 Cpx7A is the more abundant isoform and contains a C-terminal CAAX box that undergoes prenylation,14 a post-translational lipid attachment that helps localize this variant, and mammalian CPX3 and CPX4 at synapses.11,15,19 The less abundant Cpx7B lacks a prenylation motif, similar to mammalian CPX1 and CPX2. We previously demonstrated the Cpx7B C-terminus is phosphorylated by PKA at residue S126 in an activity-dependent manner, leading to reduced SV clamping and enhanced spontaneous release and synaptic growth.10 Given the important role of Cpx7B phosphorylation, it was surprising the more abundant Cpx7A lacks this PKA phosphorylation site. Unlike Cpx7B, Cpx7A is subject to RNA editing via ADAR at three adenosine residues within the mRNA sequence of exon 7A.14,17 One edit site generates an isoleucine (I) to methionine (M) substitution at amino acid 125 that is not predicted to alter protein function, but induces pre-mRNA conformational changes in exon-intron base pairing that facilitates editing of two downstream adenosine residues.14 At this downstream site, the unedited AAT codon encodes an asparagine (N) at amino acid 130. Editing of both residues (AAT to GGT) produces a glycine (N130G), while editing of only the first base (AAT to GAT) generates an aspartic acid (N130D) and editing of the second base (AAT to AGT) produces a serine (N130S) (Figure 1E). As such, RNA editing generates a potentially phospho-competent Cpx7AN130S, phosphomimetic Cpx7AN130D and phospho-incompetent Cpx7AN130G.
Figure 1. Alternative splicing and RNA editing generate diversity in the Complexin C-terminal amphipathic helix.
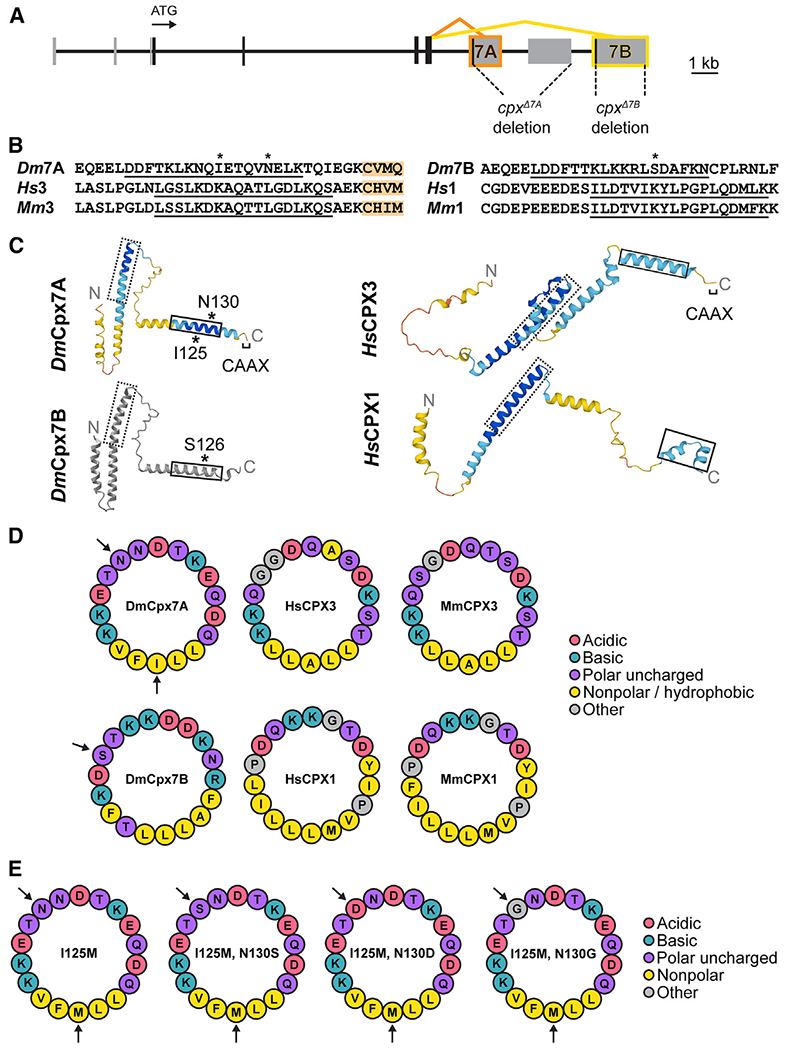
(A) Diagram of the Drosophila cpx genomic locus with protein-coding exons indicated in black and noncoding exons in gray. The ATG start codon is noted, together with 7A (orange) and 7B (yellow) alternative splicing. The location of CRISPR-gener-ated cpx deletions removing 7A (cpxΔ7A) or 7B (cpxΔ7B) are shown.
(B) Alignment of Cpx C-termini from Drosophila melanogaster (Dm), Homo sapiens (Hs), and Mus musculus (Mm) highlight the two subfamilies that contain or lack a CAAX prenylation motif (orange). The amphipathic helix is underlined, with asterisks denoting residues modified by RNA editing (I125, N130) in DmCpx7A or phosphorylation (S126) in DmCpx7B.
(C) AlphaFold predictions of Cpx homologs in Dm and Hs. The dashed box denotes the SNARE-binding central helix and the solid box highlights the C-terminal amphipathic helix. Cpx7A RNA editing sites and the Cpx7B phosphorylation site are denoted with *. AlphaFold per-residue confidence scores (pLDDT) are color-coded: blue = very high (pLDDT >90), cyan = confident (90 > pLDDT >70), yellow = low (70 > pLDDT >50), orange = very low (pLDDT <50). Cpx7B was generated using a simplified AlphaFold version without confidence scores and visualized with iCn3D.
(D) HELIQUEST predictions of the Cpx C-terminal amphipathic helix show conserved hydrophilic and hydrophobic faces, with amino acid properties noted in the legend. Arrows indicate Cpx7A edit sites and the Cpx7B phosphorylation site.
(E) Amphipathic helix models for non-edited (left) and edited (right) Cpx7A proteins.
To begin testing if these editing changes alter Cpx function, a structural comparison of Cpx7A and Cpx7B with their mammalian homologs was performed using AlphaFold.20,21 AlphaFold predictions indicate each Cpx homolog contains a conserved SNARE-binding central helix and a C-terminal alpha-helical domain (Figure 1C). Given the C-terminal amphipathic helix of Cpx binds SVs,22,23 helical wheel models were generated with HELIQUEST24 to examine hydrophilic and hydrophobic faces of the helix in relation to Cpx7A editing sites and the Cpx7B S126 phosphorylation site (Figure 1D). Both splice isoforms have phospho-competent serine and/or threonine residues in similar positions on the hydrophilic face, including the Cpx7B S126 residue. Mammalian CPXs also contain phospho-competent residues on this hydrophilic surface (Figure 1D), suggesting phosphorylation of this region may represent a conserved mechanism for modulating Cpx activity. The Cpx7A N130S edit adds another phospho-competent residue to match the paired S/T residues where Cpx7B S126 resides (Figure 1E). The N130D edit adds another negative charge to the hydrophilic face, generating a helix with a negative charge at nearly every other amino acid on this surface. The N130G edit inserts a glycine residue that matches glycine residues found at the same site in mammalian CPX3. We conclude that RNA editing alters the hydrophilic face of the Cpx7A C-terminal amphipathic helix to generate variants that more closely resemble Cpx7B or mammalian CPXs, suggesting RNA editing may alter the properties of the helix or its potential for phosphorylation.
Characterizing the functional significance of alternative splicing of Cpx exon 7
Before examining the impact of RNA editing on Cpx7A function, we first characterized Cpx alternative splicing to determine endogenous roles for Cpx7A and Cpx7B. In cpx null mutants lacking both splice isoforms (cpxSH1), spontaneous mini frequency is dramatically elevated (>50-fold), evoked release is decreased, and larval neuromuscular junction (NMJ) growth is enhanced.7 Although both variants support aspects of Cpx function when overexpressed, endogenous Cpx7A mRNA is >100-fold more abundant and hypothesized to play a more critical role in synaptic transmission.14 To test their endogenous function, CRISPR mutants disrupting 7A (cpxΔ7A) or 7B (cpxΔ7B) were generated by introducing an early stop codon at the beginning of exon 7A or 7B, respectively. Western analysis of adult brain lysates with a pan-Cpx antibody showed an 87% reduction in overall Cpx expression in cpxΔ7A (p < 0.0001) and a milder 10% reduction in cpxΔ7B (p = 0.9398), consistent with Cpx7A being the predominant isoform (Figure 2A). Immunostaining for Cpx at third instar larval NMJs showed a similar effect (Figures 2B and 2C), with a >85% reduction in Cpx at synapses in cpxΔ7A mutants (p < 0.0001) and no detectable decrease in cpxΔ7B mutants (p = 0.9567). Complete loss of Cpx in cpxSH1 severely disrupts behavior and reduces viability, with the few escaper adults displaying a profound loss of motor control and an inability to walk. Loss of Cpx7A also strongly disrupted motor behavior, though not as severely as cpxSH1. cpxΔ7A adults showed an inability to climb in a negative geotaxis assay (0% ± 0% pass rate, n = 8 cohorts of 10 flies) and had reduced larval crawling velocity (p = 0.0011, n = 10 larvae) (Figures S1A and S1B). In contrast, cpxΔ7B adults were moderately hyperactive in climbing compared with controls (n = 8 cohorts of 10 flies, p = 0.0055) and displayed only a mild decrease in crawling velocity (p = 0.1155, n = 10 larvae). Together, these data indicate Cpx7A has a more prominent role in supporting larval and adult motor behavior.
