Abstract
The magnetic resonance imaging (MRI) visible perivascular space (PVS) reportedly clears amyloid-β and metabolic waste during sleep. Previous studies reported an association between sleep and the PVS in small vessel disease, traumatic brain injury, and Alzheimer’s disease. However, this relationship in a healthy cohort is still unclear. Here, we used the Human Connectome Project Aging dataset to analyze the relationship between sleep and the PVS in cognitively healthy adults across the aging continuum. We measured sleep parameters using the self-reported Pittsburgh Sleep Quality Index questionnaire. We found that older adults who had better sleep quality and sleep efficiency presented with a larger PVS volume fraction in the basal ganglia (BG). However, sleep measures were not associated with PVS volume fraction in the centrum semiovale (CSO). In addition, we found that body mass index (BMI) influenced the BG-PVS across middle-aged and older participants. In the entire cognitively healthy cohort, the effect of sleep quality on PVS volume fraction was mediated by BMI. However, BMI did not influence this effect in the older cohort. Furthermore, there are significant differences in PVS volume fraction across racial/ethnic cohorts. In summary, the effect of sleep on the PVS volume alteration was different in the middle-aged adults and older adults.
Keywords: body mass index, magnetic resonance imaging, perivascular space, race, sleep
1. Introduction
The perivascular space (PVS), also called the Virchow-Robin space, is a fluid-filled space around blood vessels in the brain1. The PVS is thought to clear cerebral metabolic waste through the aquaporin-4 (AQP4) channel expressed on the endfeet of astrocytes2–4. AQP4, an astrocytic water channel in the glymphatic system, is one of the key factors regulating parenchymal cerebrospinal fluid (CSF) influx and interstitial amyloid-β (Aβ) deposition. The deletion of AQP4 or low AQP4 expression reportedly led to chronic sleep disruption in mice, resulting in severe neurodegeneration in the hippocampus and decreased working memory5,6.
Magnetic resonance imaging (MRI)-visible PVSs are associated with aging, body mass index (BMI), hypertension, and neuroimaging findings of small vessel disease1,7. The majority of previous MRI investigations suggest that higher PVS number and larger PVS volume in the brain white matter is either a sign of pathology, or the normal physiological process in healthy aging. Previous research has shown that PVS visibility increases with age8. Enlarged PVS volumes have also been reported to have a positive association with sleep disturbance in populations with small vessel disease, traumatic brain injury, and Alzheimer’s disease9,10.
As PVS is better visible in the basal ganglia (BG) and centrum semiovale (CSO) when PVS is enlarged11, most MRI studies on the PVS focus on the PVSs in these two regions. Moreover, findings from previous studies indicated that morphology of PVSs in specific anatomical areas of the brain have been positively associated with several neurological disorders, such as small vessel disease and Alzheimer’s disease (AD) and cognitive decline1,12,13. Some studies have demonstrated that higher MRI-visible BG-PVSs count are associated with arteriosclerosis, vascular cognitive impairment, and cognitive decline in Parkinson’s disease14–16. In addition, enlarged CSO-PVS volumes are associated with AD and cerebral amyloid angiopathy15,17–20. These findings highlight the clinical significance of the anatomical distribution and morphology of the PVS, and their importance in neurological health.
As researchers delve deeper into understanding the mechanisms that underlie brain health, recent animal model studies show that the glymphatic system plays a major role in clearing metabolic waste during sleep21,22. In animal models, it has been shown that enlargement of the PVS is linked to impaired waste clearance23 This, in turn, obstructs the removal of harmful metabolic byproducts such as Aβ, leading to neurological damage to the brain24. In human studies, one night of sleep deprivation or deep sleep interruption resulted in an increase in Aβ deposition observed under positron emission tomography imaging and an increase in Aβ in the CSF of the lumbar spine, respectively25,26 There is also evidence from both human and animal studies that sleep position is associated with brain clearance alternation. For example, head position during sleep was also found to impact the glymphatic clearance function in humans27,28. In addition, supine sleep position occurred more frequently in patients with neurodegenerative diseases compared to healthy controls27. In one animal study, the glymphatic clearance was shown to be more efficient in the lateral sleep position28. These observations have prompted researchers to investigate the relationship between the PVS and sleep in humans. The development of neurodegenerative diseases has been linked to sleep deprivation. Specifically, studies have reported associations between ‘Rapid eye movement and Behavioral sleep Disorder’ (RBD) to disorders such as Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and AD29,30. A small body of research documents a positive relationship between PVS volume and sleep interruptions31,32. Del Brutto and colleagues found enlarged BG-PVSs in populations who had poor sleep efficiency33. Polysomnography (PSG)-based studies demonstrated an association between a greater number of PVSs and a lower total sleep time32,34,35. It has also been found that patients with severe sleep apnea have larger PVSs34. On the other hand, one study found that longer time in bed was associated with larger total PVS volume in patients with cerebrovascular disease, supporting a hypothesis of compensatory regulation between the time in bed, sleep time, and sleep quality36.
These observations reflect an association between poor sleep and the structure and possibly the function of PVSs in different diseases. However, the association between sleep and PVS and the impact of this relationship on the cognitive status of a healthy adults remain unclear. Therefore, in this study we used MRI data from the Human Connectome Project Aging (HCP-Aging)37 dataset to investigate the relationship between sleep, PVS volume, and cognitive status in a healthy cohort of adults. Sleep parameters used in this study were assessed using the subjective self-reported Pittsburgh Sleep Quality Index (PSQI) questionnaire38. Most prior research has focused on diseased populations; while this study investigates the association of sleep with PVS morphology in a cognitive healthy population. Identifying and understanding the association of sleep behavior with the brain-wide distribution of brain clearance alterations in cognitively normal subjects will stimulate new avenues of research and therapeutic development and identify possible new measures to assess interventional efficacy.
Our study aimed to describe the relationship between sleep and PVS in BG and CSO regions in a cognitively normal aging population. Using the Enhanced PVS Contrast (EPC) imaging approach39, we identified and quantified PVS morphological features. Analysis of sleep quality, sleep efficiency, and PVS changes in BG and CSO was conducted using multivariable regression models. Furthermore, we examined whether the effect of sleep on PVS affected cognitive performance. We also examined the morphology of PVS in relation to body mass index (BMI), race, and ethnicity. For further research on PVS and sleep, this study will provide a normative reference.
2. Material and Methods
2.1. Participants
We used MRI data from 725 cognitively healthy participants (36–100 years old) from the HCP-Aging Lifespan Release 2.037. At the time of recruitment, participants in the sample were in good health, with no diagnosed history of neurologic or major psychiatric disorder. The HCP-Aging dataset focuses on recruiting participants who exhibit normal health status relevant to their respective age groups and excluded individuals with neurological diseases, including diagnosed AD and symptomatic stroke, that could complicate data analysis. Exclusion criteria for the HCP-Aging dataset did not include sleep behavior, so participants with the PSQI score greater than 5 (which indicates sleep disorder) were still considered cognitively healthy. For cognitive testing, participants with Montreal Cognitive Assessment (MoCA) scores below 19 were excluded from the HCP-Aging dataset. Participants who scored between 20 and 30 were still considered healthy aging, even though a MoCA score below 25 is considered to reflect mild cognitive impairment (MCI)40 in conventional scoring.
In our analysis, we included age, sex, race, body mass index (BMI), sleep measured by the PSQI38, and cognitive status measured by the MoCA40 (more details are provided in Supplement Method), and NIH toolbox working memory tests. The exclusion criteria for the current analysis were: (a) missed T1-weighted MPRAGE (T1W) and T2-weighted SPACE (T2W) MR images (n=170), (b) inferior MRI and PVS quality (n=25), and (c) missed PSQI data (n=17). Consequently, a total number of 513 participants were included in the analysis. We categorized the participants by age as middle-aged adults (36–65 years old, N=363), or older adults (above 65 years old, N=150). The demographic information of the participants is shown in Table 1.
Table 1.
