Abstract
Microsphere technology was used to develop formulations of rifampin for targeted delivery to host macrophages. These formulations were prepared by using biocompatible polymeric excipients of lactide and glycolide copolymers. Release characteristics were examined in vitro and also in two monocytic cell lines, the murine J774 and the human Mono Mac 6 cell lines. Bioassay assessment of cell culture supernatants from monocyte cell lines showed release of bioactive rifampin during a 7-day experimental period. Treatment of Mycobacterium tuberculosis H37Rv-infected monocyte cell lines with rifampin-loaded microspheres resulted in a significant decrease in numbers of CFU at 7 days following initial infection, even though only 8% of the microsphere-loaded rifampin was released. The levels of rifampin released from microsphere formulations within monocytes were more effective at reducing M. tuberculosis intracellular growth than equivalent doses of rifampin given as a free drug. These results demonstrate that rifampin-loaded microspheres can be formulated for effective sustained and targeted delivery to host macrophages.
The impact of tuberculosis on the world today can best be appreciated by the fact that on 23 April 1993, the World Health Organization declared tuberculosis a global public health emergency (38, 39). This distinction has never been given to any other disease. Tuberculosis is considered the “world’s foremost cause of death from a single infectious agent” (29). In the United States, the incidence of tuberculosis began to increase in approximately 1985 for the first time since 1953 (2). By 1992, the number of cases of tuberculosis had increased by 20%. Even though the number of tuberculosis cases began to decline slightly after 1992, it is estimated that about 10 to 15 million people in the United States are infected but are not yet sick and therefore are not infectious (1). These people still remain at risk for developing active disease, and that risk becomes even greater when the person is coinfected with the human immunodeficiency virus (22).
Treatment of tuberculosis is generally successful, except in the case of multiple-drug-resistant strains of Mycobacterium tuberculosis (MDRTB). MDRTB strains not only are difficult to treat but also are life threatening, sometimes resulting in a high mortality rate (e.g., 72 to 89%) in a short period of time (e.g., 4 to 16 weeks) (5, 6, 11). Although it may be important to search for new drugs to treat infection with MDRTB, it is also important to utilize currently available therapy to treat initial infection with M. tuberculosis so that MDRTB does not develop.
In the development of tuberculosis therapy, two points are important to consider. First, the metabolism of M. tuberculosis is slow, resulting in a generation time that is measured in hours (15). This means that drug regimens should ideally have a low level of toxicity for long-term administration, and if possible, should be bactericidal so that elimination of the organism is rapid and is not totally dependent on the immune system. Second, the tubercle bacillus is a facultative intracellular parasite (3); therefore, drugs should also be able to penetrate host cells. Thus, an ideal method for treating tuberculosis would be one that not only is able to safely deliver drugs systemically for long term, but also would be able to target drugs to the intracellular environment in which the tubercle bacilli are found (i.e., macrophages).
Microsphere technology is an established technique that has been used to deliver several different types of drugs, including antigens, by injection (13) or oral administration (14), steroids (7), peptides (28), proteins (18), and antibiotics (19, 31). The chemical composition of the microspheres is based on one that was originally used to make synthetic resorbable sutures and is known to be biocompatible in humans. The polymers are biodegradable by means of nonenzymatic degradation and can be formulated for release up to several months or years, depending on the chemical and physical properties of the specific drug to be encapsulated and the specific polymeric excipient that will be used. The purpose of this investigation was to develop small microspheres for delivery of antimycobacterial drugs to infected host macrophages. For these studies, rifampin was chosen because it is one of the established first-line drugs used to treat tuberculosis.
To assess intracellular delivery of rifampin from microsphere formulations, two macrophage cell lines, the J774 murine macrophage cell line and the Mono Mac 6 (MM6) human monocytic cell line, were used. Both of these cell lines were infected with M. tuberculosis H37Rv and were treated with rifampin-loaded microsphere formulations. Because of limitations with cell line maintenance in vitro, experimental procedures were limited to 7 days. Although this time frame is not the optimum for complete drug release from microspheres, the results reveal that sufficient rifampin was released from microsphere formulations during the first week to significantly inhibit intracellular growth of M. tuberculosis. More importantly, more than 90% of rifampin was still available for further release at the end of the 7-day experiments. These results indicate that microsphere formulations can be used for effective intracellular delivery of rifampin to host macrophages infected with M. tuberculosis.
MATERIALS AND METHODS
Preparation of rifampin microspheres for macrophage uptake.
The primary goal of this study was to prepare a microsphere formulation that could be used to target the delivery of effective doses of rifampin to macrophages, i.e., a small microsphere (1 to 10 μm). A brief description of the process used to prepare these small microspheres is as follows. First, an excipient solution was prepared by dissolving approximately 2.8 g of poly(dl-lactide-co-glycolide) (DL-PLG) in 11.0 g of either methylene chloride or ethyl acetate. To this solution, approximately 150 mg of rifampin was added and a homogeneous solution was obtained by thorough mixing. The resulting mixture was then introduced into 300 ml of an aqueous process medium consisting of polyvinyl alcohol (Air Products, Inc., Allentown, Pa.). In some formulations, carboxymethyl cellulose (Air Products, Inc.) was included. An emulsion consisting of microdroplets of appropriate size was formed with the aid of a Silverson emulsifier (Silverson Machines, East Longmeadow, Mass.). The emulsion was then transferred to 5.0 liters of water. The resulting microspheres were then concentrated by centrifugation and collected by lyophilization.
Once the small microspheres were obtained, assessment of three criteria was important in evaluating their ability to be used for macrophage targeting: (i) drug loading (i.e., percent weight), (ii) in vitro release, and (iii) diameter.
Drug content of rifampin microspheres.
The rifampin content of each lot of microspheres was determined by first extracting the rifampin from a known quantity of microspheres and by quantifying the amount of drug spectrophotometrically. More specifically, multiple samples of microspheres were weighed into volumetric flasks. Similarly, control samples were prepared by weighing rifampin and excipient into volumetric flasks. Ethyl acetate was added to the flasks to dissolve the contents. The concentration of rifampin contained in each sample was determined by measuring the absorbance on a spectrophotometer at a wavelength of 474 nm. A series of rifampin solutions of known concentrations in ethyl acetate were prepared, and absorbances were measured to generate a standard curve. The rifampin concentrations in the microsphere and control samples were then obtained by linear regression. The total amount of rifampin in each microsphere sample was calculated as (rifampin concentration [μg/ml]) (mg/1,000 μg)(sample volume [ml])/(microsphere sample weight [mg]).
