Abstract
The innate immune system is critical for inducing durable and protective T cell responses to infection, and has been increasingly recognized as a target for cancer immunotherapy. In this review, we present a framework wherein distinct innate immune signaling pathways activate five key dendritic cell activities that are important for T cell mediated immunity. We discuss molecular pathways that can agonize these activities and highlight that no single pathway can agonize all activities needed for durable immunity. The immunological distinctions between innate immunotherapy administration to the tumor microenvironment versus administration via vaccination are examined, with particular focus on the strategies that enhance dendritic cell migration, interferon expression, and interleukin-1 family cytokine production. In this context, we argue for the importance of appreciating necessity vs sufficiency when considering the impact of innate immune signaling in inflammation and protective immunity, and offer a conceptual guideline for the development of efficacious cancer immunotherapies.
eTOC
Innate immune pathways are commonly discussed targets of cancer immunotherapy. Cao and Kagan review the state of this rapidly advancing field of study. They introduce the concept that five key innate immune activities in dendritic cells are needed to stimulate durable T cell mediated anti-tumor immunity.
Introduction
Cancer immunotherapies represent a notable example of how basic scientific explorations can impact human health. A wealth of fundamental biochemistry and genetic studies have identified modulators of T cell function that impact inflammation and immunity. Examples in this area include investigations of membrane proteins that potentiate or suppress T cell receptor (TCR) signaling activities, such as CD40, CD80 and CD86, which potentiate TCR signaling activities, and PD-1 and CTLA4, which suppress TCR functions1,2. These proteins are known respectively as costimulatory and coinhibitory molecules, with the latter representing targets of therapies that promote anti-tumor immunity3–5.
Despite these successes in translating basic science discoveries into clinical treatments of disease, the benefits of T cell directed cancer immunotherapy are not comprehensive. Therapies that target T cell coinhibitory receptors (e.g. PD-1 or CTLA4) are effective at treating a minor spectrum of patients with cancer6. A contributing factor to immunotherapy unresponsiveness is the paucity of tumor T cell infiltration, characterizing non-inflamed or “cold” tumors. Mechanisms involved in the absence of T cell infiltration include the lack of tumor antigens, defects in antigen presentation, and poor T cell activation and homing into the tumor bed7. Therefore, an objective of cancer immunobiology is to identify ways to convert cold tumors to inflammatory T cell enriched “hot” tumors. Innovations towards this goal may derive from the one area of biology where the immune system has been successfully weaponized to provide life-long immunity to disease—infection.
Inquiries of how infectious agents induce durable and protective immunity have been ongoing for many years8,9. Yet, the molecular basis of pathogen detection remained elusive long after the molecular descriptions of T cell activation were underway. The descriptions of the pattern recognition receptors (PRRs) of the innate immune system, expertly reviewed in 2002 by Janeway10, provided a conceptual framework to discuss infection-mediated induction of adaptive immunity. PRRs are a structurally unrelated set of proteins that share the ability to interact with microbial products, typically cell wall components or nucleic acids. Upon microbial detection by PRRs expressed by dendritic cells (DCs), several immunostimulatory activities are triggered that promote T cell mediated immunity. Examples of PRRs include the Toll-like Receptors (TLRs), RIG-I like Receptors (RLRs), nucleotide binding domain leucine rich repeat containing proteins (NLRs), c-type lectin receptors (CLRs), and the enzyme cyclic GMP-AMP synthase (cGAS). The detailed mechanisms by which PRRs sense and respond to microbes and their products has been described in detail elsewhere11–14. In this review, we discuss PRRs that control key activities of DCs needed to stimulate T cell mediated immunity, and how PRR-targeted therapies may be utilized to advance the goal of tumor eradication.
Innate immune signaling pathways in DCs that stimulate durable T cell mediated immunity
Due to their unique ability to stimulate naïve T cells, there has been longstanding interest in targeting DCs using vaccines or cell-based immunotherapies. There are five key activities in DCs that are needed to stimulate new and long-lived antigen-specific T cell responses (Figure 1). These activities include 1) MHC-mediated presentation of protein antigens, 2) T cell costimulatory molecule expression, 3) Effector T cell activating cytokine expression, 4) DC migration to the lymph node that drains the cancerous or infected tissue, and 5) production and release of the memory inducing cytokines interleukin (IL)-1β and type I interferon (IFN). The former cytokine (IL-1β) mediates CD4+ and CD8+ T cell activities15–17 whereas the latter (IFN) primarily mediates CD8+ T cell activities18–20. Each of these five DC activities is necessary for the differentiation of naïve T cells into robust and durable mediators of anti-infective and anti-tumor immunity. PRRs have attracted much attention in this area, as chemical mimics of microbial cell wall components or nucleic acids can elicit several of these activities from DCs. For example, TLR signaling on DCs promotes antigen capture21, and loading on MHC-I and MHC-II22,23. TLRs also promote the expression of T cell costimulatory molecules, including CD40, CD80 and CD8624, and the expression of IL-1225 and type I IFNs26, which are key cytokines that induce type 1 CD4+ T cell and cytolytic CD8+ T cell effector responses to infection and cancer. RLRs27 and cGAS28 also stimulate DCs to drive the above-described T cell activities, and represent particularly potent inducers of type I IFN production (Figure 2). However, it is becoming increasingly appreciated that not all PRRs elicit similar DC activities and distinct subsets of DCs express different repertoires of PRRs29. In addition, recent studies have suggested that PRR stimulation is not sufficient to activate all five activities in DCs that are key to stimulate durable lymphocyte responses (Figure 2). For example, robust induction of DC migration does not occur when PRRs are activated. In the case of respiratory syncytia virus (RSV) infections, DC migratory activities from the lung to the draining lymph node were intact in mice lacking MyD88 and MAVS30, which regulate TLR and RLR signaling respectively13,27. In contrast, RSV-induced cytokine and costimulatory molecule expression were ablated in the absence of MyD88 and MAVS30. Similarly, while TLR ligand injection into the skin induces some migration of DCs to the draining lymph nodes, this activity is not maximal and can be substantially enhanced by other DC stimulants, as discussed below17.
Figure 1. Five key activities in DCs that are needed to stimulate new and long-lived antigen-specific T cell responses.
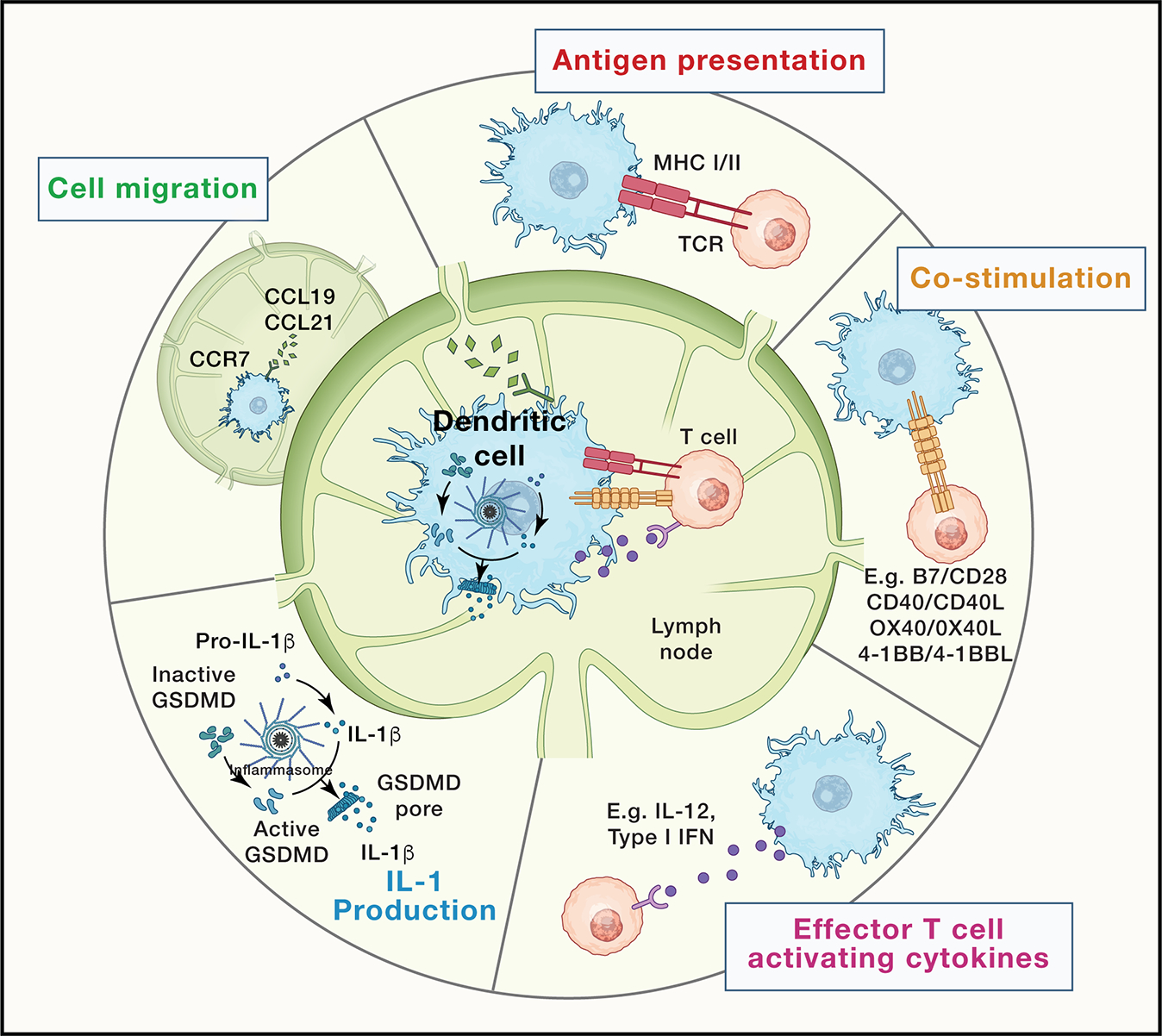
A generalized, color-coded depiction of these key activities (antigen presentation, co-stimulation, immunostimulatory cytokine production, IL-1 production, cell migration) is provided at the center of the figure and elaborated at the periphery.
