Abstract
Background.
Despite treatment with combination antiretroviral therapy (cART), persons living with HIV (PLWH) are at higher risk of cardiac structural abnormalities that may presage clinical heart failure, including myocardial fibrosis. This study assessed whether circulating cellular and soluble protein markers of immune activation cross-sectionally associate with myocardial fibrosis among cART-treated PLWH in South Africa.
Methods.
Participants were enrolled in Khayelitsha township near Cape Town, SA. Cardiac magnetic resonance imaging was performed. Plasma protein biomarkers were measured using enzyme-linked immunoassays and monocyte phenotypes were evaluated using flow cytometry. Associations were assessed using multivariable linear and logistic regression.
Results.
Among 69 cART-treated PLWH, mean (SD) age was 48 (10) years, 71% were female, and time since HIV diagnosis was 9 (6) years. Evidence of left ventricular fibrosis by late gadolinium enhancement was present in 74% of participants and mean (SD) extracellular volume fraction (ECV) was 30.9 (5.9)%. Degree of myocardial fibrosis/inflammation measured by ECV was positively associated with percentages of circulating non-classical and intermediate monocyte phenotypes reflecting inflammation and tissue injury.
Conclusion.
These data generate hypotheses on possible immune mechanisms of HIV-associated non-ischemic myocardial disease, specifically among cART-treated PLWH in sub-Saharan Africa, where the majority of the HIV burden exists globally.
Keywords: myocardial fibrosis, extracellular volume fraction, monocyte activation, monocyte phenotype, HIV infection
1. INTRODUCTION
Global efforts to improve access to combination antiretroviral therapy (cART) among persons living with HIV (PLWH) have resulted in a shift in morbidity and mortality from AIDS-defining conditions to aging-related non-communicable diseases, such as cardiovascular disease (CVD).1 Evidence suggests that PLWH receiving cART are at higher risk of myocardial disease compared to persons without HIV (PWOH), including heart failure (HF)2 and subclinical myocardial fibrosis.3,4 Contemporary data are needed to understand the pathogenesis of HIV-associated myocardial disease, particularly in regions where HIV prevalence is high and access to cART is growing, such as South Africa (SA).
Immune activation is a hallmark of both chronic HIV infection2 and the pathophysiology of fibrosis,5 in which activated monocytes play a pivotal role. Specifically, there is evidence supporting the importance of circulating monocyte mobilization during cardiac remodeling and the deleterious effects of pro-inflammatory macrophages on cardiac function. However, our understanding of this pathobiology is limited, particularly among PLWH, where underlying mechanisms may differ compared to PWOH.
Using cardiovascular magnetic resonance (CMR) imaging among residents of a peri-urban township of Cape Town, SA, we previously reported high prevalence of myocardial fibrosis/inflammation among cART-treated PLWH (measured by extracellular volume fraction [ECV]), and a greater degree of fibrosis/inflammation when compared to PWOH.3 In this report, we evaluated cross-sectional associations between ECV and levels of both circulating monocyte subsets and protein biomarkers of immune activation among the same cohort of PLWH.
2. MATERIALS AND METHODS
2.1. Study Population
Participants were enrolled at Site B health clinic in Khayelitsha—a low-income, densely populated peri-urban residential area on the outskirts of Cape Town, SA with high HIV prevalence. All participants were PLWH receiving routine care with most recent HIV viral loads of <200 RNA copies/mL, no known CVD (e.g., HF, structural heart disease, coronary heart disease, or prior peri/myocarditis), no active treatment for tuberculosis or bacterial infection, and no diagnosis of AIDS-defining illness ≤1 year preceding enrollment. The currently presented study was conducted among a randomly selected subsample of PLWH from the parent study.3 This study was approved by the University of Cape Town Human Research Ethics Committee. Informed consent was obtained from participants in English and/or Xhosa.
2.2. Clinical Data
Clinical data were collected at the time of enrollment. Confirmed data—e.g., medical diagnoses, historic laboratory monitoring, and prescribed medications—were obtained from medical records, and self-reported data—e.g., substance use—were ascertained by interview. A blood draw was performed on the day of enrollment and clinical laboratory measures were ascertained using standard assay procedures at the National Health Laboratory Service, Groote Schuur Hospital in Cape Town, SA.