In vitro release analysis of rifampin microspheres.
In order to assess the release kinetics of rifampin from the microspheres, multiple samples of each microsphere lot were weighed into glass test tubes (16 by 100 mm) equipped with serum separators (Fisher Scientific). To each tube, 3.0 ml of receiving fluid consisting of 0.05 M sodium phosphate solution was added. Note that the phosphate solution also contained a small amount of sodium azide as a preservative. The test tubes were placed in an incubator maintained at 37°C. The receiving fluid was removed and replaced with new fluid at 2, 6, and 24 h and every 24 to 48 h thereafter. Concentrations of rifampin were determined by a standard high-performance liquid chromatography (HPLC) assay described in the U.S. Pharmacopeia (32). An absorption maximum of 254 nm was used for rifampin.
Microsphere size determination and sterilization.
The size distribution for each lot of rifampin microspheres was determined with a Malvern Particle Size Analyzer (Malvern Instruments, Malvern, United Kingdom). Analysis of size distribution is important to ensure that a 1- to 10-μm diameter be maintained. This size range is necessary for targeting to macrophages. The microsphere lots were sterilized by gamma radiation (2.5-Mrad dose) by Neutron Products, Inc., Dickerson, Md. Following sterilization, microsphere formulations were stored desiccated at 4°C until use. Surface morphology of microsphere formulations was examined by scanning electron microscopy to verify uniformity and absence of cracks, holes, or irregularities in surface morphology.
Mycobacterial strains.
M. tuberculosis H37Rv (ATCC 27294, SRI no. 1345) was maintained on Middlebrook 7H10 agar slants, containing 0.5% glycerol and 10% OADC (Difco). The MIC for rifampin was determined by the Etest to be 0.06 to 0.25 μg/ml, which was identical to that reported by Wanger and Mills (37).
Monocyte cell lines.
The J774 murine macrophage cell line was obtained from the American Type Culture Collection (ATCC [TIB 67], Rockville, Md.) and maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum and 2 mM l-glutamine (J774 medium). The MM6 cell line was obtained from the German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany. The MM6 cell line was originally established by H. W. L. Ziegler-Heitbrock, Institute of Immunology, University of Munich, Munich, Germany (41), and is a human acute monocytic leukemia cell line. This cell line has been previously used by us to examine the effectiveness of antimycobacterial drugs against intracellularly replicating M. tuberculosis (40). MM6 cells were maintained in RPMI 1640 containing 10% (vol/vol) fetal calf serum, 2 mM l-glutamine, nonessential amino acids, 1 mM sodium pyruvic acid, and 9 μg of bovine insulin (Sigma Chemical Co., St. Louis, Mo.) per ml (MM6 medium). Cell lines were routinely assayed to verify the absence of mycoplasma contamination by using the Gen-Probe Mycoplasma Rapid Detection system (Gen-Probe, San Diego, Calif.).
Treatment of monocytes with microspheres.
The J774 and MM6 monocytic cell lines were treated with either placebos or rifampin-loaded microsphere formulations by the procedure described below. In one set of experiments, noninfected monocytes were used in order to obtain uptake and release data for the rifampin-loaded microspheres. In another set of experiments, M. tuberculosis H37Rv-infected monocytes were used in order to examine the intracellular effectiveness of rifampin-loaded microsphere formulations. In additional experiments, J774 macrophages were allowed to take up rifampin-loaded microspheres for 24 h and assessed for the amount of drug delivered during that uptake period. That information was then used to compare the effectiveness of rifampin given in microsphere formulations with equivalent concentrations of free drug used to treat M. tuberculosis-infected macrophages.
MM6 cells were plated in 12-well tissue culture plates (Corning) at a concentration of 4 × 105 cells/ml/well in MM6 medium containing 1% fetal bovine serum (FBS). Three plates for each formulation were set up to harvest at 2, 4, and 7 days. To nine wells per plate, 40 μg of rifampin-containing microspheres was added; three wells per plate were controls. At the time of harvest, the contents of each well were transferred to sterile microcentrifuge tubes and centrifuged at 10,000 × g for 4 min at 4°C. Supernatants from triplicate wells were removed and transferred to a sterile Pyrex tube (12 by 100 mm), frozen, and lyophilized. Microscopic observation of supernatants showed the absence of macrophages or microspheres.
The J774 macrophages were plated in Falcon 12-well tissue culture plates at a concentration of 2 × 105 cells/well/ml. At 24 h, the J774 medium was replaced with 1.0 ml of J774 medium per well containing only 1% FBS. Experimental wells had 40 μg of microspheres added. Samples were harvested at 2, 4, and 7 days in the same manner as that for MM6 samples. For bioassays, lyophilized samples were resuspended in 240 μl of sterile water (Sigma) and kept on ice. Eighty-microliter samples from each Pyrex tube, equivalent to the contents of one well of a triplicate, were absorbed onto disks and evaluated for drug activity by the bioassay described below.
Macrophage viability assays.
Cell viability was determined by means of an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide [thiazolyl blue]) cytotoxicity assay (Sigma), which was modified in our laboratory as described previously (40).
Bioassay.
A bioassay with Staphylococcus aureus (ATCC 29213) was developed to determine rifampin concentrations in macrophage culture supernatants. Stock solutions of rifampin in HPLC-grade methanol (Fisher) were prepared, aliquoted, and frozen at −70°C. For assays, standard solutions of rifampin in the appropriate macrophage cell line’s tissue culture medium were prepared in duplicate and assayed with test samples to provide a standard concentration curve.
Sterile filter paper disks (13 mm in diameter; Schleicher and Schuell) were aseptically placed into individually coded wells of 12-well tissue culture plates, and 80 μl of each sample was absorbed onto the disc. During preparation, the plates were placed on cold pack trays. The tissue culture plates containing impregnated disks were refrigerated at 4°C until application onto agar plates. By the direct colony suspension method (23a), a suspension of S. aureus (ATCC 29213) was prepared and adjusted to match a 0.5 McFarland turbidity standard. Within 15 min of suspension preparation, 150-mm Mueller Hinton agar plates were swabbed for lawn growth. The previously loaded disks of macrophage culture supernatants, as well as standard drug concentrations, were aseptically applied to the inoculated plates by using sterile forceps and were gently tapped to make uniform contact with the medium. The plates were sealed with Parafilm, inverted, and placed in a 37°C incubator (no CO2) for 18 to 20 h. At termination of incubation, zones for drug standards were measured and a standard curve was plotted. Zone diameters for test samples were measured, and values were entered into the computer for regression analysis to determine the amount of drug in 80 μl of macrophage culture supernatant. The values were converted to micrograms of drug per milliliter of macrophage culture supernatant.
