Abstract
The placenta performs essential biologic functions for fetal development throughout pregnancy. Placental dysfunction is at the root of multiple adverse birth outcomes such as intrauterine growth restriction, preeclampsia, and preterm birth. Exposure to endocrine disrupting chemicals during pregnancy can cause placental dysfunction, and many prior human studies have examined molecular changes in bulk placental tissues. Placenta-specific cell types, including cytotrophoblasts, syncytiotrophoblasts, extravillous trophoblasts, and placental resident macrophage Hofbauer cells play unique roles in placental development, structure, and function. Toxicant-induced changes in relative abundance and/or impairment of these cell types likely contribute to placental pathogenesis. Although gene expression insights gained from bulk placental tissue RNA-sequencing data are useful, their interpretation is limited because bulk analysis can mask the effects of a chemical on individual populations of placental cells. Cutting-edge single cell RNA-sequencing technologies are enabling the investigation of placental cell-type specific responses to endocrine disrupting chemicals. Moreover, in situ bioinformatic cell deconvolution enables the estimation of cell type proportions in bulk placental tissue gene expression data. These emerging technologies have tremendous potential to provide novel mechanistic insights in a complex heterogeneous tissue with implications for toxicant contributions to adverse pregnancy outcomes.
Keywords: Placenta, villous tissue, endocrine disrupting chemicals, single cell analyses, cellular deconvolution
Introduction
The placenta is a transient organ that develops during pregnancy and plays a key role in not only the health of the fetus but also the mother (Gingrich et al., 2020; Padmanabhan et al., 2021). Defects in placental development are associated with poor pregnancy outcomes, intrauterine growth restriction, and programming of offspring health and disease. Gestational exposures to endocrine-disrupting chemicals (EDCs) can perturb placental functions contributing to adverse pregnancy outcomes and later onset diseases. Understanding the molecular pathways linking EDC exposures to health outcomes via placental dysfunction is a highly active area of promising and ongoing toxicologic and epidemiologic research. These studies typically use bulk placental samples, ignoring the complex cellular heterogeneity of the placental tissue. This review summarizes the current status of EDC exposures on bulk placental tissue gene expression, as measured by RNA-sequencing, highlighting the diverse cellular functions of different placental cell types and cutting-edge technologies available for single cell measures, enabling cell type resolution of placental gene expression. Lastly, we provide actionable recommendations to advance research on EDC exposure effects on placental tissue and development.
Impact of prenatal exposure to endocrine disrupting chemicals (EDC) on perinatal and childhood outcomes
EDCs are chemicals that interfere with hormone signaling through multiple mechanisms (Ho et al., 2022; Kabir et al., 2015), including disruption of synthesis, transport, or binding of endogenous hormones in the body (Kavlock et al., 1996), as well as direct activation of hormone receptors (Kiyama and Wada-Kiyama, 2015). Environmental contaminants that are classified as EDCs include pesticides (Brander et al., 2016), heavy metals (Jia et al., 2021), plasticizers (Rattan et al., 2017), and pharmaceuticals (Ho et al., 2022). Exposure to one or more EDCs during gestation is linked to pregnancy complications including preeclampsia (Cantonwine et al., 2016), gestational diabetes (Shaffer et al., 2019) and preterm birth (Welch et al., 2022). It is also associated with offspring outcomes including hypospadias, cryptorchidism (Grady and Sathyanarayana, 2012; Sathyanarayana et al., 2016; Wu et al., 2022), male (Bonde et al., 2016) and female (Rattan et al., 2017) reproductive disorders, behavioral problems (Philippat et al., 2017), autistic traits (Day et al., 2021), decreased IQ scores (Tanner et al., 2020), liver injury (Midya et al., 2022) and cardiometabolic dysfunction (Abrantes-Soares et al., 2022). Depending on the chemical or chemical mixture studied, EDC exposure is also associated with both increased (Pearce et al., 2021) and decreased (Hu et al., 2021) birth weight, highlighting the need to improve our understanding of specific chemical impacts and the molecular mechanisms underpinning these associations. Epidemiological evidence for links between many specific chemical exposures and diseases remain mixed, however, and further research is required into specific mechanisms and thresholds of exposure that may contribute to disease outcomes.
In general, as described by the Developmental Origins of Health and Disease (DOHaD) hypothesis (Barker, 2007), exposures that disrupt growth and development in early life can contribute to lifelong health effects (Almond and Currie, 2011). The prenatal period of development represents a uniquely susceptible life stage for EDC exposure, as endocrine signals during this period are critical for fetal growth and healthy organ development (Gicquel and Le Bouc, 2006; Scott et al., 2009; Toivanen and Shen, 2017). For example, maternal exposure to EDCs is linked to birth weight in human studies and mechanistic toxicology studies, demonstrating EDC impacts body weight and energy metabolism. Pregnant mice exposed to EDCs like tributyltin have offspring with altered differentiation potential of multipotent cells, leaving them predisposed to differentiate into adipocytes (Kirchner et al., 2010). These cell-type differentiation impacts of EDCs can reveal mechanisms for their contribution to disease etiologies. For example, the EDCs that predispose multipotent cells to differentiate into adipocytes potentially contribute to obesity (Hao et al., 2012; Kirchner et al., 2010). In addition, EDCs like phthalates and bisphenol A stimulate cell proliferation in prostate (Corti et al., 2022) and breast (Williams and Darbre, 2019) cells, providing a plausible mechanism for contribution to cancer progression. Virtually all of the adverse health endpoints implicated by prenatal EDC exposures are at least partly mediated by the placenta, which may be unsurprising given the placenta’s multifunctional role in fetal development. There is therefore a great need to understand the role the placenta plays in mediating EDC effects on maternal and fetal health during pregnancy.
Placental function, structure, development, and cell types
The placenta is a transient and multifaceted organ specially adapted to carry out multiple life-supporting and regulatory functions for the fetus throughout pregnancy. Perhaps the most fundamental functions of the placenta are to facilitate fetal oxygen, nutrient, and waste exchange at the maternal-fetal interface, which consists of a selective interhaemal barrier separating maternal and fetal circulations (Caruso et al., 2012). The placenta is also responsible for regulating many physiological processes of pregnancy through its roles in hormone synthesis, immune protection, and xenobiotic metabolism, all necessary for normal fetal development during pregnancy. Additionally, placental growth, development, and function are highly dependent on maternal and fetal hormone signaling and crosstalk (Murphy et al., 2006).
Originating from the earliest stages of pregnancy, the fetal components of the placenta, including the placental disk, umbilical cord, and amniotic and chorionic membranes, derive from the blastocyst, and therefore share genetic makeup with the fetus (Caruso et al., 2012). Initial placental development, known as placentation, begins shortly after fertilization as the blastocyst undergoes implantation into the endometrial layer of the uterine wall (Kim and Kim, 2017). The placental disk originates from two tissues: the stromal extraembryonic mesoderm and the epithelial trophectoderm. Importantly, the extraembryonic mesoderm differentiates into fibroblasts, placental blood vessels and immune cells, including Hofbauer cells, the resident macrophages of the placenta (Burton and Fowden, 2015). Hofbauer cells are one of several notable placental cell types that confer unique structure and function to the organ. In addition to serving immune cell functions, Hofbauer cells promote critical angiogenesis throughout the placenta across the entire pregnancy (Loegl et al., 2016; Reyes and Golos, 2018; Seval et al., 2007). The maternal component of the placenta, the decidua, is derived from the endometrial lining of the uterus. Thus, together the placenta and associated membranes represent an example of parabiosis with cellular components derived from the fetal and maternal compartments.
As the placenta grows throughout pregnancy, finger-like projections called chorionic villous trees with extensive placental vasculature extend from the chorion of the placental disk into the interstitial space that houses the fetal-maternal interface (Figure 1). The trophectoderm, which makes up the wall of the blastocyst at the implantation face, differentiates into trophoblast. The trophoblast layer gives rise to cell types which constitute the epithelial barrier and covering of the villous trees (Burton and Fowden, 2015). Multiple cell types of trophoblast lineage perform essential placenta functions unique to the maternal-fetal interface and are often implicated in placental dysfunction. The innermost trophoblast layer covering villous trees consists of proliferative and undifferentiated cytotrophoblasts. These cells fuse to form and replenish the syncytiotrophoblast layer, a multinucleated, semi-continuous syncytium covering the surface of villi and some parts of the basal and fetal plates (Castellucci and Kaufmann, 2006; Midgley et al., 1963). The syncytium makes up the interhaemal membrane separating maternal and fetal circulations where gas and nutrient exchange takes place. Cytotrophoblasts also differentiate into extravillous trophoblasts that invade from anchoring chorionic villi that connect to the maternal component of the placenta, termed the decidua, which derives from the uterine endometrium. Extravillous trophoblasts, in conjunction with maternal immune cells, facilitate critical remodeling of the maternal spiral arteries necessary to optimize maternal blood flow to the placenta (Burton and Fowden, 2015; Maltepe and Fisher, 2015). The extravillous trophoblasts also form the outermost cell columns at the tips of chorionic villi that anchor the villi to the maternal basal plate and stabilize them in the intervillous space (Pijnenborg et al., 1981). Given the complexity of the placental tissue, to gain insight into placental function and/or dysfunction from EDC exposure, effects on placenta-specific cell types should be prioritized for interrogation.
Figure 1. Human term placental tissue cellular heterogeneity.
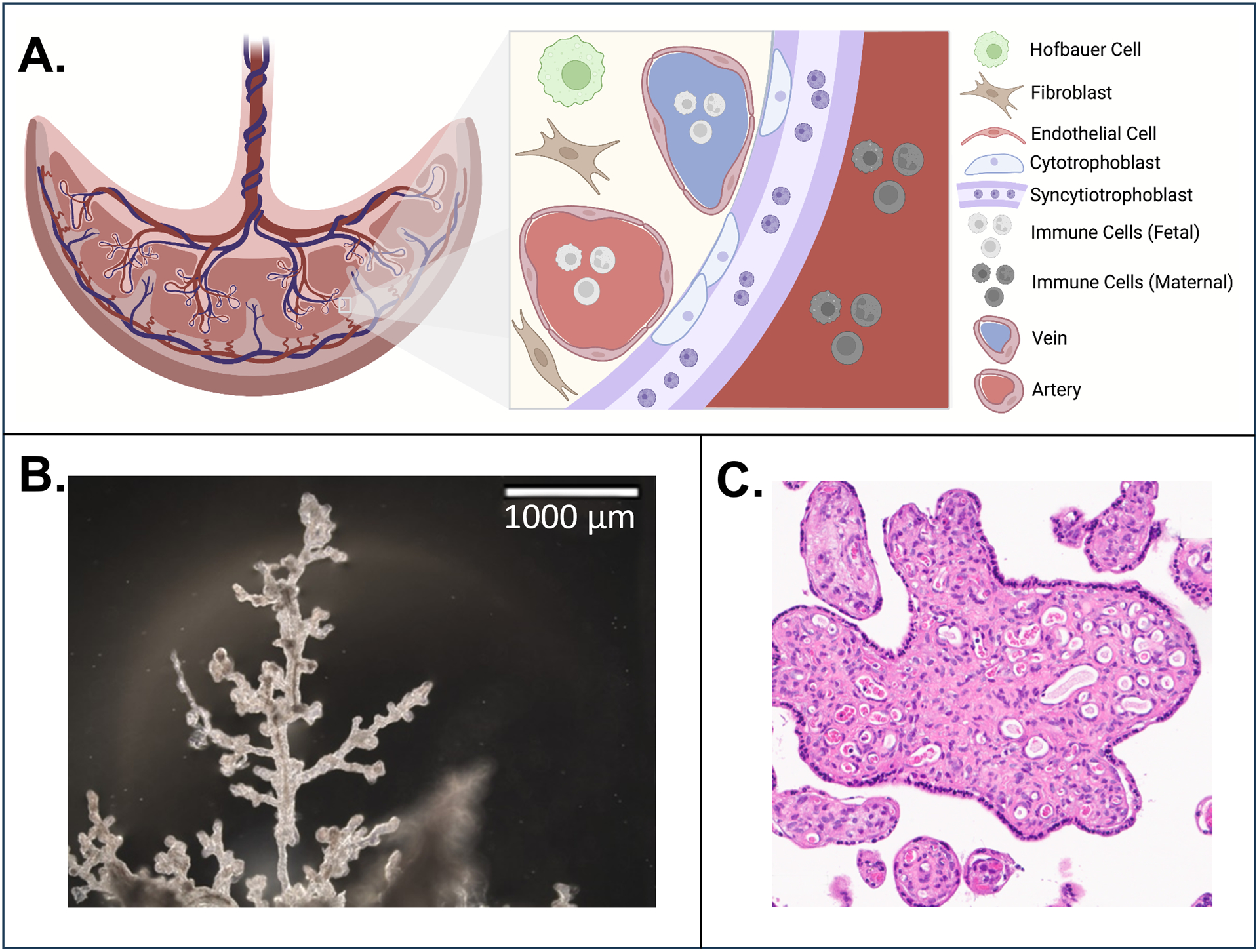
A. Major cell types of the placenta include the resident macrophage Hofbauer cells (green), mesenchymal cells including fibroblasts (brown), and endothelial cells that line the fetal vasculature (pink). The outermost layer is the multinucleated semi-continuous syncytiotrophoblast (purple), which is formed by the syncytialization of cytotrophoblasts (light blue). The syncytiotrophoblast is the direct barrier between the placental villous tissue and the intervillous space perfused with maternal blood (dark red). Created with BioRender.com. B. Human term placenta villous tree excised from human placenta in Harris Lab. C. Cross-section of human term villous tree isolated by Harris Lab and visualized using hematoxylin and eosin stain.