2.3. Cardiac Magnetic Resonance Imaging
Participants underwent a standardized CMR protocol using a 3T large bore Skyra system (Siemens Healthcare) at the University of Cape Town, described previously.3 Briefly, T1 mapping was performed using the shortened Modified Look-Locker Inversion Recovery sequence; T2 mapping using SSFP imaging; T2-weighted imaging using the black-blood short-Tau inversion recovery sequence; and late-gadolinium enhancement (LGE) using standardized methods. Global ECV fraction was calculated as [partition coefficient] × [1 – hematocrit] and did not exclude LGE, as is standard practice. CMR images were analyzed by three independent reviewers, each of whom were blinded to participant clinical characteristics.
2.4. Protein Biomarkers and Cell Phenotyping
Plasma and peripheral blood mononuclear cells (PBMCs) were processed from blood samples on the day of collection and stored at −80°C and in liquid nitrogen, respectively. Circulating levels of interleukin-6 (IL-6, R&D Systems), tumor necrosis factor receptor-1 (TNFR1, R&D Systems), sCD14 (Hycult), and sCD163 (R&D Systems) were measured in plasma by enzyme-linked immunosorbent assays. PBMCs were stained with Zombie NIR viability dye followed by CD3-FITC, HLA-DR-BV605, CD56-PE, CD14-PE/Cy7, CD16-PerCP/Cyanine 5.5, CD163-BV421, CCR5-BV711, and CCR2-PE/Dazzle 594 antibodies (all Biolegend). A Fortessa flow cytometer and FACS Diva software were used to capture 500,000 events within 24 hours of staining, then analyzed and output as percentages of HLA-DR+ myeloid cells using FlowJo (version 9.9.6).
2.5. Statistical Methods
Complete case analyses were performed to estimate the cross-sectional association between cardiac tissue characteristics measured via CMR imaging and circulating levels of protein biomarkers and monocyte cell phenotypes. Inference was made using linear and logistic regression models for continuous and dichotomous response characteristics, respectively, adjusting for age, sex at birth, body mass index (BMI), smoking, and hypertension. Further adjustment for and effect measure modification (multiplicative interaction) by prior active tuberculosis infection was evaluated. Unadjusted associations between biomarkers and HIV-related characteristics, such as CD4+ cell count, were also assessed. All analyses were conducted using R version 4.0.
3. RESULTS
Participant characteristics are presented in Table 1. Among 69 PLWH, mean (standard deviation [SD]) age was 48 (10) years; 71% were female; 30% were current smokers; 33% were hypertensive; 9% had hepatitis B; and 57% had prior active tuberculosis by history. Mean (SD) time since HIV diagnosis was 9 (6) years; current and nadir CD4+ count was 583 (261) and 266 (207) cells/μL, respectively; 45% had a prior AIDS diagnosis; and 36% were virally suppressed for ≥4 years. All participants were taking a nucleoside reverse transcriptase inhibitor combination, specifically tenofovir disoproxil fumarate and emtricitabine, and 86% were taking a non-nucleoside reverse transcriptase inhibitor, 93% of whom were prescribed efavirenz. Seventy-four percent of participants had evidence of left ventricular scarring or fibrosis, 27% of whom exhibited a diffuse or patchy pattern (19% of total participants). Mean (SD) ECV was 30.9 (5.9)% and native T1 time was 1248 (41) ms.
Table 1.
