Abstract
Background
Exercise intolerance among childhood cancer survivors substantially increases risk for early mortality, reduced cognitive function, poor quality of life, emotional distress, and sub-optimal participation in social roles. Fortunately, exercise intolerance is modifiable, even among individuals with impaired cardiopulmonary and neuromuscular health. This study aims to evaluate the impact of tailored exercise intervention remotely supervised by fitness professionals in survivors with exercise intolerance. Telehealth-based delivery of the intervention aims to enhance uptake by removing the burden of travel and allowing participants to gain confidence with exercise and physical activity at home.
Methods
This is an ongoing single-blind, two-arm, prospective, clinical trial that will randomize 160 participants 1:1 to intervention (n=80) and attention control (n=80) groups. The intervention group receives an individually tailored exercise prescription based on results from baseline assessments performed remotely via a Health Insurance Portability and Accountability Act-compliant virtual platform and personal preferences for aerobic exercise. Each prescription includes aerobic and strengthening components designed to progress gradually to 150–300-minutes of moderate aerobic activity and twice weekly strengthening exercises over 20-weeks. The first two weeks are supervised for 6 sessions, tapering to twice/week for weeks 3–4, once/week for weeks 5–8, every other week for weeks 9–16 and once midway between weeks 17–20. The schedule is modifiable depending on participant need, adherence, and response to exercise. Each session is approximately one hour.
Conclusion
This study tests the efficacy of an individually prescribed, virtually supervised exercise intervention on exercise intolerant childhood cancer survivors.
Clinicaltrials.gov registration:
Keywords: childhood cancer survivors, exercise intolerance, physical activity, fitness, remote, telehealth-based, web-based
1. Introduction
Survival following a childhood cancer diagnosis exceeds 85% in developed countries, with over 500,000 survivors living in the United States today [1]. Unfortunately, cure is not without consequences. Late effects are well-documented in this vulnerable population, who are five times more likely than siblings to report severe, disabling, life threatening or fatal chronic conditions [2], suggesting risk for accelerated aging. Chronic conditions, including impairments of cardiac, pulmonary, autonomic, musculoskeletal, and neurosensory function [3], and specific biomarkers of aging, including shortened leukocyte telomere length [4], reduced mitochondrial deoxyribonucleic acid (DNA) copy number [5] and DNA methylation-based epigenetic age acceleration (EAA) [6] are present in childhood cancer survivors, and are associated with previous cancer treatment exposures.
In the general and in diseased populations, chronic conditions [7–9] and biomarkers of aging [10–13] respond favorably to interventions designed to optimize health behaviors, including those that deliver prescribed exercise with the goal of improving exercise tolerance. Thus, although childhood cancer survivors cannot change treatment exposures, they may be able to optimize health outcomes by engaging in regular exercise. Unfortunately, nearly 33% of childhood cancer survivors report that they do not participate in any regular physical activity, and an additional 27% fail to meet weekly recommendations of 150-minutes of moderate physical activity [14]. Engaging in activity is difficult for anyone, especially in persons whose initial responses to exercise are blunted and uncomfortable from cancer or treatment related organ system dysfunction [15]. Discomfort during exercise is likely discouraging [16], and meaningful behavioral change is difficult without specific guidance [17]. Evidence to support interventions that promote exercise among childhood cancer survivors is scarce [18]; current standard of care is simply to encourage activity [19]. Interventions tailored to accommodate survivor-specific impairments are needed.
Recent meta-analyses and systematic reviews indicate that individualized home-based exercise interventions can improve exercise capacity in persons with heart failure [20], in older adults [21], in survivors of adult-onset cancers [22, 23], and that providing direct remote feedback (video or telephone) has similar positive effects on exercise capacity to supervised center-based exercise [21]. Thus, individualized interventions designed to increase exercise capacity, supervised remotely, and delivered in a home setting have potential for success in childhood cancer survivors. This study is powered on its primary aim, which is to test the impact of an individually tailored, supervised, home-delivered aerobic and strengthening intervention on exercise capacity in survivors of childhood cancer. We will further examine the effects of the intervention on cardiac, pulmonary, musculoskeletal, and neurosensory function as well as emotional health, participation in family and community activities, quality of life and cognitive function. Additionally, we will evaluate the impact of exercise on aging biomarkers. Those in the intervention (INT) group will be compared to an attention control (AC) group. Supervision is tapered over 20-weeks. If effective, this study will move the standard of care (encouraging physical activity) for exercise intolerance from its current state, ineffective for many survivors, to a clinically actionable, practical, sustainable, relatively low cost and generalizable intervention.
2. Methods
2.1. Study design and participants
This is a single-blind two-arm, prospective, randomized clinical trial currently enrolling participants from the St. Jude Lifetime Cohort (SJLIFE), a retrospective cohort study designed to assess childhood cancer survivors surviving ≥5-years from cancer diagnosis. Potentially eligible participants have exercise capacity <85% of predicted VO2peak. Figure 1 outlines the study design and overview. Consented participants are randomized after baseline testing to either the INT or AC group. Outcomes are evaluated at baseline, after the intervention (20-weeks; primary outcome assessment) and 6-months after completion of the intervention (durability outcome). This study is approved and monitored by the St. Jude Children’s Research Hospital Institutional Review and Data Safety Monitoring Boards, respectively.
Figure 1.