The placental microenvironment is an important factor in determining its structure and cell type composition. For example, placental oxygen levels change throughout pregnancy with severe hypoxic conditions prevailing during the first trimester. The first trimester is a period when implantation and cytotrophoblast invasion occurs and uterine spiral artery remodeling begins. Oxygen tension influences the destiny and function of several of the placental cell types during early pregnancy (Zhao et al., 2021). High oxygen tension in the spiral artery promotes endovascular trophoblast invasion (Sato, 2020). The ability of the placenta to cope with environmental challenges highlights its plasticity to safeguard a healthy pregnancy. For instance, hypoxia-inducible factor (HIF) (Fryer and Simon, 2006) is induced to allow for adaptation to low oxygen tension. Maternal blood pressure is another potential mediator of placental cell type composition. For example, elevated blood pressure has been associated with a reduced mesenchymal stromal cells-to-syncytiotrophoblast ratio (Broséus et al., 2022). Recent histopathologic findings indicate SARS-CoV-2 infection leads to significant placental hypoperfusion and inflammation (Di Girolamo et al., 2021). A systematic review found that 12% of pregnant women with SARS-CoV-2 infection had the virus present in syncytiotrophoblasts (Ashary et al., 2020). The consequence of this and other inflammatory disorders in modulating cell type composition is an avenue for future research.
Placenta as an endocrine organ and a target organ for EDC toxicity
One of the key roles of the placenta is its endocrine function, including the ability to synthesize and secrete hormones that are central for establishment and optimal maintenance of pregnancy, fetal development, and parturition (Costa, 2016). Early placental defects underlie several disorders of pregnancy such as miscarriage, fetal growth restriction, and pre-eclampsia (Dimitriadis et al., 2023). The placenta’s secretome comprises of a plethora of hormones, steroids (progesterone, estrogen), proteins (human chorionic gonadotropin [hCG], human placental lactogen [HPL], placental growth hormone [HPGH], leptin, adiponectin, inhibin, activin, placental growth factor [PIGF]), and cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1). The roles placental hormones play during pregnancy are myriad and beyond the scope of this review. However, some of their most important functions include regulation of growth and differentiation of trophoblasts, support of fetal growth, protection of the fetus from infection, modulation of maternal adaptations, and preparation of the uterus and mother for parturition (Mesiano, 2009).
The syncytiotrophoblast layer is the predominant site of the production of progesterone, a key player in pregnancy maintenance. The functions of progesterone include placentation, immune tolerance, inhibition of myometrium contractility, and preparing the mammary gland for lactation. During early pregnancy, the corpus luteum is the site of production of progesterone, under the influence of placental hCG (Garner and Armstrong, 1977). hCG also plays a role in placental angiogenesis, trophoblast invasion, myometrial quiescence, and immunomodulation (Nwabuobi et al., 2017). Other placental hormones such as HPL regulate maternal lipid and carbohydrate metabolism (Costa, 2016), while HPGH takes on growth hormone functions (Caufriez et al., 1993), namely, metabolic regulation, induction of insulin resistance, as well as promotion of gluconeogenesis and nutrient availability for the growing fetus (Alsat et al., 1998). Estrogens are the other major class of steroids produced by the placenta and promote embryo implantation, angiogenesis, vasodilation, and syncytialization (Berkane et al., 2017). The endocrine function of the placenta extends to the support of maternal immunologic (Daniel et al., 1987), nutritional, metabolic (Parrettini et al., 2020; Stern et al., 2021), and cardiovascular (Melzer et al., 2010) adaptations. In addition to its endocrine function, the placenta also synthesizes several chemicals that influence inflammation, oxidative stress response, angiogenesis, and innate defense mechanisms. MicroRNAs (Jin et al., 2022) and extracellular vesicles (Jin and Menon, 2018) that regulate post-transcriptional gene expression and facilitate intracellular communication between mother and placenta have the potential to serve as biomarkers of disease, and are also part of the placental secretome.
The placenta is a plausible target organ for EDC toxicity because of multiple structural and functional characteristics. The placental structure consists of a large surface area and a thin interface separating maternal and fetal circulation, which provides an ideal environment for maternal transfer of compounds (Burton and Jauniaux, 2015). Because the placenta is highly perfused at rates up to 700 ml/min by the third trimester (Wang and Zhao, 2010), systemically available toxicants are readily delivered to the placenta. Additionally, multiple different types of EDCs can cross the placenta, as previously reviewed (Yang et al., 2019). Beyond its role in material transfer, the placenta plays a role in biotransformation and chemical metabolism. The placenta is capable of metabolizing toxicants, such as EDCs, into reactive metabolites because it expresses an abundance of metabolizing enzymes such as cytochrome P450s, glutathione-s-transferases, lipases and other enzymes, thereby increasing the risk of tissue-generated metabolites (Myllynen et al., 2005). Due to the unique characteristics of the placenta, EDCs have the potential to disrupt critical signaling and cause significant placental injury, as previously reviewed (Gingrich et al., 2020; Yang et al., 2019).
Use of bulk placental tissue in environmental exposure gene expression studies
Human epidemiology studies frequently sample biopsies of placental villous tissue for measurement of molecular biomarkers of exposure or adverse pregnancy outcomes. Gene expression is frequently selected as a molecular biomarker because it is highly responsive to chemical exposure and represents a potential mechanistic link between exposure and function. In particular, bulk placental tissue gene expression has been investigated with a multitude of environmental exposures using candidate gene quantitative PCR, targeted or genome-wide microarray, and genome-wide RNA-sequencing technologies in animal models and humans (Rosenfeld, 2021). Recently, there have been several human population studies evaluating mRNA, lncRNA, or microRNA expression with RNA-sequencing in bulk placental tissue with the majority focusing on prenatal exposure to metals (Table 1) (Lapehn and Paquette, 2022). Importantly, these prior RNA-sequencing and exposure studies did not estimate cell composition or include cell composition in their analytic framework, which represents a future opportunity. RNA-sequencing analyses of prenatal chemical exposures, including EDCs, are an understudied category of environmental exposure with only three papers evaluating prenatal chemical exposures (phthalates, polycyclic aromatic hydrocarbons, and organophosphate pesticides) with the placental transcriptome at birth in a human population (Li et al., 2023; Paquette et al., 2023, 2021). Therefore, there is still a substantial breadth of knowledge to be gained through new studies of the full placental transcriptome and its associations with environmental exposures.
Table 1.
Summary of human population molecular epidemiology studies on environmental exposures and placental tissue RNA-sequencing at birth from 2017–2023.
| Exposure | Timing of exposure | Exposure matrix | RNA-sequencing type | Cohort | Largest N* | Placental Sampling | Birth Outcomes Assessed | Main RNA-seq and Birth Outcome Findings | Reference | Data Availability |
|---|---|---|---|---|---|---|---|---|---|---|
| Selenium, Cadmium | 2.8 months postpartum | Toenails | mRNA | RICHS | 173 | Fetal side near cord insertion | Fetal Growth |
|
(Everson et al., 2017) | No |
| Cadmium | Birth | Placenta | mRNA | RICHS | 200 | Fetal side adjacent to cord insertion | Birth Weight |
|
(Everson et al., 2018) | No |
| Arsenic | 2nd trimester | Urine | mRNA | NHBCS | 46 | Fetal side at base of the umbilical cord | Birth Weight |
|
(Winterbottom et al., 2019) | No |
| Cadmium | Birth | Placenta | lncRNA | RICHS | 199 | Fetal Side <2cm from cord | Birth Weight |
|
(Hussey et al., 2020) | dbGaP phs001586.v1.p1 |
| PM2.5 | 12 weeks preconception through birth | Residential Address at Birth | mRNA | RICHS | 471 | Fetal Side <2cm from cord | Fetal Growth |
|
(Deyssenroth et al., 2021) | By request |
| Phthalates | 2nd and 3rd Trimester | Maternal Urine | mRNA & lncRNA | CANDLE | 760 | Fetal Villous Tissue | - |
|
(Paquette et al., 2021) | No |
| Selenium | Birth | Placenta | miRNA | NHBCS, RICHS | 393 | Fetal Side | - |
|
(F.-Y. Tian et al., 2022) | dbGaP phs001586 |
| Cadmium | Birth | Placenta | miRNA | NHBCS, RICHS | 396 | 2cm from cord insertion | NNNS Score |
|
(Tehrani et al., 2022) | doi.org/10.15139/S3/KHXJ2G |
| Polycyclic Aromatic Hydrocarbons | 2nd Trimester | Maternal Urine | mRNA & lncRNA | CANDLE | 629 | Fetal Villous Tissue | - |
|
(Paquette et al., 2023) | By request |
| Organophosphate Pesticides | 1st, 2nd, and 3rd trimester | Maternal Urine | mRNA | SAWASDEE | 254 | Umbilical side, 2cm from cord insertion | - |
|
(Li et al., 2023) | By request |
Sample size may have varied throughout the papers based on the specific analysis presented (exposure-gene expression relationship, or gene expression-outcome relationship). This table presents the single largest N used in one of these analyses.
