Abstract
Posttraumatic stress disorder (PTSD) occurs in some people following exposure to a terrifying or catastrophic event involving actual/threatened death, serious injury, or sexual violence. PTSD is a common and debilitating mental disorder that imposes a significant burden on individuals, their families, health services, and society. Moreover, PTSD is a risk factor for chronic diseases such as coronary heart disease, stroke, diabetes, as well as premature mortality. Furthermore, PTSD is associated with dysregulated immune function. Despite the high prevalence of PTSD, the mechanisms underlying its etiology and manifestations remain poorly understood. Compelling evidence indicates that the human gut microbiome, a complex community of microorganisms living in the gastrointestinal tract, plays a crucial role in the development and function of the host nervous system, complex behaviors, and brain circuits. The gut microbiome may contribute to PTSD by influencing inflammation, stress responses, and neurotransmitter signaling, while bidirectional communication between the gut and brain involves mechanisms such as microbial metabolites, immune system activation, and the vagus nerve. In this literature review, we summarize recent findings on the role of the gut microbiome in PTSD in both human and animal studies. We discuss the methodological limitations of existing studies and suggest future research directions to further understand the role of the gut microbiome in PTSD.
Keywords: Posttraumatic stress disorder, trauma, gut microbiome, gut-microbiota-brain axis
Introduction
Posttraumatic stress disorder (PTSD) is a common and debilitating mental disorder that occurs in some persons after exposure to a traumatic event, such as physical or sexual violence, combat, or a severe accident. The cross-national lifetime prevalence of PTSD among countries (n=24) participating in the World Health Organization World Mental Health Surveys was 3.9%1. The prevalence of PTSD is higher in countries with a recent history of collective trauma (such as war and conflict) as well as in groups with higher rates of trauma exposure. In Africa, for instance, a systematic review found that current PTSD prevalence ranged from 8% in conflict unexposed regions to 30% in high conflict regions2. Moreover, PTSD is a significant risk factor for chronic diseases such as diabetes3, coronary heart disease4,5, stroke6, cardiometabolic disease7, autoimmune diseases8, and is associated with cognitive decline9 and early mortality10. Thus, comprehending the pathophysiology of PTSD is critical for us to understand risk and resilience following trauma, as well as to identify the mechanisms underlying the relationship between PTSD and chronic diseases.
Although substantial research has greatly improved our knowledge regarding the prevalence, clinical symptoms, and consequences of PTSD over the last decades, much remains to be learned regarding why some persons are more vulnerable to developing PTSD after trauma and the mechanisms driving the relationship between PTSD and chronic diseases. Notably, one area that has been understudied is the relationship between PTSD and the gut microbiome. The human body is home to trillions of microbes, especially the gastrointestinal (GI) tract. Those microbes, together with their genomes, are collectively known as the human microbiome11. Rather than simple passengers in or on our bodies, commensal microbes play critical roles in physical health, immune function, inflammation, and in disease12. A disrupted gut microbiome has been associated with brain function and various psychiatric disorders13. The ‘microbiota–gut–brain axis’ refers to the complex connections involving multiple biological systems that allow bidirectional communication between gut microbes and the brain (Figure 1). For example, psychological factors such as stress or depression may modulate the composition and activity of the gut microbial community through the limbic system (e.g., amygdala, hippocampus, fornix, thalamus, septum, and prefrontal cortex) to release stress hormones (e.g., noradrenaline)14. And the gut microbiota can influence brain activity and behavior through various pathways, including microbial products (e.g., gamma-aminobutyric acid, short-chain fatty acids (SCFA), and serotonin precursors) entering the brain via the bloodstream, cytokine release from mucosal immune cells, the release of gut hormones like serotonin from enteroendocrine cells, and afferent neural pathways, such as the vagus nerve. It is crucial to maintaining homeostasis of the gastrointestinal, central nervous, and microbial systems of the host15. The potential role of the gut microbiome in mental health and a range of chronic diseases is increasingly recognized16,17. For example, the human gut microbiome has been proposed to be involved in the interaction between inflammation and stress regulation in the brain through the cytokines and other inflammatory signaling molecules13. However, the role of the human microbiome in PTSD specifically has yet to be understood.
Figure 1: The putative bidirectional communication system of the gut–microbiota–brain axis14.
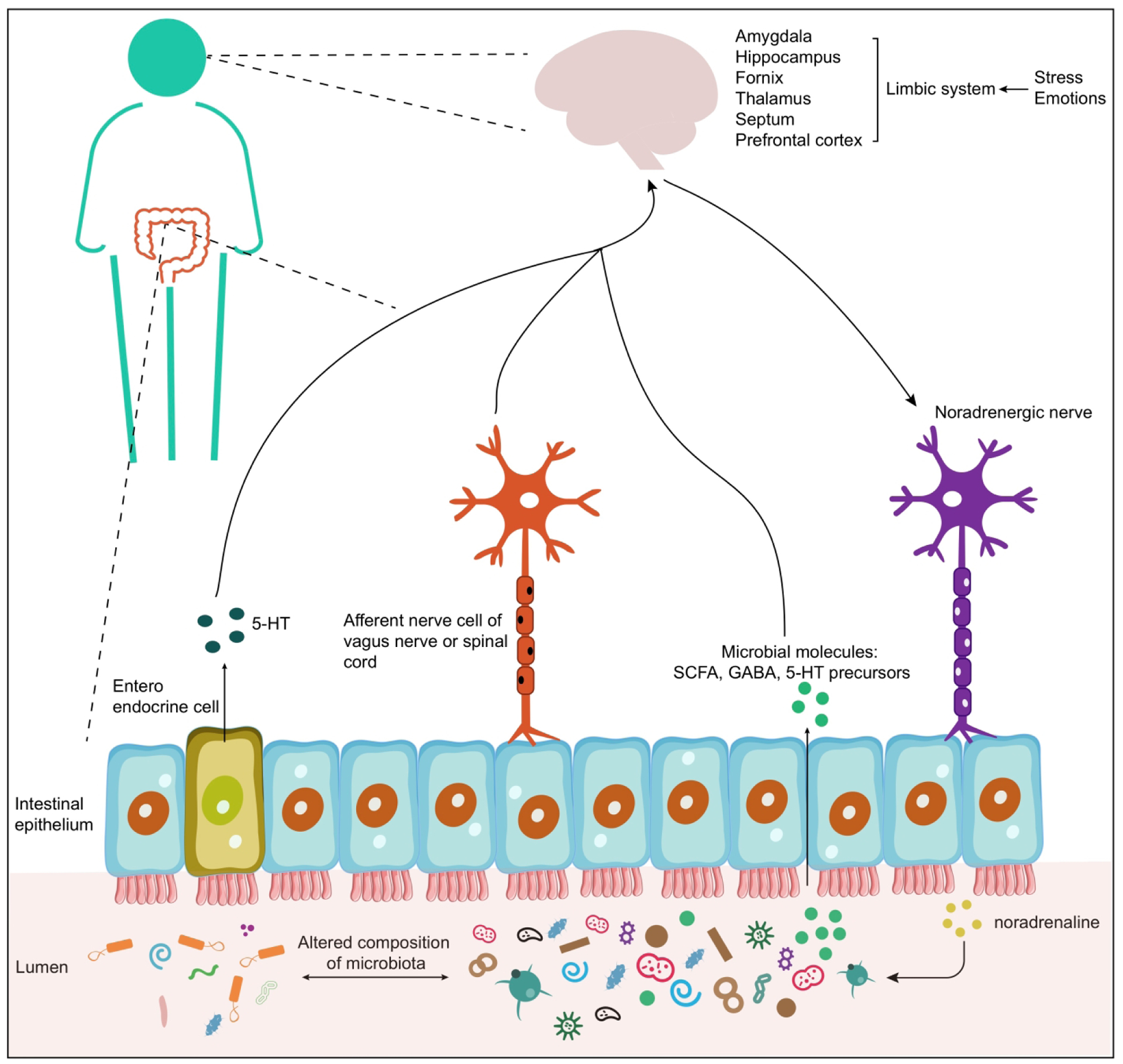
This system involves the endocrine, humoral, immune, and metabolic pathways. On the one hand, stress and emotions can affect the concentration of stress hormone (e.g., noradrenaline) and neurotransmitters in the gut lumen, and this might contribute to the changes in the composition and activity (e.g., microbial gene expression or signaling between taxa) of the gut microbial community. On the other hand, the microbiota can produce neuroactive compounds such as serotonin [5-hydroxytryptamine (5-HT)], gamma-aminobutyric acid (GABA), and microbial metabolites [short-chain fatty acids (SCFA)]. These metabolites can travel through portal circulation to interact with the host immune system, influence metabolism and/or affect local neuronal cells of the enteric nervous system and afferent pathways of the vagus nerve that signal directly to the brain. Altered composition of microbiota: it pertains to changes in response to stress and alterations in the microbiota composition, which subsequently influence emotional processing through the bidirectional communication in the gut-brain axis.
