SUMMARY
Tick-borne encephalitis virus (TBEV) is a flavivirus that causes human neuroinfections and represents a growing health problem. The human monoclonal antibody T025 targets envelope protein domain III (EDIII) of TBEV and related tick-borne flaviviruses, potently neutralizing TBEV in vitro and in preclinical models, representing a promising candidate for clinical development. We demonstrate that TBEV escape in the presence of T025 or T028 (another EDIII-targeting human monoclonal antibody) results in virus variants of reduced pathogenicity, characterized by distinct sets of amino acid changes in EDII and EDIII that are jointly needed to confer resistance. EDIII substitution K311N impairs formation of a salt bridge critical for T025-epitope interaction. EDII substitution E230K is not on the T025 epitope but likely induces quaternary rearrangements of the virus surface because of repulsion of positively charged residues on the adjacent EDI. A combination of T025 and T028 prevents virus escape and improves neutralization.
Graphical Abstract

In brief
Svoboda et al. investigate tick-borne encephalitis virus escape from two potent human monoclonal antibodies. The mutants, resistant to individual antibodies, have distinct sets of amino acid changes that are jointly needed to confer full resistance. A combination of the two monoclonal antibodies effectively prevents virus escape.
INTRODUCTION
Neutralizing monoclonal antibodies (mAbs) represent a promising strategy to combat viral infection.1–5 Because of advances in the discovery and selection of neutralizing mAbs from human memory B cells, it is possible to rapidly identify highly effective human mAbs against novel or emerging pathogens.3 Tick-borne flaviviruses (TBFVs; genus Orthoflavivirus, family Flaviviridae)6 cause orphan diseases that represent an attractive target for development of therapeutic mAbs. TBFVs infect humans worldwide, with a significant increase in the number of cases over the past two decades.7 Clinical presentation of human TBFV infection ranges from asymptomatic or mild flu-like illness to life-threating encephalitis or hemorrhagic fever.8 TBFVs include Powassan virus (POWV), Omsk hemorrhagic fever virus (OHFV), Kyasanur forest disease virus (KFDV), and louping ill virus (LIV). However, of all TBFVs, tick-borne encephalitis virus (TBEV) is considered the most medically important.8,9
TBEV causes more than 10,000 cases of tick-borne encephalitis (TBE), with numerous deaths each year.10,11 The virus is endemic throughout most of Eurasia, mainly in central, eastern, and northern Europe, as well as northern and eastern Asia, including northern Japan and South Korea.12,13 Recently, autochthonous TBE cases were also reported in the United Kingdom,14 and TBEV has been detected in North Africa.15 TBE is also imported into non-endemic countries, such as the United States, Canada, and Australia.16–18 TBEV causes infection and inflammation of the brain and/or meninges and can manifest as meningitis, meningoencephalitis, or encephalomyelitis, which is the most severe form. The mortality rate ranges from less than 1% up to 20% depending on the particular TBEV subtype.12 Importantly, a significant proportion of people with TBE suffer from long-lasting or permanent health impairments that severely affect their quality of life.19
Although there is a safe and effective vaccine to prevent TBE, vaccination coverage is low in many endemic countries, mainly because of its high cost and the need for regular boosters to maintain full protection. Apart from supportive care, there is no specific therapy for people with TBE. Therefore, development of medical countermeasures against TBE is urgently needed.12,20
Along with drugs that interfere with TBEV replication, mAbs represent a promising approach against TBE. The main target of flavivirus-neutralizing antibodies is the envelope (E) protein, which mediates virus entry into host cells. The E protein consists of three structural domains (EDI, EDII, and EDIII), a helical stem, and two anti-parallel transmembrane helices.21,22 EDIII is the receptor-binding domain and the main target of the most potent neutralizing antibodies.
We previously studied the human neutralizing antibody response to TBEV in a cohort of infected and vaccinated people.23 Potent TBEV EDIII-recognizing mAbs were derived from people who had recovered from TBEV infection. Some of these antibodies neutralized not only TBEV but also other TBFVs, such as OHFV, KFDV, POWV, LIV, and Langat virus. Notably, prophylactic or early therapeutic administration of antibodies was effective at low doses in mice challenged with a lethal dose of TBEV, suggesting that they may be effective in post-exposure prophylaxis or early therapy against human TBE.23
Similar to other RNA viruses, flaviviruses, including TBEV, undergo error-prone replication,24,25 which may favor development of virus resistance under selective pressure by single mAbs.26–34
Here, we investigated TBEV escape from two potent human mAbs that target EDIII: T025 and T028.23 Virus sequencing revealed distinct sets of amino acid substitutions, one in EDIII and the other in EDII, that were selected simultaneously by each of the two antibodies when administered individually. Importantly, the escape variants were highly attenuated. Virus escape was effectively prevented by combining T025 and T028. Mechanistic analysis by reverse genetics revealed the relative contribution of the EDII and EDIII substitutions to escape.
RESULTS
T025 and T028 escape variants bear distinct sets of amino acid changes
To determine whether TBEV escape variants emerge in the presence of T025, we passaged TBEV-Hypr in vitro in the presence of subneutralizing concentrations of T025 (Figure 1A). T025-resistant virus emerged at passage 5 (TBEV-MUT25; Figure 1B). Sequencing of the E gene revealed two nucleotide mutations (G1660A and G1905T) compared with virus passaged in parallel in the absence of antibody. Both mutations led to amino acid changes, E230K in EDII and K311N in the T025 epitope, resulting in formation of a site of potential N-linked glycosylation (N-X-S/T; Figure 1C). Interestingly, E230K was detectable already at first passage, but full resistance was acquired only in the fifth passage, when K311N appeared.
Figure 1. Generation of TBEV escape variants from T025 and T028 mAbs.

(A and D) Passaging strategy for selection of escape variants from T025 (A) and T028 (D).
(B and E) Sensitivity of TBEV escape variants (TBEV-MUT25 and TBEV-MUT28) and the parental strain (TBEV-Hypr) to the selecting mAbs T025 (B) and T028 (E), as determined by a neutralization test. Two independent experiments were performed in octuplicate.
(C and F) Surface model of the TBEV E protein with domains I, II, and III colored in red, yellow, and blue, respectively. The amino acid changes in TBEV-MUT25 (C) and TBEV-MUT28 (F) are shown.
(G) Alignment of the E protein sequences of TBEV-Hypr, both escape variants, and other notable TBFVs. Amino acid changes in the E protein sequence are highlighted in yellow for TBEV-MUT25 and in blue for TBEV-MUT28. OHFV, Omsk hemorrhagic fever virus; KFDV, Kyasanur forest disease virus; POWV, Powassan virus; AHFV, Alkhurma hemorrhagic fever virus; LGTV, Langat virus; LIV, louping ill virus.
A similar analysis of escape variants was performed for T028. TBEV-MUT28 was retrieved at the fifth passage and was associated with G1804A and A2066C nucleotide mutations, resulting in amino acid changes G278R in EDII and E365A in EDIII, respectively. E365A is within the putative epitope of T028, occurred already at the third passage, and preceded G278R in the fifth passage (Figures 1D–1F). The emergence of different sets of amino acid changes in virus propagated in the presence of T025 or T028 was consistent with the two antibodies binding distinct epitopes on EDIII, as shown by a biolayer interferometry binding assay that allowed determination of the kinetics and affinity of molecular interactions (Figure S1). Confirming virus escape, both mutant viruses were completely resistant to the mAb used to select them, whereas sensitivity to a panel of other previously described mAbs targeting EDIII was variable (Figure S2).23
EDIII (K311N in TBEV-MUT25 and E365A in TBEV-MUT28) and one of the EDII changes (G278R in TBEV-MUT28) were located at highly conserved positions compared with other TBFVs (OHFV, KFDV, POWV, Alkhurma hemorrhagic fever virus (AHFV), Langat virus (LGTV), and LIV) (Figure 1G). E230K involves a poorly conserved residue, with K230 already present in naturally occurring POWV (Figure 1G).
To evaluate the stability of the newly established virus variants, we passaged TBEV-MUT25 and TBEV-MUT28 in porcine kidney stable (PS) cells without selective pressure. After 10 passages, we observed no reversion to the parental sequence, and the c mutants preserved the mutations in EDII and EDIII for the whole period. We conclude that virus escape occurs in the presence of T025 or T028 and that the emerging virus variants display a combined set of amino acid changes, one at EDII and the other at EDIII, that are unique for each of the two antibodies. No mutations were detected in virus passaged in the absence of antibodies.