Environmental toxicology studies, similar to epidemiology studies, have also used bulk placental tissue to evaluate gene expression following in vitro EDC treatment. Placental villous explant tissue obtained from human pregnancies is a common model because it allows for measurement of toxicological endpoints in intact human tissue and has the advantage of retaining some aspects of the placental microenvironment such as cell-to-cell interactions. Use of this model generally consists of excising out villous tissue from the ex vivo placenta in small chunks, floating tissues in appropriate tissue culture media, exposing explants to toxicant(s) of interest, and flash-freezing tissues or isolating mRNA for future analysis. Multiple studies have used this model to evaluate toxicant-induced changes in gene expression for several compounds including the trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine (DCVC) measured with RNA-sequencing (Elkin et al., 2021), candidate gene PCR studies of Bisphenol A (Sieppi et al., 2016; Zou et al., 2022) and the cholesterol-lowering drug Pravastatin (Brownfoot et al., 2015), among others. Animal models have also been used extensively to study effects of toxicant exposure on placenta tissue in vivo, though most animal placentas differ in structure and/or function relative to human placentas. In these models, pregnant animals are exposed to toxicants through various methods, animals are euthanized, and bulk placental tissues are dissected out and processed for further analysis. For example, animal models have been used to assess toxicant-induced changes in gene expression for different EDCs, including phthalates (Xu et al., 2021), bisphenols A and S (Mao et al., 2020), trichlorethylene (Elkin et al., 2021), PCBs (Laufer et al., 2022) and dexamethasone (Lee et al., 2017). Similar to epidemiological studies, prior toxicology studies did not take into account differences in placental cell composition. Therefore, it is unknown which cells within the bulk placental tissues analyzed contributed to gene expression changes reported in each study. Because many of these studies have their raw data stored in data repositories such as the National Institutes of Health’s Gene Expression Omnibus, there is a {Updating}future opportunity to re-analyze these data using innovative computational methods that account for cell composition, as described in subsequent sections.
To date, bulk placental tissue makes up the vast majority of RNA-sequencing data (Table 1). Although gene expression insights gained from bulk placental tissue RNA-sequencing data are useful, their interpretation is somewhat limited because bulk analysis can mask the effects of a chemical on individual populations of placental cells which could be identified through RNA-sequencing of sorted cell types or single cells (Zhang et al., 2019). Because the placenta undergoes such rapid growth, development, and ultimately, aging over the course of a pregnancy, cell type composition of placental tissue can vary greatly depending on when sample collection occurs (Lim et al., 2017; Sitras et al., 2012; Suryawanshi et al., 2022). Another challenge of interpreting data from bulk placental tissue is the extreme heterogeneity of tissue resulting in vastly different gene expression patterns which can fluctuate solely based on the location within the tissue of sample collection (Coorens et al., 2021). Moreover, placental tissue presents a unique set of challenges for data interpretation because the organ is embedded into the maternal decidua to varying degrees throughout pregnancy, which results in the unavoidable co-mingling of placental cells and maternal cells when placental tissue is procured for experimental purposes (Heazlewood et al., 2014; Lamb et al., 2012; Sardesai et al., 2017). Due to the unique conditions of placental tissue in vivo, interpreting gene expression data from bulk placental tissue samples is particularly challenging. Use of the emerging single-cell RNA-sequencing technologies when evaluating placental tissue gene expression changes is a way to avoid some of the pitfalls of interpreting bulk placenta RNA-sequencing data.
Single-cell molecular analyses and the placenta
Single-cell RNA-sequencing is a relatively new technology that measures genome-wide gene expression in a series of individual cells, as opposed to sequencing all transcripts collected from bulk tissue homogenate. Single-cell RNA-sequencing improves on bulk RNA-sequencing by capturing cell specific and cell state specific gene expression, allowing for the detection of differences in cell composition or differential expression within cell types, which improve the potential biologic relevance of inferences of results. However, single cell technologies typically require increased cost and expertise and limit throughput and sequencing depth relative to bulk tissue RNA-sequencing (Hedlund and Deng, 2018). Single-cell RNA-sequencing data analysis typically begins by annotating individual cells using defining genes or similarity indices with established cell type-specific samples. Once cells are annotated to cell types, investigators can perform cell type specific differential expression analyses or even compare the cell type composition of their samples (Luecken and Theis, 2019). Furthermore, single-cell RNA-sequencing measures are not affected by cellular heterogeneity differences that confound bulk tissue analyses (Campbell et al., 2020). The increasing adoption of single-cell RNA-sequencing across tissues has led to the discovery of new cell types and subtypes with distinct gene expression profiles from previously discovered cell types. Most single-cell RNA-sequencing approaches require dissociation of the tissue to a single cell suspension. Consequently, cell composition and novel cell type results must be interpreted with caution as tissue dissociation bias, the differential resilience of cell types to the tissue dissociation process, may distort the appearance and abundance of cell types (Hedlund and Deng, 2018). This is especially true in the human placenta since large, multi-nucleated syncytiotrophoblasts may be less likely to remain intact through processing and are generally incompatible with microfluidic-based whole cell single-cell RNA-sequencing platforms. Single nucleus RNA-sequencing, which relies on only capturing more stable nuclei instead of whole cells, has emerged as an alternative to potentially address dissociation bias as well as capture large cells not amenable to single-cell preparation protocols (Kim et al., 2023). Though single-cell RNA-sequencing is more susceptible to dissociation bias, the technology provides key insight into cell states and distributions and overcomes critical limitations of bulk analyses.
We identified 15 studies that performed single-cell RNA-sequencing on human placental tissue (as of December 2022) (Table 2), including 9 studies involving placentas collected at the end of gestation (Campbell et al., 2023; Chen et al., 2022; Pavličev et al., 2017; Pique-Regi et al., 2019; Tsang et al., 2017; Wang et al., 2022; Yang et al., 2021; Zhang et al., 2021; Zhou et al., 2022) and 6 studies involving placentas collected earlier in pregnancy (Li et al., 2022; Liu et al., 2018; Ray et al., 2022; Sun et al., 2019; Suryawanshi et al., 2018; Vento-Tormo et al., 2018). All studies were conducted in humans, except one study which used mice (Tosevska et al., 2022). While the majority of early pregnancy placental samples were from aborted fetal tissue, Sun et al collected first trimester samples using chorionic villi sampling, which provides an opportunity to follow pregnancies to the end of gestation (Sun et al., 2019). Syncytiotrophoblasts are multinucleated cells which would be anticipated to cause inherent challenges for single-cell RNA-sequencing. Only one study attempted to directly address this issue by isolating syncytiotrophoblasts using laser microdissection followed by conventional bulk RNA-sequencing (Pavličev et al., 2017). This challenge may explain why syncytiotrophoblasts are not well represented as a major portion of cell types in clustering-based analyses of these cell types, despite their prominent role in placental physiology and function. As an alternative, single-nucleus RNA-sequencing has been successfully employed to capture transcriptomes generated from an in vitro syncytiotrophoblast model (Khan et al., 2021). Another key consideration for all placental studies is the reporting of key clinical characteristics and covariates of interest, and only a few studies reported this type of data for important factors such as maternal BMI, maternal age, and/or race/ethnicity of participants (Chen et al., 2022; Pique-Regi et al., 2019; Yang et al., 2021). Four studies also collected and generated single-cell RNA-sequencing data of the maternal decidua (Pique-Regi et al., 2019; Sun et al., 2019; Suryawanshi et al., 2018; Vento-Tormo et al., 2018), which provided opportunities to explore interactions between the maternal-fetal interface using receptor-ligand networks. While earlier single-cell RNA-sequencing studies characterized typically developing placental and decidual cell types and their interactions, more recent single-cell RNA-sequencing studies have begun to apply this approach to pregnancy complications, including preeclampsia (Campbell et al., 2023; Tsang et al., 2017; Zhou et al., 2022), preterm birth (Pique-Regi et al., 2019) and gestational diabetes (Yang et al., 2021). One study applied single-cell RNA-sequencing to investigate functional changes to the placenta related to severe COVID-19 infection ultimately resulting in fetal demise (Chen et al., 2022). Meta-analysis or other integrative analyses in single-cell RNA-sequencing studies of the placenta are currently limited. However, one study performed an integrated analysis of newly collected uncomplicated placentas alongside 2 previously published studies (Pique-Regi et al., 2019; Tsang et al., 2017) to develop a deconvolution reference for bulk placental gene expression measures (Campbell et al., 2023). However, no human placenta studies to date have utilized single-cell RNA-sequencing data in the context of environmental exposures analysis.
Table 2.
Existing single cell RNA-sequencing studies in the human placenta
| Reference | Population Information | Gestational Time Point | Number of Samples/Cells | Single cell approach and Sequencing Platform | Main Findings | Data Availability |
|---|---|---|---|---|---|---|
| (Pavličev et al., 2017) | Non-pathological pregnancies delivered via c-section in the absence of labor | Samples collected at the end of term gestation 39.14–39.28 weeks | 2 Placentas/87 Cells | Used smart-seq2 approach followed by Illumina HiSeq2500 |
|
GSE87726 |
| (Tsang et al., 2017) | Placentas were collected from 2 in patients not in active labor that were delivered via C-section at the Prince of Wales Hospital in Hong Kong Primary Outcome: Early onset preeclampsia (PE) |
Samples collected at the end of preterm/term gestation Gestational Age (PE) 28.14 weeks-32.56 weeks Gestational Age (controls) 38–38.28 weeks |
8 Placentas (4 normal/4 PE), 2 normal placentas were additionally sampled peripherally/20,518 cells | 10x genomics library preparation followed by sequencing via Illumina miseq and nextseq |
|
N/A |
| (Liu et al., 2018) | Placentas gathered in early pregnancy from participants in 306th Hospital of PLA | First and Second Trimester 8 weeks and 24 weeks | 8 Placentas /1471 cells | used smart-seq2 approach followed by Illumina Hiseq 4000 |
|
GSE89497 |
| (Suryawanshi et al., 2018) | Placentas gathered from elective termination samples. Only male samples used. | First Trimester 6–8 weeks | 8 Placentas/14,341 cells Also collected 6 decidual samples/1542 cells |
