Abstract
Introduction:
Inflammatory processes help protect the body from potential threats such as bacterial or viral invasions. However, when such inflammatory processes become chronically engaged, synaptic impairments and neuronal cell death may occur. In particular, persistently high levels of C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α) have been linked to deficits in cognition and several psychiatric disorders. Higher-order cognitive processes such as fluid intelligence (Gf) are thought to be particularly vulnerable to persistent inflammation. Herein, we investigated the relationship between elevated CRP and TNF-α and the neural oscillatory dynamics serving Gf.
Methods:
Seventy adults between the ages of 20–66 years (Mean=45.17 years, SD=16.29, 21.4% female) completed an abstract reasoning task that probes Gf during magnetoencephalography (MEG) and provided a blood sample for inflammatory marker analysis. MEG data were imaged in the time-frequency domain, and whole-brain regressions were conducted using each individual’s plasma CRP and TNF-α concentrations per oscillatory response, controlling for age, BMI, and education.
Results:
CRP and TNF-α levels were significantly associated with region-specific neural oscillatory responses. In particular, elevated CRP concentrations were associated with blunted gamma activity in the right inferior frontal gyrus and right cerebellum. In contrast, elevated TNF-α levels scaled with alpha/beta oscillations in the left anterior cingulate and left middle temporal, and gamma activity in the left intraparietal sulcus.
Discussion:
Elevated inflammatory markers such as CRP and TNF-α were associated with aberrant neural oscillations in regions important for Gf. Linking inflammatory markers with regional neural oscillations may hold promise in identifying mechanisms of cognitive and psychiatric disorders.
Keywords: Inflammation, magnetoencephalography, MEG, abstract reasoning, alpha, gamma
Introduction
When the innate immune system is activated, acute inflammatory processes engage to defend the body from potential threats such as viral or bacterial invasion (Lyman et al. 2014; Stephenson et al. 2018). However, when this regulatory system is perturbed and/or chronically engaged, inflammatory processes can lead to the downstream development of disease states (Dolsen et al. 2019), as pro-inflammatory mediators can allow leukocytes to migrate into the brain causing synaptic impairment, neuronal death, and tissue damage (de Vries et al. 1996; Lyman et al. 2014). In fact, it has been shown that such systemic inflammation can cause and accelerate long-term neurodegenerative and psychiatric diseases like dementia and depression (Lyman et al. 2014; Stephenson et al. 2018; Dolsen et al. 2019).
Several inflammatory markers, including C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α) have been widely associated with systemic inflammation and cognitive impairment (Heringa et al. 2014). CRP is a sensitive measure of the acute and non-specific response that occurs to protect the body from infection, damage to tissues, and inflammation (Pepys and Hirschfield 2003). One proinflammatory cytokine that is particularly responsible for inducing CRP expression and inflammation is TNF-α (Felger et al. 2020). Thus, TNF-α is instrumental in modulating inflammatory processes and the various agents involved (Aggarwal et al. 2012), which is beneficial for neutralizing potential infections and efficiently removing damaged cells (Probert 2015). TNF-α is also thought to have a role in regulating vital physiological processes such as synaptic plasticity (Beattie et al. 2002; Kaneko et al. 2008), cognition (Baune et al. 2008; Beste et al. 2010), and astrocyte-mediated synaptic amplification (Olmos and Lladó 2014). Abnormal TNF-α responses can also impair neuronal functioning through excessive glutamatergic activation leading to excitotoxicity, which has been implicated in cognitive dysfunction associated with psychiatric and neurodegenerative diseases (Olmos and Lladó 2014; Heringa et al. 2014; Lyman et al. 2014). However, unfortunately, the relationship between CRP and TNF-α levels and brain function remains poorly understood.
Previous studies have linked elevated peripheral CRP and TNF-α levels to systemic inflammatory disease states as seen in aging, Alzheimer’s disease, Parkinson’s disease, and HIV-infection (Hirsch and Hunot 2009; Roberts et al. 2009; Holmes et al. 2009; Noble et al. 2010; Tegeler et al. 2016; Becher et al. 2017; Spooner et al. 2021). For example, inflammatory responses such as the invasion of leukocytes to the central nervous system (CNS) or activation of CNS-based inflammatory cells (e.g., microglia) result in the overproduction of cytokines including TNF-α and CRP, which in turn, influences downstream cellular processes (e.g., redox imbalances) to attenuate neuronal cell functionality and ultimately, induce cell death and functional dependencies in numerous pathologies exhibiting neurocognitive dysfunction (Hirsch and Hunot 2009; Becher et al. 2017; Pepys and Hirschfield 2003; Barbosa et al. 2012; Doganavsargil-Baysal et al. 2013; Xiu et al. 2019). Specifically, fluid intelligence (Gf) has been shown to be particularly vulnerable to low-grade systemic inflammatory processes (Luciano et al. 2009; Lee et al. 2016; Kappelmann et al. 2019), but the brain regions and processes involved remain poorly understood. Gf is broadly defined as the ability to problem-solve in novel situations, learn new skills, and adapt to changing environments (Horn and Cattell 1966). Gf is known to rely on the prefrontal cortices (Crone and Richard Ridderinkhof 2011; Duncan 2013), as well as distributed network-level interactions between prefrontal and parietal circuits, along with input from occipital and temporal regions, the latter being central to the parieto-frontal integration theory of intelligence (Jung and Haier 2007).
Prior work focusing on the neural dynamics serving Gf have identified theta oscillations in frontoparietal regions (Neubauer et al. 2017; Taylor et al. 2020, 2022; Heinrichs-Graham et al. 2022), which is consistent with studies of other higher-order cognitive functions closely coupled with Gf, such as cognitive control, attention, and working memory (Cavanagh and Frank 2014; Rajan et al. 2019). Such studies have also consistently implicated gamma activity in frontoparietal and occipital cortices (Cohen and Ridderinkhof 2013; Rosen and Reiner 2017) and alpha oscillations, with the latter supporting inhibiting neural processes (Sadaghiani et al. 2012; Cohen and Ridderinkhof 2013; Taylor et al. 2022). These findings are further supported by a study that used transcranial direct stimulation (tDCS) of the left and right dorsolateral prefrontal cortices and found that stronger alpha/beta parieto-frontal phase coherence was associated with slower reaction times during an abstract reasoning task (Arif et al. 2021). Despite the well-known role of CRP and TNF-α in systemic inflammatory processes on the brain and behavior and ample evidence that these processes impact Gf, little is known regarding how elevated CRP and TNF-α levels affect the neural processing underlying Gf. Thus, in the current study, we quantified the relationship between peripheral CRP and TNF-α levels and the neural oscillations serving Gf using an abstract reasoning task in a sample of adults. We predicted that elevated CRP and TNF-α would be associated with weaker neural oscillatory activity in the theta, alpha/beta, and gamma range in brain regions critical for Gf.