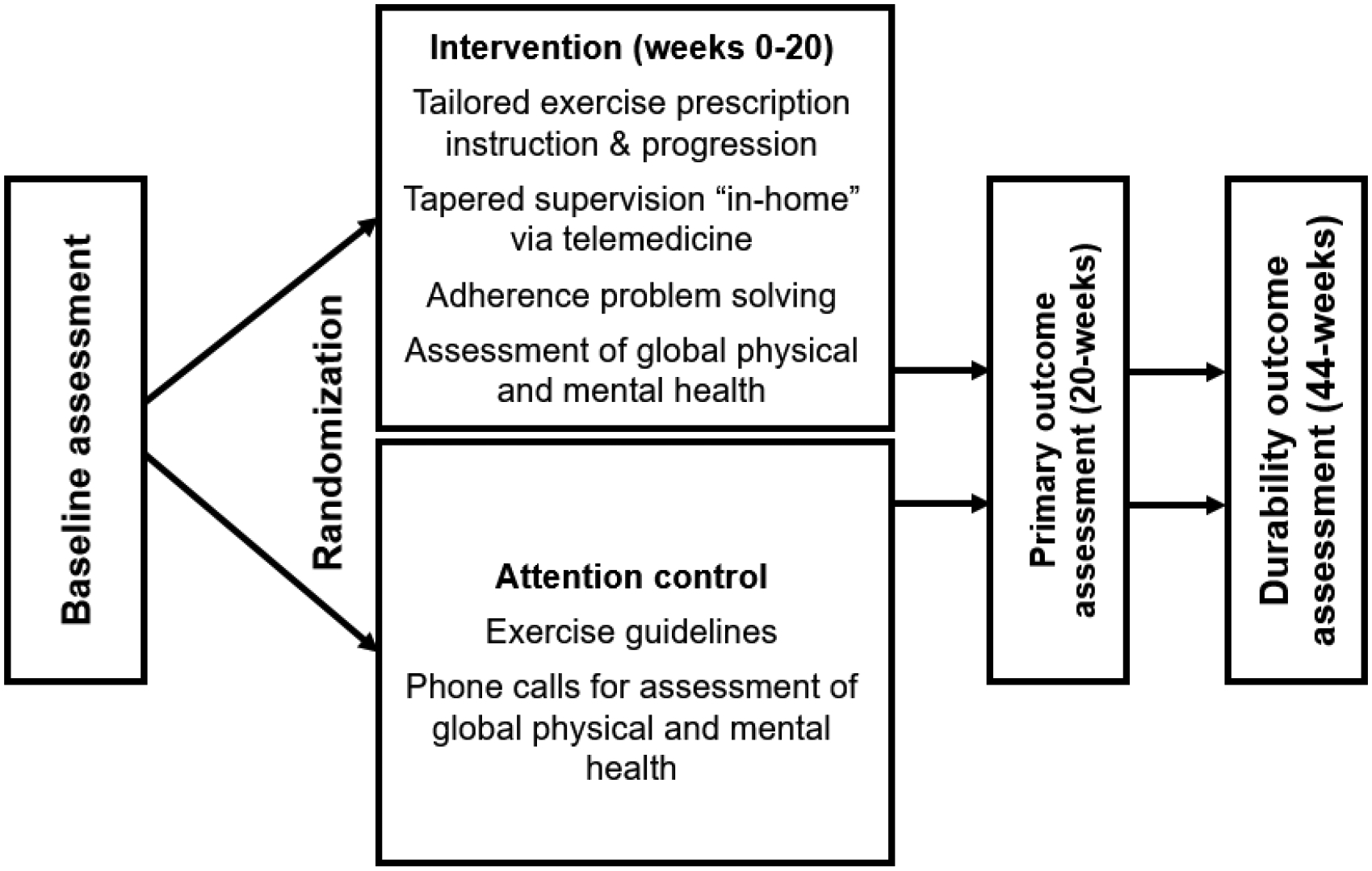
Study Design and overview
2.2. Participant eligibility and recruitment
This study will enroll 160 SJLIFE participants for approximately 11-months. Participants will undergo remote assessment to confirm eligibility, assess baseline outcomes and receive medical clearance from study physicians. Assessment team members are blinded to group assignments.
Inclusion and exclusion criteria are outlined in Table 1. Pregnant females, those enrolled in a formal exercise intervention, or who self-report engaging in >150-minutes/week of moderate physical activity, those endorsing significant psychological distress or those requiring immediate medical intervention are ineligible. The Alcohol Use Disorders Identification Test (AUDIT) and Drug Abuse Screen Test (DAST-10) are used to screen for those who have active alcohol or drug use disorders before baseline testing. If a participant indicates alcohol or drug abuse, participants are taken off study and not randomized. Participants then receives referral for local treatment services. This study was opened for recruitment on February 8, 2021, and 133 participants were enrolled as of August 18, 2023.
Table 1.
Study inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 18 to 39-years of age at enrollment | Enrolled in a formal exercise intervention |
| SJLIFE participant (have history of cancer diagnosed and treated at St. Jude Children’s Research Hospital, alive ≥5 years after cancer diagnosis) | Self-report of >150-mins/week of moderate physical activity |
| VO2peak <85% predicted calculated from 2MSPT (during remote baseline testing) | Currently pregnant |
| Understanding of directions for use of web-based fitness platform on the study provided iPad and HR monitor | Significant psychological distress (e.g., suicidal ideation) |
| Clearance for participation in exercise by a study physician | Conditions requiring immediate medical intervention (e.g., angina, decompensated heart failure) |
| Alcohol or drug abuse in the past year (AUDIT >=13/women and >=15/men or DAST - 10 >=3) |
Abbreviations: 2MSPT = 2-minute step in place test; AUDIT = Alcohol Use Disorders Identification Test; DAST = Drug Abuse Screening Test; HR, heart rate; mins, minutes; SJLIFE, St. Jude Lifetime Cohort; VO2peak, relative peak oxygen uptake
2.3. Consent and randomization
Initial eligibility criteria are confirmed before obtaining consent. If approached in person, the participant is provided with a hard copy of the consent to review. Otherwise, the consent is emailed to the participant for review before the consent discussion, where the study staff reviews the document and answers questions. All participants are informed that, if randomized to the AC group, they will be offered the intervention once the study is complete. No additional data collection is planned for AC participants who choose to receive the intervention. Upon agreement to participate, participants and the study team member sign the document. A copy of the signed consent form is provided to the participant.
Participants complete a baseline medical screening/impairment assessment, performance testing and study questionnaires remotely via a commercially available, Health Insurance Portability and Accountability Act (HIPAA) compliant online platform (Moterum Technologies Inc, Salt Lake City, UT). Participants are stratified by age (18–29 and 30–39-years), sex, race, and baseline estimate (from 2-minute step in place test; 2MSPT) of percent predicted VO2peak (<70%, >70%) and randomized 1:1 between the study arms.