DropSeq or 10X processing followed by sequencing using Illumina NextSeq 500 platform |
|
N/A |
| (Vento-Tormo et al., 2018) | Placental and decidual samples were collected from the Human Developmental Biology Resource from the UK tissue authority | First Trimester 6–14 weeks | 5 placentas/70,000 cells | Single-cell libraries prepared using 10x genomics chromium system as well as plate-based smart-seq2 followed by sequencing using Illumina HiSeq 2000 |
|
E-MTAB-6701 ; E-MTAB-6678 ; E-MTAB-7304 |
| (Sun et al., 2019) | Placental and decidual samples were collected from chorionic villi sampling of patients recruited into the Cedars-Sinai prenatal biorepository. All patients went on to deliver healthy term live births. Primary Outcome: Fetal Sex |
First trimester samples (10–13 weeks) | 10 Placentas (5 female/5 male) /7,245 cells | Single cell libraries prepared using 10x genomics kit followed by NovaSeq 6000 |
|
N/A |
| (Pique-Regi et al., 2019) | Placental and decidual samples were collected from women from Detroit Medical Center Primary outcome: Preterm and term delivery |
Samples collected at end of preterm/term gestation 6 term samples (3 in labor, 3 not in labor-Age 38–40 weeks) 3 PTL (33–35 weeks) samples |
9 samples/79,906 cells | Single-cell libraries prepared using 10x genomics chromium system followed by sequencing using Illumina HiSeq X Ten System |
|
phs001886 |
| (Yang et al., 2021) | Samples were collected from singleton full term placentas after cesarean section without any other pregnancy complications Outcome of interest: gestational diabetes mellitus |
Samples collected at the end of term gestation (38.14–40.56 weeks) | 4 Placentas (2 gestational diabetes mellitus cases/2 control)/27,220 cells | Single-cell libraries prepared using 10x genomics chromium system followed by sequencing using illumina novaseq 6000 |
|
GSE173193 |
| (Zhang et al., 2021) | Samples collected from singleton pregnancies delivered via cesarean section without other pregnancy complications at Weifang Traditional Chinese Hospital in Shandong, China Outcome of interest: PE |
Samples collected at the end of preterm/term gestation (34.7–38.7 weeks) | 6 Placentas (3 PE/3 controls)/11,518 cells | Single cell libraries prepared using Singleron GEXSCOPE system followed by sequencing via Illumina Hiseq X |
|
Data available upon request |
| (Khan et al., 2021) | Human ESCs (H1; WA01) originated from WiCell Research Institute. Cells exposed to bone morphogenetic protein 4 (BMP4) in presence of inhibitors of ACTIVIN/TGFB; A83–01 and FGF2; PD173074 (BAP) Primary Outcome; Different cells with trophoblast lineage. In low (5%) and normal (20%) oxygen conditions |
Cell from early pregnancy | 2 replicates for each treatment group/5000 nuclei | Single cell libraries prepared using 10x Genomics Chromium Next Gel Bead-in-Emulsion (GEMs) Single Cell 3′ Kit v3.1 followed by sequencing via Illumina NovaSeq S1 flow cell |
|
GSE171768 |
| (Zhou et al., 2022) | Placentas were collected from patients delivering via cesarean section at Changzhou Maternal and Child Health Care Hospital Primary outcome: PE |
Samples collected at the end of preterm/term gestation (gestational age 36.14–38.43 weeks) | 4 Placentas (2 preeclampsia cases/2 controls)/29,006 cells | Single-cell libraries prepared using 10x genomics system |
|
GSE173193 |
| (Chen et al., 2022) | Samples were collected from a patient who experienced incomplete uterine rupture and fetal demise at 28.56 weeks and 2 preterm controls. Exposure: Severe COVID-19 infection in mid-pregnancy (20–24 weeks) |
Samples collected at the end of preterm gestation (28.56–30 weeks) | 3 Placentas (1 COVID infected vs. 2 Preterm)/17,481 cells | Single-cell libraries prepared using 10x genomics chromium system followed by sequencing using illumina HiSeq X |
|
Data available upon request |
| (Ray et al., 2022) | Samples were collected from Mount Sinai hospital in Toronto, CA | First Trimester Samples (6–8 weeks) | 2 Placentas/cell number not reported | Single-cell libraries prepared using 10x genomics chromium system |
|
GSE145036 |
| (Tosevska et al., 2022) | Samples were collected from C57BL/6J mice following intranasal exposure to PM2.5 | Placentas collected Late stage pregnancy (E19) following laparotomy and hysterectomy | N=6 samples (3 exposed, 3 controls)/40,739 cells | Single-cell libraries prepared using 10x genomics chromium system |
|
GSE178233) |
| (Li et al., 2022) | Samples collected from elective terminations at Shanghai First Maternity and Infant Hospital | First Trimester Samples (6–16 weeks) | N=11 Placentas/52,179Cells | Single-cell libraries prepared using 10x genomics chromium system followed by Illumina NovaSeq 6000 |
|
Data available upon request |
| (Wang et al., 2022) | Samples collected at the end of gestation from Shenzhen Second People’s Hospital | Samples collected at the end of gestation (28–40 weeks) | N=3 placentas/13747 cells | Single-cell libraries prepared using 10x genomics chromium system followed by sequencing on MGI-seq platform |
|
Data Availability CNSA: ID # CNP0000878 |
| (Campbell et al., 2023) | Samples collected from singleton pregnancies delivered via cesarean section without complications at Von Voigtlander Women’s Hospital in Ann Arbor, MI | Samples collected at the end of term gestation | N=2 placentas (1 male, 1 female), each with a technical replicate/9,244 cells Analyzed in conjunction with N=3 term, non-laboring placentas from Pique-Regi et al, 2021 and N=4 control placentas from Tsang et al, 2017/31,250 cells |
Single-cell libraries prepared using 10x genomics chromium system followed by Illumina NovaSeq 6000 |
|
GSE182381 |
Future opportunities and recommendations in single-cell placental toxicology
The generation of bulk transcriptomics data in well powered cohorts with rich phenotypic data provides an unprecedented opportunity to study environmental exposures, but the ability to gain mechanistic insight into toxicity is limited due to a lack of cell-specific information. Generating single-cell data in a cohort setting may not be feasible due to cost, timeline constraints, and logistics of sample collection, but cellular deconvolution tools can provide an opportunity to reveal the cellular proportions of bulk sequencing data (Figure 2). Deconvolution refers to the bioinformatic process of estimating the distribution of cell types that constitute bulk tissue. Campbell et al recently developed a human placental deconvolution tissue reference panel based on cell type-specific gene expression profiles generated from new and existing single-cell RNA-sequencing data (Campbell et al., 2023). This panel was developed for use with the bioinformatic deconvolution tool CibersortX (Newman et al., 2019) but may be applied in other deconvolution algorithms and includes gene expression signatures for 8 placental cell types, 11 other fetal cell types, and 8 co-mingled maternal peripheral immune cell types. The rapid development of publicly available, cell type-specific sequencing and microarray data and conveniently packaged deconvolution algorithm software have made deconvolution increasingly available to the research community. Deconvolution references are now increasingly available across species such as mice (Marsh and Blelloch, 2020; Nelson et al., 2016), and other tissues and biological molecules, including brain, umbilical cord blood, and DNA methylation (Campbell et al., 2023). The use of deconvoluted cell composition estimates in placental research is evolving but has largely focused on modeling cell composition proportions as a primary outcome or as a covariate in genome-wide association studies of gene expression. For example, the Campbell placental RNA reference panel applied to a previously published case control microarray study of preeclampsia revealed that the proportion of extravillous trophoblasts was overrepresented among preeclampsia cases compared to controls, a cell type whose dysfunction has previously been implicated in preeclampsia. Further, a differential gene expression analysis of the same dataset suggested that upregulation of preeclampsia relevant genes FLT1, ENG, and LEP among preeclampsia cases was partially mediated by differences in cell composition (Campbell et al., 2023). Another tool developed using single cell RNA-sequencing data is “PlacentalCellEnrich”, which allows the user to characterize if a list of genes is enriched for genes with placental cell specific expression patterns (Jain and Tuteja, 2021). These approaches may be applied to bulk placenta tissue studies to test whether and how EDCs affect placental cell composition and gene expression.
Figure 2. Recommendations for placental RNA-sequencing implementation based on sample size and research question.
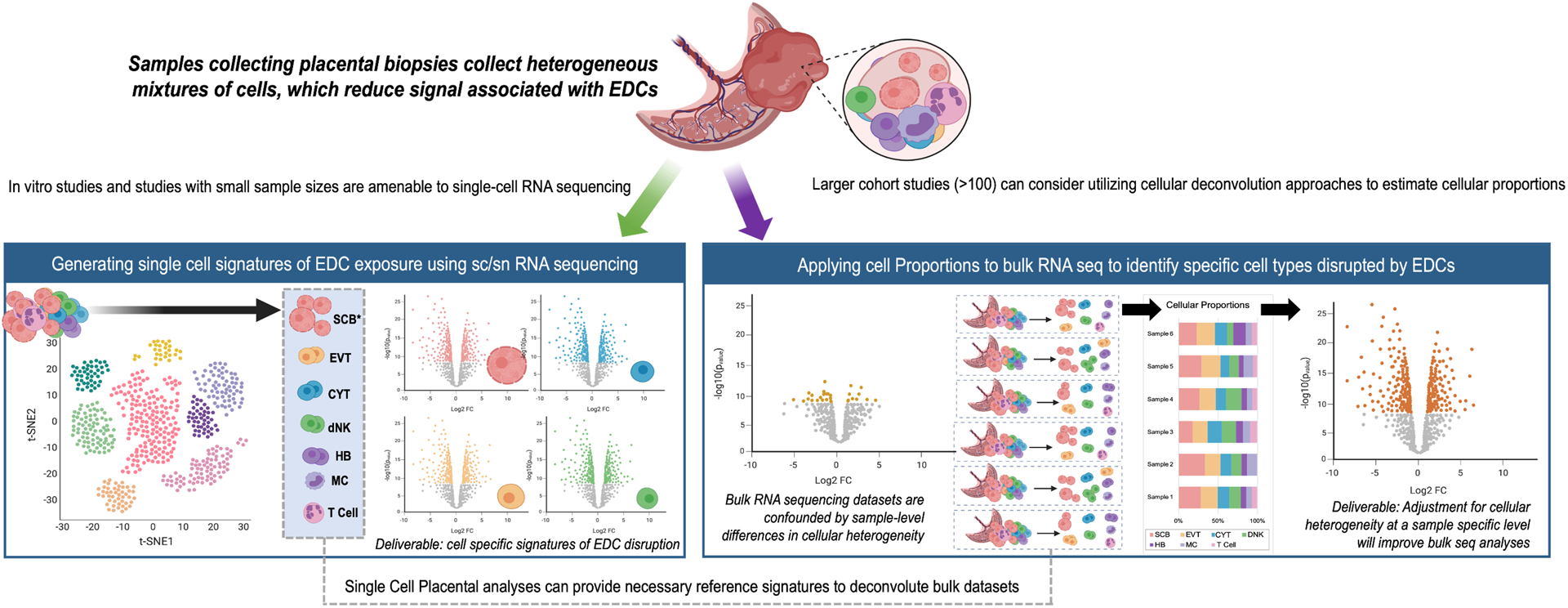
Single-cell RNA-sequencing data is most amenable to in vitro studies and studies with small sample sizes, and can produce cell specific signatures of EDC disruption. Large cohort studies may find it more challenging to deploy single-cell sequencing, but can leverage single cell analyses to adjust for cellular heterogeneity and examine cell proportions within their data. *Syncytiotrophoblasts are multi-nucleated and may be challenging to capture using standardized single-cell approaches. EDC=Endocrine Disrupting Chemicals, SC=Single Cell, SN=Single Nuclear, SCB=Syncytiotrophoblast, EVT=Extra villous trophoblast, CYT=Cytotrophoblast, dNK=decidual natural killer cell, HB=hofbauer cell, MC=macrophage”
Adopting widespread use of single-cell RNA-sequencing technologies to assess cell-specific differential gene expression in the placenta induced by toxicant exposures offers important advantages over current methods using bulk tissue or specific cell types. Similar approaches have been successfully deployed in lead (Pb) toxicology of the hippocampus brain region in mice (Bakulski et al., 2020), and has recently been applied in a mouse air pollution exposure study of the placenta (Tosevska et al., 2022). Evaluating gene expression changes at single cell resolution from tissues containing a full breadth of placental cell types will allow researchers to assess cell type-specific gene expression changes. This will lead to in-depth analysis of cell-by-cell transcriptional responses to a toxicant. Researchers will be able to pinpoint responses and/or impairment of specific cell types that likely play a role in placental pathogenesis. Single-cell approaches can even identify novel cell types and states. Moreover, changes in relative cell type abundance resulting from toxicant exposure can be determined using single-cell RNA-sequencing.
Single-cell approaches can be integrated with a chemical risk assessment framework for translational impact. One such framework is the Adverse Outcome Pathway (AOP) approach, used by researchers in regulatory and academic institutions. AOPs organize biological effects of toxicant exposure into a logical sequence of events of increasing scale of biological complexity from molecular, to cellular, and finally to organism or even population level effects (Ankley et al., 2010; Spinu et al., 2020; Vinken, 2013). Each step in an AOP is linked by Key Events that ultimately link early molecular events (e.g. toxicant binding to hormone receptor) to adverse health outcomes (e.g. fetal growth restriction). Single cell RNA-sequencing allows researchers to identify molecular (transcriptomic) responses at single cell resolution within the complex microenvironment of the placenta. Single-cell transcriptomics methods present a powerful method for identifying Key Events in specific placental cells, allowing researchers to build new AOPs that demonstrate how EDC exposure leads to adverse pregnancy outcomes. Thus, these methods have the potential to significantly improve our ability to assess the risks posed by exposure to EDCs during pregnancy.