T025 and T028 escape variants have an altered viral phenotype in vitro
To investigate the impact of virus escape on the kinetics of viral growth and plaque morphology, we compared TBEV-MUT25 and TBEV-MUT28 with parental TBEV-Hypr. The titer of parental TBEV was 1.10 × 105 plaque-forming units (PFUs)/mL and 1.30 × 106 PFUs/mL after 48 h and 72 h, respectively, whereas the titer of TBEV-MUT25 reached comparable titers earlier, 36 h and 48 h after infection (2.18 × 105 PFUs/mL and 5.74 × 106 PFUs/mL, respectively; Figure 2A). Similarly, the growth of TBEV-MUT28 was not impaired over parental TBEV, reaching comparable titers after 48 h and 72 h (5.18 × 105 PFUs/mL and 2.85 × 106 PFUs/mL, respectively; Figure 2A).
Figure 2. Characterization of T025 and T028 escape variants.

(A) Growth kinetics of TBEV-MUT25 and TBEV-MUT28 compared with TBEV-Hypr (n = 3 for each virus; mean values with standard deviation [SD] are shown).
(B and C) Plaque morphology (B; scale bar, 15.4 mm) and diameter (C) of the escape variants compared with the parental strain (****p < 0.0001, n = 104 for TBEV-MUT25, n = 101 for TBEV-Hypr, n = 101 for TBEV-MUT28, Mann-Whitney U test).
(D) Assessment of virulence in a mouse model of TBEV infection. BALB/c mice were infected with 1,000 PFUs of TBEV-Hypr (n = 12 mice) or the escape variant (n = 12 mice for TBEV-MUT25 and n = 6 mice for TBEV-MUT28). Survival curves for TBEV-MUT25 (****p < 0.0001) and TBEV-MUT28 (***p = 0.0007) were analyzed by log rank Mantel-Cox test and are shown at the top left; the other panels show disease score over time.
(E and F) Neutralization profiles of recombinant TBEV mutants (obtained by site-directed mutagenesis) with either single or combined mutations conferring resistance to T025 (E) or T028 (F) mAbs. Two independent experiments were performed in octuplicate.
In contrast, the plaque morphology of the antibody-resistant variants in PS cells was markedly different from that of the parental virus (Figure 2B). Plaques of TBEV-Hypr were large and clearly distinct 96 h post infection, with diameters ranging from 357–2,231 mm (mean, 1,115 ± 432 μm). In comparison, plaques produced by TBEV-MUT25 or TBEV-MUT28 were significantly smaller (p < 0.0001), with diameters of 222–1,460 μm (mean, 708 ± 255 μm) and 311–1,606 μm (mean, 828 ± 287 μm), respectively (Figure 2C). Thus, the virus escape variants of T025 and T028 had similar or even slightly improved growth kinetics but smaller plaques.
TBEV-MUT25 and TBEV-MUT28 are highly attenuated in vivo
Next, the neuroinvasiveness (i.e., the ability of a virus to invade the CNS after replication in peripheral tissues and induction of viremia)35 of TBEV-MUT25 and TBEV-MUT28 was compared with parental TBEV-Hypr. Three groups of 6-week-old BALB/c mice were infected subcutaneously with 1,000 PFUs of TBEV-MUT25 (n = 12), TBEV-MUT28 (n = 6), or TBEV-Hypr (n = 12) and monitored for disease and survival over 28 days. Mice infected with parental virus developed mild clinical signs starting on day 6 post infection (piloerection or hunched back), which rapidly progressed to 100% mortality by day 13 (mean survival, 9.1 ± 1.6 days).
In contrast, significantly lower mortality and longer mean survival were observed in mice infected with escape variants. With TBEV-MUT25, symptoms started on day 8, mortality was lower (42%), and survival was longer (mean survival, 21.8 ± 7.3 days). Only mild, if any, symptoms were observed in TBEV-MUT25-infected mice that survived. With TBEV-MUT28, all but one mouse developed symptoms and survived (mean survival, 25.8 ± 4.8 days; Figure 2D).
To assess reversion to the parental genotype, we collected the brains of mice that died from TBEV-MUT25 or TBEV-MUT28 infection and isolated the virus to sequence the E gene, which confirmed the persistence of both amino acid changes (K311N and E230K for TBEV-MUT25 and G278R and E365A for TBEV-MUT28). The E sequence from brains of mice infected with parental virus was also unchanged. Notably, no mutations corresponding to K311N or E230K were observed in the brains of TBEV-Hypr-infected mice treated with T025, indicating that viral escape from T025 is not observed in vivo. We conclude that T025- and T028-resistant variants are less virulent than the parental strain.
Paired amino acid changes are required to confer full resistance
To evaluate the role of each mutation in virus escape, we produced recombinant TBEV variants bearing single (E230K, K311N for TBEV-MUT25; G278R, E365A for TBEV-MUT28) or combined (E230K+K311N for TBEV-MUT25; G278R+E365A for TBEV-MUT28) amino acid changes and analyzed their sensitivity to T025 or T028. Compared with parental TBEV, E230K or K311N conferred only partial resistance to T025, with K311N having a stronger effect (half-maximal inhibitory concentration [IC50] = 9.184 μg/mL) than E230K (IC50 = 0.5998 μg/mL). Consistent with earlier experiments with TBEV-MUT25, the E230K+K311N combination conferred complete resistance to T025 (IC50 > 10 μg/mL), confirming that both mutations are required for complete virus escape from T025 (Figure 2E). Similar results were obtained for T028, where complete resistance was acquired only when both amino acid changes (G278R+E365A) were present, whereas individual changes resulted in a partial (E365A, IC50 = 0.873 μg/mL) or no change in sensitivity to T028 neutralization (G278R, IC50 = 0.023 μg/mL) compared with TBEV-Hypr (Figure 2F). Thus, both of the amino acid changes in TBEV-MUT25 and TBEV-MUT28 E are required for full escape from the respective antibodies.
TBEV-MUT25 escape is independent of 311N glycosylation
Decreased sensitivity of the virus to neutralization by T025 with substitution at K311EDIII is consistent with the structural observation that this residue is important for T025 recognition of EDIII because the T025 antibody is in direct contact with K311 of TBEV EDIII (D100HC of T025 makes a salt bridge with K311EDIII). This is based on a crystal structure of the antibody fragment (Fab) from T025 (IGVH3–IGVH30/IGVK3–IGVK15) in complex with the EDIII T025 published previously (PDB: 7LSG; Figure 3A; Figure S4).23 In addition to the predicted loss of the salt bridge, the K311N substitution in TBEV-MUT25 leads to formation of a consensus sequence for potential N-linked glycosylation (Asn-X-Ser/Thr, where X represents any amino acid except proline) (Figure 3B). To investigate the role of potential 311N glycosylation in antibody escape, we produced mutant viruses with K311Q and K311N+T313A in which the sequence was altered so that the site could not be glycosylated(Figure 3C). No significant difference in T025 neutralization was observed compared with the K311N variant, indicating that glycosylation at this position is not necessary for the virus to escape from T025 (Figure 3D).
Figure 3. Evaluation of the K311N change in EDIII of TBEV-MUT25.
(A) Binding of T025 to EDIII of the E protein on a model of the TBEV virion. T025 directly interacts with K311EDIII, but not with E230EDII.
(B) Mutation resulting in K311N forms a site of potential N-linked glycosylation.
(C) Portions of the T025 epitope in TBEV-Hypr and recombinantly produced mutants with amino acid changes in 311, 313, or both, disrupting the glycosylation sequence.
(D) Neutralization profiles of TBEV-Hypr (black) and recombinant variants at the potential glycosylation site (shades of green). The recombinant variant corresponding to TBEV-MUT25 (K311N+E230K) is shown in orange. Two independent experiments were performed in octuplicate.