Figure 2. The effect of various innate immune stimuli on the key DC activities.
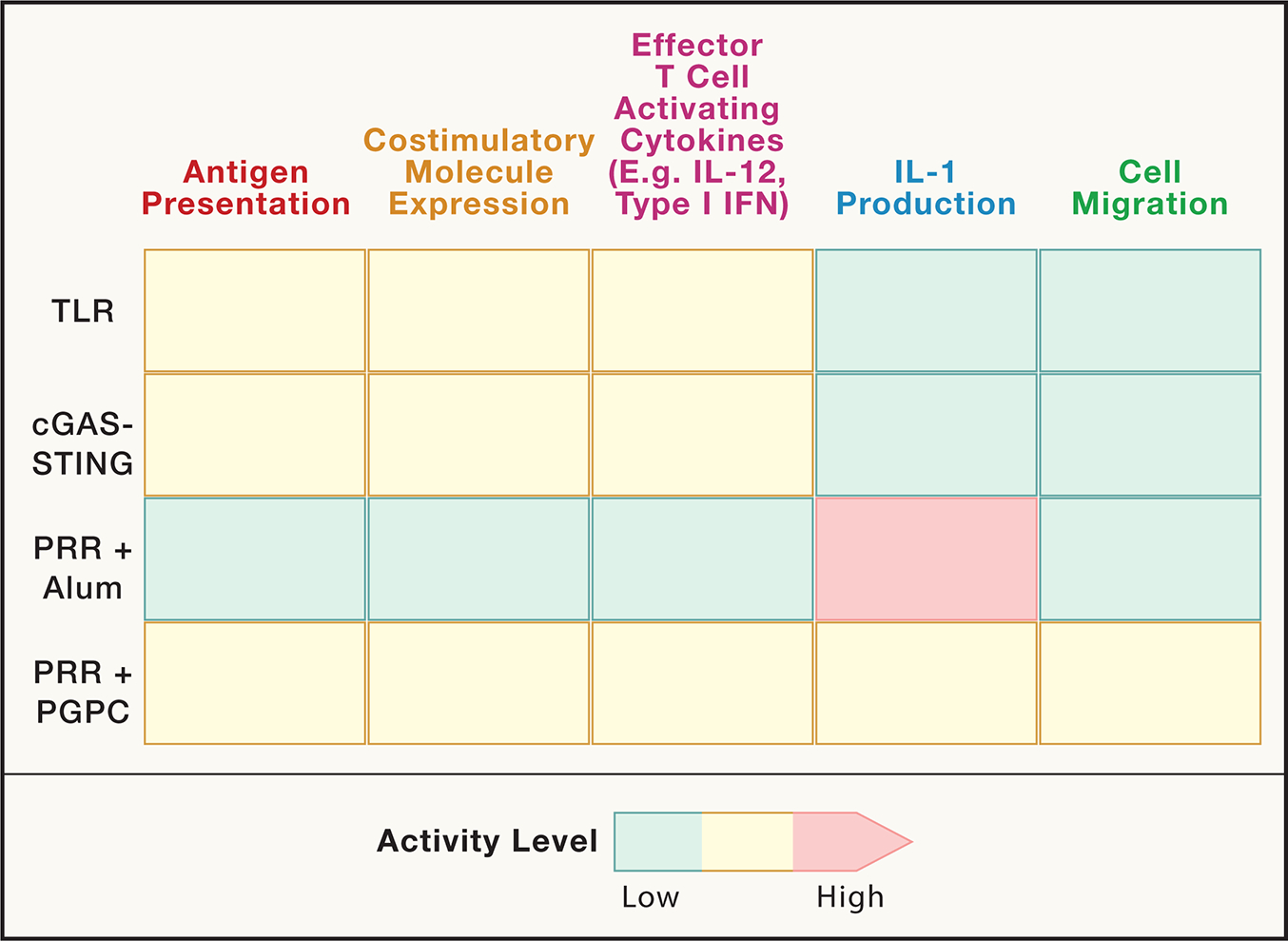
Relative capacity for different innate immune agonists (TLR, cGAS, with and without adjuvants aluminum hydroxide (alum) or PGPC) to stimulate the key DC activities that are important for eliciting optimal T cell mediated immunity. We note that not all innate immune agonists are shown (e.g. ligands for CLRs) and not all have been examined in the same studies. As such, the relative intensity of innate immune activities depicted should be considered speculative and may be context dependent.
Like migratory activities, TLRs and most other PRRs are unable to elicit IL-1β production from DCs, the absence of which results in deficiencies in memory T cell induction and re-activation16,17,31,32. Whereas TLRs are robust inducers of pro-IL-1β production, the cleavage and release of this cytokine into the extracellular space is not mediated by TLR, RLR or cGAS signaling alone33. Rather, TLR signaling must occur in conjunction with a second signal, which often represents cellular injury or dysfunction, in order for DCs to release bioactive IL-1β into the extracellular space. The cellular injury signals that promote pro-IL-1β cleavage and release are numerous, yet commonly result in the assembly of a protein complex in the DC cytosol called the inflammasome34. The inflammasome is one of several supramolecular organizing centers (SMOCs), which represent the signaling organelles of the innate immune system. In the TLR, RLR and cGAS pathways, distinct SMOCs are assembled that activate inflammatory transcription factors such as NF-κB, AP-1 and IFN regulatory factors (IRFs)35. Inflammasomes, in contrast, do not stimulate transcription, but rather serve as a subcellular site of inflammatory caspase activation, commonly caspase-136. Caspase-1 can cleave pro-IL-1β into its bioactive state and also cleave the pro-protein gasdermin D (GSDMD), which forms plasma membrane pores37–41. These GSDMD pores may serve as conduits for IL-1β secretion or (if unrepaired by the cell) may promote a lytic form of cell death known as pyroptosis37–41. As TLRs, RLRs and cGAS are not effective inducers of inflammasome assembly, these PRRs are unable to stimulate production of bioactive IL-1β.
The significance of the role of IL-1β for induction of adaptive immunity dates to the earliest descriptions of this cytokine as a lymphocyte activating factor (LAF)42–45. More recently, Paul and colleagues revealed that CD4+ and CD8+ T cell responses to protein antigens are enhanced when adjuvants are supplemented with recombinant IL-1β32,46. Genetic analysis has revealed the requirement of IL-1 receptor signaling on T cells for memory cell generation16. In the context of cancer, the use of adjuvants that induce IL-1β release from DCs was effective at inducing long-lived populations of resident memory CD8+ T cells that protect mice from multiple implantable tumor models17,31. However, not all inducers of IL-1β production from DCs are capable of stimulating T cell mediated anti-tumor immunity. Stimuli that elicit inflammasome activities that promote DC pyroptosis are robust inducers of IL-1β production, but the death of the DC interferes with all other activities for antigen-specific T cell generation17,47. Examples of pyroptosis-inducing inflammasome agonists include aluminum hydroxide and QS-21, both of which agonize the NLRP3 inflammasome and induce pyroptosis48,49. These chemicals are used clinically as adjuvants and represent robust inducers of antigen-specific antibody responses50,51. However, their utility in generating cytolytic and T helper cell type 1 (TH1) responses is limited, as the death of DCs associated with these stimuli likely undermines the days-long T cell interactions needed to stimulate adaptive immunity52. There are distinct NLRP3 agonists that promote inflammasome activities in the absence of pyroptosis. These agonists include a set of oxidized lipids, typified by the chemical PGPC (1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine), which are naturally released from damaged cells53. When murine DCs are stimulated with TLR ligands and PGPC, IL-1β is added to the repertoire of cytokines these cells can produce while maintaining viability. These DCs also display heightened migratory activities compared to DCs stimulated with TLR ligands or aluminum hydroxide alone17,54,55 (Figure 2).
The enhanced migratory and IL-1β production activities of DCs exposed to TLR ligands and PGPC demonstrate that PRR stimulations alone are not sufficient to maximally elicit all five of the key activities needed to stimulate T cell mediated immunity. As such, DCs stimulated with TLR ligands and PGPC are more immunostimulatory than DCs that have been activated with TLR ligands (or aluminum hydroxide) alone17,56 (Figure 2). In reference to the term “active”, which historically defines TLR stimulated cells, DCs exposed to TLR ligands and PGPC have been termed “hyperactive”. In vitro studies have demonstrated that murine type 1 or type 2 DCs (i.e. cDC1 or cDC2) can achieve a hyperactive state, and that CD8+ T cell mediated anti-tumor immunity is dependent on cDC1s in mice57. Studies in human monocyte-derived DCs demonstrated that similar activation states exist, and hyperactive human cDC2s are associated with an enhanced ability to elicit TH1 and TH17 cell differentiation58. Consistent with the idea that hyperactive DCs are more immunostimulatory than other DC activation states, recent work has found that TLR ligand + PGPC-based adjuvants generate CD8+ T cell responses to model tumor antigens to a greater extent than TLR ligands or aluminum hydroxide adjuvants alone17. This increased generation of antigen-specific T cells through the use of DC hyperactivating adjuvants is associated with durable protective immunity to implantable tumor models in mice17.