An additional future opportunity of single cell approaches is to model interactions between cells. Cell-cell interactions can be modeled through the use of receptor-ligand networks, which is a mathematical model detailing the relationship between different cells within a tissue, which is reflected in the ligands and their expressed receptors through both single cell and bulk RNA-sequencing data (Armingol et al., 2021). A network is a mathematical collection of objects composed of “nodes” connected by “edges”. Here, “nodes” represent cytokine ligands or receptors from specific cell types, and “edges” quantitatively encode the relationships between nodes. Since different placental cells release protein ligands, ligands bind to cell surface receptors, and receptors pass information to cells, these edges can be considered ‘directed’. Computational methods have been developed to analyze directed networks, estimating global properties of the network as indicators of the ‘immune state’. Receptor-ligand interactions have been curated in various databases, including cellphoneDB (Efremova et al., 2020), iCellNet (Noël et al., 2021), and the Human Connectome DB (Hou et al., 2020). These databases can act as a network scaffold for individual cell type networks, which can be refined through expression thresholding, expression product, expression correlation, or differential combination approaches (Armingol et al., 2021). This network architecture provides a framework to overlay tissue-specific expression levels and correlations to determine the immune cell cross talk in the decidua and placenta.
One exciting potential application of single-cell RNA-sequencing in placental toxicology is to use it to understand how EDCs disrupt the unique signaling pathways that occur at the maternal-fetal interface during pregnancy. Cellular communication within and between maternal and fetal cells is essential to the routine functioning of the maternal-fetal interface, and EDCs may disrupt these molecular signals. Placental cells and maternal cells undergo a carefully orchestrated ligand-receptor mediated crosstalk program with the specific goal of regulating some physiological processes unique to pregnancy. An example of maternal-fetal tissue coordination is the selective modulation of the maternal immune system in response to the presence of the semi-allogeneic fetus. Although complex and not fully understood, tissue and cell-specific immunomodulation is regulated by interactions between uterine natural killer cells and regulatory T cells on the maternal side and extravillous trophoblasts and syncytiotrophoblast on the placental side (Napso et al., 2018). Additionally, hormones synthesized and released by the placenta regulate maternal T cells (Morelli et al., 2015). Using single-cell RNA-sequencing to interrogate how EDCs may disrupt maternal-fetal signaling will lead to new mechanistic insights by potentially identifying new cells involved and revealing cell-specific signaling through gene expression.
Spatial transcriptomics is an emerging single-cell technology that does not require tissue dissociation and thus preserves the tissue structure and cellular geographic organization. The technology was highlighted by Nature Biotechnology as the 2021 method of the year (Marx, 2021) and is now available and scalable through several commercially available platforms. Spatial transcriptomics works by integrating existing sequencing based approaches with microscopy based techniques such as fluorescence in situ hybridization (FISH) (Tian et al., 2022). Spatial transcriptomics can provide resolution into the cell-type composition of tissues, the rules and patterns of how individual cell types spatially interact, and also elucidate molecular interactions between tissue components (L. Tian et al., 2022). Given the significance of the maternal-fetal interface in fetal development and placental function, spatial transcriptomics approaches could provide novel insight into the interactions of cells and tissues at this critical interface. Recently, two studies were published detailing spatial transcriptomics applied to chorionic villous placental tissue (Liu et al., 2022) and trophoblast development in early pregnancy (Arutyunyan et al., 2023). Eventually, spatial transcriptomics approaches should be used to provide insight into how EDCs may interfere with processes at the maternal fetal-interface, representing a critical research gap.
Looking ahead, additional recently developed genomics technologies have the potential to yield novel mechanistic insight into how EDCs may disrupt cellular functions in the placenta. Single cell CUT&TAG is a novel experimental approach that can profile single cell DNA accessibility, histone modifications, and transcription factor occupancy at a single cell resolution (Bartosovic et al., 2021), representing a major technological improvement over chromatin immunoprecipitation and sequencing (CHIP-Seq). This may be highly relevant in the field of EDCs, based on their known mechanisms of toxicity involving disruption of nuclear hormone receptor transcription factors which can result in changes to the synthesis and signaling of downstream genes (Hall and Greco, 2019). Single-cell Assay for Transposase-Accessible Chromatin (ATAC)-sequencing is another emerging technology that can be used to understand the regulatory elements that drive cell-type gene expression (Fang et al., 2021). Endocrine disrupting chemicals including phthalates have been shown to disrupt histone acetylation and chromatin accessibility (Kuhl et al., 2007; Zhang et al., 2014), but to our knowledge this has not been profiled on a genome scale or single-cell level. Researchers may also consider other applications of single-cell methodologies as they are developed, such as single cell proteomics, or epigenomics. We anticipate that these emerging technologies may further elucidate the mechanisms of toxicity for EDCs by pinpointing how they disrupt maternal-fetal communication and transcriptomic regulation of gene expression.
Conclusions
Exposures during pregnancy to EDCs can result in adverse pregnancy outcomes and postnatal complications for children. Molecular and cellular changes to the placenta are likely central to these disorders. Given the placenta is constituted of multiple cell types of unique form and function, greater biologic and mechanistic insights can be gained by incorporating measures of cell composition or cell-specific measures (Figure 2). Specifically for large epidemiologic studies using archived bulk placental tissue measures, we recommend that investigators use single cell placental reference panels to estimate cell composition in their samples. With this approach, investigators can test for differences in estimated placental cell composition by exposure or outcome, and they can incorporate measures of cell composition in their tests of differential gene expression. For smaller epidemiologic studies or in vitro toxicologic studies with fresh placental tissue available, we recommend investigators consider a single nuclei approach to assess cell type specific differential gene expression. This allows for the identification of cell type specific adverse outcome pathways. As new technologies emerge and throughput increases, innovative opportunities for placental EDC toxicology will continue to evolve. This manuscript has focused on placental villous tissues, however the placenta coordinates with additional tissues (fetal membrane, decidua). Many of the principles presented are applicable in these tissues, and fully capturing the biology will require considering tissue-tissue interactions. In addition, the concepts of this manuscript are applicable to toxicants beyond EDCs. The field of placental toxicology is in an exciting position to be able to build on prior successes in bulk tissue and in cell culture and now able to extend and consider cell specific responses and interactions in the tissue.
Funding
This work was supported by the National Institutes of Health, National Institute of Environmental Health Sciences (Grant Nos. [P42 ES017198, Harris], [P30 ES017885, Padmanabhan], [R01 ES025531, Bakulski], [R01 ES033785, Paquette] ); National Institute of Diabetes and Digestive Kidney Diseases (Grant No. [T32 DK071212, Padmanabhan]); National Institute on Aging (Grant Nos. [R01 AG055406, Balkulski] and [P30 AG053760, Balkulski]); Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant No. K99/R00 HD096112). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, NIDDK, NIA, NICHD, or NIH.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abrantes-Soares F, Lorigo M, Cairrao E, 2022. Effects of BPA substitutes on the prenatal and cardiovascular systems. Critical Reviews in Toxicology 52, 469–498. 10.1080/10408444.2022.2142514 [DOI] [PubMed] [Google Scholar]
- Almond D, Currie J, 2011. Killing Me Softly: The Fetal Origins Hypothesis. Journal of Economic Perspectives 25, 153–172. 10.1257/jep.25.3.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsat E, Guibourdenche J, Couturier A, Evain-Brion D, 1998. Physiological role of human placental growth hormone. Mol Cell Endocrinol 140, 121–127. 10.1016/s0303-7207(98)00040-9 [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL, 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29, 730–741. 10.1002/etc.34 [DOI] [PubMed] [Google Scholar]
- Armingol E, Officer A, Harismendy O, Lewis NE, 2021. Deciphering cell–cell interactions and communication from gene expression. Nature Reviews Genetics 22, 71–88. 10.1038/s41576-020-00292-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arutyunyan A, Roberts K, Troulé K, Wong FCK, Sheridan MA, Kats I, Garcia-Alonso L, Velten B, Hoo R, Ruiz-Morales ER, Sancho-Serra C, Shilts J, Handfield L-F, Marconato L, Tuck E, Gardner L, Mazzeo CI, Li Q, Kelava I, Wright GJ, Prigmore E, Teichmann SA, Bayraktar OA, Moffett A, Stegle O, Turco MY, Vento-Tormo R, 2023. Spatial multiomics map of trophoblast development in early pregnancy. Nature. 10.1038/s41586-023-05869-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashary N, Bhide A, Chakraborty P, Colaco S, Mishra A, Chhabria K, Jolly MK, Modi D, 2020. Single-Cell RNA-seq Identifies Cell Subsets in Human Placenta That Highly Expresses Factors Driving Pathogenesis of SARS-CoV-2. Front Cell Dev Biol 8, 783. 10.3389/fcell.2020.00783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakulski KM, Dou JF, Thompson RC, Lee C, Middleton LY, Perera BPU, Ferris SP, Jones TR, Neier K, Zhou X, Sartor MA, Hammoud SS, Dolinoy DC, Colacino JA, 2020. Single-Cell Analysis of the Gene Expression Effects of Developmental Lead (Pb) Exposure on the Mouse Hippocampus. Toxicol Sci 176, 396–409. 10.1093/toxsci/kfaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, 2007. The origins of the developmental origins theory. J Intern Med 261, 412–417. 10.1111/j.1365-2796.2007.01809.x [DOI] [PubMed] [Google Scholar]
- Bartosovic M, Kabbe M, Castelo-Branco G, 2021. Single-cell CUT&Tag profiles histone modifications and transcription factors in complex tissues. Nature Biotechnology 39, 825–835. 10.1038/s41587-021-00869-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkane N, Liere P, Oudinet J-P, Hertig A, Lefèvre G, Pluchino N, Schumacher M, Chabbert-Buffet N, 2017. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr Rev 38, 123–144. 10.1210/er.2016-1065 [DOI] [PubMed] [Google Scholar]
