Abstract
Trimethylsilyl chloride (TMSCl) is commonly used to “activate” metal(0) powders toward oxidative addition of organohalides, but knowledge of its mechanism remains limited by the inability to characterize chemical intermediates under reaction conditions. Here, fluorescence lifetime imaging microscopy (FLIM) overcomes these prior limitations and shows that TMSCl aids in solubilization of the organozinc intermediate from zinc(0) metal after oxidative addition, a previously unknown mechanistic role. This mechanistic role is in contrast to previously known roles for TMSCl before the oxidative addition step. To achieve this understanding, FLIM, a tool traditionally used in biology, is developed to characterize intermediates during a chemical reaction—thus revealing mechanistic steps that are unobservable without fluorescence lifetime data. These findings impact organometallic reagent synthesis and catalysis by providing a previously uncharacterized mechanistic role for a widely used activating agent, an understanding of which is suitable for revising activation models and for developing strategies to activate currently unreactive metals.
Keywords: Organozinc reagents, oxidative addition, trimethylsilyl chloride activation, fluorescence lifetime imaging microscopy, reaction mechanisms
Graphical Abstract

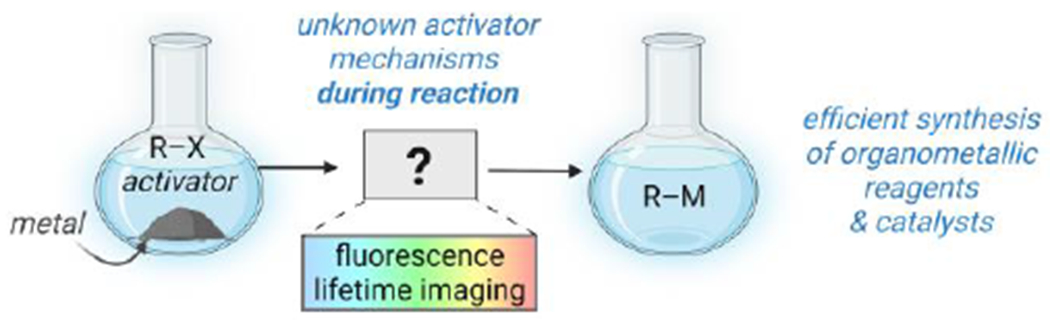
Mechanism revealed through imaging: a widely used activating agent for metal powders, trimethylsilyl chloride, creates an efficient synthesis of organometallic reagents by increasing their solubility. Fluorescence lifetime imaging microscopy (FLIM) is developed to provide molecular fingerprints that characterize intermediates and products, enabling mechanistic determination.
The addition of activating agents to metal(0) powders plays a key role in accessing a wide array of organometallics for subsequent stoichiometric and catalytic reactions.[1–5] Conceptually, the preparation of organometallic reagents by oxidative addition of organohalides to commercial metal powders provides the most direct and atom-economical method toward organometallics; however, this route currently only works for a handful of metals (e.g., zinc,[2,6–18] lithium,[19] magnesium,[20] indium,[21–23] aluminum,[24] manganese[25]). Thus, any metal-activation strategy is of high importance to understand, enabling development of activation strategies for additional metals, which in turn provides access to diverse organometallic reagents and catalysts for use in synthesis.
Trimethylsilyl chloride is used to activate multiple types of metal powders toward reaction with organic substrates.[2,5–17] In 1994 and 2016, respective studies by Utimoto14 and scientists at Merck[26] provided data that suggested two mechanistic roles for TMSCl in activating zinc toward oxidative addition to make synthetically valuable organozinc reagents (e.g., for use in Negishi cross-coupling reactions),[2,6–16] both of which occur before the oxidative addition step by exposing additional zinc(0): 1) partial removal of the inhibiting oxide layer, and 2) counteraction of metal agglomeration in the presence of lead contaminants, which appears applicable only if the TMSCl is present during the full reaction sequence and not solely used as a pretreatment.[13,26] These prior studies, however important, harnessed ex situ characterizations of the compositions of the zinc metal powders (typically under vacuum), followed by correlation with overall reaction kinetics and/or product yields on the bench scale—leaving the behaviors of the zinc powders and their associated intermediates under reaction conditions with and without activation uncharacterized. Thus, the lack of spatially resolved reactivity data under synthetic conditions is a substantial barrier to the ability to understand reaction mechanisms of activating agents, and subsequently to increase yields, generate reproducible synthetic procedures, and develop reactions of additional metals (Figure 1).