Methods
Participants
Seventy cognitively normal participants between the ages of 20 and 66 years were selected from an ongoing study (R01 MH116782) based on their completion of: (1) an abstract reasoning task during MEG, (2) structural MRI, and (3) a blood draw. Overall, the sample had an average age of 45.17 years (SD = 16.29 years; Supplemental Figure 1A), an average BMI of 29.23 (SD = 5.88; Supplemental Figure 1B), and an average education level equivalent to a bachelor’s degree (M = 16.29 years, SD = 2.04 years). Three participants were left-handed and the remaining participants were right-handed. In terms of race, 78.6% were white, 11.4% were Black and/or African American, 7.1% were Asian, and 2.9% identified as more than one race. Of these participants, 92.9% identified as not Hispanic, while 7.1% identified as Hispanic. The sample was 21.4% female at birth and 78.6% male. Exclusion criteria included a history of neurological or psychiatric diagnoses, history of head trauma, current substance use disorder, and the use of psychiatric and neurological prescription medications (e.g., anticonvulsants, benzodiazepines, antipsychotics) that are known to strongly affect neural functioning. All demographic data were obtained via self-report from participants during the intake process. The study protocol was approved by the local Institutional Review Board and each participant provided written informed consent following a detailed description of the study.
Evaluation of C-Reactive Protein and Tumor Necrosis Factor-α
Comprehensive quantification of CRP and TNF-α were conducted based on electrochemiluminescence-based multiplex immunoassays in plasma. Specifically, samples were prepared on multispot 96-well plates from the V-PLEX Human Cytokine 54-Plex Kit (Meso Scale Discovery) according to manufacturer instructions. Plates were analyzed using a Meso Scale QuickPlex SQ 120 and sample concentrations were calculated using the Discovery Workbench 4.0 software using a 4-PL curve model. Any concentrations below the lower level of detection based on 2.5 SD above the assay background blank were reported as 0 pg/mL. Because two kits were used for inflammatory cytokine quantification, values were standardized to control for potential differences in the kits.
Abstract Reasoning Task Paradigm
Participants completed an abstract reasoning task adapted from the classic Raven’s Progressive Matrices (Raven 2003), which is a widely used assessment of Gf. The specific adaptations included making the task non-progressive so that difficulty was constant across all participants and changing the stimulus presentation format so that participants could respond to each trial using a button pad. Participants were shown a centrally presented fixation cross in a 2 × 2 grid for a period of 2500 to 3000 ms (Figure 1), with either the bottom left or bottom right box highlighted by a white border. An array of four complex figures was then presented in each of the four boxes within the 2 × 2 grid for 4000 ms. Participants were instructed to respond whether the complex figure in the highlighted box accurately completed the 2 × 2 matrix based on the color, shape, and/or orientation of the patterns in the other three boxes. Participants responded by pressing a button with their right index finger if the highlighted figure correctly completed the matrix (i.e., match), or by pressing a button with their right middle finger if the highlighted figure did not correctly complete the matrix (i.e., non-match), regardless of hand dominance. There were 120 trials, equally split and pseudorandomized between correct and incorrect matrix completions. The task took approximately 14 minutes to complete.
Figure 1. Abstract Reasoning Task Paradigm.
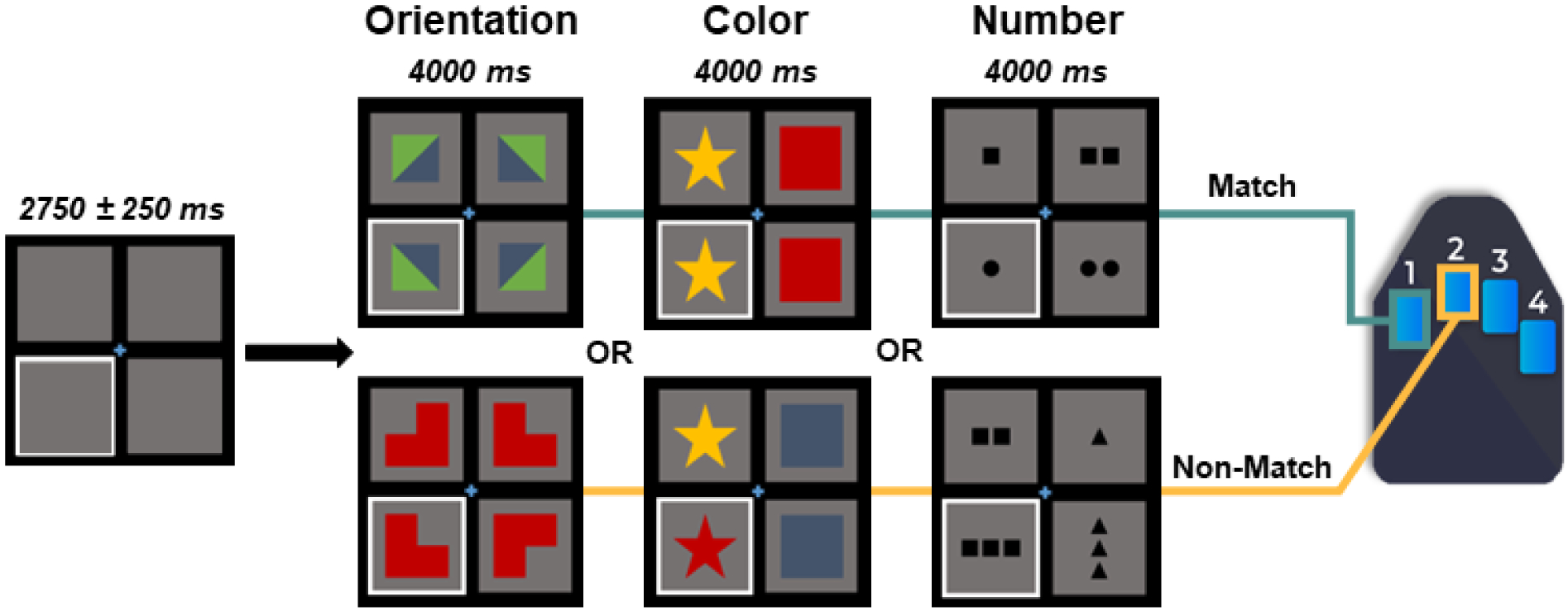
Participants were presented with an empty grid of gray boxes for 2500 to 3000 ms with either the left or right bottom square highlighted by a white border to indicate the location of the upcoming target. Complex images then populated each of the four squares within the grid for 4000 ms. Participants indicated whether the image in the highlighted square correctly completed the pattern in the grid by responding via button press (i.e., right index finger for matching patterns, 60 trials; right middle finger for non-matching patterns, 60 trials). Match and non-match trials were presented in a pseudo-randomized order for the duration of the task. Participants performed well on the task, with a mean accuracy of 85.6% (SD = 8.6%) and mean reaction time of 1309.4 ms (SD = 300.6 ms).
MEG Data Acquisition
Functional MEG data were collected using a MEGIN MEG system (Helsinki, Finland) equipped with 306 sensors (204 planar gradiometers, 102 magnetometers) that were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz. All recordings occurred in a one-layer magnetically shielded room with active shielding engaged. Prior to MEG acquisition, four coils were attached to the participant’s head and localized along with fiducial and scalp surface points using a three-dimensional (3D) digitizer (FASTRAK 3SF0002, Polhemus Navigator Sciences, Colchester, Vermont). Once the participants were positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the four coils, thus inducing a measurable magnetic field which enabled each coil to be localized in reference to the MEG sensor array throughout the recording session.
MEG and MRI Processing
MEG and MRI data processing closely followed previously reported pipelines for this cognitive task (Taylor et al. 2020, 2022; Arif et al. 2021). The structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. MEG data were subjected to environmental noise reduction and corrected for head motion using the signal space separation method with a temporal extension (Taulu and Simola 2006). Only data from the 204 planar gradiometers were used for further analysis. All MEG and MRI data were further processed in BESA (Research: Version 7.1; MRI: Version 3.0; Statistics: Version 2.1). Cardiac and ocular artifacts were removed from the MEG data using signal space projection (SSP; Uusitalo and Ilmoniemi 1997), and this correction was accounted for during source analysis.