- Bonde JP, Flachs EM, Rimborg S, Glazer CH, Giwercman A, Ramlau-Hansen CH, Hougaard KS, Høyer BB, Hærvig KK, Petersen SB, Rylander L, Specht IO, Toft G, Bräuner EV, 2016. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis. Hum Reprod Update 23, 104–125. 10.1093/humupd/dmw036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander SM, Gabler MK, Fowler NL, Connon RE, Schlenk D, 2016. Pyrethroid Pesticides as Endocrine Disruptors: Molecular Mechanisms in Vertebrates with a Focus on Fishes. Environ Sci Technol 50, 8977–8992. 10.1021/acs.est.6b02253 [DOI] [PubMed] [Google Scholar]
- Broséus L, Vaiman D, Tost J, Martin CRS, Jacobi M, Schwartz JD, Béranger R, Slama R, Heude B, Lepeule J, 2022. Maternal blood pressure associates with placental DNA methylation both directly and through alterations in cell-type composition. BMC Med 20, 397. 10.1186/s12916-022-02610-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownfoot FC, Tong S, Hannan NJ, Binder NK, Walker SP, Cannon P, Hastie R, Onda K, Kaitu’u-Lino TJ, 2015. Effects of Pravastatin on Human Placenta, Endothelium, and Women With Severe Preeclampsia. Hypertension 66, 687–697. 10.1161/HYPERTENSIONAHA.115.05445 [DOI] [PubMed] [Google Scholar]
- Burton GJ, Fowden AL, 2015. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci 370, 20140066. 10.1098/rstb.2014.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, 2015. What is the placenta? Am J Obstet Gynecol 213, S6.e1, S6–8. 10.1016/j.ajog.2015.07.050 [DOI] [PubMed] [Google Scholar]
- Campbell KA, Colacino JA, Park SK, Bakulski KM, 2020. Cell Types in Environmental Epigenetic Studies: Biological and Epidemiological Frameworks. Curr Envir Health Rpt 7, 185–197. 10.1007/s40572-020-00287-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KA, Colacino JA, Puttabyatappa M, Dou JF, Elkin ER, Hammoud SS, Domino SE, Dolinoy DC, Goodrich JM, Loch-Caruso R, Padmanabhan V, Bakulski KM, 2023. Placental cell type deconvolution reveals that cell proportions drive preeclampsia gene expression differences. Communications Biology in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF, 2016. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environ Health Perspect 124, 1651–1655. 10.1289/EHP188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso M, Evangelista M, Parolini O, 2012. Human term placental cells: phenotype, properties and new avenues in regenerative medicine. Int J Mol Cell Med 1, 64–74. [PMC free article] [PubMed] [Google Scholar]
- Castellucci M, Kaufmann P, 2006. Basic Structure of the Villous Trees, in: Benirschke K, Kaufmann Peter, Baergen R (Eds.), Pathology of the Human Placenta. Springer New York, New York, NY, pp. 50–120. 10.1007/0-387-26742-5_6 [DOI] [Google Scholar]
- Caufriez A, Frankenne F, Hennen G, Copinschi G, 1993. Regulation of maternal IGF-I by placental GH in normal and abnormal human pregnancies. Am J Physiol 265, E572–577. 10.1152/ajpendo.1993.265.4.E572 [DOI] [PubMed] [Google Scholar]
- Chen J, Du L, Wang F, Shao X, Wang X, Yu W, Bi S, Chen Dexiong, Pan X, Zeng, Huang L, Liang Y, Li Y, Chen R, Xue F, Li X, Wang S, Zhuang M, Liu M, Lin L, Yan H, He F, Yu L, Jiang Q, Xiong Z, Zhang L, Cao B, Wang Y-L, Chen Dunjin, 2022. Cellular and molecular atlas of the placenta from a COVID-19 pregnant woman infected at midgestation highlights the defective impacts on foetal health. Cell Proliferation 55, e13204. 10.1111/cpr.13204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorens THH, Oliver TRW, Sanghvi R, Sovio U, Cook E, Vento-Tormo R, Haniffa M, Young MD, Rahbari R, Sebire N, Campbell PJ, Charnock-Jones DS, Smith GCS, Behjati S, 2021. Inherent mosaicism and extensive mutation of human placentas. Nature 592, 80–85. 10.1038/s41586-021-03345-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti M, Lorenzetti S, Ubaldi A, Zilli R, Marcoccia D, 2022. Endocrine Disruptors and Prostate Cancer. Int J Mol Sci 23, 1216. 10.3390/ijms23031216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MA, 2016. The endocrine function of human placenta: an overview. Reprod Biomed Online 32, 14–43. 10.1016/j.rbmo.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Daniel K, Szurkowski M, Kokot F, Grzeszczak W, 1987. [Secretion of growth hormone, insulin and glucagon in obese persons before and after using low-calorie diet]. Pol Tyg Lek 42, 607–612. [PubMed] [Google Scholar]
- Day DB, Collett BR, Barrett ES, Bush NR, Swan SH, Nguyen RHN, Szpiro AA, Sathyanarayana S, 2021. Phthalate mixtures in pregnancy, autistic traits, and adverse childhood behavioral outcomes. Environment International 147, 106330. 10.1016/j.envint.2020.106330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyssenroth MA, Rosa MJ, Eliot MN, Kelsey KT, Kloog I, Schwartz JD, Wellenius GA, Peng S, Hao K, Marsit CJ, Chen J, 2021. Placental gene networks at the interface between maternal PM2.5 exposure early in gestation and reduced infant birthweight. Environmental Research 199, 111342. 10.1016/j.envres.2021.111342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo R, Khalil A, Alameddine S, D’Angelo E, Galliani C, Matarrelli B, Buca D, Liberati M, Rizzo G, D’Antonio F, 2021. Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 3, 100468. 10.1016/j.ajogmf.2021.100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV, Whitehead C, Hyett J, da Silva Costa F, Nicolaides K, Menkhorst E, 2023. Pre-eclampsia. Nat Rev Dis Primers 9, 8. 10.1038/s41572-023-00417-6 [DOI] [PubMed] [Google Scholar]
- Efremova M, Vento-Tormo M, Teichmann SA, Vento-Tormo R, 2020. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nature Protocols 15, 1484–1506. 10.1038/s41596-020-0292-x [DOI] [PubMed] [Google Scholar]
- Elkin ER, Bakulski KM, Colacino JA, Bridges D, Kilburn BA, Armant DR, Loch-Caruso R, 2021. Transcriptional profiling of the response to the trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine revealed activation of the eIF2α/ATF4 integrated stress response in two in vitro placental models. Arch Toxicol 95, 1595–1619. 10.1007/s00204-021-03011-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson TM, Kappil M, Hao K, Jackson BP, Punshon T, Karagas MR, Chen J, Marsit CJ, 2017. Maternal exposure to selenium and cadmium, fetal growth, and placental expression of steroidogenic and apoptotic genes. Environmental Research 158, 233–244. 10.1016/j.envres.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson TM, Punshon T, Jackson BP, Hao K, Lambertini L, Chen J, Karagas MR, Marsit CJ, 2018. Cadmium-Associated Differential Methylation throughout the Placental Genome: Epigenome-Wide Association Study of Two U.S. Birth Cohorts. Environ Health Perspect 126, 017010. 10.1289/EHP2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R, Preissl S, Li Y, Hou X, Lucero J, Wang X, Motamedi A, Shiau AK, Zhou X, Xie F, Mukamel EA, Zhang K, Zhang Y, Behrens MM, Ecker JR, Ren B, 2021. Comprehensive analysis of single cell ATAC-seq data with SnapATAC. Nature Communications 12, 1337. 10.1038/s41467-021-21583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer BH, Simon MC, 2006. Hypoxia, HIF and the placenta. Cell Cycle 5, 495–498. 10.4161/cc.5.5.2497 [DOI] [PubMed] [Google Scholar]
- Garner PR, Armstrong DT, 1977. The effect of human chorionic gonadotropin and estradiol-17beta on the maintenance of the human corpus luteum of early pregnancy. Am J Obstet Gynecol 128, 469–475. 10.1016/0002-9378(77)90026-6 [DOI] [PubMed] [Google Scholar]
- Gicquel C, Le Bouc Y, 2006. Hormonal regulation of fetal growth. Horm Res 65 Suppl 3, 28–33. 10.1159/000091503 [DOI] [PubMed] [Google Scholar]
- Gingrich J, Ticiani E, Veiga-Lopez A, 2020. Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy. Trends in Endocrinology & Metabolism 31, 508–524. 10.1016/j.tem.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady R, Sathyanarayana S, 2012. An update on phthalates and male reproductive development and function. Curr Urol Rep 13, 307–310. 10.1007/s11934-012-0261-1 [DOI] [PubMed] [Google Scholar]
- Hall JM, Greco CW, 2019. Perturbation of Nuclear Hormone Receptors by Endocrine Disrupting Chemicals: Mechanisms and Pathological Consequences of Exposure. Cells 9, 13. 10.3390/cells9010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C, Cheng X, Xia H, Ma X, 2012. The endocrine disruptor 4-nonylphenol promotes adipocyte differentiation and induces obesity in mice. Cell Physiol Biochem 30, 382–394. 10.1159/000339032 [DOI] [PubMed] [Google Scholar]
- Heazlewood CF, Sherrell H, Ryan J, Atkinson K, Wells CA, Fisk NM, 2014. High incidence of contaminating maternal cell overgrowth in human placental mesenchymal stem/stromal cell cultures: a systematic review. Stem Cells Transl Med 3, 1305–1311. 10.5966/sctm.2014-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund E, Deng Q, 2018. Single-cell RNA sequencing: Technical advancements and biological applications. Molecular Aspects of Medicine, The emerging field of single-cell analysis 59, 36–46. 10.1016/j.mam.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Ho V, Pelland-St-Pierre L, Gravel S, Bouchard MF, Verner M-A, Labrèche F, 2022. Endocrine disruptors: Challenges and future directions in epidemiologic research. Environ Res 204, 111969. 10.1016/j.envres.2021.111969 [DOI] [PubMed] [Google Scholar]
- Hou R, Denisenko E, Ong HT, Ramilowski JA, Forrest ARR, 2020. Predicting cell-to-cell communication networks using NATMI. Nature Communications 11, 5011. 10.1038/s41467-020-18873-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JMY, Arbuckle TE, Janssen P, Lanphear BP, Zhuang LH, Braun JM, Chen A, McCandless LC, 2021. Prenatal exposure to endocrine disrupting chemical mixtures and infant birth weight: A Bayesian analysis using kernel machine regression. Environ Res 195, 110749. 10.1016/j.envres.2021.110749 [DOI] [PubMed] [Google Scholar]
- Hussey MR, Burt A, Deyssenroth MA, Jackson BP, Hao K, Peng S, Chen J, Marsit CJ, Everson TM, 2020. Placental lncRNA expression associated with placental cadmium concentrations and birth weight. Environmental Epigenetics 6, dvaa003. 10.1093/eep/dvaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Tuteja G, 2021. PlacentaCellEnrich: A tool to characterize gene sets using placenta cell-specific gene enrichment analysis. Placenta 103, 164–171. 10.1016/j.placenta.2020.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Zhang L, Zhao J, Ren M, Li Zewu, Wang J, Wang S, Liu Y, An H, Li Y, Yan L, Li Zhiwen, Liu X, Pan B, Ye R, 2021. Associations between endocrine-disrupting heavy metals in maternal hair and gestational diabetes mellitus: A nested case-control study in China. Environ Int 157, 106770. 10.1016/j.envint.2021.106770 [DOI] [PubMed] [Google Scholar]