Figure 1.
Knowledge gap addressed here by FLIM: molecular imaging during on-going reaction characterizes intermediates and mechanistic pathway.
Here, a fluorescence lifetime imaging microscopy (FLIM) method is developed to address the knowledge gaps[27] from previous analytical methods. The fluorescence lifetime of an organic fluorophore (i.e., how long it remains in its excited state before relaxation by emission of a photon) is extremely sensitive to its microenvironment. This microenvironment sensitivity is now harnessed to differentiate between organozinc intermediates at different mechanistic steps in the synthesis of organozinc reagents from zinc metal, with and without TMSCl activation, under reaction conditions (i.e., in situ). A tool traditionally used in biology,[28] FLIM has been used to characterize single-electron reduction and heterogeneous catalyst carbonation products.[29–32] FLIM has not been used previously to analyze oxidative addition reactions to metal powders, or, to our knowledge, to characterize any chemical intermediates during an ongoing reaction in synthetic chemistry.
Commercial zinc powder of the same supplier and mesh as a synthetic procedure was pretreated with TMSCl[17] (Scheme 1a). This zinc contained 6.5 ppm lead according to the vendor certificate of analysis, below the quantity at which TMSCl had a documented mechanistic effect on preventing lead-induced agglomeration when present for the full reaction sequence;[13,26] thus, this effect is likely not a primary mechanistic mode of action here.
Scheme 1.

a. Reaction schematic. b. FLIM sequence with time upon sample standing; solubilization of surface organozinc complex observed. c. Intensity-only fluorescence microscopy images for comparison. d. FLIM control with imaging agent 3 (no C–I bond), showing no chemical reaction. e. FLIM comparison with 1 but without TMSCl pretreatment of zinc, showing organozinc intermediates exhibit slower solubilization.
The residual solution was decanted, and the zinc powder was rinsed with THF (3×) to remove residual TMSCl. Therefore, any differences between TMSCl-activated and unactivated zinc would derive from either lasting changes to the zinc surface or from small quantities of residual TMSCl. This powder was then treated at 60 °C with oxidative addition imaging agent boron dipyrromethene (BODIPY) aryl iodide 1. Imaging agent 1 contained a reactive C–I bond tethered through an inert linker to a spectator[33] BODIPY fluorophore. This core was chosen for the imaging agent due to its high fluorescence quantum yield and well-established chemical inertness.[33,34] The zinc powder was then rinsed with THF (3×) to remove unreacted 1 in solution.
Initial images at t = 0 showed surface organozinc oxidative addition intermediate 2, as evidenced by the bright fluorescence on the surface of individual zinc particles of ~10–30 μm (Scheme 1b and Scheme 1c).[35–40] Further consistent with the assignment of 2, a control experiment with imaging agent 3, which was identical to 1 except that it lacked a reactive C–I bond and therefore could not undergo oxidative addition, remained dark (Scheme 1d).
In the time sequences in Schemes 1b and 1c, the gradual solubilization of surface intermediate 2 to form solution organozinc reagent 4 is readily apparent. Solubilization is seen by a gradient of solution fluorescence near particles that becomes progressively brighter with time. To remove the potential for data convolution from photobleaching, the sample was moved to a new location for each timepoint.[41] Fluorescence lifetimes are reported as τavg int.
The sensitivity of FLIM toward microenvironment uniquely and unambiguously established that a molecular change accompanied the spatial change (seen as a change from blue/shorter lifetime to red/longer lifetime), consistent with progression along the reaction coordinate from solution 1 to surface intermediate 2 to final solution organozinc reagent 4. Without FLIM data (i.e., intensity only[36,37,39,40]), the molecular assignment of solution organozinc reagent 4 could not be confidently assigned as distinct from 1 (starting material) or 3 (the product of potential protodemetalation). The dramatically lower lifetime of 2 compared to 1 is attributed to quenching by the zinc surface.[37,38] The substantially increased lifetime of 4 (τsolution = 4.6 ns) compared to 2 (τsurface ~ 0.8 ns) is attributed to removal of the organozinc from its proximity to the quenching zinc metal surface; the shorter lifetime of 4 (τsolution = 4.6 ns) compared to 1 (τsolution = 5.5 ns) is attributed to the presence of zinc(II) in molecular 4.[42,43] (See Scheme S3 for the regions of interest used for each measurement. See Table S2 for a summary of molecular assignments from respective fluorescence lifetimes.) The increased intensity of the solubilized material is attributed to the reduction in quenching with reduced proximity to the metallic zinc surface. Notably, in contrast to this solubility, zinc powder not pretreated with TMSCl produced organozinc intermediate 2 with substantially higher persistence, as evidenced by the lack of equivalent solubilization at t = 45 min (Scheme 1e; full time sequence in SI). ROIs were summed for fitting, reaching greater than 160,000 counts above the noise (which was typically <1% of signal but always <5% of signal, including in the measurement of the dim solution background at t = 0; Scheme S5). Although there is heterogeneity between particles in each sample, the same trends of solubilization occur over time.