Figure 2. Morphological and physiological phenotypes in CRISPR-generated splicing mutants lacking Cpx7A or 7B.

(A) Quantification and representative western of Cpx levels from adult brain extracts normalized to loading control (anti-Tubulin) for the indicated genotypes (control white, cpxΔ7A, cpxΔ7B, and cpxSH1).
(B) Immunostaining of third instar larval segment 3 muscle 4 NMJs for the indicated genotypes with antibodies against Cpx (yellow, upper panels), Brp (red), and anti-HRP (cyan) show a large decrease in total Cpx levels in cpxΔ7A mutants lacking the predominant Cpx7A isoform. Scale bar, 10 μm.
(C) Quantification of total Cpx fluorescence within the HRP-positive area at muscle 4 NMJs for the indicated genotypes (au = arbitrary units).
(D) Quantification of mean AZ number per muscle area at muscle 4 NMJs of the indicated genotypes.
(E) Representative postsynaptic current recordings of spontaneous release at third instar muscle 6 NMJs in control (blue), cpxSH1 (gray), cpxΔ7A (orange), or cpxΔ7B (yellow).
(F) Quantification of average spontaneous release rate for the indicated genotypes. Note the y axis gap between 10 and 60 Hz due to the extreme elevation of mini frequency in cpxSH1 null mutants.
(G) Average traces of evoked EJC responses for the indicated genotypes.
(H) Quantification of average eEJC amplitude for the indicated genotypes. All recordings were performed in 2.0 mM external Ca2+ saline. Data are shown as mean ± SEM. Asterisks indicate the following p values: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. ns, not significant. See also Figure S1.
To examine synaptic morphology in cpxΔ7A and cpxΔ7B mutants, active zone (AZ) and bouton number were quantified by immunostaining for the AZ protein Bruchpilot (Brp) and neuronal membranes (anti-HRP) at third instar larval NMJs (Figures 2B, 2D, and S1C). In contrast to the large increase in AZ (65%, p < 0.0001) and bouton (72%, p = 0.0012) number in cpxSH1, synaptic growth was largely unaffected in cpxΔ7A (AZ#: p = 0.2809, bouton#: p = 0.4576) or cpxΔ7B (AZ#: p = 0.3121, bouton#: p = 0.7781). To assay synaptic function, two-electrode voltage-clamp (TEVC) was used to measure spontaneous and evoked neurotransmitter release at third instar NMJs. In contrast to the dramatic increase in spontaneous release rate observed in cpxSH1 (>55-fold), endogenously expressed Cpx7A or Cpx7B substantially rescued mini frequency (Figures 2E and 2F). The presence of Cpx7A in the cpxΔ7B mutant returned spontaneous release rates to control levels (p = 0.2641). The residual Cpx7B in cpxΔ7A mutants was not able to fully clamp spontaneous fusion, with a 2-fold increase compared with controls (p < 0.0001). In contrast, the presence of only one of the two splice isoforms was not as effective in supporting normal levels of evoked release (Figures 2G and 2H). A 71% reduction in the peak amplitude of the evoked excitatory junctional current (eEJC) was observed in cpxSH1 compared with control (p < 0.0001). Although less severe, cpxΔ7A displayed a 46% reduction (p < 0.0001) and cpxΔ7B a 25% reduction (p = 0.0269) compared with controls. In addition to the number of SVs that fuse, Cpx also modulates release kinetics by promoting fast synchronous fusion and reducing slower asynchronous release.4 Like eEJC amplitude, release kinetics in cpxΔ7B mutants were more similar to controls, while loss of Cpx7A resulted in reduced charge transfer and increased asynchronous release (Figures S1D–S1H, mean eEJC rise times: control: 1.30 ± 0.1 ms; cpxΔ7A: 1.33 ± 0.1 ms; cpxΔ7B: 1.26 ± 0.03 ms; control vs. cpxΔ7B p = 0.97, control vs. cpxΔ7A p = 0.99; mean eEJC decay times: control: 15.4 ± 0.7 ms; cpxΔ7A: 12.1 ± 0.5 ms; cpxΔ7B: 15.3 ± 0.4 ms; control vs. cpxΔ7B p = 0.61, control vs. cpxΔ7A p = 0.0002). In summary, we conclude endogenous levels of either Cpx isoform are sufficient to clamp spontaneous fusion, while both are needed to fully recapitulate evoked responses. Given the behavioral defects observed in animals lacking Cpx7A, certain neuronal subtypes are likely to be more reliant on this splice variant for supporting synaptic transmission compared with MNs.
Single-cell RNA-seq reveals stochastic RNA editing of Cpx7A
Larval muscles are innervated by tonic Type Ib and phasic Type Is glutamatergic MN subclasses that display unique morphological and functional properties.25,26 Given Ib and Is neurons have distinct presynaptic output, RNA editing of Cpx7A might contribute to these differences. To determine the abundance and diversity of Cpx7A edit variants (I125M, N130G, N130S, N130D) in larval MNs, single-cell PatchSeq RNA datasets from ~200 Ib and Is MNs27 were analyzed that allowed identification of RNA editing diversity at single neuron resolution. Strikingly, individual larval MNs showed highly stochastic RNA editing of the three adenosines (positions 375, 388, and 389) that are subject to editing in cpx exon 7A. Editing rates ranged from 0% to 100%, with 98% of Ib MNs and 94% of Is MNs showing some level of Cpx7A editing (Figures 3A and 3B). For adenosine 375, the average I125M (adenosine 375 to inosine 375) edited transcript level per neuron was 31.7% ± 2.4% in Ib (n = 95 cells) and 30.3% ± 2.4% in Is (n = 86 cells). Only 3% of Ib and 1% of Is neurons fully edited all Cpx mRNA to I125M. For all edit variants, the average Ib MN expressed 53% unedited Cpx7A, 32% Cpx7AI125M, 0.0% Cpx7AN130D, 1.5% Cpx7AN130S, 0.3% Cpx7AN130G, 0.2% Cpx7AI125M,N130D, 9.5% Cpx7AI125M,N130S, and 3.7% Cpx7AI125M,N130G (Figures 3A and 3B). The average Is MN expressed a similar ratio, suggesting differential RNA editing of Cpx7A is unlikely to drive the distinct release properties of these unique neuronal subtypes, though stochastic editing of Cpx could contribute to individual MN heterogeneity in presynaptic output.
Figure 3. Stochastic expression of Cpx7A RNA editing variants in single neurons alters Cpx localization and synaptic growth.

(A) Quantification of unedited and edited Cpx7A mRNAs from single Ib (blue, n = 95 cells) and Is (orange, n = 86 cells) MN RNA-seq datasets. Each point represents the number of edit variant reads as percent of total Cpx reads in an individual neuron.
(B) Sorted single-cell RNA editing profiles for Cpx7A across the population of Ib and Is MNs. Each neuron is displayed as a stacked bar with corresponding edit and unedited read percentages that total 100% of cpx mRNA for that cell. Cells 1–95 are Ib MNs and cells 96–181 are Is MNs. Neurons are sorted by the largest unedited percent for both Ib and Is groups.
(C) Quantification of RNA editing percentage for known edit sites in genes encoding the synaptic proteins Synapsin (Syn) and Syntaxin 1A (Syx1A) compared with Cpx7A. Each point represents the percent of editing occurring at the base position of interest in one cell. All cells included for quantification (Ib = nine cells, Is = 11 cells) contained at least 10 reads at all base positions of interest (Syn N15, Syn R19, Syn R20, Cpx I125, and Syx1A M244).
(D) Representative western from adult brain extracts stained for Cpx or Tubulin (loading control) for the indicated genotypes: control (elavC155-GAL4; cpxPE), cpxSH1 (elavC155-GAL4; cpxSH1), unedited rescue Cpx7AN130 (elavC155-GAL4; cpxSH1, UAS-Cpx7AI125,N130), Cpx7AN130S rescue (elavC155-GAL4; cpxSH1, UAS-Cpx7AI125M,N130S), and Cpx7AN130D rescue (elavC155-GAL4; cpxSH1, UAS-Cpx7AI125M,N130D).
(E) Quantification of Cpx protein levels normalized to Tubulin from westerns of the indicated genotypes.