- Jin J, Menon R, 2018. Placental exosomes: A proxy to understand pregnancy complications. Am J Reprod Immunol 79, e12788. 10.1111/aji.12788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Xu Q, Li J, Xu S, Tang C, 2022. Micro-RNAs in Human Placenta: Tiny Molecules, Immense Power. Molecules 27, 5943. 10.3390/molecules27185943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir ER, Rahman MS, Rahman I, 2015. A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 40, 241–258. 10.1016/j.etap.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA, 1996. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect 104 Suppl 4, 715–740. 10.1289/ehp.96104s4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T, Seetharam AS, Zhou J, Bivens NJ, Schust DJ, Ezashi T, Tuteja G, Roberts RM, 2021. Single Nucleus RNA Sequence (snRNAseq) Analysis of the Spectrum of Trophoblast Lineages Generated From Human Pluripotent Stem Cells in vitro. Front Cell Dev Biol 9, 695248. 10.3389/fcell.2021.695248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Kang H, Jo A, Yoo S-A, Lee H-O, 2023. Perspectives on single-nucleus RNA sequencing in different cell types and tissues. J Pathol Transl Med 57, 52–59. 10.4132/jptm.2022.12.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-M, Kim J-S, 2017. A Review of Mechanisms of Implantation. Dev Reprod 21, 351–359. 10.12717/DR.2017.21.4.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner S, Kieu T, Chow C, Casey S, Blumberg B, 2010. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol 24, 526–539. 10.1210/me.2009-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama R, Wada-Kiyama Y, 2015. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ Int 83, 11–40. 10.1016/j.envint.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Kuhl AJ, Ross SM, Gaido KW, 2007. CCAAT/Enhancer Binding Protein β, But Not Steroidogenic Factor-1, Modulates the Phthalate-Induced Dysregulation of Rat Fetal Testicular Steroidogenesis. Endocrinology 148, 5851–5864. 10.1210/en.2007-0930 [DOI] [PubMed] [Google Scholar]
- Lamb AN, Rosenfeld JA, Coppinger J, Dodge ET, Dabell MP, Torchia BS, Ravnan JB, Shaffer LG, Ballif BC, 2012. Defining the impact of maternal cell contamination on the interpretation of prenatal microarray analysis. Genet Med 14, 914–921. 10.1038/gim.2012.77 [DOI] [PubMed] [Google Scholar]
- Lapehn S, Paquette AG, 2022. The Placental Epigenome as a Molecular Link Between Prenatal Exposures and Fetal Health Outcomes Through the DOHaD Hypothesis. Curr Environ Health Rep. 10.1007/s40572-022-00354-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer BI, Neier K, Valenzuela AE, Yasui DH, Schmidt RJ, Lein PJ, LaSalle JM, 2022. Placenta and fetal brain share a neurodevelopmental disorder DNA methylation profile in a mouse model of prenatal PCB exposure. Cell Rep 38, 110442. 10.1016/j.celrep.2022.110442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Yun HJ, Kim CY, Cho YW, Lee Y, Kim MH, 2017. Prenatal exposure to dexamethasone in the mouse induces sex-specific differences in placental gene expression. Develop. Growth Differ 59, 515–525. 10.1111/dgd.12376 [DOI] [PubMed] [Google Scholar]
- Li H, Peng H, Hong W, Wei Y, Tian H, Huang X, Jia L, Zheng J, Duan T, He Q, Wang K, 2022. Human Placental Endothelial Cell and Trophoblast Heterogeneity and Differentiation Revealed by Single-Cell RNA Sequencing. Cells 12. 10.3390/cells12010087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lesseur C, Srirangam P, Kaur K, Hermetz K, Caudle WM, Fiedler N, Panuwet P, Prapamontol T, Naksen W, Suttiwan P, Baumert BO, Hao K, Barr DB, Marsit CJ, Chen J, 2023. Associations between prenatal organophosphate pesticide exposure and placental gene networks. Environ Res 224, 115490. 10.1016/j.envres.2023.115490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YC, Li J, Ni Y, Liang Q, Zhang J, Yeo GSH, Lyu J, Jin S, Ding C, 2017. A complex association between DNA methylation and gene expression in human placenta at first and third trimesters. PLoS One 12, e0181155. 10.1371/journal.pone.0181155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fan X, Wang R, Lu X, Dang Y-L, Wang Huiying, Lin H-Y, Zhu C, Ge H, Cross JC, Wang Hongmei, 2018. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Research 28, 819–832. 10.1038/s41422-018-0066-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhai M, Zhang Q, Yang T, Wan Z, Li J, Liu X, Xu B, Du L, Chan RWS, Zhang L, Yeung WSB, Cheung KW, Chiu PCN, Wang W-J, Lee C-L, Gao Y, 2022. Resolving the gene expression maps of human first-trimester chorionic villi with spatial transcriptome. Front. Cell Dev. Biol 10, 1060298. 10.3389/fcell.2022.1060298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loegl J, Hiden U, Nussbaumer E, Schliefsteiner C, Cvitic S, Lang I, Wadsack C, Huppertz B, Desoye G, 2016. Hofbauer cells of M2a, M2b and M2c polarization may regulate feto-placental angiogenesis. Reproduction 152, 447–455. 10.1530/REP-16-0159 [DOI] [PubMed] [Google Scholar]
- Luecken MD, Theis FJ, 2019. Current best practices in single-cell RNA-seq analysis: a tutorial. Molecular Systems Biology 15, e8746. 10.15252/msb.20188746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltepe E, Fisher SJ, 2015. Placenta: the forgotten organ. Annu. Rev. Cell Dev. Biol 31, 523–552. 10.1146/annurev-cellbio-100814-125620 [DOI] [PubMed] [Google Scholar]
- Mao J, Jain A, Denslow ND, Nouri M-Z, Chen S, Wang T, Zhu N, Koh J, Sarma SJ, Sumner BW, Lei Z, Sumner LW, Bivens NJ, Roberts RM, Tuteja G, Rosenfeld CS, 2020. Bisphenol A and bisphenol S disruptions of the mouse placenta and potential effects on the placenta–brain axis. Proc. Natl. Acad. Sci. U.S.A 117, 4642–4652. 10.1073/pnas.1919563117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh B, Blelloch R, 2020. Single nuclei RNA-seq of mouse placental labyrinth development. Elife 9, e60266. 10.7554/eLife.60266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx V, 2021. Method of the Year: spatially resolved transcriptomics. Nature Methods 18, 9–14. 10.1038/s41592-020-01033-y [DOI] [PubMed] [Google Scholar]
- Melzer K, Schutz Y, Boulvain M, Kayser B, 2010. Physical activity and pregnancy: cardiovascular adaptations, recommendations and pregnancy outcomes. Sports Med 40, 493–507. 10.2165/11532290-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Mesiano S, 2009. CHAPTER 11 - The Endocrinology of Human Pregnancy and Fetoplacental Neuroendocrine Development A2 - Strauss, Jerome F, in: Barbieri RL (Ed.), Yen & Jaffe’s Reproductive Endocrinology (Sixth Edition). W.B. Saunders, Philadelphia, pp. 249–281. 10.1016/B978-1-4160-4907-4.00011-5 [DOI] [Google Scholar]
- Midgley AR, Pierce GB, Deneau GA, Gosling JRG, 1963. Morphogenesis of Syncytiotrophoblast in vivo: An Autoradiographic Demonstration. Science 141, 349–350. 10.1126/science.141.3578.349 [DOI] [PubMed] [Google Scholar]
- Midya V, Colicino E, Conti DV, Berhane K, Garcia E, Stratakis N, Andrusaityte S, Basagaña X, Casas M, Fossati S, Gražuleviciene R, Haug LS, Heude B, Maitre L, McEachan R, Papadopoulou E, Roumeliotaki T, Philippat C, Thomsen C, Urquiza J, Vafeiadi M, Varo N, Vos MB, Wright J, McConnell R, Vrijheid M, Chatzi L, Valvi D, 2022. Association of Prenatal Exposure to Endocrine-Disrupting Chemicals With Liver Injury in Children. JAMA Netw Open 5, e2220176. 10.1001/jamanetworkopen.2022.20176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S, Mandal M, Goldsmith LT, Kashani BN, Ponzio NM, 2015. The maternal immune system during pregnancy and its influence on fetal development. RRB 171. 10.2147/RRB.S80652 [DOI] [Google Scholar]
- Murphy VE, Smith R, Giles WB, Clifton VL, 2006. Endocrine Regulation of Human Fetal Growth: The Role of the Mother, Placenta, and Fetus. Endocrine Reviews 27, 141–169. 10.1210/er.2005-0011 [DOI] [PubMed] [Google Scholar]
- Myllynen P, Pasanen M, Pelkonen O, 2005. Human placenta: a human organ for developmental toxicology research and biomonitoring. Placenta 26, 361–371. 10.1016/j.placenta.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN, 2018. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front Physiol 9, 1091. 10.3389/fphys.2018.01091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AC, Mould AW, Bikoff EK, Robertson EJ, 2016. Single-cell RNA-seq reveals cell type-specific transcriptional signatures at the maternal-foetal interface during pregnancy. Nat Commun 7, 11414. 10.1038/ncomms11414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA, Steiner D, Diehn M, Alizadeh AA, 2019. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol 37, 773–782. 10.1038/s41587-019-0114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël F, Massenet-Regad L, Carmi-Levy I, Cappuccio A, Grandclaudon M, Trichot C, Kieffer Y, Mechta-Grigoriou F, Soumelis V, 2021. Dissection of intercellular communication using the transcriptome-based framework ICELLNET. Nature Communications 12, 1089. 10.1038/s41467-021-21244-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwabuobi C, Arlier S, Schatz F, Guzeloglu-Kayisli O, Lockwood CJ, Kayisli UA, 2017. hCG: Biological Functions and Clinical Applications. Int J Mol Sci 18, 2037. 10.3390/ijms18102037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Song W, Puttabyatappa M, 2021. Praegnatio Perturbatio—Impact of Endocrine-Disrupting Chemicals. Endocrine Reviews 42, 295–353. 10.1210/endrev/bnaa035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Lapehn S, Freije S, MacDonald J, Bammler T, Day DB, Loftus CT, Kannan K, Alex Mason W, Bush NR, LeWinn KZ, Enquobahrie DA, Marsit C, Sathyanarayana S, 2023. Placental Transcriptomic Signatures of Prenatal Exposure to Hydroxy-Polycyclic Aromatic Hydrocarbons. Environment International 107763. 10.1016/j.envint.2023.107763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, MacDonald J, Lapehn S, Bammler T, Kruger L, Day DB, Price ND, Loftus C, Kannan K, Marsit C, Mason WA, Bush NR, LeWinn KZ, Enquobahrie DA, Prasad B, Karr CJ, Sathyanarayana S, on behalf of program collaborators for Environmental influences on Child Health Outcomes, 2021. A Comprehensive Assessment of Associations between Prenatal Phthalate Exposure and the Placental Transcriptomic Landscape. Environ Health Perspect 129, 097003. 10.1289/EHP8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrettini S, Caroli A, Torlone E, 2020. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front Endocrinol (Lausanne) 11, 611929. 10.3389/fendo.2020.611929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavličev M, Wagner GP, Chavan AR, Owens K, Maziarz J, Dunn-Fletcher C, Kallapur SG, Muglia L, Jones H, 2017. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Research 27, 349–361. 10.1101/gr.207597.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JL, Neelon B, Bloom MS, Buckley JP, Ananth CV, Perera F, Vena J, Hunt K, program collaborators for Environmental influences on Child Health Outcomes, 2021. Exploring associations between prenatal exposure to multiple endocrine disruptors and birth weight with exposure continuum mapping. Environ Res 200, 111386. 10.1016/j.envres.2021.111386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, Slama R, EDEN Mother–Child Study Group, 2017. Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ Health Perspect 125, 097014. 10.1289/EHP1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Dixon G, Brosens I, 1981. The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta 2, 303–316. 10.1016/s0143-4004(81)80027-6 [DOI] [PubMed] [Google Scholar]