When considering the above-described data from the perspective of therapeutic development, a critical theme emerges. No single innate immune pathway can elicit all five of the key activities in DCs needed to stimulate durable T cell immunity. Additionally, we consider that much effort in vaccine development has rightfully focused on the identification of cancer-associated (or microbial) antigens. These antigens may serve as guides for the induction of T cell mediated immunity59. However, antigen identification is but one component of the process needed for efficient induction of adaptive immunity. The ability for a vaccine to induce protective immunity is not only dependent on the antigen(s) selected, but also on the DC stimulant used. DC stimulants, i.e. adjuvants, that stimulate all key activities in DCs may confer more robust and durable T cell responses to said antigen, and may be the missing puzzle piece that drives the immunogenic conversion of cold tumors to hot tumors. In the following sections, we discuss this concept further by describing current efforts at harnessing innate immune pathways as tools for cancer immunotherapy. In each instance, we discuss the approach in terms of reported efficacy, and how the approach relates to the five key DC activities needed to stimulate T cell mediated immunity. By using this five activity guideline, we aim to explain successes and disappointments in immunotherapy approaches, and potentially to provide a logic-based operating plan for future immunotherapy development.
Using growth factors to increase intra-tumoral DC abundance
Conventional type I (cDC1) and type 2 (cDC2) DCs represent the principal antigen presenting cells that generate new T cell responses to cancer antigens, yet the abundance of these cells in tumors is often low60–62. The low abundance of DCs within a cancerous (or infected) tissue is considered a bottleneck for antigen delivery to the lymph node in the context needed for naïve T cell stimulation63. To alleviate this bottleneck, efforts have been taken to increase DC abundance through the use of the DC differentiation factor FMS-like tyrosine kinase 3 ligand (FLT3L)64. While FLT3L-based immunotherapies have efficacy in several models of cancer, and in some trials in humans, these approaches also demonstrated that new DC generation is not sufficient to confer immunity. FLT3L-based approaches need to be combined with DC stimulatory approaches to ensure the increase in DC abundance correlates with an increase in the types of T cell responses needed for anti-tumor immunity.
FLT3L treatments inhibit the growth of murine solid tumors including colon carcinoma, prostate cancer, Lewis lung carcinoma, melanoma, and lymphoma65–68. In addition, adoptive cellular therapy with T cells expressing FLT3L triggered DC proliferation within tumors and lymphoid tissues, enhanced type I IFN pro-inflammatory signatures, and promoted antitumor activity in solid tumor models in mice69. Studies that use FLT3L as part of combined therapies have advanced us a step further. For instance, a multipronged approach involving in situ immunomodulation with FLT3L along with TLR3 and CD40 co-stimulation (and radiotherapy) enhanced DC-mediated T cell recruitment and triggered regression of multiple orthotopic tumor models in mice70. In murine melanoma models, systemic administration of FLT3L followed by intra-tumoral treatment with TLR3 ligands expanded and activated DC progenitors in the tumor proper, sensitized the tumors to antibodies that blocked the interactions between the coinhibitory receptors PD-1 and PD-L1, and protected these mice from tumor re-challenge62. Immunostimulatory gene therapy using adenoviruses expressing FLT3L and thymidine kinase promoted antitumor immunity and improved survival in murine model of brainstem glioma, and is currently being tested in the clinic71. Finally, in a clinical trial, in situ vaccination with a combination of FLT3L, radiotherapy, and a TLR3 agonist induced anti-tumor T cell responses and cancer remission in patients with advanced stage indolent non-Hodgkin’s lymphomas72. These collective studies support the idea that innate immune cell numbers are key to enhance anti-tumor immunity, but that increasing DC abundance is not sufficient for protection. Innate immune activities within these DCs are required to extract the benefit of FLT3L-based therapies.
Using DC stimulants (i.e. adjuvants) to increase T cell stimulatory cytokine production
Stimuli of PRRs, including TLRs and cGAS, have been explored as means to increase inflammatory DC activities and enhance anti-tumor immunity73,74. These efforts can be grouped into two categories: direct immunostimulation via injection of PRR ligands into the tumor microenvironment (TME) or indirect immunostimulation through the use of PRR ligands as adjuvants in cancer vaccines. A distinction between these approaches is the tissue in which the immunotherapy is delivered. In the case of cancer vaccines, the therapy is typically delivered via injection into a healthy region of the body distal to the diseased (i.e. cancerous) tissue. Injection into the healthy skin, for example, may stimulate cells other than DCs. Local macrophage, fibroblast or endothelial cell responses that exist at the site of injection may result in reactogenicity (swelling, pain), but these symptoms are usually resolved without consequence75. The stimulated DCs at the injection site, in contrast, migrate to the lymph node that drains the injection site and represent the key agents of T cell stimulation. The exposure of non-DCs to innate immune agonists at the site of vaccine injection may therefore have a temporary impact, in terms of local reactogenicity, but the long-term effects of the vaccine are largely mediated by other cell types. This statement may not apply when considering injections of innate immune stimuli into the TME. In the TME, innate immune agonists may impact cancer, stroma, and immune cells in ways that could either potentiate or undermine anti-tumor immune responses. Furthermore, as discussed in the accompanying review by Pittet and colleages76, complex environmental conditioning cues result in significant DC heterogeneity within the TME. DC subsets have differential and overlapping capacity to capture, traffic, and present tumor antigens to naïve T cells in tumor draining lymph nodes77. There is also accumulating evidence of impactful intra-tumoral DC-T cell crosstalk during the development of anti-tumor immunity. For instance, there are subsets of tumor resident DCs that express chemokines and costimulatory signals that facilitate homing and differentiation of immature T cells into antigen-specific effector T cells within the tumor proper78,79. Intra-tumoral DCs are also thought to be important in re-stimulating previously activated effector T cells. Therefore, DC-subset specificity and compartmentalization sculpt T-cell immunity. Importantly, this idea means that the variable nature of the TME, between patients as well as throughout the disease course in an individual, translates into unpredictability in response to intra-tumoral therapeutic delivery of innate immune stimuli (compared to vaccination approaches). In the following section, we discuss examples of beneficial and potentially non-beneficial effects of intra-tumoral delivery of innate immune stimuli.
Within the TME, normal or cancerous cells are abundant, as are cell death events. The factors released by damaged cells in the TME can stimulate TLRs, cGAS and likely other PRRs. Examples of such factors include heat shock proteins, ATP, nucleic acids, uric acid, calcium regulatory protein S100 family, and nuclear protein high mobility group box 180. Activation of TLRs by this diverse repertoire of damage associated molecular patterns (DAMPs) modulates signaling pathways in a cell and context-specific manner. For instance, TLR stimulation in DCs is pivotal for priming antigen presentation and inducing cytotoxic T cell responses. In macrophages, TLR stimulation promotes M2 to M1 phenotypic switching, expression of costimulatory molecules and immunostimulatory cytokines, and consequently antitumor immunity81. Furthermore, TLR activation facilitates differentiation of myeloid-derived suppressor cells (MDSC) towards an M1 phenotype and enhances tumor regression in mice82. TLR ligation on T and B cells may promote their survival, as well as cytokine, antibody, costimulatory molecule expression, and effector functions83,84. Interestingly, in tumor cells, TLR signaling can have conflicting functions. TLRs may facilitate interactions with immune cells to reverse immune suppression85 and promote tumor apoptosis86. However, TLRs on tumor cells can also promote tumor stemness, resistance to cytotoxic lymphocyte attack, and tumor cell proliferation and metastasis87.
As evidenced above, the predominant effect of TLR simulation on the diverse population of cells in the TME is anti-tumorigenic, prompting TLR agonists to be studied as immunotherapies. For instance, the TLR2 agonists Pam3Cys and SMP-105 are under investigation in bladder cancer88. The TLR3 agonists polyI:C and ARNAX have been shown to enhance effector T cell responses and tumor suppression89–91. The TLR4 activators AS04, MPLA, and GLA-SE have been tested in experimental and clinical trials to treat cervical cancer and lymphoma92. Flagellin, an agonist of TLR5 and multiple NAIPs in the NLR family, has been studied in the context of head/neck and prostate cancers93,94. The TLR7 agonist imiquimod has been tested in several gynecologic cancers. Finally, several variations of CpG oligodeoxynucleotides have been tested as TLR9 agonists in the treatment of a range of tumors95. A central feature of all TLR-based immunotherapies is their ability to induce several key activities described in Figure 1. These activities include the induction of antigen-presentation, T cell co-stimulation, T effector cell cytokines, and type I IFNs from responding DCs (and macrophages). The impact of type I IFNs is notable here, as these cytokines are key drivers of cytolytic T cell activities in the TME, as well as analogous Natural Killer (NK) cell activities96. As such, type I IFN production has emerged as a functional biomarker of an effective intra-tumor innate immunotherapy. This need for a robust type I IFN response in the TME even extends to chemotherapies, where IFN gene expression profiles are associated with protective immunity97.
Based on the emerging importance of type I IFNs as a key aspect of intra-tumoral protective immunity, non-TLR pathways that drive IFN responses have attracted attention. Notable examples include the pathways activated by cGAS and its downstream effector protein (which is also a PRR) STING. These proteins induce inflammatory responses typified by type I IFNs to double stranded DNA. Under normal circumstances, DNA is sequestered from the cytosolic space. However, tumor cells are prone to leak DNA into the cytosol, due to a combination of genomic instability, oxidative stress, and metabolism dysregulation74. This leaked DNA may be detected by cGAS, which consequently activates its latent enzymatic activity to produce a cyclic dinucleotide (CDN) known as cyclic 2’3’ GMP-AMP (cGAMP). cGAMP, as well as other CDNs, represent ligands for STING that activate inflammatory and IFN responses that are key to stimulate cytolytic and inflammatory T and NK cell responses to cancer. cGAS or STING activation and type I IFN release from DCs have been shown to augment DC maturation, antigen processing and presentation, migration, tumor antigen-specific T cell priming and activation, and maintenance of cytotoxic T cell stemness98–102. In addition, cGAS-STING activation in tumor cells can be anti-tumorigenic by inducing apoptosis103,104. STING activation has beneficial effects in several preclinical cancer models105–108, leading to strong clinical interest in the development of cGAS and STING agonists.
