Abstract
The vestibular cerebellum plays an essential role in maintaining our balance and ensuring perceptual stability during activities of daily living. Here I examine three key regions of the vestibular cerebellum: the floccular lobe, anterior vermis (lobules I-V), and nodulus and ventral uvula (lobules X-IX of the posterior vermis). These cerebellar regions encode vestibular information and combine it with extra-vestibular signals to create internal models of eye, head, and body movements, as well as their spatial orientation with respect to gravity. To account for changes in the external environment and/or biomechanics during self-motion, the neural mechanisms underlying these computations are continually updated to ensure accurate motor behavior. To date, studies on the vestibular cerebellum have predominately focused on passive vestibular stimulation, whereas in actuality, most stimulation is the result of voluntary movement. Accordingly, I also consider recent research exploring these computations during active self-motion and emerging evidence establishing the cerebellum’s role in building predictive models of self-generated movement.
Keywords: proprioception, visual, multimodal, multisensory, efference copy, corollary discharge, spatial orientation
Fundamental neural computations for self-motion
The vestibular system comprises five distinct sensory organs that detect the head’s motion in three translational and three rotational dimensions to ensure accurate behavior and perceptual stability. The two otolith organs - the saccule and utricle - sense linear acceleration (i.e., gravity and translational motion), while the three semicircular canals sense angular acceleration. The receptor cells of the otoliths and semicircular canals send signals via the vestibular nerve to vestibular nuclei of the brainstem (reviewed in[1,2]). In turn, three main regions of the cerebellar cortex receive vestibular input from the vestibular nuclei as well as, in one case, directly from peripheral vestibular nerve afferents (Figure 1A): i) the floccular lobe, ii) lobules I-V of the anterior vermis, and iii) the nodulus and ventral uvula (lobules X-IX) of the posterior vermis.
Figure 1. Overview of the vestibular cerebellum and its circuitry.

(A) Schematic of the vestibular cerebellum showing the anterior vermis (green), nodulus and uvula of the posterior vermis (blue), and flocculonodular lobes (light red) along the rostral–caudal axis. Perpendicular to this, along the medial–lateral axis, the cerebellum can also be divided into three longitudinal zones, including the vermis (I–X), intermediate hemispheres, and lateral hemispheres, which project to the fastigial, interpositus, and dentate deep cerebellar nuclei respectively, as well as to the vestibular nuclei. The lateral hemispheres are also commonly referred to as the cerebro-cerebellum. The fastigial deep cerebellar nucleus and vestibular nuclei (yellow) receive direct input from the Purkinje cells of the vestibular cerebellar cortex. (B) Purkinje cells receive two specific sources of input each of which differentially affect the Purkinje cell responses. Mossy fiber (MFs) inputs act via interneurons to induce high frequency simple spike responses. In contrast, climbing fiber inputs induce low frequency complex spikes. Purkinje cells provide the sole output of the cerebellar cortex and project to the deep cerebellar and vestibular nuclei. Unipolar brush cells, a class of local circuit neurons that are abundant in the nodulus / uvula and flocculonodular lobes are thought to amplify the mossy fiber input via feedforward excitation. (C) Schematic of an extracellular recording from a Purkinje cell showing high-frequency simple spike as well as a complex, climbing fiber response. Note that climbing fiber excitation is followed by a pause in simple spike firing.
Collectively these regions, herein referred to as the vestibular cerebellum, play an essential role in coordinating movement and balance during our everyday activities. As a result, patients with cerebellar dysfunction display impairments in balance and postural stability[3–5]. Moreover, in addition to its well-known contributions to maintaining postural stability and controlling gaze, the vestibular cerebellum plays a key role in our perception of self-motion and spatial orientation. For instance, patients with cerebellar loss demonstrate impairments in their ability to navigate through space[6], consistent with their difficulty in judging distances and scale (dysmetria). In addition, their perception of sustained self-motion is impaired[7,8]. Patients with cerebellar impairments also display worse head motion detection thresholds compared to healthy individuals[9] and comprised perception of verticality relative to the world[10] characterized by an increased influence of visual versus vestibular cues[11].
Like all regions of the cerebellar cortex, the vestibular cerebellum receives two distinct sources of input. Mossy fibers originate in diverse sensory and motor regions and innervate granule cells, which in turn, send their vast parallel fiber projection to synapse on the distal dendrites of Purkinje cells. Climbing fibers arise from the inferior olivary nucleus and synapse on the proximal dendrites of Purkinje cells (Figure 1B). Accordingly, Purkinje cells - the sole output of the cerebellar cortex – fire two types of spikes, simple spikes that are responsible for the majority of their firing activity, and complex spikes that are far less frequent and are generated by their climbing fiber input (Figure 1C).
A common principle across the vestibular cerebellum is that vestibular information is integrated with extra-vestibular self-motion cues (i.e., proprioceptive and visual sensory inputs as well as motor signals). This integration underlies the computation of essential internal models of self-motion for accurate motor control. These internal models are required to coordinate eye, head, and body movements in response to unexpected versus expected changes in head motion, and to encode vital information regarding the head’s orientation relative to gravity. Neurophysiological studies have identified distinct computations in each of these three regions. Floccular lobe Purkinje cells integrate vestibular with visual signals to calculate an internal models of the eye movements required for stable gaze (reviewed in[12,13]). Anterior vermis Purkinje cells integrate vestibular with proprioceptive signals to generate internal models of body versus head movement, essential for the robust control of posture and balance (reviewed in[1]). And the Purkinje cells of the nodulus/ventral uvula integrate vestibular semicircular canal and otolith signals to compute an internal model of the head’s position with respect to gravity (reviewed in[14,15]).
Below, I review the vestibular cerebellum from this functional, neural circuit, and neural coding perspective. I consider the distinct computations performed by each of these three main regions of the vestibular cerebellar cortex in experiments where vestibular stimulation was passively applied. However, in everyday life most vestibular stimulation arises due to our own voluntary movement. Thus, I also review recent work that has advanced our understanding of how the cerebellum integrates extra-vestibular motor and vestibular signals to discriminate actively generated stimulation (termed vestibular reafference) from externally applied stimulation (termed vestibular exafference). To date, our understanding of the vestibular cerebellum is based largely on studies performed in macaque monkeys, and to some extent humans. Additionally, the mouse has proven to be an increasingly advantageous model for bridging the gap between neuronal responses, circuits, and behavior due to advances in genetically engineered mouse lines. Finally, I consider how the computations performed in each region mediate the long-term learning required to ensure accurate motor control and perceptual stability during our everyday activities and consider evidence for predictive models of actively generated self-motion.
The Floccular Lobe: Adaptive Plasticity Ensures Gaze Stability.
The flocculus and adjoining ventral paraflocculus (i.e., the floccular lobe) are essential for the motor learning required to keep the vestibulo-ocular reflex (VOR) calibrated across our life span. The VOR ensures stable gaze during head motion by generating compensatory eye movements that are equal and opposite to ongoing head movements (Figure 2A, left panel). Continuous calibration of this vital reflex is required to provide visual stability during our everyday activities. For example, after putting on a pair of glasses with a new prescription, initially head motion will result in retinal image motion (Figure 2A, center panel). However, over time the VOR is modified to account for the new relationship between eye and head motion (Figure 2A, right panel). The floccular lobe also plays a key role in mediating the VOR compensation that occurs following peripheral vestibular loss due to aging, disease, surgery, and/or injury.
Figure 2. VOR motor learning and computations underlying gaze stability.