Determination of rifampin content in phagocytosed microspheres.
J774 macrophages were plated at a concentration of 2 × 105 cells/ml/well and placed in a CO2 incubator at 37°C. The cells were incubated overnight, after which medium was replaced with medium containing 1% serum. After an additional incubation of 24 h, one set of macrophages (one 12-well plate) was dosed with 150 μg of 1.4% rifampin-loaded microspheres, a second set (one 12-well plate) was dosed with 150 μg of placebo microspheres, and a third set (one 12-well plate) was maintained as a reagent control. After incubation at 37°C for 24 h, adherent macrophages were washed three times to remove unphagocytosed microspheres (as evidenced by microscopic observation). Three hundred microliters of sterile 0.125% sodium dodecyl sulfate (SDS) in water (Sigma) was added to each well, and the plates were shaken briefly by hand. Following incubation at 37°C for 20 min, the contents of four wells were pooled in sterile Pyrex screw-cap tubes. The wells were washed three times with sterile water (Sigma), which was added to the tubes. This resulted in three pooled samples per plate. The samples were frozen and lyophilized.
Following lyophilization, 400 μl of ethyl acetate (certified by the American Chemical Society [Fisher]) was added to each tube, and the tubes were shaken overnight. Ethyl acetate dissolved the microsphere polymer and released free rifampin. The tubes were then placed in a desiccator, and the atmosphere was evacuated with a vacuum pump for 6 h. Samples were then reconstituted in 320 μl of J774 medium containing 1% serum, and the rifampin bioassay was performed as described above.
Preparation of mycobacteria for infection.
Before infection of monocyte cell lines, M. tuberculosis H37Rv was initially grown in Middlebrook 7H9 (Difco Laboratories, Detroit, Mich.) containing 0.5% Tween 80 (Sigma) and 10% albumin-dextrose-catalase (Difco). After the mycobacteria had reached exponential phase, they were dispersed by vortexing with glass beads, and clumps were allowed to settle for 30 min (23). Supernatant was removed, aliquoted, frozen at −70°C, and then thawed and used for infection by resuspension in appropriate cell culture medium. Actual numbers of CFU/milliliter were determined by preparation of serial dilutions in Dulbecco’s phosphate-buffered saline (DPBS; Mediatech, Inc., Herndon, Va.) and plating on 7H10 agar.
Infection of monocytes.
Prior to infection, J774 cells were plated at a concentration of 2 × 105 cells per ml per well in 12-well tissue culture dishes (Falcon). After allowing cells to adhere overnight, the medium was replaced with fresh medium containing 1% serum in order to reduce cell proliferation (27). After 48 h, the adherent cells were enumerated by means of an ocular grid to determine the number of macrophages (40). Mycobacteria were suspended in RPMI 1640 containing 1% FBS, and the suspension was dispensed into individual wells at a density of five mycobacteria per macrophage (40). Infected J774 cells were then incubated at 37°C in an atmosphere containing 5% carbon dioxide for 4 h. Following incubation, the supernatant was aspirated, and the cells were washed twice with DPBS (Mediatech) to remove unphagocytosed mycobacteria. Fresh medium (1.0 ml), with or without microsphere preparations, or free rifampin was then added to each well, and the experiments were continued to completion. One milliliter of fresh medium was added at day 4.
Before infection, MM6 cells were adjusted to 8 × 105 cells per ml, and 0.5 ml per well was dispensed in 12-well tissue culture dishes (Corning Costar Corp., Cambridge, Mass.). The mycobacteria were then added to the MM6 cells to achieve a final ratio of 20 mycobacteria per macrophage, with a density of 4 × 105 MM6 cells per 1.0 ml per well (40). After infection for 4 h, the infected MM6 cells were collected by centrifugation (200 × g) and washed twice with DPBS (Mediatech) to remove any unphagocytosed mycobacteria. The cells were then replated at a density of 4 × 105 cells per 1.0 ml per well, with appropriate wells containing microsphere preparations, and the plates were incubated at 37°C with 5% carbon dioxide. One milliliter of fresh medium was added at day 4, and infection was continued until CFU assays.
Determination of CFU.
Determination of CFU at zero hour was conducted by first lysing the monocytes with 0.25% (wt/vol) SDS in DPBS and then plating serial dilutions onto 7H10 agar plates (40). As a means of decreasing viscosity, 5 μl (5 U of activity) of RQ DNase (Promega Corp., Madison, Wis.) with MgSO4 (5 mM) was added to each well following addition of SDS, and the plates were incubated at 37°C for 20 min (40). The plating procedure was repeated at 7 days, and numbers of CFU were enumerated after 10 to 14 days of incubation of cultures.
RESULTS
Characteristics of microsphere formulations.
Although numerous formulations of rifampin-loaded microspheres were prepared in this study, only representative ones are listed in Table 1. These are the primary formulations that eventually led to the development of the final preparation that gave the best results in macrophage studies. Because of size distribution and in vitro release characteristics, formulations 5 and 6 were eventually chosen for further studies involving macrophages. The benefit of using a combination of standard-molecular-weight DL-PLG and a low-molecular-weight DL-PLG (e.g., formulations 5 and 6 [Table 1]) is that the low-molecular-weight formulation degrades at a faster rate, thus resulting in optimum release over the length of any particular experiment. This was particularly important with regard to the macrophage cell lines, because experimental constraints allowed us to extend an experiment for only 7 days. Formulation 7 was developed in order to improve release characteristics in macrophages by incorporating carboxymethyl cellulose. However, as discussed later in this article (see Table 2), this formulation was not adequate for continued studies.
TABLE 1.