E230K in EDII changes the quaternary structure of E based on structural model analysis
Although located in EDII, changing residue 230 (E → K) alone conferred partial resistance to T025 and other mAbs binding to EDIII (Figure 4A).23 We performed structural analyses of models of TBEV alone or in complex with T025 to determine the most probable binding position of T025 on the surface of the TBEV virion and found that, in addition to direct contact with EDIII, it also interacts with EDII of the neighboring E protein subunit (Figure 4B). Moreover, E230 sits in a positively charged electrostatic pocket formed by three adjacent lysines of the neighboring subunit (K38, K296, and K298), interacting directly with K298 (Figures 4C and S5). In the E230K variant, a negatively charged glutamic acid is replaced by a positively charged lysine, possibly leading to repulsion of K230 by K298 and changes to the subunit’s quaternary structure. According to this in silico analysis, E230 does not interact directly with T025 or affect the tertiary structure of the subunit to which it belongs. However, the E230K substitution changes the quaternary structure of the subunit, affecting T025 binding.
Figure 4. Evaluation of the E230K change in EDII of TBEV-MUT25.
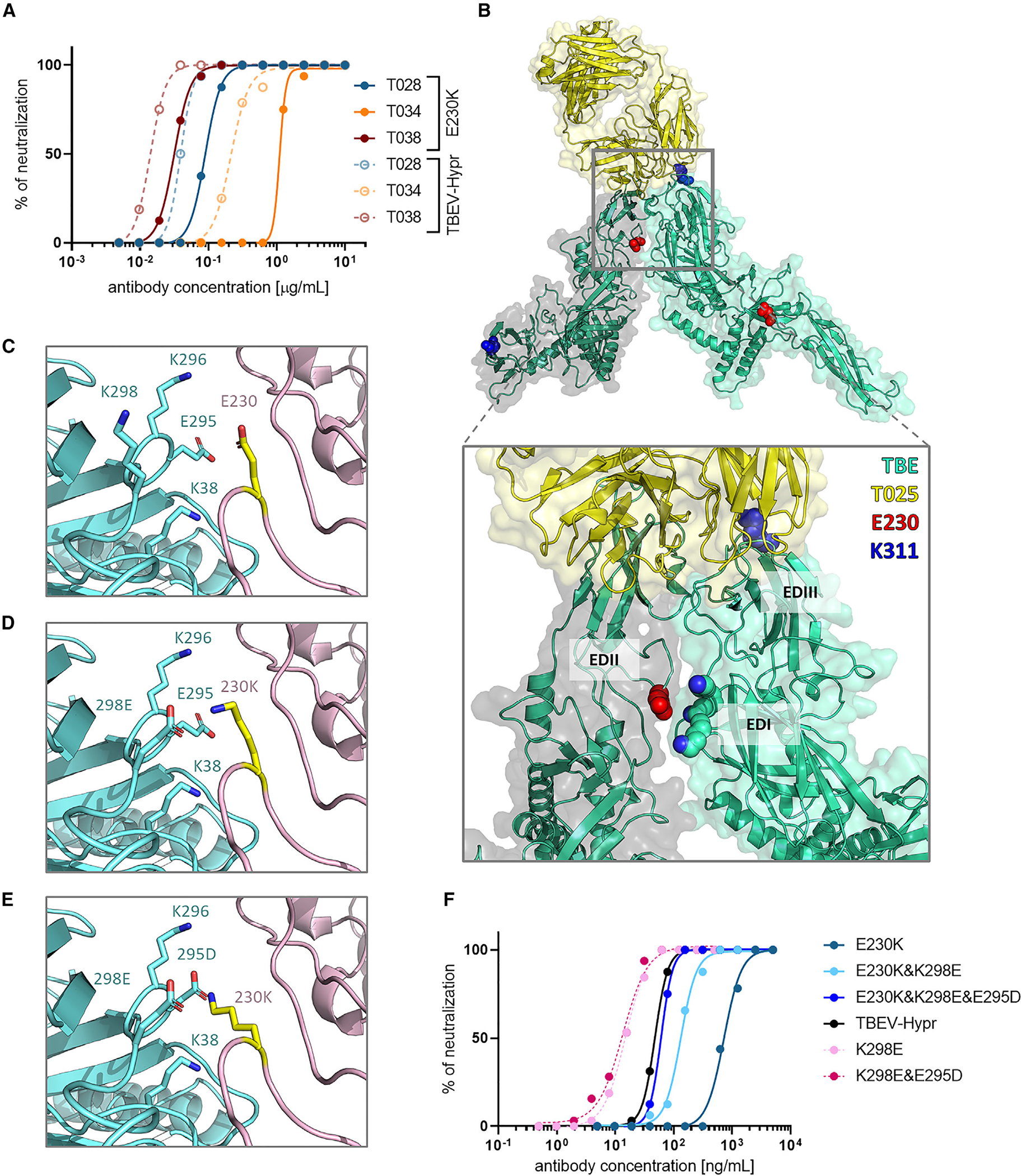
(A) Comparison of mAb neutralization against TBEV-Hypr and the E230K recombinant variant. Two independent experiments were performed in octuplicate.
(B) Most probable position of T025 binding to the TBEV E protein. T025 binds the EDIII epitope while interacting with EDII of the neighboring E protein subunit.
(C) Interaction between E230 in EDII and EDI. E230 sits in a positively charged electrostatic pocket formed by K38, K298, and K296 of the adjacent E protein subunit, interacting directly with K298. The E230K mutation possibly leads to repulsion and change in the quaternary structure.
(D and E) Introduction of counterchanges K298E (D) or K298E and E295D (E) to restore the electrostatic interactions.
(F) Neutralization profiles of recombinant variants before (E230K) and after (E230+K298E, E230+K298E+E295D) introduction of counterchanges (shades of blue). Variants without E230K are shown in shades of pink, and the parental strain (TBEV-Hypr) is shown in black. Two independent experiments were performed in octuplicate.
To corroborate this model, we produced a recombinant TBEV with E230K combined with an amino acid change at position 298 (K → E) (E230K+K298E). This would abrogate repulsion while preserving K230 (Figure 4D). Compared with E230K (IC50 = 724.8 ng/mL), the introduction of E230K+K298E to restore the electrostatic interactions increased the sensitivity to T025 neutralization (IC50 = 132.1 ng/mL). Remarkably, adding another amino acid change at position 295 (E → D) (E230K+K298E+E295D) restored complete sensitivity to T025 neutralization (IC50 = 60.76 ng/mL). Interestingly, K298E and K298E+E295D variants lacking K230 exhibited increased sensitivity to neutralization by T025 compared with wild-type virus (IC50 = 14.86 ng/mL and 13.42 ng/mL, respectively) (Figure 4F).
The combination of two EDIII antibodies prevents TBEV escape and improves neutralization
In vitro selection of resistant variants during monotherapy prompted us to investigate whether a combination of two anti-EDIII antibodies could prevent TBEV-Hypr escape. The TBEV-MUT25 and TBEV-MUT28 variants preserved partial sensitivity to the antibody for which the other mutant was selected against (TBEV-MUT25 to T028 and TBEV-MUT28 to T025) (Figure S2A). Therefore, we hypothesized that their combination could impair or delay escape. After five passages of TBEV-Hypr in the presence of T025/T028 antibody cocktail (1:1 ratio) at subneutralizing concentrations (i.e., the lowest antibody concentration preventing development of cytopathic effect [CPE]), we observed no increase in resistance or any changes in the E protein, suggesting that the combination of these antibodies may be resistant to escape (Figure 5A). Furthermore, the T025/T028 cocktail (IC50 = 17.7 ng/mL) revealed significantly improved neutralization over the two antibodies alone (T025: IC50 = 29.27 ng/mL, p = 0.0148; T028: IC50 = 22.0 ng/mL, p = 0.0479) (Figures 5B and 5C).
Figure 5. Combination of T025 and T028 mAbs prevents escape and improves neutralization.

(A) Comparison of the passaging of TBEV-Hypr in the presence of a single mAb (T025 or T028) or combination (T025+T028). Antibody concentration refers to the highest concentration of mAb with visible CPE. For T025+T028, the concentration corresponds to the total amount of antibody added at a 1:1 ratio (i.e., 100 ng/mL contains 50 ng/mL of T025 and 50 ng/mL of T028). Passaging of the virus in the presence of a combination of the antibodies was performed in triplicate (n = 3); error bars indicate SD. Selection of mutants resistant to single antibodies was performed in monoplicates.