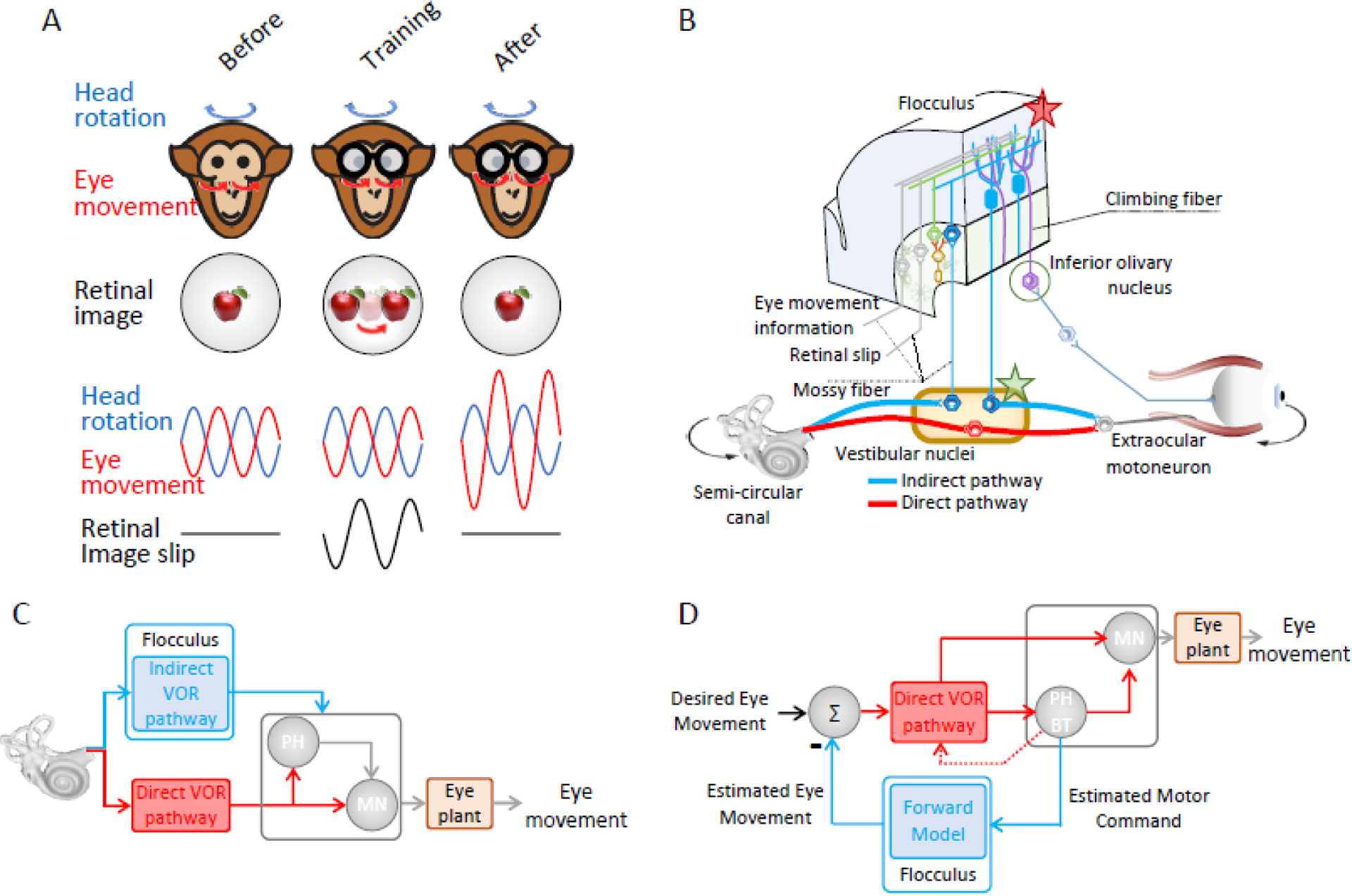
(A) Schematic of a training paradigm in primates designed to induce an increase in VOR gain. Initially, the VOR gain is ~1 (eyes move with the same amplitude, but in the opposite direction from the head). The subject then wears 2X magnifying spectacles, which initially cause retinal image slip in the direction opposite that of head motion. Over time, this visual error signal induces an increase in VOR gain and as a result the retinal image slip is reduced. (B) Two pathways control the gain of the VOR. First, the direct reflex pathway (red) comprises a three-neuron arc: vestibular afferents project to vestibular nuclei neurons that in turn project to the eye muscle motoneurons. This direct pathway is paralleled by an inhibitory side loop (blue) through the floccular lobe. As a result, the total gain is determined by the net drive from these two pathways. During visually induced VOR motor learning, climbing fibers encode a visual error signal which induces learning at the synapses of vestibular parallel fibers onto the Purkinje cells (red star). Then over time, motor learning is consolidated via modification of synapses in the target neurons of the vestibular nuclei (green star). (C) Schematic diagram of the inverse model of eye movement generation the floccular lobe. Its drive must be matched to that of premotor centers, such as the nucleus prepositus hypoglossi (PH) controlling the direct VOR, to generate the proper net command to drive motoneurons (MN) and account for the biomechanics of the eye plant. (D) Because mossy fiber projections to the floccular lobe encode eye movement as well as vestibular information, it has been proposed that the floccular lobe could serve as a “forward” as well as an inverse model.
Lesions of the floccular lobe impair the adaptive plasticity that underlies VOR motor learning and compensation across animal species including humans[16]. In addition, this cerebellar region plays a more general role in the control of eye movements including the maintenance of stable gaze (i.e., gaze holding) and the generation of smooth pursuit and optokinetic eye movements (reviewed in[17,18]). Accordingly, patients with cerebellar disease affecting the floccular lobe display deficits in gaze stability during their everyday activities (reviewed in[1]). Targeted lesions in monkeys further suggest that the relative contribution of ventral paraflocculus may be more significant than that of the flocculus in mediating VOR motor learning[19].
Like all regions of cerebellar cortex, the floccular lobe receives two major sources of input: mossy fiber and climbing fiber inputs (Figure 1B). Mossy fiber projections to the floccular lobe originate in a variety of nuclei in the oculomotor and vestibular brainstem nuclei, and predominately encode vestibular and eye movement information[20,21], while its climbing fiber inputs originate in the inferior olive and predominately relay image motion on the retina (retinal slip) to the floccular lobe[22]. Mossy fiber projections from the dorsolateral pontine nuclei also relay visual information to the floccular lobe (reviewed in[17]), consistent with it playing a key role, together with the cerebellar oculomotor vermis, in the control of visually driven eye movements, as has been comprehensively reviewed elsewhere [17,18]. Indeed, the commands that drive these visually driven eye movements can be used to cancel the VOR when the behavioral goal is to redirect rather than stabilize gaze. Because the eye movement produced by the VOR keep gaze stationary relative to the world, it must be overridden (“cancelled”) to redirect gaze in the direction of on-going head motion[23].
The VOR motor learning and computations underlying gaze stability
For the last half-century, research on the floccular lobe has been pivotal in advancing our understanding of the fundamental mechanisms that govern cerebellar motor learning. David Marr[24] and James Albus[25] initially proposed an influential theoretical model of the cerebellum. This model hypothesized that sensorimotor learning was driven by errors signals carried by climbing-fibers that, in turn, induced synaptic plasticity at the parallel fiber-Purkinje cell synapses. It further posited that climbing fibers signal movement errors that, in turn, reduce the strength of the parallel fiber-Purkinje cell synapses. Since then, numerous groups have leveraged the well-characterized circuitry that generates the VOR to test the Marr-Albus model and have significantly advanced our understanding of the neural mechanisms that underlie cerebellar learning.
Our current knowledge of the neurophysiological mechanisms underlying the adaptive plasticity compensation of the VOR has greatly benefitted from different animal models, spanning from the Xenopus tadpole to monkey (reviewed in[26]). Figure 2B illustrates the two main parallel pathways mediate the VOR. First, a direct pathway comprising a 3-neuron arc is shown; vestibular afferents send head motion signals to vestibular nuclei neurons that, in turn, directly project to extraocular motoneurons. The second pathway is mediated by projections from the vestibular nuclei to the cerebellar floccular lobe, which are subsequently followed by projections from Purkinje cells in the floccular lobe directly back to the vestibular nuclei. This pathway effectively creates an “adjustable gain” side loop that regulates the gain of the VOR[27–29]. An early study in rabbit [30] aimed to directly test Marr-Albus’s theoretical model by leveraging this well-characterized circuitry. Consistent with the Marr-Albus model, the authors experimentally demonstrated that stimulation of the climbing fiber input to the flocculus reduced the strength of the parallel fiber-Purkinje cell synapse when parallel fibers were simultaneously activated. Specifically, climbing fiber spiking induced profound depolarization in target Purkinje cells that resulted in long term depression (LTD) and induction of plasticity in parallel fiber-Purkinje cell synapses. An earlier publication [22] showed, additionally, that individual floccular climbing fibers signal retinal image motion. Importantly, this latter result established that climbing fiber input indeed provides a visual error signal. Consequently, floccular climbing fibers signal when the VOR is no longer compensatory, consistent with the predictions of the Marr-Albus model.
Collectively, these and related findings led to the prevailing view that visual error induces a modification in the strength of the parallel-fiber-Purkinje-cell synapses (Figure 2B, red star). In turn, this resultant change in synaptic strength alters the contribution of the indirect VOR pathway. Indeed, investigations in transgenic mice lacking key elements of the signaling pathways required for LTD have confirmed its role in cerebellar plasticity (reviewed in[31,32]). However, more recent studies have served to further refine our understanding of the cellular mechanisms underlying cerebellar VOR motor learning (BOX 1). Notably, while climbing fiber-induced weakening of parallel fiber - Purkinje cell synapses (LTD) leads to a VOR gain increase, climbing fiber-induced strengthening of this same synapse can lead to a VOR gain decrease. Additionally, synergistic plasticity at other synapses within granular and molecular layers of the floccular lobe as well as vestibular nuclei can further optimize learning and behavior (reviewed[33,34]). Lastly, plasticity at the level of the vestibular nuclei is thought to be largely responsible for the long-term consolidation of motor learning. Specifically gain changes, which are initially stored in the floccular cerebellum during motor learning, subsequently drive the formation of long-term synaptic changes at the level of the vestibular nuclei (Figure 2B, green star). This proposal - termed the transfer or consolidation hypothesis - is consistent with existing inactivation and lesion studies (reviewed in[35]). Computational modeling studies have provided a powerful means to investigate the properties that emerge from the dynamic interplay of the multiple forms of plasticity observed across different sites within the floccular cerebellum during VOR motor learning[36,37].
Box 1: Cellular mechanisms underlying cerebellar VOR motor learning.
The long-standing view is that LTD at the parallel fiber–Purkinje cell synapse is the principal form of plasticity mediating VOR motor learning. However, other forms of plasticity also make substantive contributions. Electrophysiological experiments have established that long term potentiation (LTP) as well as LTD can be induced at parallel fiber-Purkinje cell synapses[109,110]. Furthermore, the induction of synaptic plasticity at other synapses within granular and molecular layers of the floccular lobe (reviewed in[33,34]) can work in a coordinated manner with the parallel fiber–Purkinje cell LTD to enhance VOR motor learning. As a result, VOR motor learning deficits, while present, are not complete in transgenic mice with impaired cerebellar LTD[111,112], and VOR motor learning can still occur in the absence of complex spiking in Purkinje cells[113,114].