Several in vitro and murine tumor models have supported the benefits of natural CDNs that activate STING-induced type I IFN responses in controlling tumor growth and prolonging survival109–111. CDNs have also been tested as a cancer vaccine adjuvant and shown antitumor effects in murine models of colon, pancreatic, and upper airway squamous cell carcinoma112. The use of these agonists has been limited however, by their instability and low bioavailability. New strategies have explored how to overcome these limitations, including optimizing delivery of CDNs (e.g. in biopolymer implants or liposomal nanoparticles)113,114, as well as structurally modifying the molecules to enhance their stability and potency115. Non-CDN STING agonists are also being researched. For instance, a CDN analogue called lavone-8-acetic acid derivative 5,6-dimethylxanthenone-4-acetic acid (DMXAA) suppresses the growth of many mouse models of cancer, including B16 melanoma, in a STING dependent manner115. Unfortunately, DMXAA clinical trials have been limited because its interaction is restricted to mouse STING. Therefore, recent studies are exploring DMXAA analogues that may be more efficient at activating human STING116. There is also a growing body of research that supports the use of STING agonists as adjuvants with chemotherapy and radiotherapy110,117. In addition, STING agonists have been shown in preclinical tumor models to increase the efficacy of T cell directed immunotherapies, such as those targeting coinhibitory receptors118–120. However, as was discussed for TLRs, emerging evidence has revealed that cGAS-STING signaling may have pro-tumorigenic functions. This pathway can contribute to a immune-suppressive tumor environment by mobilizing regulatory T cells and myeloid derived suppressor cells (MDSCs), some of the most important suppressors of anti-tumor immunity121. In addition, STING signaling is reported to promote tumor cell metastasis by activating noncanonical NF-kB signaling and epithelial-to-mesenchymal transition122.
Targeting CCR7 to direct DC migration to lymph nodes
After activation, DCs must traffic to the tumor draining lymph nodes, rich in T cells, to initiate adaptive immune responses. The CCR7-CCL19/CCL21 axis guides DCs to their lymph node destination. CCR7 is a G protein-coupled chemokine receptor that can be upregulated by PRRs on DCs and activated by PRR-induced lymph node-homing chemokines CCL19 and CCL21123. PRR-induced expression of CCR7 ligands throughout the lymphatic system establishes a gradient that facilitates directional movement of DCs toward their cognate T cells within lymph nodes124,125. CCR7 oligomerization and stimulation results in downstream phosphorylation by Src and activation of a variety of molecular pathways including P13K/AKT, MAPK/NF-kB, and HIF-1a signaling126–128. The molecular targets of many of these pathways in the regulation of immune cell migration remain elusive, but likely regulate actin cytoskeleton rearrangement, metabolic reprogramming, as well as protein and epigenetic modifications that collectively influence the migration of DCs toward their destination129–133.
Aside from DCs, CCR7 can be expressed by cancer cells and potentiate metastasis. In the setting of this dichotomy, studies on the role of CCR7 in tumorigenesis have led to discrepant results134. CCR7 expression in lung cancer seems to correlate with better survival prognosis135, whereas in other circumstances (breast, pancreatic, gastric, colorectal) correlate with metastasis and poor survival prognosis136–140. Therefore, CCR7 and its ligands play two important but conflicting roles in tumorigenesis; whether targeting CCR7 using agonists or antagonists is more appropriate for cancer intervention remains debatable. Subsets of pre-clinical studies have shown that CCR7 agonism using intra-tumoral administration of CCL21 or CCL19 ligands potentiate DC and T cell influx into the tumor proper, and antitumor immune response141,142. Direct delivery of chemokines, however, has been challenging due to system toxicities. Therefore, recent studies have focused on targeted and controlled delivery of these chemokines using nanoparticles, gene modification, and incorporation into CAR T cell therapy. For instance, a vault nanoparticle encapsulating CCL21 has been developed with promising in vitro and in vivo results143. Murine B16-BL6 melanoma cells transfected to express CCL19 had a slower rate of growth after transplantation, as compared with control counterparts144. In vivo transfection of CCL19 or CCL21 via intra-tumoral injection of adenoviral vectors encoding these chemokines into murine B16-BL6 melanoma and colon carcinoma reduced tumor growth145,146. Co-expression of CCL19 in CAR T cells reduced growth of solid tumors and prolonged survival in mice147. Finally, DCs transfected in vitro to express CCR7 demonstrated enhanced ability to migrate to draining lymph nodes, and to mediate an anti-tumor response in melanoma and lung cancer models148–150. Clinical relevance of this approach was assessed in a phase I trial involving intra-tumoral injection of CCL21-gene modified DCs into patients with lung cancer, which resulted in an enhanced tumor-specific CD8 T cell response151. These are just a few of many examples illustrating the clinical potential of exploiting CCR7 in immunotherapy.
Targeting IL-1 signaling to stimulate memory T cells
While TLR and STING agonists are becoming increasingly sophisticated in therapeutic use, a fundamental aspect of immune system function may undermine the utility of these PRR ligands as agents of immunotherapy. This aspect relates to the aforementioned inability of TLR or cGAS-STING agonists alone to induce IL-1β production (Figure 2). IL-1β is a cytokine that has the potential to act both as a general inflammatory agonist in the TME and to maximize memory T cell responses to cancer antigens31,32. The receptor for IL-1β is a heterodimer of IL-1R1 and IL-1RacP, which is referred hereafter as IL-1R. IL-1R is expressed by a variety of cell types, including cancer cells, T and B cells, fibroblasts and endothelial cells. IL-1R signaling via its downstream adaptor protein MyD88 is essential to generate memory CD4+ or CD8+ T cells152. Past studies have hinted at the potential anti-tumor benefits of IL-1β. For instance, enhanced IL-1β production in mice vaccinated with irradiated melanoma or with ex vivo matured/antigen-loaded DCs is associated with enhanced antigen presentation by DCs, antigen-specific T cell activity, and ultimately control of tumor growth153,154. Chemotherapy activates NLRP3 inflammasome in DCs, IL-1β release, and cytotoxic T cell responses that suppress tumor growth155. Based on this evidence, vaccination approaches that seek to stimulate long-lived T cell responses would likely benefit from the use of DC hyperactivators as adjuvants to potentiate IL-1β production. In the TME, agonists of IL-1β production may also be beneficial, as IL-1R signaling on memory T cells is key to reactivating their effector functions, including TH1, TH2 and TH17 cells16. Consistent with this idea, supplementing T cell therapy with IL-1β improves anti-tumor responses, such as in adoptive T cell therapy of a murine melanoma model156.
However, as was the case of TLR and cGAS-STING agonists, IL-1R signaling in the TME may also have pro-tumor functions. For instance, IL-1β can enhance recruitment of MDSCs and stimulate IL-17 production by CD4+ T cells that in turn promote tumor growth155,157–159. IL-1β can also promote endothelial cell activities that enhance angiogenesis, leading to metastasis160. These potential pro-tumor functions of IL-1β have led to speculation that neutralization of this cytokine would promote anti-tumor immunity. Suggestive clinical data to support this theory was offered by a clinical trial initially designed to study Canakinumab (an IL-1β neutralizing antibody) in heart disease. In this trial, circumstantial evidence suggested the ability of the drug to lower lung cancer incidence and mortality161. However, a trial to formally assess Canakinumab as an anti-tumor therapy did not yield promising results162. This lack of clinical efficacy may be explained by the need for IL-1R signaling to promote T cell responses in cancer. Immunotherapies that promote IL-1 mediated T cell responses may be required to further explore this possibility. Ultimately, the complex role of IL-1 signaling in tumor immunity is likely reflected by its diverse function in a background of heterogenetic tumor environments.
Concluding Remarks
Strategies of inducing anti-tumor immunity are diverse, yet all derive from the focal point of how our body responds to infectious agents. Here we have focused on distinct innate immune agonists and signaling pathways, and how they may be used as agents of cancer immunotherapy. We discussed datasets illustrating that not all innate immune pathways and DC activation states are equivalent. Different DC agonists (and tissues of agonism) may impact the effectiveness of an immunotherapy. Much of what we discussed can be considered prophetic, as we have far more pre-clinical data to interpret than clinical data for innate immunotherapies. Despite this prophetic nature, the lessons learned on how one can use our basic understanding of DC and T cell biology to create new cancer therapies will likely guide the future of innate immunity. As our knowledge of innate immune pathways increases, so will therapeutic opportunities. Importantly, this knowledge will not only inform the future, but will also help explain the past. For example, the paradigm of the five key DC activities needed for T cell immunity (Figure 1) may explain the successes and disappointments of prior approaches to host defense.