(B and C) Comparison of the neutralization profiles of T025, T028, or their combination for TBEV-Hypr (B) and calculated IC50 (C). Five independent experiments performed in octuplicate (*p < 0.05, Student’s t test). Mean values with SD (error bars) are shown.
DISCUSSION
T025 and T028 were selected among 776 antibodies obtained from the memory B cells of six people who had recovered from TBE and three vaccinees.23 Both antibodies were found to be highly effective neutralizers with broad activity against numerous TBFVs and represent lead candidates for TBE immunotherapy.23 To evaluate the risk of developing resistance, we serially passaged TBEV in cell culture in the presence of T025 or T028 and investigated the functional significance and mechanism of action of amino acid changes in escape mutants as well as their effect on virus fitness and virulence.
Escape virus mutants readily emerged in vitro, with each of the two antibodies leading to paired amino acid changes in the viral envelope, one each in EDII and EDIII. However, this contrasts the in vivo situation because no escape was observed in TBEV-infected mice treated with T025, suggesting that, although the virus can be pushed to resistance in tissue culture, the same may be difficult, or even impossible, in a more biologically relevant situation. This in vivo property of T025 may not be generalizable to all antibodies targeting the flavivirus EDIII because a single anti-EDIII antibody selects for resistant variants in Zika virus (ZIKV)-infected macaques.34
The E protein plays numerous critical roles during virus maturation and host cell entry. Therefore, this protein is under intense evolutionary pressure, and mutations are often associated with a loss of viral fitness.36,37 Consistent with this view, though the parental strain invariably kills mice after peripheral inoculation, TBEV-MUT25 and TBEV-MUT28 mutants presented strikingly reduced pathogenicity, suggesting that the escape mutations affected key structural elements of the E protein that are functionally important for viral neuroinvasiveness. A significant reduction in virulence of antibody escape mutants has been observed previously observed for TBEV as well as a number of other flaviviruses.31,36–43 TBEV-MUT25 and TBEV-MUT28 produced smaller plaques, but their growth rate in cell culture was not affected, underscoring the fact that the in vitro growth properties of TBEV do not necessarily correspond to virulence in vivo.44 These findings are also consistent with our observation that no escape is observed when sequencing the virus from the brains of TBEV-infected mice treated with T025.
The mechanism of TBEV escape from T025, particularly the effect of the two identified escape mutations K311D and E230K, was investigated in more detail. T025 binds the EDIII lateral ridge near the EDI-EDIII hinge region in a similar orientation as reported previously for mouse mAb 19/1,786, which also neutralizes TBEV.22,23,45 T025 inserts D100HC and W94LC into a cleft in EDIII, forming a salt bridge (D100HC-K311EDIII) and hydrogen bonds to EDIII, suggesting that K311 is a critical amino acid residue of the EDIII epitope.23 The K311N substitution observed in the TBEV-MUT25 E protein eliminates formation of the D100HC-K311EDIII salt bridge, contributing to virus escape from T025.
The K311N substitution also creates a potential N-linked glycosylation consensus sequence (i.e., N-X-S/T). A glycan at this position would likely sterically hinder antibody access to the epitope, contributing to the escape. Interestingly, a similar potential glycosylation site has been observed at amino acid residue D308N in the E protein of the experimentally selected neutralizing antibody-resistant mutant of LIV.38 However, the D308E substitution (i.e., the absence of the potential glycosylation site observed in a natural LIV isolate) also resulted in resistance to the antibody, suggesting that glycosylation is not required for the virus to escape the antibody.38 Similarly, formation of a potential N-linked glycosylation site has been observed in EDII of the antibody escape mutant of West Nile virus, and the presence of an additional N-linked glycan has been confirmed biochemically.46 However, substitutions at the same position by other amino acid residues that did not introduce this additional glycan showed that antibody escape occurs independent of glycan occupancy.46 Therefore, our results are consistent with previous findings by showing that removal of the potential glycosylation site at position 311 by an alternative amino acid that cannot be glycosylated or by modifying the N-X-S/T consensus sequence does not affect resistance of the virus to T025. This suggests that shielding of the epitope by a glycan is not the primary mechanism of antibody escape.
E230K is located in EDII, outside of the T025 epitope, indicating an indirect contribution to antibody escape. Our results suggest that the E230K substitution leads to repulsion of positively charged residues on the neighboring E protein, resulting in rearrangement or destabilization of the quaternary structure of E proteins on the viral surface. Based on structural information, T025 does not make direct contact with residue 230.23 However, based on structural comparisons with 19/1,786,22 it is likely that T025 recognizes EDI and EDII from neighboring subunits. E230 sits in a positively charged electrostatic pocket formed by three adjacent lysines (K38, K296, and K298) and interacts directly with K298 of the subunit with EDIII recognized by T025. This electrostatic interaction between neighboring subunits may stabilize the quaternary structure of the virion, stabilizing the T025 epitope. Because the quaternary structure is important for binding of T025, the potency of this antibody is largely affected by E230K. Replacing the positively charged residues in EDI with negatively charged residues to restore the electrostatic interactions with K230 rescued the sensitivity of TBEV for neutralization by T025, demonstrating that E230K contributed to TBEV escape from T025 via repulsion of the positively charged residues of the neighboring E protein. These data highlight the importance of the quaternary organization of the E protein for interactions with neutralizing antibodies and show that substitutions distant from the epitope can have major effects on virus neutralization by mAbs.47–49 Remarkably, the K311N and E230K substitutions contributed only partially; full resistance to T025 required the simultaneous presence of both. Thus, mechanistically, amino acid changes that are within and distal to the antibody epitope can synergize to confer full resistance.
Limitations of the study
One of the limitations of the study is that the main results were obtained only with T025. Similar to T025, we identified two amino acid substitutions in the E protein of T028-resistant TBEV. However, the mechanisms of escape were characterized only for T025. We cannot rule out the possibility that other escape mechanism(s) are involved in the case of T028-resistant TBEV. Another limitation is that the quaternary rearrangements of the virus surface in T025-resistant TBEV have only been proposed based on in silico simulations. Cryoelectron microscopy (cryo-EM) analysis of the virion structure could provide more detailed insight into the structural changes in the T025-resistant mutant. However, the obtained titers of the T025-resistant virus were not high enough for cryo-EM analysis.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Daniel Ruzek (ruzekd@paru.cas.cz).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Sequencing data of E gene of TBEV-MUT25 and TBEV-MUT28 are deposited in GenBank database under accession numbers GenBank: OR460195 and OR460196.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Cell lines
Porcine kidney stable (PS) were grown in Leibovitz medium (L-15) supplemented with fetal bovine serum (3%), a mixture of antibiotics and antimycotics (100 U/mL penicillin and 100 μg/mL streptomycin), and L-glutamine (1%) at 37°C. Baby hamster kidney (BHK-21) cells (ATCC CCL-10) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, L-glutamine, and a mixture of antibiotics as described above at 37°C and 5% CO2.
Mice
The animal experiments complied with all relevant European Union guidelines for work with animals and was carried out in accordance with Czech national law guidelines on the use of experimental animals and protection of animals against cruelty (Animal Welfare Act No. 246/1992 Coll.). The protocol was approved by the Committee on the Ethics of Animal Experimentation of the Institute of Parasitology and the Departmental Expert Committee for the Approval of Projects of Experiments on Animals of the Czech Academy of Sciences (permit no. 111/2020). BALB/cOlaHsd mice (6-week-old, female) were purchased from Envigo and housed in BSL-3 facility in standard cages (6 mice/cage) with water and food supply ad libitum.
METHOD DETAILS
Selection of antibody-resistant TBEV
Antibody-resistant variants were selected by culturing TBEV-Hypr in PS cells at increasing concentrations of T025 or T028. Antibodies were diluted in L-15 medium and incubated with 100 PFU TBEV-Hypr per well for 90 min at 37°C, followed by the addition of 3 × 104 cells per well. Wild-type virus was passaged in parallel without the antibody as a mock selection. After 5 days of incubation, the culture media was harvested from the wells with a microscopically visible cytopathic effect and used for the next passage with an increased antibody concentration (Figures 1A and 1D). To ensure that the same number of viral particles was used for the next passage, we measured the genomic copy number by quantitative RT-PCR, using the Advanced kit for Tick-borne encephalitis (Genesig) and Lyophilised OneStep qRT-PCR (Oasig) following manufactures manufacturer’s instructions.52 After each passage, we examined the E protein sequence. The data were compared with that of the mock-selected virus and TBEV-Hypr E gene sequence from the GenBank database (GenBank Accession Number U39292.1).