- Pique-Regi R, Romero R, Tarca AL, Sendler ED, Xu Y, Garcia-Flores V, Leng Y, Luca F, Hassan SS, Gomez-Lopez N, 2019a. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 8. 10.7554/eLife.52004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique-Regi R, Romero R, Tarca AL, Sendler ED, Xu Y, Garcia-Flores V, Leng Y, Luca F, Hassan SS, Gomez-Lopez N, 2019b. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 8, e52004. 10.7554/eLife.52004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA, 2017. Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol 233, R109–R129. 10.1530/JOE-17-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Saha A, Ghosh A, Roy N, Kumar RP, Meinhardt G, Mukerjee A, Gunewardena S, Kumar R, Knöfler M, Paul S, 2022. Hippo signaling cofactor, WWTR1, at the crossroads of human trophoblast progenitor self-renewal and differentiation. Proceedings of the National Academy of Sciences 119, e2204069119. 10.1073/pnas.2204069119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes L, Golos TG, 2018. Hofbauer Cells: Their Role in Healthy and Complicated Pregnancy. Front. Immunol 9, 2628. 10.3389/fimmu.2018.02628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, 2021. Transcriptomics and Other Omics Approaches to Investigate Effects of Xenobiotics on the Placenta. Front Cell Dev Biol 9, 723656. 10.3389/fcell.2021.723656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardesai VS, Shafiee A, Fisk NM, Pelekanos RA, 2017. Avoidance of Maternal Cell Contamination and Overgrowth in Isolating Fetal Chorionic Villi Mesenchymal Stem Cells from Human Term Placenta. Stem Cells Transl Med 6, 1070–1084. 10.1002/sctm.15-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Grady R, Barrett ES, Redmon B, Nguyen RHN, Barthold JS, Bush NR, Swan SH, 2016. First trimester phthalate exposure and male newborn genital anomalies. Environ Res 151, 777–782. 10.1016/j.envres.2016.07.043 [DOI] [PubMed] [Google Scholar]
- Sato Y, 2020. Endovascular trophoblast and spiral artery remodeling. Mol Cell Endocrinol 503, 110699. 10.1016/j.mce.2019.110699 [DOI] [PubMed] [Google Scholar]
- Scott HM, Mason JI, Sharpe RM, 2009. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 30, 883–925. 10.1210/er.2009-0016 [DOI] [PubMed] [Google Scholar]
- Seval Y, Korgun ET, Demir R, 2007. Hofbauer Cells in Early Human Placenta: Possible Implications in Vasculogenesis and Angiogenesis. Placenta 28, 841–845. 10.1016/j.placenta.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Shaffer RM, Ferguson KK, Sheppard L, James-Todd T, Butts S, Chandrasekaran S, Swan SH, Barrett ES, Nguyen R, Bush N, McElrath TF, Sathyanarayana S, TIDES Study team, 2019. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ Int 123, 588–596. 10.1016/j.envint.2018.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieppi E, Vähäkangas K, Rautio A, Ietta F, Paulesu L, Myllynen P, 2016. The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures. Molecular and Cellular Endocrinology 429, 41–49. 10.1016/j.mce.2016.03.034 [DOI] [PubMed] [Google Scholar]
- Sitras V, Fenton C, Paulssen R, Vårtun Å, Acharya G, 2012. Differences in gene expression between first and third trimester human placenta: a microarray study. PLoS One 7, e33294. 10.1371/journal.pone.0033294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinu N, Cronin MTD, Enoch SJ, Madden JC, Worth AP, 2020. Quantitative adverse outcome pathway (qAOP) models for toxicity prediction. Arch Toxicol 94, 1497–1510. 10.1007/s00204-020-02774-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C, Schwarz S, Moser G, Cvitic S, Jantscher-Krenn E, Gauster M, Hiden U, 2021. Placental Endocrine Activity: Adaptation and Disruption of Maternal Glucose Metabolism in Pregnancy and the Influence of Fetal Sex. Int J Mol Sci 22, 12722. 10.3390/ijms222312722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Gonzalez TL, Deng N, DiPentino R, Clark EL, Lee B, Tang J, Wang Y, Stripp BR, Yao C, Tseng H-R, Karumanchi SA, Koeppel AF, Turner SD, Farber CR, Rich SS, Wang ET, Williams J, Pisarska MD, 2019. The maternal-fetal interface of successful pregnancies and impact of fetal sex using single cell sequencing. bioRxiv 641118. 10.1101/641118 [DOI] [Google Scholar]
- Suryawanshi H, Max K, Bogardus KA, Sopeyin A, Chang MS, Morozov P, Castano PM, Tuschl T, Williams Z, 2022. Dynamic genome-wide gene expression and immune cell composition in the developing human placenta. J Reprod Immunol 151, 103624. 10.1016/j.jri.2022.103624 [DOI] [PubMed] [Google Scholar]
- Suryawanshi H, Morozov P, Straus A, Sahasrabudhe N, Max KEA, Garzia A, Kustagi M, Tuschl T, Williams Z, 2018. A single-cell survey of the human first-trimester placenta and decidua. Sci Adv 4, eaau4788. 10.1126/sciadv.aau4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner EM, Hallerbäck MU, Wikström S, Lindh C, Kiviranta H, Gennings C, Bornehag C-G, 2020. Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environ Int 134, 105185. 10.1016/j.envint.2019.105185 [DOI] [PubMed] [Google Scholar]
- Tehrani JM, Kennedy E, Tung PW, Burt A, Hermetz K, Punshon T, Jackson BP, Hao K, Chen J, Karagas MR, Koestler DC, Lester B, Marsit CJ, 2022. Human placental microRNAs dysregulated by cadmium exposure predict neurobehavioral outcomes at birth. Pediatr Res. 10.1038/s41390-022-02201-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F-Y, Kennedy EM, Hermetz K, Burt A, Everson TM, Punshon T, Jackson BP, Hao K, Chen J, Karagas MR, Koestler DC, Marsit C, 2022. Selenium-associated differentially expressed microRNAs and their targeted mRNAs across the placental genome in two U.S. birth cohorts. Epigenetics 17, 1234–1245. 10.1080/15592294.2021.2003044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Chen F, Macosko EZ, 2022. The expanding vistas of spatial transcriptomics. Nature Biotechnology. 10.1038/s41587-022-01448-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivanen R, Shen MM, 2017. Prostate organogenesis: tissue induction, hormonal regulation and cell type specification. Development 144, 1382–1398. 10.1242/dev.148270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosevska A, Ghosh S, Ganguly A, Cappelletti M, Kallapur SG, Pellegrini M, Devaskar SU, 2022. Integrated analysis of an in vivo model of intra-nasal exposure to instilled air pollutants reveals cell-type specific responses in the placenta. Sci Rep 12, 8438. 10.1038/s41598-022-12340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang JCH, Vong JSL, Ji L, Poon LCY, Jiang P, Lui KO, Ni Y-B, To KF, Cheng YKY, Chiu RWK, Lo YMD, 2017a. Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics. PNAS 114, E7786–E7795. 10.1073/pnas.1710470114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang JCH, Vong JSL, Ji L, Poon LCY, Jiang P, Lui KO, Ni Y-B, To KF, Cheng YKY, Chiu RWK, Lo YMD, 2017b. Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics. Proc Natl Acad Sci USA 114, E7786. 10.1073/pnas.1710470114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park J-E, Stephenson E, Polański K, Goncalves A, Gardner L, Holmqvist S, Henriksson J, Zou A, Sharkey AM, Millar B, Innes B, Wood L, Wilbrey-Clark A, Payne RP, Ivarsson MA, Lisgo S, Filby A, Rowitch DH, Bulmer JN, Wright GJ, Stubbington MJT, Haniffa M, Moffett A, Teichmann SA, 2018. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 563, 347–353. 10.1038/s41586-018-0698-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M, 2013. The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology 312, 158–165. 10.1016/j.tox.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Wang Q, Li J, Wang S, Deng Q, An Y, Xing Y, Dai X, Li Z, Ma Q, Wang K, Liu C, Yuan Y, Dong G, Zhang T, Yang H, Du Y, Hou Y, Ke W, Shang Z, 2022. Single-cell transcriptional profiling reveals cellular and molecular divergence in human maternal-fetal interface. Sci Rep 12, 10892. 10.1038/s41598-022-14516-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao S, 2010. Vascular Biology of the Placenta, Integrated Systems Physiology: from Molecules to Function to Disease. Morgan & Claypool Life Sciences, San Rafael (CA). [PubMed] [Google Scholar]
- Welch BM, Keil AP, Buckley JP, Calafat AM, Christenbury KE, Engel SM, O’Brien KM, Rosen EM, James-Todd T, Zota AR, Ferguson KK, Pooled Phthalate Exposure and Preterm Birth Study Group, Alshawabkeh AN, Cordero JF, Meeker JD, Barrett ES, Bush NR, Nguyen RHN, Sathyanarayana S, Swan SH, Cantonwine DE, McElrath TF, Aalborg J, Dabelea D, Starling AP, Hauser R, Messerlian C, Zhang Y, Bradman A, Eskenazi B, Harley KG, Holland N, Bloom MS, Newman RB, Wenzel AG, Braun JM, Lanphear BP, Yolton K, Factor-Litvak P, Herbstman JB, Rauh VA, Drobnis EZ, Sparks AE, Redmon JB, Wang C, Binder AM, Michels KB, Baird DD, Jukic AMZ, Weinberg CR, Wilcox AJ, Rich DQ, Weinberger B, Padmanabhan V, Watkins DJ, Hertz-Picciotto I, Schmidt RJ, 2022. Associations Between Prenatal Urinary Biomarkers of Phthalate Exposure and Preterm Birth: A Pooled Study of 16 US Cohorts. JAMA Pediatr 176, 895–905. 10.1001/jamapediatrics.2022.2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GP, Darbre PD, 2019. Low-dose environmental endocrine disruptors, increase aromatase activity, estradiol biosynthesis and cell proliferation in human breast cells. Mol Cell Endocrinol 486, 55–64. 10.1016/j.mce.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Winterbottom EF, Ban Y, Sun X, Capobianco AJ, Marsit CJ, Chen X, Wang L, Karagas MR, Robbins DJ, 2019. Transcriptome-wide analysis of changes in the fetal placenta associated with prenatal arsenic exposure in the New Hampshire Birth Cohort Study. Environ Health 18, 100. 10.1186/s12940-019-0535-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang J, Wei Y, Chen J, Kang L, Long C, Wu S, Shen L, Wei G, 2022. Contribution of prenatal endocrine-disrupting chemical exposure to genital anomalies in males: The pooled results from current evidence. Chemosphere 286, 131844. 10.1016/j.chemosphere.2021.131844 [DOI] [PubMed] [Google Scholar]
- Xu W, Wu H, Shang L, 2021. Gene expression in rat placenta after exposure to di(2-ethylhexyl) phthalate. Hum Exp Toxicol 40, 504–514. 10.1177/0960327120954259 [DOI] [PubMed] [Google Scholar]
- Yang C, Song G, Lim W, 2019. A mechanism for the effect of endocrine disrupting chemicals on placentation. Chemosphere 231, 326–336. 10.1016/j.chemosphere.2019.05.133 [DOI] [PubMed] [Google Scholar]
- Yang Y, Guo F, Peng Y, Chen R, Zhou W, Wang H, OuYang J, Yu B, Xu Z, 2021. Transcriptomic Profiling of Human Placenta in Gestational Diabetes Mellitus at the Single-Cell Level. Front Endocrinol (Lausanne) 12, 679582. 10.3389/fendo.2021.679582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Lai J, Hu B, Zhang S, Zhao J, Li W, 2014. A chromatin modifier regulates Sertoli cell response to mono-(2-ethylhexyl) phthalate (MEHP) via tissue inhibitor of metalloproteinase 2 (TIMP2) signaling. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 1839, 1170–1182. 10.1016/j.bbagrm.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Caudle WM, Pi J, Bhattacharya S, Andersen ME, Kaminski NE, Conolly RB, 2019. Embracing Systems Toxicology at Single-Cell Resolution. Curr Opin Toxicol 16, 49–57. 10.1016/j.cotox.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Bian Q, Chen Y, Wang X, Yu S, Liu S, Ji P, Li L, Shrestha M, Dong S, Guo R, Zhang H, 2021. Dissecting human trophoblast cell transcriptional heterogeneity in preeclampsia using single-cell RNA sequencing. Molecular Genetics & Genomic Medicine 9, e1730. 10.1002/mgg3.1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Wong RJ, Stevenson DK, 2021. The Impact of Hypoxia in Early Pregnancy on Placental Cells. IJMS 22, 9675. 10.3390/ijms22189675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang H, Yang Y, Guo F, Yu B, Su Z, 2022. Trophoblast Cell Subtypes and Dysfunction in the Placenta of Individuals with Preeclampsia Revealed by Single-Cell RNA Sequencing. Mol Cells 45, 317–328. 10.14348/molcells.2021.0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Harris LK, Forbes K, Heazell AEP, 2022. Sex-specific effects of bisphenol A on the signaling pathway of ESRRG in the human placenta. Biology of Reproduction 106, 1278–1291. 10.1093/biolre/ioac044 [DOI] [PMC free article] [PubMed] [Google Scholar]