In considering immunotherapy approaches of the past, a central point of consideration is that there exists a fundamental distinction between pathways that are necessary and pathways that are sufficient to induce protective immunity. Several pathways have been described as necessary for inflammatory activities in diverse contexts of disease. Inhibition of any necessary pathway will result in immunosuppression and represents a potential treatment for autoimmune or autoinflammatory disease. For example, TNFα, IL-1R and IL-23 inhibition all offer protection (to varying extents) against autoimmune or autoinflammatory diseases in mice and humans. However, while a pathway may be necessary for inflammation, it may not be sufficient to induce protective immunity. This concept may explain why single target immunotherapies are more effective as tools of immunosuppression than immunostimulation. For example, strategies that target individual molecules and pathways among the five key DC activities have been used clinically as a means of immunostimulation. IL-12R agonists (using recombinant IL-12p70), costimulatory molecule agonists (using CD40 antibodies, recombinant CD40 ligand, or OX40 antibodies), and inducers of type I IFNs have all been attempted for use in a therapeutic setting against cancer, as have individual PRR agonists163–165. None of these approaches would be expected to elicit all five of the key DC activities needed to orchestrate robust T cell mediated immunity. In considering the future, approaches that directly agonize all five of the key DC pathways may prove useful. Yet much remains to be learned. We do not yet know the therapeutic potential of distinct DC activation states for most murine models of cancer, particularly in genetically engineered and spontaneous models which may better represent human disease. We also have not fully appreciated the complexity of the DC-T cell crosstalk during tumorigenesis, and how this translates into potential differences in the efficacy and side-effect profile of intra-tumoral versus vaccination approaches of delivering different innate immunostimulants. Another unknown is whether immune responses against leukemia depend on these five key dendritic cell activities to the same extent as solid tumors, especially given the disseminated nature of the disease and lack of an obvious tumor draining lymph node. DCs play an important role in the elimination of leukemic cells166, and research has explored the use of TLR and STING agonists in the treatment of leukemia105,167. However, clinical trials testing DC stimulants in the treatment of blood cancers is only in the preliminary phases (NCT01842139, NCT01834248). Finally, as Pittet and colleagues discuss in their accompanying review76, we do not know the degree of T cell clonality resulting from DC agonistic treatment, and the relationship between innate and adaptive immunotherapies. Addressing these unknowns will require time, and an increased investment in the basic understanding of immune system functions is necessary. The value of such an investment cannot be overstated, as the impact of human health may be felt for generations to come.
Highlights.
Lessons from infection-induced immunity inform cancer immunotherapy development
Five key dendritic cell (DC) activities stimulate long-lived anti-tumor T cells
No single innate immune pathway stimulates all five protective DC activities
Next-generation cancer vaccines and intra-tumoral immunizations are in development
Acknowledgments
We thank all members of the Kagan lab for helpful discussions. This work was supported by NIH grants AI167993, AI116550, and DK34854 to J.C.K. L.L.C. was supported by NIH grant DK007477.
Footnotes
Declaration of interests
J.C.K consults and holds equity in Corner Therapeutics, Larkspur Biosciences and Neumora Therapeutics. J.C.K. is listed as an inventor on patents filed by Boston Children’s Hospital on the use of novel dendritic cell stimuli in a therapeutic setting. None of these relationships influenced this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matson CA, and Singh NJ (2020). Manipulating the TCR signaling network for cellular immunotherapy: Challenges & opportunities. Mol Immunol 123, 64–73. 10.1016/j.molimm.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pauken KE, Lagattuta KA, Lu BY, Lucca LE, Daud AI, Hafler DA, Kluger HM, Raychaudhuri S, and Sharpe AH (2022). TCR-sequencing in cancer and autoimmunity: barcodes and beyond. Trends Immunol 43, 180–194. 10.1016/j.it.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldman AD, Fritz JM, and Lenardo MJ (2020). A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 20, 651–668. 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi Y, Shi Y, Haymaker CL, Naing A, Ciliberto G, and Hajjar J (2020). T-cell agonists in cancer immunotherapy. J Immunother Cancer 8. 10.1136/jitc-2020-000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saibil SD, and Ohashi PS (2020). Targeting T cell activation in immuno-oncology. Curr Oncol 27, S98–S105. 10.3747/co.27.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagchi S, Yuan R, and Engleman EG (2021). Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 16, 223–249. 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 7.Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C, and Depil S (2019). Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front Immunol 10, 168. 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medzhitov R, and Janeway CA Jr. (1999). Innate immune induction of the adaptive immune response. Cold Spring Harb Symp Quant Biol 64, 429–435. 10.1101/sqb.1999.64.429. [DOI] [PubMed] [Google Scholar]
- 9.Paul WE (2011). Bridging innate and adaptive immunity. Cell 147, 1212–1215. 10.1016/j.cell.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 10.Janeway CA Jr., and Medzhitov R (2002). Innate immune recognition. Annu Rev Immunol 20, 197–216. 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 11.Ablasser A, and Chen ZJ (2019). cGAS in action: Expanding roles in immunity and inflammation. Science 363. 10.1126/science.aat8657. [DOI] [PubMed] [Google Scholar]
- 12.Chen YG, and Hur S (2022). Cellular origins of dsRNA, their recognition and consequences. Nat Rev Mol Cell Biol 23, 286–301. 10.1038/s41580-021-00430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald KA, and Kagan JC (2020). Toll-like Receptors and the Control of Immunity. Cell 180, 1044–1066. 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton K, Dixit VM, and Kayagaki N (2021). Dying cells fan the flames of inflammation. Science 374, 1076–1080. 10.1126/science.abi5934. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Sasson SZ, Wang K, Cohen J, and Paul WE (2013). IL-1beta strikingly enhances antigen-driven CD4 and CD8 T-cell responses. Cold Spring Harb Symp Quant Biol 78, 117–124. 10.1101/sqb.2013.78.021246. [DOI] [PubMed] [Google Scholar]
- 16.Jain A, Song R, Wakeland EK, and Pasare C (2018). T cell-intrinsic IL-1R signaling licenses effector cytokine production by memory CD4 T cells. Nat Commun 9, 3185. 10.1038/s41467-018-05489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhivaki D, Borriello F, Chow OA, Doran B, Fleming I, Theisen DJ, Pallis P, Shalek AK, Sokol CL, Zanoni I, and Kagan JC (2020). Inflammasomes within Hyperactive Murine Dendritic Cells Stimulate Long-Lived T Cell-Mediated Anti-tumor Immunity. Cell Rep 33, 108381. 10.1016/j.celrep.2020.108381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, and Mescher MF (2005). Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol 174, 4465–4469. 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 19.Kolumam GA, Thomas S, Thompson LJ, Sprent J, and Murali-Krishna K (2005). Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med 202, 637–650. 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Lee A, Grigoryan L, Arunachalam PS, Scott MKD, Trisal M, Wimmers F, Sanyal M, Weidenbacher PA, Feng Y, et al. (2022). Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol 23, 543–555. 10.1038/s41590-022-01163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, and Watts C (2004). Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science 305, 1153–1157. 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 22.Blander JM, and Medzhitov R (2006). Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812. 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 23.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, and Reis e Sousa C (2005). Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892. 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 24.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, and Medzhitov R (2001). Toll-like receptors control activation of adaptive immune responses. Nat Immunol 2, 947–950. 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G (2003). Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3, 133–146. 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 26.Noppert SJ, Fitzgerald KA, and Hertzog PJ (2007). The role of type I interferons in TLR responses. Immunol Cell Biol 85, 446–457. 10.1038/sj.icb.7100099. [DOI] [PubMed] [Google Scholar]
- 27.Rehwinkel J, and Gack MU (2020). RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol 20, 537–551. 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Wu J, Du F, Chen X, and Chen ZJ (2013). Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791. 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skold AE, van Beek JJ, Sittig SP, Bakdash G, Tel J, Schreibelt G, and de Vries IJ (2015). Protamine-stabilized RNA as an ex vivo stimulant of primary human dendritic cell subsets. Cancer Immunol Immunother 64, 1461–1473. 10.1007/s00262-015-1746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres JP, Mejias A, Gomez AM, Jafri H, Ramilo O, and Chen ZJ (2008). MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci U S A 105, 14046–14051. 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Den Eeckhout B, Tavernier J, and Gerlo S (2020). Interleukin-1 as Innate Mediator of T Cell Immunity. Front Immunol 11, 621931. 10.3389/fimmu.2020.621931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, Caucheteux S, Ratner-Hurevich M, Berzofsky JA, Nir-Paz R, and Paul WE (2013). IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med 210, 491–502. 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamkanfi M, and Dixit VM (2014). Mechanisms and functions of inflammasomes. Cell 157, 1013–1022. 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Swanson KV, Deng M, and Ting JP (2019). The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19, 477–489. 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagan JC, Magupalli VG, and Wu H (2014). SMOCs: supramolecular organizing centres that control innate immunity. Nat Rev Immunol 14, 821–826. 10.1038/nri3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinon F, Burns K, and Tschopp J (2002). The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10, 417–426. 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 37.Chan AH, and Schroder K (2020). Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med 217. 10.1084/jem.20190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, and Shao F (2016). Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116. 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 39.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, and Kagan JC (2018). The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48, 35–44 e36. 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, and Broz P (2018). The Gasdermin-D pore acts as a conduit for IL-1beta secretion in mice. Eur J Immunol 48, 584–592. 10.1002/eji.201747404. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, and Lieberman J (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158. 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrar WL, Mizel SB, and Farrar JJ (1980). Participation of lymphocyte activating factor (Interleukin 1) in the induction of cytotoxic T cell responses. J Immunol 124, 1371–1377. [PubMed] [Google Scholar]