RNA isolation, RT-PCR, and sequencing
Viral RNA was isolated from the culture supernatant or brain tissue homogenate using the QIAmp Viral RNA Mini Kit according to the manufacturer’s instructions. The isolated RNA was amplified using the QIAGEN OneStep Ahead RT-PCR Kit. The total reaction volume of 25 μL consisted of 10 μL OneStep Ahead RT-PCR Master Mix (2.5×), 1 μL OneStep Ahead RT-Mix (25×), 1.25 μL of each primer (10 μM, Table S1), 2.3 μL RNase-Free Water, 0.2 μL OneStep Ahead Master Mix Tracer (125×), 5 μL 5× Q-Solution, and 4 μL template RNA. The RT-PCR cycle was initiated by reverse transcription (10 min at 50°C), followed by initial PCR activation (5 min at 95°C) and 40 cycles of denaturation (10 s at 95°C), annealing (10 s at 54 or 55°C), and extension (10 s at 72°C), and ended with a final extension (2 min at 72°C). Final products were separated in a 1.2% agarose gel using EliDNA PS Green in Tris-acetate EDTA buffer and visualized using Azure Biosystems C300 (Azure Biosystems, USA). The products were purified using Wizard SV Gel and PCR Clean-Up System (Promega, USA) and sequenced by a commercial service provider (Eurofins Genomics). The sequences were analyzed using Geneious Prime software (version 2021.2.2).
Virus neutralization test (VNT) and immunofluorescent staining
The virus neutralization test was performed similarly to the previous study with slight modifications.23 The mAbs (T025, T028, T034, and T038) were diluted in L-15 medium to an initial concentration of 10 μg/mL and then serially diluted in a 2-fold manner in 96-well plates. Antibodies were incubated with 100 PFU/well of TBEV-Hypr, antibody-resistant mutants (TBEV-MUT25, TBEV-MUT28) or recombinant variants produced by site-directed mutagenesis for 90 min at 37°C. Next, 3 × 104 cells per well were added and the plates incubated at 37°C. The cytopathic effect was monitored 4–5 days after infection using an inverted microscope (Olympus). VNT was performed in octuplicate for each concentration and the result expressed as the percentage of neutralization for each concentration.
A similar procedure was used for the fluorescence-based neutralization assay. After incubation, cell monolayers were fixed and stained.23 Briefly, cell monolayers were fixed with cold acetone/methanol (1:1) and blocked with 10% FBS. Cells were then incubated with a 1:250 diluted mouse antiflavivirus antibody, washed, and fluorescently labeled with a 1:500 diluted secondary goat anti-mouse antibody. After another wash, we used 1:2000 diluted DAPI to stain the nuclei. Finally, images were acquired using an Olympus IX81 epifluorescence microscope with Olympus Xcellence software and processed using ImageJ.
Growth kinetics
The PS cells were seeded at a density of 3 × 104 per well in a 96-well plate and incubated overnight. Subsequently, the cell monolayers were infected with 100 PFU of wild-type or resistant TBEV variant (TBEV-MUT25, TBEV-MUT28) per well. After 60 min, the medium was replaced with fresh medium and the plate incubated for 72 h. The experiment was performed in triplicate and the medium collected 12, 24, 36, 48, and 72 h after infection. The viral titers determined by the plaque assay were used to generate the growth curves.
Plaque assay
Viral titers, growth kinetics, or plaque morphology were determined using the plaque assay.53,54 Briefly, 10-fold dilutions of virus (TBEV-Hypr, TBEV-MUT25, or TBEV-MUT28) and PS cells (1.8 × 105 per well) were incubated at 37°C for 3–4 h and then overlaid with 1.5% carboxymethylcellulose in L-15 medium. After the 4-day incubation, plates were washed in phosphate-buffered saline (PBS) and stained with naphthalene black solution. Viral titer was expressed as PFU per milliliter. Images of plaques were scanned on a BioVendor C-series array reader specially modified for plaque assays. Imaging was conducted well by well in a sequential manner, employing bottom illumination for the sample, and utilizing a full high-definition camera system. Subsequently, each captured image underwent analysis through the BioVendor Analytics software. This software employs image processing algorithms specifically tailored for plaque counting.55
Mouse infections
Three groups of 6-week-old female BALB/c mice were infected subcutaneously with a lethal dose of TBEV-Hypr (1000 PFU/mouse) or the same dose of TBEV-MUT25 or TBEV-MUT28. During the 28-day experimental period, we monitored the health status of the mice, the clinical symptoms of the disease, and the survival rate. The brains of the diseased mice were collected and homogenized in 1000 μL PBS. The homogenate was clarified by centrifugation (10,000 × g for 15 min). Total RNA was isolated from the homogenate and used for RT-PCR targeting the E gene. The amplified DNA was then purified and sequenced as described above.
Site-directed mutagenesis
We produced recombinant TBEV variants carrying a single (E230K or K311N) or double (E230K&K311N) mutation to investigate the role of the mutations in TBEV-MUT25 resistance. Similarly, recombinant variants were produced for TBEV-MUT28 (G278R, E365A, and G278R&E365A). In addition, other variants were prepared to investigate the mechanism of viral escape of TBEV-MUT25 by glycosylation of positions 311–313 (K311Q, K311N&T313A) or changes in the quaternary structure of the virion (K298E, E230K&K298E, E295D&K298E, E230K&E295D&K298E) (Figure S3).
To produce recombinant TBEV variants with the specific mutations in the envelope protein sequence, we used a reverse genetics system derived from wild-type TBEV (strain Hypr). This system is based on the generation of infectious, subgenomic, overlapping DNA fragments spanning the entire viral genome.50,56,57 A total of three fragments covering the TBEV genome were synthesized and cloned into pUC57 or pC11 (GenScript, Piscataway, NJ, USA) plasmid vector. Fragment I covers nucleotide positions 1 to 3,662, fragment II from 3,545 to 8,043, and fragment III from 7961 to 11,100. Fragments I and III are flanked by the human cytomegalovirus promoter (pCMV) and hepatitis delta ribozyme, followed by the simian virus 40 polyadenylation signal (HDR/SV40pA).
Fragments I, II, and III were used as templates for amplification of overlapping sequences using the original ISA method.56 Primers with modified sequences targeting regions 230 and/or 295, 298, 311–313 of fragment I were used to amplify two (E230K, K311N, K311Q, K298E, E295D&K298E, K311N&T313A, G278R, and E365A) or three (E230K&K311N, G278R&E365A, E230K&K298E, E230K&E295D&K298E) modified amplicons, i.e., Ia and Ib or Ia, Ib, and Ic, respectively (Figure S3). Together with unmodified fragments II and III, these sequences were used to produce variants with the indicated mutations in the envelope protein (Table S2).
For the transfection, BHK-21 cells were seeded in a 24-well plate at a density of 2 × 105 cells per well (400 μL) and incubated overnight at 37°C and 5% CO2 to form a monolayer. A DNA master solution containing 700 ng of each fragment was mixed with 2 μL X-tremeGENE Transfection Reagent and 200 μL Opti-MEM and incubated for 15 min at room temperature. The entire mixture was added to the wells containing the formed monolayers and incubated at 37°C and 5% CO2 for 3 to 4 days. The supernatant was collected and viral titer determined by plaque assay. Successful mutagenesis was confirmed by sequencing the envelope protein genes of the acquired mutant viruses (Sanger method).