(F) Representative staining of third instar muscle 4 NMJs and axons of the indicated genotypes from segment A3 with antibodies against Cpx (yellow) and HRP (cyan). Cpx staining in axons is denoted with white arrows. The brightness of anti-Cpx staining was enhanced in controls to highlight the lower amounts of Cpx normally found in non-synaptic regions of the axon. Scale bar, 10 μm.
(G) Quantification of the Cpx NMJ/axon fluorescence ratio for the indicated genotypes.
(H) Quantification of mean AZ number per muscle area at muscle 4 NMJs of the indicated genotypes. Data are shown as mean ± SEM. Asterisks indicate the following p values: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. ns, not significant. See also Figure S2.
Prior studies suggested editing at adenosine 375 serves to enhance exon-intron base pairing within the pre-spliced mRNA to generate a more favorable double-stranded RNA template for editing at the downstream adenosines 388 and 389 that form the AAT codon (N130).14 Consistent with this model, single MNs that showed editing for both I125 and N130 were far more common than single edits to N130 alone (Figure 3A). Ib neurons expressing Cpx7AI125M,N130S mRNA were 6-fold more abundant that those expressing Cpx7AN130S (I125M, N130S: 9.5 ± 1.5 edit % per cell; N130S: 1.47 ± 0.51 edit % per cell, p = 0.0035). A similar ratio was observed in Is neurons (Figures 3A and 3B). In the 56% of Ib cells expressing Cpx7AI125M,N130S edited transcripts, an average of 17% of total cpx mRNA were of this variant, similar to the 16% of total cpx mRNA in Is cells that expressed Cpx7AI125M,N130S. Rarely, Cpx7AI125M,N130S represented the only cpx mRNA detected within an MN (Figure 3A). In contrast to the more abundant Cpx7AI125M,N130S, only 6% of Ib neurons expressed Cpx7AI125M,N130D and it represented just 3% of the total cpx mRNA in these cells. The Cpx7AI125M,N130G variant, which requires A-to-I editing at all three adenosines, was observed in 40% of Ib MNs, representing 9% of total cpx mRNA in cells in which it was expressed, similar to Is (Figures 3A and 3B). For the most abundant edit variant (Cpx7AI125M), no correlation of Cpx7A editing percentage and adar mRNA expression level in that cell was observed for MNs (Figures S2A and S2B). We conclude that Cpx7AI125M,N130S is the highest expressed variant in larval MNs that alters the N130 residue on the amphipathic helix.
To determine if RNA editing of other synaptic genes showed similar stochastic single neuron editing, additional mRNAs were examined. RNA editing percentages at three sites (N15D, R19G, R20G) within Synapsin (Syn) and one site (M244V) in Syntaxin 1A (Syx1A) were compared in the same MNs that edited Cpx7A to I125M (Figure 3C). Both Syn and Syx1A displayed stochastic editing rates that ranged from 0% to 100% across MNs. For example, Syn R20G editing was observed at an average of 55% ± 11.6% per cell (n = 11 Is cells), while Syx1A M244V was edited in the same neurons at a rate of only 5.4% ± 3.8% per cell (n = 11 Is cells). Similar to Cpx, no significant difference in editing percent of Syn or Syx1A was observed between Ib and Is (Figure 3C). We conclude ADAR-mediated RNA editing is not all-or-none in individual Drosophila MNs, with stochastic editing at single adenosines across multiple mRNAs having the potential to generate unique synaptic proteomes within the same population of neurons.
RNA editing of Cpx7A alters its subcellular localization and functional properties
Given N130 resides near the Cpx7B S126 phosphorylation site, computational analysis of candidate phosphorylation motifs in exon 7A were performed that predicted a Casein Kinase 2 (CK2) consensus sequence of S/T E/D found in some CK2 targets.28 To determine if Cpx7AI125M,N130S can be phosphorylated by CK2, in vitro phosphorylation assays were performed. Indeed, Cpx7AI125M,N130S was phosphorylated by CK2 (p = 0.0003), while unedited Cpx7AI125,N130 was not (Figures S2C and D; p = 0.9554), pinpointing N130S as a potential target for CK2 phosphorylation in vivo. Although it is unknown if CK2 phosphorylation alters Cpx7AI125M,N130S function, it provides a potential additional regulatory layer downstream of RNA editing.
To examine if RNA editing alters Cpx7A function, transgenic UAS rescue lines expressing Cpx7AI125M,N130S, Cpx7AI125M,N130D, or unedited Cpx7AI125,N130 were generated and expressed panneuronally using elavC155-Gal4 in the cpxSH1 null. All three Cpx7A transgenic proteins were overexpressed at similar levels when assayed by western analysis of adult brain lysates (Figures 3D and 3E) or anti-Cpx immunostaining at larval NMJs (Figures S2E and S2F). Given N130S and N130D alter the hydrophilic face of the C-terminal amphipathic helix that regulates SV binding (Figure 1E), Cpx subcellular distribution at the NMJ was assayed. Endogenous Cpx accumulates along the periphery of presynaptic boutons (Figure 3F), co-localizing with other SV proteins.14 Lower Cpx levels are found in non-synaptic regions of the axon, resulting in a 3.6-fold synaptic enrichment (Cpx synapse/axon fluorescence ratio) at control NMJs (Figures 3F and 3G). Expression of unedited Cpx7AI125,N130 in nulls resulted in a shift to more Cpx enrichment at synapses, increasing the synapse/axon ratio to 4.7 (p = 0.1218). Expression of Cpx7AI125M,N130S or Cpx7AI125M,N130D in nulls had the opposite effect, with a greater fraction of Cpx in non-synaptic regions of the axon. Cpx7AI125M,N130D was enriched 2-fold at synapses, decreasing ~44% compared with controls (p = 0.0024) and ~57% compared with unedited Cpx7AI125,N130 (p < 0.0001). Cpx7AI125M,N130S had a more striking change, with a nearly one-to-one ratio of its abundance at synapses and along axons (1.16 ± 0.09, p < 0.0001 to control). As such, unedited Cpx7AI125,N130 is more strongly enriched at synapses, while the edited variant Cpx7AI125M,N130S distributes equally between axons and synapses.
Quantification of synaptic AZ and bouton number revealed significant differences in the ability of different Cpx7A edited proteins to rescue synaptic overgrowth in cpxSH1 (Figures 3H and S2G). Cpx7AI125M,N130D fully rescued the increased number of AZs (p = 0.3658) and boutons (p = 0.3722), returning synaptic morphology to control levels. In contrast, Cpx7AI125M,N130S displayed the weakest rescue, with ~80% more AZs and boutons than controls (p < 0.0001), slightly less than the doubling observed in cpxSH1. Expression of unedited Cpx7AI125,N130 in the null background resulted in partial rescue (AZ#: p = 0.0013, bouton#: p < 0.0001). Electrophysiological analysis of spontaneous fusion rates revealed a similar pattern of rescue as observed for synaptic growth (Figures 4A and 4B), consistent with enhanced mini frequency being the primary driver for synaptic over-proliferation in cpx mutants. Like the full rescue of synaptic overgrowth, Cpx7AI125M,N130D returned mini frequency to control levels (p > 0.9999). In contrast, Cpx7AI125M,N130S expression failed to properly clamp spontaneous release, similar to its inability to rescue synaptic overgrowth. Compared with the 102 Hz mini frequency at cpxSH1 null NMJs, Cpx7AI125M,N130S expression was able to reduce spontaneous fusion by only ~50% to 47 Hz (p < 0.0001 to control). Rescue with unedited Cpx7AI125,N130 created a more effective fusion clamp, decreasing mini frequency to 13 Hz (p = 0.0034 to control), although this rate was still ~3-fold higher than controls that contain endogenous Cpx7B or the Cpx7AI125M,N130D rescue. Both unedited and edited Cpx7A improved evoked release amplitude and kinetics compared with null mutants (Figures 4C–4F). Similar to spontaneous release, Cpx7AI125M,N130D showed the strongest rescue, while Cpx7AI125M,N130S and Cpx7AI125M,N130 displayed intermediate rescues. We conclude that RNA editing of Cpx7A regulates its functional properties, with N130D and N130S displaying several opposing effects compared with unedited Cpx7A. The N130D edit improved Cpx’s ability to clamp spontaneous release, in contrast to the N130S edit, which reduced clamping and failed to prevent synaptic overgrowth. Based on the distinct phenotypes of N130S and N130D, the primary effect of N130S may not be downstream of phosphorylation, or the N130D edit may not act as a phosphomimetic in the case of Cpx.
Figure 4. RNA editing of Cpx7A alters its ability to regulate neurotransmitter release.