- 43.Larsson EL, Iscove NN, and Coutinho A (1980). Two distinct factors are required for induction of T-cell growth. Nature 283, 664–666. 10.1038/283664a0. [DOI] [PubMed] [Google Scholar]
- 44.Mizel SB (1979). Physicochemical characterization of lymphocyte-activating factor (LAF). J Immunol 122, 2167–2172. [PubMed] [Google Scholar]
- 45.Raulet DH, and Bevan MJ (1982). A differentiation factor required for the expression of cytotoxic T-cell function. Nature 296, 754–757. 10.1038/296754a0. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, and Paul WE (2009). IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A 106, 7119–7124. 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDaniel MM, Kottyan LC, Singh H, and Pasare C (2020). Suppression of Inflammasome Activation by IRF8 and IRF4 in cDCs Is Critical for T Cell Priming. Cell Rep 31, 107604. 10.1016/j.celrep.2020.107604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, and Flavell RA (2008). Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126. 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marty-Roix R, Vladimer GI, Pouliot K, Weng D, Buglione-Corbett R, West K, MacMicking JD, Chee JD, Wang S, Lu S, and Lien E (2016). Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J Biol Chem 291, 1123–1136. 10.1074/jbc.M115.683011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He P, Zou Y, and Hu Z (2015). Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother 11, 477–488. 10.1080/21645515.2014.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ragupathi G, Gardner JR, Livingston PO, and Gin DY (2011). Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev Vaccines 10, 463–470. 10.1586/erv.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mempel TR, Henrickson SE, and Von Andrian UH (2004). T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159. 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 53.Di Gioia M, and Zanoni I (2021). Dooming Phagocyte Responses: Inflammatory Effects of Endogenous Oxidized Phospholipids. Front Endocrinol (Lausanne) 12, 626842. 10.3389/fendo.2021.626842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, and Kagan JC (2016). An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236. 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zanoni I, Tan Y, Di Gioia M, Springstead JR, and Kagan JC (2017). By Capturing Inflammatory Lipids Released from Dying Cells, the Receptor CD14 Induces Inflammasome-Dependent Phagocyte Hyperactivation. Immunity 47, 697–709 e693. 10.1016/j.immuni.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatscher L, Kaszubowski T, Amon L, Dudziak D, and Heger L (2023). Circumventing pyroptosis via hyperactivation shapes superior immune responses of human type 2 dendritic cells compared to type 3 dendritic cells. Eur J Immunol, e2250123. 10.1002/eji.202250123. [DOI] [PubMed] [Google Scholar]
- 57.Ferris ST, Durai V, Wu R, Theisen DJ, Ward JP, Bern MD, Davidson J.T.t., Bagadia P, Liu T, Briseno CG, et al. (2020). cDC1 prime and are licensed by CD4(+) T cells to induce anti-tumour immunity. Nature 584, 624–629. 10.1038/s41586-020-2611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatscher L, Lehmann CHK, Purbojo A, Onderka C, Liang C, Hartmann A, Cesnjevar R, Bruns H, Gross O, Nimmerjahn F, et al. (2021). Select hyperactivating NLRP3 ligands enhance the T(H)1- and T(H)17-inducing potential of human type 2 conventional dendritic cells. Sci Signal 14. 10.1126/scisignal.abe1757. [DOI] [PubMed] [Google Scholar]
- 59.Schumacher TN, and Schreiber RD (2015). Neoantigens in cancer immunotherapy. Science 348, 69–74. 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 60.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, et al. (2014). Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652. 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. (2017). Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 169, 750–765 e717. 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, et al. (2016). Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 44, 924–938. 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, and Vivier E (2019). Harnessing innate immunity in cancer therapy. Nature 574, 45–56. 10.1038/s41586-019-1593-5. [DOI] [PubMed] [Google Scholar]
- 64.Watowich SS, and Liu YJ (2010). Mechanisms regulating dendritic cell specification and development. Immunol Rev 238, 76–92. 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Somers KD, Brown RR, Holterman DA, Yousefieh N, Glass WF, Wright GL Jr., Schellhammer PF, Qian J, and Ciavarra RP (2003). Orthotopic treatment model of prostate cancer and metastasis in the immunocompetent mouse: efficacy of flt3 ligand immunotherapy. Int J Cancer 107, 773–780. 10.1002/ijc.11464. [DOI] [PubMed] [Google Scholar]
- 66.Chakravarty PK, Guha C, Alfieri A, Beri V, Niazova Z, Deb NJ, Fan Z, Thomas EK, and Vikram B (2006). Flt3L therapy following localized tumor irradiation generates long-term protective immune response in metastatic lung cancer: its implication in designing a vaccination strategy. Oncology 70, 245–254. 10.1159/000096288. [DOI] [PubMed] [Google Scholar]
- 67.Riediger C, Wingender G, Knolle P, Aulmann S, Stremmel W, and Encke J (2013). Fms-like tyrosine kinase 3 receptor ligand (Flt3L)-based vaccination administered with an adenoviral vector prevents tumor growth of colorectal cancer in a BALB/c mouse model. J Cancer Res Clin Oncol 139, 2097–2110. 10.1007/s00432-013-1532-z. [DOI] [PubMed] [Google Scholar]
- 68.Esche C, Subbotin VM, Maliszewski C, Lotze MT, and Shurin MR (1998). FLT3 ligand administration inhibits tumor growth in murine melanoma and lymphoma. Cancer Res 58, 380–383. [PubMed] [Google Scholar]
- 69.Lai J, Mardiana S, House IG, Sek K, Henderson MA, Giuffrida L, Chen AXY, Todd KL, Petley EV, Chan JD, et al. (2020). Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat Immunol 21, 914–926. 10.1038/s41590-020-0676-7. [DOI] [PubMed] [Google Scholar]
- 70.Oba T, Long MD, Keler T, Marsh HC, Minderman H, Abrams SI, Liu S, and Ito F (2020). Overcoming primary and acquired resistance to anti-PD-L1 therapy by induction and activation of tumor-residing cDC1s. Nat Commun 11, 5415. 10.1038/s41467-020-19192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faisal SM, Mendez FM, Nunez F, Castro MG, and Lowenstein PR (2020). Immune-stimulatory (TK/Flt3L) gene therapy opens the door to a promising new treatment strategy against brainstem gliomas. Oncotarget 11, 4607–4612. 10.18632/oncotarget.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, Zhan Y, Ostrowski D, Yellin M, Marsh H, et al. (2019). Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med 25, 814–824. 10.1038/s41591-019-0410-x. [DOI] [PubMed] [Google Scholar]
- 73.Pahlavanneshan S, Sayadmanesh A, Ebrahimiyan H, and Basiri M (2021). Toll-Like Receptor-Based Strategies for Cancer Immunotherapy. J Immunol Res 2021, 9912188. 10.1155/2021/9912188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng J, Mo J, Zhu T, Zhuo W, Yi Y, Hu S, Yin J, Zhang W, Zhou H, and Liu Z (2020). Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer 19, 133. 10.1186/s12943-020-01250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zepp F (2016). Principles of Vaccination. Methods Mol Biol 1403, 57–84. 10.1007/978-1-4939-3387-7_3. [DOI] [PubMed] [Google Scholar]
- 76.Pittet MJ, Di Pilato M, Garris C, Mempel TR (2023). Dendritic cells shape T-cell immunity at priming and effector sites. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marciscano AE, and Anandasabapathy N (2021). The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol 52, 101481. 10.1016/j.smim.2021.101481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prokhnevska N, Cardenas MA, Valanparambil RM, Sobierajska E, Barwick BG, Jansen C, Reyes Moon A, Gregorova P, delBalzo L, Greenwald R, et al. (2023). CD8(+) T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity 56, 107–124 e105. 10.1016/j.immuni.2022.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spranger S, Dai D, Horton B, and Gajewski TF (2017). Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 31, 711–723 e714. 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng R, and Ma J (2022). Immunotherapeutic Implications of Toll-like Receptors Activation in Tumor Microenvironment. Pharmaceutics 14. 10.3390/pharmaceutics14112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vidyarthi A, Khan N, Agnihotri T, Negi S, Das DK, Aqdas M, Chatterjee D, Colegio OR, Tewari MK, and Agrewala JN (2018). TLR-3 Stimulation Skews M2 Macrophages to M1 Through IFN-alphabeta Signaling and Restricts Tumor Progression. Front Immunol 9, 1650. 10.3389/fimmu.2018.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, Mao H, Yu M, and Wang X (2020). TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett 469, 173–185. 10.1016/j.canlet.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 83.Buchta CM, and Bishop GA (2014). Toll-like receptors and B cells: functions and mechanisms. Immunol Res 59, 12–22. 10.1007/s12026-014-8523-2. [DOI] [PubMed] [Google Scholar]