MEG Pre-Processing
The continuous magnetic time series was then filtered with a 60 Hz notch filter. Epochs were 6500 ms, with the baseline extending from −1800 to −800 ms prior to visual stimulus onset to minimize any anticipation effects. Only trials with correct responses were considered for further analysis. Epochs containing artifacts were rejected using a fixed threshold method that was set per participant and supplemented with visual inspection. Briefly, in MEG, the raw signal amplitude is strongly affected by the distance between the brain and the MEG sensor array, as the magnetic field strength falls off sharply as the distance from the current source (i.e., brain) increases. To account for this source of variance across participants, as well as other sources of variance, we used an individualized threshold based on the signal distribution for both amplitude and gradient to reject artifacts. The average amplitude threshold across all participants was 1384.47 (SD = 707.59) fT/cm, the average gradient threshold was 337.77 (SD = 315.66) fT/(cm*ms), and an average of 109.71 (SD = 8.19) trials out of the original 120 were used for further analysis. The number of trials included in the final MEG analyses was not significantly associated with CRP (r = −0.16, p = 0.210) or TNF-α (r = −0.02, p = 0.879) levels.
MEG Time-Frequency Transformation & Sensor-Level Statistics
We then transformed the artifact-free epochs into the time-frequency domain (resolution: 2 Hz, 25 ms) using complex demodulation (Papp and Ktonas 1977; Kovach and Gander 2016). Each sensor’s spectral power estimations were averaged over trials to produce time-frequency plots of mean spectral density, which were then normalized by the baseline power of each respective bin, calculated as the mean power from −1800 to −800 ms. The time-frequency windows for subsequent source imaging were identified using a stringent two-stage statistical approach that utilized paired-sample t-tests against baseline on each pixel in the spectrogram (per sensor) at the first stage, followed up with cluster-based nonparametric permutation testing at the second level. This testing was conducted across all participants and the entire frequency range (4 – 100 Hz) and used an initial cluster threshold of p < 0.05 and 5000 permutations. These methods are described in depth in our recent publications (Wiesman and Wilson 2020; Wiesman et al. 2021).
MEG Source Imaging
Time-frequency resolved source images were computed using the dynamic imaging of coherent sources (DICS) beamformer to image oscillatory activity in the time-frequency windows of interest per participant (Van Veen et al. 1997; Gross et al. 2001). Following convention, we used task and baseline periods of equal duration and bandwidth for each time-frequency window identified in the sensor-level statistical analyses to derive noise-normalized source power per voxel for each participant. The resulting pseudo-t maps represent noise-normalized source power differences (i.e., active versus baseline) per participant and voxel (resolution: 4 × 4 × 4 mm). These maps were then transformed into standardized space and spatially resampled by applying the same transform that was applied to the native space structural MRI per participant.
Whole-Brain Statistics
To probe whole-brain relationships between neural oscillatory power serving abstract reasoning and CRP and TNF-α levels, spectrally specific whole-brain maps for each participant were used. Briefly, we first computed whole-brain voxel-wise regression analyses between spectrally specific neural activity and CRP and TNF-α, separately, controlling for age, BMI, and education (Taylor et al. 2020; Schantell et al. 2022). Individual participant-level maps containing significant artifacts were excluded from analysis. To account for multiple comparisons, a significance threshold of p < 0.005 was used for the identification of significant clusters in all whole-brain statistical maps, accompanied by a cluster (k) threshold of at least 25 contiguous voxels (i.e., 1600 mm3 of brain tissue) based on the theory of Gaussian random fields (Poline et al. 1995; Worsley et al. 1996). To visualize effects, pseudo-t values were extracted from the peak voxel of the resultant significant clusters of neural activity (i.e., the voxel with the highest statistical value per cluster) in each participant. All whole-brain statistical analyses were computed using SPM12 in MATLAB (MathWorks; Natick, Massachusetts) and other statistical analyses were conducted in IBM SPSS v.25.
Results
Participant Characteristics and Behavioral Results
A total of 70 participants successfully completed the abstract reasoning task and provided a blood sample for inflammatory marker analysis. Participants had an average peripheral CRP level of 3.10 mg/L (SD = 3.38 mg/L; CRPNormed M = 0.84, CRPNormed SD = 0.91) and an average TNF-α level of 5.51 pg/mL (SD = 3.49 pg/mL; TNF-αNormed M = 0.95, TNF-αNormed SD = 0.30). Overall, participants performed well on the abstract reasoning task in terms of accuracy 85.6% (SD = 8.6%) and mean reaction time of 1309.4 ms (SD = 300.6 ms). There were no significant relationships between CRP and TNF-α levels and task behavior, controlling for age, BMI, and education. There was a significant relationship between reaction time and accuracy in which those with a faster reaction time responded more accurately to the abstract reasoning task, controlling for age, BMI, and education (r = −0.61, p < 0.001). Further, age was associated with both reaction time (r = 0.52, p < 0.001) and accuracy (r = −0.40, p = 0.001), and accuracy was associated with BMI (r = −0.26, p = 0.033) and education (r = 0.33, p = 0.007). Thus, we adjusted for age, BMI, and education in all subsequent whole-brain analyses.
Oscillatory Neural Responses
We observed robust neural oscillatory responses in three temporally and spectrally defined windows during the abstract reasoning task (Figure 2). These included statistically significant increases in power relative to the baseline period in the theta band (0 – 250 ms; 4 – 8 Hz), a decrease in power in the alpha/beta band (400 – 1300 ms; 8 – 22 Hz), and an increase in power in the gamma band (175 – 500 ms; 62 – 74 Hz). All MEG sensor-level responses were significant at p < 0.05 following multiple comparisons correction using nonparametric permutation testing. At the anatomical level, the theta, alpha/beta, and gamma responses were numerically strongest in the bilateral occipital regions (Figure 2, bottom panel), but robust multispectral changes were also seen across widespread cortical regions in the parietal, temporal, frontal, and prefrontal cortices (see Supplemental Materials).
Figure 2. Neural Oscillatory Responses to the Abstract Reasoning Task.