Binding assay by biolayer interferometry
Biolayer interferometry (BLI) measurements were made on an Octet instrument (Sartorius Octet R8 System) at 30°C with shaking at 1000 rpm with AR2G Biosensors following the manufacturer’s protocol ‘premix assay’. Biosensors were hydrated with dH2O 10 min prior to the experiment. For activation, sensors were immersed for 5 min in a mix of EDC and s-NHS (20 and 10 mM, respectively). To capture the first antibody, sensors were immersed for 10 min in 200 nM Ab1 diluted in sodium acetate (10 mM, pH 4.5). Quenching was achieved by immersing sensors for 5 min in Octet 1M Ethanolamine pH 8.5. Baseline comprised sensors being immersed for 5 min in PBS. For antigen association, sensors were immersed for 15 min with 400 nM EDIII or a premix of EDIII-Ab2 (Ab2 at 400 nM). Sensors were immersed for 15 min with Ab3 at 200 nM as a positive control. Curve fitting was performed using the Octet Analysis Studio 12.2 software. The TBEV EDIII protein was produced in E. coli and refolded as detailed23
Quaternary structure analysis
The model of T025 bound to the mature TBEV, was made starting from the two structures, a crystal structure of T025 bound to envelope protein domain III (EDIII) (PDB code: 7LSG),23 and the cryo-EM structure of TBEV mature particle (PDB code: 5O6A).22 The crystal structure of T025 in a complex with EDIII was aligned onto the EDIII of the envelope protein of mature TBEV cryo-EM structure.
Conformer analysis was performed with Schrodinger Maestro version 13.2.128. The input structure was three adjacent envelope protein chains taken from the PDB code 7Z51.58 After the protein preparation was performed, the conformer analysis took place, keeping all the residues fixed except the E230 and K298.
Graphics
The crystal structure of the envelope glycoprotein from TBEV (1SVB) was obtained from the Protein DataBank (PDB) database and used for visualization of identified mutations by PyMol software. Further graphical changes were obtained using the InkScape program (version 1.0). Envelope protein sequences are available from GenBank database and were aligned in BioEdit software: TBEV (U39292.1), OHFV (NP_932085), KFDV (YP_009513189), POWV (NP_775516), AHFV (AAL08421), LGTV (NP_620108.1), LIV (NP_740271).
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical tests were performed in GraphPad Prism. The Shapiro-Wilk test was used to test the normality assumption. Differences in plaque diameter between TBEV-Hypr and mutated viruses were evaluated by the Mann-Whitney U test. Survival rates were analyzed by log rank Mantel-Cox test and IC50s by Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Detailed statistical analysis information can also be found in each figure legend. The number of animals or samples per group (n) for each experiment is indicated either in the figure legend or within the figure itself.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Anti-EDIII monoclonal antibodies T025, T028, T034 and T038 | Agudelo et al.23 | N/A |
| Mouse Anti-Flavivirus Group Antigen Antibody, clone D1-4G2-4-15 | Sigma-Aldrich | Cat#MAB10216; RRID: AB_827205 |
| Goat Anti-Mouse IgG Antibody, FITC conjugate | Sigma-Aldrich | Cat#AP181F; RRID: AB_92580 |
|
| ||
| Bacterial and virus strains | ||
|
| ||
| Tick-borne encephalitis virus (TBEV); strain Hypr | Collection of Arboviruses, Institute of Parasitology, Biology Center of the Czech Academy of Sciences, Ceske Budejovice, Czech Republic | N/A |
| TBEV-MUT25 | this paper | N/A |
| TBEV-MUT28 | this paper | N/A |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Leibovitz (L-15) medium | Biosera | Cat#LM-L1050 |
| Dulbecco’s Modified Eagle’s Medium (DMEM) | Biosera | Cat#LM-D1112 |
| Antibiotic-Antimycotic 100X | Biosera | Cat#XC-A4110 |
| Glutamine stable 100X, 200mM | Biosera | Cat#XC-T1750 |
| Fetal Bovine Serum (FBS) South America | Biosera | Cat#FB-1001B |
| Dulbecco’s Phosphate Buffer Saline | Sigma-Aldrich | Cat#D8537 |
| Opti-MEM | Gibco | Cat#31985062 |
| X-tremeGENE™ HP DNA Transfection Reagent | Roche | Cat#06366244001 |
| EliDNA PS Green | Elisabeth Pharmacon | Cat#ED01 |
| DAPI (4’,6-diamidino-2-phenylindole) | Sigma-Aldrich | Cat#D9542 |
| Octet 1M Ethanolamine | Sartoirus | Cat#18-1071 |
| Dulbecco’s Phosphate Buffer Saline | Sigma-Aldrich | Cat#D8537 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Advanced kit for Tick-borne encephalitis | Genesig | Cat#Path-TBEV |
| Lyophilised OneStep qRT-PCR | Oasig | Cat#oasig-onestep-150 |
| QIAmp Viral RNA mini kit | Qiagen | Cat#52906 |
| QIAGEN OneStep Ahead RT-PCR Kit | Qiagen | Cat#220211 |
| Wizard® SV Gel and PCR Clean-Up System | Promega | Cat#A9281 |
|
| ||
| Deposited data | ||
|
| ||
| TBEV-MUT25 E gene nucleotide sequence | This paper | OR460195 |
| TBEV-MUT28 E gene nucleotide sequence | This paper | OR460196 |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| Porcine stable (PS) kidney cells | National Cell Culture Collection, National Institute of Public Health, Prague, Czech Republic | N/A |
| BHK-21 [C-13] | ATCC | Cat#CCL-10 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| Mouse: Balb/cOlaHsd | Envigo | RRID:IMSR_ENV:HSD-162 |
|
| ||
| Oligonucleotides | ||
|
| ||
| Primers for TBEV E protein; see Table S1 | this paper | N/A |
| Primers for site-directed mutagenesis; see Table S2 | this paper | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| Plasmids: Fragment I, II and III of TBEV genome | Eyer et al.50 | N/A |
| Software and algorithms | ||
| Geneious Prime® 2021.2.2 | Biomatters Ltd. | RRID:SCR_010519 |
| BioEdit Sequence Alignment Editor | Hall51 | RRID:SCR_007361 |
| PyMol Molecular Graphics System | Schrodinger, LLC | RRID:SCR_000305 |
| GraphPad Prism 9.3.1 | GraphPad | RRID:SCR_002798 |
| InkScape 1.2.2 | InkScape | RRID:SCR_014479 |
| ImageJ | NIH | RRID:SCR_003070 |
| Octet Analysis Studio 12.2 | Sartorius | N/A |
| Olympus Xcellence | Olympus | N/A |
| BioRender | BoRender | https://www.biorender.com/ |
| BioVendor Analytics software | BioVendor | N/A |
|
| ||
| Other | ||
|
| ||
| Sartorius Octet® R8 System | Sartorius | RRID:SCR_023267 |
| Octet® Amine Reactive 2ND | Sartorius | Cat#18-5093 |
| Generation (AR2G) Biosensors | ||
| Azure Biosystems C300 | Azure Biosystems | N/A |
| Olympus IX81 epifluorescence microscope | Olympus | N/A |
| Array Reader C-series | BioVendor | Cat# ARCXI096 |
Highlights.
TBEV escape from T025 or T028 results in virus variants with reduced pathogenicity
Escape mutants display a combination of amino acid changes essential for resistance
Mutations within and distal to the antibody epitope can synergize for the resistance
A combination of T025 and T028 prevents virus escape and improves neutralization
ACKNOWLEDGMENTS
This study was supported by a Czech-Swiss collaborative project funded jointly by the Czech Science Foundation (GF21-05445L to D.R.) and Swiss National Science Foundation (310030L_196866 to D.F.R.) and in part by the Swiss Vaccine Research Institute (SVRI) and NIH grants U01 AI151698 (United World Antiviral Research Network, UWARN), P01 AI138938, and U19 AI111825 (to D.F.R.). This work was also supported by the project National Institute of Virology and Bacteriology (Program EXCELES, ID Project LX22NPO5103), funded by the European Union-Next Generation EU (to D.R.). The study was also possible thanks to the IRB-Rockefeller University Stavros Niarchos Foundation Partnership for Global Infectious Disease Research.
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
Footnotes
DECLARATION OF INTERESTS
The Rockefeller University filed a patent application in connection with antibodies T025 and T028, on which M.A., D.F.R., and M.C.N. are inventors.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.113149.