Representative formulations of rifampin-loaded microspheres
| Formulation no. | Excipient | Excipient Solvent | Rifampin content (wt%)
|
Microsphere size (μm)a | In vitro release (%) after 2 days | |
|---|---|---|---|---|---|---|
| Theoretical | Observed | |||||
| 1 | 60:40 DL-PLG | Methylene chloride | 2 | 0.62 | 15 | 0 |
| 2 | 60:40 DL-PLG | Ethyl acetate | 5 | 0.25 | 8.3 | 5 |
| 3 | 60:40 DL-PLG | Methylene chloride | 5 | 3.1 | 10 | 2 |
| 4 | 60:40 DL-PLG | Methylene chloride | 5 | 2.9 | 8.9 | 3.8 |
| 5 | 60:40/50:50 DL-PLGb | Methylene chloride | 5 | 1.4 | 7.5 | 8.0 |
| 6 | 60:40/50:50 DL-PLGb | Methylene chloride | 5 | 1.8 | 8.8 | 7.0 |
| 7 | 60:40/50:50 DL-PLG | Methylene chloride/CMCc | 5 | 3.2 | 14.7 | NDd |
Data are reported as 90 volume percentiles
Excipient is a blend of 60:40 DL-PLG and low-molecular-weight 50:50 DL-PLG.
CMC, sodium carboxymethyl cellulose used as a surfactant.
ND, not done.
TABLE 2.
Release characteristics of rifampin-loaded microsphere formulations in MM6 and J774 monocytic cell linesa
| Time (days) | Value for MM6 cellsb
|
Value for J774 cellsb
|
||||
|---|---|---|---|---|---|---|
| Formulation 5 | Formulation 6 | Formulation 7 | Formulation 5 | Formulation 6 | Formulation 7 | |
| 2 | <0.008c | <0.008c | <0.008 | 0.026 ± 0.006 | <0.008 | <0.008c |
| 4 | 0.037 ± 0.005 | 0.011 ± 010 | 0.009 ± 0.0002 | 0.026 ± 0.004 | 0.0084 ± 0.0006 | 0.0097 ± 0.0008 |
| 7 | 0.044 ± 0.001 | 0.017 ± 0.002 | 0.005 ± 0.001 | 0.040 ± 0.005 | 0.015 ± 0.001 | 0.026 ± 0.002 |
Experiments were conducted for 7 days as described in Materials and Methods. Rifampin concentrations in cell supernatants were quantitated by means of a bioassay, which is also described in Materials and Methods. Each experiment was conducted in triplicate, and values are reported as the means ± standard errors of the means.
Values are in micrograms of rifampin per milliliter.
Detectable level of the bioassay was 0.008 μg/ml.
In vitro release of microsphere formulations.
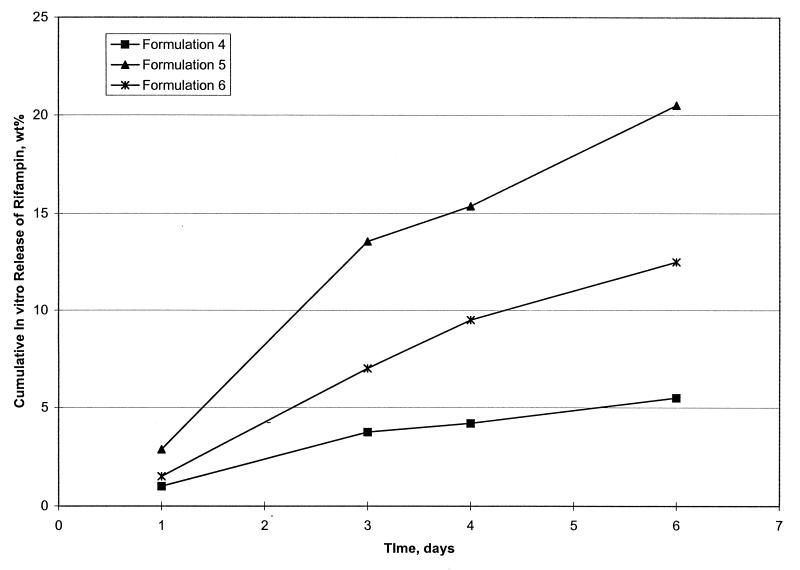
Initially, rifampin-loaded microsphere formulations were evaluated for in vitro release at 2 days. Based on this information and other characteristics, including percent loading and size, formulations were then chosen for extended in vitro release evaluation (Table 1). To conform to the 7-day macrophage experiments, extended release was evaluated for 6 days. Formulations 4, 5, and 6 were chosen for these extended studies, which are shown in Fig. 1. Formulations 5 and 6 produced the best in vitro release pattern, resulting in 21 and 12% cumulative in vitro drug release, respectively, after 6 days (Fig. 1).
FIG. 1.
In vitro release characteristics of rifampin-loaded microsphere formulations over a 6-day period in receiving fluid. Microsphere formulations 4, 5, and 6 are shown. The method used is described in Materials and Methods.
Microsphere size distribution and surface morphology.
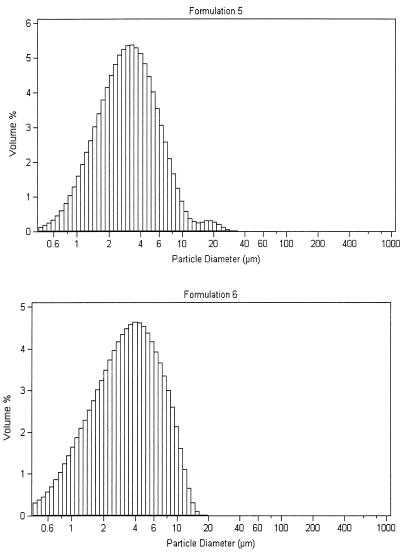
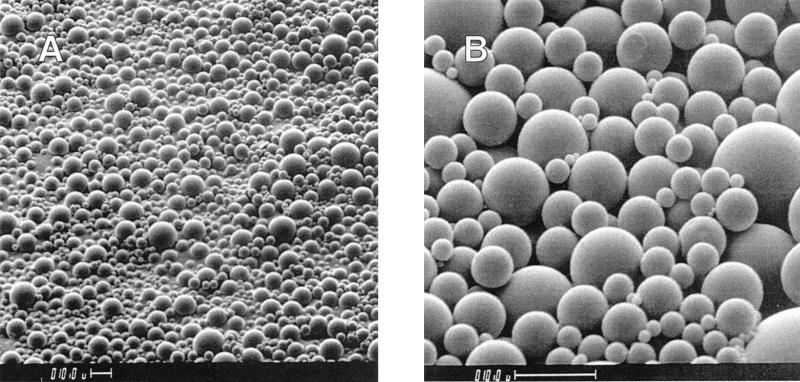
Each microsphere formulation was evaluated for size distribution and surface morphology to ensure optimum delivery to macrophages. For this reason, it was important to maintain a 1- to 10-μm size. Size distributions for formulations 5 and 6 are given in Fig. 2 (top and bottom). The average size for these formulations was 3 to 4 μm, and distribution demonstrated a Gaussian curve. The surface morphology of a typical microsphere formulation is shown in Fig. 3, with formulation 5 as an example. There was no evidence of cracks, holes, or major defects in the outer film of the formulations.