Together, the data in Scheme 1 establish that TMSCl causes organometallic intermediates to experience accelerated solubilization, plausibly through changes to the zinc surface. Solubilization of this otherwise persistent organometallic intermediate is the rate-determining step in similar synthetic preparations,[37] therefore its facilitated solubilization may be an underlying mechanistic contributor to acceleration of bulk reaction kinetics with TMSCl pretreatment similar to systems which are accelerated via salt effects[17,35]. Thus, contrary to previously known reaction mechanisms, an acceleration of solution organozinc formation that occurs after the oxidative addition step is revealed as a contributing mechanistic role for TMSCl.
To better understand the mechanism of TMSCl treatment on the behavior of zinc surface intermediates, the order of addition was reversed. First, zinc powder was treated with imaging agent alkyl iodide 5 or alkyl 6 (identical except no C–I bond), producing FLIM images in Scheme 2. The faint fluorescence on the surface of the zinc from 6 derives from the minor physisorption of this compound to the zinc surface, 8.[35–37,39,40] Second, TMSCl was added, the samples were swirled to mix, and then immediately imaged. Trimethylsilyl chloride produced an immediate solubilization of the surface organometallic intermediate 7, as seen by removal of the fluorescent material from zinc surface (image representative of spatial survey of sample shown). The impact on physisorbed 8 was harder to interpret given the faintness of the starting fluorescence: Most regions remained dark after TMSCl treatment, but some showed a brightening and shift to longer lifetimes, consistent with capturing on-going solubilization (example, Scheme 2). Control experiments showed that TMSCl does not quench or otherwise alter the fluorescence of BODIPY (see SI) and therefore confirmed that the cause of loss of fluorescence in Scheme 2 was solubilization of material from the zinc surface. These results point to a mechanism where the TMSCl activates zinc powder toward formation of solution organozinc reagents by causing shedding of the top surface layers and concurrent, plausibly indiscriminate, solubilization of material attached to those layers, including otherwise persistent reaction intermediates.
Scheme 2.

FLIM shows that TMSCl causes rapid and likely indiscriminate solubilization of surface materials.
It is currently unclear if this activation mechanism functions by way of manipulation of the zinc oxide surface, and therefore is limited to initial reaction stages when the surface still contains zinc oxide, or if the mechanism functions instead (or also) by way of manipulation of the zinc metal. A previous report detailed that TMSI reacts favorably with zinc in the presence of tetramethylethylenediamine to produce TMSZnI(L2),[44] which raises the possibility of partial or reversible redox reactivity of Zn with TMSCl, a process which may etch and weaken the surface, leading to increased solubilization.[45]
The bench-scale impact of TMSCl pretreatment (without rinse) on the rate of oxidative addition of (2-iodoethyl)benzene to zinc powder to form organozinc reagent 9 was next examined (Scheme 3). Utimoto previously showed rate acceleration of oxidative addition of dodecyl iodide to zinc upon TMSCl pretreatment, but those prior studies used HCl-preactivated zinc prior to the additional treatment of zinc with TMSCl.[13] Use of this extra pretreatment (HCl) could obscure rate enhancement from the TMSCl alone. 1H NMR spectroscopy kinetics confirmed an accelerative effect of TMSCl on the overall reaction that was stoichiometry-dependent, with a notable reduction in the induction period (Scheme 3). Despite TMSCl being added as a 5 mol % pretreatment, residual unreacted TMSCl remained present throughout the full reaction profile, as determined by 1H NMR spectroscopy. The continued presence of TMSCl supported the plausibility of ongoing reactions with the zinc powder that contribute to activation; further, the residual activator may impact downstream reactions with organozinc reagents synthesized through these published methods.