Mechanistically distinct plasticity pathways within the flocculus also play differential roles in producing learned increases versus decreases in VOR gain (reviewed in[35]). Cerebellar LTD at the parallel fiber-Purkinje cell synapse mediates increases in VOR gain, while LTP of the same synapse can mediate decreases in VOR gain[111,115–117]. Furthermore, gain decrease learning produces relatively small changes in simple spike activity[46,118,119], suggesting that climbing fiber signaling preferentially influence plasticity at the level of the vestibular and cerebellar nuclei, rather than at the parallel fiber-Purkinje cell synapse. Indeed, mutant mice with both impaired cerebellar LTD and LTP can produce gain–decrease but not gain–increase learning[116]. Such underlying mechanistic differences can account for several experimental observations, including the finding that VOR gain decrease learning decays more slowly[120], is less easily reversed[121], and displays more specificity for the training frequency[122,123], compared to gain-increase learning.
Finally, in addition to the multiple sites and mechanisms of plasticity within the cerebellar flocculus, plasticity at downstream sites within the vestibular nuclei also ultimately shapes VOR motor performance. In-vitro studies have demonstrated that either LTD or LTP can be induced in the vestibular nuclei depending on membrane potential and/or stimulation conditions[124,125]. Moreover, behaviorally-relevant patterns of vestibular nerve activation can produce LTD at the afferent-vestibular nuclei neuron synapse in alert monkeys[126]. Plasticity within the vestibular nuclei also makes substantive contributions to the VOR motor learning required to compensate for the loss of vestibular nerve input[127]. Such loss of vestibular sensory input also evokes rapid homeostatic plasticity that unmasks and upweights extra-vestibular signals such as neck proprioceptive and efference copy signals in the vestibular nuclei during active behaviors[70,128–130].
In the context of VOR motor learning, it is further noteworthy that climbing fibers appear to convey nonvisual as well as visual signals to the flocculus. In particular, there is evidence that climbing fibers transmit vestibular signals and/or motor efference copy signals (e.g.,[38–40]) that elicits robust modulation in Purkinje cell responses[41]. Thus, the error signals guiding VOR motor learning are most likely multimodal; however, implications of this possibility are not yet well understood. Furthermore, recent investigations have established that the climbing fiber-induced complex spikes in floccular Purkinje cells are not binary events, as has been generally assumed, but are instead graded in nature. Increased complex spike duration has been reported to correlate with higher single-trial plasticity[42]. This result is interesting since an increase in duration would theoretically lead to more calcium entry into Purkinje cells - producing larger synaptic depression, and ultimately stronger learning (reviewed in[43]).
Internals models of eye movement and neural coding strategies
At a functional and computational level, the cerebellum is generally considered to be essential for the computation of ‘internal models’ required for accurate behavior during self-motion. In response to passively applied vestibular stimulation, it has been proposed that floccular Purkinje cells encode a neural representation of eye movements consistent with a class of internal models called inverse models (reviewed in[13,44]). Inverse models account for the motor command that is required to generate a particular movement. Floccular Purkinje cells project directly to neurons in the vestibular nuclei that in turn project to eye muscle motoneurons. Thus, the simple spike output of floccular Purkinje cells is required to match the dynamics of the brainstem premotor circuits and biomechanics of the eyeball – together referred to as the “oculomotor plant” to generate accurate compensatory VOR eye movements. Thus, it has been proposed that the indirect pathway through the flocculus encodes an inverse model of these “plant” dynamics (Figure 2C) (see[13]). However, the situation is more complex since, as reviewed above, mossy fiber projections to the floccular lobe encode eye movement as well as vestibular information. For this reason, it has been argued that the floccular lobe could serve as a “forward” as well as an inverse model (Figure 2D;[12,13]). Forward models make predictions of the expected sensory input based on estimates of the motor commands that have been sent to the muscles. Theoretically, the difference between this prediction and the actual sensory feedback can be calculated to compute a “sensory prediction error signal”. Such an error signal could be used to update the VOR command generated by the primary brainstem pathway via Floccular Purkinje cell projections onto vestibular nuclei.
To date most studies have focused on the responses of individual floccular Purkinje cells. However, several recent studies have investigated how the patterning of action potentials across populations of neurons influences coding and motor performance. Indeed, there is longstanding evidence for synchronous firing in the cerebellum; the climbing fiber input to the cerebellum is well synchronized[45] and Purkinje cell simple spiking can be synchronized, in vivo[46,47]. Moreover, the level of synchronization across Purkinje cells can alter the action potential timing and patterning of their target neurons in the deep cerebellar and vestibular nuclei (reviewed in[48]). A recent study reported that the vestibular and eye movement information encoded by individual Purkinje cells is invariant to changes in spike irregularity induced by optogenetic stimulation[49]. However, at the population level, a negative interaction has been reported between induction of synchronized Purkinje cell firing via optogenetics and coding fidelity[50]. Future studies will be required to understand the population response of floccular Purkinje cells and how it contributes to optimizing VOR motor learning.
The Anterior Vermis: lobules I–V of the anterior cerebellar vermis
Lobules I-V of the anterior vermis are involved in two essential computations. First, this region transforms the vestibular signals experienced during self-motion from the head-centered reference frame of the vestibular sensory organs (i.e., the semicircular canals and otoliths are in the head), into the body-centered reference frame required for accurate postural control. Second, it plays a critical role in distinguishing self-generated from externally applied vestibular inputs. In everyday life, our vestibular system is simultaneously activated by stimuli that result from changes in the external world (exafference) and those that are the result of our own motor actions (reafference). The brain’s ability to distinguish between vestibular exafference and reafference is essential for both perceptual stability and for accurate motor control. Patients with cerebellar disease affecting the anterior vermis display symptoms consistent with disruption of these two computations, including postural imbalance, unsteady gait, and impaired motor coordination[4,5,51,52].
Mossy fiber inputs to the anterior vermis originate in vestibular, cuneate, as well as other brainstem nuclei and robustly drive Purkinje cell simple spike activity in response to vestibular and proprioceptive stimulation (reviewed in[2]). Cutaneous stimulation can evoke complex spikes in Purkinje cells[53], but whether vestibular inputs can similarly drive complex spikes is not known. Anterior vermis Purkinje cells, in turn, directly project to both the vestibular nuclei and the rostral portion of the fastigial nucleus — the most medial of the deep cerebellar nuclei. Accordingly, targeted lesions of the rostral fastigial nucleus in monkeys produce impaired postural control and balance[54–56], consistent with what is observed in patients with compromised anterior vermis function.
Transformation head-centered vestibular signals into a body-centered referenced frame
Vestibular spinal reflexes are essential for generating postural adjustments to ensure the maintenance of balance and posture during our everyday activities. Importantly, however, these pathways must account for the head’s position relative to the body in order to generate the appropriate postural control commands. To account for head position, the brain integrates neck proprioceptive information with vestibular input. This integration results in the transformation of head-centered vestibular information into a body-centric reference frame (reviewed in[57]). The anterior vermis plays an essential role in the computations that underlie this required transformation. Inhibition of cerebellar vermis function impairs the normal modulation of vestibulospinal pathways that occurs to account for changes in the head’s position relative to the body[3,58]. Moreover, single unit recording studies in both cats and monkeys have shown that the simple spike activity of most anterior vermis Purkinje cells encodes both vestibular and neck proprioceptive-related information[59,60].
Recent studies in primates have further established how these two streams of sensory information are ultimately transformed into an estimate of body motion (Figure 3A). Anterior vermis Purkinje cells respond to vestibular and neck proprioceptive stimulation with considerable heterogeneity (Figure, 3B, gray shading;[60]) and encode an intermediate representation of self-motion in between head and body motion. In contrast, their target neurons in the deep cerebellar nuclei fall into two discrete classes-termed unimodal versus bimodal - that unambiguously encode distinct representations of head versus body motion, respectively[61–63](Figure 3B, compare magenta and orange, with gray shaded areas). Furthermore, the vestibular and proprioceptive responses of Purkinje cells and deep cerebellar nuclei neurons both show tuning as a function of static head position (Figure 3C). Computational approaches have demonstrated that responses of ~40 anterior vermis Purkinje cells can explain the fully transformed responses of body motion encoding neurons deep cerebellar nuclei (Figure 3A; [60]). Interestingly, such a convergence ratio is consistent with the results of prior estimates based on anatomical analyses[48,64]. It is likely that the heterogeneity of Purkinje cell responses to vestibular and/or neck proprioceptive stimulation constitutes a basis set for sensorimotor errors that provides the plasticity required to generate accurate movements[65] as well as ensure robust calibration over time.
Figure 3. The neural encoding of body motion: the integration of vestibular and neck proprioceptive signals.