FIG. 2.
Size distributions of rifampin-loaded microsphere formulations 5 (top) and 6 (bottom) as determined with a Malvern particle size analyzer. Data are plotted as volume percentile versus particle diameter.
FIG. 3.
Scanning electron micrographs of rifampin-loaded microsphere formulation 5 taken at scope magnifications of ×500 (A) and ×2,000 (B). Bars, 10 μm.
Release of rifampin from macrophages treated with various microsphere formulations.
Before experiments were conducted to determine effectiveness of rifampin-loaded microspheres on intracellularly replicating mycobacteria, it was necessary to determine release characteristics of microsphere formulations within macrophages. Three formulations were chosen for this purpose, i.e., formulations 5, 6, and 7 (Table 2). Each of these formulations is described in Table 1.
Of the three formulations, formulation 5 gave the best release pattern in both the MM6 and the J774 monocytic cell lines. At the end of 7 days, release from this formulation resulted in approximately 2.6 and 8.1 times greater concentrations of rifampin in MM6 cells than with formulations 6 and 7, respectively (Table 2). In J774 cells, release from formulation 5 was 2.7 and 1.5 times greater than that from formulations 6 and 7, respectively, at the end of 7 days (Table 2). For this reason, formulation 5 was chosen for more extensive studies in macrophages.
Macrophage viability assays.
To assess the effects of microsphere formulations on macrophages, viability assays were conducted with the MM6 human monocytic cell line. The effects of free drug and equivalent drug delivered with microspheres on macrophage viability are indicated in Table 3. As discussed above, the MIC for the M. tuberculosis H37Rv strain used in this investigation is 0.06 to 0.25 μg/ml. Three doses were tested based on the higher MIC and were 0.25, 0.75, and 2.0 μg/ml. For treatment with microsphere formulations, equivalent doses were used, based on the loading percent for that particular formulation. In this case, the formulation contained 1.4 wt% rifampin (formulation 5 [see above]); therefore, 18, 54, and 143 μg of the microsphere formulation were necessary to deliver sufficient rifampin for the MIC, three times the MIC (3× MIC), and eight times the MIC (8× MIC), respectively (Table 3).
TABLE 3.
Effect of rifampin-loaded microspheres on viability of macrophages at the end of a 7-day dosinga
| Factor | Amt of rifampin (μg/ml) | Effect on MM6 (% viability after 7 days) | Amt of microspheres necessary to deliver equivalent concn of rifampin (μg/ml) | Effect on MM6 (% viability after 7 days) |
|---|---|---|---|---|
| MIC | 0.25 | 91 | 18 | 100 |
| 3× MIC | 0.75 | 77 | 54 | 100 |
| 8× MIC | 2.0 | 42 | 143 | 92 |
Microsphere formulation 5 (1.4 wt%) was used in these experiments.
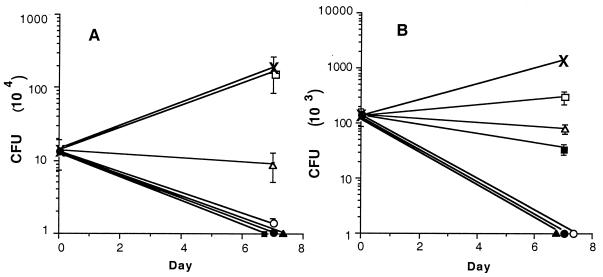
In preliminary experiments (data not presented), the lower end of the MIC range (0.06 μg/ml) was used for rifampin-loaded microspheres. However, because of percent release from microspheres during the experimental time period, reduction in mycobacterial intracellular growth was observed, but it was not significant. With the higher MIC equivalents presented in Table 3, it was possible to obtain significant reductions in mycobacterial growth with the microsphere formulations (Fig. 4). At these concentrations, it was observed that rifampin delivered by microspheres had reduced toxicity compared to rifampin delivered at equivalent concentrations extracellularly (Table 3).
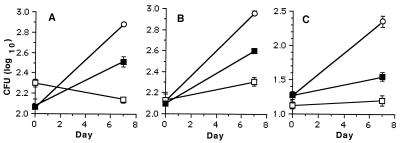
FIG. 4.
Effectiveness of rifampin-loaded microspheres (formulation 5) at reducing intracellular growth of M. tuberculosis H37Rv in murine J774 (A) and human MM6 (B) monocytic cell lines. Cell lines were infected with M. tuberculosis H37Rv as described in Materials and Methods and treated with rifampin-loaded microspheres containing 1.4 wt% rifampin (formulation 5) for 7 days. Samples were plated at 0 and 7 days to determine numbers of CFU. Rifampin concentrations for free drug were 0 (×), MIC (■), 3× MIC (▴), and 8× MIC (•). Rifampin concentrations of drug-loaded microspheres were MIC (□), 3× MIC (▵), and 8× MIC (○).
Effectiveness of rifampin-loaded microspheres on intracellular growth of M. tuberculosis.
The antimycobacterial effectiveness of rifampin-loaded microspheres in both the murine J774 and the human MM6 monocytic cells lines was examined. Following infections of monocytic cell lines with M. tuberculosis H37Rv, cells were treated with rifampin added either directly to culture medium or by means of drug-loaded microspheres. Infected cells were treated with rifampin concentrations equal to the MIC (i.e., 0.25 μg of rifampin/ml) and to 3× and 8× MICs (i.e., 0.75 and 2.0 μg of rifampin/ml, respectively) (Fig. 4). The total amounts of microspheres added in these experiments were 18, 54, and 143 μg/well, for MIC, 3× MIC, and 8× MIC, respectively.