2.4. Study materials
Participants are provided with written instructions, videos of strengthening exercises, a Bluetooth enabled heart rate (HR) monitor worn on the arm (OH1+ or Verity, Polar Electro, Kempele, Republic of Finland) and an iPad (Generation 9 or 10, OS 15.3.1 or 16.5, Apple Inc., Cupertino, CA) pre-loaded with a shortcut to Moterum. This platform provides a secure log-in where participants access their personalized account to connect with their exercise specialist via video session or access their individualized exercise plans. Participants in the intervention group receive exercise equipment (bands, weights). Once participants receive the testing equipment, they are instructed to familiarize themselves with each item and set up their web-based fitness platform account. An assessment only exercise specialist schedules an orientation video session to familiarize the participant with the online platform and all testing equipment. The baseline assessment is scheduled at the end of the virtual orientation session. After baseline testing, the participant is contacted by an intervention exercise specialist to review the results of their baseline assessment and receive group assignment. The first supervised exercise session is then scheduled and includes reviewing the components and proposed progression of their exercise program. Aerobic and strengthening exercises are demonstrated, the participant practices, receives feedback and is encouraged to ask questions.
2.5. Intervention
Exercise specialists (American College of Sports Medicine Certified Clinical Exercise Physiologist (ACSM-CEP)) establish visit times with each participant for initial video communication supervised visits. During the first two weeks, supervision and guidance is provided for six sessions (3/week, divided between aerobic and strengthening sessions) via the web-based fitness platform, allowing for visualization of exercise performance and two-way communication. Supervision is tapered to twice/week in weeks 3–4, once/week in weeks 5–8, every other week in weeks 9–16 and once midway between weeks 17–20. Participants’ goal is to exercise 3x/week for the entire intervention duration so as supervised sessions taper, they exercise independently (following individualized exercise programs written by the exercise specialist) with increasing frequency [12, 24]. For resistance training, the goal is a load that elicits fatigue after 3 sets of 10–12 repetitions on 8–10 exercises 2 days/week. Participants who have difficulty with their exercises or adherence to their program are provided with additional sessions as needed as part of the tailored approach in the study design. Each visit lasts approximately one-hour. The progression of the intervention is estimated a priori but is monitored by the specialist during visits and delayed or accelerated as appropriate. The intervention exercise specialists have cell phones and email accounts, so they can be contacted during working hours (including evenings) to answer questions.
At each visit, ability to adhere to the program is reviewed. Participants having difficulty with adherence (<80% of the prescribed intervention over a rolling two-week interval) complete a specific problem-solving session with the exercise specialist, and with assistance from the study psychologist. The exercise specialist helps identify potential barriers to adherence and discusses possible solutions. Upon identification of potential barriers, the participant selects one solution that they are willing to implement over the next month and it is operationalized. Participants who need more frequent supervision because of difficulties performing an exercise or because of a lapse in adherence may select additional supervision as a potential solution. Participants are not removed from the intervention trial due to poor adherence. Intervention sessions are monitored via the web-based platform. iPads are provided by the study, so remote technology support is available as needed. Replacement iPads and HR monitor devices are provided to participants within 24-hours of failure notification.
Participants randomized to AC are provided with results of their baseline assessment and a handout outlining the Physical Activity Guidelines for Americans. The exercise specialist answers general questions about their performance and encourages them to engage in physical activity. A specific prescription is not provided. Participants are asked to answer general questions about recent exercise habits and to complete the PROMIS ability to participate 8a Short Forms.
2.6. Study Outcomes
The primary outcome of this study is exercise capacity, remotely assessed by the 2MSPT. Testing also includes measures of organ system dysfunction, emotional health, ability to participate in family, social and work activities, and quality of life. Before any fitness testing begins, study staff asks physical activity related health history questions, as well as questions from the Physical Activity Readiness Questionnaire (PAR-Q) [25–27], to ensure exercise is appropriate and safe. If further follow up is needed, a study physician will contact the participant to obtain additional information to determine the safety of further testing and participation in exercise. Data collection is completed at baseline, 20-weeks, and six-months after the intervention is complete in both INT and AC groups. Participants complete PROMIS forms each week during the first eight-weeks and every four-weeks during weeks 9–20. Study outcomes include exercise capacity (2MSPT), resting HR, HR variability (HRV), oxygen saturation and blood pressure (BP), body weight, lung function (spirometry), neurosensory integrity, measurements of physical function (muscle strength) and PA, and questionnaires related to alcohol and drug abuse, PA readiness, health history, anxiety, and activity impairment (Table 2).
Table 2.
Outcome collection timeline
| Measure | Time point | ||
|---|---|---|---|
| BL | 20-wk | 6-mo | |
| Informed Consent | X | ||
| Screening Questionnaires: | X | ||
| Alcohol Use Disorders Identification Test (AUDIT) | |||
| Drug Abuse Screen Test (DAST-10) | |||
| Pregnancy test (urine), if applicable | X | ||
| 2-minute step in place test | X | X | X |
| Resting HR, oxygen saturation and blood pressure | X | X | X |
| Weight | X | X | X |
| Spirometry | X | X | X |
| Muscle Strength | X | X | X |
| Neurosensory integrity | X | X | X |
| Physical activity and HR/HR variability | X | X | X |
| Questionnaire: | X | X | X |
| Physical activity Readiness Questionnaire (PAR-Q) | |||
| Questionnaires: | X | X | X |
| Physical Activity Related Health History | |||
| Patient Health Questionnaire (PHQ-8) | |||
| Generalized Anxiety Disorder (GAD-7) | |||
| PROMIS Ability to Participate 8a (SF v2.0) | |||
| Work Productivity and Activity Impairment Questionnaire (WPAI) | |||
| Work and Social Adjustment Scale (WSAS) | |||
| Medical Outcomes Survey Short Form (SF-36) | |||
| Childhood Cancer Survivor Study Neurocognitive | |||
| Questionnaire – Revised (CCSS-NCQ) | |||
| Questionnaire (Acceptability Measures): | X | X | |
| Service User Technology Acceptability Questionnaire (SUTAQ) | |||
| System Usability Scale (SUS) | |||
| OPTIONAL: Biomarkers (saliva), if applicable | X | X | X |
Abbreviations: BL, baseline; HR, heart rate; mo, month; wk, week
2.7. Vital signs, sensory testing, and spirometry
Body weight is obtained twice on an electronic scale without shoes. Oxygen saturation (SpO2) and resting HR are measured using a pulse oximeter (FL400, FaceLake, Buffalo Grove, IL) placed on the index finger. The participant is seated and rests for 5-minutes before documentation. Once HR and SpO2 have stabilized, the participant reports the values. Blood pressure is measured using a wireless BP monitor (7350, Omron Healthcare, Kyoto, Japan). The exercise specialist instructs the participant to place the BP monitor on the left upper arm and to start the device after 5-minutes of seated rest. A second reading is taken on the left arm after a one-minute rest. If both readings are >10 mmHg different for either systolic or diastolic pressures, a third measurement is taken. Peripheral sensation and motor function are evaluated using the sensory and motor symptom questions from the modified total neuropathy scale [28]. A total score out of 8 is documented. Scores of >2 are considered impaired. Spirometry is assessed with a portable flowmeter (PF100, Microlife, Widnau, Switzerland). Peak expiratory flow (PEF) and forced expiratory volume in one-second (FEV1) are measured. Participants are instructed to take a deep breath and fully exhale into the mouthpiece as forcefully and quickly as possible. Peak expiratory flow and FEV1 are measured digitally on the screen. Participants have three attempts, and the highest values are documented.