The hypothalamic-pituitary-adrenal (HPA) axis is the central coordinator of how humans respond to stressful situations, and PTSD is often associated with alterations in the HPA axis18. Furthermore, the HPA axis affects both immune and inflammatory responses (e.g., cortisol is a primary suppressor of inflammation), and inflammatory cytokines can activate the HPA system. Recent accumulating evidence indicates that the gut microbiome can affect the development and regulation of the HPA axis and thus affect the neuroendocrine system in the brain19. Hence, one direct mechanism by which the gut microbiome may play an important role in the development and progression of PTSD is via its interaction with the HPA neuroendocrine stress axis.
Currently, there are a few review studies on this field. For example, Leclercq et al. suggested that the gut microbiome may be key in determining an individual’s susceptibility to developing PTSD after a traumatic event20. Brenner et al. conducted a systematic review to evaluate existing studies on prebiotic and probiotic interventions for traumatic brain injury and PTSD patients21. Malan-Muller et al. undertook a review study that highlights emerging microbiome research in psychiatric disorders, with a specific emphasis on anxiety- and trauma-related disorders, and explores the gut microbiome as a potential therapeutic target22. In another study, the effects of trauma on the gut microbiome, the influence of the gut microbiome on brain function, and the role of the bidirectional Microbiota-Gut-Brain axis in determining individual resilience or vulnerability to PTSD development after trauma exposure were summarized23. Here we review the latest existing studies on the PTSD–microbiome association and interventional studies that examined the effect of microbiome-targeted supplementation on PTSD. We further explore the notion that the gut microbiome may play an important role in mediating symptoms of PTSD. We also point out both encouraging findings and methodological limitations in those studies, and we suggest future directions for systematically investigating the role of the gut microbiome in PTSD.
The Microbiota-Gut-Brain Axis.
A growing body of basic science indicates the presence of a ‘microbiota-gut-brain axis’ linking gut microbiota and mental health24,25. A bidirectional communication between the brain, gut, and gut microbiome acts via various routes including nervous, endocrine, and immune signaling pathways26. From the ‘top down’, via the central nervous system, acute stress can alter the composition and function of the gut microbiome, the control of gastrointestinal motility, permeability, and the release of luminal neurotransmitters27. From the ‘bottom up’, rodent models have shown that the gut microbiome influences stress-related neurocircuitry and behaviors, feeding behavior, and obesity through neuro-immune-neuroendocrine pathways24,26,28–35, and the gut microbiome can regulate neuronal function and fear extinction learning36. Clinical studies also suggest a key role of the gut microbiome in depression37,38. Moreover, clinical studies using prebiotics (microbiota-targeting diets)39,40, as well as probiotics (bacterial strains)41–47, have shown promise to reduce stress, improve cognitive function, and/or alleviate depressive symptoms. With regard to PTSD specifically, previous human correlational studies and animal experiments suggest a brain-gut connection48–65, whereby gut microbiota influences both amygdala development and response66. Neuroimaging studies demonstrate dysregulation in brain circuits involved in learned fear, with an emphasis on the amygdala, which plays a central role in PTSD67–71. Despite these important observations, they remain primarily correlational, and the mechanisms by which the gut-brain axis directly affects the neural circuitry of fear and stress circuitry remain largely unknown.
Immune system, microbiome, and PTSD.
The human immune system is a sophisticated network comprising both innate and adaptive components, serving as a critical defense mechanism against external threats and internal disruptions to maintain homeostasis72. The microbiome plays an important role in training the host immune system and modulating inflammation, while reciprocally, the immune system shapes the composition and activity of the microbiome73. Accumulating evidence highlights the involvement of both innate and adaptive immune systems in the pathophysiology of PTSD74–76. Previous evidence indicates that PTSD is connected with the immune system through multiple inflammatory markers, including C-reactive protein, interferon-gamma, interleukin-6, interleukin-10, and tumor necrosis factor-alpha77–79. Importantly, the gut microbiome plays a key role in host inflammation. For example, several gut bacterial genera (e.g., Roseburia and Odoribacter) have been reported to possess potential anti-inflammatory properties through producing some metabolites such as SCFA80,81. Therefore, exploring the intricate interplay between the immune system and the gut microbiome in the context of PTSD is of significant importance to unravel the underlying mechanisms of this disorder.
Existing human studies examining the role of the gut microbiome in PTSD.
Epidemiologic studies have found that PTSD is associated with an increased likelihood of developing gastrointestinal disorders82–84, such as inflammatory bowel disease (IBD)85. Indeed, previous studies have shown that patients with IBD are more susceptible to PTSD, and PTSD exacerbates IBD symptoms86–88. It is widely recognized that dysbiosis of the human gut microbiome is associated with various inflammation-related health conditions also associated with PTSD, such as IBD89,90, cardiometabolic disease91,92, and diabetes93. Given these findings, the potential bidirectional effects between the gut microbiome and the stress and immune systems, and extant evidence from animal94–97 and human studies showing gut dysbiosis associated with depression and anxiety98–100, the potential role of the gut microbiome in PTSD warrants further attention. In fact, a recent meta-analysis of the microbiome and psychiatric disorders was unable to evaluate the PTSD–microbiome relation specifically because of the paucity of studies directly examining the microbiome in patients with PTSD38.
To date, only a limited number of observational human studies have examined the PTSD-microbiome association34,101–103. The detailed information and key findings of these studies are summarized in Table 1. The first exploratory study101 (n=30), in a South African cohort, found that three specific phyla (Actinobacteria, Lentisphaerae, and Verrucomicrobia) were able to distinguish individuals with PTSD (n=18) from those who were trauma-exposed but did not have a PTSD diagnosis (n=12). This study also found that a lower total relative abundance of these phyla correlated with higher PTSD symptoms. The second study34 (n=93), in a combat-exposed male cirrhotic veteran cohort, found that individuals with combat-related PTSD had decreased levels of microbial diversity and commensal taxa from Lachnospiraceaeae and Ruminococcaceae, and increased levels of pathobionts (e.g., Enterococcus and Escherichia/Shigella) compared with non-PTSD combat-exposed patients. The third study102, also in a South African cohort, compared PTSD cases (n=79) to trauma-exposed controls (n=58) and identified a group of periodontal disease-related genera (i.e., Mitsuokella, Odoribacter, Catenibacterium, and Olsenella), which could distinguish PTSD from trauma-exposed controls with an accuracy of 66.4%. The relative abundance of this consortium of genera was positively correlated with PTSD severity and childhood trauma. In addition, one study (n=189) conducted on a unique cohort of Israeli veterans who participated in the 1982 Lebanon war reported the associations between the oral microbiota and PTSD103. The study found that the specific microbial signatures, especially the abundance of the bacteria sp_HMT_914, 332 and 871 and Noxia) were associated with PTSD severity, as indicated by symptoms such as intrusiveness, arousal, reactivity, anxiety, hostility, memory difficulties, and idiopathic pain. In a recent study (n=148)104, PTSD was reliably identified using the microbiome profile. The authors found that the genera Dialister and Veillonella were negatively and positively correlated with PTSD, respectively. Moreover, they transplanted the fecal samples from trauma-exposed adolescents (PTSD vs. No PTSD) into germ-free mice. Fecal microbiota transplantation of trauma-exposed youth with PTSD led to increased neophobia behavior (assessed in the elevated plus maze) in germ-free mice. This result implies that the gut microbiome may play a role in the stress- and anxiety-related symptoms seen in PTSD pathology.
