Abstract
Introduction:
Resident memory T (TRM) cells are embedded in peripheral tissue and capable of acting as sentinels that can respond quickly to repeat pathogen exposure as part of an endogenous anti-microbial immune response. Recent evidence suggests that chronic antigen exposure and other microenvironment cues may promote the development of TRM cells within solid tumors as well, and that this TRM phenotype can sequester tumor-specific T cells into tumors and out of circulation resulting in limited systemic antitumor immunity. Here, we perform a review of the published English literature and describe tissue-specific mediators of TRM cell differentiation in states of infection and malignancy with special focus on the role of TGF-β and how targeting TGF-β signaling could be used as a therapeutical approach to promote tumor systemic immunity.
Discussion:
The presence of TRM cells with antigen specificity to neoepitopes in tumors associates with positive clinical prognosis and greater responsiveness to immunotherapy. Recent evidence indicates that solid tumors may act as reservoirs for tumor specific TRM cells and limit their circulation – possibly resulting in impaired systemic antitumor immunity. TRM cells utilize specific mechanisms to egress from peripheral tissues into circulation and other peripheral sites, and emerging evidence indicates that immunotherapeutic approaches may initiate these processes and increase systemic antitumor immunity.
Conclusions:
Reversing tumor sequestration of tumor-specific T cells prior to surgical removal or radiation of tumor may increase systemic antitumor immunity. This finding may underlie the improved recurrence free survival observed with neoadjuvant immunotherapy in clinical trials.
Introduction
One of the hallmarks of adaptive immunity is the capacity for memory, which enables rapid clearance of pathogens upon re-exposure to the initial antigen1. T cells that remain after being licensed by the primary antigen exposure exist as central, effector, and resident memory T cell subsets (TCM, TEM, and TRM, respectively) and occupy different anatomic spaces where they fulfill distinct roles in protective immunity2,3. TRM cells are classically described as non-recirculating memory T cells that remain positioned at common sites of re-exposure, including barrier tissues such as the skin4 and mucosa5. In these barrier tissues, abundant CD4 and CD8 TRM clonotypes that target a variety of antigens can be found6,7. These tissue-retained T cells are distinguished by expression of the integrins CD698,9 and CD10310,11. CD69 is a membrane-bound type II C-lectin receptor that acts primarily via S1PR1 (Sphingosine-1-Phosphate Receptor 1) to promote tissue residency8,9. S1PR1 receptor signaling overrides retention mediated by G alpha i-coupled receptors. CD69, whose expression is rapidly induced on the surface of T lymphocytes following T cell activation, binds the S1PR1 receptor to induce receptor activation and internalization without lipid ligands, thus promoting retention12,13. Integrin, alpha E (ITGAE), also known as CD103, is highly expressed at mucosal sites and binding to E-cadherin promotes retention in peripheral tissues14. Other markers such as CD49a15,16 and CD4417,18 have also been extensively explored to distinguish TRM cells from other T cell subsets, both in mice and humans. CD44 is a type I transmembrane glycoprotein that has long been used to differentiate memory and effector T cells from their naïve counterparts19. CD49d/α4-integrin is selectively expressed in mucosal endothelium and mediates both cellular rolling and firm adhesion by binding MCAM-1, thus functioning as a T cell retention receptor in mucosal lymphoid tissues20,21. Local tissue environments supply a diverse array of cytokines22,23 that mediate expression of distinct chemokine receptors such as CCR8 in the skin24 and CXCR6 in respiratory epithelium25 that contribute to maintenance of a tissue residence phenotype in T cells. TRM cells undergo a distinct differentiation program that discriminates them from circulating TCM cells and TEM cells4,26,27 even when these subsets share a common progenitor, as evidenced by T cell receptor (TCR) repertoire overlap in distinct tissue compartments28,29.
This tissue resident program develops to populate barrier tissues such as skin and mucosa with highly protective T cells specific against the pathogens most commonly present in those environments. Importantly, similar TRM cell phenotypes have also been observed in solid tumors and found to be enriched in T cell clonotypes specific for tumor antigens30. Therefore, a better understanding of the cues that drive the development of a TRM cell phenotype amongst tumor-infiltrating T cells and the implications of such programs on the compartmentalization of anti-tumor immunity is needed. For example, if development of a TRM cell phenotype occurs upon chronic TCR stimulation and cytokine cues in tumors such that the tumor acts as a reservoir for a significant proportion of the tumor specific T cells in a patient, definitive surgical removal or radiation ablation treatment may remove most of the patient’s antitumor immunity. Here we discuss the environmental factors and transcriptional programming steps that drive the differentiation of naïve T cells to a TRM cell phenotype, with a specific focus on CD8+ T cells and the role of the multi-functional cytokine transforming growth factor-beta (TGF-β), summarized in Figure 1. We additionally discuss existing evidence suggesting that tumor specific T cell tumor residency can be therapeutically manipulated, and the clinical implications of these observations.
Figure 1: Mechanisms of development and maintenance of TRM cells.
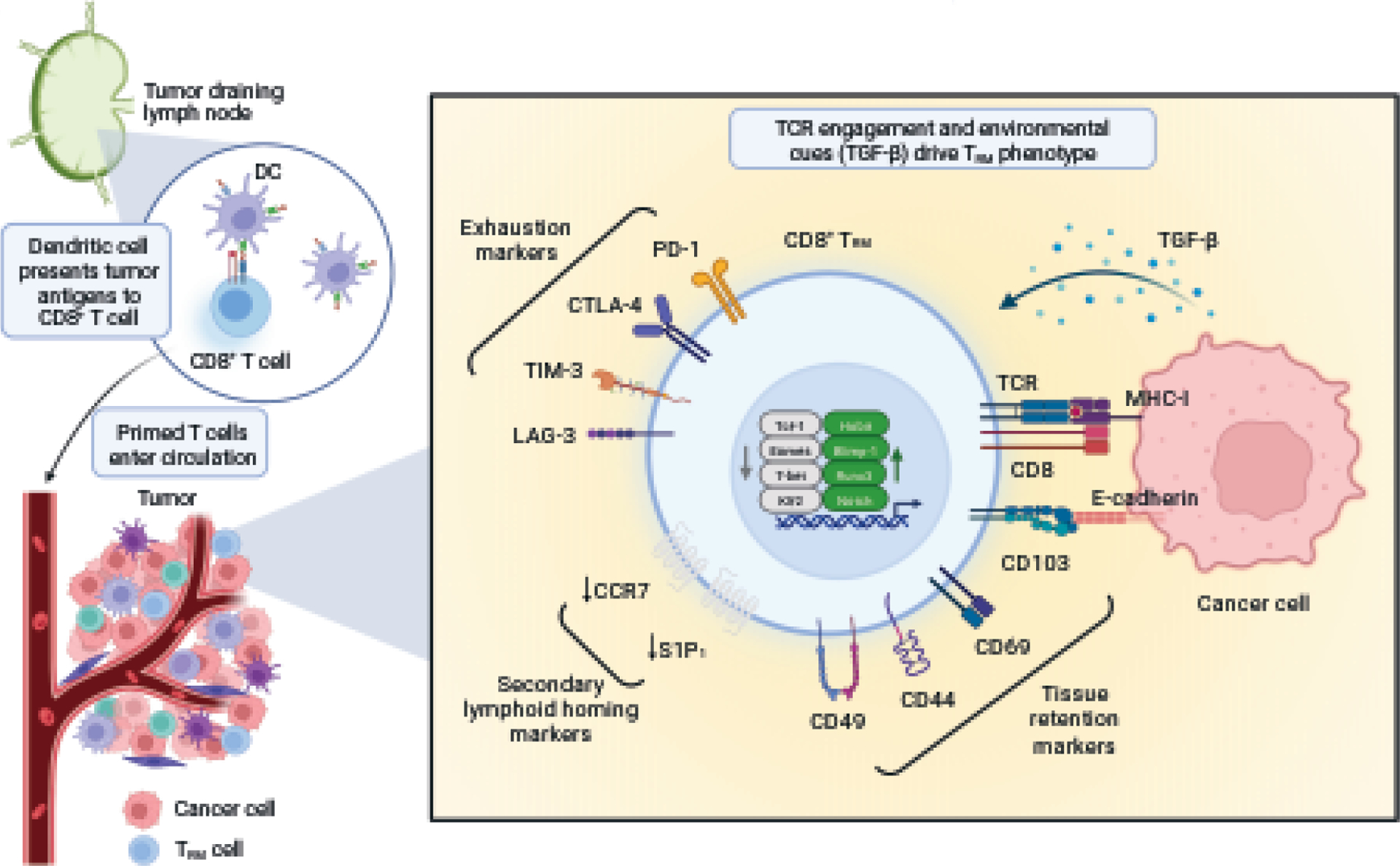
Tissue-specific dendritic cells (DCs) migrate to the draining lymph nodes (dLN) where they present antigens to naïve T cells. Once primed, these T cells have the capacity to become CD8+ TRM precursors and migrate to the injured tissue, following a chemotactic gradient. In the tissue, exposure to transforming growth factor beta (TGF-β) initiates the resident memory program, namely by driving the expression of the integrin alpha E (ITGAE/CD103) that binds to E-cadherin expressed by the tissue, enforcing retention. TGF-β is also important in driving the general transcriptional profile of TRM cells, which requires the expression of Hobit, Blimp-1, Runx3 and Notch and downregulation of Tcf-1, Eomes, T-bet and Klf2. TRM cells are often characterized by the expression of immune checkpoint receptors associated with T cell exhaustion such as Lag-3, TIM-3, CTLA-4, and PD-1.