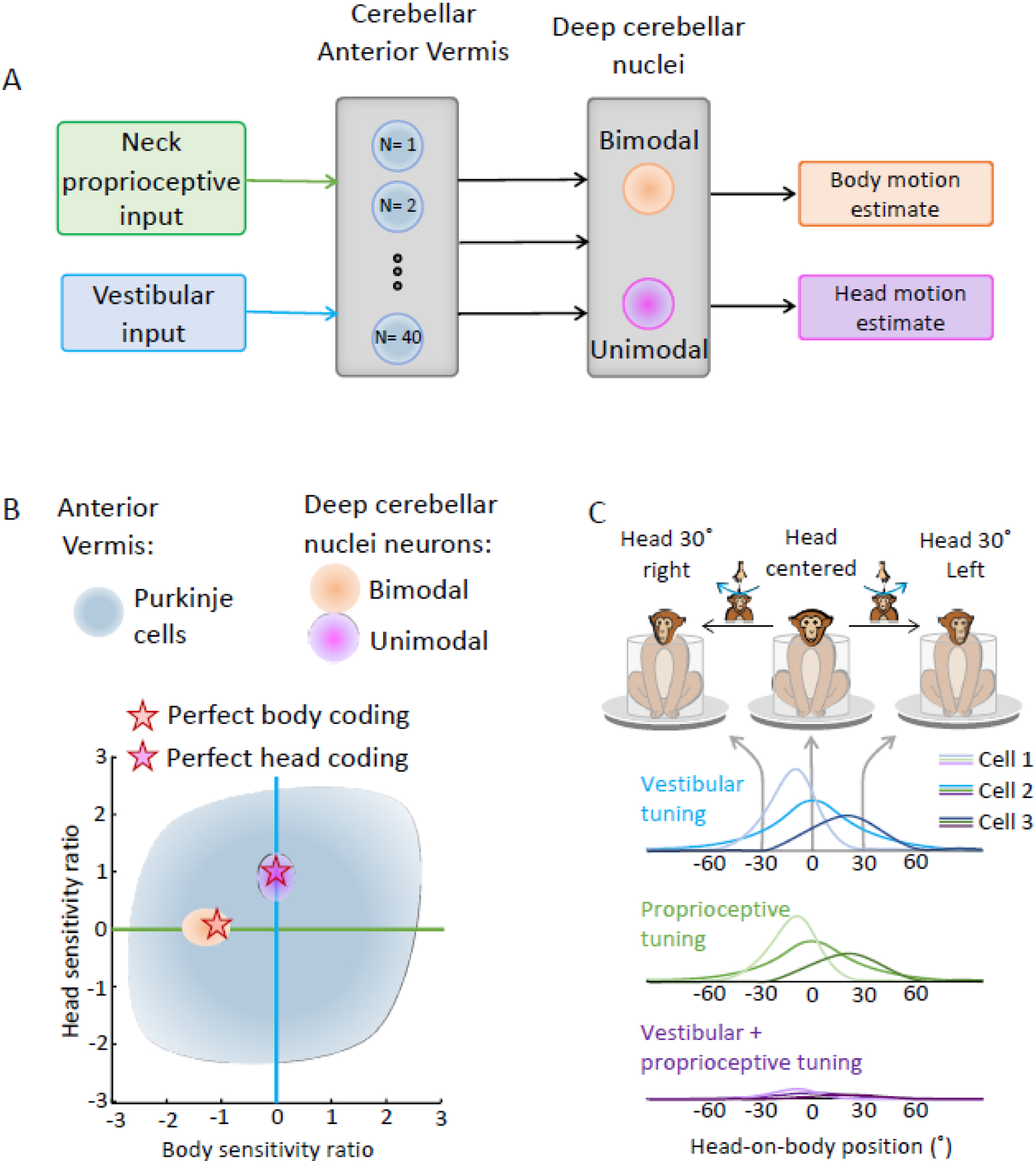
(A) Schematic of the proposed mechanisms for vestibular–neck convergence in the anterior vermis of the cerebellum, which then projects to the rostral fastigial nucleus (see text for details). (B) Scatter plot of the relationship between the head versus body sensitivity ratios of anterior vermis Purkinje cells[60] versus unimodal and bimodal rostral fastigial nucleus (rFN) neurons[61]. Orange versus magenta stars indicate ideal encoding of body versus head movement in space, respectively. (C) Cerebellar processing of both neck proprioceptive and vestibular inputs is characterized by a nonlinear operation in which head-on-body position modulates the gain of vestibular and dynamic neck proprioceptive responses. These two inputs sum linearly on rostral fastigial nucleus neurons during combined stimulation, and because a given neuron has similar tuning to both sensory inputs, it encodes body motion relative to space.
An internal model that predicts the sensory consequences of active self-motion
The anterior vermis is also involved in a second essential computation, namely the construction of an internal model that predicts the sensory consequences of actively generated self-motion. In everyday life, our sensory systems are simultaneously activated by stimuli that result from changes in the external world (i.e., exafference) and by stimuli that are the result of our own motor actions (i.e., reafference). This fact has implications for the vestibular system, where the ability to distinguish between expected vestibular reafference and exafference is critical for ensuring postural and perceptual stability. This is because, while robust vestibulo-spinal reflexes are essential for stabilizing our balance in response to unexpected vestibular stimuli, these same reflexes would be counter-productive if they remained intact when our behavioral goal is to instead move through space.
Indeed, the vestibular responses of neurons at the first central stage of processing in vestibulo-spinal reflexes (i.e., the “vestibular-only” or “VO” neurons of the vestibular nuclei) are markedly suppressed (~70–80%) during active movements. Over the past several decades, a systematic series of studies have been performed where the correspondence between intended and actual head movement was experimentally controlled (Figure 4A). Taken together, this work has established that this reafferent vestibular suppression only occurs when the brain’s estimate (internal model) of the expected proprioceptive consequences of active self-motion matches the actual sensory proprioceptive feedback that is experienced – as is the case during normal voluntary self-motion (Fig. 4B, left yellow box; for review, see[1,66]).
Figure 4.

Computations that suppress the encoding of active self-motion. (A) Vestibular nuclei VO neurons but not vestibular afferents display attenuated responses to active versus passive self-motion. The first 3 columns compare a typical vestibular afferent’s versus VO neuron’s responses during passive, active, and simultaneous active and passive self-motion. In the latter condition, head-in-space motion equals the sum of the passive rotation generated by the turntable and voluntary head-on-body movements. While afferents encode total head motion (black superimposed traces), VO neurons selectively encoded the passive component of the motion (blue superimposed traces). The fourth column illustrates an experiment in which the vestibular feedback of an active movement is cancelled by passively counterrotating the whole body in real time via a servo-loop. In this condition, there is no head motion and so the afferent is unresponsive as expected. However, because there is a match between actual and expected proprioceptive input, VO neurons encode a negative image of expected vestibular feedback (bottom right). This negative image can be predicted by the suppression observed during active movements (red superimposed trace). (B) Current model of the proposed mechanisms for the computation of vestibular exafference. Neck proprioceptive inputs are compared with the expected sensory consequence of neck motor command (internal model) in a putative matching center in the cerebellum. If these signals match, a cancellation signal is sent to VO neurons in the vestibular nuclei to remove exafference. In turn, vestibular thalamocortical pathway neurons also selectively encode unexpected motion (bottom right box).
What is the source of the cancellation signal required to suppress neurons at the first stage of central processing in vestibulo-spinal reflexes? (Figure 4B, red line) Vestibular afferents are not a candidate because they faithfully relay information to these neurons during active movements (Figure 4A, third row), including locomotion ([67–70]). In fact, their coding is identical in passive and active conditions. Instead, there is emerging evidence that predictive cerebellum-based mechanisms mediate the observed reafferent vestibular suppression. First, neurons in the rostral portion of the most medial deep cerebellar nucleus (the fastigial nucleus), demonstrate suppressed modulation for active versus passive vestibular stimulation[61,71]. Overall, the level of suppression is comparable to that of VO neurons of the vestibular nuclei (Figure 4B, bottom right box). Second, both the vestibular and fastigial nuclei receive direct projections from anterior vermis Purkinje cells. And third, the anterior vermis receives motor related information from areas including primary motor cortex (Figure 4B;[72]), in addition to the vestibular and proprioceptive sensory inputs detailed above. It is further noteworthy that posterior thalamocortical vestibular pathway neurons also selectively encode unexpected passive self-motion, most likely as a result of this cerebellar-based computation (Fig. 4B, top right box;[73]). The selective coding of exafference in thalamocortical vestibular pathway, which is even more pronounced (Figure 4B, bottom right inset), likely provides a neural correlate for ensuring perceptual stability during active versus externally generated motion.
Finally and importantly, the anterior vermis-based internal model predicting the sensory consequences of active motion, also allows the motor system to learn to expect unexpected sensory inputs if they persist to remain calibrated over time (see[74,75], for a review). Taken together, current understanding of the anterior vermis is consistent with a class of internal model called a forward model (Figure 4B). Forward models account for the causal relationship between the generated motor command and the sensory consequences of the action. Thus, in this context, the anterior vermis computes a forward internal model of the expected sensory consequences of active self-motion.
Nodulus/uvula of the posterior cerebellar vermis
The nodulus and ventral uvula (lobules X -IX) of the posterior cerebellar vermis integrate vestibular afferent input from the semicircular canals and otoliths to compute a representation of our self-motion relative to gravity (reviewed in[76,77]). Lesions of this cerebellar region produce impairments in the three-dimensional orientation of vestibular-induced eye movements relative to gravity. In the case of healthy monkeys, an eye movement, termed the “post-rotatory response” occurs when there is a sudden cessation of constant velocity head motion. The offset of head motion produces an VOR response that mirrors the initial response to the onset of rotation but is in the opposite direction. When the head’s orientation is altered relative to space, this response normally serves to realign the eyes relative to gravity. However, in monkeys with lesions of the nodulus and ventral uvula, this compensatory gravity-dependent response is absent[78–80].