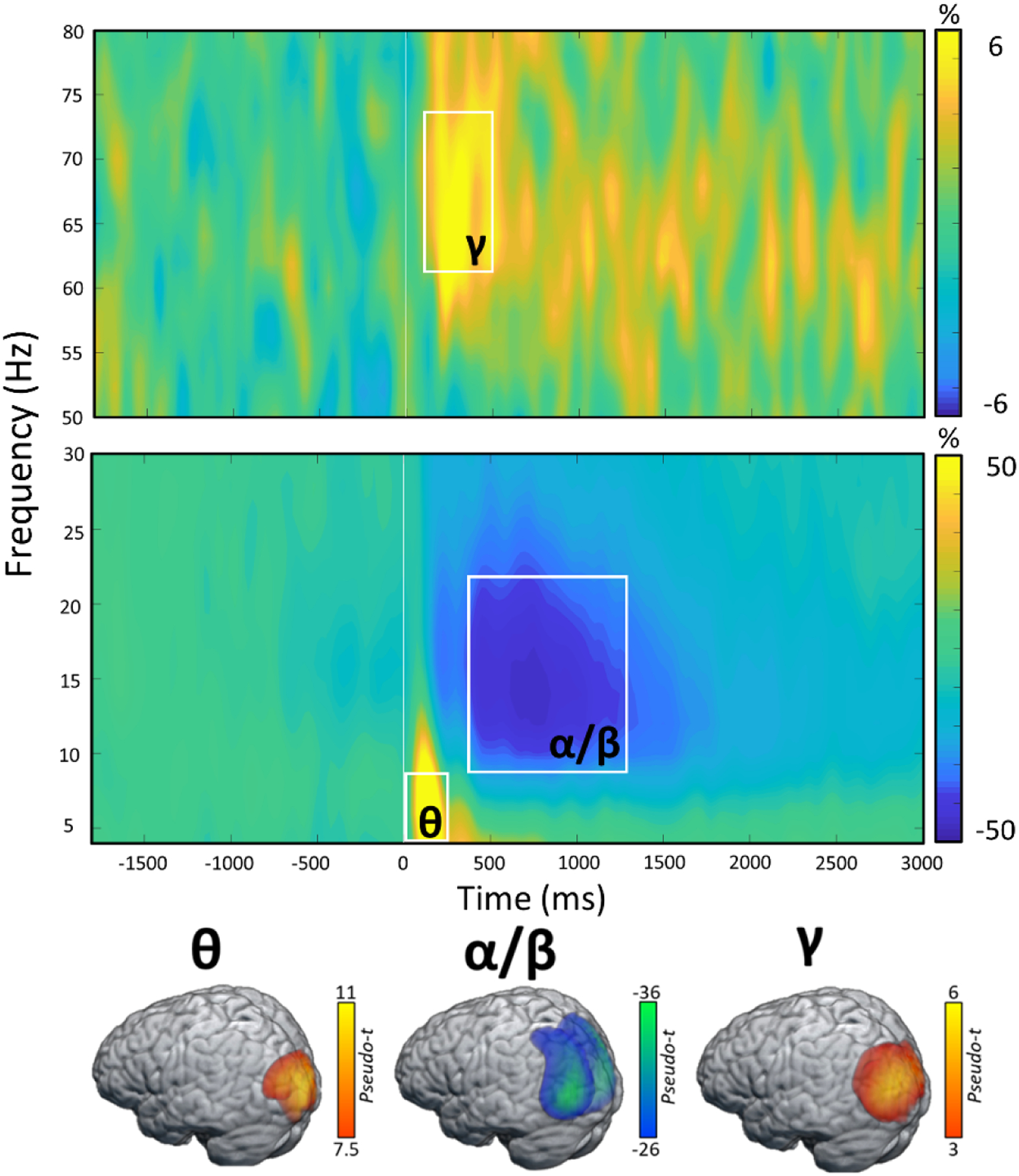
(Top): Grand-averaged time-frequency spectrograms of MEG sensors exhibiting one or more significant oscillatory responses. Shown from top to bottom: gamma (62–74 Hz, 175–500 ms); alpha/beta (8–22 Hz, 400–1300 ms); and theta (4–8 Hz, 0–250 ms). Each spectrogram displays frequency (Hz) on the y-axis and time (ms) on the x-axis. Signal power data are expressed as a percent difference from the baseline period (−1800 to- −800 ms) with color legends shown with each spectrogram. Time 0 on the x-axis represents the onset of the complex images in each of the four boxes. (Bottom): Grand-averaged beamformer images (pseudo-t) across all participants for each time-frequency component across all correct trials.
Whole-Brain Regressions with Inflammatory Cytokines
To address our primary hypotheses, we assessed the relationship between peripheral CRP and TNF-α concentrations separately for each spectrally specific oscillatory response using voxel-wise regressions, controlling for age, BMI, and education. Whole-brain regression analyses revealed that higher TNF-αNormed concentrations were associated with weaker alpha/beta neural oscillations (i.e., less negative) in the left anterior cingulate cortex (r = 0.49, p < 0.005, k = 468 voxels; Figure 3A), weaker alpha/beta neural oscillations in the left middle temporal gyrus (r = 0.40, p < 0.005, k = 37 voxels; Figure 3B), and weaker gamma power (i.e., less positive) in the left intraparietal sulcus (r = −0.46, p < 0.005, k = 26 voxels; Figure 3C) during the abstract reasoning task. Additionally, we found that higher CRPNormed concentrations were associated with stronger gamma oscillatory activity in the right cerebellum (r = 0.44, p < 0.005, k = 95 voxels; Figure 4A) and weaker gamma oscillatory activity in the right inferior frontal gyrus (r = −0.45, p < 0.005, k = 34 voxels; Figure 4B) during task performance. Finally, we assessed whether inflammation-related alterations in neural oscillatory activity scaled with behavioral performance in terms of reaction time (in ms) and accuracy (% correct). These analyses indicated that weaker alpha/beta oscillations (i.e., less negative) in the left middle temporal gyrus were associated with faster reaction times during the abstract reasoning task, adjusting for the effects of age, BMI, and education (r = −0.25, p = 0.050; Figure 5).
Figure 3. Higher Levels of TNF-α are Associated with Blunted Neural Oscillatory Activity Serving Abstract Reasoning.
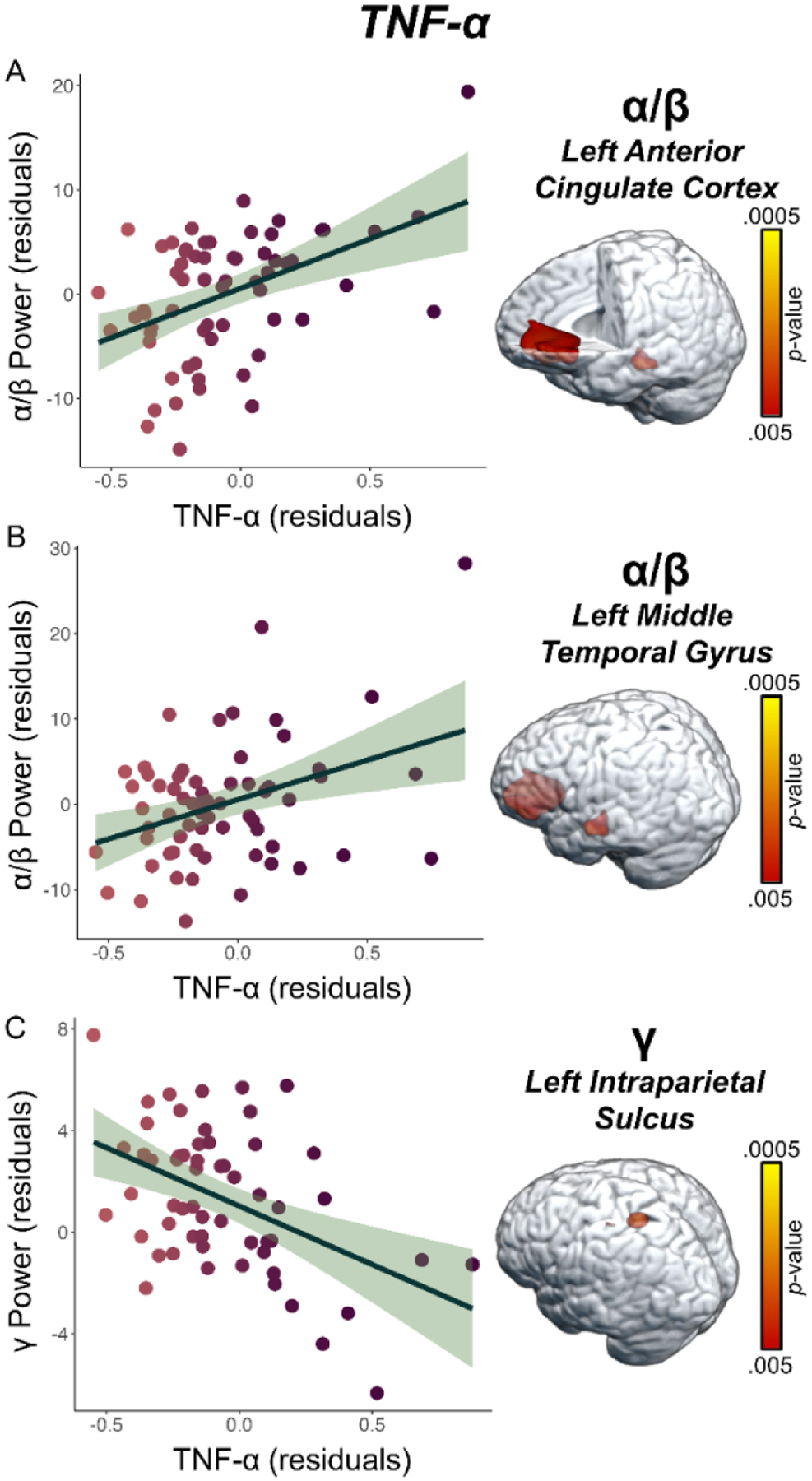
(A) Unstandardized residuals of extracted pseudo-t values from the peak voxel in the left anterior cingulate cortex illustrate the significant relationship between TNF-αNormed (x-axis) and weaker oscillatory alpha/beta power (y-axis) serving abstract reasoning, controlling for age, BMI, and education (p < 0.005). (B) Similarly, weaker alpha/beta power (y-axis) in the left middle temporal gyrus was associated with elevated levels of TNF-αNormed (x-axis). (C) Finally, higher TNF-αNormed (x-axis) levels were also associated with weaker gamma power in the left intraparietal sulcus, controlling for age, BMI, and education (y-axis; p < 0.005). Unstandardized residuals adjusted for age, BMI, and education for TNF-αNormed and oscillatory alpha/beta and gamma power are shown on the x- and y-axes, respectively, of all scatterplots. The green shaded area depicts the standard error of the mean.