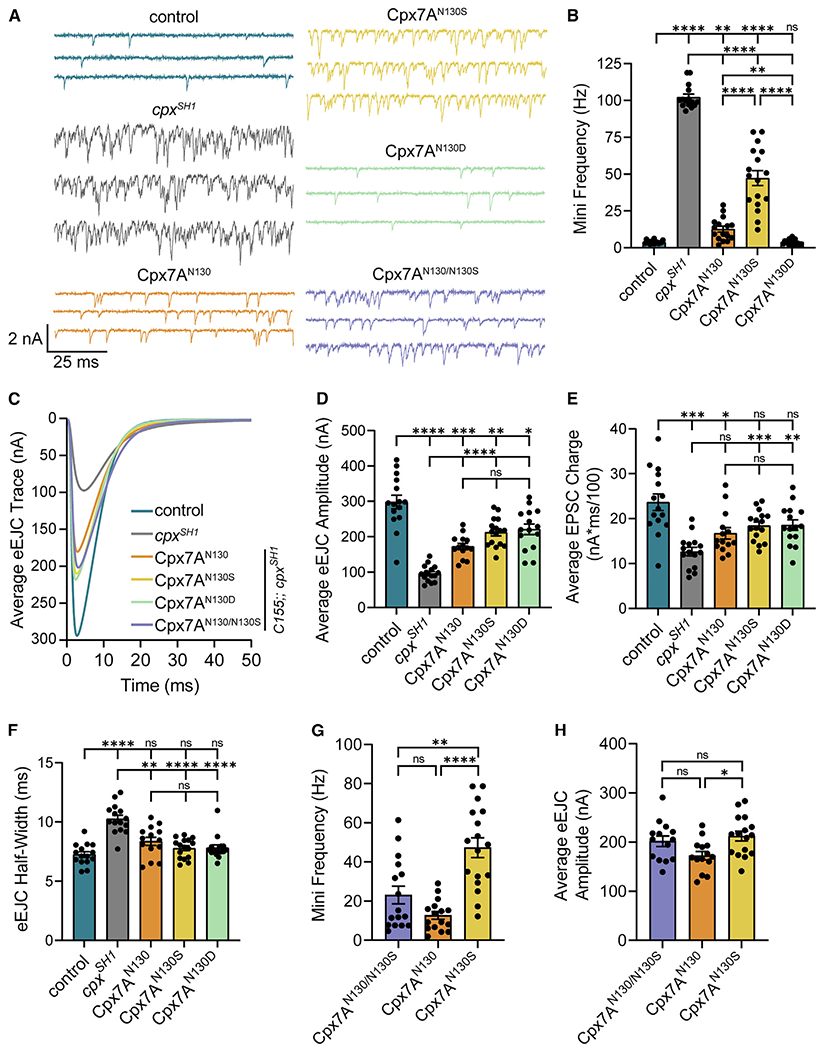
(A) Representative postsynaptic current recordings of spontaneous release at third instar muscle 6 NMJs in control (blue, elavC155-GAL4; cpxPE), cpxSH1 (gray, elavC155-GAL4; cpxSH1), unedited Cpx7AN130 rescue (orange, elavC155-GAL4; cpxSH1, UAS-Cpx7AI125,N130), Cpx7AN130S rescue (yellow, elav-GAL4; cpxSH1 UAS-Cpx7AI125M,N130S), Cpx7AN130D rescue (green, elavC155-GAL4; cpxSH1, UAS-Cpx7AI125M,N130D), and co-expression of Cpx7AN130/N130S rescue (purple, elavC155-GAL4; cpxSH1, UAS-Cpx7AI125,N130/cpxSH1, UAS-Cpx7AI125M,N130S).
(B) Quantification of average spontaneous release rate for the indicated genotypes.
(C) Average traces of evoked EJC responses for the indicated genotypes.
(D) Quantification of average eEJC amplitude for the indicated genotypes.
(E) Quantification of average evoked release charge obtained by measuring total release over time following single action potentials.
(F) Average evoked EJC half-width change for each genotype.
(G) Quantification of average spontaneous release rate for co-expression of Cpx7AN130/N130S rescue compared with Cpx7AN130 rescue and Cpx7AN130S rescue alone in the cpxSH1 mutant background.
(H) Quantification of average eEJC amplitude for co-expression of Cpx7AN130/N130S rescue compared with Cpx7AN130 rescue and Cpx7AN130S rescue alone in the cpxSH1 mutant background. All recordings were performed in 2.0 mM external Ca2+ saline. Data are shown as mean ± SEM. Asterisks indicate the following p values: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. ns, not significant.
RNA-seq analysis demonstrated most MNs express a combination of edited Cpx7A proteins (Figures 3A and 3B), with Cpx7AI125M,N130S representing the most abundant edit to the N130 residue. To determine if co-expression of unedited Cpx7AI125,N130 with Cpx7AI125M,N130S results in potential competition for interactions with the SNARE fusion machinery, the two proteins were co-expressed in the cpxSH1 null background. If unedited Cpx7A restored mini frequency to baseline rates, RNA editing would likely have effects on synaptic transmission only when neurons predominantly express edited Cpx. Alternatively, if co-expression of Cpx7AI125M,N130S increased mini frequency beyond unedited Cpx7AI125,N130 rescue alone, a combinatorial role for unique Cpx edit variants to modulate presynaptic output would be more likely. Indeed, an intermediate effect on spontaneous fusion was observed when unedited Cpx7AI125,N130 and Cpx7AI125M,N130S were co-expressed in cpxSH1. Co-expression of the two proteins resulted in a spontaneous release rate of 23 Hz (Figures 4A and 4G), distinct from the 47 Hz in Cpx7AI125M,N130S (p = 0.0036). Co-expression of unedited Cpx7AI125,N130 and Cpx7AI125M,N130S also displayed a larger evoked response than unedited Cpx7AI125,N130 alone, suggesting N130S acts in a semi-dominant fashion for promoting evoked release (Figures 4C and 4H). These data suggest co-expression of different Cpx edited and unedited variants independently interface with the release machinery, indicating stochastic RNA editing can generate release heterogeneity across individual neurons.
DISCUSSION
Mechanisms controlling SNARE complex assembly dynamics provide attractive sites for regulatory control of presynaptic neurotransmitter release. Several SNARE-binding proteins act at multiple steps of the SV cycle to chaperone SNARE proteins and regulate their ability to zipper and form the four-stranded alpha-helical bundle that drives fusion.1 Cpx and Syt1 play key roles at a late stage of SNARE assembly to control whether SVs fuse through the evoked or spontaneous release pathway. The Cpx C-terminal amphipathic helix has emerged as an important site for regulatory control of the protein.13 In addition to acting as a membrane curvature sensor that can tether Cpx to SVs,22,23,29 the C-terminal domain can clamp fusion by blocking SNARE assembly,30 remodel membranes to regulate fusion pore dynamics,31 directly stimulate SNARE-mediated assembly,32 or compete with Syt1 for membrane binding.33 In addition, several post-translational modifications to this domain can alter Cpx function,10,11,34 defining presynaptic plasticity mechanisms that directly impinge on SNARE-mediated fusion.
In the current study, we examined the consequences of alternative splicing and RNA editing to the C-terminus of Drosophila Cpx. Endogenous expression of either Cpx7A or Cpx7B was sufficient to prevent synaptic overgrowth and the dramatic elevation of spontaneous release rates observed in null mutants. Indeed, across all manipulations of Cpx splicing and RNA editing, large increases in mini frequency were always associated with synaptic overgrowth, supporting the linkage between spontaneous fusion and NMJ growth in Drosophila.10,35 Endogenously expressed Cpx7A or Cpx7B alone were not sufficient to fully recapitulate evoked release observed in controls, suggesting both are required in vivo. Given differences in the expression level between Cpx7A and Cpx7B, it was surprising that endogenous Cpx7B could support Cpx function at larval NMJs. We considered the possibility that a truncated Cpx might still be produced that lacked the 7A exon in cpxΔ7A mutants. However, western analysis showed that only traces of a truncated Cpx7A protein, observed as a much fainter lower molecular weight band that was barely detectable in cpxΔ7A, dramatically less than the already reduced levels of Cpx7B. These data indicate loss of the C-terminal domain destabilizes Cpx and leads to degradation, similar to prior observations on a truncating mutant (cpx572) containing a stop codon at the end of exon 6.14 Given Cpx7B clamped spontaneous fusion and promoted evoked release at only ~15% of the Cpx7A expression level, the two splice variants likely have intrinsic differences in their activity. The estimated requirements for zippering of 3–11 SNARE complexes in a radial assembly for a single SV to undergo action potential-triggered fusion36–39 provides a candidate mechanism for Cpx expression to differentially impact these two release pathways. For spontaneous release, a smaller number of Cpx proteins could block enough SNARE zippering events to prevent reaching the minimum required for fusion. For Ca2+-triggered release, excess Cpx or increased mobility might be needed to bind more SNARE complexes in the radial assembly to control Syt1 activity and SNARE zippering, potentially explaining why low levels of Cpx7B in cpxΔ7A mutants were more effective at clamping spontaneous release than promoting evoked fusion.