- 84.Geng D, Zheng L, Srivastava R, Asprodites N, Velasco-Gonzalez C, and Davila E (2010). When Toll-like receptor and T-cell receptor signals collide: a mechanism for enhanced CD8 T-cell effector function. Blood 116, 3494–3504. 10.1182/blood-2010-02-268169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye J, Ma C, Hsueh EC, Dou J, Mo W, Liu S, Han B, Huang Y, Zhang Y, Varvares MA, et al. (2014). TLR8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO Mol Med 6, 1294–1311. 10.15252/emmm.201403918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meyer T, Nindl I, Schmook T, Ulrich C, Sterry W, and Stockfleth E (2003). Induction of apoptosis by Toll-like receptor-7 agonist in tissue cultures. Br J Dermatol 149 Suppl 66, 9–14. 10.1046/j.0366-077x.2003.05632.x. [DOI] [PubMed] [Google Scholar]
- 87.Huang B, Zhao J, Unkeless JC, Feng ZH, and Xiong H (2008). TLR signaling by tumor and immune cells: a double-edged sword. Oncogene 27, 218–224. 10.1038/sj.onc.1210904. [DOI] [PubMed] [Google Scholar]
- 88.Simons MP, O’Donnell MA, and Griffith TS (2008). Role of neutrophils in BCG immunotherapy for bladder cancer. Urol Oncol 26, 341–345. 10.1016/j.urolonc.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kyi C, Roudko V, Sabado R, Saenger Y, Loging W, Mandeli J, Thin TH, Lehrer D, Donovan M, Posner M, et al. (2018). Therapeutic Immune Modulation against Solid Cancers with Intratumoral Poly-ICLC: A Pilot Trial. Clin Cancer Res 24, 4937–4948. 10.1158/1078-0432.CCR-17-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marquez-Rodas I, Longo F, Rodriguez-Ruiz ME, Calles A, Ponce S, Jove M, Rubio-Viqueira B, Perez-Gracia JL, Gomez-Rueda A, Lopez-Tarruella S, et al. (2020). Intratumoral nanoplexed poly I:C BO-112 in combination with systemic anti-PD-1 for patients with anti-PD-1-refractory tumors. Sci Transl Med 12. 10.1126/scitranslmed.abb0391. [DOI] [PubMed] [Google Scholar]
- 91.Seya T, Takeda Y, and Matsumoto M (2019). A Toll-like receptor 3 (TLR3) agonist ARNAX for therapeutic immunotherapy. Adv Drug Deliv Rev 147, 37–43. 10.1016/j.addr.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 92.Lambert SL, Yang CF, Liu Z, Sweetwood R, Zhao J, Cheng L, Jin H, and Woo J (2012). Molecular and cellular response profiles induced by the TLR4 agonist-based adjuvant Glucopyranosyl Lipid A. PLoS One 7, e51618. 10.1371/journal.pone.0051618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haderski GJ, Kandar BM, Brackett CM, Toshkov IM, Johnson CP, Paszkiewicz GM, Natarajan V, Gleiberman AS, Gudkov AV, and Burdelya LG (2020). TLR5 agonist entolimod reduces the adverse toxicity of TNF while preserving its antitumor effects. PLoS One 15, e0227940. 10.1371/journal.pone.0227940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mett V, Komarova EA, Greene K, Bespalov I, Brackett C, Gillard B, Gleiberman AS, Toshkov IA, Aygun-Sunar S, Johnson C, et al. (2018). Mobilan: a recombinant adenovirus carrying Toll-like receptor 5 self-activating cassette for cancer immunotherapy. Oncogene 37, 439–449. 10.1038/onc.2017.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frank MJ, Reagan PM, Bartlett NL, Gordon LI, Friedberg JW, Czerwinski DK, Long SR, Hoppe RT, Janssen R, Candia AF, et al. (2018). In Situ Vaccination with a TLR9 Agonist and Local Low-Dose Radiation Induces Systemic Responses in Untreated Indolent Lymphoma. Cancer Discov 8, 1258–1269. 10.1158/2159-8290.CD-18-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klement JD, Redd PS, Lu C, Merting AD, Poschel DB, Yang D, Savage NM, Zhou G, Munn DH, Fallon PG, and Liu K (2023). Tumor PD-L1 engages myeloid PD-1 to suppress type I interferon to impair cytotoxic T lymphocyte recruitment. Cancer Cell 41, 620–636 e629. 10.1016/j.ccell.2023.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, and Kroemer G (2015). Type I interferons in anticancer immunity. Nat Rev Immunol 15, 405–414. 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 98.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. (2011). Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 208, 1989–2003. 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klarquist J, Hennies CM, Lehn MA, Reboulet RA, Feau S, and Janssen EM (2014). STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J Immunol 193, 6124–6134. 10.4049/jimmunol.1401869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li W, Lu L, Lu J, Wang X, Yang C, Jin J, Wu L, Hong X, Li F, Cao D, et al. (2020). cGAS-STING-mediated DNA sensing maintains CD8(+) T cell stemness and promotes antitumor T cell therapy. Sci Transl Med 12. 10.1126/scitranslmed.aay9013. [DOI] [PubMed] [Google Scholar]
- 101.Lorenzi S, Mattei F, Sistigu A, Bracci L, Spadaro F, Sanchez M, Spada M, Belardelli F, Gabriele L, and Schiavoni G (2011). Type I IFNs control antigen retention and survival of CD8alpha(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J Immunol 186, 5142–5150. 10.4049/jimmunol.1004163. [DOI] [PubMed] [Google Scholar]
- 102.Papewalis C, Jacobs B, Wuttke M, Ullrich E, Baehring T, Fenk R, Willenberg HS, Schinner S, Cohnen M, Seissler J, et al. (2008). IFN-alpha skews monocytes into CD56+expressing dendritic cells with potent functional activities in vitro and in vivo. J Immunol 180, 1462–1470. 10.4049/jimmunol.180.3.1462. [DOI] [PubMed] [Google Scholar]
- 103.Ranoa DRE, Widau RC, Mallon S, Parekh AD, Nicolae CM, Huang X, Bolt MJ, Arina A, Parry R, Kron SJ, et al. (2019). STING Promotes Homeostasis via Regulation of Cell Proliferation and Chromosomal Stability. Cancer Res 79, 1465–1479. 10.1158/0008-5472.CAN-18-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vanpouille-Box C, Demaria S, Formenti SC, and Galluzzi L (2018). Cytosolic DNA Sensing in Organismal Tumor Control. Cancer Cell 34, 361–378. 10.1016/j.ccell.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 105.Curran E, Chen X, Corrales L, Kline DE, Dubensky TW Jr., Duttagupta P, Kortylewski M, and Kline J (2016). STING Pathway Activation Stimulates Potent Immunity against Acute Myeloid Leukemia. Cell Rep 15, 2357–2366. 10.1016/j.celrep.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Falahat R, Perez-Villarroel P, Mailloux AW, Zhu G, Pilon-Thomas S, Barber GN, and Mule JJ (2019). STING Signaling in Melanoma Cells Shapes Antigenicity and Can Promote Antitumor T-cell Activity. Cancer Immunol Res 7, 1837–1848. 10.1158/2326-6066.CIR-19-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, Watkins SC, Sarkar SN, and Okada H (2014). STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res 2, 1199–1208. 10.1158/2326-6066.CIR-14-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thomsen MK, Skouboe MK, Boularan C, Vernejoul F, Lioux T, Leknes SL, Berthelsen MF, Riedel M, Cai H, Joseph JV, et al. (2020). The cGAS-STING pathway is a therapeutic target in a preclinical model of hepatocellular carcinoma. Oncogene 39, 1652–1664. 10.1038/s41388-019-1108-8. [DOI] [PubMed] [Google Scholar]
- 109.Karaolis DK, Cheng K, Lipsky M, Elnabawi A, Catalano J, Hyodo M, Hayakawa Y, and Raufman JP (2005). 3’,5’-Cyclic diguanylic acid (c-di-GMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochem Biophys Res Commun 329, 40–45. 10.1016/j.bbrc.2005.01.093. [DOI] [PubMed] [Google Scholar]
- 110.Li T, Cheng H, Yuan H, Xu Q, Shu C, Zhang Y, Xu P, Tan J, Rui Y, Li P, and Tan X (2016). Antitumor Activity of cGAMP via Stimulation of cGAS-cGAMP-STING-IRF3 Mediated Innate Immune Response. Sci Rep 6, 19049. 10.1038/srep19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang CH, Zundell JA, Ranatunga S, Lin C, Nefedova Y, Del Valle JR, and Hu CC (2016). Agonist-Mediated Activation of STING Induces Apoptosis in Malignant B Cells. Cancer Res 76, 2137–2152. 10.1158/0008-5472.CAN-15-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, et al. (2015). STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 7, 283ra252. 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng N, Watkins-Schulz R, Junkins RD, David CN, Johnson BM, Montgomery SA, Peine KJ, Darr DB, Yuan H, McKinnon KP, et al. (2018). A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. JCI Insight 3. 10.1172/jci.insight.120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang X, Pillarisetty VG, Pillai SPS, Wittrup KD, and Stephan MT (2017). Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J Clin Invest 127, 2176–2191. 10.1172/JCI87624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, et al. (2015). Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep 11, 1018–1030. 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y, Sun Z, Pei J, Luo Q, Zeng X, Li Q, Yang Z, and Quan J (2018). Identification of alpha-Mangostin as an Agonist of Human STING. ChemMedChem 13, 2057–2064. 10.1002/cmdc.201800481. [DOI] [PubMed] [Google Scholar]
- 117.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al. (2014). STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 41, 843–852. 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ager CR, Reilley MJ, Nicholas C, Bartkowiak T, Jaiswal AR, and Curran MA (2017). Intratumoral STING Activation with T-cell Checkpoint Modulation Generates Systemic Antitumor Immunity. Cancer Immunol Res 5, 676–684. 10.1158/2326-6066.CIR-17-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ding L, Kim HJ, Wang Q, Kearns M, Jiang T, Ohlson CE, Li BB, Xie S, Liu JF, Stover EH, et al. (2018). PARP Inhibition Elicits STING-Dependent Antitumor Immunity in Brca1-Deficient Ovarian Cancer. Cell Rep 25, 2972–2980 e2975. 10.1016/j.celrep.2018.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, and Chen ZJ (2017). cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci U S A 114, 1637–1642. 10.1073/pnas.1621363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.An X, Zhu Y, Zheng T, Wang G, Zhang M, Li J, Ji H, Li S, Yang S, Xu D, et al. (2019). An Analysis of the Expression and Association with Immune Cell Infiltration of the cGAS/STING Pathway in Pan-Cancer. Mol Ther Nucleic Acids 14, 80–89. 10.1016/j.omtn.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TBK, Taunk NK, et al. (2018). Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472. 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sokol CL, and Luster AD (2015). The chemokine system in innate immunity. Cold Spring Harb Perspect Biol 7. 10.1101/cshperspect.a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, and Sixt M (2013). Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339, 328–332. 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 125.Schmid MA, Takizawa H, Baumjohann DR, Saito Y, and Manz MG (2011). Bone marrow dendritic cell progenitors sense pathogens via Toll-like receptors and subsequently migrate to inflamed lymph nodes. Blood 118, 4829–4840. 10.1182/blood-2011-03-344960. [DOI] [PubMed] [Google Scholar]