Scheme 3.

1H NMR spectroscopy kinetics of oxidative addition establish concentration-dependent macroscale rate acceleration with TMSCl pretreatment. Data with no TMSCl has been previously published.[36]

In conclusion, FLIM data revealed an additional contributing and previously unknown pathway for a widely used activating agent, TMSCl. These data show that TMSCl increases the solubility of reaction intermediates, after oxidative addition, from the surface of zinc. The power of FLIM to enable molecular assignments of reaction intermediates in synthetic chemistry is demonstrated. The demonstration of this solubilization mechanism raises the possibility of extending broader surface-shedding activation strategies—for example through catalytic and/or reversible surface redox etching—to develop synthetic methods that enable oxidative addition of organohalides to currently recalcitrant metals.
Supplementary Material
Acknowledgements
We thank the National Institutes of Health (R01GM131147) and the UCI for funding and a dissertation fellowship to E.M.H, Hannah Peacock for collecting Stern–Volmer data, Dr. Ich C. Tran for collecting SEM and EDS data, and the UC Irvine Materials Research Institute (IMRI) for instrumentation, which is supported by the National Science Foundation (DMR-2011967, CHE-0802913, and CHE-1338173). Figures were created with Biorender.
Footnotes
Supporting information for this article is given via a link at the end of the document.
Institute and/or researcher Twitter usernames: @BlumLaboratory
References
- [1].Takai K, Ueda T, Hayashi T, Moriwake T, Tetrahedron Lett. 1996, 37, 7049–7052. [Google Scholar]
- [2].Bourne-Branchu Y, Gosmini C, Danoun G, Chem. Eur. J 2017, 23, 10043–10047. [DOI] [PubMed] [Google Scholar]
- [3].Fuerstner A, Hupperts A, J. Am. Chem. Soc 1995, 117, 4468–4475. [Google Scholar]
- [4].Fürstner A, Tesche B, Chem. Mater 1998, 10, 1968–1973. [Google Scholar]
- [5].Lim S, Cho H, Jeong J, Jang M, Kim H, Hwan Cho S, Lee E, Org. Lett 2020, 22, 7387–7392. [DOI] [PubMed] [Google Scholar]
- [6].Picotin G, Miginiac P, J. Org. Chem 1987, 52, 4796–4798. [Google Scholar]
- [7].Gawroński JK, Tetrahedron Lett. 1984, 25, 2605–2608. [Google Scholar]
- [8].Coutant EP, Janin YL, Synthesis (Stuttg) 2015, 47, 511–516. [Google Scholar]
- [9].Harvey NL, Krysiak J, Chamni S, Cho SW, Sieber SA, Romo D, Chem. Eur. J 2015, 21, 1425–1428. [DOI] [PubMed] [Google Scholar]
- [10].Knochel P, Chang M Yeh P, Berk SC, Talbert J, J. Org. Chem 2002, 53, 2390–2392. [Google Scholar]
- [11].Chen ZD, Chen Z, Wang QE, Si CM, Wei BG, Tetrahedron Lett. 2020, 61, 152051. [Google Scholar]
- [12].Meador RIL, Anderson RE, Chisholm JD, Org. Biomol. Chem 2021, 19, 6233–6236. [DOI] [PubMed] [Google Scholar]
- [13].Takai K, Kakiuchi T, Utimoto K, J. Org. Chem 1994, 59, 2671–2673. [Google Scholar]
- [14].Erdik E, Tetrahedron 1987, 43, 2203–2212. [Google Scholar]
- [15].Hatano B, Tachikawa T, Mori T, Nagahashi K, Kijima T, Tetrahedron Lett. 2011, 52, 3467–3469. [Google Scholar]
- [16].Wang X-M, Liu Y-W, Ma R-J, Si C-M, Wei B-G, J. Org. Chem 2019, 84, 11261–11267. [DOI] [PubMed] [Google Scholar]
- [17].Krasovskiy A, Malakhov V, Gavryushin A, Knochel P, Angew. Chem. Int. Ed 2006, 45, 6040–6044. [DOI] [PubMed] [Google Scholar]