Review Methods
Literature was identified by searching PubMed for “resident memory T cell” in combination with terms including differentiation, immunotherapy, cancer, and recirculation, searching publication dates from March 1993 to March 2023. Both pre-clinical and clinical reports were included. The relative contribution of reach report was described within the context of the larger field along with additional questions that it raised. One reviewer independently screened each report and synthesized findings, which were verified by all authors.
Discussion
TGF-β and other mediators of TRM differentiation
The tissue resident memory programming may be initiated in draining lymph nodes (dLN) when dendritic cells (DCs) present naïve T cells with antigens from the initial site of exposure31. Before migrating out of the dLN and entering circulation, activated T cells undergo extensive proliferation in the days following antigenic exposure32. Yoon and colleagues reported that activated CD8+ T cells undergo up to 5–6 divisions within 2–4 days post antigen encounter32. More recently, Kurd et al. performed a time course experiment in LCMV-infected mice and, using single-cell RNA-sequencing (scRNA-seq) to track individual T cell clonotypes over time and location, were able to identify rapid induction of a TRM signature in T cells that traffic into peripheral tissues33. There is some evidence, although, that CD8+ T cells can be preconditioned in the dLN for TRM differentiation upon interaction with integrins on migratory DCs34. Another study tracing lineage patterns of adoptively transferred barcode-labeled ovalbumin-specific transgenic CD8+ T (OT-I) cells in immunized mice found a subset of antigen-specific CD8+ effector T cells that began expressing a TRM transcriptional signature while still in the dLN35. This demonstrates the potential for CD8+ T cells to commit to a memory and possibly tissue resident fate prior to peripheral tissue entry. Acquisition of a TRM cell phenotype in a subset of CD8+ T cells upon entry into peripheral tissues is well established, but whether commitment to TRM cell fate necessarily begins in the dLN priming phase requires further investigation.
The TRM program in peripheral tissues is promoted by engagement with chemokine receptors that drive expression of adhesion molecules like integrins36–39. After initial chemotaxis to tissues mediated at least by the chemokines CXCL9 and CXCL1040, T cell tissue entry begins when signals such as IL-141, TNFα41–43, LPS44, and IL-442 promote expression of the cell adhesion molecules E-selectin and P-selectin on endothelial cells at site of active inflammation that facilitate rolling and firm adhesion within vessels. Following T cell extravasation through a process that requires at least CD4445, T cells within tissues are exposed to TGF-β secreted by fibroblasts, epithelial cells, and leukocytes46,47. TGF-β promotes tissue residency by upregulating adhesion molecules including the integrins CD10314,48,49, αEβ7, α1, CD6948, αvβ6, αvβ850, and CD49a16. Interestingly, IL-12, which has also been reported to induce CD49a in vitro16, negatively affects the expression of CD10351,52. This might help explain why IL-12, together with IFN-β, in highly proinflammatory microenvironments promotes the differentiation of a subset of TRM cells that are phenotypically CD103−CD69+23. CD103− CD8 TRM cells develop in the intestinal LP where they cluster in areas of infection together with other inflammatory immune cells such as CX3CR1+ macrophages and dendritic cells22. Of note, CD103− TRM cells seem to display distinct functional capabilities and tissue specificities when compared with their CD103+ counterparts. CD103− TRM cells display a transcriptional profile similar to circulating T cells and display higher migratory potential, making them the primary responders in sites of secondary infection53. Consequently, CD103− TRM cells exhibit more plasticity, being capable of modifying their phenotype following migration, whereas CD103+ TRM cells may be more resistant to transdifferentiation54.
Recent work has also highlighted the importance of TGF-β signaling in maintaining a subset of stem-like CD8+ T cells that is crucial for long-term T cell response in tissues55–57. These cells, termed precursor of exhausted T cells (TPEX), have a high proliferative capacity in lymphoid tissues where its progeny is committed to an exhausted T cell fate. Although they share functional similarities with CD8+ memory T cells formed during acute infection, chronic antigen exposure enforces a distinct transcriptional and epigenetic program that characterizes this population as TCF-1+PD-1+CXCR5+Tim-3lo58–61. In a model of chronic LCMV infection, parabiosed mice were used to track virus-specific CD8+ T cells, leading to the observation that stem-like TPEX CD8+ cells proliferated in LNs to give rise to terminally differentiated progeny that formed a resident memory population. Unlike in the acute setting, there was minimal trafficking of stem-like cells between mice, which further supports the concept of acquired residency62. Using a mouse model of melanoma, Li et al demonstrated that TGF-β signaling was the main driver of differentiation of tumor-specific stem-like CD8+ T cells into TRM phenotype in the tumor dLN. Furthermore, the authors were able to show that stem-like CD8+ T cells also differentiate into TRM-like cells in dLN of human head and neck squamous cell carcinoma (HNSCC) patients56. These TCF1+ tumor specific CD8+ T cells seem to maintain antitumor responses by giving rise to effector cells that take up residency in tumors63,64. They expand in the dLN and traffic to the tumor63 or proliferate in regions of the tumor that are enriched with antigen presenting cells (APCs)64, processes that involve TGF-β-mediated upregulation of CD103. Clearly TGF-β plays a crucial role in the development of TRM phenotype once activated T cells enter peripheral tissues, such as skin and mucosa.
TRM transcriptional program
The transcriptional signature of TRM cells was initially established through adoptive transfer of gBT-I cells, which express a transgenic TCR specific for herpes simplex virus (HSV), into HSV-immunized mice. Microarray analysis of CD103+ gBT-I cells isolated from skin epithelium revealed that TRM cells develop from KLGR1− precursors that upregulate CXCR327. KLRG1 (Killer Lectin-like Receptor G1) is a co-inhibitory receptor that has been used to identify antigen-experienced T cells and to distinguish memory precursor cells from effector T cells65. During effector differentiation, CD8 T cells gain expression of KLRG1. KLRG1 is then downregulated as cells further differentiate into memory T cell lineages, including TRM cells, a process mediated by the transcriptional repressor Bach2. In the gBT-I model, after entering the epidermis, KLGR1− precursors are signaled by TGF-β and IL-15 to enhance retention and survival of TRM cells by promoting CD103 and Bcl-2 expression, respectably27. A transcriptional analysis comparing circulating TEM and TCM cells to different TRM subsets revealed that TRM cells selectively upregulate the expression of the aryl hydrocarbon receptor (Ahr) which acts as a promoter of TRM cell differentiation and function66, the G protein signaling genes RGS1 and RGS2 that modulate T cell trafficking67, and the receptor of the immunoglobulin (Ig) superfamily CD244 (SLAMF4; 2B4), known to increase tissue infiltration of not only CD8+ T cells but also dendritic cells (DCs) and myeloid-derived suppressor cells (MDSCs)68. Inversely, the authors found that the transcriptional signature was characterized by the downregulation of S1PR1 and Fam65b69, an inhibitor of the small GTPase RHOA70. Interestingly, expression of both S1PR171 and Fam65b69 has been shown to be negatively affected by TGF-β signaling. As mentioned, at the protein level, surface expression of S1PR1 is disabled upon forming a complex with CD6913,72. In HSV-immunized mice, adoptively transferred gBT-I cells with deficient CD69 expression (CD69−/−) failed to maintain tissue residency to the same extent as wildtype cells, but upon treatment with the S1P receptor agonist, FTY720, accumulation of CD69−/− cells in infected skin was restored13. Transcriptionally, CD8 TRM cells in the parenchyma of non-lymphoid tissues (NLTs) have also been shown to lack expression of the transcription factor KLF2 and its target S1pr173. These two different mechanisms highlight the importance of S1PR1 downregulation for tissue retention and maintenance of long-term immunity in peripheral tissues.
Although the transcriptomic signature of TRM cells is heterogenous across tissues74, several transcription factors have been identified as master regulators of TRM cell fate. The T-box transcription factors Eomesodermin (Eomes) and T-bet, for instance, have been described as key regulators of TRM cell differentiation75,76. Eomes seems to play tissue-specific roles, having been reported to repress TRM cell formation in the skin, liver, and kidney75,77, while supporting maintenance of established TRM cells in the small intestine, partially by inducing the antiapoptotic molecule Bcl-278. T-bet, whose expression is proportional to the strength of the TCR signaling, has been widely described to promote TE/TEM formation79 and suppress TRM cell differentiation75. Like T-bet, the transcriptional repressor Blimp1 is also upregulated by inflammation and/or TCR triggering. However, contrary to T-bet, Blimp1 works to maintain the TRM phenotype while also being able to promote the differentiation of TE/TEM cells80–82. T-bet also mediates the expression of the transcription factor Hobit, which is jointly upregulated with Blimp1 to promote TRM cell differentiation80,82,83. Hobit has been found to be upregulated in a subset of LCMV-specific CD8+ T cells located within peripheral tissues that were identified to be TRM cell precursors, since depletion of these cells substantially decreased TRM cell development77. Hobit, as Blimp1, was shown to bind and regulate the expression of Tcf7, S1pr1, Klf2 and Ccr7, and loss of these transcription factors might enable TRM cells to leave the tissue and re-enter circulation77. Hobit and Blimp1 are at the core of transcriptional program of tissue residency, mostly working together but with some exceptions, as is the case for the differentiation of CD8+ TRM cell in the lungs which has been reported to exclusively dependent on Blimp184. KLF2 is a member of the Krüppel-like transcription factor family of proteins that directly controls the expression of CD62L and S1P185. KLF2 is downregulated during TRM cell development, leading to decreased expression of S1P1 and facilitating the retention of these cells in peripheral tissue11,71,86. More recently, the transcription factor Runx3 has also been shown mediate TRM cell differentiation and survival87,88. In a murine melanoma model, adoptively transferred tumor specific CD8+ T cells deficient in Runx3 were unable to maintain tumor residency resulting in uncontrolled tumor growth88. Interestingly, Runx3 enforces tissue residency of CD8+ T cells through the promotion of chromatin accessibility at TGF-β regulated genes88. Another regulator of the TRM cell formation that acts on the TGF-β-driven residency program is the SKI proto-oncogene. SKI negatively regulates TGF-β signaling by directly interacting with Smads and repressing the transcription of TGF-β responsive genes such as CD10389.