2.8. Physical performance and biospecimen collection
Exercise capacity is measured using the 2MSPT [29]. The participant stands directly next to a blank wall keeping a stable chair nearby for safety. They place two pieces of tape; one at the patella and another at the iliac crest. The exercise specialist verbally assists the participant with palpating the anatomical sites. Using the supplied measuring tape, the participant measures the distance between the two tape marks, then places the 3rd piece of tape at the halfway point of the patella and iliac crest tape marks. The participant then removes the patella and iliac crest marks leaving only the halfway point tape. The exercise specialist instructs the participant to start and counts the number of times the right knee completes a step in two-minutes, ensuring the knee meets or passes the tape mark each time. Feedback is provided if the participant needs to lift the knees higher to meet the tape mark. Pulse oximetry is used to capture HR, documented prior to and immediately after the test, and one- and two-minutes into recovery. The participant is asked to rate how hard they felt they were working during the test using the RPE scale.
Handgrip strength is measured using a digital dynamometer (Jamar, Patterson, Warrenville, IL). The participant holds the dynamometer with fingertips in the grooves, with the elbow bent to 90 degrees, and squeezes as hard as possible for 2–3-seconds. Peak force is recorded in kilograms. Participants perform the sit-to-stand test to measure leg strength using a standard-sized chair next to a table or wall for safety. With arms crossed at the shoulders, participants attempt to stand and sit as quickly as possible for 30-seconds. The number of times the participant stands and sits again are recorded. Physical activity, HR and HRV are measured using an accelerometer (xGT3X-BT, ActiGraph, LLC, Pensacola, FL) and a Polar HR monitor. Participants wear the accelerometer on an elastic belt around the waist and the HR monitor around the chest, snug against the skin, to collect physical activity data, HR and HRV over 3-days at each assessment timepoint.
Optionally, after signing the appropriate consent forms, participants in both groups provide saliva samples at baseline, after the intervention and 6-months after completion of the intervention. Deoxyribonucleic acid is then extracted from saliva samples, and a DNA methylation EPIC array is used for methylation profiling [30]. Quantitative polymerase chain reaction (qPCR) is then used to simultaneously quantify telomere length and mitochondrial DNA copy number [31, 32].
Participants complete global physical and mental health (PROMIS) short form measures (2-items each) weekly during weeks 1–8 and every fourth week during weeks 9–20. Social and work participation is evaluated with the PROMIS ability to participate in social roles and activities Short Form [33], the work productivity and activity impairment questionnaire (WPAI) [34, 35] and the work and social adjustment scale (WSAS) [36, 37]. Emotional health is assessed using the Patient Health Questionnaire 8 (PHQ-8) for depression [38, 39]. The Generalized Anxiety Disorder 7 (GAD-7) questionnaire is used to assess mild, moderate and severe anxiety [40, 41], and the Medical Outcomes Survey Short Form (SF-36) to assess quality of life [42, 43]. Cognitive function is evaluated via the Childhood Cancer Survivor Study Neurocognitive Questionnaire (CCSS-NCQ) – Revised. The CCSS-NCQ is a 32 item self-report questionnaire evaluating four domains of neurocognitive function: (1) memory; (2) task efficiency; (3) organization; and (4) emotional regulation [44]. Lastly, two constructs related to participant experience with the intervention are assessed at the end of and six months after the intervention is complete. The Service User Technology Acceptability Questionnaire (SUTAQ) [45] includes 22 statements to which participants use a 6-point Likert-type scale to rate agreement. The System Usability Scale (SUS) is a 10-item questionnaire, which measures agreement (i.e., strongly agree to strongly disagree) on a five-point scale [46].
2.9. Data management
Registration, enrollment, randomization, and data collection/entry are done at St. Jude Children’s Research Hospital. Data from the assessment and the survey are recorded by study personnel by participant identification number on optical recognition forms and downloaded daily onto a computer server with restricted access to the research team. The medical records are abstracted, and the data are recorded using an established database. All files are password protected. Identifying information is stored separately and the files are linked by identification number. Paper forms are stored in a locked file cabinet.
2.10. Sample size calculation
With a sample size of 129, we have 80% power using a two-sided two sample t-test and an alpha value of 0.05 to detect a difference of 3.75 ml/kg/min (0.5 standard deviation (SD)) between groups on the VO2peak assessed with the 2MSPT. We estimate 20% missing data at week 20. We plan to enroll a total of 163 participants on the study to ensure adequate power. This sample size accounts for interim analysis when 65 survivors have been randomized and followed for 20-weeks. We will only stop for futility if the p-value is larger than 0.724 in the interim analysis. During the interim analysis, we will also obtain estimates of the variability and correlation in peak VO2/2MSPT measurements and missingness proportions. We will use this information to revise the sample size upwards if required. The sample size will not be reduced.
2.11. Data analysis
For each of the continuous outcomes in Aims 1 and 3, differences between two groups will be compared using two-sample t-tests, where the two peak VO2/2MSPT (or other outcomes) measurements at end of intervention and baseline post randomization are denoted by and for the INT group and and for the AC group, and , and for i = 1, 2,…64 denote differences b the 2 groups. It is important to note that even when original distributions are not normal (distributions of Yi’s), the distribution of the differences (Di’s) are often close to normal. However, we will test assumptions of normality for the differences, and if normality assumptions are not met, we will appropriately transform the data before applying t-tests for analysis.