Table 1:
Human studies that have identified associations between the human microbiome and PTSD
| Study | Population | Sample types | Sequencing platform | Main findings |
|---|---|---|---|---|
| Hemmings et al. (2017)101 | South African cohort (n=30) | Fecal sample | 16S rRNA gene sequencing | Three phyla (Actinobacteria, Lentisphaerae, and Verrucomicrobia) can distinguished those with PTSD from those who were trauma exposed but did not have a PTSD diagnosis. |
| Bajaj et al. (2019)34 | Combat-exposed male cirrhotic veterans (n=93) | Fecal sample | 16S rRNA gene sequencing | Combat-related PTSD is associated with lower microbial diversity, higher pathobionts, and lower autochthonous taxa composition. |
| Malan-Muller et al. (2022)102 | South African cohort (n=137) | Fecal sample | 16S rRNA gene sequencing | The relative abundance of a consortium of four genera (Mitsuokella, Odoribacter, Catenibacterium, and Olsenella) was higher in the PTSD group than in the trauma-exposed controls and correlated positively with PTSD severity. |
| Levert-Levitt et al. (2022)103 | Israeli veterans (n=189) | Saliva sample | 16S rRNA gene sequencing | Decreased levels of the bacteria sp_HMT_914, 332 and 871 and Noxia were correlated with PTSD severity. |
| Feldman et al. (2022)104 | Mother-child dyads from Sderot, Israel (n=148) | Fecal sample | 16S rRNA gene sequencing | The genera Dialister and Veillonella were negatively and positively correlated with PTSD, respectively. This study provides causative evidence that the microbial trauma profile is at least partially responsible for the trauma-related phenotype. |
In addition, two interventional studies examined the effect of microbiome-targeted supplementation on PTSD symptoms in combat veterans105,106. The first study105, conducted on 10 combat veterans with PTSD, found that regular consumption of fermented soy formulation (FSWW08) for a period of 6 months led to a reduction in anxiety, derealization/detachment, general infection, headache, loss of appetite, panic, upper gastrointestinal burning, and upper respiratory infection. However, causal inference from this study is limited by the lack of a control group as well as the small sample size, selection factors, and reporting biases. In the second study106 involving 31 combat veterans with PTSD, participants were randomized to either an intervention group receiving Lactobacillus reuteri DSM 17938 supplementation or a placebo group (daily for 8 weeks +/− 2 weeks) at a 1:1 ratio, stratified by irritable bowel syndrome status. Probiotic supplementation showed a trend towards a decrease in plasma C-reactive protein (CRP) concentrations compared to the placebo group, and although no significant between-group differences were observed in stress responsivity during the Trier Social Stress Test post-supplementation, the placebo group exhibited a significantly larger increase in mean heart beats per minute between baseline and the math task compared to the probiotic group.
Current Barriers to Progress.
The progress in the field of the PTSD gut microbiome has been constrained by several methodological limitations: (1) Reliance on small and clinically ascertained samples. (2) Risk of confounding bias, such that the relation between PTSD and the microbiome may be explained by a third host factor (e.g., IBD, socioeconomic status, diet, or depression). (3) Failure to distinguish the effects of trauma vs. PTSD in some studies. (4) Using only 16S rRNA gene sequencing, which only offers taxonomic profiles with low resolution (e.g., family and genus levels) and does not offer functional profiles of the gut microbiome. (5) Lack of causal analysis and rational design of microbiota-targeted interventions. (6) The only U.S.-based microbiome studies in PTSD have been conducted in male veterans; the other large microbiome initiatives are all male veteran-focused. Notably, PTSD is more common among women than men107, and civilians differ from veterans on many important characteristics important for health108. Thus, larger and more well-controlled samples, as well as studies on women and civilians, are needed. An additional common limitation of these studies is, with one exception, the lack of integration of insights from animal experiments to test whether microbiome-targeted interventions can alter stress-induced behavior related to neural circuits implicated in PTSD. Such translational studies are necessary to understand mechanisms and to demonstrate causality.
Animal models used in studying PTSD.
Animal models have been extensively utilized to study the role of the gut microbiome in various brain disorders, e.g., autism50–52,55,56, Parkinson’s Disease48,49,62, and Alzheimer’s Disease53,54. Although PTSD is a complex heterogeneous phenotype that is difficult to model in animals109–111, animal studies remain a critical tool for advancing our understanding of PTSD and the neurobiology of stress112–114. In fact, animal models are useful complements to human studies to investigate causal relations where such human studies are neither ethical nor feasible. We simply cannot randomly assign humans to trauma exposure and assess their gut microbiome before and after trauma. Moreover, rodents have demonstrated biologically preserved behavioral and neurobiological responses to stress, which further underlie the use of rodent models in studying the threat- and stress-related neurocircuitry relevant to PTSD115,116.
Note that there are no animal models of PTSD, but animal models that address neurobiological questions relevant to PTSD do exist. In particular, given that PTSD results from stressful, traumatic experiences, rodent models can simulate stress induction (by applying physical, social, and psychological stressors individually or in combination) and certain aspects of disorder development, in particular those focused on the neural circuitry of threat-related behaviors117. Thus, animal models are an indispensable tool to investigate underlying neurocircuitry and pathways and, in particular, the complex interaction of environmental, genetic, neuroendocrine, and gut microbiome factors that may contribute to the development of PTSD.
Currently, multiple stress paradigms, including physical stressors (e.g., footshock, restraint/immobilization stress, underwater stress, and single prolonged stress), social stressors (e.g., housing instability, early life stress, and social defeat), and psychological stressors (e.g., predator and predator odor) represent some of the most etiologically relevant approaches in preclinical research109,110,112,113,115,116,118–121. The different stressors can lead to different behavioral changes in animals. Each model has its advantages and disadvantages, and it can address a different neurobiological question. There is no single model that serves all purposes effectively.
Given the fact that only some people exposed to stress ultimately develop PTSD122, understanding of mechanisms of susceptibility and resilience is important for understanding the pathophysiology of PTSD. Therefore, it is essential to develop an animal model capable of discerning the factors that contribute to susceptibility or resilience to PTSD. This model is important for understanding the mechanisms that determine the individual variability in the development of PTSD.
Existing rodent studies examining the role of the gut microbiome in the neurocircuits of PTSD.
The role of the microbiome in neurocircuits implicated in PTSD has been investigated in animal studies (Table 2). For example, there are several animal studies that investigated the relationship between the gut microbiome and such neurocircuits using a social defeat stress model. Gautam et al.33 investigated how the gut microbiome of male mice responded to exposure to aggressor mice using a cage-within-cage resident-intruder all-male model. They found that the genus Oscillospira, Lactobacillus, Akkermansia, and Anaeroplasma are the top differential abundant genera between control and stressor-exposed mice. Similarly, Hoke et al.123 reported that the exposure to aggressor-exposed social stress caused a notable shift in the time-resolved ratios of Firmicutes and Bacteroidetes and an alternation in the relative abundances of Verrucomicrobia and Actinobacteria. Pearson-Leary et al.124 demonstrated that immune-modulating microbiota, such as Clostridia, were upregulated in short-defeat latencies rats compared to long-defeat latencies rats or control rats. Specifically, they transplanted fecal samples from the three groups to naïve and non-stressed recipient rats via oral gavage and found that fecal transplants from short-defeat latencies/vulnerable rats to naive, non-stressed rats can decrease the latency to immobility and increase the time of mouse spent immobile in the forced swim test. Using a chronic subordinate colony housing (CSC) stress paradigm, Reber et al. showed that the administration of a heat-killed preparation of Mycobacterium vaccae can prevent stress-induced pathology120. Additionally, their findings indicated that the CSC paradigm led to increased abundance of Helicobacter. Langgartner et al.125 demonstrated that transplanting feces from non-stressed single-housed control mice had mild stress-protective effects on mice exposed to a CSC stress paradigm. Specifically, this was evident in the improvement of CSC-induced thymus atrophy, systemic low-grade inflammation, alterations in bone homeostasis, and a decrease in the time spent in the corners during the open-field test.