In addition to these highly conserved pathways, tissue-specific transcriptional regulators of TRM cells have been identified by comparing gene expression patterns of tissue isolated from different compartments of immunized mice. In the small intestinal epithelium, unique regulators include Nr4a2, Junb, Fosl233 and Hic176. In the respiratory epithelium, signaling through the TNF receptor family members CD137 (4–1BB)90 and GITR91 is required for the generation of CD4+ and CD8+ TRM cells.
TRM cells can, therefore, be identified using certain core transcriptional signatures and several cell surface protein markers. However, some circulating T cells also express genes associated with tissue-residency at levels comparable to T cells infiltrating NLTs, including canonical TRM markers such as CXCR6, CD69 and ITGA111,92,93. A key feature that distinguishes TRM cells in tissue from TRM-like cells in circulation may be their functional status. Recently, Noé and colleagues investigated liver-infiltrating TRM cells following malaria prime-target vaccination (PTV) and compared them to CD69+ TRM-like circulating cells and found that both subsets shared expression of conventional-residency markers but differed metabolically and functionally94. Specifically, TRM-like cells in circulation upregulated the zinc transporter SLC39A7 and were metabolically active, while tissue infiltrating TRM cells were metabolically quiescent but ready for rapid effector function upon activation. As such, although T cells expressing markers associated with tissue residency can be found both in tissue and in circulation, these cell population may be distinguished from each other functionally.
Recirculation of TRM cells
Although TRM cells were initially portrayed as T lymphocytes permanently embedded in nonlymphoid peripheral tissue like skin and mucosa, under certain conditions TRM cells can leave the tissue of residency and recirculate95,96. Often these circulating TRM cells are described as ex-TRM cells and express some EM phenotype markers such as KLRG1 and CX3CR197. In murine models, local restimulation of TRM cells results in the egress of cells from tissues of residency97–99. Using a (VSV)-expressing ovalbumin (VSVova) infection model and resorting to skin-grafts, Fonseca et al. demonstrated that upon reactivation of TRM cells within the previously immunized mice, OT-I cells started accumulating in the dLN and circulating OT-I cells with TCM and TEM signatures were observed in distant LNs 2–3 weeks later98. This data indicates that TRM cells are capable of not only tissue egress, but also transdifferentiation into other memory phenotypes in circulation, which is suggestive of developmental plasticity. Behr and colleagues also observed recirculation of tissue-resident OT-I cells that developed after oral infection with Listeria monocytogenes-expressing OVA (Lm-OVA). Hobit expression, which is necessary to enact the TRM program in T cells within this model, was downregulated following secondary antigen recognition97. Consequently, reduced or abrogated Hobit expression following TRM cell reactivation might be crucial for TRM cell tissue egress and differentiation into other T cell phenotypes in circulating cells.
Another example of systemic immunity arising from egressed TRM cells comes from the work of Klicznik et al100. Using transcriptional profiling of rare cell populations by RNA sequencing (RNA-seq) the authors were able to identify a population of skin TRM CD4+ T cells that recirculate through blood and thoracic duct lymphatics in the steady state. These CD4+ T cells express the cutaneous lymphocyte antigen (CLA), characteristic of skin–resident memory T cells, together with CD103, and their exit from tissue was associated with downregulation of CD69. Interestingly, these circulating CD4+CLA+CD103+ TRM cells were able to reseed distant skin sites, regaining CD69 expression upon reentering the tissue100. The Klicznik work did not definitively identify the mechanism of egress of TRM cells from the skin, but a later study proposed that regular tissue-damaging conditions result in the activation of TRM cells and subsequent emigration. Specifically, this study observed an increased percentage of circulating TRM cells of skin origin in the blood of patients with graft-versus-host disease (GVHD) and cutaneous infections, implicating local inflammation in re-activation and tissue egress of TRM cells101.
Tissue residency in malignancy
In addition to their role in pathogen defense, cells phenotypically similar to TRM have been identified within epithelial malignancies (carcinomas) where they constitute an important part of the antitumor immune response. The gene expression signature of subset of CD4+ and CD8+ T cells infiltrating carcinomas parallels that of TRM cells present in healthy barrier tissue like skin and mucosa30, with these tumor infiltrating lymphocytes (TILs) expressing CD103 and CD69102,103. However, chronic antigen persistence in the tumor results in a fundamentally different microenvironment compared to the setting of resolution of infection and inflammation where classic TRMs have been described. Chronic TCR signaling and other features such as TGF-β exposure in tumors drive a transcriptional program in T cells that shares hallmark features of TRM generated after infection104. To date, these CD103+ TILs have mostly been described simply as TRM cells, but recent evidence suggests that they may more accurately be referred to a ‘TRM-like’ cells105–107.
Steele and colleagues demonstrated that TCR affinity may play an important role in the accumulation of CD8+ T cells in tumors108. In their study, interaction with high-affinity antigens, but not low-affinity, led to the downregulation CXCR4 and upregulation of the CXCL12 decoy receptor, ACKR3. CXCR4 is a chemokine receptor that binds to CXCL12, also known as SDF-1, which is produced by Bone Marrow (BM) stroma, thus functioning as a homing signal to the BM109. CXCR4 expression can therefore prevent infiltration of T cells into tumors because the surrounding stroma expresses CXCL12.110 In the YUMMER1.7 mouse melanoma model, reduction in the sensitivity to CXCL12 promoted T cell tumor infiltration and improved tumor control in the context of PD-L1 immunotherapy. Objectively, entry of T cells into tumors seems to be a critical step in the development of an effective T cell response.
Accumulation of TRM cells in tumors is associated with increased survival30,103,111 and positive response to immunotherapies, including anti-PD-1 and anti-CTLA-4 immune checkpoint blockade (ICB)112,113. Immunotherapy strategies such as ICB may reverse an exhausted (and dysfunctional) state in T cells caused by chronic antigen exposure, characterized by the expression of high levels of the inhibitory receptors PD-1, Lag-3, TIM-3, CTLA4, and Tigit114–117. Indeed, gene expression programs indicating exhaustion and tissue residency appear to be closely associated in tumor specific T cells. Although it is clear that the development of exhaustion and tissue residency gene expression programs occurs downstream of TCR signaling118, additional understanding of the complex similar or dissimilar signals from the tumor microenvironment that drive either program are needed and may help inform the development of novel therapeutics beyond immune checkpoint blockade that modulate one or both programs.
Examples of the importance of TRM-like cells in response to ICB exist across several cancer types. In a non-small cell lung cancer (NSCLC) patient cohort, Djenidi et al. demonstrated that CD8+ tumor-infiltrating lymphocytes (TIL) upregulate genes encoding adhesion molecules including CD69, CD103, RGS1, ICOS1 and downregulate egress mediators S1P1 and ITGB2. Besides markers of residency, these TILs also acquired the expression of PD-1 and Tim-330, consistent with the idea of persistent antigen exposure and T cell exhaustion in the tumor microenvironment (TME). Furthermore, the authors observed that the presence of CD103+ TILs associated with increased survival of early-stage NSCLC patients and that neutralization of PD-1–PD-L1 interactions improved the specific cytotoxic activity of CD8+CD103+ T cells against autologous tumor cells30. In early stage primary triple negative breast cancer, enrichment in CD8+CD103+ TRM cells has also been associated with improved overall survival after standard chemotherapy111. In their study, Savas and colleagues only reported on the contribution of infiltrating T cells to clinical outcomes, but more recently Egelston et al. suggested that this effect may be mediated by enhanced infiltration into the tumor parenchyma rather than the stroma by a subset of CD8+CD103+ TILs relative to their CD103− counterparts119. Hence, spatial localization of CD8+CD103+ TILs within cancer islands is an important determinant of patient survival.
In patients with hepatocellular carcinoma, pre-treatment biopsies with a higher proportion of CD8+ TRM cells to exhausted CD8+ T (TEX) cells demonstrated enhanced responsiveness to anti-PD-1 therapy113. Similarly, in patients with advanced stage melanoma, pre-treatment enrichment of CD8+ TRM cells in the tumor was associated with responsiveness to immune-checkpoint blockade (ICB) and recurrence free survival (RFS). Multiplex immunohistochemistry showed that CD8+ TRM cells localized in closer proximity to melanoma cells compared to other CD8+ T cells, supporting the idea that spatial localization matters for cytolytic effects112.