For each of the outcomes in aim 2, because they can be represented both as continuous measures and dichotomies (impaired/normal), we will evaluate both mean changes in the differences between groups as described above and compare the proportions of those whose values indicate impairment at end of intervention between groups, adjusting for the baseline prevalence of the specific impairment. These analyses will utilize Generalized Estimating Equation (GEE) [47], specifying a binomial distribution in PROC GENMOD in SAS v9.4 with compound symmetry as the working covariance structure to account for correlation among repeated observations.
Analyses to also be conducted to determine the sustainability of changes in our outcome measures (6 months post-intervention) in a similar manner, as discussed above, by taking the differences between the measurement obtained at 6 months after end of intervention and baseline measurement and comparing the proportions of those whose values indicate impairment 6 months after end of intervention between groups. In addition, since we will have observations at three time points for all outcome measures, and at many time points for the PROMIS Global Health Instruments, we will also use GEE approaches to assess patterns of change over time using PROC GENMOD in SAS v9.4, with compound symmetry as the working covariance structure to account for correlation among repeated observations.
For analysis of the biomarkers, let and , j=1, 2,…n denote differences from after the intervention to baseline in the marker level and outcome measure for n individuals on the trial. Similarly, and , j=1, 2,…n denote differences from 6 months after the intervention to after the intervention in the marker level and outcome measure for n individuals on the trial. Generalized linear regression will be used to compare the changes in the biomarker levels between the intervention and the control group with baseline values of the biomarker as a covariate in the model. Generalized linear regression will be used to assess the relationship between changes in biomarker levels and changes in outcome measures and the models will be appropriately adjusted for other covariates of interest.
2.12. Missing and incomplete data
We do not expect >20% missing data but have increased the sample size to ensure adequate power. However, if there are missing data, irrespective of the missingness percentage, a multiple imputation approach will be established, assuming that data are missing at random, as described in Fitzmaurice, et al. [47], to test the null hypothesis and assess sensitivity of the results.
3. Discussion
Evidence suggests that regular exercise is difficult and/or uncomfortable for childhood cancer survivors because of underlying organ system impairments [14, 15], reducing motivation to perform physical activity, maintain quality of life and substantially increases mortality risk. This study employs a telehealth-based strategy to test the efficacy of individually prescribed exercise training on exercise tolerance in childhood cancer survivors. Results from this study will highlight the importance of improving cardiorespiratory fitness to reduce late effects as well as increase motivation, confidence, and independence to encourage lifelong physical activity.
This study is innovative for various reasons. It engages an individualized, progressive exercise prescription that accommodates survivor-specific organ-based impairments within the context of American Heart Association and/or American College of Sports Medicine Exercise guidelines for persons with chronic disease. This study also provides supervision and/or guidance from an exercise specialist to reassure survivors lacking confidence that they can be successful, and that starting an exercise program is naturally progressive. Importantly, this study tapers supervision over time, allowing survivors to become progressively independent with the exercises. Further, both intervention and assessments are designed to be completely home-based and all materials (equipment, written and video instruction, supervision/monitoring devices) are provided, removing barriers of fitness center travel, access, recurring cost, and self-consciousness. This study is also flexible, addressing adherence concerns, and engages the survivor in identifying barriers and selecting solutions that work for them. This study is also assessing the sustainability of outcomes six months post- intervention, testing long-term adherence to the exercise program.
This intervention is not without limitations. Using telehealth-based assessment and intervention strategies requires internet access. To counteract this, internet hot spots are sent to participants with unreliable internet access. Scheduling limitations can also hinder participant progress. It may become difficult to schedule live sessions for those working nightshifts or multiple jobs. However, our exercise specialists have successfully worked through scheduling conflicts in the past. Lastly, like all intervention studies, adherence to the regimen can be a challenge when conducting virtual sessions. Since supervision is tapered over time, participants become more inclined to miss or skip sessions. Participants will not be dropped for poor adherence. Instead, we have participants identify barriers that are causing poor adherence and help find solutions.
4. Conclusion
This study is an ongoing, telehealth-based intervention designed to improve overall fitness and mental health to perform exercise among exercise intolerant survivors of childhood cancer. Exercise intolerance substantially increases their risk for early mortality, reduced cognitive function, poor quality of life, emotional distress, and sub-optimal participation in social roles [48–56]. Fortunately, exercise intolerance is modifiable, even among individuals with impaired cardiac [57, 58], pulmonary [59], and neuromuscular health [60]. Nevertheless, survivors do not, on average, engage in exercise [14]. Findings from this study will shed light on the efficacy of using a telehealth-based system to improve exercise tolerance and motivate childhood cancer survivors to engage in physical activity and improve long-term health.
Acknowledgements
The authors would like to thank Tracie Gatewood for contributions in manuscript formatting and editing.
Funding
This trial is supported by funding from the National Cancer Institute [U01 CA195547 (Hudson, Ness), P30 CA021765 (Roberts), U01 CA246570 (Ness, Jefferies)] and the American Lebanese Syrian Associated Charities. The sponsors have no involvement in the study design, collection, analysis, and interpretation of data, writing the report or the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 2MSPT
2-minute step in place test
- AC
attention control group
- AUDIT
alcohol use disorders identification test
- BP
blood pressure
- CCSS-NCQ
Childhood Cancer Survivor Study Neurocognitive Questionnaire
- DAST
drug abuse screening test
- DNA
Deoxyribonucleic acid
- EAA
epigenetic age acceleration
- FEV1
forced expiratory volume in 1 second
- GAD-7
Generalized Anxiety Disorder – 7
- HR
heart rate
- HRV
heart rate variability
- INT
intervention group
- PAR-Q
physical activity readiness questionnaire
- PEF
peak expiratory flow
- PHQ-8
patient health questionnaire-8
- PROMIS
Patient Reported Outcomes Measurement Information System global physical and mental health short forms
- SF v2.0
PROMIS ability to participate
- SF-36
medical outcomes survey short form
- SJLIFE
St. Jude Lifetime Cohort
- SPO2
oxygen saturation
- SUS
system usability scale
- SUTAQ
Service User Technology Questionnaire
- VO2peak
relative peak oxygen uptake
- WASA
work and social adjustment scale
- WPAI
work productivity and activity impairment questionnaire
Footnotes
CRediT authorship contribution statement
Arun Maharaj: Writing – original draft, Writing – review & editing. John L. Jefferies: Writing – review & editing, Conceptualization, Investigation, Supervision. Daniel A. Mulrooney: Writing – review & editing, Conceptualization, Supervision. Gregory T. Armstrong: Writing – review & editing, Conceptualization. Tara M. Brinkman: Writing – review & editing, Conceptualization. Sean T. O’Neil: Writing – review & editing, Investigation. Sarah Terrell: Writing – Conceptualization. Robyn E. Partin: Writing – review & editing, Investigation. Deo Kumar Srivastava: Writing – review & editing, Conceptualization, Formal analysis, Methodology. Melissa M. Hudson: Writing – review & editing, Conceptualization, Supervision. Zhaoming Wang: Writing – review & editing, Conceptualization. Kirsten K. Ness: Writing – review & editing, Conceptualization, Supervision, Funding acquisition, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Protocol documents are available upon request to the corresponding author. Study data will be made publicly available when the study is complete at https://www.stjude.cloud/ [61] and www.zenodo.com [62].