Demographic information of the study cohort.
| Middle-aged Adult 36–65 years old (N=363) | Older Adult Above 65 years old (N=150) | p-value | |
|---|---|---|---|
| Age | 49.5±8.3 | 73.9±6.2 | <0.0001*** |
| Sex (M/F) | 147(40%)/216(60%) | 68(45%)/82(55%) | 0.031* |
| BMI (kg/ m2) | 27.12±4.95 | 26.68+4.47 | 0.33 |
| Race (Asian/Black/White/Other groups) | 33(9%)/69(19%)/207(57%)/53(14%) | 9(6%)/6(4%)/132(88%)/3(2%) | |
| Ethnicity (Hispanic or Latino/ Non-) | 46(13%)/317(87%) | 1(0.6%)/149(99.4%) | |
| Intracranial Volume | 1463660.96±201010.38 | 1554716.80±169200.00 | <0.0001*** |
| Region of Interest | |||
| BG Volume (mm3) | 39257.30±3539.08 | 36282.97±3541.92 | <0.0001*** |
| CSO Volume (mm3) | 251966.27±31961.19 | 233757.27±31058.74 | <0.0001*** |
| PVS | |||
| BG-PVS Volume (mm3) | 758.78±331.18 | 1085.95±445.37 | <0.0001*** |
| CSO-PVS Volume (mm3) | 6022.79±2792.79 | 7090.70±2613.59 | <0.0001*** |
| PSQI | |||
| PSQI Total Score | 4.56±2.69 | 4.81±2.72 | 0.34 |
| Sleep Efficiency Score | 0.43±0.75 | 0.47±0.81 | 0.57 |
| Sleep Quality Score | 0.82±0.69 | 0.70±0.61 | 0.046* |
| Sleep Latency Score | 0.80±0.85 | 0.86±0.83 | 0.49 |
| Duration of Sleep (h) | 6.78±1.04 | 7.03±1.08 | 0.017* |
| Time in bed (h) | 8.45±3.17 | 8.43±2.45 | 0.95 |
| Daytime dysfunction | 0.47±0.50 | 0.53±0.50 | 0.25 |
| Sleep medication | 0.42±0.91 | 0.71±1.15 | 0.003* |
| Cognitive performance | |||
| MoCA score | 26.77±2.35 | 26.19±2.38 | 0.012* |
| NIH toolbox Working Memory score | 102.93±14.45 | 106.81±13.11 | 0.005* |
Data are presented as mean value ± standard deviation (SD). Mann-Whitney U, and t-tests were used to compare PVS volume fraction and sleep measurements between individuals of different ages. The ANOVA test was applied to compare PVS volume fraction between different races. BMI: body mass index; BG: basal ganglia; CSO: centrum semiovale; MoCA: Montreal Cognitive Assessment; PSQI: Pittsburgh Sleep Quality Index; PVS: perivascular space.
Significant *p<0.05; ***p<0.0001
2.2. MRI Acquisition
All participants were scanned using a customized Siemens 3T Prisma scanner housed at Washington University in St. Louis, using a standard 32-channel Siemens receive head coil. The T1W image was acquired with repetition time (TR)/inversion time (TI) = 2500/1000 ms, time to echo (TE) = 1.8/3.6/5.4/7.2 ms, field of view (FOV) = 256 × 240 × 166 mm and the T2W image with TR= 3200 ms, TE= 564 ms, FOV= 256 × 240 × 166 mm.
2.3. MRI pre-processing
Structural T1w MPRAGE and T2w SPACE images were preprocessed in parallel with a LONI pipeline41 using the HCP minimal processing pipeline version 4.0.142 and Freesurfer version 6. The preprocessing steps started by gradient nonlinearity corrections. Structural images were registered together, then brought into native space anterior commissure-posterior commissure alignment, and then registered to MNI space using FSL’s FNIRT43. The native space images were used to generate individual regional subcortical PVS features for white and pial surfaces using FreeSurfer44. Extensive description of the minimal preprocessing applied can be found in a prior publication42.
2.4. PVS segmentation and quality control
PVS segmentation was performed as explained in Sepehrband et al.39 In brief, T1w and T2w images were adaptatively filtered to remove the high-frequency noise and then co-registered and combined to obtain enhanced PVS contrast (EPC). EPC is shown to provide superior visibility of PVS compared with T1w or T2w alone39. PVSs were segmented from EPC images (Figure 1a) by applying Frangi filter45 using Quantitative Imaging Toolkit46 and a vesselness threshold, which was optimized for the HCP data.37 To verify the accuracy of PVS segmentation, PVS segmentation quality control was performed by four trained analysts. Evaluation criteria included motion, image quality, and white matter hyperintensity (WMH) severity. The rating scale was from 1 to 3. 1 indicates good quality without motion and ringing, and 3 shows poor image contrast or severe motion and ringing. Images with a score of 3 were excluded from this study.
Figure 1. Examples of perivascular space (PVS) and centrum semiovale (CSO) mask.
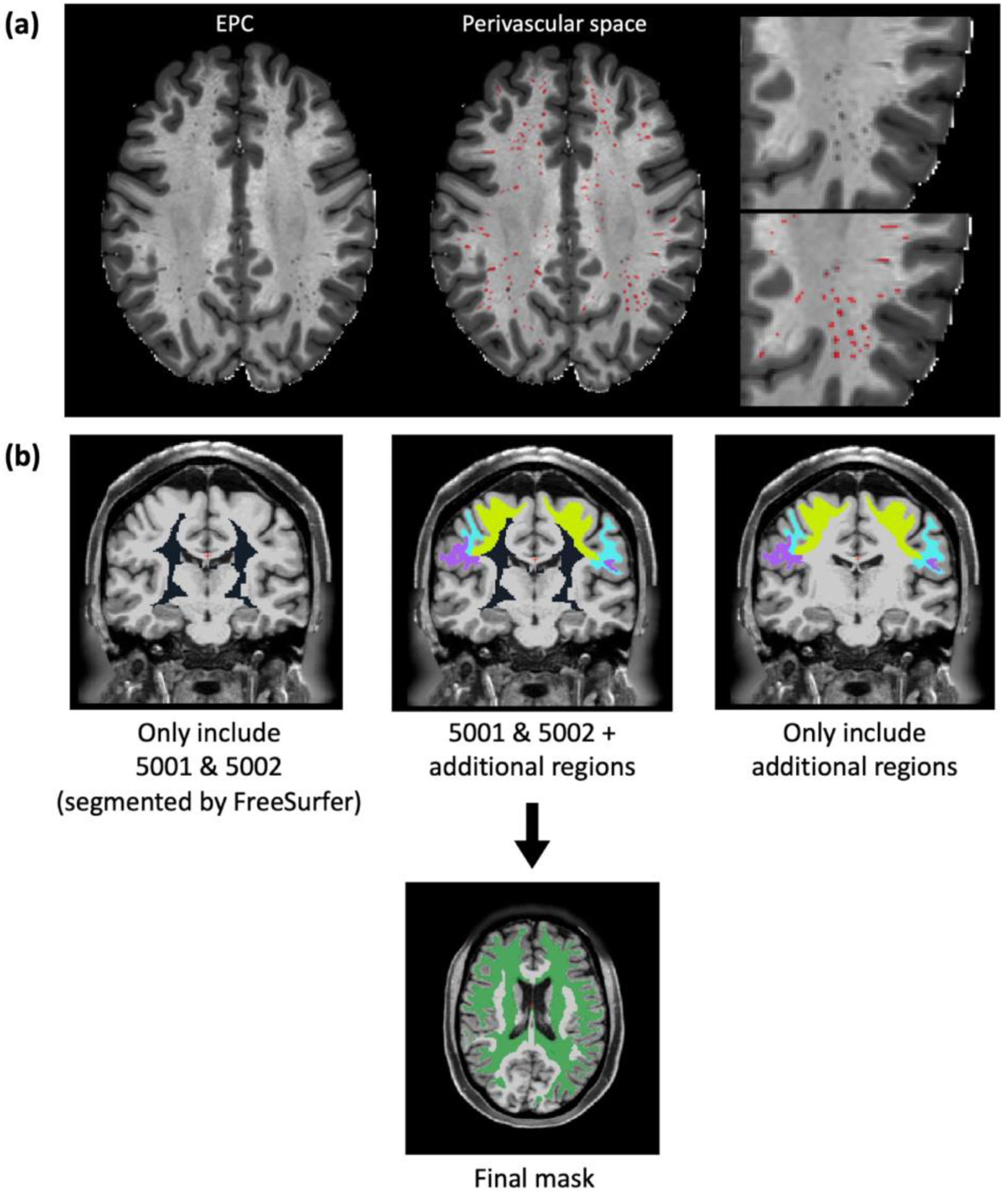
(a) Enhanced PVS contrast (EPC) image overlaps with the PVS mask (red area). (b) Mask of the centrum semiovale (CSO).
2.5. Regions of interest (ROIs)
We used the BG and CSO as our regions of interest (ROIs), which are the most common regions where enlarged PVS are visible on MRI, as segmented by FreeSurfer. Based on the guideline of FreeSurfer ‘Desikan-Killiany’ cortical atlas47, areas “5001-Left- UnsegmentedWhiteMatter” and “5002-Right-UnsegmentedWhiteMatter” are the regions corresponding to the CSO. Although these two regions contain most of the CSO, they do not include most of the white matter areas underlying the cortex (Figure 1b). On MRI, the CSO-PVSs become visible immediately inferior to the cortical layer, where they run centripetally from the external part of the white matter towards the lateral ventricles1; we therefore added to the FreeSurfer CSO mask the white matter areas underlying the following cortical regions: the caudal middle frontal, inferior parietal, pars opercularis, pars orbitalis, pars triangularis, postcentral, precentral, rostral middle frontal, superior frontal, superior parietal, and supramarginal regions in both the right and left hemispheres (Figure 1b). We have previously demonstrated that these regions also contain a significant amount of MRI-visible PVSs in healthy young adults7. BG were segmented using an atlas-based approach48,49. Brain volume and white matter masks were derived from the Desikan-Killiany atlas47.