Quantity, however, is not the only determinant of improved prognosis. Quality, particularly diversity of T cell specificity, is also a crucial aspect of TRM cells association with favorable clinical outcomes. T cell responses depend on antigen recognition and the type of tumor antigens presented by cancer cells may shape the TCR repertoire observed. Notably, evaluation of the TCR repertoire of TILs has been a major focus of predictive work in cancer prognostics120–122. TCR repertoire analysis of CD8+ T cells within the mucosa of patients with gastric cancer revealed a positive association between clonotype diversity and survival123. In patients with pancreatic cancer, repertoire diversity has been linked to enhanced survival following neoadjuvant therapy and surgical resection124. The degree of clonal infiltration is not a one-size-fits-all phenomenon, with tumor control being shaped by the functional attributes of tumor-specific TILs. In ovarian cancer, Tsuji et al. found that favorable prognosis was associated with strong monoclonal TCR repertoires125. This might be due to the presence of a significant number of bystander TILs that recognize non-cancer antigens103. In patients with metastatic melanoma, TCRs with neoantigen specificity are more likely to be identified amongst clonotypes present in higher frequencies126. Investigation of the TCR repertoire of CD8+ T cells that harbor a TRM phenotype has thus facilitated the identification of TCR clones that recognize tumor-associated (TAAs) and tumor-specific (TSAs) antigens.
Building systemic immunity against cancer through egress of TRM cells
As in the setting of infection, recirculated TRM-like cells may have the potential to confer enhanced protection against distant disease or locoregional disease relapse. Given this potential, mechanisms that regulate T cell trafficking are of interest since enhanced systemic antitumor immunity could be achieved by egress of TRM cells from the primary tumor or dLN prior to surgical removal or radiation ablation. Initially, responsiveness to ICB was related to early expansion of circulating CD8+ TEM with diverse repertoire clonality127–130. Recent studies suggest that ICB can also induce the egress of reinvigorated, exhausted, TRM-like cells. In a study by Luoma and colleagues, tumor-infiltrating CD8+ T cells that expressed elevated tissue-resident memory and cytotoxicity signatures clonally expanded during neoadjuvant anti-PD-1 or anti-PD-1/CTLA-4 immunotherapy. Treatment also resulted in the identification of increased frequencies of the same tumor-infiltrating CD8+ T cell clonotypes in the blood of the patients after treatment compared to before, demonstrating that ICB therapy may drive egress of tissue-infiltrating CD8+ T cells and enhance systemic immunity95. More recently, in a neoadjuvant immunotherapy clinical study using dual PD-L1 blockade and TGF-β neutralization, Sievers et al. demonstrated expansion of antigen-specific exhausted and proliferating T cells that harbored markers of residency including CD10396. Dual PD-L1 blockade and TGF-β neutralization reduced intratumoral expression of CD103, a TGF-β-induced integrin whose downregulation was validated in vitro to be linked to TGF-β neutralization. This treatment established a pool of neoepitope-specific CD8+ T cells that were undetectable in the blood prior to treatment. Whether tumor specific T cells identified at greater frequency in circulation after treatment with ICB egress form the primary tumor, tumor dLN or both, requires further study. Concomitantly, whether TGF-β neutralization is required in addition to ICB to facilitate efficient egress of TRM cells from peripheral tissues into circulation or whether this can be achieved with ICB alone also requires further study. Figure 2 illustrates the concept of using immunotherapy to induce recirculation of TRM-like cells in patients with cancer prior to surgical removal.
Figure 2: Neoadjuvant therapy as a strategy to induce recirculation of TRM-like cells and systemic immunity against cancer.
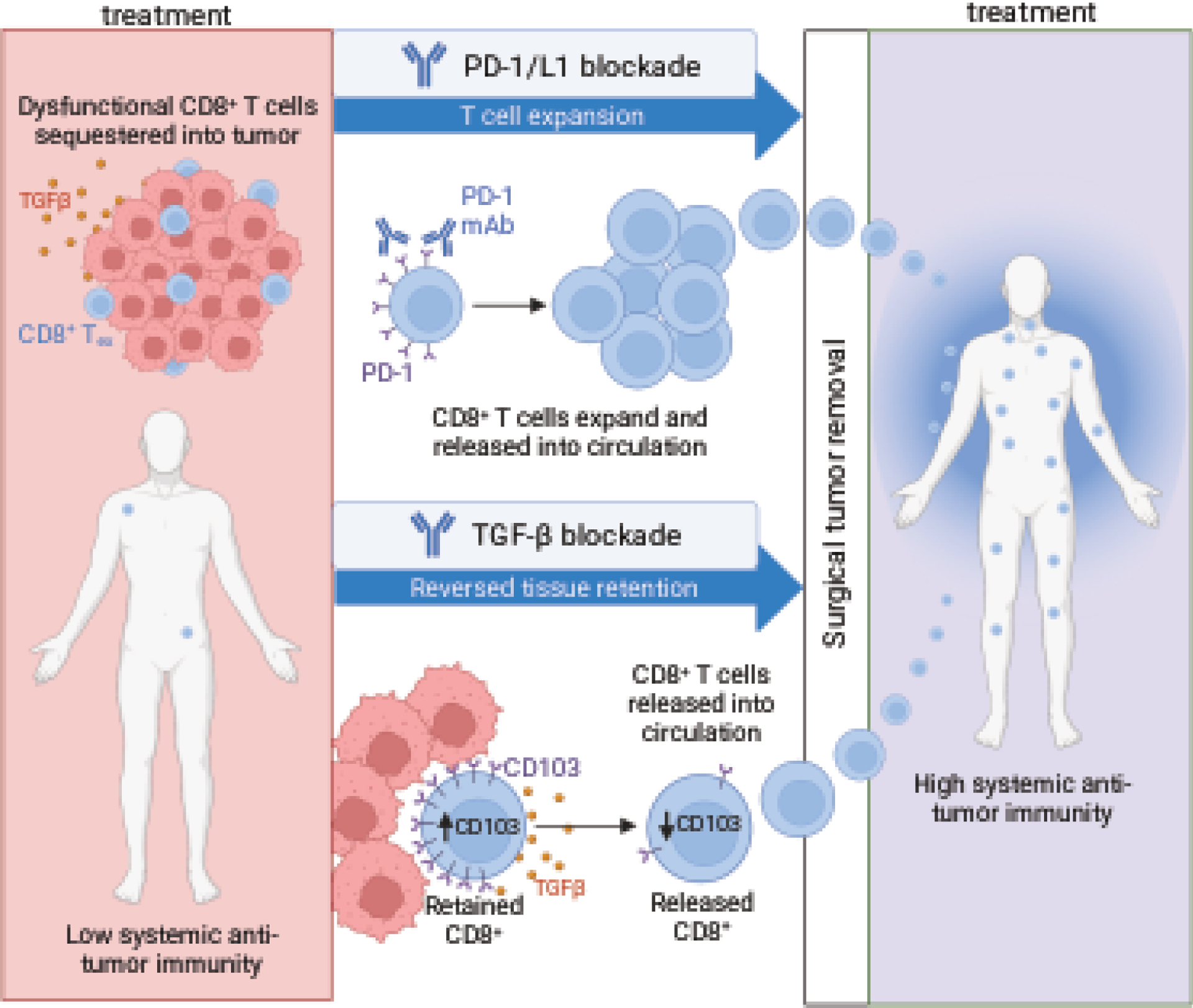
Through expression of tissue resident markers such as CD103, exhausted, dysfunctional CD8+ T cells reside in tumor tissue. PD-1 targeting immune checkpoint blockade with or without TGF- β blockade enhance egress of CD8+ T cells out of the tumor and into circulation prior to surgical removal of the tumor. This treatment result may increase the systemic anti-tumor immunity of the patient and improve recurrence free survival.
These findings have important clinical implications because CD8+ TRM cells, at least in the context of prior infection, have the potential to sustain a long-term systemic antitumor immunity131,132. Some early evidence exists that this may also be true for TRM cells that develop in the contact of malignancy. In a patient with dormant metastatic melanoma, CD8+CD103+ T cells were enriched in micrometastases near melanoma cells, providing control of cancer growth131. In a patient with vaginal melanoma refractory to ICB, tumor specific CD8+ TRM cells were found only in metastatic lesions that shared TCRs with TEM in the primary tumor. Although the isolated CD8+ TRM cells showed strong functional responsiveness to melanoma antigens in vitro, loss of major histocompatibility complex (MHC) class I expression blunted tumor responsiveness in vivo133. In a cohort of patients with metastatic melanoma who had a durable response to immunotherapy, scRNA-seq and paired scTCR-seq were performed across tissues at time points that spanned up to 9 years. Interestingly, tumor-reactive clonotypes were found not only as TRM clones in the tumor and distant skin, but also as TEM cells in circulation132 . This supports a growing body of literature indicating that TRM cells expand locally in response to ICB, but then traffic through the blood to confer systemic antitumor immunity95,134. How ICB alters tissue retention of other TRM cells in non-malignant tissue (intestines, for example) remains to be determined.
Another route of protection against metastasis may be driven by precursor cells that differentiate into TRM cells following recirculation from the tumor. Using a murine model of triple negative breast cancer (TNBC), Christian et al. performed TCR-β repertoire sequencing on CD8+ T cells isolated from the tumor and benign distant tissues and found that TRM clonotypes in the distant tissue that overlapped with those in the tumor shared developmental ontogeny. The distant mucosal TRM cells originated from a subset of CXCR6− intratumoral effector T cells (Teff)/ TEM135. CXCR6 promotes tissue residency through interaction with its unique ligand CXCL16 expressed by epithelial cells136, and immune cells such as dendritic cells (DCs)137. Disrupting CXCR6-mediated retention through intratumoral injection of a CXCL16 neutralizing antibody resulted in the reduction of the metastatic disease burden in distal tissues135. This suggests that a subset of tumor infiltrated Teff/TEM cells retains potential to egress from tumors and fully differentiate into TRM cells at distant sites, thereby conferring protection from metastasis.