Table 2:
Animal studies that have identified associations between the microbiome and PTSD
| Study | Animal models | Stressor | Sample sizes | Sequencing platform | Main findings |
|---|---|---|---|---|---|
| Yang et al. (2017)127 | Male, C57BL/6 mice | Social defeat: male, CD1 aggressor mice | Social defeat: n=14; Control: n=8 | 16S rRNA gene sequencing | Bifidobacterium may benefit chronic social defeat stress exposed mice. |
| Szyszkowicz et al., (2017)126 | Male, C57BL/6 | Social defeat: male, CD1 aggressor mice | Social defeat: n=18; Control: n=6 | 16S rRNA gene sequencing | The mRNA expression of interleukin (IL)-1β and IL-6 within the prefrontal cortex were associated with Flavobacterium spp. and Turicibacter spp., which were also strongly correlated to social interaction ratios using social defeat procedures. |
| Bharwani et al., (2017)128 | Male, C57BL/6J mice | Social defeat: male, CD1 aggressor mice | Social defeat: n=32; Control: n33 | 16S rRNA gene sequencing | The treatment with Lactobacillus rhamnosus decreased CSDS-induced anxiety-like behavior and prevented deficits in social interaction with conspecifics |
| Gautam et al. (2018)33 | Male, C57BL/6J mice | CCRISD: male, SJL albino mice | Agg-E: n=5; Control: n=5 | 16S rRNA gene sequencing | The genus Oscillospira, Lactobacillus, Akkermansia and Anaeroplasma were the top four genera that differed between control and stressor-exposed mice. |
| Pearson-Leary et al. (2019)124 | Male, Long-Evans rats | Social defeat: male Sprague-Dawley rats | Social defeat: n=19; Control: n=8 | Shotgun metagenome sequencing | An increased expression of immune-modulating microbiota, such as Clostridia, in short-defeat latencies rats was observed. |
| Zhou et al. (2020)132 | Male, Sprague-Dawley rats | SPS | SPS: n=8; Control: n=8 | 16S rRNA gene sequencing | Changes in Firmicutes, Bacteroidetes, Cyanobacteria, and Proteobacteria levels were most pronounced after SPS exposure. |
| Pascual Cuadrado et al. (2021)135 | Male, Arc-CreERT2 x R26-CAGLSL-Sun1-sfGFP-myc mice | FS | FS: n= 33; Control: n=6 | 16S rRNA gene sequencing | Several microbial taxa (e.g., Akkermansia muciniphila and Parabacteroides distasonis) were significantly enriched in mice behaving similarly to non-stressed mice when compared to the mice that showed extreme responses after foot shock stress. |
| Kelly et al. (2021)134 | Male, Sprague-Dawley rats | HS | Control: n=8; LCHS: n=8; LCHS/CS 7/7: n=8; LCHS/CS 14: n=8. | 16S rRNA gene sequencing | They identified altered microbiota composition among those groups subjected to chronic restraint stress, then went further to identify a temporal effect over the course of two weeks which improved after removal of stressful stimuli. |
| Hoke et al. (2022)123 | Male, C57BL/6J mice | CCRISD: male, SJL albino mice | Agg-E: n=5 control: n=5 | 16S rRNA sequencing | Agg-E SS caused a significant shift in the time-resolved ratios of Firmicutes and Bacteroidetes abundance. Furthermore, Agg-E SS caused diverging shifts in the relative abundances of Verrucomicrobia and Actinobacteria. |
| Tanelian et al. (2022)131 | Male, Sprague-Dawley outbred rats | SPS | SPS: n=14; Control: n=10 | 16S rRNA sequencing | After SPS, the gut microbial communities and their predictive functionality shifted especially in SPS cohorts, with volatility at the genus level correlated with the time spent in the center of OF and on the open arms of EPM |
| Bastiaanssen et al. (2022)129 | Male, C57BL/6J mice | Social defeat: CD1 aggressor mice | Social defeat: n=19; Control: n=41 | 16S rRNA gene sequencing | The volatility (the degree of compositional change over time) of the microbiome significantly associated with social interaction ratio and corticosterone response. |
| Laudani et al. (2023)130 | Male, C57BL/6J mice | 24-hour restraint | Resilient: n=8; Susceptible: n=9; Control: n=41 | 16S rRNA gene sequencing | The trauma (24-hour restraint) significantly affected the microbial structure in mice with a long-lasting hyperarousal. And the mice with ephemeral hyperarousal displayed reduced volatility after trauma exposure compared to long-lasting hyperarousal mice. |
| Tanelian et al., (2023)133 | Female, female Sprague-Dawley rats | SPS | Stressed: n=16; Control: n=10 | 16S rRNA gene sequencing | Differences in the gut microbial composition, functionality, and metabolites were observed in outbred female rats before and after trauma. |
Agg-E: aggressor-exposed; CCRISD: Cage-within-cage resident intruder social defeat; SD: Social defeat; SPS: Single prolonged stress; HS: Hemorrhagic shock; LCHS: lung contusion with hemorrhagic shock; LCHS/CS 7/7: LCHS plus 7 days of restraint cylinder stress for 2 hours daily; LCHS/CS 14: LCHS plus 14 days of restraint cylinder stress for 2 hours daily; OF: Open Field; EPM: Elevated Plus Maze.
In a separate molecular approach, Szyszkowicz et al.126 indicated that changes in mRNA expression of interleukin (IL)-1β and IL-6 within the prefrontal cortex were correlated with the abundance of Flavobacterium spp. and Turicibacter spp in male C57BL/6 mice previously exposed to chronic social defeat stress (CSDS). Furthermore, these bacterial species were highly associated with social avoidance severity in a social interaction test. Yang et al.127 demonstrated that Bifidobacterium might benefit CSDS-exposed mice as it significantly increases the time mouse spent in the interaction zone during a social interaction test compared with the vehicle group. Interestingly, another study reported that the treatment with Lactobacillus rhamnosus decreased CSDS-induced anxiety-like behavior and prevented deficits in social interaction with conspecifics128. Bastiaanssen et al.129 demonstrated that the volatility (the degree of compositional change over time) of the microbiome was significantly associated with the social interaction ratio and corticosterone responses in male mice using a chronic psychosocial stress paradigm. In addition, Laudani et al.130 found that 24-hour restraint stress significantly affected the microbial structure in mice with long-lasting hyperarousal. Also, the mice with ephemeral hyperarousal displayed reduced volatility after restraint stress exposure compared to long-lasting hyperarousal mice. In a different stress paradigm (i.e., single prolonged stress: SPS, consisting of 2 hours restraint stress followed by 20 min forced swim stress and subsequent ether exposure), researchers observed various SPS-induced changes in the gut microbiome of male Sprague-Dawley rats131,132. In a recent study using SPS133, differences in the gut microbial composition, functionality, and metabolites were observed in outbred female rats before and after trauma, and these differences correlated with their coping abilities in response to traumatic stress experiences.
With a different type of stress approach - a rat model of trauma and hemorrhagic shock coupled with either 0, 7, or 14 days of restraint cylinder stress - a previous study suggests that persistent stress may be a driving factor associated with the continued changes in microbial diversity after trauma134. Moreover, a recent study demonstrated that several microbial species (e.g., Akkermansia muciniphila and Parabacteroides distasonis) were significantly enriched in mice behaving similarly to non-stressed mice when compared to the mice that showed extreme responses after foot shock stress135. However, no consistent overall picture of the microbiome has yet emerged from these animal studies.