2.6. Sleep parameters
All sleep parameters were assessed using the PSQI38, which is a 19-item self-rated questionnaire for evaluating subjective sleep quality based on the participant’s sleep pattern during the previous month. PSQI items are scored from 0 to 3, with higher scores representing poor sleep behavior. We computed the sleep efficiency score based on component 4 of the PSQI, which evaluates the participant’s habitual sleep efficiency. Sleep efficiency is calculated as the proportion of total sleep time divided by the duration spent in bed. The 4-level sleep efficiency score was derived from this as: 0 (proportion> 85%), 1 (proportion 75–84%), 2 (proportion 65–74%), and 3 (proportion<65%). The sleep quality score is computed by component 1 of the PSQI, which evaluates the subjective sleep quality, scored as 0 (sleep quality very good), 1 (sleep quality fairly good), 2 (sleep quality fairly poor), and 3 (very poor sleep quality). The duration of sleep is derived by question 4 of the PSQI, scored as 0 (sleep time>7 hours), 1 (6–7 hours), 2 (5–6 hours), and 3 (<5 hours). The sleep latency score is computed by component 2 of the PSQI, which evaluates the time required to initiate sleep, alongside its frequency. The 4-level derived score is 0 (less than 15 minutes to fall asleep, or no problem falling asleep during the past month), 1 (16–30 minutes to fall asleep, or if have problem falling asleep less than once per week), 2 (31–60 minutes to fall asleep, or if have problem falling asleep occurs once or twice a week), and 3 (>60 minutes to initiate sleep or experiences delayed sleep 3 or more times per week). Sleep medication is computed by component 6 of the PSQI, which evaluates use of sleep medication, scored as 0 (did not use sleep medication in the past month), 1 (used sleep medication less than once a week), 2 (sleep medication taken once or twice weekly), and 3 (used sleep medication 3 or more times per week). The time in bed is computed by question 1 and 3 of the PSQI. Daytime dysfunction is computed by component 7 of the PSQI, which evaluates the ability to stay awake and maintain enthusiasm, scored as 0 (no problem to keep awake and enthusiasm ), 1 (have very slight problem), 2 (somewhat of a problem), and 3 (have a very big problem). The PSQI total score is computed by adding each sleep components. The score ranges from 0 to 21; a higher overall scores indicates worse sleep quality.
2.7. Statistics
We used the fraction of the ROI volume that is occupied by the PVS as our measure to calculate the PVS burden in the BG and the CSO. Owing to the non-normal distribution of the PVS in the BG, the log-transformed value of the volume fraction of the PVS was used.
We used SPSS software version 26.0 (IBM SPSS Inc., Armonk, NY, USA) to perform the correlation, multivariable linear regression, and used R software to conduct the mediation analysis. First, the Mann-Whitney U, and t-tests were used to compare PVS volume fraction and sleep measurements between individuals of different ages. The ANOVA test was applied to compare PVS volume fraction by race (African American, Asian, White, and more than one race) and ethnicity (participants who self-identified as Hispanic /Latino or not). All multiple comparison p-values were corrected via Tukey’s HSD multiple comparisons adjustment. Two-sided p-values < 0.05 were considered statistically significant (Table 1).
Next, we used multivariable linear regression analysis to investigate the association between PVS volume fraction (dependent variable) and sleep measurements (independent variables), including sleep efficiency score, sleep quality score, and sleep duration. Age, sex, race/ethnicity, and BMI were used as covariates in the regression models. Aging plays an important role in the PVS volume50, and aging is also associated with a decreased ability to maintain sleep51. On the other hand, the amount of sleep required by people of different age groups also varies52. In our initial analysis, we used age as a continuous variable to investigate the relationship between sleep measurements (from PSQI) and PVS in a regression. We found a significant interaction between sleep measurements and age (data not shown). Therefore, we hypothesized that the magnitude of association between sleep and PVS might differ according to age. To better illustrate the differential sleep-PVS associations by age in the subsequent analysis, we categorized the participants by age into the following two groups: middle-aged adults (36–65 years old, N=363), and older adults (above 65 years old, N=150). We followed the cutoff age range used in papers53–55 in our analysis. We used dummy variables to analyze the influence of other explanatory variables, including age group, sex, and race/ethnicity categories on the PVS volume fraction; we used age group as a binary variable (middle-aged represented with 1 vs. older aged represented with 0). To test if the association between sleep and PVS differed in middle-aged adults versus older adults, we added interaction terms between age group and sleep measurements into the regression model.
Furthermore, we used the R causal mediation analysis package to investigate the mediation pathways between sleep, PVS volume fraction, MoCA score (cognitive status) and BMI. We used model 4 in the mediation model of Preacher and Hayes56 to evaluate whether the sleep measured by the PSQI and cognitive performance measured by the MoCA test were mediated by the PVS variable. We calculated the percent mediation, which assesses the change in the direct sleep-cognitive performance effect with the inclusion of a mediator, PVS volume fraction (Figure 2a). In addition, we noted that BMI was also significantly associated with PVS volume fraction in the regression model. A previous study found that poor sleep was associated with a higher BMI over time, and high BMI was associated with shorter sleep duration57. Therefore, we also explored the mediation pathways between sleep, PVS volume fraction, and BMI. We calculated the percent mediation, which assesses the change in the direct Sleep-PVS effect with the inclusion of a mediator, BMI (Figure 2b).
Figure 2. The simple mediation model was conducted in this study.
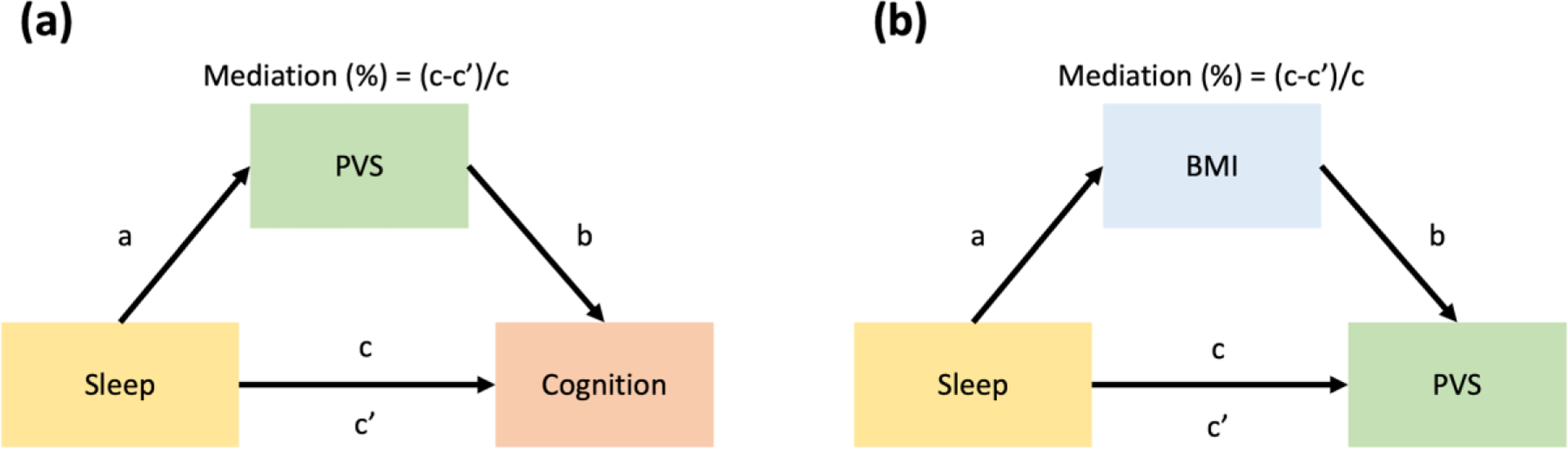
In (a), cognitive performance is regarded as a dependent variable, sleep measurement is regarded as an independent variable, and PVS volume fraction is regarded as a mediator; In (b), PVS volume fraction is considered the dependent variable, sleep measurement is considered an independent variable, and BMI is considered the mediator. Mediation (%) = (c-c’)/c.
3. Results
The demographic characteristics of the study cohort are shown in Table 1. There are more female participants than male (p=0.031). We found that older participants had significantly larger intracranial volume (p<0.0001), BG-PVS volume (p<0.0001) and CSO-PVS volume (p<0.0001) than middle-aged participants. In contrast, middle-aged participants had significantly larger BG volume (p<0.0001) and CSO volume (p<0.0001) than older participants. For sleep measurements, middle-aged participants had higher sleep quality score (p=0.046), longer sleep duration (p=0.017), and lower score for sleep medication (p<0.003). PSQI total score, sleep efficiency score, sleep latency score, time in bed, and daytime dysfunction were not significantly different between middle-aged and older participants. With respect to cognitive performance, older participants had significantly lower MoCA score (p=0.012) and higher working memory score (p=0.005) than middle-aged participants.