In a study using a mouse model of melanoma-associated vitiligo, Molodtsov and colleagues138 demonstrated that the presence of CD8+ TRM cells in regional lymph nodes confers immunity to metastatic melanoma. Through scRNA sequencing, the authors found that some of the most expanded clonotypes in vitiligo-affected skin also occurred in the LNs where they were overwhelmingly maintained as TRM cells. In turn, these CD8+ TRM cells afforded long-lived protection against melanoma seeding in LNs. They further expanded these findings to humans, finding CD8+CD69+ T cells within human melanoma-infiltrated sentinel lymph nodes (SLNs) that exhibited TRM features138. This suggests that the potential to generate TRM cells in the SLNs of melanoma patients could confer protection against metastasis.
The clinical implications of TRM cells in cancer
Multiple independent reports now indicate that tumor specific T cells harboring a TRM phenotype are sequestered in solid tumors and likely dLN and are present at very low frequencies or undetectable in circulation in patients with cancer95,96. If this reservoir of tumor specific T cells in the tumor and dLN is removed with surgery or ablated with radiation treatment, a significant proportion of the patient’s antitumor immunity could be lost. Multiple reports also indicate that peripheral blood frequencies of tumor specific T cells that display a TRM phenotype in the tumor can be substantially increased with ICB-based immunotherapy prior to definitive treatment95,96. Additionally, multiple lines of pre-clinical and clinical evidence suggest that enhancement of systemic anti-tumor immunity confers protection against disease relapse or metastasis131–133,135. Together, these data indicate that for patients with newly diagnosed cancer, tissue egress and re-circulation of tumor specific TRM cells may underlie the substantially improved recurrence free survival observed in patients that receive neoadjuvant ICB. Proceeding with definitive surgical resection or radiation treatment without first activating and inducing tissue egress of tumor specific TRM cells into circulation may remove a substantial proportion of the patient’s antitumor immunity and reduce the chance of long-term relapse free survival. In addition to surgical stress139 and surgery associated NK cell dysfunction140, reduced systemic antitumor immunity after surgical removal of a tumor harboring tumor specific TRM cells may also result from so called ‘surgery-induced immunosuppression’, which is consistent with observations of outgrowth of metastatic deposits following removal of a primary tumor across many cancer types141.
The rationale for aiming to therapeutically induce TRM cell egress from primary tumors into circulation may be clinical context dependent. For example, inducing tissue egress of tumor specific TRM cells from tumors and dLN into circulation may be desirable in patients with newly diagnosed cancer to maintain systemic anti-tumor immunity following definitive surgical resection or radiation ablation, but may be undesirable in a patient with relapsed disease where the goal is to maintain the greatest possible density of tumor specific T cells within disease deposits. Accordingly, use of therapeutic interventions that aim to induce tissue egress of TRM cells into circulation may have the strongest scientific rationale in the neoadjuvant or induction setting.
Conclusions
Recognition of compartmentalization of tumor specific T cells into tumors through induction of a TRM-like phenotype may have significant implications for how we treat cancer. Yet, mature clinical studies definitively linking improved recurrence free survival to tissue egress of functional TRM cells that can detect and eliminate residual or circulating cancer cells following ICB in neoadjuvant or induction studies in patients with newly diagnosed cancer are still needed. Investigation into the additional contribution of therapeutically inhibiting TGF-β or other environmental cues that promote TRM cell differentiation to tissue egress and enhanced systemic antitumor immunity is specifically important because of the roles that TGF-β signaling also plays in tumorigenesis. Early in tumor progression, TGF-β inhibits epithelial cell proliferation, mainly through cell cycle arrest and induction of apoptosis. In late-stage cancers, accumulation of genomic alterations within the epithelial compartment often results in the loss of TGF-β-associated anti-proliferative signaling142,143. As a result, the tumor promoting and immunosuppressive properties of TGF-β dominate in established malignancies, making it an attractive therapeutic target to both directly inhibit tumor cell proliferation and survival as well as to enhance anti-tumor immunity. In this review, we mainly explored the role of TGF-β in the differentiation of tumor-resident CD8 T cells and how targeting TGF-β signaling could lead to the egress of tumor-reactive T cells from the tumor into circulation and the resulting enhanced systemic immunity. Considering this mechanism, TGF-β inhibition is rational in the neoadjuvant setting prior to surgical removal of the tumor. The decision to inhibit TGF-β in other setting such as relapsed or metastatic disease must consider other effects of TGF-β blockade on the tumor cell and immune compartments. What is clear, however, is that failing to ‘use the tumor as a vaccine’ and promote systemic antitumor immunity through therapeutic reversal of tumor sequestration of tumor specific T cells prior to definitive treatment is failing to offer patients with newly diagnosed cancers a better chance at long term recurrence free survival.
Highlights.
Tissue resident T cells (TRM) express adhesion molecules and embed within tissues
TRM promotion in tumors leads to sequestration of T cells out of circulation
Definitive surgery or tumor ablation may remove most tumor-specific T cells
Neoadjuvant immunotherapy promotes egress of TRM from tumors into circulation
This effect may underlie the clinical benefit observed with neoadjuvant immunotherapy
Acknowledgements
The authors thank Dr. Cem Sievers for his critical review of this manuscript. Research support was provided by the Intramural Research Program of the National Cancer Institute (Project number ZIA BC012131) and the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the American Association for Dental Research and the Colgate-Palmolive Company.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None declared
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. Feb 2010;125(2 Suppl 2):S33–40. doi: 10.1016/j.jaci.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 2.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. May 2013;13(5):309–20. doi: 10.1038/nri3442 [DOI] [PubMed] [Google Scholar]
- 3.Jameson SC, Masopust D. Understanding Subset Diversity in T Cell Memory. Immunity. Feb 20 2018;48(2):214–226. doi: 10.1016/j.immuni.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. May 2009;10(5):524–30. doi: 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- 5.Masopust D, Vezys V, Usherwood EJ, et al. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. Apr 15 2004;172(8):4875–82. doi: 10.4049/jimmunol.172.8.4875 [DOI] [PubMed] [Google Scholar]
- 6.Mackay LK, Kallies A. Transcriptional Regulation of Tissue-Resident Lymphocytes. Trends Immunol. Feb 2017;38(2):94–103. doi: 10.1016/j.it.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Park SL, Zaid A, Hor JL, et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol. Feb 2018;19(2):183–191. doi: 10.1038/s41590-017-0027-5 [DOI] [PubMed] [Google Scholar]
- 8.Walsh DA, Borges da Silva H, Beura LK, et al. The Functional Requirement for CD69 in Establishment of Resident Memory CD8(+) T Cells Varies with Tissue Location. J Immunol. Aug 15 2019;203(4):946–955. doi: 10.4049/jimmunol.1900052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cibrián D, Sánchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol. Jun 2017;47(6):946–953. doi: 10.1002/eji.201646837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma C, Mishra S, Demel EL, Liu Y, Zhang N. TGF-β Controls the Formation of Kidney-Resident T Cells via Promoting Effector T Cell Extravasation. J Immunol. Jan 15 2017;198(2):749–756. doi: 10.4049/jimmunol.1601500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar BV, Ma W, Miron M, et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. Sep 19 2017;20(12):2921–2934. doi: 10.1016/j.celrep.2017.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. Jan 22 2004;427(6972):355–60. doi: 10.1038/nature02284 [DOI] [PubMed] [Google Scholar]
- 13.Mackay LK, Braun A, Macleod BL, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol. Mar 1 2015;194(5):2059–63. doi: 10.4049/jimmunol.1402256 [DOI] [PubMed] [Google Scholar]
- 14.Casey KA, Fraser KA, Schenkel JM, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. May 15 2012;188(10):4866–75. doi: 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheuk S, Schlums H, Gallais Sérézal I, et al. CD49a Expression Defines Tissue-Resident CD8(+) T Cells Poised for Cytotoxic Function in Human Skin. Immunity. Feb 21 2017;46(2):287–300. doi: 10.1016/j.immuni.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bromley SK, Akbaba H, Mani V, et al. CD49a Regulates Cutaneous Resident Memory CD8(+) T Cell Persistence and Response. Cell Rep. Sep 1 2020;32(9):108085. doi: 10.1016/j.celrep.2020.108085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan RJ, Usherwood EJ, Zhong W, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. Feb 1 2001;166(3):1813–22. doi: 10.4049/jimmunol.166.3.1813 [DOI] [PubMed] [Google Scholar]