Although animal models offer an opportunity to understand the interaction between the microbiome and neurocircuits implicated in PTSD, several challenges remain. And despite that animal studies are a key step to address neurobiological questions relevant to PTSD, animal models cannot replicate human illness. Additionally, experimental protocols in animal studies are often not standardized, complicating inter-study comparison and validation of findings. Moreover, differences in animal (e.g., age, sex, species/strain, and vendor), housing conditions (e.g., co-housing and individually ventilated cages), incubation time (i.e., the period between stress stimuli and testing), acclimation time (to the testing room), specific behavioral tests conducted and their order, etc., further aggravate the problem of variability across animal studies. Recent advances in sequencing techniques and computational tools have allowed an increasing number of metagenomics studies to be performed. However, the analysis of the microbiome can be affected by multiple factors, including sample collection (e.g., collection tubes containing preservative media or not containing preservative media), sample transportation (e.g., ice pack, liquid nitrogen, and collection tubes containing preservative media), DNA extraction (e.g., sample volume, preprocessing, and DNA collection kit), genome sequencing (e.g., sequencing platform, sequencing depth, sequencing errors, and genomic repeats), and cumbersome downstream analysis (e.g. quality control and statistical analyses), etc. Together, these factors have significant effects on results, their consistency and reproducibility, highlighting the need to establish a uniform experimental protocol.
Sex differences in understanding the interactions between the microbiome and PTSD.
As we mentioned above, PTSD is more prevalent in women than men107. Previous studies in humans and animal models suggested that differences between the way that the gonadal hormones testosterone or estrogen interact with the HPA axis or modulate hippocampal functioning may contribute to this112,136–139. In particular, the expression of pathological symptoms of PTSD in females may vary from those observed in males140. Sex is also one of the important host factors affecting the human microbiome141,142 and animal microbiome143,144. Sex-related differences, such as sex hormones, may interact with the gut microbiome, potentially influencing the gut-brain axis and contributing to the development of PTSD. Further research is needed to understand the effects of sex differences on PTSD in both human and animal studies.
Potential Future Directions.
To address the limitations identified above and enhance the scientific rigor of studies in this field, we suggest the following directions for future research: (1) leveraging large human cohorts (especially community dwelling civilian women) with both gut microbiome and PTSD data, as well as numerous potential host factors including demographic, socioeconomic, and health variables available; (2) prospective assessment of trauma/PTSD prior to microbiome sample collection in human cohorts to examine whether PTSD is related to the microbiome over and above trauma exposure alone; (3) using whole-metagenome shotgun (WMS) sequencing data that offers both taxonomic and functional profiles of the gut microbiome; (4) integrating multi-omics data (metagenomics, metatranscriptomic, and metabolomics) to systematically understand the mechanisms linking the intestine and PTSD as previous studies revealed that abnormal intestinal environment (e.g., intestinal barrier dysfunction, concentration of short-chain fatty acids (SCFA), and various microbial metabolites)145,146 is associated with PTSD. (4) improving causal inference using novel computational/statistical methods to identify putative PTSD-causal species/pathways, which will help us rationally design synbiotics (the combination of specific probiotics and prebiotics) that may prevent or ameliorate PTSD; and (5) incorporating a combination of uniform and diverse animal models to investigate the causation between stress-related alterations in behavior and gut microbiome.
We expect that the directions proposed above will have three direct and meaningful implications for future research and practice. First, they would represent an important advance by providing information on whether PTSD (versus trauma alone) is associated with gut microbiome differences, with careful adjustment for numerous potential host factors. Second, emerging evidence suggests that PTSD is associated with immune and inflammatory dysfunction, a range of chronic diseases, as well as cognitive deficits all of which have been associated with a disrupted microbiome. The proposed research strategies will provide the foundation for future studies examining whether PTSD-associated gut microbiome dysbiosis is a contributor to chronic diseases. Third, clinical trials are expensive and time consuming and there is limited evidence to support prebiotic or probiotic supplementation trials for PTSD. Using an animal model with an intervention design can help us disentangle the causal relationships. Overall, the proposed research strategies hold the potential to fundamentally alter our understanding of the biological basis of PTSD and trigger future studies on microbiota-targeted interventions for preventing or mitigating PTSD.
Concluding Remarks
Conducting research on the microbiome and PTSD benefits from collaboration among multidisciplinary research teams with complementary expertise in trauma and PTSD epidemiology, microbiome data analysis, animal models, and clinical microbiology. Given the early stage of this field, there are many opportunities for early career researchers to contribute to its advance. Moreover, cross-cultural variation in the microbiome makes collaborator with the global community including researchers from low income and other countries less represented in PTSD research as well as indigenous researchers whose communities face a higher burden of PTSD. Findings will point to new avenues for research and treatment regarding both vulnerabilities to developing - and the consequences of having - PTSD, providing a highly cost-effective set of strategies for determining if additional investment in randomized controlled trials and developing microbiome-based therapies for individuals with PTSD is worthwhile regarding disrupting the negative physical health consequences of PTSD.
Highlights.
PTSD is a common and debilitating mental disorder.
The human microbiome affects the nervous system, behaviors, and brain circuits.
A microbiome perspective will aid in understanding the pathogenesis of PTSD.
Animal models aids microbiome-PTSD study.
Acknowledgments
Y.Y. L. is supported by grants R01AI141529, R01HD093761, R01AG067744, UH3OD023268, U19AI095219, and U01HL089856 from the National Institutes of Health, USA. K.C.K is supported by R01MH101269 from the National Institutes of Health, USA. This work was also supported by at Harvard TH Chan School of Public Health Dean’s Fund for Scientific Advancement Incubation Award to Y.-Y.L. and K.C.K.