In the J774 murine cell line, a significant reduction in numbers of CFU (P < 0.001) was observed for all three concentrations of rifampin given as a free drug (Fig. 4A). For rifampin delivered in microsphere formulation 5 (1.4 wt%), 3× and 8× MICs both resulted in significant reductions in numbers of CFU (P < 0.001) at 7 days (Fig. 4A). The rifampin-loaded microspheres, which were given at a concentration equivalent to the MIC (i.e., 0.25 μg/ml), did not cause significant reduction in CFU at 7 days (Fig. 4A). However, as extrapolated from the data presented in Table 2, only 8% of the rifampin had been released from the microsphere formulations by day 7. Even so, significant reduction in the numbers of CFU was observed with 3× and 8× MIC equivalent rifampin-loaded microspheres. With the MM6 cell line, a significant reduction in numbers of CFU was observed for all concentrations of free rifampin (P < 0.001) as well as equivalent concentrations of rifampin-loaded microspheres (P < 0.05, < 0.001, and < 0.001 for MIC, 3× MIC, and 8× MIC, respectively) (Fig. 4B). In parallel experiments, placebo microspheres were also added to MM6 and J774 cells at concentrations of 4, 40, and 400 μg of placebo/ml. In these experiments, no significant reduction in numbers of CFU was observed at 7 days following infection with M. tuberculosis H37Rv.
Effectiveness of drug delivery by microspheres versus free drug.
The following experiments were conducted in order (i) to determine the amount of rifampin that is delivered to macrophages with microspheres following 24 h of feeding prior to infection and (ii) to compare the effectiveness of that dose to an equivalent dose of free drug. The J774 murine macrophage cell line was first allowed to phagocytose rifampin-loaded microspheres (formulation 5, 1.4 wt% rifampin) for 24 h. By the bioassay, the mean concentration of rifampin that was delivered with the microspheres was determined to be 0.094 ± 0.003 μg/well (n = 9). This information was then used to set up three successive experiments, in triplicate, to compare the effectiveness of equivalent doses of rifampin given extracellularly. In each group, J774 cells were infected with M. tuberculosis H37Rv and either not treated, treated with free rifampin, or treated with rifampin-loaded microsphere formulation 5. The experimental data for all three experiments are given in Fig. 5A to C. The mean free drug concentration used to treat infected macrophages was 0.09 ± 0.002 μg of rifampin/well (n = 9), and the mean concentration of rifampin delivered by total release from microspheres was 0.097 ± 0.008 μg/well (n = 9). In each experiment, doses of rifampin delivered by means of the microsphere formulation were able to significantly reduce CFU compared to the nontreated control (P was determined to be ≤0.001 for each experiment) (Fig. 5A to C). More importantly, doses of rifampin delivered by microspheres were able to significantly reduce CFU compared to equivalent doses delivered as free drug (P was determined to be ≤0.001 for each experiment) (Fig. 5A to C).
FIG. 5.
Comparison of levels of antimycobacterial effectiveness of free rifampin versus equivalent doses delivered with rifampin-loaded microspheres. Three experiments were performed, i.e., experiments 1 (A), 2 (B), and 3 (C). J774 macrophages were infected with M. tuberculosis H37Rv and treated with no rifampin (○), 0.09 ± 0.002 μg of rifampin/well (n = 9) (■) as free drug, or 0.097 ± 0.008 μg of rifampin/well (n = 9) delivered in microsphere formulation no. 5 (□). Numbers of CFU were determined in triplicate at 0 and 7 days, as described in Materials and Methods. In each experiment, the difference between the mean value for the nontreated control and that for drug-treated sets was significant at P ≤ 0.001. Also, in each experiment the difference between the mean values for the cells treated with free rifampin versus those treated with rifampin-loaded microspheres was significant at P ≤ 0.001. Significance was determined by one-way analysis of variance, and a correction for multiple comparisons (posttest) was performed by the Tukey-Kramer multiple-comparisons test.
DISCUSSION
This report describes the development and use of microsphere formulations that can be used for delivery of rifampin to host macrophages. Delivery of the drug results in intracellular release that is capable of producing significant reduction in numbers of CFU of M. tuberculosis actively dividing in host macrophages. In the particular series described in this study, the 60:40/50:50 DL-PLG formulation, use of methylene chloride as the excipient solvent appears to be the best for delivery to macrophages. Also important for extended release in macrophages is the percent loading. Even though formulation 6 was prepared with the same formulation and contained a slightly higher concentration of rifampin (1.8 versus 1.4%), the lower percent loading in formulation 5 produced a slightly higher release in macrophages over a 7-day period (about 2.5 times greater). However, it should be noted that in this study macrophage cell line experiments were limited to 7 days. Even so, significant reductions in numbers of CFU were observed during this time period though only about 8% of the rifampin had been released. This suggests that under experimental conditions allowing for extended periods of study (e.g., an animal model), efficient release of the drug would continue for even longer time intervals. Furthermore, in an appropriate animal model or human host, higher percent loadings that would achieve optimum release over an even longer period of time may be possible.
Also important is the fact that delivery of rifampin to host macrophages by means of the microsphere formulations produces significantly greater reduction in intracellular replication of M. tuberculosis than does an equivalent concentration of rifampin given as free drug. Targeting to macrophages was achieved by maintaining a size distribution of less than 10 μm for microsphere formulations and careful attention to microsphere morphology. In addition, delivery of rifampin by means of microsphere formulations reduced the toxicity of rifampin for the human monocytic cell line MM6. This is an important property that will be necessary for extended studies involving the use of these formulations to treat tuberculosis in appropriate animal models and eventually humans. Thus, not only can microsphere technology deliver rifampin more efficiently to host macrophages, it can deliver the drug at higher concentrations and with reduced toxicity. This may prove to be even more important when considering other antimycobacterial drugs that have toxicities greater than rifampin.
The biocompatibility of microspheres prepared from poly(dl-lactide) and DL-PLG has been studied and reported by Visscher et al. (33–35). Moreover, these polymers have been used for other medical applications; the earliest biomedical uses of these polymers was in synthetic resorbable sutures (17). These polymers biodegrade by undergoing random, nonenzymatic, hydrolytic scissioning of their ester linkages to form lactic acid and glycolic acid, which are normal metabolic compounds (30). Lactic acid and glycolic acid then break down further into carbon dioxide and water (30). Encapsulated drugs are released over an extended period of time, depending on several factors associated with both the drug and the microsphere. Thus, the biocompatability of these microsphere formulations has been well established.