3.1. Association between sleep and PVS in different age groups
We investigated the relationship between sleep measures and the PVS volume fraction among each age group using multivariable linear regression. We found that the sleep quality score was significantly negatively associated with the BG-PVS volume fraction among the older (beta= −0.048, p=0.001), and in middle-aged adults (beta=−0.001, p=0.86) (Figure 3a), indicating that poorer sleep quality (higher sleep quality score) is associated with smaller PVS volume fraction. The statistically significant interaction by age group (p=0.005) showed that the sleep quality-BG-PVS association is stronger for older persons than for middle-aged persons (Figure 3b).
Figure 3. Forest plots illustrating the association between the PVS volume fraction and sleep measures in healthy participants.
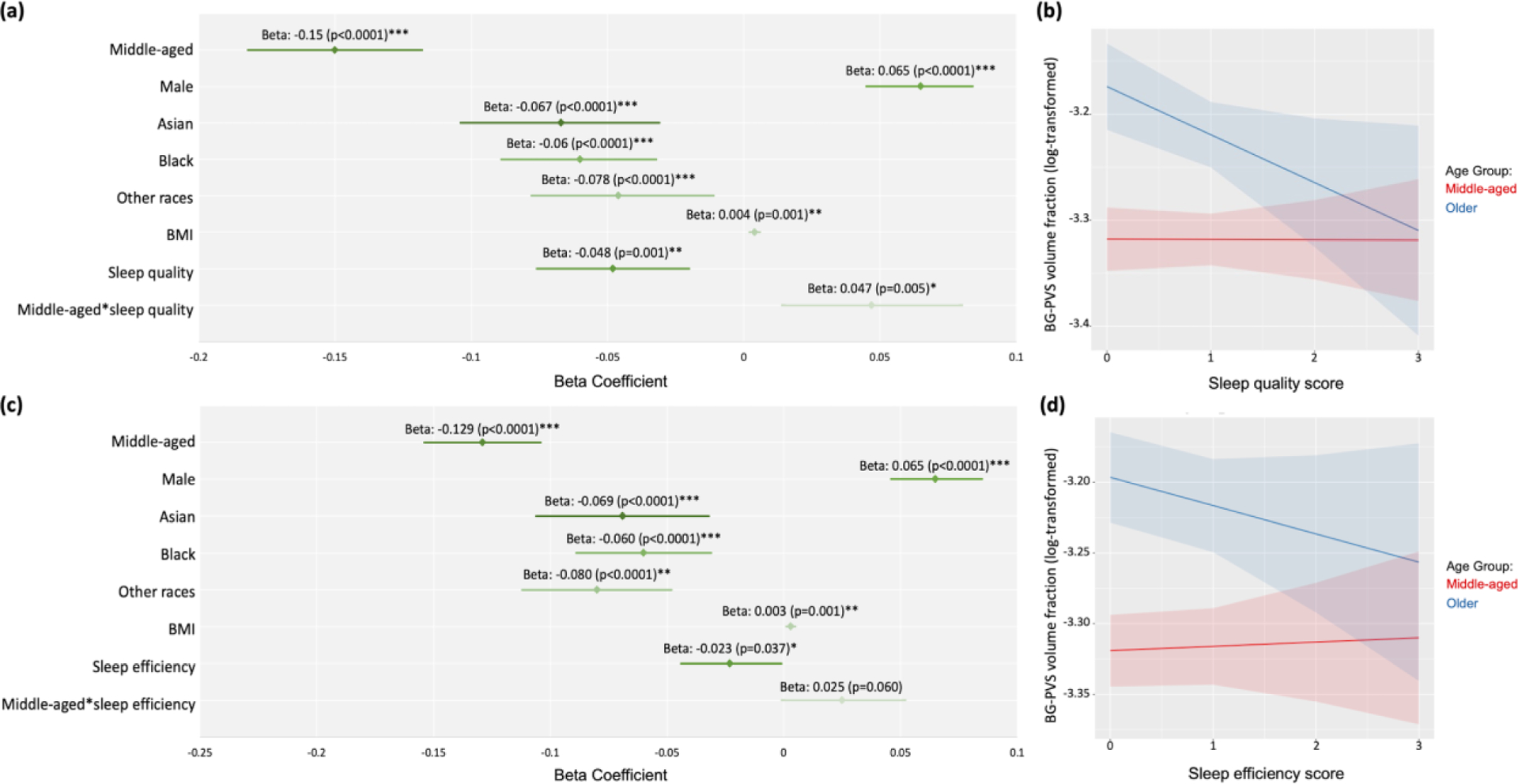
(a) Multiple linear regression analysis was conducted to investigate the relationship between the PVS volume fraction in basal ganglia (BG) and sleep quality score. (b) The interaction between age groups and sleep quality. (c) The relationship between the PVS volume fraction in BG and sleep efficiency score. (d) The interaction between age groups and sleep efficiency. The red line represents the middle-aged group (mean of age= 49.50 years old); the blue line represents the older age group (mean of age= 70.19 years old). Significant *p<0.05; **p<0.001; ***p<0.0001
We also found that the sleep efficiency score was significantly negatively associated with the BG-PVS volume fraction in the older age group (beta=−0.023, p=0.037), indicating that poorer sleep efficiency (higher sleep efficiency score) was associated with lower PVS volume. In contrast, sleep efficiency score was positively associated with BG-PVS in the middle-aged group (beta=0.002, p=0.64) (Figure 3c). However, the interaction coefficient was not statistically significant (interaction beta = 0.025, p=0.060) (Figure 3d).
Our analysis showed no significant association between the BG-PVS volume fraction and sleep duration, sleep latency score, sleep medication, daytime dysfunction, or total PSQI score (Supplement Table 1). There were also no significant associations between the CSO-PVS volume fraction and sleep measurements (Supplement Table 1).
3.2. The association between sleep quality, sleep efficiency, and BG-PVS in the older age group
Analyzed in the older age group alone (above 65 years old), the BG-PVS volume fraction had a significant negative association with the sleep quality score (beta=−0.046, p= 0.027) and sleep efficiency score (beta=−0.023, p=0.037) (Figure 3). According to the PSQI, a higher sleep measure score corresponds to poorer sleep behavior. Therefore, these results indicate that older participants who had better sleep quality and sleep efficiency has higher BG-PVS volume fraction.
Our next step was to conduct a mediation analysis to uncover the relationship between sleep quality, BG-PVS, and cognitive status. Results of the mediation analysis indicated that there was no mediating effect of the BG-PVS volume fraction in the association between the sleep quality and MoCA scores (Figure 4a, average causal mediation effect (95% CI)= −0.0024 (−0.03,0.03), p=0.94). In addition, no mediating effect of the BG-PVS volume fraction on the association between the sleep efficiency and MoCA scores was observed (Figure 4b, average causal mediation effect (95% CI)= −0.0010 (−0.03,0.02), p=0.83).
Figure 4. Demonstration of the mediation analysis of the perivascular space (PVS) in the basal ganglia (BG), sleep quality/efficiency and cognitive performance in the older participants.