We previously found that PKA phosphorylation of S126 in the Cpx7B C-terminus enhances spontaneous release and promotes structural and functional synaptic plasticity at larval NMJs.10 Although Cpx7A lacks this phosphorylation site, it undergoes RNA editing to generate up to eight unique C-terminal sequence variants (I125 with N130, S130, D130, or G130 and M125 with N130, S130, D130 or G130). Single-cell RNA-seq revealed multiple Cpx7A editing variants are simultaneously expressed in MNs. As such, ADAR-mediated RNA editing does not act in an “all-or-none” fashion, but instead stochastically deaminates A-to-I residues with distinct efficiencies. To determine if Cpx7A edit variants within a single MN alter presynaptic output, transgenic rescues were used to assay their function. The most prominent variant Cpx7AI125M,N130S displayed altered synaptic distribution, with more of the protein observed in axons. It also failed to clamp spontaneous fusion, resulting in synaptic overgrowth. Although Cpx7AI125M,N130S can be phosphorylated by CK2 in vitro, it remains unclear if this is relevant to Cpx function in vivo. CK2 phosphorylates multiple synaptic proteins, including Syx1A,40,41 Syt1,42 and mammalian CPX1.43 Phosphorylation of the C-terminus of mammalian CPX1 by CK2 alters its SNAREbinding affinity43 and mutation of the site (S115) prevents CPX1 from stimulating liposomal fusion.32 In Drosophila, presynaptic CK2 controls synaptic stability by regulating Ankyrin2 function,28 preventing a detailed analysis of its role in neurotransmitter release due to synapse loss. Given a putative phosphomimetic edit variant (Cpx7AI125M,N130D) was able to fully clamp spontaneous release and support normal synaptic growth, outperforming even unedited Cpx7A, the N130S change may instead alter the structure or binding properties of the amphipathic helix.
Given stochastic expression of Cpx edited proteins within single MNs, and the requirement for multiple SNARE complexes to drive fusion, we assayed if co-expression of Cpx7AI125M,N130S with unedited Cpx7AI125,N130 could allow distinct Cpx proteins to independently alter release output. Indeed, the N130S isoform acted in a semi-dominant manner, preventing unedited Cpx7A from fully clamping spontaneous fusion and supporting higher levels of evoked release. These data suggest each Cpx variant is likely to have access to assembling SNAREs, allowing them to fine tune presynaptic output in unique ways. Beyond stochastic RNA editing of Cpx7A, similar heterogeneity in the percent of RNA editing in Synapsin and Syx1A mRNAs was observed across MNs. As such, stochastic RNA editing across multiple mRNAs is likely to generate unique synaptic proteomes within the same neuronal population that contributes to heterogeneous properties of individual cells with similar transcriptomes. Such a mechanism would be a robust way to change multiple features of neuronal output given ADAR editing alters the function of proteins that contribute to synaptic release and membrane excitability.17,44–47
In addition to stochastic RNA editing observed in MNs, external stimuli or internal neuronal dynamics could also alter the landscape of RNA editing. Recent evidence demonstrates temperature recodes the octopus neuronal proteome by increasing RNA editing.48 Likewise, studies of RNA editing of AMPA receptors in mammals revealed an essential developmental editing switch that restricts Ca2+ permeability as development progresses.49 Within Drosophila, overall RNA editing levels increase across development.50 Although we observed Cpx editing variants were similarly expressed in Ib and Is MNs, analysis of FACS-sorted neuronal populations from adult Drosophila brains revealed RNA editing of Cpx7A was more robust and diverse in these neurons, with 22% of cpx mRNAs encoding N130S and 23% encoding N130G.51 As Drosophila ADAR itself is subject to developmentally regulated auto-editing that changes its enzymatic activity,52 the frequency of Cpx editing may also be regulated by intrinsic activity or cell-type identity, allowing even more dynamic changes to Cpx7A function within single neurons. Editing dysregulation has also been observed in brain samples from autistic patients,53 consistent with changes in the RNA editing landscape occurring during development, across neuronal subtypes, and in response to external factors such as temperature or disease.
Limitations of the study
Because we focused on a single snapshot of the RNA editing profile in third instar larval MNs, RNA editing of Cpx7A could dynamically change over time in this neuronal population. Whether external cues or intrinsic neuronal excitability differences contribute to differences in Cpx RNA editing is unknown. In addition, the use of the Gal4 system to express Cpx7A edit variants results in overexpression of these proteins compared with their in vivo levels, as shown in Figure 3D. Although we cannot exclude that expression levels impact Cpx7A’s role in neurotransmitter release, the edited versions all express at similar levels when driven by elav-Gal4. In spite of similar expression, the N130S and N130D versions displayed profound differences in their ability to support synaptic transmission in the cpx null background. It will also be important to examine editing profiles for the entire transcriptome across individual neurons to determine if stochastic RNA editing is a feature for all edited loci or only apparent for a subset of targets. Finally, further studies will be required to characterize evolutionary advantages that stochastic RNA editing of specific genes within the same neuronal population provides for neuronal function or circuit dynamics.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and be fulfilled by the lead contact, J. Troy Littleton (troy@mit.edu).
Materials availability
Drosophila stocks generated in this study are available from the lead contact upon request without restriction.
Data and code availability
This paper analyzes existing, publicly available RNAseq data. All Isoform-Patchseq RNA profiling raw data is available at NCBI under the GEO accession #GSE222976. All other data reported in this paper will be shared by the lead contact upon request.
This paper does not report any original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Animals
Drosophila melanogaster were cultured on standard medium and maintained at 25°C. Late third-instar larvae were used for imaging and electrophysiological experiments. Western blots were performed on adult brain extracts. Behavior was conducted on late third-instar larvae and adults (aged 2–3 days). Males were used for experiments unless otherwise noted. Experiments were performed in a w1118 (Bloomington Drosophila Stock Center (BDSC) #3605) genetic background unless otherwise noted. Genotypes of the strains used are reported in the figure legends and indicated in the resource table. For electrophysiology of Cpx7A editing lines described in Figure 4, experimenters were blinded to genotype for both data collection and analysis. No vertebrate animals were used in this study, so institutional permission and oversight, health/immune status, participants involvement in prior procedures, and drug or naive state, is not applicable.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-Brp | Developmental Studies Hybridoma Bank | Cat# nc82; RRID: AB_2314866 |
| Rabbit anti-Cpx | Described in Huntwork and Littleton7 | RRID:AB_2568068 |
| Goat anti-Rabbit IgG (H + L), Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-11008; RRID:AB_143165 |
| Goat anti-Mouse IgG (H + L), Alexa Fluor 546 | Thermo Fisher Scientific | Cat# A-11030; RRID:AB_2534089 |
| Goat anti-Horseradish Peroxidase, Alexa Fluor 647 | Jackson ImmunoResearch Labs | Cat# 123-605-021; RRID:AB_2338967 |
| Mouse anti-Tubulin | Sigma-Aldrich | Cat# T5168; RRID:AB_477579 |
| IRDye 680LT-conjugated Goat anti-Mouse | LI-COR Biosciences | Cat# 926–68020; RRID:AB_10706161 |
| IRDye 800CW-conjugated Goat anti-Rabbit | LI-COR Biosciences | Cat# 926–32211; RRID:AB_621843 |
| Bacterial and virus strains | ||
| E. coli: BL21 Competent cells | New England BioLabs | Cat# C2530H |
| Chemicals, peptides, and recombinant proteins | ||
| Vectashield mounting medium | Vector Laboratories | Cat# H-1000; RRID:AB_2336789 |
| Glutathione Sepharose 4B | Thermo Fisher Scientific | Cat# 45-000-139 |
| CK2 catalytic subunit | SignalChem | Cat# C70-10G |
| [32P]ATP | PerkinElmer | Cat# BLU502H250UC |
| Bio-Safe Coomassie Stain | Bio-Rad | Cat# 1610787 |
| Critical commercial assays | ||
| QuikChange Lightning Site-Directed Mutagenesis Kit | Agilent | Cat# 210518 |
| NEBuilder HighFidelity DNA Assembly Cloning Kit | New England BioLabs | Cat# E5520 |
| Deposited data | ||
| Single-cell RNAseq dataset | NCBI; Described in Jetti et al.27 | GEO: GSE222976 |