REFERENCES
- [1].Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feurer EJ, Cronin KA (Eds.), SEER Cancer Statistics Review (CSR) 1975–2018, 2021. https://seer.cancer.gov/archive/csr/1975_2018/. (Accessed Jan 1 2023). [Google Scholar]
- [2].Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, Sklar CA, Robison LL, Oeffinger KC, Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study, J. Clin. Oncol 32(12) (2014) 1218. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, Clinical ascertainment of health outcomes among adults treated for childhood cancer, JAMA 309(22) (2013) 2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Song N, Li Z, Qin N, Howell CR, Wilson CL, Easton J, Mulder HL, Edmonson MN, Rusch MC, Zhang J, Shortened leukocyte telomere length associates with an increased prevalence of chronic health conditions among survivors of childhood cancer: A report from the St. Jude Lifetime CohortTelomere and Health Conditions in Childhood Cancer Survivors, Clin. Cancer Res 26(10) (2020) 2362–2371. doi: 10.1158/1078-0432.CCR-19-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCastlain K, Howell CR, Welsh CE, Wang Z, Wilson CL, Mulder HL, Easton J, Mertens AC, Zhang J, Yasui Y, The association of mitochondrial copy number with sarcopenia in adult survivors of childhood cancer, J. Natl. Cancer Inst 113(11) (2021) 1570–1580. doi: 10.1093/jnci/djab084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Qin N, Li Z, Song N, Wilson CL, Easton J, Mulder H, Plyler E, Neale G, Walker E, Zhou X, Epigenetic age acceleration and chronic health conditions among adult survivors of childhood cancer, J. Natl. Cancer Inst 113(5) (2021) 597–605. doi: 10.1093/jnci/djaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ostman C, Smart N, Morcos D, Duller A, Ridley W, Jewiss D, The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta-analysis, Cardiovasc. Diabetol 16(1) (2017) 1–11. doi: 10.1186/s12933-017-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rao S, Pandey A, Garg S, Park B, Mayo H, Despres J-P, Kumbhani D, de Lemos JA, Neeland IJ, Effect of exercise and pharmacological interventions on visceral adiposity: a systematic review and meta-analysis of long-term randomized controlled trials, Mayo Clin. Proc 94(2) (2019) 211–224. doi: 10.1016/j.mayocp.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sharman JE, La Gerche A, Coombes JS, Exercise and cardiovascular risk in patients with hypertension, Am. J. Hypertens 28(2) (2015) 147–158. doi: 10.1093/ajh/hpu191. [DOI] [PubMed] [Google Scholar]
- [10].Bryan AD, Magnan RE, Hooper AEC, Harlaar N, Hutchison KE, Physical activity and differential methylation of breast cancer genes assayed from saliva: a preliminary investigation, Ann. Behav. Med 45(1) (2013) 89–98. doi: 10.1007/s12160-012-9411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chang YK, Cho SH, Kim J-H, Association between leukocyte mitochondrial DNA copy number and regular exercise in postmenopausal women, Korean J. Fam. Med 37(6) (2016) 334. doi: 10.4082/kjfm.2016.37.6.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fitzgerald KN, Hodges R, Hanes D, Stack E, Cheishvili D, Szyf M, Henkel J, Twedt MW, Giannopoulou D, Herdell J, Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial, Aging 13(7) (2021) 9419. doi: 10.18632/aging.202913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sindi S, Solomon A, Kåreholt I, Hovatta I, Antikainen R, Hänninen T, Levälahti E, Laatikainen T, Lehtisalo J, Lindström J, Telomere length change in a multidomain lifestyle intervention to prevent cognitive decline: A randomized clinical trial, J. Gerontol. - Biol. Sci. Med. Sci 76(3) (2021) 491–498. doi: 10.1093/gerona/glaa279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Scott JM, Li N, Liu Q, Yasui Y, Leisenring W, Nathan PC, Gibson T, Armenian SH, Nilsen TS, Oeffinger KC, Association of exercise with mortality in adult survivors of childhood cancer, JAMA Oncol 4(10) (2018) 1352–1358. doi: 10.1001/jamaoncol.2018.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lansing RW, Gracely RH, Banzett RB, The multiple dimensions of dyspnea: review and hypotheses, Respir. Physiol. Neurobiol 167(1) (2009) 53–60. doi: 10.1016/j.resp.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Flack KD, Johnson L, Roemmich JN, Aerobic and resistance exercise reinforcement and discomfort tolerance predict meeting activity guidelines, Physiol. Behav 170 (2017) 32–36. doi: 10.1016/j.physbeh.2016.11.032. [DOI] [PubMed] [Google Scholar]
- [17].Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput J-P, Chastin S, Chou R, World Health Organization 2020 guidelines on physical activity and sedentary behaviour, Br. J. Sports. Med 54(24) (2020) 1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chow EJ, Leger KJ, Bhatt NS, Mulrooney DA, Ross CJ, Aggarwal S, Bansal N, Ehrhardt MJ, Armenian SH, Scott JM, Paediatric cardio-oncology: epidemiology, screening, prevention, and treatment, Cardiovasc. Res 115(5) (2019) 922–934. doi: 10.1093/cvr/cvz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Children’s Oncology Group, Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers Version 5.0, 2018. http://www.survivorshipguidelines.org/pdf/2018/cog_ltfu_guidelines_v5.pdf. (Accessed Jan 1 2023).