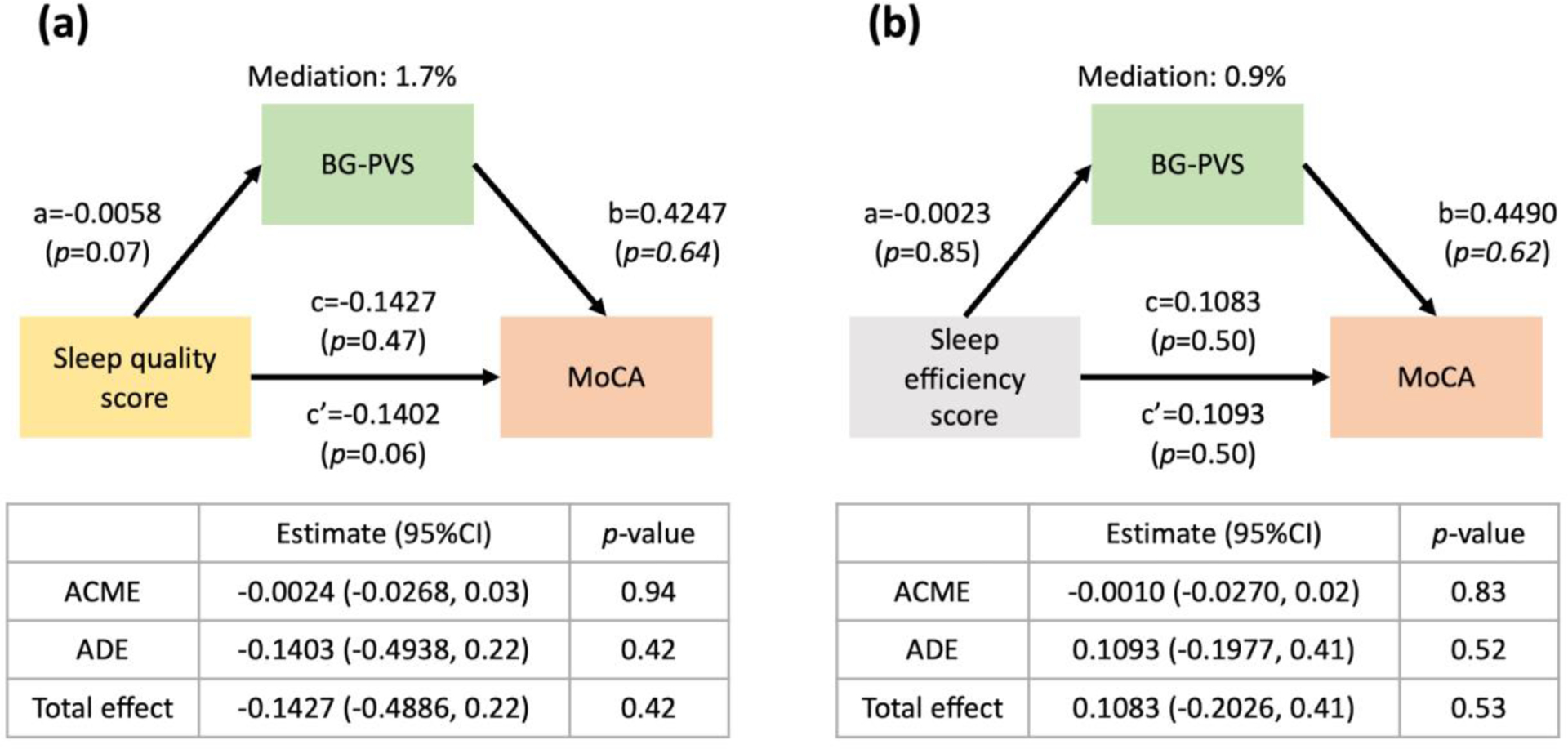
(a) There is no mediating effect of BG-PVS volume fraction on the association between sleep quality and the Montreal Cognitive Assessment (MoCA) score. (b) There is no mediating effect of BG-PVS volume fraction on the association between sleep efficiency and Montreal Cognitive Assessment (MoCA) score. Mediation (%) = (c-c’)/c. ACME: Average Causal Mediated Effect; ADE: Average Direct Effect. Significant *p<0.05
3.3. The relationship between sleep quality, BG-PVS, and BMI
In the regression model, we found a statistically significant positive association between BMI and BG-PVS volume fraction (Figure 3a, 3c) and between BMI and CSO-PVS volume fraction (data not shown). Therefore, we hypothesized that BMI might have a mediating effect on the relationship between sleep quality and PVS volume fraction. In the mediation analysis including all participants of the study (N=513), and adding age as a covariate, the sleep quality score was significantly and positively associated with BMI (p=0.0005). In addition, BMI was significantly and positively associated with BG-PVS volume fraction (p<0.0001). The mediation analysis showed a significant indirect effect of the sleep quality score on the BG-PVS volume fraction through BMI (Figure 5a; average causal mediation effect (95% CI)= 0.0027 (0.0005,0.01), p=0.012). However, the direct association between sleep quality score and BG-PVS was not significant (p=0.17). When we limited the analysis to the middle-aged cohort (N=361), there was no mediation effect of sleep quality on BG-PVS, mediated through BMI (Figure 5b, mediation effect (95% CI)= 0.0024 (−0.0008,0.01), p=0.14), but BMI was significantly positively associated with BG-PVS volume fraction (p=0.0005). In contrast, among the older participants (N=150), the sleep quality score had a significant positive association with BG-PVS volume fraction (p=0.016). Similarly, the BMI had a significant positive effect on BG-PVS volume fraction (p=0.011). However, there was no significant mediation effect through BMI in the older cohort on the association between sleep quality score and BG-PVS volume fraction (Figure 5c, average causal mediation effect (95% CI) = 0.0062 (−0.0007, 0.01), p=0.07). There was not any mediation effect of BMI observed in the association between sleep efficiency and BG-PVS volume fraction. (Supplement Figure 1).
Figure 5. Demonstration of the mediation analysis of the perivascular space (PVS) in the basal ganglia (BG), sleep quality and BMI in the whole sample (N=513), middle-aged participants (N=363) and older participants (N=150).
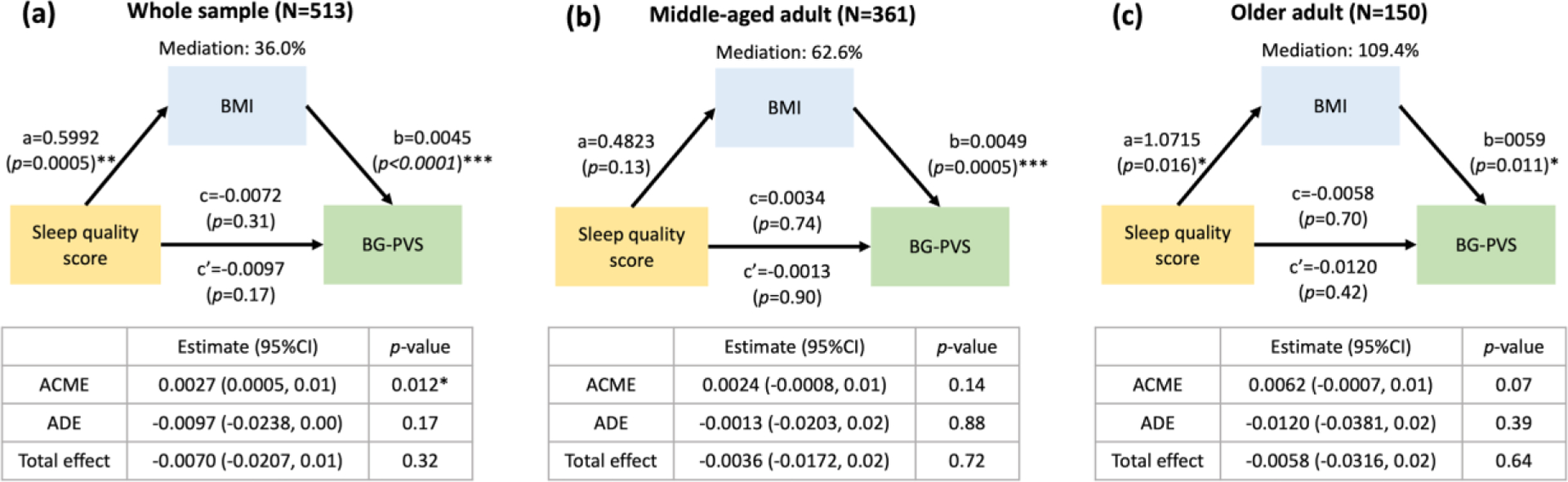
(a) The relationship between sleep quality score, body mass index (BMI), and BG-PVS volume fraction in the whole sample. It depicted the significant indirect effect of sleep quality on the BG-PVS volume fraction through the BMI in whole sample. (b) In the middle-aged group, there is no mediation effect of sleep quality on BG-PVS volume fraction through BMI in the middle-aged cohort. The BMI is directly positively associated with BG-PVS volume fraction. (c) In the older age group, the sleep quality is significantly associated with BMI. BMI is significantly associated with PVS. But, the effect of sleep quality on PVS was not mediated through BMI significantly.. The direct effect of the path is the coefficient a, b, and c. The indirect effect is the coefficient c’. Mediation (%) = (c-c’)/c. ACME: Average Causal Mediated Effect; ADE: Average Direct Effect. Significant *p<0.05
3.4. PVS across race and ethnicity
PVS volume fractions were also observed to significantly differ among racial and ethnic groups (Figure 3a, 3c). As the incidence of cerebrovascular diseases is significantly higher in African Americans compared to Caucasians58, we further investigated the effect of race/ethnicity on the association between the PVS volume fraction and sleep measurements. We did not find significant interactions between sleep parameters and races/ethnicity (p>0.21). In more detail, the results of ANOVA showed that Asians (p=0.006), African Americans (p<0.0001), and other races (p=0.040) all had significantly smaller BG-PVS volume fraction than Whites. (Figure 6a). CSO-PVS volume fractions did not differ across racial/ethnic groups (data not shown).
Figure 6. The perivascular space (PVS) in the basal ganglia (BG) by race/ethnicity.