| Experimental models: Organisms/strains | ||
| D. melanogaster: CRISPR control: w1118 | Bloomington Drosophila Stock Center (BDSC) | RRID:BDSC_3605 |
| D. melanogaster: elavC155-GAL4 | BDSC | RRID:BDSC_8765 |
| D. melanogaster: Cpx null deletion: cpxSH1 | Described in Huntwork and Littleton7 | FlyBase:FBal0241995 |
| D. melanogaster: Transgenic rescue control: cpxPE | Described in Huntwork and Littleton7 | N/A |
| D. melanogaster: Cpx exon 7A deletion: cpxΔ7A | This paper | N/A |
| D. melanogaster: Cpx exon 7B deletion: cpxΔ7B | This paper | N/A |
| D. melanogaster: cpxSH1, UAS-Cpx7AI125,N130 | This paper | N/A |
| D. melanogaster: cpxSH1, UAS-Cpx7AI125M,N130S | This paper | N/A |
| D. melanogaster: cpxSH1, UAS-Cpx7AI125M,N130D | This paper | N/A |
| D. melanogaster: Transgenic docking strain: yw;;attP2 | BDSC | RRID:BDSC_8622 |
| D. melanogaster: CRISPR injection strain: vas-Cas9 | BDSC | RRID:BDSC_56552 |
| Recombinant DNA | ||
| pCFD5: Expression vector gRNA | Addgene | RRID:Addgene_73914 |
| P3>dsRed: Reporter cassette | Addgene | RRID:Addgene_51434 |
| pVALIUM20: UAS plasmid | Drosophila Genomics Resource Center | RRID:DGRC_1467 |
| pGEX-2T: GST-fusion plasmid | GE Healthcare Life Sciences | https://www.addgene.org/vector-database/2868/ |
| Software and algorithms | ||
| Zen software | Zeiss | https://www.zeiss.com/microscopy/en/products/software/zeiss-zen.html; RRID:SCR_018163 |
| LI-COR Odyssey Classic Imager | LI-COR Biosciences | RRID:SCR_023765 |
| Master-8 | AMPI | https://www.ampi.co.il/master-8 |
| Axoscope 10.0 | Molecular Devices, pClamp10 | https://www.moleculardevices.com/products/axon-patch-clamp-system/acquisition-and-analysis-software/pclamp-software-suite; RRID:SCR_011323 |
| Clampfit 10.0 | Molecular Devices, pClamp10 | https://www.moleculardevices.com/products/axon-patch-clamp-system/acquisition-and-analysis-software/pclamp-software-suite; RRID:SCR_011323 |
| Prism | Graphpad | https://www.graphpad.com/; RRID:SCR_002798 |
| ImageJ/Fiji | ImageJ; Described in Schindelin et al.54 | https://imagej.net/software/fiji/downloads; RRID:SCR_002285 |
| Integrative Genomics Viewer (IGV) | Integrative Genomics Viewer; Described in Robinson et al.55 | https://software.broadinstitute.org/software/igv/download; RRID:SCR_011793 |
| AlphaFold | Described in Jumper et al.; Varadi et al.20,21 | https://alphafold.ebi.ac.uk/; RRID:SCR_023662 |
| HELIQUEST | Described in Gautier et al.24 | https://heliquest.ipmc.cnrs.fr/ |
| iCn3D | NCBI; Described in Wang et al.56 | https://www.ncbi.nlm.nih.gov/Structure/icn3d/icn3d.html |
| CRISPR Optimal Target Finder | Described in Gratz et al.57 | http://targetfinder.flycrispr.neuro.brown.edu/ |
METHOD DETAILS
Transgenic constructs
QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent) was used for site-directed mutagenesis on unedited Cpx7A to generate specified Cpx edit variants that were subcloned into a pVALIUM20 construct (Drosophila Genomics Resource Center (DGRC) #1467), as previously described.10 The resulting constructs were injected into a yw;;attP2 third chromosome docking strain by BestGene Inc (BDSC #8622). UAS lines were recombined into the cpxSH1 null mutant background and elavC155-GAL4 (BDSC #8765) was used for pan-neuronal expression of transgenes.
Generation of CRISPR-modified Cpx strains
Two endogenous Cpx truncation lines were generated (cpxΔ7A and cpxΔ7B) using a CRISPR genome engineering approach. Four guide RNAs (gRNAs) flanking the splice acceptor site of exon 7A or 7B were selected using the CRISPR Optimal Target Finder.57 gRNAs were cloned into the pCFD5 expression vector (Addgene #73914)58 and donor constructs were generated to encode a floxed P3>DsRed reporter cassette (Addgene #51434) in the reverse orientation flanked with one kb homology arms upstream and downstream of the splice acceptor site of either exon 7A or 7B by Gibson assembly protocol using NEBuilder HighFidelity DNA Assembly Cloning Kit (E5520). An early stop codon was inserted between homology arms for each respective exon construct, with several amino acid coding sequences maintained to preserve proper exon splicing. gRNA binding sites of donor template were mutated using silent mutations that did not alter amino acid sequence. Template and gRNA plasmids were co-injected into vas-Cas9 embryos (BDSC #56552) by BestGene Inc and Ds>Red positive transformants were selected by BestGene Inc. The modified locus with stop codons inserted into exon 7A or 7B were confirmed by sequencing.
Locomotion analysis
Adult geotaxis was measured in adult male flies aged 2–3 days as previously described.59 Eight cohorts of ten adult males (80 total flies) per genotype were separated after eclosion and allowed to recover from CO2 for 24 h on standard fly medium. After 24 h, each cohort was moved to a chamber made from two clear plastic vials taped together, with a line drawn around the lower vial 8 cm from the bottom. Each cohort was allowed to acclimate in the chamber for 5 min before assays began. Negative geotaxis was measured as percent of the cohort that crossed the 8 cm line within 10 s after being tapped to the bottom of the chamber. Each cohort was subjected to ten rounds of negative geotaxis assay, with a 1-min rest period between each. The percent of flies that crossed the 8 cm line after each round was averaged to produce a pass rate per cohort. Larval crawling was assayed in third-instar larvae of both sexes, as previously described.60,61 Larvae were briefly washed in room temperature water before placing onto the center of a 5 cm Petri dish containing 2% agarose, with five animals from a single genotype placed together (n = 10 larvae per genotype). The Petri dish was placed over a grid and velocity was measured as average distance (in mm) traveled during the first 30 s following placement.
Immunohistochemistry
Larvae were dissected in hemolymph-like HL3.1 solution (in mM: 70 NaCl, 5 KCl, 4 MgCl2, 10 NaHCO3, 5 trehalose, 115 sucrose, 5 HEPES, pH 7.2) and fixed in 4% paraformaldehyde for 18 min. Larvae were washed three times for 5 min with PBST (PBS containing 0.1% Triton X-100), followed by a 30-min incubation in block solution (5% NGS (normal goat serum) in PBST). Fresh block solution and primary antibodies were then added. Samples were incubated overnight at 4°C and washed with two short washes and three extended 20 min washed in PBST. PBST was replaced with block solution and fluorophore-conjugated secondary antibodies were added. Samples were incubated at room temperature for 2 h. Finally, larvae were rewashed with PBST and mounted in Vectashield (Vector Laboratories). Antibodies used for this study include: mouse anti-Brp, 1:500 (NC82; Developmental Studies Hybridoma Bank (DSHB)); rabbit anti-Cpx, 1:50007; goat anti-rabbit Alexa Fluor 488, 1:500 (A-11008; Thermo Fisher Scientific); goat anti-mouse Alexa Fluor 546, 1:500 (A-11030; Thermo Fisher Scientific); Alexa Fluor 647 conjugated anti-HRP, 1:500 (#123-605-021; Jackson ImmunoResearch).
Confocal imaging and imaging data analysis
Imaging was performed on a Zeiss Pascal confocal microscope (Carl Zeiss Microscopy) using a 63×1.3 NA oil-immersion objective (Carl Zeiss Microscopy). Images were processed with the Zen (Zeiss) software. A 3D image stack was acquired for each NMJ imaged (muscle 4 Ib NMJ of abdominal segment A3) and merged into a single plane for 2D analysis using FIJI image analysis software.54 No more than two NMJs were analyzed per larva. Anti-HRP labeling was used to identify neuronal anatomy (axons and NMJs) and quantify synaptic bouton number and NMJ area. Brp puncta quantification was used to measure AZ number. Muscle 4 area was used to normalize quantifications for muscle surface area. For Cpx fluorescence quantification, the HRP-positive area was used to outline NMJs and axons. Total Cpx fluorescent intensity was measured in the outlined area, with background fluorescence of mean pixel intensity of non-HRP areas subtracted. For NMJ/axon ratios, background subtracted mean NMJ Cpx fluorescence was compared to background subtracted mean axon Cpx fluorescence within the same image.
Two-electrode voltage-clamp electrophysiology
Postsynaptic currents were recorded from third instar muscle 6 at segment A3 using two-electrode voltage clamp with a −80 mV holding potential. Experiments were performed in room temperature HL3.1 saline solution (in mM, 70 NaCl, 5 KCl, 10 NaHCO3, 4 MgCl2, 5 trehalose, 115 sucrose, 5 HEPES, pH 7.2). Final Ca2+ was adjusted to 2 mM unless otherwise noted. Motor axon bundles were cut and suctioned into a glass electrode and action potentials were stimulated at 0.5 Hz (unless indicated) using a programmable stimulator (Master-8, AMPI). Data acquisition and analysis was performed using Axoscope 10.0 and Clampfit 10.0 software (Molecular Devices) and inward currents were labeled on a reverse axis for clarity.