- [20].Zwisler A-D, Norton RJ, Dean SG, Dalal H, Tang LH, Wingham J, Taylor RS, Home-based cardiac rehabilitation for people with heart failure: A systematic review and meta-analysis, Int. J. Cardiol 221 (2016) 963–969. doi: 10.1016/j.ijcard.2016.06.207. [DOI] [PubMed] [Google Scholar]
- [21].Geraedts H, Zijlstra A, Bulstra SK, Stevens M, Zijlstra W, Effects of remote feedback in home-based physical activity interventions for older adults: a systematic review, Patient Educ. Couns 91(1) (2013) 14–24. doi: 10.1016/j.pec.2012.10.018. [DOI] [PubMed] [Google Scholar]
- [22].Fuller JT, Hartland MC, Maloney LT, Davison K, Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials, Br. J. Sports. Med 52(20) (2018) 1311–1311. doi: 10.1136/bjsports-2017-098285. [DOI] [PubMed] [Google Scholar]
- [23].Stout NL, Baima J, Swisher AK, Winters-Stone KM, Welsh J, A systematic review of exercise systematic reviews in the cancer literature (2005–2017), PM. R 9(9) (2017) S347–S384. doi: 10.1016/j.pmrj.2017.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ, Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention, Circulation 107(8) (2003) 1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- [25].Adams R, Revised physical activity readiness questionnaire, Can. Fam. Physician 45 (1999) 992. doi, https://www.ncbi.nlm.nih.gov/pubmed/10216799. [PMC free article] [PubMed] [Google Scholar]
- [26].Cardinal BJ, Esters J, Cardinal MK, Evaluation of the revised physical activity readiness questionnaire in older adults, Med. Sci. Sports Exerc 28(4) (1996) 468–472. doi: 10.1097/00005768-199604000-00011. [DOI] [PubMed] [Google Scholar]
- [27].Thomas S, Reading J, Shephard RJ, Revision of the physical activity readiness questionnaire (PAR-Q), Can. J. Sport. Sci 17(4) (1992) 338–345. doi, https://www.ncbi.nlm.nih.gov/pubmed/1330274. [PubMed] [Google Scholar]
- [28].Wampler MA, Miaskowski C, Hamel K, Byl N, Rugo H, Topp KS, The modified total neuropathy score: a clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer, J. Support. Oncol 4(8) (2006) 9–16. doi, https://www.researchgate.net/publication/294685541_The_modified_Total_Neuropathy_Score_a_clinically_feasible_and_valid_measure_of_taxane-induced_peripheral_neuropathy_in_women_with_breast_cancer. [Google Scholar]
- [29].Rikli RE, Jones CJ, Functional fitness normative scores for community-residing older adults, ages 60–94, J. Aging. Phys.Act 7(2) (1999) 162–181. doi: 10.1123/japa.7.2.162. [DOI] [Google Scholar]
- [30].Middleton LY, Dou J, Fisher J, Heiss JA, Nguyen VK, Just AC, Faul J, Ware EB, Mitchell C, Colacino JA, Saliva cell type DNA methylation reference panel for epidemiological studies in children, Epigenetics 17(2) (2022) 161–177. doi: 10.1080/15592294.2021.1890874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lapham K, Kvale MN, Lin J, Connell S, Croen LA, Dispensa BP, Fang L, Hesselson S, Hoffmann TJ, Iribarren C, Automated assay of telomere length measurement and informatics for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort, Genetics 200(4) (2015) 1061–1072. doi: 10.1534/genetics.115.178624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen Y, Hill HZ, Lange G, Falvo MJ, Salivary mitochondrial DNA copy number is associated with exercise ventilatory efficiency, J. Strength Cond. Res 31(7) (2017) 2000–2004. doi: 10.1519/Jsc.0000000000001932. [DOI] [PubMed] [Google Scholar]
- [33].Hahn EA, Kallen MA, Jensen RE, Potosky AL, Moinpour CM, Ramirez M, Cella D, Teresi JA, Measuring social function in diverse cancer populations: Evaluation of measurement equivalence of the Patient Reported Outcomes Measurement Information System®(PROMIS®) ability to participate in social roles and activities short form, Psychol. Test Assess. Model 58(2) (2016) 403. doi, https://www.ncbi.nlm.nih.gov/pubmed/30221102. [PMC free article] [PubMed] [Google Scholar]
- [34].Ortega CCF, Veiga DF, Camargo K, Juliano Y, Neto MS, Ferreira LM, Breast reconstruction may improve work ability and productivity after breast cancer surgery, Ann. Plast. Surg 81(4) (2018) 398–401. doi: 10.1097/SAP.0000000000001562. [DOI] [PubMed] [Google Scholar]
- [35].Reilly MC, Zbrozek AS, Dukes EM, The validity and reproducibility of a work productivity and activity impairment instrument, Pharmacoeconomics 4(5) (1993) 353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- [36].Jansson-Fröjmark M, The work and social adjustment scale as a measure of dysfunction in chronic insomnia: reliability and validity, Behav. Cogn. Psychother 42(2) (2014) 186–198. doi: 10.1017/S135246581200104X. [DOI] [PubMed] [Google Scholar]
- [37].Thandi G, Fear NT, Chalder T, A comparison of the Work and Social Adjustment Scale (WSAS) across different patient populations using Rasch analysis and exploratory factor analysis, J. Psychosom. Res 92 (2017) 45–48. doi: 10.1016/j.jpsychores.2016.11.009. [DOI] [PubMed] [Google Scholar]
- [38].Dhingra SS, Kroenke K, Zack MM, Strine TW, Balluz LS, PHQ-8 Days: a measurement option for DSM-5 Major Depressive Disorder (MDD) severity, Popul. Health Metr 9(1) (2011) 1–8. doi: 10.1186/1478-7954-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH, The PHQ-8 as a measure of current depression in the general population, J. Affect. Disord 114(1–3) (2009) 163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- [40].Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, Holland JC, Partridge AH, Bak K, Somerfield MR, Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation, J. Clin. Oncol 32(15) (2014) 1605. doi: 10.1200/Jco.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Esser P, Hartung TJ, Friedrich M, Johansen C, Wittchen HU, Faller H, Koch U, Härter M, Keller M, Schulz H, The Generalized Anxiety Disorder Screener (GAD‐7) and the anxiety module of the Hospital and Depression Scale (HADS‐A) as screening tools for generalized anxiety disorder among cancer patients, Psychooncology 27(6) (2018) 1509–1516. doi: 10.1002/pon.4681. [DOI] [PubMed] [Google Scholar]