There are several advantages to using controlled-release microspheres. These have been summarized in a review by Tice and Cowsar (30) and are given here briefly. First, patient compliance is no longer a problem, because dosing is automatic and the levels in blood are preprogrammed. These properties would be useful in the treatment of tuberculosis, because of the chronic nature of the disease. Second, the first-pass effect, which is inherent in the peroral route, is not a factor. Third, targeting of the drug is possible. This is extremely important in tuberculosis because drugs can be targeted to the macrophages where the tubercle bacilli are replicating and persisting. Also, it may be possible to use more toxic drugs to treat infection with MDRTB strains, because these drugs would be released in optimum amounts over an extended period of time at the site of infection (i.e., within macrophages). Microencapsulation techniques might also offer an effective method to treat latent tuberculosis. Lastly, microspheres can be given by conventional routes, such as intravenous injection, and because of their size (i.e., ≤10 μm) (21) it is conceivable that the small microcapsules could be delivered by aerosolization directly to the alveolar macrophages in the lungs. These parameters will have to be tested in appropriate animal models. The results reported here are an important first step in designing these studies and suggest that use of this technology can result in important improvements in therapeutic regimens used in the treatment of tuberculosis.
A comparable technology for drug delivery is the use of liposomes. This technology has been used to deliver various drugs to treat M. avium (4, 8, 10, 12, 16, 20, 24, 26) and M. tuberculosis infections (9, 25, 36). Although there have been some promising results, more studies are necessary to improve liposome technology in this area. The microsphere technology reported here is not meant to replace liposome technology; however, there are some distinct differences that make microsphere technology more ideal for clinical use. First, the microspheres are more stable for prolonged storage; second, microspheres can be formulated for sustained release for several weeks to months; and third, microspheres can be formulated in sizes for targeting macrophages (reported in this article) as well as larger sizes with increased percent loading for sustained systemic release (studies in progress). These attributes make microsphere technology applicable to use in developing countries and for improving drug compliance during treatment of mycobacterial infections.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grant AI38185.
We thank Lloyd Carlson for the scanning electron micrographs and W. Suling, curator of SRI Bacteriological Stock Collection, for use of M. tuberculosis H37Rv (ATCC 27294, SRI no. 1345).
REFERENCES
- 1.American T S. Control of tuberculosis in the United States. Am Rev Respir Dis. 1992;1992:1623–1633. doi: 10.1164/ajrccm/146.6.1623. [DOI] [PubMed] [Google Scholar]
- 2.Amin N M. Let’s stop the comeback of tuberculosis. Postgrad Med. 1990;88:107–124. doi: 10.1080/00325481.1990.11716425. [DOI] [PubMed] [Google Scholar]
- 3.Barksdale L, Kim K S. Mycobacterium. Bacteriol Rev. 1977;41:217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez L E M, Wu M, Young L S. Intracellular killing of Mycobacterium avium complex by rifapentine and liposome-encapsulated amikacin. J Infect Dis. 1987;156:510–513. doi: 10.1093/infdis/156.3.510. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. Nosocomial transmission of multidrug-resistant tuberculosis to health-care workers and HIV-infected patients in an urban hospital. Florida Morbid Mortal Weekly Rep. 1990;39:718–722. [PubMed] [Google Scholar]
- 6.Centers for Disease Control. Nosocomial transmission of multidrug-resistant tuberculosis among HIV-infected persons. Florida and New York Morbid Mortal Weekly Rep. 1991;40:649–652. [PubMed] [Google Scholar]
- 7.Cowsar D R, Tice T R, Gilley R M, English J P. Poly(lactide-co-glycolide) microcapsules for controlled release of steroids. Methods Enzymol. 1985;112:101–116. doi: 10.1016/s0076-6879(85)12010-0. [DOI] [PubMed] [Google Scholar]
- 8.Cynamon M H, Swenson C E, Palmer G S, Ginsberg R S. Liposome-encapsulated-amikacin therapy of Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1989;33:1179–1183. doi: 10.1128/aac.33.8.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deol P, Khuller G K, Joshi K. Therapeutic efficacies of isoniazid and rifampin encapsulated in lung-specific stealth liposomes against Mycobacterium tuberculosis infection induced in mice. Antimicrob Agents Chemother. 1997;41:1211–1214. doi: 10.1128/aac.41.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duzgunes N, Perumal V K, Kesavalu L, Goldstein J A, Debs R J, Gangadharam P R J. Enhanced effect of liposome-encapsulated amikacin on Mycobacterium avium-M. intracellulare complex infection in beige mice. Antimicrob Agents Chemother. 1988;32:1404–1411. doi: 10.1128/aac.32.9.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edlin B R, Tokars J I, Grieco M H, Crawford J T, Williams J, Sordillo E M, Ong K R, Kilburn J O, Dooley S W, Castro K G, Jarvis W R, Holmberg S D. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers S, Bucke W, Leitzke S, Fortmann L, Smith D, Hansch H, Hahn H, Bancroff G, Muller R. Liposomal amikacin for treatment of M. avium infections in clinically relevant experimental settings. Zentralbl Bakteriol. 1996;284:218–231. doi: 10.1016/s0934-8840(96)80097-1. [DOI] [PubMed] [Google Scholar]
- 13.Eldridge J H, Hammond C J, Meulbroek J A, Staas J K, Gilley R M, Tice T R. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. J Control Release. 1990;11:205–214. [Google Scholar]
- 14.Eldridge J H, Staas J K, Meulbroek J A, Tice T R, Gilley R M. Biodegradable and biocompatible poly(dl-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun. 1991;59:2978–2986. doi: 10.1128/iai.59.9.2978-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine P. Leprosy and tuberculosis—an epidemiological comparison. Tubercle. 1984;65:137–153. doi: 10.1016/0041-3879(84)90067-9. [DOI] [PubMed] [Google Scholar]