Participant demographic and clinical characteristics (n=69)
| Mean (SD) or % (n) | |
|---|---|
|
| |
| Demographics | |
|
| |
| Age, years | 48 (10) |
| Female sex at birth | 71% (49) |
| Black African race | 100% (69) |
|
| |
| Clinical Characteristics | |
|
| |
| Current smoker | 30% (21) |
| Body mass index, kg/m2 | 26.5 (6.7) |
| Systolic blood pressure, mmHg | 132 (18) |
| Diastolic blood pressure, mmHg | 79 (12) |
| Total cholesterol, mg/dL | 175 (30) |
| High-density lipoprotein cholesterol, mg/dL | 63 (23) |
| Low-density lipoprotein cholesterol, mg/dL | 86 (29) |
| Hypertension diagnosis | 33% (23) |
| Diabetes diagnosis | 6% (4) |
| Prior active tuberculosis infection | 57% (39) |
|
| |
| HIV-Related Characteristics | |
|
| |
| CD4+ count, cells/μL | 583 (261) |
| CD4+ nadir count, cells/μL | 266 (207) |
| HIV viral load <200 copies/mL | 97% (67) |
| HIV diagnosis duration, years | 9.3 (6.1) |
| Antiretroviral regimen contains | -- |
| Nucleoside reverse transcriptase inhibitor | 96% (66) |
| Non-nucleoside reverse transcriptase inhibitor | 86% (59) |
| Protease inhibitor | 9% (6) |
| HIV suppression duration ≥4 years | 36% (25) |
| Prior AIDS | 45% (31) |
|
| |
| Myocardial Tissue Characteristics | |
|
| |
| LGE presence | 74% (49) |
| LGE presence, diffuse or patchy | 19% (13) |
| ECV fraction, % | 30.9 (5.9) |
| Native T1 time, ms | 1248 (41) |
| T1, weighted signal intensity ratio | 0.90 (0.11) |
| Native T2 time, ms | 39.1 (2.4) |
| T2W, signal intensity ratio | 1.48 (0.21) |
| Pericardial effusion | 20% (14) |
|
| |
| Circulating Protein Biomarkers | |
|
| |
| IL-6, pg/mL | 2.50 (2.01) |
| TNFRI, pg/mL | 420 (138) |
| sCD14, ng/mL | 6637 (7736) |
| sCD163, ng/mL | 798 (273) |
|
| |
| Circulating Monocyte Subsets | |
|
| |
| CD14++ CD16− (Classical), % | 25.4 (15.4) |
| CD16+ (Intermediate or Non-Classical), % | 2.74 (2.60) |
| CD14++ CD16+ (Intermediate), % | 1.89 (2.35) |
| CD14++ CD16+ CD163+ | 0.42 (0.51) |
| CD14++ CD16+ CCR2+ | 0.59 (0.65) |
| CD14++ CD16+ CCR5+ | 0.32 (0.45) |
| CD14+ CD16++ (Non-Classical), % | 0.85 (0.90) |
| CD14+ CD16++ CD163+ | 0.06 (0.05) |
| CD14+ CD16++ CCR2+ | 0.09 (0.13) |
| CD14+ CD16++ CCR5+ | 0.14 (0.13) |
Monocyte subsets are expressed as percentages of myeloid (HLADR+) cells. Total HLADR+CD14+/dimCD16++ combines both intermediate (CD14+CD16++) and non-classical (CD14dimCD16++) monocyte subsets.
SD=standard deviation; LGE=late gadolinium enhancement; IL6=interleukin-6; TNFRI=tumor necrosis factor receptor I; sCD14=soluble cluster of differentiation; HLADR=human leukocyte antigen-DR isotype; CD=cluster of differentiation.
Given we have previously shown ECV fraction differs by HIV status in this population,3 Figure 1 displays the adjusted mean difference in ECV fraction per SD increment in markers of inflammation and immune activation. The strongest associations were observed for the following monocyte subsets: CD16+ (2.21 ECV unit difference per SD increment in cellular phenotype percentage [95% confidence interval: 0.98–3.43]; p=0.001); CD14++CD16+ (2.09 [0.86–3.33]; p=0.001); CD14++CD16+CCR2+ (2.50 [1.23–3.77]; p<0.001); CD14++CD16+CD163+ (1.35 [−0.01–2.71]; p=0.05); and CD14+CD16++CCR5+ (1.56 [0.19–2.93]; p=0.03). Associations between other measures of inflammation—e.g., plasma proteins—and other myocardial tissue characteristics—e.g., LGE, T1, and T2 times—were either small and/or imprecise. Further adjustment for prior active tuberculosis did not substantially alter parameter estimates or overall inference, and there was no evidence of effect measure modification by multiplicative interaction (all p>0.20). Some monocyte subsets associated with ECV were also inversely associated with current CD4+ count—specifically CD14++CD16+ (p=0.01), CD14++CD16+CD163+ (p=0.03), and CD14+CD16++ (p=0.02).