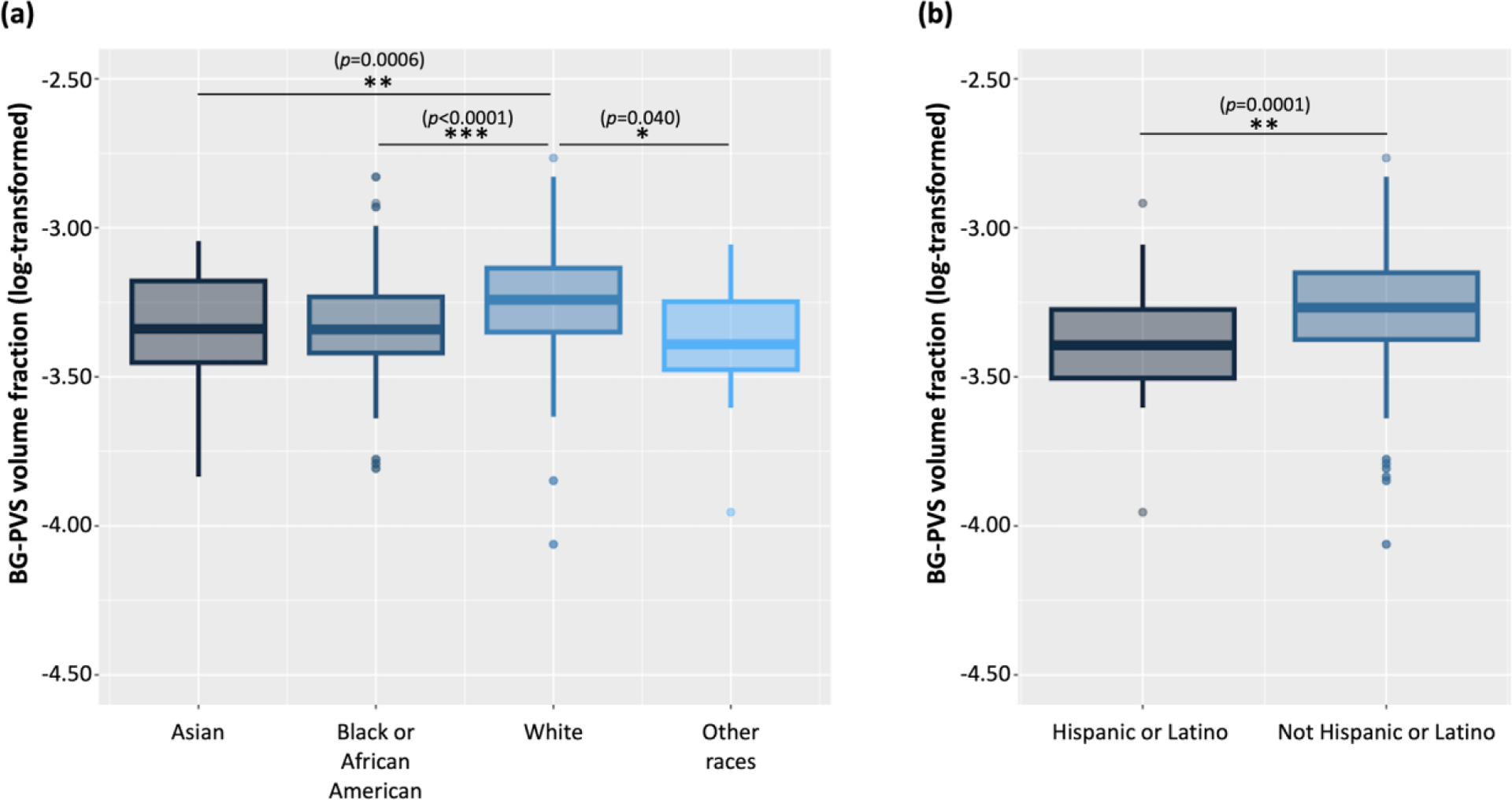
(a) Asians had significantly smaller BG-PVS volume fraction than Whites. African Americans had significantly smaller BG-PVS volume fraction than Whites. Participants of other races had significantly smaller BG-PVS volume fraction than Whites. (b) Hispanic or Latino participants had smaller BG-PVS than non-Hispanic persons. Significant *p<0.05; **p<0.001; ***p<0.0001
4. Discussion
Many studies have shown the different mechanisms of glymphatic clearance during sleep27,33,36. In the current study, variation in PVS volume alterations were observed across age and racial/ethnic groups. However, the actual mechanisms causing the different patterns of PVS morphology across age, race, and ethnicity that we observed are still unknown. We plan to study these mechanisms in the future using larger scale datasets and investigate the relationship between PVS and sleep in subregions of white matter CSO.
In this study, we analyzed the relationship between sleep and PVS volume fraction in the BG and CSO in a healthy aging cohort. Overall, we found that different sleep behaviors were associated with the PVS volume fraction in the BG in the older age group but not in the middle-aged group. In addition, we did not find an association between sleep and PVS in the CSO. After categorizing our population into middle-aged (36–65 years old) and older groups (above 65 years old), we found that there were different patterns of association between sleep measures and PVS volume fraction in the BG by age. We found that better sleep quality and sleep efficiency, as indicated by lower scores in the corresponding PSQI components, were associated with larger BG-PVS volume fraction. Our results in the healthy older group were in contrast to the results in the previous PVS studies with diseased samples, where poor sleep was found to be associated with enlarged BG-PVS32,36. Our hypothesis is that there are pathological and physiological PVS such that the pattern of change in PVS may differ. In healthy persons, larger PVS may be a sign of better brain clearance.
One possible explanation for the contradictory results could be related to the different mechanisms of PVS for healthy adults on removing metabolic waste from the brain. A recent review article suggested that sleep and glymphatic function may interact differently in healthy adults than in animal models59. As sleep has been shown to drive metabolite clearance from the adult brain60, it is possible that a large amount of fluid in BG-PVS might indicate a higher rate of fluid exchange and an efficient waste clearance, as previously proposed61. On the other hand, in patients with cerebrovascular diseases32,36, the negative correlation between BG-PVS volume and sleep efficiency might indicate either a compensatory mechanism where accumulation of fluid is a consequence of the perivascular/glymphatic system dysfunction determined by lower sleep efficiency. Lysen et al. found that higher sleep efficiency was associated with higher CSO-PVS counts in a middle- to old-aged participants61. However, the association of the sleep efficiency and the CSO-PVS volume fraction in the middle-aged healthy population was not significant in our study. Another possible reason for the contrary results we found could be due to our mapping all PVSs rather than only PVSs larger than a certain diameter. Using the high-resolution images of the HCP-Aging dataset and the accuracy of PVS segmentation by EPC technique, we detected higher PVS volume fraction compared to other studies39. Moreover, it is also possible that the findings might be impacted by variations across studies in sleep assessment methods and PVS measurement methods59.
We did not find a significant association between sleep and CSO-PVS. One explanation could be that CSO is a large brain region, and some subregions may have an association with sleep, while others do not, thus cancelling one another out on the final outcome. Therefore, future research is needed on the association between sleep and PVS in subregions of the CSO.
In our mediation analysis, we noted that BMI plays a role in mediating the relationship between sleep and BG-PVS volume in the entire healthy cohort (N=513). This cohort shows that BMI has a larger association to BG-PVS than sleep. This is consistent with a previous study showing a significant positive correlation between BMI and PVS volume fraction in a young participants (22–37 years old)7. Recent research found a positive association between visceral fat, MRI-visible PVS and white matter lesions in the brain62. Currently, it is unknown exactly how BMI is linked to PVS, but it may be related to higher intracranial pressure, since CSF pressure correlates linearly with BMI11. Alternatively, the BMI-PVS association may be linked to reduced vascular contractility and vascular dysfunction that is often associated with obesity11. It is worth noting that the relationship between the BG-PVS volume fraction and sleep was not mediated by BMI status in the older population (N=150), although the sample size was smaller than the whole sample, there was the trend towards marginal significant (p=0.07). One potential explanation is that body fat mass increases while lean body mass and bone mineral density decrease in the older adults, so that body fat may be underestimated by BMI in the older study participants63. In another mediation model between sleep, BMI, and BG-PVS, we tested sleep quality as the mediator and BMI as the predictor of BG-PVS. However, no mediation was found (data not shown). These findings suggest that BMI may influence the association of the PVS and sleep in various ways.
We also investigated the association of the sleep measurements on the PVS by race and ethnicity. Sleep quality did not significantly interact with race/ethnicity; however, the BG-PVS volume fraction did significantly differ by race. One previous study reported larger dilated BG-PVS volume in Whites, compared to African Americans, Asians, and other racial participants64. In line with this result, we found that Whites had significantly larger BG-PVS volume fraction than Asians, African Americans, and other racial groups (Figure 6a). In addition, Hispanics or Latinos had smaller BG-PVS than other ethnic groups (Figure 6b). There was no difference for CSO-PVS volume fraction by race. This is the first study to demonstrate the association of the PVS volume fraction and sleep in a racially/ethnically diverse sample. These findings should be investigated cautiously with additional social and biological factors, as the mechanism and association between race and PVS is still not fully investigated. These data from the HCP-Aging dataset provide initial insight of the potential differences, and more studies are necessary.