- 16.Gangadharam P R, Ashtekar D R, Flasher D L, Duzgunes N. Therapy of Mycobacterium avium complex infections in beige mice with streptomycin encapsulated in sterically stabilized liposomes. Antimicrob Agents Chemother. 1995;39:725–730. doi: 10.1128/AAC.39.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilding D K, Reed A M. Biodegradable polymers for use in surgery polyglycolic/poly(lactic acid) homo- and copolymers. Polymer. 1979;20:1459–1464. [Google Scholar]
- 18.Hora M S, Rana R K, Nunberg J H, Tice T R, Gilley R M, Hudson M E. Release of human serum albumin from poly(lactide-co-glycolide) microspheres. Pharm Res. 1990;7:1190–1194. doi: 10.1023/a:1015948829632. [DOI] [PubMed] [Google Scholar]
- 19.Jacob E, Setterstrom J A, Bach D E, Heath J R, McNiesh L M, Cierny I G. Evaluation of biodegradable ampicillin anhydrate microcapsules for local treatment of experimental staphylococcal osteomyelitis. Clin Orthop Relat Res. 1991;267:237–244. [PubMed] [Google Scholar]
- 20.Leitzke S, Bucke W, Borner K, Muller R, Hahn H, Ehlers S. Rationale for and efficacy of prolonged-interval treatment using liposome-encapsulated amikacin in experimental Mycobacterium avium infection. Antimicrob Agents Chemother. 1998;42:459–461. doi: 10.1128/aac.42.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow P E. Aerosol characterization and deposition. Am Rev Respir Dis. 1974;110:88–102. doi: 10.1164/arrd.1974.110.6P2.88. [DOI] [PubMed] [Google Scholar]
- 22.National Action Plan To Combat Multidrug-Resistant Tuberculosis. Meeting the challenge of multidrug-resistant tuberculosis: summary of a conference. Management of persons exposed to multidrug-resistant tuberculosis. Morbid Mortal Weekly Rep. 1992;41:1–71. [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Antimycobacterial susceptibility testing for Mycobacterium tuberculosis: tentative standard. Report M24-T. 15, no. 16. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 23a.National Committee for Clinical Laboratory Standards. Document M2-A5. 13, no. 24. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 24.Nightingale S D, Saletan S L, Swenson C E, Lawrence A J, Watson D A, Pilkiewicz F G, Silverman E G, Cal S X. Liposome-encapsulated gentamicin treatment of Mycobacterium avium-Mycobacterium intracellulare complex bacteremia in AIDS patients. Antimicrob Agents Chemother. 1993;37:1869–1872. doi: 10.1128/aac.37.9.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orozco L C, Quintana F O, Beltrán R M, de Moreno I, Wasserman M, Rodriguez G. The use of rifampicin and isoniazid entrapped in liposomes for the treatment of murine tuberculosis. Tubercle. 1986;67:91–97. doi: 10.1016/0041-3879(86)90002-4. [DOI] [PubMed] [Google Scholar]
- 26.Petersen E A, Grayson J B, Hersh E M, Dorr R T, Chiang S M, Oka M, Proffitt R T. Liposomal amikacin: improved treatment of Mycobacterium avium complex infection in the beige mouse model. J Antimicrob Chemother. 1996;38:819–828. doi: 10.1093/jac/38.5.819. [DOI] [PubMed] [Google Scholar]
- 27.Rastogi N, Potar M-C, David H L. Intracellular growth of pathogenic mycobacteria in the continuous murine macrophage cell line J774: ultrastructure and drug-susceptibility studies. Curr Microbiol. 1987;16:79–92. [Google Scholar]
- 28.Redding T W, Schally A V, Tice T R, Meyers W E. Long-acting delivery systems for peptides: inhibition of rat prostate tumors by controlled release of [d-Trp6]luteinizing hormone-releasing hormone from injectable microcapsules. Proc Natl Acad Sci USA. 1984;81:5845–5848. doi: 10.1073/pnas.81.18.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snider D E J, Raviglione M, Kochi A. Global burden of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 3–11. [Google Scholar]
- 30.Tice T R, Cowsar D R. Biodegradable controlled-release parenteral systems. J Pharm Technol. 1984;8:26–36. [Google Scholar]
- 31.Tice T R, Rowe C E, Gilley R M, Setterstrom J A, Mirth D D. Proceedings of the 13th International Symposium on Controlled Release of Bioactive Materials. Lincolnshire, Ill: Controlled Release Society, Inc.; 1986. Development of microencapsulated antibiotics for topical administration; pp. 169–170. [Google Scholar]
- 32.United States Pharmacopeial Convention. U.S. Pharmacopeia. 22nd ed. Rockville, Md: U.S. Pharmacopeial Convention, Inc.; 1990. [Google Scholar]
- 33.Visscher G E, Robison R L, Argentieri G I. Tissue response to biodegradable injectable microcapsules. J Biomater Appl. 1987;2:118–131. doi: 10.1177/088532828700200103. [DOI] [PubMed] [Google Scholar]
- 34.Visscher G E, Robison R L, Maulding H V, Fong J W, Pearson J E, Argentieri G I. Biodegradation of and tissue reaction to 50:50 poly(dl-lactide-co-glycolide) microcapsules. J Biomed Mater Res. 1985;19:349–365. doi: 10.1002/jbm.820190315. [DOI] [PubMed] [Google Scholar]
- 35.Visscher G E, Robison R L, Maulding H V, Fong J W, Pearson J E, Argentieri G I. Biodegradation of and tissue reaction to microcapsules. J Biomed Mater Res. 1986;20:667–676. doi: 10.1002/jbm.820200510. [DOI] [PubMed] [Google Scholar]
- 36.Vladimirsky M A, Ladigina G A. Antibacterial activity of liposome-entrapped streptomycin in mice infected with Mycobacterium tuberculosis. Biomedicine. 1982;36:375–377. [PubMed] [Google Scholar]
- 37.Wanger A, Mills K. Testing of Mycobacterium tuberculosis susceptibility to ethambutol, isoniazid, rifampin, and streptomycin by using Etest. J Clin Microbiol. 1996;34:1672–1676. doi: 10.1128/jcm.34.7.1672-1676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. WHO declares tuberculosis a global emergency. Soz-Praevmed. 1993;38:251–52. [PubMed] [Google Scholar]
- 39.World Health Organization. TB: a global emergency. Report 94.177. Geneva, Switzerland: World Health Organization; 1994. [Google Scholar]
- 40.Wright E L, Quenelle D C, Suling W J, Barrow W W. Use of Mono Mac 6 human monocytic cell line and J774 murine macrophage cell line in parallel antimycobacterial drug studies. Antimicrob Agents Chemother. 1996;40:2206–2208. doi: 10.1128/aac.40.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler-Heitbrock H W L, Thiel E, Fütterer A, Herzog V, Wirtz A. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988;41:456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]