REFERENCES
- 1.Walsh SR, and Seaman MS (2021). Broadly Neutralizing Antibodies forHIV-1 Prevention. Front. Immunol. 12, 712122. 10.3389/fimmu.2021.712122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavda VP, Prajapati R, Lathigara D, Nagar B, Kukadiya J, Redwan EM, Uversky VN, Kher MN, and Patel R (2022). Therapeutic monoclonal antibodies for COVID-19 management: an update. Expert Opin. Biol. Ther. 22, 763–780. 10.1080/14712598.2022.2078160. [DOI] [PubMed] [Google Scholar]
- 3.Dibo M, Battocchio EC, Dos Santos Souza LM, da Silva MDV, Banin-Hirata BK, Sapla MMM, Marinello P, Rocha SPD, and Faccin-Galhardi LC (2019). Antibody Therapy for the Control of Viral Diseases: An Update. Curr. Pharm. Biotechnol. 20, 1108–1121. 10.2174/1389201020666190809112704. [DOI] [PubMed] [Google Scholar]
- 4.Gupta SL, and Jaiswal RK (2022). Neutralizing antibody: a savior in theCovid-19 disease. Mol. Biol. Rep. 49, 2465–2474. 10.1007/s11033-021-07020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caskey M, Klein F, and Nussenzweig MC (2019). Broadly neutralizinganti-HIV-1 monoclonal antibodies in the clinic. Nat. Med. 25, 547–553. 10.1038/s41591-019-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zerbini FM, Siddell SG, Lefkowitz EJ, Mushegian AR, Adriaenssens EM, Alfenas-Zerbini P, Dempsey DM, Dutilh BE, García ML, Hendrickson RC, et al. (2023). Changes to virus taxonomy and the ICTV Statutes ratified by the International Committee on Taxonomy of Viruses (2023). Arch. Virol. 168, 175. 10.1007/s00705-023-05797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabowski JM, and Hill CA (2017). A Roadmap for Tick-Borne Flavivirus Research in the “Omics” Era. Front. Cell. Infect. Microbiol. 7, 519. 10.3389/fcimb.2017.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould EA, and Solomon T (2008). Pathogenic flaviviruses. Lancet 371, 500–509. 10.1016/s0140-6736(08)60238-x. [DOI] [PubMed] [Google Scholar]
- 9.Gritsun TS, Nuttall PA, and Gould EA (2003). Tick-borne flaviviruses. Adv. Virus Res. 61, 317–371. 10.1016/s0065-3527(03)61008-0. [DOI] [PubMed] [Google Scholar]
- 10.Chiffi G, Grandgirard D, Leib SL, Chrdle A, and Růžek D (2023). Tick-borne encephalitis: A comprehensive review of the epidemiology, virology, and clinical picture. Rev. Med. Virol.e2470 10.1002/rmv.2470. [DOI] [PubMed] [Google Scholar]
- 11.Yoshii K (2019). Epidemiology and pathological mechanisms of tick-borne encephalitis. J. Vet. Med. Sci. 81, 343–347. 10.1292/jvms.18-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruzek D, Avšič Županc T, Borde J, Chrdle A, Eyer L, Karganova G, Kholodilov I, Knap N, Kozlovskaya L, Matveev A, et al. (2019). Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res. 164, 23–51. 10.1016/j.antiviral.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Yoshii K, Song JY, Park SB, Yang J, and Schmitt HJ (2017). Tick-borne encephalitis in Japan, Republic of Korea and China. Emerg. Microbes Infect. 6, e82. 10.1038/emi.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansbridge CT, Osborne J, Holding M, Dryden M, Aram M, Brown K, and Sutton J (2022). Autochthonous tick-borne encephalitis in the United Kingdom: A second probable human case and local eco-epidemiological findings. Ticks Tick. Borne. Dis. 13, 101853. 10.1016/j.ttbdis.2021.101853. [DOI] [PubMed] [Google Scholar]
- 15.Fares W, Dachraoui K, Cherni S, Barhoumi W, Slimane TB, Younsi H, and Zhioua E (2021). Tick-borne encephalitis virus in Ixodes ricinus (Acari: Ixodidae) ticks, Tunisia. Ticks Tick. Borne. Dis. 12, 101606. 10.1016/j.ttbdis.2020.101606. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri A, and Růžek D (2013). First documented case of imported tick-borne encephalitis in Australia. Intern. Med. J. 43, 93–96. 10.1111/imj.12017. [DOI] [PubMed] [Google Scholar]
- 17.Artsob H, and Spence L (1991). Imported arbovirus infections in Canada1974-89. Can. J. Infect Dis. 2, 95–100. 10.1155/1991/678906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention CDC (2010). Tick-borne encephalitis among U.S. travelers to Europe and Asia - 2000–2009. MMWR Morb. Mortal. Wkly. Rep. 59, 335–338. [PubMed] [Google Scholar]
- 19.Bogovic P, Lotric-Furlan S, and Strle F (2010). What tick-borne encephalitis may look like: clinical signs and symptoms. Travel Med. Infect. Dis. 8, 246–250. 10.1016/j.tmaid.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Eyer L, Seley-Radtke K, and Ruzek D (2023). New directions in the experimental therapy of tick-borne encephalitis. Antiviral Res. 210, 105504. 10.1016/j.antiviral.2022.105504. [DOI] [PubMed] [Google Scholar]
- 21.Rey FA, Heinz FX, Mandl C, Kunz C, and Harrison SC (1995). The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375, 291–298. 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 22.Füzik T, Formanová P, Růžek D, Yoshii K, Niedrig M, and Plevka P (2018). Structure of tick-borne encephalitis virus and its neutralization by a monoclonal antibody. Nat. Commun. 9, 436. 10.1038/s41467-018-02882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agudelo M, Palus M, Keeffe JR, Bianchini F, Svoboda P, Salát J, Peace A, Gazumyan A, Cipolla M, Kapoor T, et al. (2021). Broad and potent neutralizing human antibodies to tick-borne flaviviruses protect mice from disease. J. Exp. Med. 218, e20210236. 10.1084/jem.20210236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauring AS, Frydman J, and Andino R (2013). The role of mutational robustness in RNA virus evolution. Nat. Rev. Microbiol. 11, 327–336. 10.1038/nrmicro3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolan PT, Whitfield ZJ, and Andino R (2018). Mechanisms and Concepts in RNA Virus Population Dynamics and Evolution. Annu. Rev. Virol. 5, 69–92. 10.1146/annurev-virology-101416-041718. [DOI] [PubMed] [Google Scholar]
- 26.Holzmann H, Mandl CW, Guirakhoo F, Heinz FX, and Kunz C (1989). Characterization of antigenic variants of tick-borne encephalitis virus selected with neutralizing monoclonal antibodies. J. Gen. Virol. 70, 219–222. 10.1099/0022-1317-70-1-219. [DOI] [PubMed] [Google Scholar]
- 27.Lin B, Parrish CR, Murray JM, and Wright PJ (1994). Localization of a neutralizing epitope on the envelope protein of dengue virus type 2. Virology 202, 885–890. 10.1006/viro.1994.1410. [DOI] [PubMed] [Google Scholar]
- 28.Lok SM, Ng ML, and Aaskov J (2001). Amino acid and phenotypic changes in dengue 2 virus associated with escape from neutralisation by IgM antibody. J. Med. Virol. 65, 315–323. 10.1002/jmv.2036. [DOI] [PubMed] [Google Scholar]
- 29.Zou G, Kukkaro P, Lok SM, Ng JKW, Tan GK, Hanson BJ, Alonso S, MacAry PA, and Shi PY (2012). Resistance analysis of an antibody that selectively inhibits dengue virus serotype-1. Antiviral Res. 95, 216–223. 10.1016/j.antiviral.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Beasley DWC, and Barrett ADT (2002). Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J. Virol. 76, 13097–13100. 10.1128/jvi.76.24.13097-13100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers TJ, Halevy M, Nestorowicz A, Rice CM, and Lustig S (1998). West Nile virus envelope proteins: nucleotide sequence analysis of strains differing in mouse neuroinvasiveness. J. Gen. Virol. 79, 2375–2380. 10.1099/0022-1317-79-10-2375. [DOI] [PubMed] [Google Scholar]
- 32.Daffis S, Kontermann RE, Korimbocus J, Zeller H, Klenk HD, and Ter Meulen J (2005). Antibody responses against wild-type yellow fever virus and the 17D vaccine strain: characterization with human monoclonal antibody fragments and neutralization escape variants. Virology 337, 262–272. 10.1016/j.virol.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 33.Shimoda H, Mahmoud HYAH, Noguchi K, Terada Y, Takasaki T, Shimojima M, and Maeda K (2013). Production and characterization of monoclonal antibodies to Japanese encephalitis virus. J. Vet. Med. Sci. 75, 1077–1080. 10.1292/jvms.12-0558. [DOI] [PubMed] [Google Scholar]