- 18.Walzer T, Arpin C, Beloeil L, Marvel J. Differential in vivo persistence of two subsets of memory phenotype CD8 T cells defined by CD44 and CD122 expression levels. J Immunol. Mar 15 2002;168(6):2704–11. doi: 10.4049/jimmunol.168.6.2704 [DOI] [PubMed] [Google Scholar]
- 19.Baaten BJ, Tinoco R, Chen AT, Bradley LM. Regulation of Antigen-Experienced T Cells: Lessons from the Quintessential Memory Marker CD44. Front Immunol. 2012;3:23. doi: 10.3389/fimmu.2012.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka T Leukocyte Adhesion Molecules. In: Ratcliffe MJH, ed. Encyclopedia of Immunobiology. Academic Press; 2016:505–511. [Google Scholar]
- 21.Tissino E, Benedetti D, Herman SEM, et al. Functional and clinical relevance of VLA-4 (CD49d/CD29) in ibrutinib-treated chronic lymphocytic leukemia. J Exp Med. Feb 5 2018;215(2):681–697. doi: 10.1084/jem.20171288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol. Apr 2015;16(4):406–14. doi: 10.1038/ni.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergsbaken T, Bevan MJ, Fink PJ. Local Inflammatory Cues Regulate Differentiation and Persistence of CD8(+) Tissue-Resident Memory T Cells. Cell Rep. Apr 4 2017;19(1):114–124. doi: 10.1016/j.celrep.2017.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCully ML, Ladell K, Andrews R, et al. CCR8 Expression Defines Tissue-Resident Memory T Cells in Human Skin. J Immunol. Mar 1 2018;200(5):1639–1650. doi: 10.4049/jimmunol.1701377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wein AN, McMaster SR, Takamura S, et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J Exp Med. Dec 2 2019;216(12):2748–2762. doi: 10.1084/jem.20181308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ariotti S, Beltman JB, Chodaczek G, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A. Nov 27 2012;109(48):19739–44. doi: 10.1073/pnas.1208927109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nature Immunology. 2013/12/01 2013;14(12):1294–1301. doi: 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- 28.Gaide O, Emerson RO, Jiang X, et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. Jun 2015;21(6):647–53. doi: 10.1038/nm.3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerlach C, van Heijst JW, Swart E, et al. One naive T cell, multiple fates in CD8+ T cell differentiation. J Exp Med. Jun 7 2010;207(6):1235–46. doi: 10.1084/jem.20091175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djenidi F, Adam J, Goubar A, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. Apr 1 2015;194(7):3475–86. doi: 10.4049/jimmunol.1402711 [DOI] [PubMed] [Google Scholar]
- 31.Khan TN, Mooster JL, Kilgore AM, Osborn JF, Nolz JC. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J Exp Med. May 30 2016;213(6):951–66. doi: 10.1084/jem.20151855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon H, Legge KL, Sung SS, Braciale TJ. Sequential activation of CD8+ T cells in the draining lymph nodes in response to pulmonary virus infection. J Immunol. Jul 1 2007;179(1):391–9. doi: 10.4049/jimmunol.179.1.391 [DOI] [PubMed] [Google Scholar]
- 33.Kurd NS, He Z, Louis TL, et al. Early precursors and molecular determinants of tissue-resident memory CD8(+) T lymphocytes revealed by single-cell RNA sequencing. Sci Immunol. May 15 2020;5(47)doi: 10.1126/sciimmunol.aaz6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mani V, Bromley SK, Äijö T, et al. Migratory DCs activate TGF-β to precondition naïve CD8(+) T cells for tissue-resident memory fate. Science. Oct 11 2019;366(6462)doi: 10.1126/science.aav5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kok L, Dijkgraaf FE, Urbanus J, et al. A committed tissue-resident memory T cell precursor within the circulating CD8+ effector T cell pool. J Exp Med. Oct 5 2020;217(10)doi: 10.1084/jem.20191711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oo YH, Shetty S, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. Dig Dis. 2010;28(1):31–44. doi: 10.1159/000282062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohli K, Pillarisetty VG, Kim TS. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. Jan 2022;29(1):10–21. doi: 10.1038/s41417-021-00303-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laufer JM, Legler DF. Beyond migration-Chemokines in lymphocyte priming, differentiation, and modulating effector functions. J Leukoc Biol. Aug 2018;104(2):301–312. doi: 10.1002/jlb.2mr1217-494r [DOI] [PubMed] [Google Scholar]
- 39.Krummel MF, Bartumeus F, Gérard A. T cell migration, search strategies and mechanisms. Nat Rev Immunol. Mar 2016;16(3):193–201. doi: 10.1038/nri.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. Nov 15 2012;491(7424):463–7. doi: 10.1038/nature11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyble CW, Hynes KL, Kuchibhotla J, Marcus BC, Hallahan D, Gewertz BL. TNF-alpha and IL-1 upregulate membrane-bound and soluble E-selectin through a common pathway. J Surg Res. Dec 1997;73(2):107–12. doi: 10.1006/jsre.1997.5207 [DOI] [PubMed] [Google Scholar]
- 42.Stocker CJ, Sugars KL, Harari OA, Landis RC, Morley BJ, Haskard DO. TNF-alpha, IL-4, and IFN-gamma regulate differential expression of P- and E-selectin expression by porcine aortic endothelial cells. J Immunol. Mar 15 2000;164(6):3309–15. doi: 10.4049/jimmunol.164.6.3309 [DOI] [PubMed] [Google Scholar]
- 43.Weller A, Isenmann S, Vestweber D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor alpha. Journal of Biological Chemistry. 1992/07/25/ 1992;267(21):15176–15183. doi: 10.1016/S0021-9258(18)42162-X [DOI] [PubMed] [Google Scholar]
- 44.Gotsch U, Jäger U, Dominis M, Vestweber D. Expression of P-selectin on endothelial cells is upregulated by LPS and TNF-alpha in vivo. Cell Adhes Commun. Apr 1994;2(1):7–14. doi: 10.3109/15419069409014198 [DOI] [PubMed] [Google Scholar]
- 45.DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. Oct 24 1997;278(5338):672–5. doi: 10.1126/science.278.5338.672 [DOI] [PubMed] [Google Scholar]
- 46.Thompson EA, Darrah PA, Foulds KE, et al. Monocytes Acquire the Ability to Prime Tissue-Resident T Cells via IL-10-Mediated TGF-β Release. Cell Rep. Jul 30 2019;28(5):1127–1135.e4. doi: 10.1016/j.celrep.2019.06.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira C, Barros L, Baptista M, et al. Type 1 T(reg) cells promote the generation of CD8(+) tissue-resident memory T cells. Nat Immunol. Jul 2020;21(7):766–776. doi: 10.1038/s41590-020-0674-9 [DOI] [PubMed] [Google Scholar]
- 48.Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. Oct 17 2013;39(4):687–96. doi: 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. Dec 2013;14(12):1294–301. doi: 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- 50.Mohammed J, Beura LK, Bobr A, et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nat Immunol. Apr 2016;17(4):414–21. doi: 10.1038/ni.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allakhverdi Z, Fitzpatrick D, Boisvert A, et al. Expression of CD103 identifies human regulatory T-cell subsets. J Allergy Clin Immunol. Dec 2006;118(6):1342–9. doi: 10.1016/j.jaci.2006.07.034 [DOI] [PubMed] [Google Scholar]
- 52.Sigmundsdóttir H, Johnston A, Gudjónsson JE, Valdimarsson H. Differential effects of interleukin 12 and interleukin 10 on superantigen-induced expression of cutaneous lymphocyte-associated antigen (CLA) and alphaEbeta7 integrin (CD103) by CD8+ T cells. Clin Immunol. Apr 2004;111(1):119–25. doi: 10.1016/j.clim.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 53.Fung HY, Teryek M, Lemenze AD, Bergsbaken T. CD103 fate mapping reveals that intestinal CD103(−) tissue-resident memory T cells are the primary responders to secondary infection. Sci Immunol. Nov 11 2022;7(77):eabl9925. doi: 10.1126/sciimmunol.abl9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christo SN, Evrard M, Park SL, et al. Discrete tissue microenvironments instruct diversity in resident memory T cell function and plasticity. Nat Immunol. Sep 2021;22(9):1140–1151. doi: 10.1038/s41590-021-01004-1 [DOI] [PubMed] [Google Scholar]
- 55.Hu Y, Hudson WH, Kissick HT, et al. TGF-β regulates the stem-like state of PD-1+ TCF-1+ virus-specific CD8 T cells during chronic infection. J Exp Med. Oct 3 2022;219(10)doi: 10.1084/jem.20211574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li G, Srinivasan S, Wang L, et al. TGF-beta-dependent lymphoid tissue residency of stem-like T cells limits response to tumor vaccine. Nat Commun. Oct 13 2022;13(1):6043. doi: 10.1038/s41467-022-33768-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma C, Wang L, Liao W, et al. TGF-beta promotes stem-like T cells via enforcing their lymphoid tissue retention. J Exp Med. Oct 3 2022;219(10)doi: 10.1084/jem.20211538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zehn D, Thimme R, Lugli E, de Almeida GP, Oxenius A. ‘Stem-like’ precursors are the fount to sustain persistent CD8(+) T cell responses. Nat Immunol. Jun 2022;23(6):836–847. doi: 10.1038/s41590-022-01219-w [DOI] [PubMed] [Google Scholar]
- 59.Kallies A, Zehn D, Utzschneider DT. Precursor exhausted T cells: key to successful immunotherapy? Nature Reviews Immunology. 2020;20(2):128–136. doi: 10.1038/s41577-019-0223-7 [DOI] [PubMed] [Google Scholar]
- 60.Philip M, Fairchild L, Sun L, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545(7655):452–456. doi: 10.1038/nature22367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Utzschneider DT, Legat A, Fuertes Marraco SA, et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nature Immunology. 2013;14(6):603–610. doi: 10.1038/ni.2606 [DOI] [PubMed] [Google Scholar]