Figure 4. Elevated CRP Levels are Associated with Alterations in Gamma Power During the Abstract Reasoning Task.
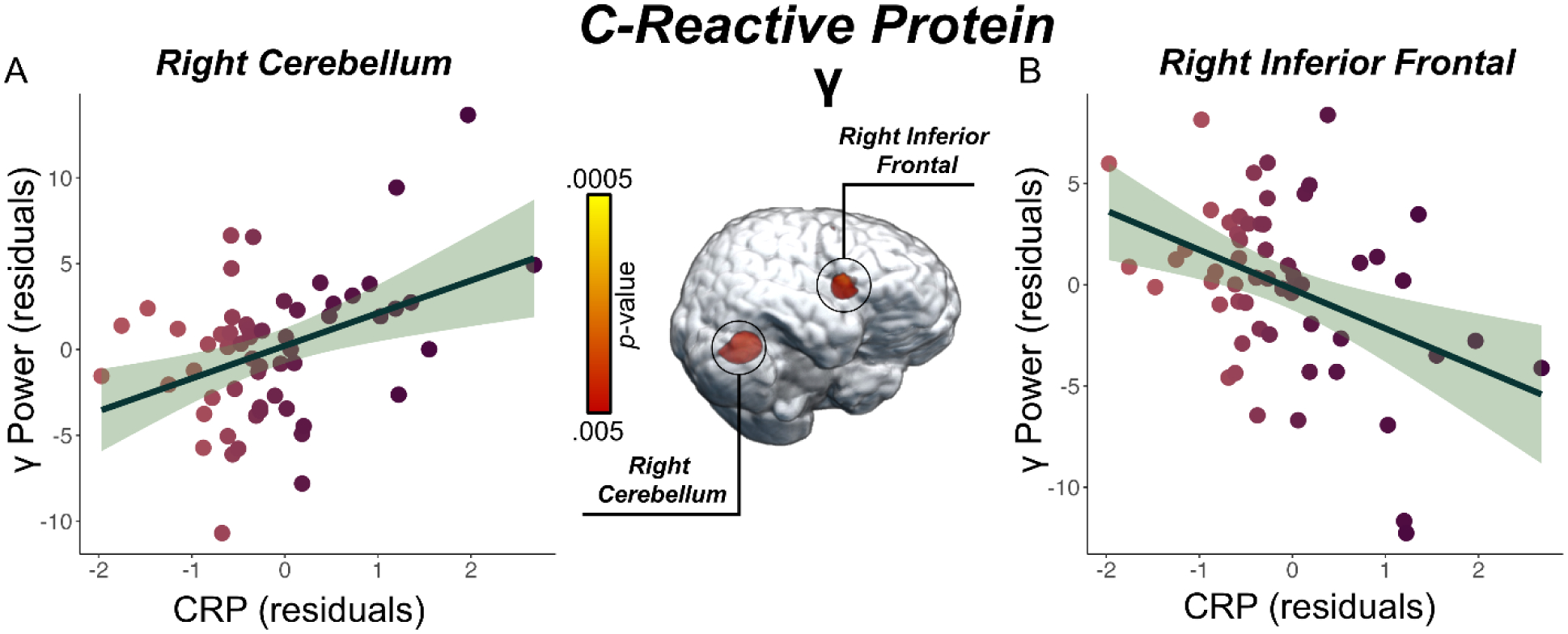
(A) Extracted pseudo-t values from the peak voxel in the right cerebellum show the significant relationship between CRPNormed (x-axis) and stronger oscillatory gamma power (pseudo-t, y-axis) serving abstract reasoning, controlling for age and BMI (p < 0.005). (B) CRPNormed (x-axis) levels were also associated with weaker gamma power in right inferior frontal gyrus, controlling for age, BMI, and education (y-axis; p < 0.005). Unstandardized residuals adjusted for age, BMI, and education for CRPNormed and oscillatory gamma power (pseudo-t) are shown on the x- and y-axes, respectively, of all scatterplots. The green shaded area depicts the standard error of the mean.
Figure 5. Oscillatory Activity Associated with Elevated TNF-α Scales with Performance in the Abstract Reasoning Task.