- 34.Keeffe JR, Van Rompay KKA, Olsen PC, Wang Q, Gazumyan A, Azzopardi SA, Schaefer-Babajew D, Lee YE, Stuart JB, Singapuri A, et al. (2018). A Combination of Two Human Monoclonal Antibodies Prevents Zika Virus Escape Mutations in Non-human Primates. Cell Rep. 25, 1385–1394.e7. 10.1016/j.celrep.2018.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMinn PC (1997). The molecular basis of virulence of the encephalitogenic flaviviruses. J. Gen. Virol. 78, 2711–2722. 10.1099/0022-1317-78-11-2711. [DOI] [PubMed] [Google Scholar]
- 36.Holzmann H, Heinz FX, Mandl CW, Guirakhoo F, and Kunz C(1990). A single amino acid substitution in envelope protein E of tick-borne encephalitis virus leads to attenuation in the mouse model. J. Virol. 64, 5156–5159. 10.1128/jvi.64.10.5156-5159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holzmann H, Stiasny K, Ecker M, Kunz C, and Heinz FX (1997). Characterization of monoclonal antibody-escape mutants of tick-borne encephalitis virus with reduced neuroinvasiveness in mice. J. Gen. Virol. 78, 31–37. 10.1099/0022-1317-78-1-31. [DOI] [PubMed] [Google Scholar]
- 38.Gao GF, Hussain MH, Reid HW, and Gould EA (1994). Identification of naturally occurring monoclonal antibody escape variants of louping ill virus. J. Gen. Virol. 75, 609–614. 10.1099/0022-1317-75-3-609. [DOI] [PubMed] [Google Scholar]
- 39.Kale SR, Shah PS, and Gadkari DA (1991). Characterization of temperature sensitive mutants of Japanese encephalitis virus isolated from persistently infected mammalian cells. Indian J. Med. Res. 93, 121–130. [PubMed] [Google Scholar]
- 40.Sultana H, Foellmer HG, Neelakanta G, Oliphant T, Engle M, Ledizet M, Krishnan MN, Bonafé N, Anthony KG, Marasco WA, et al. (2009). Fusion loop peptide of the West Nile virus envelope protein is essential for pathogenesis and is recognized by a therapeutic cross-reactive human monoclonal antibody. J. Immunol. 183, 650–660. 10.4049/jimmunol.0900093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiramatsu K, Tadano M, Men R, and Lai CJ (1996). Mutational analysis of a neutralization epitope on the dengue type 2 virus (DEN2) envelope protein: monoclonal antibody resistant DEN2/DEN4 chimeras exhibit reduced mouse neurovirulence. Virology 224, 437–445. 10.1006/viro.1996.0550. [DOI] [PubMed] [Google Scholar]
- 42.Jiang WR, Lowe A, Higgs S, Reid H, and Gould EA (1993). Single amino acid codon changes detected in louping ill virus antibody-resistant mutants with reduced neurovirulence. J. Gen. Virol. 74, 931–935. 10.1099/0022-1317-74-5-931. [DOI] [PubMed] [Google Scholar]
- 43.Cecilia D, and Gould EA (1991). Nucleotide changes responsible for loss of neuroinvasiveness in Japanese encephalitis virus neutralization-resistant mutants. Virology 181, 70–77. 10.1016/0042-6822(91)90471-m. [DOI] [PubMed] [Google Scholar]
- 44.Muto M, Bazartseren B, Tsevel B, Dashzevge E, Yoshii K, and Kariwa H (2015). Isolation and characterization of tick-borne encephalitis virus from Ixodes persulcatus in Mongolia in 2012. Ticks Tick. Borne. Dis. 6, 623–629. 10.1016/j.ttbdis.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Niedrig M, Klockmann U, Lang W, Roeder J, Burk S, Modrow S, and Pauli G (1994). Monoclonal antibodies directed against tick-borne encephalitis virus with neutralizing activity in vivo. Acta Virol. 38, 141–149. [PubMed] [Google Scholar]
- 46.Goo L, Debbink K, Kose N, Sapparapu G, Doyle MP, Wessel AW, Richner JM, Burgomaster KE, Larman BC, Dowd KA, et al. (2019). A protective human monoclonal antibody targeting the West Nile virus E protein preferentially recognizes mature virions. Nat. Microbiol. 4, 71–77. 10.1038/s41564-018-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiermayr S, Stiasny K, and Heinz FX (2009). Impact of quaternary organization on the antigenic structure of the tick-borne encephalitis virus envelope glycoprotein. J. Virol. 83, 8482–8491. 10.1128/jvi.00660-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma A, Zhang X, Dejnirattisai W, Dai X, Gong D, Wong wiwat W, Duquerroy S, Rouvinski A, Vaney MC, Guardado-Calvo P, et al. (2021). The epitope arrangement on flavivirus particles contributes to Mab C10’s extraordinary neutralization breadth across Zika and dengue viruses. Cell 184, 6052–6066.e18. 10.1016/j.cell.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medits I, Vaney MC, Rouvinski A, Rey M, Chamot-Rooke J, Rey FA, Heinz FX, and Stiasny K (2020). Extensive flavivirus E trimer breathing accompanies stem zippering of the post-fusion hairpin. EMBO Rep. 21, e50069. 10.15252/embr.202050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eyer L, Nougairède A, Uhlířová M, Driouich JS, Zouharová D, Valdés JJ, Haviernik J, Gould EA, De Clercq E, de Lamballerie X, and Ruzek D (2019). An E460D Substitution in the NS5 Protein of Tick-Borne Encephalitis Virus Confers Resistance to the Inhibitor Galidesivir (BCX4430) and Also Attenuates the Virus for Mice. J. Virol. 93, e00367–19. 10.1128/jvi.00367-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall T A (1999). BioEdit : a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98. [Google Scholar]
- 52.Salát J, Formanova P, Huňady M, Eyer L, Palus M, and Ruzek D (2018). Development and testing of a new tick-borne encephalitis virus vaccine candidate for veterinary use. Vaccine 36, 7257–7261. 10.1016/j.vaccine.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 53.De Madrid AT, and Porterfield JS (1969). A simple micro-culture method for the study of group B arboviruses. Bull. World Health Organ. 40, 113–121. [PMC free article] [PubMed] [Google Scholar]
- 54.Růzek D, Gritsun TS, Forrester NL, Gould EA, Kopecký J, Golovchenko M, Rudenko N, and Grubhoffer L (2008). Mutations in the NS2B and NS3 genes affect mouse neuroinvasiveness of a Western European field strain of tick-borne encephalitis virus. Virology 374, 249–255. 10.1016/j.virol.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Eyer L, Kondo H, Zouharova D, Hirano M, Valdés JJ, Muto M, Kastl T, Kobayashi S, Haviernik J, Igarashi M, et al. (2017). Escape of Tick-Borne Flavivirus from 2’-C-Methylated Nucleoside Antivirals Is Mediated by a Single Conservative Mutation in NS5 That Has a Dramatic Effect on Viral Fitness. J. Virol. 91, e01028–17. 10.1128/jvi.01028-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aubry F, Nougairède A, de Fabritus L, Querat G, Gould EA, and de Lamballerie X (2014). Single-stranded positive-sense RNA viruses generated in days using infectious subgenomic amplicons. J. Gen. Virol. 95, 2462–2467. 10.1099/vir.0.068023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Driouich JS, Ali SM, Amroun A, Aubry F, de Lamballerie X, and Nougairède A (2018). SuPReMe: a rapid reverse genetics method to generate clonal populations of recombinant RNA viruses. Emerg. Microbes Infect. 7, 40. 10.1038/s41426-018-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pulkkinen LIA, Barrass SV, Domanska A, Överby AK, Anastasina M, and Butcher SJ (2022). Molecular Organisation of Tick-Borne Encephalitis Virus. Viruses 14. 10.3390/v14040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data of E gene of TBEV-MUT25 and TBEV-MUT28 are deposited in GenBank database under accession numbers GenBank: OR460195 and OR460196.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.