Figure 1.
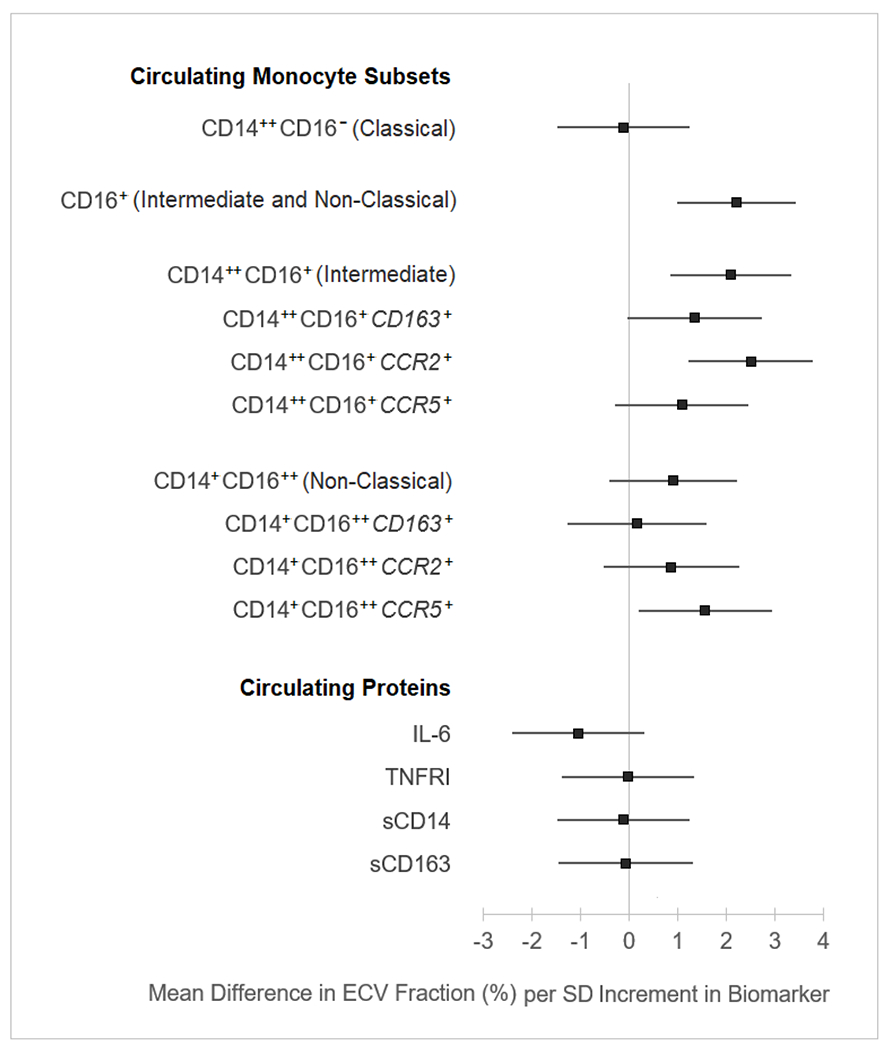
Cross-sectional associations between ECV fraction and cellular and soluble markers of immune activation among cART-treated PLWH in South Africa (n=69)
Estimated using linear regression, adjusted for age, sex at birth, body mass index, smoking, and hypertension. ECV=extracellular volume; cART=combination antiretroviral therapy; PLWH=persons living with HIV; SD=standard deviation HLA-DR=human leukocyte antigen-DR isotype; IL6=interleukin-6; TNFRI=tumor necrosis factor receptor I.
4. DISCUSSION
Among asymptomatic cART-treated PLWH receiving routine care in a peri-urban township near Cape Town, SA, we observed positive associations between the degree of myocardial fibrosis—estimated by ECV fraction—and percentages of circulating monocyte subsets reflecting inflammation and tissue injury—specifically percentages of total CD14+/dimCD16++ monocytes, total CD14+CD16++, CD14+CD16++CCR2+, CD14+CD16++CD163+, and CD14dimCD16++CCR5+. Levels of three of these monocyte subsets were also inversely associated with CD4+ count. However, associations with ECV or other myocardial tissue characteristics were not apparent with soluble plasma proteins reflecting systemic inflammation.