- [18].Rieke RD, Science 1989, 246, 1260–1264. [DOI] [PubMed] [Google Scholar]
- [19].Crockett MP, Aguirre LS, Jimenez LB, Hsu H-H, Thomas AA, J. Am. Chem. Soc 2022, 144, 16631–16637. [DOI] [PubMed] [Google Scholar]
- [20].Peltzer RM, Gauss J, Eisenstein O, Cascella M, J. Am. Chem. Soc 2020, 142, 2984–2994. [DOI] [PubMed] [Google Scholar]
- [21].Chen YH, Knochel P, Angew. Chem. Int. Ed 2008, DOI 10.1002/anie.200802292. [DOI] [Google Scholar]
- [22].Papoian V, Minehan T, J. Org. Chem 2008, 73, 7376–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Adak L, Yoshikai N, J. Org. Chem 2011, 76, 7563–7568. [DOI] [PubMed] [Google Scholar]
- [24].Blümke T, Chen Y-H, Peng Z, Knochel P, Nat. Chem 2010, 2, 313–318. [DOI] [PubMed] [Google Scholar]
- [25].Peng Z, Knochel P, Org. Lett 2011, 13, 3198–3201. [DOI] [PubMed] [Google Scholar]
- [26].Yin J, Maguire CK, Yasuda N, Brunskill APJ, Klapars A, Org. Process Res. Dev 2017, 21, 94–97. [Google Scholar]
- [27].Fürstner A, Angew. Chem. Int. Ed 1993, 32, 164–189. [Google Scholar]
- [28].Joo C, Balci H, Ishitsuka Y, Buranachai C, Ha T, Annu. Rev. Biochem 2008, 77, 51–76. [DOI] [PubMed] [Google Scholar]
- [29].Omori N, Candeo A, Mosca S, Lezcano-Gonzalez I, Robinson IK, Li L, Greenaway AG, Collier P, Beale AM, Angew. Chem. Int. Ed 2021, 60, 5125–5131. [DOI] [PubMed] [Google Scholar]
- [30].Lee H, Kim K, Mu Kang C, Choo A, Han D, Kim J, Anal. Chem 2022, 95, 1038–1046. [DOI] [PubMed] [Google Scholar]
- [31].Lezcano-González I, Oord R, Rovezzi M, Glatzel P, Botchway SW, Weckhuysen BM, Beale AM, Angew. Chem. Int. Ed 2016, 128, 5301–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tachikawa T, Yamashita S, Majima T, J. Am. Chem. Soc 2011, 133, 7197–7204. [DOI] [PubMed] [Google Scholar]
- [33].Cordes T, Blum SA, Nat. Chem 2013, 5, 993–999. [DOI] [PubMed] [Google Scholar]
- [34].Easter QT, Blum SA, Acc. Chem. Res 2019, 52, 2244–2255. [DOI] [PubMed] [Google Scholar]
- [35].Hanada EM, Tagawa TKS, Kawada M, Blum SA, J. Am. Chem. Soc 2022, 144, 12081–12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hanada EM, Jess K, Blum SA, Chem. Eur. J 2020, 26, 15094–15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Feng C, Easter QT, Blum SA, Organometallics 2017, 36, 2389–2396. [Google Scholar]
- [38].Jess K, Hanada EM, Peacock H, Blum SA, Organometallics 2020, 39, 2575–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feng C, Cunningham DW, Easter QT, Blum SA, J. Am. Chem. Soc 2016, 138, 11156–11159. [DOI] [PubMed] [Google Scholar]
- [40].Jess K, Kitagawa K, Tagawa TKS, Blum SA, J. Am. Chem. Soc 2019, 141, 9879–9884. [DOI] [PubMed] [Google Scholar]
- [41].López PA, Pham VHB, Blum SA, Angew. Chem. Int. Ed 2023, 62, e202304168. [DOI] [PubMed] [Google Scholar]
- [42].Anger P, Bharadwaj P, Novotny L, Phys. Rev. Lett 2006, 96, 3–6. [DOI] [PubMed] [Google Scholar]
- [43].George Thomas K, Kamat PV, Acc. Chem. Res 2003, 36, 888–898. [DOI] [PubMed] [Google Scholar]
- [44].Chandrasekaran R, Pulikkottil FT, Elama KS, Rasappan R, Chem. Sci 2021, 12, 15719–15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hill CL, Vander Sande JB, Whitesides GM, J. Org. Chem 2002, 45, 1020–1028. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