In this study, we investigated how PVS alterations and sleep were associated with cognitive status. In the mediation analysis for older age group, we observed the trend of higher BG-PVS volume fraction and better cognitive status in older participants who had poor sleep quality; however, there was no significant causal mediation effect between the BG-PVS volume fraction and cognitive status. Moreover, there was no significant association between the PVS and the results of the NIH toolbox working memory test (data not shown). Other findings in the literature also showed that the higher PVS burden is not associated with cognitive dysfunction in older adults65,66. However, recent findings from our previous study showed that cognitively impaired females from the ADNI had higher PVS volume fraction in the white matter compared to males, and the PVS changes in the anterosuperior medial temporal lobe in persons with mild cognitive impairment were associated with tau uptake12. These findings encourage more research on the effect of the PVS burden on cognition.
The presence of WMH was evaluated through the quality control process by trained PVS analysts, and WMH was not excluded using Fluid Attenuated Inversion Recovery (FLAIR) sequences since FLAIR was not part of the HCPA dataset. However, the effect of WMH on PVS segmentation in our previous study was evaluated in a random sample of 200 participants from the entire HCP datasets (100 HCP-Aging, 50 HCP-Young Adults, and 50 HCP-Developing participants), but no significant correlation was observed8. Therefore, the altered PVS structure in this study cannot be attributed to the presence of WMH in the PVS segmentation method.
As a mean of removing metabolic waste from the brain, the glymphatic system has gained increasing attention in recent years67,68. There is also evidence that this clearance mechanism is cyclic, notably more prominent during non-rapid eye movement (NREM) sleep than during waking hours68. With age, sleep fragmentation increases, which causes less NREM sleep to occur51. In this sense, sleep deprivation may contribute to waste protein accumulation in the brain. In the aging population, occurrence of sleep disturbances during NREM happens many years before the clinical diagnosis of AD. NREM sleep disruption has also been linked to the aggregated amyloid and tau proteins associated with Alzheimer’s disease, and sleep disruption in normal older adults increases risk of AD67,69. In our study of PVS in a healthy aging cohort, we established a baseline against which pathological PVS changes can be compared against, thus helping us gain a better understanding of how sleep affects PVS in healthy adults and in those with neurodegenerative diseases.
There are three advancements in this study compared to previous research in this area. First, we calculated the actual PVS volume rather than the traditional PVS five-point rating70. The rating system is based on counting the visible PVSs in the ROI region and rating individuals accordingly; thus, this technique is only useful for the large PVSs, and it may miss subtle important information of global PVS alteration. Second, we analyzed data from a large healthy cohort study, thereby providing a normative map. Third, PSQI included several broad domains of sleep: sleep duration, sleep disturbance, sleep latency, daytime dysfunction, sleep efficiency, sleep quality, and sleep medication use. These measures cover many different sleep problems, mostly related to insomnia, and sleep quality. In addition, some studies also suggest that removing sleep medication and daytime dysfunction can help achieve more reliable results from the PSQI questionnaire71,72. Therefore, we used individual aspects of the PSQI questionnaire in this study to investigate if there were associations between a range of sleep behaviors and PVS.
However, there are also certain limitations to this study. First, the PSQI is a self-reported questionnaire; thus, biases might exist in the responses to the PSQI items73,74. Objective validation of the PSQI measures could include polysomnography in future studies to ensure validity and precision in sleep findings. Second, the MoCA and NIH toolbox working memory questionnaires used in this study are not thorough assessments of memory performance; therefore, more extensive cognitive measurements are needed. Nevertheless, these questionnaires were the only data available in the HCP-Aging dataset. Since this study is a cross-sectional study, the direction of causation of the reported associations are hard to assess; therefore, additional tests and longitudinal assessments are warranted to structure causal relationships in future studies. Exploring other biophysical characteristics of PVS, such as diffusion75,76, and its relationship with sleep quality is another future direction to this study.
In conclusion, we investigated the relationship between the PVS volume fraction and sleep measures in the HCP-Aging healthy cohort. The effect of sleep on the PVS volume fraction varied in different ages. PVS volume fraction differed by race/ethnicity. In the future, this study could provide a baseline of comparison for the relationship between physiological changes in PVS and sleep behavior. Our study advances our understanding on the role of sleep and brain clearance in cognitively healthy aging adults
Supplementary Material
Highlights.
The link between sleep and PVS volume fraction varies by age.
Older adults who had better sleep quality and sleep efficiency have larger BG-PVS.
BMI mediated the effect of sleep on BG-PVS in the whole cognitively healthy cohort.
There are differences in PVS volume fraction across racial/ethnic cohorts.
Acknowledgments
The research reported in this publication was supported by the National Institute of Mental Health, and National Institute on Aging of the NIH under Award Numbers RF1MH123223, and R01AG070825. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
HCP-Aging:
Dataset used in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number U01AG052564. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: There are no financial conflicts of interest.
Non-financial Disclosure: The perivascular space mapping technology is part of a pending patent owned by Farshid Sepehrband and Jeiran Choupan, with no financial interest/conflict.
Credit author statement
Nien-Chu Shih: Data curation, Investigation, Formal analysis, Writing- Original draft preparation
Giuseppe Barisano: Methodology, Data acquisition, Writing- Reviewing and Editing
Karen D. Lincoln: Formal analysis, Writing- Reviewing and Editing
Wendy J. Mack: Formal analysis, Writing- Reviewing and Editing
Farshid Sepehrband: Methodology, Data acquisition, Writing- Reviewing and Editing
Jeiran Choupan: Data curation, Formal analysis, Project administration, Writing- Reviewing and Editing, Supervision, Funding acquisition
References
- 1.Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nature Reviews Neurology 2020; 16: 137–153. [DOI] [PubMed] [Google Scholar]
- 2.Asgari M, De Zélicourt D, Kurtcuoglu V. How astrocyte networks may contribute to cerebral metabolite clearance. Sci Rep 2015; 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomolka RS, Hablitz L, Mestre H, et al. Loss of aquaporin-4 results in glymphatic system dysfunction via brain-wide interstitial fluid stagnation. Elife 2023; 12: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iliff JJ, Wang M, Liao Y, et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci Transl Med 2012; 4: 147ra111–147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R, Liu Y, Chen Y, et al. Aquaporin 4 deletion exacerbates brain impairments in a mouse model of chronic sleep disruption. CNS Neurosci Ther 2020; 26: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen SMU, Landolt HP, Berger W, et al. Haplotype of the astrocytic water channel AQP4 is associated with slow wave energy regulation in human NREM sleep. PLoS Biol 2020; 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barisano G, Sheikh-Bahaei N, Law M, et al. Body mass index, time of day and genetics affect perivascular spaces in the white matter. J Cereb Blood Flow Metab 2021; 41: 1563– 1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch KM, Sepehrband F, Toga AW, et al. Brain perivascular space imaging across the human lifespan. bioRxiv 2022; 2022.01.25.475887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piantino J, Schwartz DL, Luther M, et al. Link between Mild Traumatic Brain Injury, Poor Sleep, and Magnetic Resonance Imaging: Visible Perivascular Spaces in Veterans. J Neurotrauma 2021; 38: 2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barisano G, Lynch KM, Sibilia F, et al. Imaging perivascular space structure and function using brain MRI. Neuroimage 2022; 257: 119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sepehrband F, Barisano G, Sheikh-Bahaei N, et al. Volumetric distribution of perivascular space in relation to mild cognitive impairment. Neurobiol Aging 2021; 99: 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepehrband F, Cabeen RP, Barisano G, et al. Nonparenchymal fluid is the source of increased mean diffusivity in preclinical Alzheimer’s disease. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2019; 11: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee G, Kim HJ, Fox Z, et al. MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain 2017; 140: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 15.Park YW, Shin NY, Chung SJ, et al. Magnetic Resonance Imaging–Visible Perivascular Spaces in Basal Ganglia Predict Cognitive Decline in Parkinson’s Disease. Mov Disord 2019; 34: 1672–1679. [DOI] [PubMed] [Google Scholar]