Footnotes
Declaration of interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koenen KC et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol Med 47, 2260–2274, doi: 10.1017/S0033291717000708 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng LC et al. National and regional prevalence of posttraumatic stress disorder in sub-Saharan Africa: A systematic review and meta-analysis. PLoS Med 17, e1003090, doi: 10.1371/journal.pmed.1003090 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koenen KC et al. Post-traumatic stress disorder and cardiometabolic disease: Improving causal inference to inform practice. Psychological Medicine 47, 209–225, doi: 10.1017/S0033291716002294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edmondson D, Kronish IM, Shaffer JA, Falzon L & Burg MM Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 166, 806–814, doi: 10.1016/j.ahj.2013.07.031 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seligowski AV, Misganaw B, Duffy LA, Ressler KJ & Guffanti G Leveraging Large-Scale Genetics of PTSD and Cardiovascular Disease to Demonstrate Robust Shared Risk and Improve Risk Prediction Accuracy. Am J Psychiatry 179, 814–823, doi: 10.1176/appi.ajp.21111113 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumner JA et al. Trauma Exposure and Posttraumatic Stress Disorder Symptoms Predict Onset of Cardiovascular Events in Women. Circulation 132, 251–259, doi: 10.1161/CIRCULATIONAHA.114.014492 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine AB, Levine LM & Levine TB Posttraumatic stress disorder and cardiometabolic disease. Cardiology 127, 1–19, doi: 10.1159/000354910 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Boscarino JA Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci 1032, 141–153, doi: 10.1196/annals.1314.011 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Roberts AL et al. Association of Posttraumatic Stress Disorder With Accelerated Cognitive Decline in Middle-aged Women. JAMA Netw Open 5, e2217698, doi: 10.1001/jamanetworkopen.2022.17698 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts AL, Kubzansky LD, Chibnik LB, Rimm EB & Koenen KC Association of Posttraumatic Stress and Depressive Symptoms With Mortality in Women. JAMA Netw Open 3, e2027935, doi: 10.1001/jamanetworkopen.2020.27935 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thursby E & Juge N Introduction to the human gut microbiota. Biochem J 474, 1823–1836, doi: 10.1042/BCJ20160510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho I & Blaser MJ The human microbiome: at the interface of health and disease. Nat Rev Genet 13, 260–270, doi: 10.1038/nrg3182 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers GB et al. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry 21, 738–748, doi: 10.1038/mp.2016.50 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins SM, Surette M & Bercik P The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10, 735–742, doi: 10.1038/nrmicro2876 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Morais LH, Schreiber H. L. t. & Mazmanian SK The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol 19, 241–255, doi: 10.1038/s41579-020-00460-0 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Hao Y, Fan F & Zhang B The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front Psychiatry 9, 669, doi: 10.3389/fpsyt.2018.00669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skonieczna-Zydecka K, Marlicz W, Misera A, Koulaouzidis A & Loniewski I Microbiome-The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J Clin Med 7, doi: 10.3390/jcm7120521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher S et al. HPA axis regulation in posttraumatic stress disorder: A meta-analysis focusing on potential moderators. Neurosci Biobehav Rev 100, 35–57, doi: 10.1016/j.neubiorev.2019.02.005 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Sudo N Microbiome, HPA axis and production of endocrine hormones in the gut. Adv Exp Med Biol 817, 177–194, doi: 10.1007/978-1-4939-0897-4_8 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Leclercq S, Forsythe P & Bienenstock J Posttraumatic Stress Disorder: Does the Gut Microbiome Hold the Key? Can J Psychiatry 61, 204–213, doi: 10.1177/0706743716635535 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner LA et al. Growing literature but limited evidence: A systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brain Behav Immun 65, 57–67, doi: 10.1016/j.bbi.2017.06.003 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Malan-Muller S et al. The Gut Microbiome and Mental Health: Implications for Anxiety- and Trauma-Related Disorders. OMICS 22, 90–107, doi: 10.1089/omi.2017.0077 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Burnet PWJ, Loupy KM & Lowry CA Posttraumatic Stress Disorder and the Gut Microbiome. doi: 10.1093/oxfordhb/9780190931544.013.10 (2020). [DOI] [Google Scholar]
- 24.Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG & Cryan JF Making Sense of … the Microbiome in Psychiatry. Int J Neuropsychopharmacol 22, 37–52, doi: 10.1093/ijnp/pyy067 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adan RAH et al. Nutritional psychiatry: Towards improving mental health by what you eat. Eur Neuropsychopharmacol 29, 1321–1332, doi: 10.1016/j.euroneuro.2019.10.011 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Cryan JF et al. The Microbiota-Gut-Brain Axis. Physiol Rev 99, 1877–2013, doi: 10.1152/physrev.00018.2018 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Martin CR, Osadchiy V, Kalani A & Mayer EA The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol 6, 133–148, doi: 10.1016/j.jcmgh.2018.04.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lach G, Schellekens H, Dinan TG & Cryan JF Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 15, 36–59, doi: 10.1007/s13311-017-0585-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cryan JF & Dinan TG Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13, 701–712, doi: 10.1038/nrn3346 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Dinan TG & Cryan JF Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37, 1369–1378, doi: 10.1016/j.psyneuen.2012.03.007 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Foster JA, Rinaman L & Cryan JF Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress 7, 124–136, doi: 10.1016/j.ynstr.2017.03.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galley JD et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol 14, 189, doi: 10.1186/1471-2180-14-189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautam A et al. Altered fecal microbiota composition in all male aggressor-exposed rodent model simulating features of post-traumatic stress disorder. J Neurosci Res 96, 1311–1323, doi: 10.1002/jnr.24229 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Bajaj JS et al. Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am J Physiol Gastrointest Liver Physiol 317, G661–G669, doi: 10.1152/ajpgi.00194.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szala-Rycaj J, Szewczyk A, Zagaja M & Andres-Mach M Influence of pre- pro- and synbiotics on the proper functioning of the Gut-Brain Axis and cognition in exposure to stress – review of the latest scientific reports from in vivo studies. Annals of Agricultural and Environmental Medicine 29, 326–335, doi: 10.26444/aaem/153448 (2022). [DOI] [Google Scholar]
- 36.Chu C et al. The microbiota regulate neuronal function and fear extinction learning. Nature 574, 543–548, doi: 10.1038/s41586-019-1644-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madison A & Kiecolt-Glaser JK Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Current opinion in behavioral sciences 28, 105–110, doi: 10.1016/j.cobeha.2019.01.011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikolova VL et al. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 78, 1343–1354, doi: 10.1001/jamapsychiatry.2021.2573 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dinan TG et al. Feeding melancholic microbes: MyNewGut recommendations on diet and mood. Clin Nutr 38, 1995–2001, doi: 10.1016/j.clnu.2018.11.010 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Berding K et al. A specific dietary fibre supplementation improves cognitive performance-an exploratory randomised, placebo-controlled, crossover study. Psychopharmacology (Berl) 238, 149–163, doi: 10.1007/s00213-020-05665-y (2021). [DOI] [PubMed] [Google Scholar]
- 41.Allen AP et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry 6, e939, doi: 10.1038/tp.2016.191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amat-Bou M et al. Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader-Willi Syndrome: A Randomized Crossover Trial. Nutrients 12, doi: 10.3390/nu12103123 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reininghaus EZ et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression-A Randomized Controlled Trial. Nutrients 12, doi: 10.3390/nu12113422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reininghaus EZ et al. Probiotic Treatment in Individuals with Euthymic Bipolar Disorder: A Pilot-Study on Clinical Changes and Compliance. Neuropsychobiology 79, 71–79, doi: 10.1159/000493867 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Rudzki L et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 100, 213–222, doi: 10.1016/j.psyneuen.2018.10.010 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Schellekens H et al. Bifidobacterium longum counters the effects of obesity: Partial successful translation from rodent to human. EBioMedicine 63, 103176, doi: 10.1016/j.ebiom.2020.103176 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran N, Zhebrak M, Yacoub C, Pelletier J & Hawley D The gut-brain relationship: Investigating the effect of multispecies probiotics on anxiety in a randomized placebo-controlled trial of healthy young adults. J Affect Disord 252, 271–277, doi: 10.1016/j.jad.2019.04.043 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Perez-Pardo P et al. Gut bacterial composition in a mouse model of Parkinson’s disease. Benef Microbes 9, 799–814, doi: 10.3920/BM2017.0202 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Gorecki AM et al. Altered Gut Microbiome in Parkinson’s Disease and the Influence of Lipopolysaccharide in a Human alpha-Synuclein Over-Expressing Mouse Model. Front Neurosci 13, 839, doi: 10.3389/fnins.2019.00839 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nithianantharajah J, Balasuriya GK, Franks AE & Hill-Yardin EL Using Animal Models to Study the Role of the Gut-Brain Axis in Autism. Curr Dev Disord Rep 4, 28–36, doi: 10.1007/s40474-017-0111-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi Z et al. A Novel and Reliable Rat Model of Autism. Front Psychiatry 12, 549810, doi: 10.3389/fpsyt.2021.549810 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu F, Horton-Sparks K, Hull V, Li RW & Martinez-Cerdeno V The valproic acid rat model of autism presents with gut bacterial dysbiosis similar to that in human autism. Mol Autism 9, 61, doi: 10.1186/s13229-018-0251-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J Alzheimers Dis 60, 1241–1257, doi: 10.3233/JAD-170020 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Chen Y et al. Gut Microbiome Alterations Precede Cerebral Amyloidosis and Microglial Pathology in a Mouse Model of Alzheimer’s Disease. Biomed Res Int 2020, 8456596, doi: 10.1155/2020/8456596 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buffington SA et al. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 165, 1762–1775, doi: 10.1016/j.cell.2016.06.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsiao EY et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463, doi: 10.1016/j.cell.2013.11.024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh V et al. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci 36, 7428–7440, doi: 10.1523/JNEUROSCI.1114-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schretter CE et al. A gut microbial factor modulates locomotor behaviour in Drosophila. Nature 563, 402–406, doi: 10.1038/s41586-018-0634-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532, doi: 10.1038/nature23910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olson CA et al. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 174, 497, doi: 10.1016/j.cell.2018.06.051 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Vadder F et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96, doi: 10.1016/j.cell.2013.12.016 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Sampson TR et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 167, 1469–1480 e1412, doi: 10.1016/j.cell.2016.11.018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clarke G et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18, 666–673, doi: 10.1038/mp.2012.77 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Sudo N et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558, 263–275, doi: 10.1113/jphysiol.2004.063388 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yano JM et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276, doi: 10.1016/j.cell.2015.02.047 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cowan CSM et al. Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling. Bioessays 40, doi: 10.1002/bies.201700172 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Jovanovic T & Ressler KJ How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry 167, 648–662, doi: 10.1176/appi.ajp.2009.09071074 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahan AL & Ressler KJ Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 35, 24–35, doi: 10.1016/j.tins.2011.06.007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ressler KJ et al. Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol 18, 273–288, doi: 10.1038/s41582-022-00635-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alexandra Kredlow M, Fenster RJ, Laurent ES, Ressler KJ & Phelps EA Prefrontal cortex, amygdala, and threat processing: implications for PTSD. Neuropsychopharmacology 47, 247–259, doi: 10.1038/s41386-021-01155-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levy I & Schiller D Neural Computations of Threat. Trends Cogn Sci 25, 151–171, doi: 10.1016/j.tics.2020.11.007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng D, Liwinski T & Elinav E Interaction between microbiota and immunity in health and disease. Cell Res 30, 492–506, doi: 10.1038/s41422-020-0332-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belkaid Y & Hand TW Role of the microbiota in immunity and inflammation. Cell 157, 121–141, doi: 10.1016/j.cell.2014.03.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hori H & Kim Y Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci 73, 143–153, doi: 10.1111/pcn.12820 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Michopoulos V, Powers A, Gillespie CF, Ressler KJ & Jovanovic T Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 42, 254–270, doi: 10.1038/npp.2016.146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katrinli S, Oliveira NCS, Felger JC, Michopoulos V & Smith AK The role of the immune system in posttraumatic stress disorder. Transl Psychiatry 12, 313, doi: 10.1038/s41398-022-02094-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan N, Chen Y, Xia Y, Dai J & Liu C Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry 9, 233, doi: 10.1038/s41398-019-0570-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang JJ & Jiang W Immune biomarkers alterations in post-traumatic stress disorder: A systematic review and meta-analysis. J Affect Disord 268, 39–46, doi: 10.1016/j.jad.2020.02.044 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Passos IC et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2, 1002–1012, doi: 10.1016/S2215-0366(15)00309-0 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Karlsson FH et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3, 1245, doi: 10.1038/ncomms2266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z et al. Gut microbiota, circulating inflammatory markers and metabolites, and carotid artery atherosclerosis in HIV infection. Microbiome 11, 119, doi: 10.1186/s40168-023-01566-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gradus JL et al. Posttraumatic Stress Disorder and Gastrointestinal Disorders in the Danish Population. Epidemiology 28, 354–360, doi: 10.1097/EDE.0000000000000622 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hendrix J et al. Early adverse life events and post-traumatic stress disorder in patients with constipation and suspected disordered defecation. Neurogastroenterol Motil, e14195, doi: 10.1111/nmo.14195 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malhotra D et al. Self-reported gastrointestinal disorders among veterans with gulf war illness with and without posttraumatic stress disorder. Neurogastroenterol Motil, e14548, doi: 10.1111/nmo.14548 (2023). [DOI] [PubMed] [Google Scholar]
- 85.Glynn H et al. Prevalence and Impact of Post-traumatic Stress Disorder in Gastrointestinal Conditions: A Systematic Review. Dig Dis Sci 66, 4109–4119, doi: 10.1007/s10620-020-06798-y (2021). [DOI] [PubMed] [Google Scholar]
- 86.Taft TH et al. Initial Assessment of Post-traumatic Stress in a US Cohort of Inflammatory Bowel Disease Patients. Inflamm Bowel Dis 25, 1577–1585, doi: 10.1093/ibd/izz032 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Camara RJ, Gander ML, Begre S, von Kanel R & Swiss Inflammatory Bowel Disease Cohort Study, G. Post-traumatic stress in Crohn’s disease and its association with disease activity. Frontline Gastroenterol 2, 2–9, doi: 10.1136/fg.2010.002733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taft TH et al. Posttraumatic Stress in Patients With Inflammatory Bowel Disease: Prevalence and Relationships to Patient-Reported Outcomes. Inflamm Bowel Dis, doi: 10.1093/ibd/izab152 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halfvarson J et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol 2, 17004, doi: 10.1038/nmicrobiol.2017.4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franzosa EA et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol 4, 293–305, doi: 10.1038/s41564-018-0306-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmadmehrabi S & Tang WHW Gut microbiome and its role in cardiovascular diseases. Curr Opin Cardiol 32, 761–766, doi: 10.1097/HCO.0000000000000445 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang WH, Kitai T & Hazen SL Gut Microbiota in Cardiovascular Health and Disease. Circ Res 120, 1183–1196, doi: 10.1161/CIRCRESAHA.117.309715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gurung M et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51, 102590, doi: 10.1016/j.ebiom.2019.11.051 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bravo JA et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America 108, 16050–16055, doi: 10.1073/pnas.1102999108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelly JR et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82, 109–118, doi: 10.1016/j.jpsychires.2016.07.019 (2016). [DOI] [PubMed] [Google Scholar]
- 96.O’Mahony SM et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biological psychiatry 65, 263–267, doi: 10.1016/j.biopsych.2008.06.026 (2009). [DOI] [PubMed] [Google Scholar]
- 97.Liu WH et al. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav Brain Res 298, 202–209, doi: 10.1016/j.bbr.2015.10.046 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Maes M, Kubera M & Leunis JC The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 29, 117–124 (2008). [PubMed] [Google Scholar]
- 99.Jiang H et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain, behavior, and immunity 48, 186–194, doi: 10.1016/j.bbi.2015.03.016 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Naseribafrouei A et al. Correlation between the human fecal microbiota and depression. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 26, 1155–1162, doi: 10.1111/nmo.12378 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Hemmings SMJ et al. The Microbiome in Posttraumatic Stress Disorder and Trauma-Exposed Controls: An Exploratory Study. Psychosom Med 79, 936–946, doi: 10.1097/PSY.0000000000000512 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malan-Muller S et al. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur Neuropsychopharmacol 56, 24–38, doi: 10.1016/j.euroneuro.2021.11.009 (2022). [DOI] [PubMed] [Google Scholar]