The nodulus and ventral uvula are unique among regions of the vestibular cerebellum, in that they receive substantial direct vestibular input directly from the afferents; both otolith and canal afferents send direct mossy fiber projections [81,82]. Thus, this region receives mossy fiber inputs transmitting signals directly from single vestibular sensory organs in addition to integrated vestibular input[83–85] from the vestibular nuclei[86]. Nodulus and ventral uvula Purkinje cells also respond to proprioceptive stimulation, indicating that mossy fibers relay proprioceptive as well as vestibular signals to this region[87]. In contrast, climbing fibers relay full-field visual-motion information to nodulus and ventral uvula. This visual input is encoded in a coordinate frame aligned with that of the three semicircular canal axes (reviewed in[88]). In turn, Purkinje cells of the nodulus and ventral uvula, like those of the anterior vermis, directly target neurons in both the vestibular nuclei and rostral portion of the fastigial nucleus.
Computing an internal representation of spatial orientation and self-motion relative to gravity
The nodulus and ventral uvula play an important role in computing the brain’s representation of our orientation relative to gravity, by transforming self-motion signals from a head reference frame to an Earth reference frame. In this context, it is important to emphasize that there is no individual sensory input to the brain that itself encodes gravity. This is because the otolith afferents encode the linear forces produced during tilts and translations, which are physically indistinguishable from each other as indicated by Einstein’s equivalence principle. Thus, the otolith afferents transmit ambiguous information to the brain (reviewed in[1,76]). However, head tilts, unlike head translations, comprise head rotations which will activate semicircular canal as well as otolith afferents. As a result, the brain can combine otolith and canal signals to distinguish head tilts from head translations.
Theoretically, translation information can be computed by subtracting tilt position information originating in the semicircular canals from the net gravito-inertial acceleration signal that is encoded by the otolith input (reviewed in[14]). The nodulus and ventral uvula are thought to play an essential role in this computation. Single-unit recordings in primates have provided insights into the signal processing in the nodulus and ventral uvula; simple spike activity of many neurons is influenced by static roll tilt indicative of input from otolith pathways[87,89–91]. As illustrated in Figure 5A, some nodulus / ventral uvula Purkinje cells selectively encode head tilts [92,93], whereas others selectively encode head translations[91]. Furthermore, Purkinje cell responses resemble those of otolith afferents, following the ablation of canal function. Overall, the dynamic responses of tilt-selective cells predominately encode tilt velocity, whereas those of translation-selective cells predominately encode linear acceleration[92]. In primates, the distributions of neurons that encode tilts, translations, and linear forces are comparable for both Purkinje cells in the nodulus/ventral uvula and neurons in the deep cerebellar and vestibular nuclei that are their targets ([94]; Figure 5B).
Figure 5. Computing an internal representation of spatial orientation and self-motion relative to gravity.

(A) Schematic of three hypothetical Purkinje cells sensitive to tilt, translation, and net acceleration. The tilt sensitive Purkinje cell responds robustly to tilt while showing minimal sensitivity to translation, whereas the translation sensitive Purkinje cell responds robustly to translation while showing minimal sensitivity to tilt. Note that the response of each neuron is well predicted by its sensitivity to tilt and translation, respectively in the combined conditions (i.e., during the tilt + translation versus tilt - translation conditions, where net acceleration is double versus nulled, respectively). In contrast, the net acceleration sensitive neuron is strongly modulated by the net acceleration produced in all four conditions. (B) Percentage distributions of tilt, translation versus net acceleration neurons in the nodulus and ventral uvula, versus target neurons in deep cerebellar (rostral fastigial) and vestibular nuclei. (C) Schematic diagram of the computation thought to solve the tilt/translation ambiguity problem, where (∫) represents temporal integration.
Figure 5C illustrates a representation of the computation thought to solve the tilt/translation ambiguity problem. However, how the brain computes a translation signal remains an open question. One proposal is that the signal is computed by subtracting the gravity signal from the otolith input[14,92]. If this is the case, it would be necessary to temporally integrate the output of tilt Purkinje cells (i.e., angular velocity to position).
Recent studies in the mouse however suggest that canal- and otolith-related information is actually temporally well matched for individual Purkinje cells[95,96], leading to the proposal that the required alignment may be done upstream by interneurons in the cerebellar cortex (e.g., molecular layer interneurons, unipolar Brush cells). Unipolar brush cells (UBCs) are found in especially high density in the vestibular cerebellum, and effectively form a processing layer prior to the well-studied granule cells. Rather than integrating multiple inputs like granule cells[97,98], UBCs process direct versus indirect vestibular inputs via two segregated pathways[99]. Overall, nodulus and ventral uvula Purkinje cells appear to have substantially more complex responses in mice than monkeys, with most cells displaying some sensitivities to both tilt and translation. This suggests that, if the complete dissociation of tilt from translation ever occurs in the mouse brain, it happens downstream of nodulus / ventral uvula– for example in the neurons that they target in the deep cerebellar and vestibular nuclei. Further work will be required to understand the implications of the segregation of primary and secondary vestibular input in this circuit, as well as the apparent differences observed across species.
The neural encoding of active self-motion
As we actively interact with our environment during our everyday activities, self-motion cues are provided by proprioceptive, tactile, and visual systems and motor-related signals, as well as by the vestibular system. Yet, to date, relatively little is known about how nodulus and ventral uvula Purkinje cells integrate these extra-vestibular cues during active self-motion. A study in freely moving rats [100] identified two main populations of Purkinje cells: (i) one that combined canal and otolith information to encode a tilt-dependent representation of active head rotation, and (ii) a second that encoded active head rotation about an axis defined relative to gravity. Overall, the authors concluded that these Purkinje cells effectively encode a “re-encoded, gravitationally polarized” representation of self-generated head kinematics. The study further reported that neurons were, on average, less responsive to active than passive self-motion. However, methodological considerations limit the ability to draw firm conclusions regarding encoding of head rotation in relation to gravity, particularly the unmatched dynamics for active versus passive movements. Thus, future work is required to more fully understand how the nodulus/ventral uvula encodes active versus passive self-motion (see Outstanding questions).
Outstanding questions.
Recent studies have provided key insights into how the vestibular cerebellum integrates sensory information to perform fundamental neural computations during passively applied self-motion. Yet our understanding of these computations for actively generated self-motion is surprisingly limited. How does the vestibular cerebellum process self-motion information during voluntary movements? How do computations differ across regions?
Purkinje cells fire both climbing fiber spikes and simple spikes. Climbing fiber spike are thought to encode error signals that are essential for motor learning. How do the error signals encoded across different regions of the vestibular cerebellum complement their specific computations?
We have a fundamental understanding of the synaptic mechanisms in the flocculus that underlie VOR motor learning. However, the mechanisms underlying plasticity likely vary across cerebellar regions. What synaptic mechanisms function to ensure the maintenance of postural and perceptual stability within other essential regions of the vestibular cerebellum – notably, the anterior and posterior vermis?
Behavior ultimately requires the coordinated activity of populations of Purkinje and cerebellar output neurons, yet most studies to date have focused on the responses of single neurons. How do the large-scale dynamics of population activity, especially across the different regions of the vestibular cerebellum, shape behavior and what computations are performed at the population level?
Purkinje cells in the lateral cerebellum encode reward signals as well as sensorimotor information. However, it is not known how vestibular, extra-vestibular sensorimotor information, and reward signals are integrated in this evolutionarily more recent part of the cerebellum during self-motion. How does reward-related information modulate vestibular processing at the single cell and population levels?
The posterior and anterior vestibular thalamocortical pathways transmit self-motion information to cortical regions that mediate high-level functions such as the computation of spatial orientation and perception of self-motion. How do the computations that are performed by the vestibular cerebellum shape upstream processing?
Concluding remarks
Significant advances have been made in our understanding of computations performed by the vestibular cerebellum during self-motion. Neurophysiological and theoretical studies, focused on the responses of Purkinje cells during passive self-motion, have established that the vestibular cerebellum computes internal models that are essential for ensuring the maintenance of accurate motor control. Recent studies have further provided key insights into how cerebellar processing modifies and shapes descending pathways to optimize performance during active self-motion. This latter line of research has revealed that the vestibular vermis computes a forward internal model of the expected sensory consequences of voluntarily generated head movements. Abstract algorithmic approaches have been used to model how the brain may theoretically integrate sensory and motor information (e.g., [15,101]). Nonetheless, current understanding of the neural computations carried out by the vestibular cerebellum during active movements remains far from complete (refer to Outstanding questions). For example, recent human studies using externally applied galvanic stimulation of the vestibular sensors (GVS) suggest that vestibular balance stabilizing mechanisms are systematically modulated during locomotion throughout the gait cycle[102,103]. The application of transcranial alternating current stimulation (tACS) has further provided evidence that the vestibular–cerebellar network plays a central role this modulation[104]. However, conducting single unit experiments will be necessary to establish underlying neural computations.