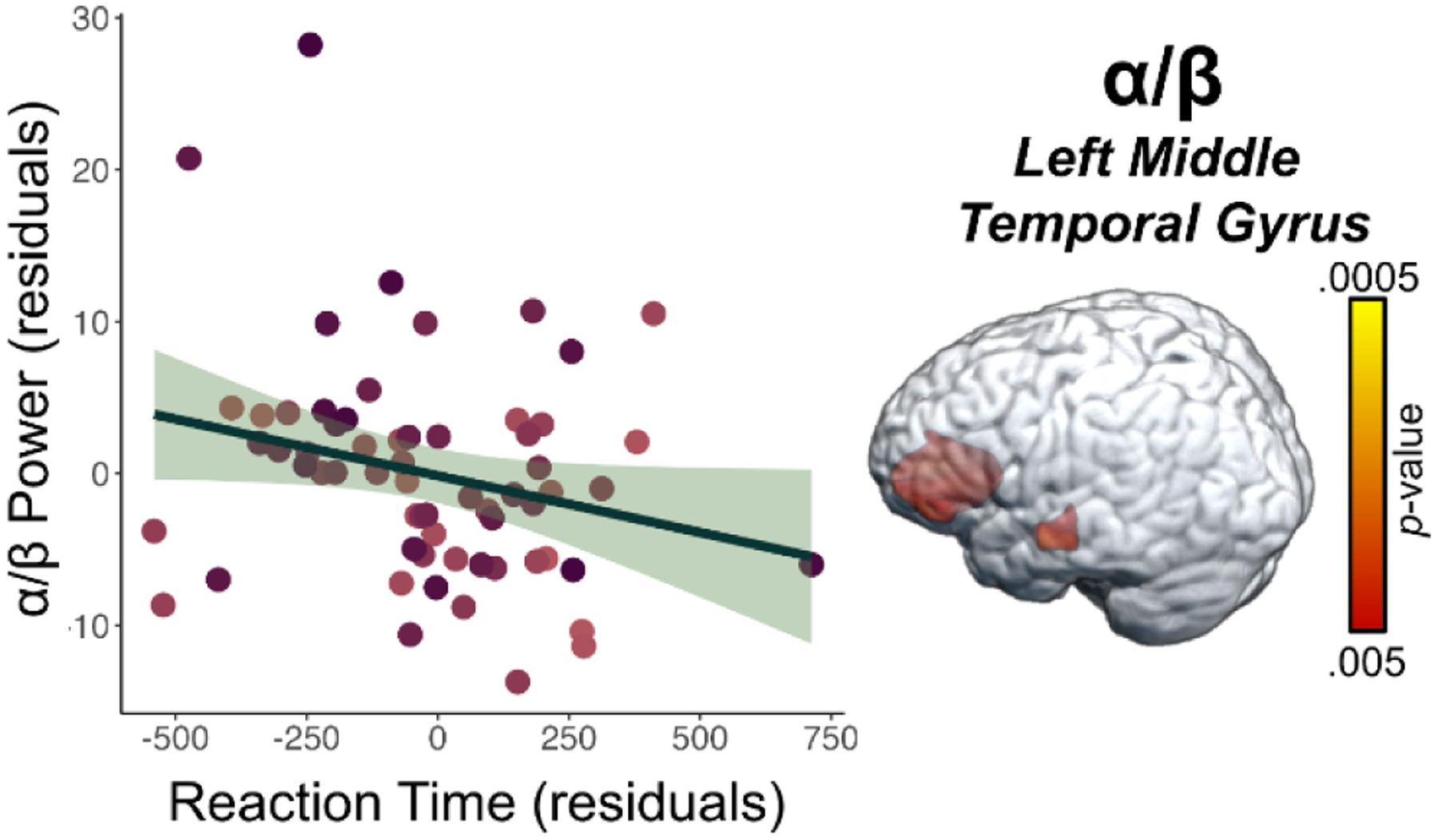
Extracted pseudo-t values from the peak voxel in the left middle temporal gyrus show the significant relationship between reaction time (ms) during the abstract reasoning task and oscillatory alpha/beta power (pseudo-t, y-axis) in the left middle temporal gyrus, controlling for age, BMI, and education (p = 0.050). Unstandardized residuals adjusted for age, BMI, and education for reaction time (ms) and oscillatory alpha/beta power (pseudo-t) are shown on the x- and y-axes, respectively. The green shaded area depicts the standard error of the mean.
Discussion
In the present study, we examined whether TNF-α and CRP were associated with the neural oscillatory dynamics serving abstract reasoning. Our key findings were that higher levels of CRP and TNF-α were associated with altered oscillatory responses in the alpha/beta and gamma bands in regions critical for abstract reasoning among a sample of adults. Specifically, higher TNF-α concentrations were associated with weaker alpha/beta oscillations (i.e., less negative) in the left anterior cingulate and left middle temporal gyrus, as well as weaker gamma oscillations (i.e., less positive) in the left intraparietal sulcus. In addition, higher CRP levels were associated with stronger oscillatory gamma activity in the right cerebellum and weaker oscillatory gamma activity in the right inferior frontal gyrus.
Behaviorally, there were no relationships between peripheral TNF-α or CRP concentrations with performance in terms of accuracy or reaction time during the abstract reasoning task, controlling for age, BMI, and education. However, there was a significant relationship in which participants who responded faster were also more accurate during the task. Previous studies have shown that fluid intelligence is associated with enhanced higher-order cognitive abilities such as attention, working memory, processing speed, and executive functions (Meiran and Shahar 2018). Thus, participants with optimal fluid intelligence abilities, as probed by the abstract reasoning task, would be expected to respond faster. Therefore, the association between reaction time and accuracy identified in the present study is consistent with the extant Gf literature.
A well-established theory of intelligence is the parieto-frontal integration theory (P-FIT) model, which posits that a distributed network consisting of the parietal and frontal cortices is paramount for intelligence abilities, with activity in these regions being further supplemented with input from the occipital cortices to aid in sensory processing. Key regions in the P-FIT model include the dorsolateral prefrontal cortices, the inferior and superior parietal lobules, the anterior cingulate, and regions within the temporal and occipital lobes (Jung and Haier 2007). Our study found that elevated TNF-α levels were significantly associated with weaker oscillatory alpha/beta activity in the left anterior cingulate and left middle temporal gyrus, and weaker gamma power in the left intraparietal sulcus, after controlling for the effects of age, BMI, and education. Thus, our findings link elevated TNF-α levels with aberrant oscillatory alpha/beta power in the anterior cingulate and left middle temporal gyrus, which have both been strongly tied to intelligence and abstract reasoning through the P-FIT model (Knauff et al. 2002; Goel and Dolan 2004; Yuan et al. 2018). Specifically, the literature broadly suggests alpha oscillations directly support higher-order cognitive abilities and the anterior cingulate is known to be a hub for such high-order processing (Palva and Palva 2011; Langer et al. 2012). Further, we found that oscillatory alpha/beta power in the left middle temporal gyrus scaled with behavioral performance in terms of reaction time, underscoring the importance of temporal contributions to task performance on fluid intelligence tasks (Basten et al. 2015), especially given their role in the early processing of sensory information in the P-FIT model (Yuan et al. 2018). This finding builds upon previous work which found that stronger alpha/beta dynamics in association cortices serving abstract reasoning was associated with slower behavioral performance (Arif et al. 2021).
Previous research has also implicated the intraparietal sulcus as a critical interface between perceptive and motor systems, specifically in terms of motor planning for advanced movements (Champod and Petrides 2010); thus, weaker gamma responses in the intraparietal sulcus, as observed in those with elevated TNF-α concentrations, may have a negative impact on sensorimotor integration, which would be critical for this complex problem-solving task. Prior work has also demonstrated that stronger recruitment of oscillatory gamma power is necessary for successfully processing novel visual stimuli (Fries 2009), and recent work using transcranial direct current stimulation (tDCS) has showed that gamma oscillations are critical for both Gf and logical thinking (Arif et al. 2021).
Similarly, we found that weaker oscillatory gamma activity in the inferior frontal gyrus was associated with higher CRP levels, and this may indicate inhibition of the neural processes required for Gf. Specifically, prior work has shown that the inferior frontal gyrus plays a key role in attention processing, which is essential for coordinating and executing goal-directed behavior (Cazzoli et al. 2021). Therefore, weaker oscillatory gamma power in the inferior frontal gyrus and elevated concentrations of CRP may be associated with the early breakdown of attentional control processes. Additionally, in further support of the P-FIT model, we found stronger gamma power in the right cerebellum was associated with elevated CRP concentrations, which is a region known for its role in orchestrating particular cognitive functions into an appropriate system, further highlighting its importance during a matrix reasoning task (Lee et al. 2005; Geier et al. 2009). Of note, the directionality of this cerebellar finding (i.e., stronger neural oscillations with higher CRP or TNF-α levels) differed from the other relationships we observed between markers and neural responses. While this could reflect compensatory processing for the weaker activity observed in the right inferior frontal region, or a related mechanism, additional work is needed to fully understand the significance.