- 126.Hauser MA, Schaeuble K, Kindinger I, Impellizzieri D, Krueger WA, Hauck CR, Boyman O, and Legler DF (2016). Inflammation-Induced CCR7 Oligomers Form Scaffolds to Integrate Distinct Signaling Pathways for Efficient Cell Migration. Immunity 44, 59–72. 10.1016/j.immuni.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 127.Riol-Blanco L, Sanchez-Sanchez N, Torres A, Tejedor A, Narumiya S, Corbi AL, Sanchez-Mateos P, and Rodriguez-Fernandez JL (2005). The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J Immunol 174, 4070–4080. 10.4049/jimmunol.174.7.4070. [DOI] [PubMed] [Google Scholar]
- 128.Kohler T, Reizis B, Johnson RS, Weighardt H, and Forster I (2012). Influence of hypoxia-inducible factor 1alpha on dendritic cell differentiation and migration. Eur J Immunol 42, 12261236. 10.1002/eji.201142053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lammermann T, Renkawitz J, Wu X, Hirsch K, Brakebusch C, and Sixt M (2009). Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood 113, 5703–5710. 10.1182/blood-2008-11-191882. [DOI] [PubMed] [Google Scholar]
- 130.Guak H, Al Habyan S, Ma EH, Aldossary H, Al-Masri M, Won SY, Ying T, Fixman ED, Jones RG, McCaffrey LM, and Krawczyk CM (2018). Glycolytic metabolism is essential for CCR7 oligomerization and dendritic cell migration. Nat Commun 9, 2463. 10.1038/s41467-018-04804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu J, Zhang X, Chen K, Cheng Y, Liu S, Xia M, Chen Y, Zhu H, Li Z, and Cao X (2019). CCR7 Chemokine Receptor-Inducible lnc-Dpf3 Restrains Dendritic Cell Migration by Inhibiting HIF-1alpha-Mediated Glycolysis. Immunity 50, 600–615 e615. 10.1016/j.immuni.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 132.Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, and Struthers RS (2004). Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem 279, 23214–23222. 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- 133.Moran TP, Nakano H, Kondilis-Mangum HD, Wade PA, and Cook DN (2014). Epigenetic control of Ccr7 expression in distinct lineages of lung dendritic cells. J Immunol 193, 4904–4913. 10.4049/jimmunol.1401104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brandum EP, Jorgensen AS, Rosenkilde MM, and Hjorto GM (2021). Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. Int J Mol Sci 22. 10.3390/ijms22158340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Itakura M, Terashima Y, Shingyoji M, Yokoi S, Ohira M, Kageyama H, Matui Y, Yoshida Y, Ashinuma H, Moriya Y, et al. (2013). High CC chemokine receptor 7 expression improves postoperative prognosis of lung adenocarcinoma patients. Br J Cancer 109, 1100–1108. 10.1038/bjc.2013.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gunther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, and Forster R (2005). Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int J Cancer 116, 726–733. 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 137.Liu Y, Ji R, Li J, Gu Q, Zhao X, Sun T, Wang J, Li J, Du Q, and Sun B (2010). Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res 29, 16. 10.1186/1756-9966-29-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, and Mori M (2002). Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res 62, 2937–2941. [PubMed] [Google Scholar]
- 139.Nakata B, Fukunaga S, Noda E, Amano R, Yamada N, and Hirakawa K (2008). Chemokine receptor CCR7 expression correlates with lymph node metastasis in pancreatic cancer. Oncology 74, 69–75. 10.1159/000139126. [DOI] [PubMed] [Google Scholar]
- 140.Sperveslage J, Frank S, Heneweer C, Egberts J, Schniewind B, Buchholz M, Bergmann F, Giese N, Munding J, Hahn SA, et al. (2012). Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int J Cancer 131, E371–381. 10.1002/ijc.26502. [DOI] [PubMed] [Google Scholar]
- 141.Hillinger S, Yang SC, Batra RK, Strieter RM, Weder W, Dubinett SM, and Sharma S (2006). CCL19 reduces tumour burden in a model of advanced lung cancer. Br J Cancer 94, 1029–1034. 10.1038/sj.bjc.6603061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Turnquist HR, Lin X, Ashour AE, Hollingsworth MA, Singh RK, Talmadge JE, and Solheim JC (2007). CCL21 induces extensive intratumoral immune cell infiltration and specific anti-tumor cellular immunity. Int J Oncol 30, 631–639. [PubMed] [Google Scholar]
- 143.Kar UK, Srivastava MK, Andersson A, Baratelli F, Huang M, Kickhoefer VA, Dubinett SM, Rome LH, and Sharma S (2011). Novel CCL21-vault nanocapsule intratumoral delivery inhibits lung cancer growth. PLoS One 6, e18758. 10.1371/journal.pone.0018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Okada N, Gao JQ, Sasaki A, Niwa M, Okada Y, Nakayama T, Yoshie O, Mizuguchi H, Hayakawa T, Fujita T, et al. (2004). Anti-tumor activity of chemokine is affected by both kinds of tumors and the activation state of the host’s immune system: implications for chemokine-based cancer immunotherapy. Biochem Biophys Res Commun 317, 68–76. 10.1016/j.bbrc.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 145.Okada N, Sasaki A, Niwa M, Okada Y, Hatanaka Y, Tani Y, Mizuguchi H, Nakagawa S, Fujita T, and Yamamoto A (2006). Tumor suppressive efficacy through augmentation of tumor-infiltrating immune cells by intratumoral injection of chemokine-expressing adenoviral vector. Cancer Gene Ther 13, 393–405. 10.1038/sj.cgt.7700903. [DOI] [PubMed] [Google Scholar]
- 146.Shao Z, Gaurav R, and Agrawal DK (2015). Intermediate-conductance calcium-activated potassium channel KCa3.1 and chloride channel modulate chemokine ligand (CCL19/CCL21)-induced migration of dendritic cells. Transl Res 166, 89–102. 10.1016/j.trsl.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, and Tamada K (2018). IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 36, 346–351. 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 148.Okada N, Mori N, Koretomo R, Okada Y, Nakayama T, Yoshie O, Mizuguchi H, Hayakawa T, Nakagawa S, Mayumi T, et al. (2005). Augmentation of the migratory ability of DC-based vaccine into regional lymph nodes by efficient CCR7 gene transduction. Gene Ther 12, 129–139. 10.1038/sj.gt.3302358. [DOI] [PubMed] [Google Scholar]
- 149.Yang SC, Batra RK, Hillinger S, Reckamp KL, Strieter RM, Dubinett SM, and Sharma S (2006). Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res 66, 3205–3213. 10.1158/0008-5472.CAN-05-3619. [DOI] [PubMed] [Google Scholar]
- 150.Yang SC, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M, Atianzar K, Kuo BY, Gardner B, Batra RK, et al. (2004). Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res 10, 2891–2901. 10.1158/1078-0432.ccr-03-0380. [DOI] [PubMed] [Google Scholar]
- 151.Lee JM, Lee MH, Garon E, Goldman JW, Salehi-Rad R, Baratelli FE, Schaue D, Wang G, Rosen F, Yanagawa J, et al. (2017). Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8(+) T-cell Infiltration. Clin Cancer Res 23, 4556–4568. 10.1158/1078-0432.CCR-16-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garlanda C, Dinarello CA, and Mantovani A (2013). The interleukin-1 family: back to the future. Immunity 39, 1003–1018. 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fotaki G, Jin C, Ramachandran M, Kerzeli IK, Karlsson-Parra A, Yu D, and Essand M (2018). Pro-inflammatory allogeneic DCs promote activation of bystander immune cells and thereby license antigen-specific T-cell responses. Oncoimmunology 7, e1395126. 10.1080/2162402X.2017.1395126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bjorkdahl O, Dohlsten M, and Sjogren HO (2000). Vaccination with B16 melanoma cells expressing a secreted form of interleukin-1beta induces tumor growth inhibition and an enhanced immunity against the wild-type B16 tumor. Cancer Gene Ther 7, 1365–1374. 10.1038/sj.cgt.7700248. [DOI] [PubMed] [Google Scholar]
- 155.Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, and Smyth MJ (2011). Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 71, 4809–4820. 10.1158/0008-5472.CAN-11-0753. [DOI] [PubMed] [Google Scholar]
- 156.Lee PH, Yamamoto TN, Gurusamy D, Sukumar M, Yu Z, Hu-Li J, Kawabe T, Gangaplara A, Kishton RJ, Henning AN, et al. (2019). Host conditioning with IL-1beta improves the antitumor function of adoptively transferred T cells. J Exp Med 216, 2619–2634. 10.1084/jem.20181218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. (2009). Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 15, 1170–1178. 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 158.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. (2013). Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 19, 57–64. 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 159.Rebe C, and Ghiringhelli F (2020). Interleukin-1beta and Cancer. Cancers (Basel) 12. 10.3390/cancers12071791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saijo Y, Tanaka M, Miki M, Usui K, Suzuki T, Maemondo M, Hong X, Tazawa R, Kikuchi T, Matsushima K, and Nukiwa T (2002). Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol 169, 469–475. 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]
- 161.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, and Group CT (2017). Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842. 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 162.Lythgoe MP, and Prasad V (2022). Repositioning canakinumab for non-small cell lung cancer-important lessons for drug repurposing in oncology. Br J Cancer 127, 785–787. 10.1038/s41416-022-01893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chyuan IT, and Lai JH (2020). New insights into the IL-12 and IL-23: From a molecular basis to clinical application in immune-mediated inflammation and cancers. Biochem Pharmacol 175, 113928. 10.1016/j.bcp.2020.113928. [DOI] [PubMed] [Google Scholar]
- 164.Bullock TNJ (2022). CD40 stimulation as a molecular adjuvant for cancer vaccines and other immunotherapies. Cell Mol Immunol 19, 14–22. 10.1038/s41423-021-00734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu R, Zhu B, and Chen D (2022). Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell Mol Life Sci 79, 191. 10.1007/s00018-022-04219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kline DE, MacNabb BW, Chen X, Chan WC, Fosco D, and Kline J (2018). CD8alpha(+) Dendritic Cells Dictate Leukemia-Specific CD8(+) T Cell Fates. J Immunol 201, 3759–3769. 10.4049/jimmunol.1801184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smits EL, Ponsaerts P, Van de Velde AL, Van Driessche A, Cools N, Lenjou M, Nijs G, Van Bockstaele DR, Berneman ZN, and Van Tendeloo VF (2007). Proinflammatory response of human leukemic cells to dsRNA transfection linked to activation of dendritic cells. Leukemia 21, 1691–1699. 10.1038/sj.leu.2404763. [DOI] [PubMed] [Google Scholar]