To maintain calibration over time, the internal models of the vestibular cerebellum must be continually updated to accommodate new constraints imposed by changes in the external environment and biomechanical dynamics (such as changes in load or muscle state[71]). It is currently an open question whether and how the rules underlying synaptic plasticity differ across regions. While we have a deep understanding of adaptive plasticity in the flocculus, much less is known about the vermis. It has been reported, for instance, that associative plasticity is characterized by much broader temporal tuning in the anterior vermis compared to the flocculus[105], but further work on the vermis is needed. Additionally, the more recently evolved lateral regions of cerebellar cortex (Fig 1: cerebro-cerebellum), integrate reward signals with sensorimotor inputs. However, whether and how these signals are integrated during self-motion is currently largely unknown.
Finally, recent innovations in high-density silicon probes are poised to rapidly advance our understanding of how large-scale population dynamics shape behavior. For example, the anterior vermis exhibits zebrin-negative expression associated with temporal coding, while the posterior vermis exhibits zebrin-positive expression, associated with rate coding[106]. Given that both regions project downstream to the same deep cerebellar nucleus (i.e., fastigial) and that behavior requires the coordinated activity of populations of cerebellar neurons, understanding the large-scale dynamics of population activity across the vestibular cerebellum is an exciting avenue for future research. Moreover, the vestibular nuclei and cerebellar nuclei send direct projections to ventral posterior lateral thalamus (VPL), which projects to cortical areas involved in self-motion processing (reviewed in[107]). VPL neuron responses to self-motion are markedly suppressed during active motion[108], in a manner consistent with a cerebellum-based forward model (Fig. 4B). These observations lead to one of the fundamental and largely unexplored questions in vestibular research: how does the vestibular cerebellum shape our sense of agency during self-motion?
Highlights.
The vestibular cerebellum integrates multimodal sensory and motor information with vestibular input to compute internal models of self-motion.
Adaptive plasticity in the floccular lobe underlies the vital computation that maintains vestibulo-ocular reflex calibration to ensure gaze stability.
The anterior vermis plays a key role in two essential computations: (i) it transforms head-centered vestibular signals into the body-centered reference frame required for postural control, and (ii) it constructs a prediction of the sensory consequences of active self-motion.
The nodulus and ventral uvula of the posterior vermis compute an internal representation of our spatial orientation and self-motion relative to gravity.
The internal models underlying each of these computations are continually updated to account for constraints imposed by changes in the environment and/or neural mechanical dynamics.
Acknowledgements:
I would like to thank Faisal Karmali, Jerome Carriot, as well as members of the Cullen lab including Robyn Mildren, Lex Gomez, Oliver Stanley, and Pum Wiboonsaksakul for their helpful feedback on the review. This work was funded by the National Institute on Deafness and Other Communication Disorders at the National Institutes of Health (Grants R01-DC002390, R01- DC018061, and R01-DC018304) and Brain Initiative Grant 1UF1NS111695.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: The author declares no competing interests in relation to this work.
References
- 1.Cullen KE (2019) Vestibular processing during natural self-motion: implications for perception and action. Nat Rev Neurosci 20, 346–363. 10.1038/s41583-019-0153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg JM et al. (2012) The Vestibular System: A Sixth Sense OUP USA
- 3.Lam CK et al. (2016) The direction of the postural response to a vestibular perturbation is mediated by the cerebellar vermis. Exp Brain Res 234, 3689–3697. 10.1007/s00221-016-4766-6 [DOI] [PubMed] [Google Scholar]
- 4.Kammermeier S et al. (2013) Disturbed vestibular-neck interaction in cerebellar disease. J Neurol 260, 794–804. 10.1007/s00415-012-6707-z [DOI] [PubMed] [Google Scholar]
- 5.Ilg W et al. (2008) The influence of focal cerebellar lesions on the control and adaptation of gait. Brain 131, 2913–2927. 10.1093/brain/awn246 [DOI] [PubMed] [Google Scholar]
- 6.Rondi-Reig L et al. (2014) How the cerebellum may monitor sensory information for spatial representation. Front Syst Neurosci 8, 205. 10.3389/fnsys.2014.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronstein AM et al. (2008) Reduced self-motion perception in patients with midline cerebellar lesions. Neuroreport 19, 691–693. 10.1097/WNR.0b013e3282fbf9f6 [DOI] [PubMed] [Google Scholar]
- 8.Bertolini G et al. (2012) Is vestibular self-motion perception controlled by the velocity storage? Insights from patients with chronic degeneration of the vestibulo-cerebellum. Plos One 7, e36763. 10.1371/journal.pone.0036763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlem K et al. (2016) Cerebellar contributions to self-motion perception: evidence from patients with congenital cerebellar agenesis. J Neurophysiol 115, 2280–2285. 10.1152/jn.00763.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarnutzer AA et al. (2015) The cerebellar nodulus: perceptual and ocular processing of graviceptive input. Ann Neurol 77, 343–347. 10.1002/ana.24329 [DOI] [PubMed] [Google Scholar]
- 11.Dakin CJ et al. (2018) Cerebellar Degeneration Increases Visual Influence on Dynamic Estimates of Verticality. Curr Biol 28, 3589–3598 e3583. 10.1016/j.cub.2018.09.049 [DOI] [PubMed] [Google Scholar]
- 12.Green AM and Angelaki DE (2010) Multisensory integration: resolving sensory ambiguities to build novel representations. Curr Opin Neurobiol 20, 353–360. 10.1016/j.conb.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisberger SG (2009) Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience 162, 763–776. 10.1016/j.neuroscience.2009.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurens J (2022) The otolith vermis: A systems neuroscience theory of the Nodulus and Uvula. Front Syst Neurosci 16, 886284. 10.3389/fnsys.2022.886284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurens J and Angelaki DE (2017) A unified internal model theory to resolve the paradox of active versus passive self-motion sensation. Elife 6. 10.7554/eLife.28074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leigh JR, Zee DS (2015.) The Neurology of Eye Movements (Vol. 5th edn Oxford University Press [Google Scholar]
- 17.Kheradmand A and Zee DS (2011) Cerebellum and ocular motor control. Front Neurol 2, 53. 10.3389/fneur.2011.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shemesh AA and Zee DS (2019) Eye Movement Disorders and the Cerebellum. J Clin Neurophysiol 36, 405–414. 10.1097/WNP.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rambold H et al. (2002) Partial ablations of the flocculus and ventral paraflocculus in monkeys cause linked deficits in smooth pursuit eye movements and adaptive modification of the VOR. J Neurophysiol 87, 912–924. 10.1152/jn.00768.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung HC et al. (2000) Cerebellar flocculus and paraflocculus Purkinje cell activity during circular pursuit in monkey. J Neurophysiol 83, 13–30. 10.1152/jn.2000.83.1.13 [DOI] [PubMed] [Google Scholar]
- 21.Suh M et al. (2000) Cerebellar flocculus and ventral paraflocculus Purkinje cell activity during predictive and visually driven pursuit in monkey. J Neurophysiol 84, 1835–1850. 10.1152/jn.2000.84.4.1835 [DOI] [PubMed] [Google Scholar]
- 22.Maekawa K and Simpson JI (1973) Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J Neurophysiol 36, 649–666. 10.1152/jn.1973.36.4.649 [DOI] [PubMed] [Google Scholar]
- 23.Cullen KE (2009) VOR Suppression. In Encyclopedia of Neuroscience. (Binder MD, Hirokawa N, Windhorst U, ed), Springer [Google Scholar]
- 24.Marr D (1969) A theory of cerebellar cortex. J. Physiol 202, 437–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albus JS (1971) A theory of cerebellar function. Math. Biosci 10, 25–61 [Google Scholar]
- 26.Straka H et al. (2016) Vestibular animal models: contributions to understanding physiology and disease. J Neurol 263 Suppl 1, S10–23. 10.1007/s00415-015-7909-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker R et al. (1972) Cerebellar modulatory action on the vestibulo-trochlear pathway in the cat. Exp Brain Res 15, 364–385. 10.1007/BF00234124 [DOI] [PubMed] [Google Scholar]
- 28.Highstein SM (1973) Synaptic linkage in the vestibulo-ocular and cerebello-vestibular pathways to the VIth nucleus in the rabbit. Exp Brain Res 17, 301–314. 10.1007/BF00234668 [DOI] [PubMed] [Google Scholar]
- 29.Lisberger SG et al. (1994) Neural basis for motor learning in the vestibuloocular reflex of primates. I. Changes in the responses of brain stem neurons. J Neurophysiol 72, 928–953. 10.1152/jn.1994.72.2.928 [DOI] [PubMed] [Google Scholar]
- 30.Ito M and Kano M (1982) Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett 33, 253–258. 10.1016/0304-3940(82)90380-9 [DOI] [PubMed] [Google Scholar]
- 31.De Zeeuw CI et al. (2021) Diversity and dynamism in the cerebellum. Nat Neurosci 24, 160–167. 10.1038/s41593-020-00754-9 [DOI] [PubMed] [Google Scholar]
- 32.Lisberger SG (2021) The Rules of Cerebellar Learning: Around the Ito Hypothesis. Neuroscience 462, 175–190. 10.1016/j.neuroscience.2020.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Zeeuw CI (2021) Bidirectional learning in upbound and downbound microzones of the cerebellum. Nat Rev Neurosci 22, 92–110. 10.1038/s41583-020-00392-x [DOI] [PubMed] [Google Scholar]