- 16.Donahue EK, Murdos A, Jakowec MW, et al. Global and Regional Changes in Perivascular Space in Idiopathic and Familial Parkinson’s Disease. Mov Disord 2021; 36: 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charidimou A, Jäger RH, Peeters A, et al. White matter perivascular spaces are related to cortical superficial siderosis in cerebral amyloid angiopathy. Stroke 2014; 45: 2930–2935. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Cho H, Park M, et al. Mri-visible perivascular spaces in the centrum semiovale are associated with brain amyloid deposition in patients with alzheimer disease-related cognitive impairment. Am J Neuroradiol 2021; 42: 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez J, Berezuk C, McNeely AA, et al. Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer’s disease patients and normal elderly from the Sunnybrook dementia study. J Alzheimer’s Dis 2015; 43: 415–424. [DOI] [PubMed] [Google Scholar]
- 20.Charidimou A, Hong YT, Jäger HR, et al. White Matter Perivascular Spaces on Magnetic Resonance Imaging: Marker of Cerebrovascular Amyloid Burden? Stroke 2015; 46: 1707–1709. [DOI] [PubMed] [Google Scholar]
- 21.He XF, Liu DX, Zhang Q, et al. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci 2017; 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science (80-) 2019; 366: 628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue Y, Liu N, Zhang M, et al. Concomitant enlargement of perivascular spaces and decrease in glymphatic transport in an animal model of cerebral small vessel disease. Brain Res Bull 2020; 161: 78–83. [DOI] [PubMed] [Google Scholar]
- 24.Keable A, Fenna K, Yuen HM, et al. Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim Biophys Acta 2016; 1862: 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shokri-Kojori E, Wang G-J, Wiers CE, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A 2018; 115: 4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju YES, Ooms SJ, Sutphen C, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 2017; 140: 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levendowski DJ, Gamaldo C, St Louis EK, et al. Head position during sleep: Potential implications for patients with neurodegenerative disease. J Alzheimer’s Dis 2019; 67: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Xie L, Yu M, et al. The effect of body posture on brain glymphatic transport. J Neurosci 2015; 35: 11034–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Zhang J, Lam SP, et al. Clinical Biomarkers of Neurodegeneration in REM Sleep Behavior Disorder. J Sleep Med 2015; 12: 27–33. [Google Scholar]
- 30.Zhang F, Niu L, Liu X, et al. Rapid eye movement sleep behavior disorder and neurodegenerative diseases. Aging Dis 2020; 11: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aribisala BS, Riha RL, Valdes Hernandez M, et al. Sleep and brain morphological changes in the eighth decade of life. Sleep Med 2020; 65: 152–158. [DOI] [PubMed] [Google Scholar]
- 32.Berezuk C, Ramirez J, Gao F, et al. Virchow-Robin spaces: Correlations with polysomnography-derived sleep parameters. Sleep 2015; 38: 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Brutto OH, Mera RM, Del Brutto VJ, et al. Enlarged basal ganglia perivascular spaces and sleep parameters. A population-based study. Clin Neurol Neurosurg 2019; 182: 53–57. [DOI] [PubMed] [Google Scholar]
- 34.Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30: 36–42. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Chen X, Liao J, et al. Non breathing-related sleep fragmentation and imaging markers in patients with atherosclerotic cerebral small vessel disease (CSVD): A cross-sectional case-control study. BMC Neurol; 20. Epub ahead of print 17 March 2020. DOI: 10.1186/s12883-020-01647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez J, Holmes MF, Berezuk C, et al. MRI-visible perivascular space volumes, sleep duration and daytime dysfunction in adults with cerebrovascular disease. Sleep Med 2021; 83: 83–88. [DOI] [PubMed] [Google Scholar]
- 37.Bookheimer SY, Salat DH, Terpstra M, et al. The Lifespan Human Connectome Project in Aging: An overview. Neuroimage 2019; 185: 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 39.Sepehrband F, Barisano G, Sheikh-Bahaei N, et al. Image processing approaches to enhance perivascular space visibility and quantification using MRI. Sci Rep 2019; 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 41.Dinov ID, Van Horn JD, Lozev KM, et al. Efficient, distributed and interactive neuroimaging data analysis using the LONI Pipeline. Front Neuroinform 2009; 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013; 80: 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkinson M, Beckmann CF, Behrens TEJ, et al. FSL. Neuroimage 2012; 62: 782–790. [DOI] [PubMed] [Google Scholar]
- 44.Fischl B FreeSurfer. Neuroimage 2012; 62: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frangi AF, Niessen WJ, Vincken KL, et al. Multiscale vessel enhancement filtering. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics) 1998; 1496: 130–137. [Google Scholar]
- 46.Cabeen RP, Laidlaw DH, Toga AW. Quantitative Imaging Toolkit : Software for Interactive 3D Visualization , Data Exploration , and Computational Analysis of Neuroimaging Datasets. ISMRM-ESMRMB Abstr 2018; 12–14. [Google Scholar]
- 47.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 48.Fischl B, Salat DH, Van Der Kouwe AJW, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004; 23: 69–84. [DOI] [PubMed] [Google Scholar]
- 49.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 50.Heier LA, Bauer CJ, Schwartz L, et al. Large Virchow-Robin Spaces : MR-Ciinical Correlation. 1987; 20: 929–936. [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Vitiello MV, Gooneratne N. Sleep in Normal Aging. Sleep Med Clin 2018; 13: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaput JP, Dutil C, Sampasa-Kanyinga H. Sleeping hours: What is the ideal number and how does age impact this? Nat Sci Sleep 2018; 10: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C, Chen Q, Wang Y, et al. Risk factors of dilated Virchow-Robin Spaces are different in various brain regions. PLoS One; 9. Epub ahead of print 2014. DOI: 10.1371/journal.pone.0105505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: The Sleep Heart Health Study. Am J Epidemiol 2001; 154: 50–59. [DOI] [PubMed] [Google Scholar]
- 55.Cai Y, Chen B, Zeng X, et al. The Triglyceride Glucose Index Is a Risk Factor for Enlarged Perivascular Space. Front Neurol 2022; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications, 2017. [Google Scholar]
- 57.Koolhaas CM, Kocevska D, te Lindert BHW, et al. Objectively measured sleep and body mass index: a prospective bidirectional study in middle-aged and older adults. Sleep Med 2019; 57: 43–50. [DOI] [PubMed] [Google Scholar]
- 58.Gorelick PB. Cerebrovascular disease in African Americans. Stroke 1998; 29: 2656–2664. [DOI] [PubMed] [Google Scholar]
- 59.Sangalli L, Boggero IA. The impact of sleep components, quality and patterns on glymphatic system functioning in healthy adults: A systematic review. Sleep Med. Epub ahead of print 2022. DOI: 10.1016/j.sleep.2022.11.012. [DOI] [PubMed] [Google Scholar]
- 60.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science (80- ) 2013; 342: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lysen TS, Yilmaz P, Dubost F, et al. Sleep and perivascular spaces in the middle-aged and elderly population. J Sleep Res 2021; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozato N, Saitou S, Yamaguchi T, et al. Association between Visceral Fat and Brain Structural Changes or Cognitive Function. Brain Sci 2021; 11: 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponti F, Santoro A, Mercatelli D, et al. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front Endocrinol (Lausanne); 10. Epub ahead of print 2020. DOI: 10.3389/fendo.2019.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi Y, Nam Y, Choi Y, et al. MRI-visible dilated perivascular spaces in healthy young adults: A twin heritability study. Hum Brain Mapp 2020; 41: 5313–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hilal S, Tan CS, Adams HHH, et al. Enlarged perivascular spaces and cognition. Neurology 2018; 91: e832–e842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valdés Hernández M del C, Ballerini L, Glatz A, et al. Perivascular spaces in the centrum semiovale at the beginning of the 8th decade of life: effect on cognition and associations with mineral deposition. Brain Imaging Behav 2020; 14: 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science (80- ) 2020; 370: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reddy OC, van der Werf YD. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sciences 2020; 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mander BA, Winer JR, Jagust WJ, et al. Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci 2017; 39: 552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Potter GM, Chappell FM, Morris Z, et al. Cerebral perivascular spaces visible on magnetic resonance imaging: Development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis 2015; 39: 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Wu YX, Lin YQ, et al. Reliability and validity of the Pittsburgh Sleep Quality Index among frontline COVID-19 health care workers using classical test theory and item response theory. J Clin Sleep Med 2022; 18: 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang C, Zhang H, Zhao M, et al. Reliability, Validity, and Factor Structure of Pittsburgh Sleep Quality Index in Community-Based Centenarians. Front Psychiatry 2020; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lauderdale DS, Knutson KL, Yan LL, et al. Self-reported and measured sleep duration: How similar are they? Epidemiology 2008; 19: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zak RS, Zitser J, Jones HJ, et al. Sleep Self-Report and Actigraphy Measures in Healthy Midlife Women: Validity of the Pittsburgh Sleep Quality Index. J Women’s Heal 2022; 31: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sepehrband F, Cabeen RP, Choupan J, et al. Perivascular space fluid contributes to diffusion tensor imaging changes in white matter. Neuroimage 2019; 197: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barisano G, Law M, Custer RM, et al. Perivascular Space Imaging at Ultrahigh Field MR Imaging. Magn Reson Imaging Clin N Am 2021; 29: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