- 103.Levert-Levitt E et al. Oral microbiota signatures in post-traumatic stress disorder (PTSD) veterans. Mol Psychiatry, doi: 10.1038/s41380-022-01704-6 (2022). [DOI] [PubMed] [Google Scholar]
- 104.Ruth Feldman KY, Sondra Turjeman, Oshrit Shtossel, Orna Zagoory-Sharon, Lelyan Moadi, Yoram Louzoun, Omry Koren. Microbiome Mediates Development of PTSD and Resilience. Research Square, doi: 10.21203/rs.3.rs-1940296/v1 (2022). [DOI] [Google Scholar]
- 105.Gocan AG, Bachg D, Schindler AE & Rohr UD Balancing steroidal hormone cascade in treatment-resistant veteran soldiers with PTSD using a fermented soy product (FSWW08): a pilot study. Horm Mol Biol Clin Investig 10, 301–314, doi: 10.1515/hmbci-2011-0135 (2012). [DOI] [PubMed] [Google Scholar]
- 106.Brenner LA et al. Evaluation of an Immunomodulatory Probiotic Intervention for Veterans With Co-occurring Mild Traumatic Brain Injury and Posttraumatic Stress Disorder: A Pilot Study. Front Neurol 11, 1015, doi: 10.3389/fneur.2020.01015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tolin DF & Foa EB Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological bulletin 132, 959 (2006). [DOI] [PubMed] [Google Scholar]
- 108.Hoerster KD et al. Health and health behavior differences: U.S. Military, veteran, and civilian men. Am J Prev Med 43, 483–489, doi: 10.1016/j.amepre.2012.07.029 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Aspesi D & Pinna G Animal models of post-traumatic stress disorder and novel treatment targets. Behav Pharmacol 30, 130–150, doi: 10.1097/FBP.0000000000000467 (2019). [DOI] [PubMed] [Google Scholar]
- 110.Deslauriers J, Toth M, Der-Avakian A & Risbrough VB Current Status of Animal Models of Posttraumatic Stress Disorder: Behavioral and Biological Phenotypes, and Future Challenges in Improving Translation. Biol Psychiatry 83, 895–907, doi: 10.1016/j.biopsych.2017.11.019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mobbs D et al. Viewpoints: Approaches to defining and investigating fear. Nat Neurosci 22, 1205–1216, doi: 10.1038/s41593-019-0456-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Richter-Levin G, Stork O & Schmidt MV Animal models of PTSD: a challenge to be met. Mol Psychiatry 24, 1135–1156, doi: 10.1038/s41380-018-0272-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verbitsky A, Dopfel D & Zhang N Rodent models of post-traumatic stress disorder: behavioral assessment. Transl Psychiatry 10, 132, doi: 10.1038/s41398-020-0806-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simmons JM, Winsky L, Zehr JL & Gordon JA Priorities in stress research: a view from the U.S. National Institute of Mental Health. Stress 24, 123–129, doi: 10.1080/10253890.2020.1781084 (2021). [DOI] [PubMed] [Google Scholar]
- 115.Toropova KA & Anokhin KV Modeling of Post-Traumatic Stress Disorder in Mice: Nonlinear Relationship with the Strength of the Traumatic Event. Neuroscience and Behavioral Physiology 49, 875–886, doi: 10.1007/s11055-019-00814-z (2019). [DOI] [Google Scholar]
- 116.Pinna G Animal Models of PTSD: The Socially Isolated Mouse and the Biomarker Role of Allopregnanolone. Front Behav Neurosci 13, 114, doi: 10.3389/fnbeh.2019.00114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Borghans B & Homberg JR in World Journal of Psychiatry Vol. 5 387 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yehuda R & Antelman SM Criteria for rationally evaluating animal models of postraumatic stress disorder. Biological Psychiatry 33, 479–486, doi: 10.1016/0006-3223(93)90001-t (1993). [DOI] [PubMed] [Google Scholar]
- 119.Whitaker AM, Gilpin NW & Edwards S Animal models of post-traumatic stress disorder and recent neurobiological insights. Behav Pharmacol 25, 398–409, doi: 10.1097/FBP.0000000000000069 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reber SO et al. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci U S A 113, E3130–3139, doi: 10.1073/pnas.1600324113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reber SO et al. Chronic subordinate colony housing paradigm: A mouse model for mechanisms of PTSD vulnerability, targeted prevention, and treatment-2016 Curt Richter Award Paper. Psychoneuroendocrinology 74, 221–230, doi: 10.1016/j.psyneuen.2016.08.031 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Atwoli L, Stein DJ, Koenen KC & McLaughlin KA Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr Opin Psychiatry 28, 307–311, doi: 10.1097/YCO.0000000000000167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoke A, Chakraborty N, Gautam A, Hammamieh R & Jett M Acute and Delayed Effects of Stress Eliciting Post-Traumatic Stress-Like Disorder Differentially Alters Fecal Microbiota Composition in a Male Mouse Model. Front Cell Infect Microbiol 12, 810815, doi: 10.3389/fcimb.2022.810815 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pearson-Leary J et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry 25, 1068–1079, doi: 10.1038/s41380-019-0380-x (2020). [DOI] [PubMed] [Google Scholar]
- 125.Langgartner D et al. The Role of the Intestinal Microbiome in Chronic Psychosocial Stress-Induced Pathologies in Male Mice. Front Behav Neurosci 12, 252, doi: 10.3389/fnbeh.2018.00252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Szyszkowicz JK, Wong A, Anisman H, Merali Z & Audet MC Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain Behav Immun 66, 45–55, doi: 10.1016/j.bbi.2017.06.009 (2017). [DOI] [PubMed] [Google Scholar]
- 127.Yang C et al. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep 7, 45942, doi: 10.1038/srep45942 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bharwani A, Mian MF, Surette MG, Bienenstock J & Forsythe P Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med 15, 7, doi: 10.1186/s12916-016-0771-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bastiaanssen TFS et al. Volatility as a Concept to Understand the Impact of Stress on the Microbiome. Psychoneuroendocrinology 124, 105047, doi: 10.1016/j.psyneuen.2020.105047 (2021). [DOI] [PubMed] [Google Scholar]
- 130.Laudani S et al. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain Behav Immun 107, 385–396, doi: 10.1016/j.bbi.2022.11.004 (2023). [DOI] [PubMed] [Google Scholar]
- 131.Tanelian A, Nankova B, Miari M, Nahvi RJ & Sabban EL Resilience or susceptibility to traumatic stress: Potential influence of the microbiome. Neurobiology of Stress 19, 100461, doi: 10.1016/j.ynstr.2022.100461 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Q et al. Correlation of gut microbiota and neurotransmitters in a rat model of post-traumatic stress disorder. Journal of Traditional Chinese Medical Sciences 7, 375–385, doi: 10.1016/j.jtcms.2020.10.005 (2020). [DOI] [Google Scholar]
- 133.Tanelian A et al. Differences in gut microbiota associated with stress resilience and susceptibility to single prolonged stress in female rodents. Neurobiol Stress 24, 100533, doi: 10.1016/j.ynstr.2023.100533 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kelly LS et al. Stress-related changes in the gut microbiome after trauma. J Trauma Acute Care Surg 91, 192–199, doi: 10.1097/TA.0000000000003209 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pascual Cuadrado D et al. Long-term molecular differences between resilient and susceptible mice after a single traumatic exposure. Br J Pharmacol 179, 4161–4180, doi: 10.1111/bph.15697 (2022). [DOI] [PubMed] [Google Scholar]
- 136.Briscione MA, Michopoulos V, Jovanovic T & Norrholm SD Neuroendocrine Underpinnings of Increased Risk for Posttraumatic Stress Disorder in Women. Vitam Horm 103, 53–83, doi: 10.1016/bs.vh.2016.08.003 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Maeng LY & Milad MR Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav 76, 106–117, doi: 10.1016/j.yhbeh.2015.04.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fenchel D et al. Beyond the HPA-axis: The role of the gonadal steroid hormone receptors in modulating stress-related responses in an animal model of PTSD. Eur Neuropsychopharmacol 25, 944–957, doi: 10.1016/j.euroneuro.2015.02.004 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Lilly MM, Pole N, Best SR, Metzler T & Marmar CR Gender and PTSD: What can we learn from female police officers? J Anxiety Disord 23, 767–774, doi: 10.1016/j.janxdis.2009.02.015 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Horovitz O, Tsoory MM, Yovell Y & Richter-Levin G A rat model of pre-puberty (juvenile) stress-induced predisposition to stress-related disorders: sex similarities and sex differences in effects and symptoms. World J Biol Psychiatry 15, 36–48, doi: 10.3109/15622975.2012.745604 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Dominianni C et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One 10, e0124599, doi: 10.1371/journal.pone.0124599 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim YS, Unno T, Kim BY & Park MS Sex Differences in Gut Microbiota. World J Mens Health 38, 48–60, doi: 10.5534/wjmh.190009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elderman M et al. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol Sex Differ 9, 26, doi: 10.1186/s13293-018-0186-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Org E et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7, 313–322, doi: 10.1080/19490976.2016.1203502 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Voigt RM et al. Abnormal intestinal milieu in posttraumatic stress disorder is not impacted by treatment that improves symptoms. Am J Physiol Gastrointest Liver Physiol 323, G61–G70, doi: 10.1152/ajpgi.00066.2022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mellon SH et al. Metabolomic analysis of male combat veterans with post traumatic stress disorder. PLoS One 14, e0213839, doi: 10.1371/journal.pone.0213839 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