- 34.Gao Z et al. (2012) Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci 13, 619–635. 10.1038/nrn3312 [DOI] [PubMed] [Google Scholar]
- 35.Cullen KEM, D.M. (2016) Procedural learning: VOR. The Curated Reference Collection in Neuroscience and Biobehavioral Psychology, [Google Scholar]
- 36.Inagaki K and Hirata Y (2017) Computational Theory Underlying Acute Vestibulo-ocular Reflex Motor Learning with Cerebellar Long-Term Depression and Long-Term Potentiation. Cerebellum 16, 827–839. 10.1007/s12311-017-0857-6 [DOI] [PubMed] [Google Scholar]
- 37.Nagao S (2021) Ocular Reflex Adaptation as an Experimental Model of Cerebellar Learning -- In Memory of Masao Ito. Neuroscience 462, 191–204 [DOI] [PubMed] [Google Scholar]
- 38.Larry N et al. (2019) Cerebellar climbing fibers encode expected reward size. Elife 8. 10.7554/eLife.46870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowan MJM et al. (2018) Graded Control of Climbing-Fiber-Mediated Plasticity and Learning by Inhibition in the Cerebellum. Neuron 99, 999–1015 e1016. 10.1016/j.neuron.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkelman BH et al. (2014) Nonvisual complex spike signals in the rabbit cerebellar flocculus. J Neurosci 34, 3218–3230. 10.1523/JNEUROSCI.3080-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanning AS et al. (2021) Population calcium responses of Purkinje cells in the oculomotor cerebellum driven by nonvisual input. J Neurophysiol 126, 1391–1402. 10.1152/jn.00715.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y and Lisberger SG (2014) Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature 510, 529–532. 10.1038/nature13282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suvrathan A and Raymond JL (2018) Depressed by Learning-Heterogeneity of the Plasticity Rules at Parallel Fiber Synapses onto Purkinje Cells. Cerebellum 17, 747–755. 10.1007/s12311-018-0968-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa T et al. (2016) The cerebro-cerebellum: Could it be loci of forward models? Neurosci Res 104, 72–79. 10.1016/j.neures.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 45.Llinas R et al. (1974) Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol 37, 560–571. 10.1152/jn.1974.37.3.560 [DOI] [PubMed] [Google Scholar]
- 46.De Zeeuw CI et al. (2011) Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci 12, 327–344. 10.1038/nrn3011 [DOI] [PubMed] [Google Scholar]
- 47.Shin SL and De Schutter E (2006) Dynamic synchronization of Purkinje cell simple spikes. J Neurophysiol 96, 3485–3491. 10.1152/jn.00570.2006 [DOI] [PubMed] [Google Scholar]
- 48.Person AL and Raman IM (2012) Synchrony and neural coding in cerebellar circuits. Front Neural Circuits 6, 97. 10.3389/fncir.2012.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Payne HL et al. (2019) Cerebellar Purkinje cells control eye movements with a rapid rate code that is invariant to spike irregularity. Elife 8. 10.7554/eLife.37102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stahl JS et al. (2022) Impact of Purkinje Cell Simple Spike Synchrony on Signal Transmission from Flocculus. Cerebellum 21, 879–904. 10.1007/s12311-021-01332-w [DOI] [PubMed] [Google Scholar]
- 51.Mitoma H et al. (2020) Consensus Paper. Cerebellar Reserve: From Cerebellar Physiology to Cerebellar Disorders. Cerebellum 19, 131–153. 10.1007/s12311-019-01091-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan EV et al. (2006) Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex 16, 1077–1086. 10.1093/cercor/bhj048 [DOI] [PubMed] [Google Scholar]
- 53.Robertson LT et al. (1982) Organization of climbing fiber input from mechanoreceptors to lobule V vermal cortex of the cat. Exp Brain Res 46, 281–291. 10.1007/BF00237186 [DOI] [PubMed] [Google Scholar]
- 54.Kurzan R et al. (1993) The effect of muscimol micro-injections into the fastigial nucleus on the optokinetic response and the vestibulo-ocular reflex in the alert monkey. Exp Brain Res 94, 252–260. 10.1007/BF00230293 [DOI] [PubMed] [Google Scholar]
- 55.Pelisson D et al. (1998) Contribution of the rostral fastigial nucleus to the control of orienting gaze shifts in the head-unrestrained cat. J Neurophysiol 80, 1180–1196. 10.1152/jn.1998.80.3.1180 [DOI] [PubMed] [Google Scholar]
- 56.Thach WT et al. (1992) The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15, 403–442. 10.1146/annurev.ne.15.030192.002155 [DOI] [PubMed] [Google Scholar]
- 57.Cullen KE (2012) The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 35, 185–196. 10.1016/j.tins.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manzoni D et al. (1998) Neck influences on the spatial properties of vestibulospinal reflexes in decerebrate cats: role of the cerebellar anterior vermis. J Vestib Res 8, 283–297 [PubMed] [Google Scholar]
- 59.Manzoni D et al. (2004) Coupling sensory inputs to the appropriate motor responses: new aspects of cerebellar function. Arch Ital Biol 142, 199–215 [PubMed] [Google Scholar]
- 60.Zobeiri OA and Cullen KE (2022) Distinct representations of body and head motion are dynamically encoded by Purkinje cell populations in the macaque cerebellum. Elife 11. 10.7554/eLife.75018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brooks JX and Cullen KE (2009) Multimodal integration in rostral fastigial nucleus provides an estimate of body movement. J Neurosci 29, 10499–10511. 10.1523/JNEUROSCI.1937-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brooks JX and Cullen KE (2014) Early vestibular processing does not discriminate active from passive self-motion if there is a discrepancy between predicted and actual proprioceptive feedback. J Neurophysiol 111, 2465–2478. 10.1152/jn.00600.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin CZ et al. (2018) Role of Rostral Fastigial Neurons in Encoding a Body-Centered Representation of Translation in Three Dimensions. J Neurosci 38, 3584–3602. 10.1523/JNEUROSCI.2116-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palkovits M et al. (1977) Quantitative histological analysis of the cerebellar nuclei in the cat. I. Numerical data on cells and on synapses. Exp Brain Res 28, 189–209. 10.1007/BF00237096 [DOI] [PubMed] [Google Scholar]
- 65.Sohn H et al. (2021) A Network Perspective on Sensorimotor Learning. Trends Neurosci 44, 170–181. 10.1016/j.tins.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cullen KE and Zobeiri OA (2021) Proprioception and the predictive sensing of active self-motion. Curr Opin Physiol 20, 29–38. 10.1016/j.cophys.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cullen KE and Minor LB (2002) Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci 22, RC226. 20026418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jamali M et al. (2009) Response of vestibular nerve afferents innervating utricle and saccule during passive and active translations. J Neurophysiol 101, 141–149. 10.1152/jn.91066.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mackrous I et al. (2022) Context-independent encoding of passive and active self-motion in vestibular afferent fibers during locomotion in primates. Nat Commun 13, 120. 10.1038/s41467-021-27753-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadeghi SG et al. (2007) Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97, 1503–1514. 10.1152/jn.00829.2006 [DOI] [PubMed] [Google Scholar]
- 71.Brooks JX et al. (2015) Learning to expect the unexpected: rapid updating in primate cerebellum during voluntary self-motion. Nat Neurosci 18, 1310–1317. 10.1038/nn.4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rathelot JA and Strick PL (2009) Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci U S A 106, 918–923. 10.1073/pnas.0808362106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dale A and Cullen KE (2015) Local population synchrony and the encoding of eye position in the primate neural integrator. J Neurosci 35, 4287–4295. 10.1523/JNEUROSCI.4253-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brooks JX and Cullen KE (2019) Predictive Sensing: The Role of Motor Signals in Sensory Processing. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 842–850. 10.1016/j.bpsc.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldberg JM and Cullen KE (2011) Vestibular control of the head: possible functions of the vestibulocollic reflex. Exp Brain Res 210, 331–345. 10.1007/s00221-011-2611-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Angelaki DE and Cullen KE (2008) Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci 31, 125–150. 10.1146/annurev.neuro.31.060407.125555 [DOI] [PubMed] [Google Scholar]
- 77.Cullen KE (2016) Physiology of central pathways. Handb Clin Neurol 137, 17–40. 10.1016/B978-0-444-63437-5.00002-9 [DOI] [PubMed] [Google Scholar]
- 78.Angelaki DE and Hess BJ (1995) Inertial representation of angular motion in the vestibular system of rhesus monkeys. II. Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol 73, 1729–1751. 10.1152/jn.1995.73.5.1729 [DOI] [PubMed] [Google Scholar]
- 79.Waespe W et al. (1985) Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science 228, 199–202. 10.1126/science.3871968 [DOI] [PubMed] [Google Scholar]
- 80.Wearne S et al. (1998) Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol 79, 2690–2715. 10.1152/jn.1998.79.5.2690 [DOI] [PubMed] [Google Scholar]
- 81.Korte GE and Mugnaini E (1979) The cerebellar projection of the vestibular nerve in the cat. J Comp Neurol 184, 265–277. 10.1002/cne.901840204 [DOI] [PubMed] [Google Scholar]
- 82.Maklad A and Fritzsch B (2003) Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Brain Res Dev Brain Res 140, 223–236. 10.1016/s0165-3806(02)00609-0 [DOI] [PubMed] [Google Scholar]
- 83.Barmack NH and Shojaku H (1992) Vestibularly induced slow oscillations in climbing fiber responses of Purkinje cells in the cerebellar nodulus of the rabbit. Neuroscience 50, 1–5. 10.1016/0306-4522(92)90376-d [DOI] [PubMed] [Google Scholar]