An expansion of activated CD16+ monocyte subpopulations has been described with HIV infection, including with cART treatment and among long-term non-progressors.6–9 This is in part due to expression of CCR5, an important HIV entry co-receptor.10 These monocytes perpetuate inflammation among cART-treated PLWH via cytokine production and immune regulation, 11,12 as well as likely serving as a latent HIV reservoir, contributing to residual levels of viral replication with cART.13
A recent study among women living with HIV in the United States suggested expression levels of CCR2 (a chemokine receptor that mediates chemotaxis during inflammation) and CD163 (a marker of monocyte/macrophage activation and tissue response to inflammation) on CD16+ monocyte subsets were associated with HIV seropositivity.14 In the same study, CCR2 expression on CD14++CD16+ monocytes was associated with ECV among women with HIV.14
Studies among the general population have linked these monocyte expression patterns to cardiovascular pathologies, as well, including increased expression of CD16, CD163, CCR2, and CCR5.15–18 This overlap between HIV seropositivity and CVD in both (i) expansion of CD16+ monocytes and (ii) upregulation of CD163, CCR2, and CCR5 is consistent with our observations in this report and the hypothesis that these monocyte subsets are an important factor in the pathogenesis of HIV-associated myocardial fibrosis.
There are limitations to this study. First, analyses are cross-sectional, so these relationships have temporal ambiguity. Second, sample size is small, limiting power to detect moderate effect sizes. Third, interquartile ranges of some proteins and cell phenotype levels are relatively narrow, limiting prediction power and possibly threatening analysis validity. This study was also exploratory and involved modeling multiple associations, including those of highly correlated biomarkers, leading to inflation of type I error. Lastly, we are unable to evaluate the heterogeneity of monocyte subsets we’ve described, which recent work has highlighted and should be appreciated.19
5. CONCLUSION
In summary, among asymptomatic cART-treated PLWH receiving routine care in SA, we observed positive associations between ECV and percentages of circulating monocyte subsets reflecting tissue inflammation and injury, some of which were also inversely associated with CD4+ count. These data generate hypotheses on possible mechanisms of HIV-associated non-ischemic myocardial disease, specifically among PLWH in sub-Saharan Africa, where the majority of HIV burden exists globally.
HIGHLIGHTS.
Higher myocardial ECV is associated with higher percentages of circulating monocyte subsets reflecting tissue inflammation and injury among cART-treated persons living with HIV in South Africa.
Lower current CD4+ count was also associated with higher percentages of some of these circulating activated monocyte subsets among this population.
Levels of plasma proteins reflecting systemic inflammation were not associated with ECV among this population.
ACKNOWLEDGEMENTS
We thank all the study participants for their commitment and support of this project. In addition, we thank Rene Goliath and research staff who assisted with recruitment at Site B, as well as CIDRI-Africa.
Funding.
This study was funded by the National Heart Lung and Blood Institute, National Institutes of Health (R21 HL137435) and the American Heart Association (17IRG33350064). This work was also supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. TP was supported by institutional training grants T32 HL007779 and T32 HL007227 from the National Institutes of Health. GM was supported by the Wellcome Trust (098316, 214321/Z/18/Z, and 203135/Z/16/Z) and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant Number 64787).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest. None.