- 62.Im SJ, Konieczny BT, Hudson WH, Masopust D, Ahmed R. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc Natl Acad Sci U S A. Feb 25 2020;117(8):4292–4299. doi: 10.1073/pnas.1917298117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Connolly KA, Kuchroo M, Venkat A, et al. A reservoir of stem-like CD8(+) T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci Immunol. Oct 2021;6(64):eabg7836. doi: 10.1126/sciimmunol.abg7836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jansen CS, Prokhnevska N, Master VA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. Dec 2019;576(7787):465–470. doi: 10.1038/s41586-019-1836-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henson SM, Akbar AN. KLRG1--more than a marker for T cell senescence. Age (Dordr). Dec 2009;31(4):285–91. doi: 10.1007/s11357-009-9100-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dean JW, Helm EY, Fu Z, et al. The aryl hydrocarbon receptor cell intrinsically promotes resident memory CD8(+) T cell differentiation and function. Cell Rep. Jan 31 2023;42(1):111963. doi: 10.1016/j.celrep.2022.111963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Druey KM. Emerging Roles of Regulators of G Protein Signaling (RGS) Proteins in the Immune System. Adv Immunol. 2017;136:315–351. doi: 10.1016/bs.ai.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 68.Agresta L, Lehn M, Lampe K, et al. CD244 represents a new therapeutic target in head and neck squamous cell carcinoma. J Immunother Cancer. Mar 2020;8(1)doi: 10.1136/jitc-2019-000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nath AP, Braun A, Ritchie SC, et al. Comparative analysis reveals a role for TGF-β in shaping the residency-related transcriptional signature in tissue-resident memory CD8+ T cells. PLoS One. 2019;14(2):e0210495. doi: 10.1371/journal.pone.0210495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Megrelis L, El Ghoul E, Moalli F, et al. Fam65b Phosphorylation Relieves Tonic RhoA Inhibition During T Cell Migration. Front Immunol. 2018;9:2001. doi: 10.3389/fimmu.2018.02001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nature Immunology. 2013/12/01 2013;14(12):1285–1293. doi: 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shiow LR, Rosen DB, Brdicková N, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. Mar 23 2006;440(7083):540–4. doi: 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- 73.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. Dec 2013;14(12):1285–93. doi: 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milner JJ, Toma C, He Z, et al. Heterogenous Populations of Tissue-Resident CD8(+) T Cells Are Generated in Response to Infection and Malignancy. Immunity. May 19 2020;52(5):808–824.e7. doi: 10.1016/j.immuni.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mackay LK, Wynne-Jones E, Freestone D, et al. T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity. Dec 15 2015;43(6):1101–11. doi: 10.1016/j.immuni.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 76.Crowl JT, Heeg M, Ferry A, et al. Tissue-resident memory CD8(+) T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat Immunol. Jul 2022;23(7):1121–1131. doi: 10.1038/s41590-022-01229-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parga-Vidal L, Behr FM, Kragten NAM, et al. Hobit identifies tissue-resident memory T cell precursors that are regulated by Eomes. Sci Immunol. Aug 20 2021;6(62)doi: 10.1126/sciimmunol.abg3533 [DOI] [PubMed] [Google Scholar]
- 78.Lin YH, Duong HG, Limary AE, et al. Small intestine and colon tissue-resident memory CD8(+) T cells exhibit molecular heterogeneity and differential dependence on Eomes. Immunity. Jan 10 2023;56(1):207–223.e8. doi: 10.1016/j.immuni.2022.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. Aug 2007;27(2):281–95. doi: 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xin A, Masson F, Liao Y, et al. A molecular threshold for effector CD8(+) T cell differentiation controlled by transcription factors Blimp-1 and T-bet. Nat Immunol. Apr 2016;17(4):422–32. doi: 10.1038/ni.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. Aug 21 2009;31(2):283–95. doi: 10.1016/j.immuni.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 82.Mackay LK, Minnich M, Kragten NA, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. Apr 22 2016;352(6284):459–63. doi: 10.1126/science.aad2035 [DOI] [PubMed] [Google Scholar]
- 83.Vieira Braga FA, Hertoghs KM, Kragten NA, et al. Blimp-1 homolog Hobit identifies effector-type lymphocytes in humans. Eur J Immunol. Oct 2015;45(10):2945–58. doi: 10.1002/eji.201545650 [DOI] [PubMed] [Google Scholar]
- 84.Behr FM, Kragten NAM, Wesselink TH, et al. Blimp-1 Rather Than Hobit Drives the Formation of Tissue-Resident Memory CD8(+) T Cells in the Lungs. Front Immunol. 2019;10:400. doi: 10.3389/fimmu.2019.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Preston GC, Feijoo-Carnero C, Schurch N, Cowling VH, Cantrell DA. The impact of KLF2 modulation on the transcriptional program and function of CD8 T cells. PLoS One. 2013;8(10):e77537. doi: 10.1371/journal.pone.0077537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlson CM, Endrizzi BT, Wu J, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. Jul 20 2006;442(7100):299–302. doi: 10.1038/nature04882 [DOI] [PubMed] [Google Scholar]
- 87.Fonseca R, Burn TN, Gandolfo LC, et al. Runx3 drives a CD8(+) T cell tissue residency program that is absent in CD4(+) T cells. Nat Immunol. Aug 2022;23(8):1236–1245. doi: 10.1038/s41590-022-01273-4 [DOI] [PubMed] [Google Scholar]
- 88.Milner JJ, Toma C, Yu B, et al. Runx3 programs CD8(+) T cell residency in nonlymphoid tissues and tumours. Nature. Dec 14 2017;552(7684):253–257. doi: 10.1038/nature24993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu B, Zhang G, Guo Z, et al. The SKI proto-oncogene restrains the resident CD103(+)CD8(+) T cell response in viral clearance. Cell Mol Immunol. Oct 2021;18(10):2410–2421. doi: 10.1038/s41423-020-0495-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou AC, Wagar LE, Wortzman ME, Watts TH. Intrinsic 4–1BB signals are indispensable for the establishment of an influenza-specific tissue-resident memory CD8 T-cell population in the lung. Mucosal Immunol. Sep 2017;10(5):1294–1309. doi: 10.1038/mi.2016.124 [DOI] [PubMed] [Google Scholar]
- 91.Chu KL, Batista NV, Wang KC, Zhou AC, Watts TH. GITRL on inflammatory antigen presenting cells in the lung parenchyma provides signal 4 for T-cell accumulation and tissue-resident memory T-cell formation. Mucosal Immunol. Mar 2019;12(2):363–377. doi: 10.1038/s41385-018-0105-5 [DOI] [PubMed] [Google Scholar]
- 92.Szabo PA, Levitin HM, Miron M, et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat Commun. Oct 17 2019;10(1):4706. doi: 10.1038/s41467-019-12464-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hombrink P, Helbig C, Backer RA, et al. Programs for the persistence, vigilance and control of human CD8(+) lung-resident memory T cells. Nat Immunol. Dec 2016;17(12):1467–1478. doi: 10.1038/ni.3589 [DOI] [PubMed] [Google Scholar]
- 94.Noe A, Datoo MS, Flaxman A, et al. Deep Immune Phenotyping and Single-Cell Transcriptomics Allow Identification of Circulating TRM-Like Cells Which Correlate With Liver-Stage Immunity and Vaccine-Induced Protection From Malaria. Front Immunol. 2022;13:795463. doi: 10.3389/fimmu.2022.795463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luoma AM, Suo S, Wang Y, et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. Aug 4 2022;185(16):2918–2935.e29. doi: 10.1016/j.cell.2022.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sievers C, Craveiro M, Friedman J, et al. Phenotypic plasticity and reduced tissue retention of exhausted tumor-infiltrating T cells following neoadjuvant immunotherapy in head and neck cancer. Cancer Cell. Apr 12 2023;doi: 10.1016/j.ccell.2023.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Behr FM, Parga-Vidal L, Kragten NAM, et al. Tissue-resident memory CD8(+) T cells shape local and systemic secondary T cell responses. Nat Immunol. Sep 2020;21(9):1070–1081. doi: 10.1038/s41590-020-0723-4 [DOI] [PubMed] [Google Scholar]
- 98.Fonseca R, Beura LK, Quarnstrom CF, et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat Immunol. Apr 2020;21(4):412–421. doi: 10.1038/s41590-020-0607-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beura LK, Wijeyesinghe S, Thompson EA, et al. T Cells in Nonlymphoid Tissues Give Rise to Lymph-Node-Resident Memory T Cells. Immunity. Feb 20 2018;48(2):327–338 e5. doi: 10.1016/j.immuni.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klicznik MM, Morawski PA, Hollbacher B, et al. Human CD4(+)CD103(+) cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci Immunol. Jul 5 2019;4(37)doi: 10.1126/sciimmunol.aav8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Strobl J, Gail LM, Kleissl L, et al. Human resident memory T cells exit the skin and mediate systemic Th2-driven inflammation. J Exp Med. Nov 1 2021;218(11)doi: 10.1084/jem.20210417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corgnac S, Boutet M, Kfoury M, Naltet C, Mami-Chouaib F. The Emerging Role of CD8(+) Tissue Resident Memory T (T(RM)) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin. Front Immunol. 2018;9:1904. doi: 10.3389/fimmu.2018.01904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duhen T, Duhen R, Montler R, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. Jul 13 2018;9(1):2724. doi: 10.1038/s41467-018-05072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lopez de Rodas M, Schalper KA. Tumour antigen-induced T cell exhaustion - the archenemy of immune-hot malignancies. Nat Rev Clin Oncol. Dec 2021;18(12):749–750. doi: 10.1038/s41571-021-00562-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gavil NV, Scott MC, Weyu E, et al. Chronic antigen in solid tumors drives a distinct program of T cell residence. Sci Immunol. Jun 8 2023;8(84):eadd5976. doi: 10.1126/sciimmunol.add5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lange J, Rivera-Ballesteros O, Buggert M. Human mucosal tissue-resident memory T cells in health and disease. Mucosal Immunol. Mar 2022;15(3):389–397. doi: 10.1038/s41385-021-00467-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Virassamy B, Caramia F, Savas P, et al. Intratumoral CD8(+) T cells with a tissue-resident memory phenotype mediate local immunity and immune checkpoint responses in breast cancer. Cancer Cell. Mar 13 2023;41(3):585–601 e8. doi: 10.1016/j.ccell.2023.01.004 [DOI] [PubMed] [Google Scholar]