- 84.Ono S et al. (2000) Properties of utricular and saccular nerve-activated vestibulocerebellar neurons in cats. Exp Brain Res 134, 1–8. 10.1007/s002210000424 [DOI] [PubMed] [Google Scholar]
- 85.Shojaku H et al. (1991) Influence of vestibular and visual climbing fiber signals on Purkinje cell discharge in the cerebellar nodulus of the rabbit. Acta Otolaryngol Suppl 481, 242–246. 10.3109/00016489109131391 [DOI] [PubMed] [Google Scholar]
- 86.Carriot J et al. (2015) Rapid adaptation of multisensory integration in vestibular pathways. Front Syst Neurosci 9, 59. 10.3389/fnsys.2015.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheliga BM et al. (1999) Control of spatial orientation of the angular vestibulo-ocular reflex by the nodulus and uvula of the vestibulocerebellum. Ann N Y Acad Sci 871, 94–122. 10.1111/j.1749-6632.1999.tb09178.x [DOI] [PubMed] [Google Scholar]
- 88.Barmack NH and Pettorossi VE (2021) Adaptive Balance in Posterior Cerebellum. Front Neurol 12, 635259. 10.3389/fneur.2021.635259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barmack NH and Shojaku H (1995) Vestibular and visual climbing fiber signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol 74, 2573–2589. 10.1152/jn.1995.74.6.2573 [DOI] [PubMed] [Google Scholar]
- 90.Marini G et al. (1976) Gravity responses of Purkinje cells in the nodulus. Exp Brain Res 24, 311–323. 10.1007/BF00235018 [DOI] [PubMed] [Google Scholar]
- 91.Yakusheva TA et al. (2007) Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron 54, 973–985. 10.1016/j.neuron.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 92.Laurens J and Angelaki DE (2020) Simple spike dynamics of Purkinje cells in the macaque vestibulo-cerebellum during passive whole-body self-motion. Proc Natl Acad Sci U S A 117, 3232–3238. 10.1073/pnas.1915873117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laurens J et al. (2013) Neural representation of orientation relative to gravity in the macaque cerebellum. Neuron 80, 1508–1518. 10.1016/j.neuron.2013.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mackrous I et al. (2019) Cerebellar Prediction of the Dynamic Sensory Consequences of Gravity. Curr Biol 29, 2698–2710 e2694. 10.1016/j.cub.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hernandez RG et al. (2020) Translation information processing is regulated by protein kinase C-dependent mechanism in Purkinje cells in murine posterior vermis. Proc Natl Acad Sci U S A 117, 17348–17358. 10.1073/pnas.2002177117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stay TL et al. (2019) Genetically eliminating Purkinje neuron GABAergic neurotransmission increases their response gain to vestibular motion. Proc Natl Acad Sci U S A 116, 3245–3250. 10.1073/pnas.1818819116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chabrol FP et al. (2015) Synaptic diversity enables temporal coding of coincident multisensory inputs in single neurons. Nat Neurosci 18, 718–727. 10.1038/nn.3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang CC et al. (2013) Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. Elife 2, e00400. 10.7554/eLife.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balmer TS and Trussell LO (2019) Selective targeting of unipolar brush cell subtypes by cerebellar mossy fibers. Elife 8. 10.7554/eLife.44964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dugue GP et al. (2017) Cerebellar re-encoding of self-generated head movements. Elife 6. 10.7554/eLife.26179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paulin MG (2005) Evolution of the cerebellum as a neuronal machine for Bayesian state estimation. J Neural Eng 2, S219–234. 10.1088/1741-2560/2/3/S06 [DOI] [PubMed] [Google Scholar]
- 102.Tisserand R et al. (2018) Down regulation of vestibular balance stabilizing mechanisms to enable transition between motor states. Elife 7. 10.7554/eLife.36123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forbes PA et al. (2017) Rapid limb-specific modulation of vestibular contributions to ankle muscle activity during locomotion. J Physiol 595, 2175–2195. 10.1113/JP272614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakazono H et al. (2022) Phase-dependent modulation of the vestibular-cerebellar network via combined alternating current stimulation influences human locomotion and posture. Front Neurosci 16, 1057021. 10.3389/fnins.2022.1057021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suvrathan A et al. (2016) Timing Rules for Synaptic Plasticity Matched to Behavioral Function. Neuron 92, 959–967. 10.1016/j.neuron.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Zeeuw CI and Ten Brinke MM (2015) Motor Learning and the Cerebellum. Cold Spring Harb Perspect Biol 7, a021683. 10.1101/cshperspect.a021683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cullen KE and Taube JS (2017) Our sense of direction: progress, controversies and challenges. Nat Neurosci 20, 1465–1473. 10.1038/nn.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dale A and Cullen KE (2019) The Ventral Posterior Lateral Thalamus Preferentially Encodes Externally Applied Versus Active Movement: Implications for Self-Motion Perception. Cereb Cortex 29, 305–318. 10.1093/cercor/bhx325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coesmans M et al. (2004) Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44, 691–700. 10.1016/j.neuron.2004.10.031 [DOI] [PubMed] [Google Scholar]
- 110.Lev-Ram V et al. (1995) Long-term depression in cerebellar Purkinje neurons results from coincidence of nitric oxide and depolarization-induced Ca2+ transients. Neuron 15, 407–415. 10.1016/0896-6273(95)90044-6 [DOI] [PubMed] [Google Scholar]
- 111.Boyden ES et al. (2006) Selective engagement of plasticity mechanisms for motor memory storage. Neuron 51, 823–834. 10.1016/j.neuron.2006.08.026 [DOI] [PubMed] [Google Scholar]
- 112.Schonewille M et al. (2011) Reevaluating the role of LTD in cerebellar motor learning. Neuron 70, 43–50. 10.1016/j.neuron.2011.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ke MC et al. (2009) Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci 12, 1171–1179. 10.1038/nn.2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen-Vu TD et al. (2013) Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci 16, 1734–1736. 10.1038/nn.3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bonnan A et al. (2021) Autonomous Purkinje cell activation instructs bidirectional motor learning through evoked dendritic calcium signaling. Nat Commun 12, 2153. 10.1038/s41467-021-22405-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Galliano E et al. (2013) Silencing the majority of cerebellar granule cells uncovers their essential role in motor learning and consolidation. Cell Rep 3, 1239–1251. 10.1016/j.celrep.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 117.Jang DC et al. (2020) Intrinsic Plasticity of Cerebellar Purkinje Cells Contributes to Motor Memory Consolidation. J Neurosci 40, 4145–4157. 10.1523/JNEUROSCI.1651-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pugh JR and Raman IM (2006) Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron 51, 113–123. 10.1016/j.neuron.2006.05.021 [DOI] [PubMed] [Google Scholar]
- 119.Voges K et al. (2017) Mechanisms underlying vestibulo-cerebellar motor learning in mice depend on movement direction. J Physiol 595, 5301–5326. 10.1113/JP274346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miles FA and Eighmy BB (1980) Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol 43, 1406–1425. 10.1152/jn.1980.43.5.1406 [DOI] [PubMed] [Google Scholar]
- 121.Boyden ES and Raymond JL (2003) Active reversal of motor memories reveals rules governing memory encoding. Neuron 39, 1031–1042. 10.1016/s0896-6273(03)00562-2 [DOI] [PubMed] [Google Scholar]
- 122.Kimpo RR et al. (2005) Distinct patterns of stimulus generalization of increases and decreases in VOR gain. J Neurophysiol 94, 3092–3100. 10.1152/jn.00048.2005 [DOI] [PubMed] [Google Scholar]
- 123.Lisberger SG et al. (1983) Frequency-selective adaptation: evidence for channels in the vestibulo-ocular reflex? J Neurosci 3, 1234–1244. 10.1523/JNEUROSCI.03-06-01234.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McElvain LE et al. (2010) Bidirectional plasticity gated by hyperpolarization controls the gain of postsynaptic firing responses at central vestibular nerve synapses. Neuron 68, 763–775. 10.1016/j.neuron.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scarduzio M et al. (2012) The repetition timing of high frequency afferent stimulation drives the bidirectional plasticity at central synapses in the rat medial vestibular nuclei. Neuroscience 223, 1–11. 10.1016/j.neuroscience.2012.07.039 [DOI] [PubMed] [Google Scholar]
- 126.Mitchell DE et al. (2016) Plasticity within non-cerebellar pathways rapidly shapes motor performance in vivo. Nat Commun 7, 11238. 10.1038/ncomms11238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beraneck M et al. (2008) Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol 100, 945–958. 10.1152/jn.90319.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jamali M et al. (2014) Neuronal detection thresholds during vestibular compensation: contributions of response variability and sensory substitution. J Physiol 592, 1565–1580. 10.1113/jphysiol.2013.267534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sadeghi SG et al. (2010) Neural correlates of motor learning in the vestibulo-ocular reflex: dynamic regulation of multimodal integration in the macaque vestibular system. J Neurosci 30, 10158–10168. 10.1523/JNEUROSCI.1368-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sadeghi SG et al. (2012) Neural correlates of sensory substitution in vestibular pathways following complete vestibular loss. J Neurosci 32, 14685–14695. 10.1523/JNEUROSCI.2493-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