CRediT Author Statement
Tess E Peterson: data curation, formal analysis, software, methodology, visualization, writing-original draft, writing-review & editing
Muki Shey: investigation, methodology, supervision, resources, data curation, visualization, writing-original draft
Nomawethu Masina: investigation
Lye-Yeng Wong: investigation, project administration, resources
Scott R Shuldiner: investigation, project administration, resources
Julian Wolfson: methodology, funding acquisition
Stephen Jermy: investigation, visualization
Hadil Saad: investigation
Mbalabu A J Lumbamba: investigation, supervision, resources
Achita Singh: investigation
Graeme Meintjes: conceptualization, funding acquisition, methodology, supervision, project administration, writing-original draft
Ntobeko A B Ntusi: conceptualization, funding acquisition, methodology, investigation, supervision, project administration, writing-original draft, writing-review & editing
Mpiko Ntsekhe: conceptualization, funding acquisition, methodology, supervision, project administration, writing-original draft
Jason V Baker: conceptualization, funding acquisition, methodology, supervision, project administration, writing-original draft, writing-review & editing
REFERENCES
- 1.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214–20. doi: 10.1016/j.amjcard.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98–e124. doi: 10.1161/CIR.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shuldiner SR, Wong LY, Peterson TE, et al. Myocardial Fibrosis Among Antiretroviral Therapy-Treated Persons With Human Immunodeficiency Virus in South Africa. Open Forum Infect Dis. 2021;8(1):ofaa600. doi: 10.1093/ofid/ofaa600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu KC, Haberlen SA, Plankey MW, et al. Human immunodeficiency viral infection and differences in interstitial ventricular fibrosis and left atrial size. Eur Heart J Cardiovasc Imaging. 2021;doi: 10.1093/ehjci/jeab037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López B, Ravassa S, Moreno MU, et al. Diffuse myocardial fibrosis: mechanisms, diagnosis and therapeutic approaches. Nat Rev Cardiol. 2021;18(7):479–498. doi: 10.1038/s41569-020-00504-1 [DOI] [PubMed] [Google Scholar]
- 6.Tippett E, Cheng WJ, Westhorpe C, et al. Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PLoS One. 2011;6(5):e19968. doi: 10.1371/journal.pone.0019968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120(23):4599–608. doi: 10.1182/blood-2012-05-433946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prabhu VM, Singh AK, Padwal V, Nagar V, Patil P, Patel V. Monocyte Based Correlates of Immune Activation and Viremia in HIV-Infected Long-Term Non-Progressors. Front Immunol. 2019;10:2849. doi: 10.3389/fimmu.2019.02849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teer E, Joseph DE, Driescher N, et al. HIV and cardiovascular diseases risk: exploring the interplay between T-cell activation, coagulation, monocyte subsets, and lipid subclass alterations. Am J Physiol Heart Circ Physiol. 2019;316(5):H1146–H1157. doi: 10.1152/ajpheart.00797.2018 [DOI] [PubMed] [Google Scholar]
- 10.Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178(10):6581–9. doi: 10.4049/jimmunol.178.10.6581 [DOI] [PubMed] [Google Scholar]
- 11.Kedzierska K, Crowe SM, Turville S, Cunningham AL. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13(1):39–56. doi: 10.1002/rmv.369 [DOI] [PubMed] [Google Scholar]
- 12.Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS. Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J Immunol Res. 2014;2014:569819. doi: 10.1155/2014/569819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan CJ, Russell RA, Sattentau QJ. High multiplicity HIV-1 cell-to-cell transmission from macrophages to CD4+ T cells limits antiretroviral efficacy. AIDS. 2013;27(14):2201–6. doi: 10.1097/QAD.0b013e3283632ec4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanni MV, Awadalla M, Toribio M, et al. Immune Correlates of Diffuse Myocardial Fibrosis and Diastolic Dysfunction Among Aging Women With Human Immunodeficiency Virus. J Infect Dis. 2020;221(8):1315–1320. doi: 10.1093/infdis/jiz184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barisione C, Garibaldi S, Ghigliotti G, et al. CD14CD16 monocyte subset levels in heart failure patients. Dis Markers. 2010;28(2):115–24. doi: 10.3233/DMA-2010-0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shantsila E, Wrigley B, Tapp L, et al. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. J Thromb Haemost. 2011;9(5):1056–66. doi: 10.1111/j.1538-7836.2011.04244.x [DOI] [PubMed] [Google Scholar]
- 17.Jones KL, Maguire JJ, Davenport AP. Chemokine receptor CCR5: from AIDS to atherosclerosis. Br J Pharmacol. 2011;162(7):1453–69. doi: 10.1111/j.1476-5381.2010.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L, Akahori H, Harari E, et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest. 2018;128(3):1106–1124. doi: 10.1172/JCI93025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718 [DOI] [PMC free article] [PubMed] [Google Scholar]