Western blot analysis
Western blotting of adult brain lysates (three heads per sample with ~one brain loaded per lane) was performed using standard laboratory procedures with mouse anti-Tubulin (T5168; Sigma-Aldrich) at 1:10000 (UAS rescue experiments) or 1:1000000 (CRISPR experiments) and rabbit anti-Cpx at 1:5000. IRDye 680LT-conjugated goat anti-mouse, 1:5000 (926–68020; LI-COR Biosciences) and IRDye 800CW-conjugated goat anti-rabbit, 1:5000 (926–32211; LI-COR Biosciences) were used as secondary antibodies. Blocking was performed in a solution containing four parts TBS (10 mM Tris Base pH 7.5, 150 mM NaCl) to one part Blocking Buffer (Rockland Immunochemicals) for 1 h. Antibody incubations were performed in a solution containing four parts TBST (1X TBS with 1% Tween 20) to one part Blocking Buffer. An LI-COR Odyssey Imaging System (LI-COR Biosciences) was used for visualization and analysis was performed using FIJI image analysis software. Relative Cpx expression was calculated by normalizing to Tubulin intensity.
Phosphorylation assays
QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent) was used for site-directed mutagenesis of unedited Cpx7A to generate Cpx7AI125M,N130S (termed N130S). Cpx variants were subcloned into a pGEX-2T construct (GE Healthcare Life Sciences) and the recombinant Cpx variants fused with GST were expressed in BL21 E. coli cells (New England BioLabs) and purified using glutathione Sepharose 4B (Thermo Fisher Scientific). Peak fractions were concentrated and further purified by gel filtration as previously described.10 In vitro kinase assays were performed using purified recombinant Cpx proteins and the catalytic subunit of CK2 (C70-10G; SignalChem). Briefly, 10 mg of purified GST-fusion protein (unedited Cpx7AI125,N130 or edited Cpx7AI125M,N130S) was used per reaction and incubated with 2,500 units of recombinant kinase and [32P]ATP (PerkinElmer). Reaction products were separated by SDS-PAGE and gels were stained with Bio-Safe Coomassie Blue (Bio-Rad), dried, and exposed to autoradiography film at room temperature. Mean integrated density of each band was quantified using FIJI and relative density of phospho-Cpx (pCpx) was calculated by normalizing to input band intensity determined by Coomassie staining.
RNAseq analysis of RNA editing
RNAseq data from 105 single MN1-Ib and 101 single MNISN-Is third instar larval MNs obtained using isoform Patchseq protocols27 were analyzed using the Integrative Genomics Viewer (IGV).55 To create single-cell Cpx RNA editing expression profiles, single RNA reads were analyzed for Cpx and included in the analysis if all three C-terminal Cpx7A edited bases were represented on a continuous single read. The percent of each Cpx7A edit variant was determined by the number of edited variant reads divided by total RNA reads for each cell, creating an RNA editing profile for each neuron. To compare single base editing across different genes, the edit percent at each base of interest was analyzed and compared to known edits in other genes within the same neurons. Neurons were excluded if each base of interest did not contain ten or more reads for all edits of interest.
Data exclusion criteria
For RNAseq analysis of Cpx editing variant expression per cell, single RNA reads were excluded if all three C-terminal Cpx7A edited bases were not represented on a continuous single read, as shown in Figures 3A and 3B. Single neurons were excluded from analysis if each base of interest did not contain ten or more reads for all edits of interest, as shown in Figures 3A–3C and Figures S2A and S2B.
Replication of results
Western blot experiments were carried out in at least six biological replicates containing three adult fly brains per sample. Phosphorylation assays were carried out in at least four in vitro technical replicates. For confocal imaging and TEVC recordings, no more than two NMJs were analyzed per larva. Adult locomotion was measured in eight cohorts of ten flies per genotype and larval locomotion was measured in ten larvae per genotype. All genetic crosses were set up at least twice to obtain reproducible results from replicate to replicate.
QUANTIFICATION AND STATISTICAL ANALYSIS
Experimental design and statistical analysis
Statistical analysis and plot generation was performed using GraphPad Prism software version 9.5.1. Appropriate sample size was determined using a normality test. Statistical significance for comparisons of two groups was determined by a two-tailed Student’s t-test. For comparisons of three or more groups of data, a one-way ANOVA followed by Tukey’s Multiple Comparisons test was used to determine significance when the largest sample standard deviation was no more than twice as large as the smallest sample standard deviation. When the largest sample standard deviation was more than twice as large as the smallest sample standard deviation, a Brown-Forsythe and Welch’s one-way ANOVA followed by Dunnett’s T3 Multiple Comparisons test was used to determine significance. For comparisons of two factors with three or more groups of data, as described in Figure 3A and S2D, a two-way ANOVA followed by Tukey’s Multiple Comparisons test was used. For Figures S2A and S2B, a line of best-fit was generated with 95% confidence intervals displayed. The mean of each distribution is plotted in figures with individual datapoints (n) also shown. Error bars represent ±SEM. Asterisks indicate the following p values: *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001, with ns = not significant.
Supplementary Material
Highlights.
RNA-seq identifies stochastic RNA editing of synaptic genes in single neurons
Multiple Complexin edit variants are expressed in single Drosophila motoneurons
Complexin editing alters the protein’s ability to clamp synaptic vesicle fusion
Co-expression of Complexin edit variants fine tune presynaptic output
ACKNOWLEDGMENTS
We thank the Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN; NIH P40OD018537), the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA), Dina Volfson and Richard Cho for assistance with methods and strain generation, and members of the Littleton lab for helpful discussions. This work was supported by The JPB Foundation and a National Institutes of Health grant (NS40296) to J.T.L. and an NSF grant (1122374) to E.A.B.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.113152.
DECLARATION OF INTERESTS
The authors declare no competing interests.
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
REFERENCES
- 1.Sauvola CW, and Littleton JT (2021). SNARE regulatory proteins in synaptic vesicle fusion and recycling. Front. Mol. Neurosci 14, 733138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizo J. (2022). Molecular mechanisms underlying neurotransmitter release. Annu. Rev. Biophys 51, 377–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quiñones-Frías MC, and Littleton JT (2021). Function of Drosophila Synaptotagmins in membrane trafficking at synapses. Cell. Mol. Life Sci 78, 4335–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorquera RA, Huntwork-Rodriguez S, Akbergenova Y, Cho RW, and Littleton JT (2012). Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. J. Neurosci 32, 18234–18245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bera M, Ramakrishnan S, Coleman J, Krishnakumar SS, and Rothman JE (2022). Molecular determinants of complexin clamping and activation function. Elife 11, e71938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malsam J, Bärfuss S, Trimbuch T, Zarebidaki F, Sonnen AF-P, Wild K, Scheutzow A, Rohland L, Mayer MP, Sinning I, et al. (2020). Complexin Suppresses Spontaneous Exocytosis by Capturing the Membrane-Proximal Regions of VAMP2 and SNAP25. Cell Rep. 32, 107926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huntwork S, and Littleton JT (2007). A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat. Neurosci. 10, 1235–1237. [DOI] [PubMed] [Google Scholar]
- 8.Hobson RJ, Liu Q, Watanabe S, and Jorgensen EM (2011). Complexin maintains vesicles in the primed state in C. elegans. Curr. Biol 21, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin JA, Hu Z, Fenz KM, Fernandez J, and Dittman JS (2011). Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr. Biol 21, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho RW, Buhl LK, Volfson D, Tran A, Li F, Akbergenova Y, and Littleton JT (2015). Phosphorylation of Complexin by PKA Regulates Activity-Dependent Spontaneous Neurotransmitter Release and Structural Synaptic Plasticity. Neuron 88, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson SW, Bourgognon J-M, Spiers JG, Breda C, Campesan S, Butcher A, Mallucci GR, Dinsdale D, Morone N, Mistry R, et al. (2018). Nitric oxide-mediated posttranslational modifications control neurotransmitter release by modulating complexin farnesylation and enhancing its clamping ability. PLoS Biol. 16, e2003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi BJ, Imlach WL, Jiao W, Wolfram V, Wu Y, Grbic M, Cela C, Baines RA, Nitabach MN, and McCabe BD (2014). Miniature neurotransmission regulates Drosophila synaptic structural maturation. Neuron 82,618–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lottermoser JA, and Dittman JS (2023). Complexin membrane interactions: implications for synapse evolution and function. J. Mol. Biol 435, 167774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhl LK, Jorquera RA, Akbergenova Y, Huntwork-Rodriguez S, Volfson D, and Littleton JT (2013). Differential regulation of evoked and spontaneous neurotransmitter release by C-terminal modifications of complexin. Mol. Cell. Neurosci 52, 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho RW, Song Y, and Littleton JT (2010). Comparative analysis of Drosophila and mammalian complexins as fusion clamps and facilitators of neurotransmitter release. Mol. Cell. Neurosci 45, 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikura K. (2016). A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol 17, 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoopengardner B, Bhalla T, Staber C, and Reenan R (2003). Nervous system targets of RNA editing identified by comparative genomics. Science 301, 832–836. [DOI] [PubMed] [Google Scholar]