Before closing, it is important to recognize several limitations of this work. First, we focused on cognitively normal adults and future studies should probe clinical populations (e.g., patients with Alzheimer’s disease, people with HIV) to ascertain whether elevated systemic inflammation is directly related to the degree of amyloid burden and/or cognitive decline. Second, we focused on specific inflammatory markers with known associations to cognitive functioning in humans, albeit future work should consider a broader range of markers. Third, we only examined fluid intelligence and future work will need to expand this to other critical areas of cognitive processing. Finally, the only theta window that survived cluster-based permutation testing at the sensor level was an early theta window that may have missed crucial aspects of higher-level theta processing. This may be why we did not identify any relationships between oscillatory theta power with CRP and TNF-α as observed in the alpha/beta and gamma bands. In sum, the current, highly novel study identified that elevated levels of TNF-α and CRP in heathy adults was associated with altered oscillatory responses in critical brain regions serving higher-order cognitive processes, including the anterior cingulate, middle temporal gyrus, intraparietal sulcus, cerebellum, and inferior frontal gyrus. Such findings may suggest that markers of inflammation could serve as promising indicators of early-stage disruptions in cognition. Longer-term alterations of these eloquent neural circuits via systemic inflammation may result in the breakdown of higher-order brain regions, therefore leading to degradations in fluid intelligence. Future work should validate the results found in this study and further delineate the role of systemic inflammation in cognitive decline and whether chronic levels of inflammation modulate the trajectory of age-related cognitive decline in otherwise cognitively normal adults.
Supplementary Material
Highlights.
Persistently high inflammatory levels are known to lead to major health problems.
CRP and TNF-α in particular have been linked to cognitive impairment and decline.
Neural oscillations serving abstract reasoning were linked to CRP and TNF-α.
Elevations in CRP and TNF-α scaled with weaker oscillations during task performance.
CRP and TNF- α levels may be pre-clinical markers of future cognitive decline.
Funding
This research was supported by grants R01-MH116782 (TWW), R01-MH118013 (TWW), P20-GM144641 (TWW), R01-DA047828 (TWW), R01-DA056223 (TWW), F31-DA056296 (MS), and R36-DA059323 (MS) from the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Gupta SC, Kim JH (2012) Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 119:651–665. 10.1182/blood-2011-04-325225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif Y, Spooner RK, Heinrichs-Graham E, Wilson TW (2021) High-definition transcranial direct current stimulation modulates performance and alpha/beta parieto-frontal connectivity serving fluid intelligence. The Journal of Physiology 599:5451–5463. 10.1113/JP282387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa IG, Rocha NP, Huguet RB, et al. (2012) Executive dysfunction in euthymic bipolar disorder patients and its association with plasma biomarkers. Journal of Affective Disorders 137:151–155. 10.1016/j.jad.2011.12.034 [DOI] [PubMed] [Google Scholar]
- Basten U, Hilger K, Fiebach CJ (2015) Where smart brains are different: A quantitative meta-analysis of functional and structural brain imaging studies on intelligence. Intelligence 51:10–27. 10.1016/j.intell.2015.04.009 [DOI] [Google Scholar]
- Baune BT, Wiede F, Braun A, et al. (2008) Cognitive dysfunction in mice deficient for TNF- and its receptors. Am J Med Genet 147B:1056–1064. 10.1002/ajmg.b.30712 [DOI] [PubMed] [Google Scholar]
- Becher B, Spath S, Goverman J (2017) Cytokine networks in neuroinflammation. Nat Rev Immunol 17:49–59. 10.1038/nri.2016.123 [DOI] [PubMed] [Google Scholar]
- Beste C, Baune BT, Falkenstein M, Konrad C (2010) Variations in the TNF- α Gene (TNF-α −308G→A) Affect Attention and Action Selection Mechanisms in a Dissociated Fashion. Journal of Neurophysiology 104:2523–2531. 10.1152/jn.00561.2010 [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ (2014) Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences 18:414–421. 10.1016/j.tics.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzoli D, Kaufmann BC, Paladini RE, et al. (2021) Anterior insula and inferior frontal gyrus: where ventral and dorsal visual attention systems meet. Brain Communications 3:fcaa220. 10.1093/braincomms/fcaa220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champod AS, Petrides M (2010) Dissociation within the Frontoparietal Network in Verbal Working Memory: A Parametric Functional Magnetic Resonance Imaging Study. J Neurosci 30:3849–3856. 10.1523/JNEUROSCI.0097-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR (2013) EEG Source Reconstruction Reveals Frontal-Parietal Dynamics of Spatial Conflict Processing. PLoS ONE 8:e57293. 10.1371/journal.pone.0057293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Richard Ridderinkhof K (2011) The developing brain: From theory to neuroimaging and back. Developmental Cognitive Neuroscience 1:101–109. 10.1016/j.dcn.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Blom-Roosemalen MCM, Oosten M van, et al. (1996) The influence of cytokines on the integrity of the blood-brain barrier in vitro. Journal of Neuroimmunology 64:37–43. 10.1016/0165-5728(95)00148-4 [DOI] [PubMed] [Google Scholar]
- Doganavsargil-Baysal O, Cinemre B, Aksoy UM, et al. (2013) Levels of TNF-α, soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder: TNF-α AND COGNITION IN BIPOLAR DISORDER. Hum Psychopharmacol Clin Exp 28:160–167. 10.1002/hup.2301 [DOI] [PubMed] [Google Scholar]
- Dolsen EA, Crosswell AD, Prather AA (2019) Links Between Stress, Sleep, and Inflammation: Are there Sex Differences? Curr Psychiatry Rep 21:8. 10.1007/s11920-019-0993-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J (2013) The Structure of Cognition: Attentional Episodes in Mind and Brain. Neuron 80:35–50. 10.1016/j.neuron.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Patel TA, et al. (2020) What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry 25:1301–1311. 10.1038/s41380-018-0096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P (2009) Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32:209–224. 10.1146/annurev.neuro.051508.135603 [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B (2009) Development of Working Memory Maintenance. J Neurophysiol 101:84–99. 10.1152/jn.90562.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, Dolan RJ (2004) Differential involvement of left prefrontal cortex in inductive and deductive reasoning. Cognition 93:B109–121. 10.1016/j.cognition.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, et al. (2001) Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A 98:694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Walker EA, Taylor BK, et al. (2022) Auditory experience modulates fronto-parietal theta activity serving fluid intelligence. Brain Communications 4:fcac093. 10.1093/braincomms/fcac093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringa SM, van den Berg E, Reijmer YD, et al. (2014) Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population – the Hoorn Study. Psychoneuroendocrinology 40:108–118. 10.1016/j.psyneuen.2013.11.011 [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397. 10.1016/S1474-4422(09)70062-6 [DOI] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, et al. (2009) Systemic inflammation and disease progression in Alzheimer disease. Neurology 73:768–774. 10.1212/WNL.0b013e3181b6bb95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL, Cattell RB (1966) Refinement and test of the theory of fluid and crystallized general intelligences. Journal of Educational Psychology 57:253–270. 10.1037/h0023816 [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ (2007) The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci 30:135–154. 10.1017/S0140525X07001185 [DOI] [PubMed] [Google Scholar]