- [42].Ware JE Jr, Sherbourne CD, The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection, Med. Care 30(6) (1992) 473–483. doi, https://www.ncbi.nlm.nih.gov/pubmed/1593914. [PubMed] [Google Scholar]
- [43].Reulen RC, Zeegers MP, Jenkinson C, Lancashire ER, Winter DL, Jenney ME, Hawkins MM, The use of the SF-36 questionnaire in adult survivors of childhood cancer: evaluation of data quality, score reliability, and scaling assumptions, Health Qual. Life Outcomes 4(1) (2006) 1–8. doi: 10.1186/1477-7525-4-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kadan-Lottick NS, Zeltzer LK, Liu Q, Yasui Y, Ellenberg L, Gioia G, Robison LL, Krull KR, Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers, J. Natl. Cancer Inst 102(12) (2010) 881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hirani SP, Rixon L, Beynon M, Cartwright M, Cleanthous S, Selva A, Sanders C, Newman SP, Quantifying beliefs regarding telehealth: development of the whole systems demonstrator service user technology acceptability questionnaire, J. Telemed. Telecare 23(4) (2017) 460–469. doi: 10.1177/1357633x16649531. [DOI] [PubMed] [Google Scholar]
- [46].Lewis JR, The system usability scale: past, present, and future, Int. J. Hum-Comput. Int 34(7) (2018) 577–590. doi: 10.1080/10447318.2018.1455307. [DOI] [Google Scholar]
- [47].Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, Handbooks of modern statistical methods: Longitudinal data analysis, Taylor & Francis Group, New York, NY, 2008. [Google Scholar]
- [48].Hayes SM, Forman DE, Verfaellie M, Cardiorespiratory fitness is associated with cognitive performance in older but not younger adults, J. Gerontol. B Psychol. Sci. Soc. Sci 71(3) (2016) 474–482. doi: 10.1093/geronb/gbu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jenney ME, Faragher EB, Jones PHM, Woodcock A, Lung function and exercise capacity in survivors of childhood leukaemia, Med. Pediatr. Oncol. Suppl 24(4) (1995) 222–230. doi: 10.1002/mpo.2950240403. [DOI] [PubMed] [Google Scholar]
- [50].Miller AM, Lopez‐Mitnik G, Somarriba G, Lipsitz SR, Hinkle AS, Constine LS, Lipshultz SE, Miller TL, Exercise capacity in long‐term survivors of pediatric cancer: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study, Pediatr. Blood Cancer 60(4) (2013) 663–668. doi: 10.1002/pbc.24410. [DOI] [PubMed] [Google Scholar]
- [51].Ness KK, DeLany JP, Kaste SC, Mulrooney DA, Pui C-H, Chemaitilly W, Karlage RE, Lanctot JQ, Howell CR, Lu L, Energy balance and fitness in adult survivors of childhood Acute Lymphoblastic leukemia, Blood 125(22) (2015) 3411–3419. doi: 10.1182/blood-2015-01-621680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].van Brussel M, Takken T, Lucia A, van der Net J, Helders PJ, Is physical fitness decreased in survivors of childhood leukemia? A systematic review, Leukemia 19(1) (2005) 13–17. doi: 10.1038/sj.leu.2403547. [DOI] [PubMed] [Google Scholar]
- [53].Christensen JF, Bandak M, Campbell A, Jones LW, Højman P, Treatment-related cardiovascular late effects and exercise training countermeasures in testicular germ cell cancer survivorship, Acta. Oncol 54(5) (2015) 592–599. doi: 10.3109/0284186X.2014.995776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Morey MC, Pieper CF, Cornoni-Huntley J, Is there a threshold between peak oxygen uptake and self-reported physical functioning in older adults?, Med. Sci. Sports Exerc 30(8) (1998) 1223–1229. doi: 10.1097/00005768-199808000-00007. [DOI] [PubMed] [Google Scholar]
- [55].Ladenvall P, Persson CU, Mandalenakis Z, Wilhelmsen L, Grimby G, Svärdsudd K, Hansson P-O, Low aerobic capacity in middle-aged men associated with increased mortality rates during 45 years of follow-up, Eur. J. Prev. Cardiol 23(14) (2016) 1557–1564. doi: 10.1177/2047487316655466. [DOI] [PubMed] [Google Scholar]
- [56].Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis, JAMA 301(19) (2009) 2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- [57].Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD, Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials, Circ. Heart. Fail 8(1) (2015) 33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal H, Lough F, Rees K, Singh SJ, Mordi IR, Exercise‐based rehabilitation for heart failure, Cochrane Database Syst. Rev 2014(4) (2014) CD003331. doi: 10.1002/14651858.CD003331.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Leite MR, Ramos EMC, Kalva-Filho CA, Freire APCF, de Alencar Silva BS, Nicolino J, de Toledo-Arruda AC, Papoti M, Vanderlei LCM, Ramos D, Effects of 12 weeks of aerobic training on autonomic modulation, mucociliary clearance, and aerobic parameters in patients with COPD, Int. J. Chron. Obstruct. Pulmon. Dis 10 (2015) 2549–2447. doi: 10.2147/COPD.S81363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rampello A, Franceschini M, Piepoli M, Antenucci R, Lenti G, Olivieri D, Chetta A, Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study, Phys. Ther 87(5) (2007) 545–555. doi: 10.2522/ptj.20060085. [DOI] [PubMed] [Google Scholar]
- [61].St. Jude Children’s Research Hospital, St. Jude Cloud, 2023. https://www.stjude.cloud/. [Google Scholar]
- [62].CERN European Organization for Nuclear Research, Zenodo, 2023. https://zenodo.org/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Protocol documents are available upon request to the corresponding author. Study data will be made publicly available when the study is complete at https://www.stjude.cloud/ [61] and www.zenodo.com [62].