- 108.Steele MM, Jaiswal A, Delclaux I, et al. T cell egress via lymphatic vessels is tuned by antigen encounter and limits tumor control. Nat Immunol. Apr 2023;24(4):664–675. doi: 10.1038/s41590-023-01443-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaix J, Nish SA, Lin WH, et al. Cutting edge: CXCR4 is critical for CD8+ memory T cell homeostatic self-renewal but not rechallenge self-renewal. J Immunol. Aug 1 2014;193(3):1013–6. doi: 10.4049/jimmunol.1400488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seo YD, Jiang X, Sullivan KM, et al. Mobilization of CD8(+) T Cells via CXCR4 Blockade Facilitates PD-1 Checkpoint Therapy in Human Pancreatic Cancer. Clin Cancer Res. Jul 1 2019;25(13):3934–3945. doi: 10.1158/1078-0432.CCR-19-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Savas P, Virassamy B, Ye C, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nature Medicine. 2018/07/01 2018;24(7):986–993. doi: 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- 112.Attrill GH, Owen CN, Ahmed T, et al. Higher proportions of CD39+ tumor-resident cytotoxic T cells predict recurrence-free survival in patients with stage III melanoma treated with adjuvant immunotherapy. J Immunother Cancer. Jun 2022;10(6)doi: 10.1136/jitc-2022-004771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barsch M, Salié H, Schlaak AE, et al. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J Hepatol. Aug 2022;77(2):397–409. doi: 10.1016/j.jhep.2022.02.032 [DOI] [PubMed] [Google Scholar]
- 114.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. Jun 18 2015;6(6):e1792. doi: 10.1038/cddis.2015.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang W, He Y, He W, et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front Immunol. 2020;11:622509. doi: 10.3389/fimmu.2020.622509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer. Jan 2021;124(2):359–367. doi: 10.1038/s41416-020-01048-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. Apr 2020;20(4):218–232. doi: 10.1038/s41568-019-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Battistello E, Hixon KA, Comstock DE, et al. Stepwise activities of mSWI/SNF family chromatin remodeling complexes direct T cell activation and exhaustion. Mol Cell. Apr 20 2023;83(8):1216–1236 e12. doi: 10.1016/j.molcel.2023.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Egelston CA, Avalos C, Tu TY, et al. Resident memory CD8+ T cells within cancer islands mediate survival in breast cancer patients. JCI Insight. Oct 3 2019;4(19)doi: 10.1172/jci.insight.130000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aran A, Garrigós L, Curigliano G, Cortés J, Martí M. Evaluation of the TCR Repertoire as a Predictive and Prognostic Biomarker in Cancer: Diversity or Clonality? Cancers (Basel). Mar 31 2022;14(7)doi: 10.3390/cancers14071771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kidman J, Principe N, Watson M, et al. Characteristics of TCR Repertoire Associated With Successful Immune Checkpoint Therapy Responses. Front Immunol. 2020;11:587014. doi: 10.3389/fimmu.2020.587014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valpione S, Mundra PA, Galvani E, et al. The T cell receptor repertoire of tumor infiltrating T cells is predictive and prognostic for cancer survival. Nat Commun. Jul 2 2021;12(1):4098. doi: 10.1038/s41467-021-24343-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jia Q, Zhou J, Chen G, et al. Diversity index of mucosal resident T lymphocyte repertoire predicts clinical prognosis in gastric cancer. Oncoimmunology. Apr 2015;4(4):e1001230. doi: 10.1080/2162402X.2014.1001230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murthy P, Saini P, Russell K, et al. 260 T cell infiltrating repertoire diversity is associated with enhanced survival following neoadjuvant therapy in patients with resectable pancreatic cancer. Journal for ImmunoTherapy of Cancer. 2020;8(Suppl 3):A158. doi: 10.1136/jitc-2020-SITC2020.0260 [DOI] [Google Scholar]
- 125.Tsuji T, Eng KH, Matsuzaki J, et al. Clonality and antigen-specific responses shape the prognostic effects of tumor-infiltrating T cells in ovarian cancer. Oncotarget. Jul 7 2020;11(27):2669–2683. doi: 10.18632/oncotarget.27666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pasetto A, Gros A, Robbins PF, et al. Tumor- and Neoantigen-Reactive T-cell Receptors Can Be Identified Based on Their Frequency in Fresh Tumor. Cancer Immunol Res. Sep 2 2016;4(9):734–43. doi: 10.1158/2326-6066.Cir-16-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. May 9 2017;114(19):4993–4998. doi: 10.1073/pnas.1705327114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Valpione S, Galvani E, Tweedy J, et al. Immune-awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat Cancer. Feb 2020;1(2):210–221. doi: 10.1038/s43018-019-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holm JS, Funt SA, Borch A, et al. Neoantigen-specific CD8 T cell responses in the peripheral blood following PD-L1 blockade might predict therapy outcome in metastatic urothelial carcinoma. Nat Commun. Apr 11 2022;13(1):1935. doi: 10.1038/s41467-022-29342-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carlisle JW, Jansen CS, Cardenas MA, et al. Clinical outcome following checkpoint therapy in renal cell carcinoma is associated with a burst of activated CD8 T cells in blood. J Immunother Cancer. Jul 2022;10(7)doi: 10.1136/jitc-2022-004803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hochheiser K, Aw Yeang HX, Wagner T, et al. Accumulation of CD103(+) CD8(+) T cells in a cutaneous melanoma micrometastasis. Clin Transl Immunology. 2019;8(12):e1100. doi: 10.1002/cti2.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han J, Zhao Y, Shirai K, et al. Resident and circulating memory T cells persist for years in melanoma patients with durable responses to immunotherapy. Nat Cancer. Mar 2021;2(3):300–311. doi: 10.1038/s43018-021-00180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pizzolla A, Keam SP, Vergara IA, et al. Tissue-resident memory T cells from a metastatic vaginal melanoma patient are tumor-responsive T cells and increase after anti-PD-1 treatment. J Immunother Cancer. May 2022;10(5)doi: 10.1136/jitc-2022-004574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Virassamy B, Caramia F, Savas P, et al. Intratumoral CD8(+) T cells with a tissue-resident memory phenotype mediate local immunity and immune checkpoint responses in breast cancer. Cancer Cell. Feb 8 2023;doi: 10.1016/j.ccell.2023.01.004 [DOI] [PubMed] [Google Scholar]
- 135.Christian LS, Wang L, Lim B, et al. Resident memory T cells in tumor-distant tissues fortify against metastasis formation. Cell Rep. May 11 2021;35(6):109118. doi: 10.1016/j.celrep.2021.109118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Day C, Patel R, Guillen C, Wardlaw AJ. The chemokine CXCL16 is highly and constitutively expressed by human bronchial epithelial cells. Exp Lung Res. May 2009;35(4):272–83. doi: 10.1080/01902140802635517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vella JL, Molodtsov A, Angeles CV, Branchini BR, Turk MJ, Huang YH. Dendritic cells maintain anti-tumor immunity by positioning CD8 skin-resident memory T cells. Life Sci Alliance. Oct 2021;4(10)doi: 10.26508/lsa.202101056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Molodtsov AK, Khatwani N, Vella JL, et al. Resident memory CD8(+) T cells in regional lymph nodes mediate immunity to metastatic melanoma. Immunity. Sep 14 2021;54(9):2117–2132.e7. doi: 10.1016/j.immuni.2021.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ananth AA, Tai LH, Lansdell C, et al. Surgical Stress Abrogates Pre-Existing Protective T Cell Mediated Anti-Tumor Immunity Leading to Postoperative Cancer Recurrence. PLoS One. 2016;11(5):e0155947. doi: 10.1371/journal.pone.0155947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tai LH, de Souza CT, Bélanger S, et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. Jan 1 2013;73(1):97–107. doi: 10.1158/0008-5472.Can-12-1993 [DOI] [PubMed] [Google Scholar]
- 141.Tohme S, Simmons RL, Tsung A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. Apr 1 2017;77(7):1548–1552. doi: 10.1158/0008-5472.Can-16-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Batlle E, Massague J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity. Apr 16 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Redman JM, Friedman J, Robbins Y, et al. Enhanced neoepitope-specific immunity following neoadjuvant PD-L1 and TGF-beta blockade in HPV-unrelated head and neck cancer. J Clin Invest. Jun 1 2023;133(11)doi: 10.1172/JCI172059 [DOI] [PMC free article] [PubMed] [Google Scholar]