- Kappelmann N, Khandaker GM, Dal H, et al. (2019) Systemic inflammation and intelligence in early adulthood and subsequent risk of schizophrenia and other non-affective psychoses: a longitudinal cohort and co-relative study. Psychol Med 49:295–302. 10.1017/S0033291718000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauff M, Mulack T, Kassubek J, et al. (2002) Spatial imagery in deductive reasoning: a functional MRI study. Brain Res Cogn Brain Res 13:203–212. 10.1016/s0926-6410(01)00116-1 [DOI] [PubMed] [Google Scholar]
- Kovach CK, Gander PE (2016) The demodulated band transform. J Neurosci Methods 261:135–154. 10.1016/j.jneumeth.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer N, Pedroni A, Gianotti LRR, et al. (2012) Functional brain network efficiency predicts intelligence. Hum Brain Mapp 33:1393–1406. 10.1002/hbm.21297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Lyoo IK, Kim S-U, et al. (2005) Intellect declines in healthy elderly subjects and cerebellum. Psychiatry Clin Neurosci 59:45–51. 10.1111/j.1440-1819.2005.01330.x [DOI] [PubMed] [Google Scholar]
- Lee SE, West KP, Cole RN, et al. (2016) General intelligence is associated with subclinical inflammation in Nepalese children: A population-based plasma proteomics study. Brain, Behavior, and Immunity 56:253–263. 10.1016/j.bbi.2016.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Marioni RE, Gow AJ, et al. (2009) Reverse Causation in the Association Between C-Reactive Protein and Fibrinogen Levels and Cognitive Abilities in an Aging Sample. Psychosomatic Medicine 71:404–409. 10.1097/PSY.0b013e3181a24fb9 [DOI] [PubMed] [Google Scholar]
- Lyman M, Lloyd DG, Ji X, et al. (2014) Neuroinflammation: The role and consequences. Neuroscience Research 79:1–12. 10.1016/j.neures.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Meiran N, Shahar N (2018) Working memory involvement in reaction time and its contribution to fluid intelligence: An examination of individual differences in reaction-time distributions. Intelligence 69:176–185. 10.1016/j.intell.2018.06.004 [DOI] [Google Scholar]
- Neubauer AC, Wammerl M, Benedek M, et al. (2017) The influence of transcranial alternating current stimulation (tACS) on fluid intelligence: An fMRI study. Personality and Individual Differences 118:50–55. 10.1016/j.paid.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JM, Manly JJ, Schupf N, et al. (2010) Association of C-Reactive Protein to Cognitive Impairment. Arch Neurol 67:87–92. 10.1001/archneurol.2009.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos G, Lladó J (2014) Tumor Necrosis Factor Alpha: A Link between Neuroinflammation and Excitotoxicity. Mediators of Inflammation 2014:1–12. 10.1155/2014/861231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Palva JM (2011) Functional Roles of Alpha-Band Phase Synchronization in Local and Large-Scale Cortical Networks. Front Psychology 2:. 10.3389/fpsyg.2011.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp N, Ktonas P (1977) Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum 13:135–145 [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM (2003) C-reactive protein: a critical update. J Clin Invest 111:1805–1812. 10.1172/JCI200318921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Holmes AP, et al. (1995) Estimating smoothness in statistical parametric maps: variability of p values. J Comput Assist Tomogr 19:788–796. 10.1097/00004728-199509000-00017 [DOI] [PubMed] [Google Scholar]
- Probert L (2015) TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience 302:2–22. 10.1016/j.neuroscience.2015.06.038 [DOI] [PubMed] [Google Scholar]
- Rajan A, Siegel SN, Liu Y, et al. (2019) Theta Oscillations Index Frontal Decision-Making and Mediate Reciprocal Frontal–Parietal Interactions in Willed Attention. Cerebral Cortex 29:2832–2843. 10.1093/cercor/bhy149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JJ (2003) Raven Progressive Matrices. In: McCallum RS (ed) Handbook of Nonverbal Assessment. Springer; US, Boston, MA, pp 223–237 [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, et al. (2009) Association of C-reactive protein with mild cognitive impairment. Alzheimers Dement 5:398–405. 10.1016/j.jalz.2009.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A, Reiner M (2017) Right frontal gamma and beta band enhancement while solving a spatial puzzle with insight. International Journal of Psychophysiology 122:50–55. 10.1016/j.ijpsycho.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, et al. (2012) Alpha-Band Phase Synchrony Is Related to Activity in the Fronto-Parietal Adaptive Control Network. Journal of Neuroscience 32:14305–14310. 10.1523/JNEUROSCI.1358-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell M, Taylor BK, Spooner RK, et al. (2022) Epigenetic aging is associated with aberrant neural oscillatory dynamics serving visuospatial processing in people with HIV. Aging (Albany NY) 14:. 10.18632/aging.204437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Taylor BK, Moshfegh CM, et al. (2021) Neuroinflammatory profiles regulated by the redox environment predicted cognitive dysfunction in people living with HIV: A cross-sectional study. EBioMedicine 70:103487. 10.1016/j.ebiom.2021.103487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J, Nutma E, van der Valk P, Amor S (2018) Inflammation in CNS neurodegenerative diseases. Immunology 154:204–219. 10.1111/imm.12922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J (2006) Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51:1759–1768. 10.1088/0031-9155/51/7/008 [DOI] [PubMed] [Google Scholar]
- Taylor BK, Embury CM, Heinrichs-Graham E, et al. (2020) Neural oscillatory dynamics serving abstract reasoning reveal robust sex differences in typically-developing children and adolescents. Developmental Cognitive Neuroscience 42:100770. 10.1016/j.dcn.2020.100770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Heinrichs-Graham E, Eastman JA, et al. (2022) Longitudinal changes in the neural oscillatory dynamics underlying abstract reasoning in children and adolescents. NeuroImage 253:119094. 10.1016/j.neuroimage.2022.119094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeler C, O’Sullivan JL, Bucholtz N, et al. (2016) The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function--data from the Berlin Aging Study II. Neurobiol Aging 38:112–117. 10.1016/j.neurobiolaging.2015.10.039 [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ (1997) Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35:135–140. 10.1007/BF02534144 [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A (1997) Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44:867–880. 10.1109/10.623056 [DOI] [PubMed] [Google Scholar]
- Wiesman AI, Christopher-Hayes NJ, Eastman JA, et al. (2021) Response certainty during bimanual movements reduces gamma oscillations in primary motor cortex. NeuroImage 224:117448. 10.1016/j.neuroimage.2020.117448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW (2020) Attention modulates the gating of primary somatosensory oscillations. Neuroimage 211:116610. 10.1016/j.neuroimage.2020.116610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, et al. (1996) A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4:58–73. [DOI] [PubMed] [Google Scholar]
- Xiu MH, Wang DM, Du XD, et al. (2019) Interaction of BDNF and cytokines in executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology 108:110–117. 10.1016/j.psyneuen.2019.06.006 [DOI] [PubMed] [Google Scholar]
- Yuan P, Voelkle MC, Raz N (2018) Fluid Intelligence and Gross Structural Properties of the Cerebral Cortex in Middle-Aged and Older Adults: A Multi-Occasion Longitudinal Study. Neuroimage 172:21–30. 10.1016/j.neuroimage.2018.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