- 18.Radhakrishnan A, Li X, Grushin K, Krishnakumar SS, Liu J, and Rothman JE (2021). Symmetrical arrangement of proteins under release-ready vesicles in presynaptic terminals. Proc. Natl. Acad. Sci. USA 118, e2024029118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reim K, Wegmeyer H, Brandstätter JH, Xue M, Rosenmund C, Dresbach T, Hofmann K, and Brose N (2005). Structurally and functionally unique complexins at retinal ribbon synapses. J. Cell Biol 169, 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Židek A, Potapenko A, et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yorda-nova G, Yuan D, Stroe O, Wood G, Laydon A, et al. (2022). Alpha-Fold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snead D, Wragg RT, Dittman JS, and Eliezer D (2014). Membrane curvature sensing by the C-terminal domain of complexin. Nat. Commun 5, 4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong J, Lai Y, Li X, Wang M, Leitz J, Hu Y, Zhang Y, Choi UB, Cipriano D, Pfuetzner RA, et al. (2016). C-terminal domain of mammalian complexin-1 localizes to highly curved membranes. Proc. Natl. Acad. Sci. USA 113, E7590–E7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautier R, Douguet D, Antonny B, and Drin G (2008). HELIQUEST: a web server to screen sequences with specific alpha-helical properties. Bioinformatics 24, 2101–2102. [DOI] [PubMed] [Google Scholar]
- 25.Aponte-Santiago NA, and Littleton JT (2020). Synaptic properties and plasticity mechanisms of invertebrate tonic and phasic neurons. Front. Physiol 11,611982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansen J, Halpern ME, Johansen KM, and Keshishian H (1989). Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J. Neurosci 9, 710–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jetti SK, Crane AB, Akbergenova Y, Aponte-Santiago NA, Cunningham KL, Whittaker CA, and Littleton JT (2023). Molecular logic of synaptic diversity between Drosophila tonic and phasic motoneurons. Neuron. 10.1016/j.neuron.2023.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Bulat V, Rast M, and Pielage J (2014). Presynaptic CK2 promotes synapse organization and stability by targeting Ankyrin2. J. Cell Biol 204, 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wragg RT, Snead D, Dong Y, Ramlall TF, Menon I, Bai J, Eliezer D, and Dittman JS (2013). Synaptic vesicles position complexin to block spontaneous fusion. Neuron 77, 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makke M, Mantero Martinez M, Gaya S, Schwarz Y, Frisch W, Silva-Bermudez L, Jung M, Mohrmann R, Dhara M, and Bruns D (2018). A mechanism for exocytotic arrest by the Complexin C-terminus. Elife 7, e38981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courtney KC, Wu L, Mandal T, Swift M, Zhang Z, Alaghemandi M, Wu Z, Bradberry MM, Deo C, Lavis LD, et al. (2022). The complexin C-terminal amphipathic helix stabilizes the fusion pore open state by sculpting membranes. Nat. Struct. Mol. Biol 29, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malsam J, Seiler F, Schollmeier Y, Rusu P, Krause JM, and Söllner TH (2009). The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc. Natl. Acad. Sci. USA 106, 2001–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang Q, Ofosuhene AP, Kiessling V, Liang B, Kreutzberger AJB, Tamm LK, and Cafiso DS (2022). Complexin-1 and synaptotagmin-1 compete for binding sites on membranes containing PtdInsP2. Biophys. J 121, 3370–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulgari D, Cavolo SL, Schmidt BF, Buchan K, Bruchez MP, Deitcher DL, and Levitan ES (2023). Ca2+ and cAMP open differentially dilating synaptic fusion pores. J. Cell Sci 136, jcs261026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshihara M, Adolfsen B, Galle KT, and Littleton JT (2005). Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science 310, 858–863. [DOI] [PubMed] [Google Scholar]
- 36.Shi VH, Craig TJ, Bishop P, Nakamura Y, Rocca D,Wilkinson KA, and Henley JM (2021). Phosphorylation of Syntaxin-1a by casein kinase 2α regulates pre-synaptic vesicle exocytosis from the reserve pool. J. Neurochem 156, 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao H, Das D, Courtney NA, Jiang Y, Briguglio JS, Lou X, Roston D, Cui Q, Chanda B, and Chapman ER (2018). Dynamics and number of trans-SNARE complexes determine nascent fusion pore properties. Nature 554, 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domanska MK, Kiessling V, Stein A, Fasshauer D, and Tamm LK (2009). Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J. Biol. Chem 284, 32158–32166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hua Y, and Scheller RH (2001). Three SNARE complexes cooperate to mediate membrane fusion. Proc. Natl. Acad. Sci. USA 98, 8065–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foletti DL, Lin R, Finley MA, and Scheller RH (2000). Phosphorylated syntaxin 1 is localized to discrete domains along a subset of axons. J. Neurosci 20, 4535–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi L, Shen Q-T, Kiel A, Wang J, Wang H-W, Melia TJ, Rothman JE, and Pincet F (2012). SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science 335, 1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett MK, Miller KG, and Scheller RH (1993). Casein kinase II phosphorylates the synaptic vesicle protein p65. J. Neurosci 13, 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shata A, Saisu H, Odani S, and Abe T (2007). Phosphorylated synaphin/complexin found in the brain exhibits enhanced SNARE complex binding. Biochem. Biophys. Res. Commun 354, 808–813. [DOI] [PubMed] [Google Scholar]
- 44.Robinson JE, Paluch J, Dickman DK, and Joiner WJ (2016). ADAR-mediated RNA editing suppresses sleep by acting as a brake on glutamatergic synaptic plasticity. Nat. Commun 7, 10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Overton IM, Baines RA, Keegan LP, and O’Connell MA (2014). The ADAR RNA editing enzyme controls neuronal excitability in Drosophila melanogaster. Nucleic Acids Res. 42, 1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maldonado C, Alicea D, Gonzalez M, Bykhovskaia M, and Marie B (2013). Adar is essential for optimal presynaptic function. Mol. Cell. Neurosci 52, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shumate KM, Tas ST, Kavalali ET, and Emeson RB (2021). RNA editing-mediated regulation of calcium-dependent activator protein for secretion (CAPS1) localization and its impact on synaptic transmission. J. Neurochem 158, 182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Birk MA, Liscovitch-Brauer N, Dominguez MJ, McNeme S, Yue Y, Hoff JD, Twersky I, Verhey KJ, Sutton RB, Eisenberg E, et al. (2023). Temperature-dependent RNA editing in octopus extensively recodes the neural proteome. Cell 186, 2544–2555.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sommer B, Köhler M, Sprengel R, and Seeburg PH (1991). RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67, 11–19. [DOI] [PubMed] [Google Scholar]
- 50.Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, et al. (2011). The developmental transcriptome of Drosophila melanogaster. Nature 471, 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sapiro AL, Shmueli A, Henry GL, Li Q, Shalit T, Yaron O, Paas Y, Billy Li J, and Shohat-Ophir G (2019). Illuminating spatial A-to-I RNA editing signatures within the Drosophila brain. Proc. Natl. Acad. Sci. USA 116, 2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palladino MJ, Keegan LP, O’Connell MA, and Reenan RA (2000). A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell 102, 437–449. [DOI] [PubMed] [Google Scholar]
- 53.Tran SS, Jun H-I, Bahn JH, Azghadi A, Ramaswami G, Van Nostrand EL, Nguyen TB, Hsiao Y-HE, Lee C, Pratt GA, et al. (2019). Widespread RNA editing dysregulation in brains from autistic individuals. Nat. Neurosci 22, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, and Mesirov JP (2011). Integrative genomics viewer. Nat. Biotechnol 29, 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Youkharibache P, Zhang D, Lanczycki CJ, Geer RC, Madej T, Phan L, Ward M, Lu S, Marchler GH, et al. (2020). iCn3D, a web-based 3D viewer for sharing 1D/2D/3D representations of biomolecular structures. Bioinformatics 36, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, and O’Connor-Giles KM (2014). Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196, 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Port F, and Bullock SL (2016). Augmenting CRISPR applications in Drosophila with tRNA-flanked sgRNAs. Nat. Methods 13, 852–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali YO, Escala W, Ruan K, and Zhai RG (2011). Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. J. Vis. Exp 2504. 10.3791/2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kashima R, Redmond PL, Ghatpande P, Roy S, Kornberg TB, Hanke T, Knapp S, Lagna G, and Hata A (2017). Hyperactive locomotion in a Drosophila model is a functional readout for the synaptic abnormalities underlying fragile X syndrome. Sci. Signal 10, eaai8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nichols CD, Becnel J, and Pandey UB (2012). Methods to assay Drosophila behavior. J. Vis. Exp 3795. 10.3791/3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This paper analyzes existing, publicly available RNAseq data. All Isoform-Patchseq RNA profiling raw data is available at NCBI under the GEO accession #GSE222976. All other data reported in this paper will be shared by the lead contact upon request.
This paper does not report any original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


