Abstract
Background:
In children with CKD, certain risk factors are associated with faster eGFR decline and earlier kidney failure. Whether these factors have lingering effects on post-transplant eGFR trajectory remains unclear. We characterized pre- and post-transplant eGFR trajectories in pediatric kidney transplant recipients by their pre-kidney replacement therapy (KRT) risk factors.
Methods:
We studied eGFR trajectories before KRT initiation and after transplantation among Chronic Kidney Disease in Children (CKiD) Study participants. We used mixed-effects models to compare the pre-KRT versus post-transplant eGFR trajectories within individual participants by 7 pre-KRT risk factors: glomerular/non-glomerular etiology, race, preemptive transplant, proteinuria, albuminuria, and systolic/diastolic blood pressure (SBP/DBP).
Results:
We analyzed 1602 pre-KRT and 592 post-transplant eGFR measurements from 246 transplant recipients. Mean annual eGFR decline was decreased from 18.0% pre-KRT (95%CI, 16.1%−19.8%) to 5.0% post-transplant (95%CI, 3.3%−6.7%). All 7 pre-KRT risk factors showed strong associations with faster pre-KRT eGFR decline, but not with post-transplant eGFR decline; only albuminuria, high SBP, and high DBP reached statistical significance with notably attenuated associations. In our multivariable model of the pre-KRT risk factors, post-transplant eGFR decline was more rapid only when albuminuria and high SBP were both present.
Conclusions:
EGFR decline substantially slows down after transplant even among children with rapidly progressing forms of CKD. Nonetheless, those who had albuminuria and high SBP before KRT might continue to show faster eGFR decline after transplant, specifically when both risk factors were present. This subgroup might benefit from intensive pre-transplant management for at least one of the two risk factors.
Keywords: transplantation, estimated glomerular filtration rate, risk factors
INTRODUCTION
Slowing the loss of kidney function is a central goal in managing chronic kidney disease (CKD) in children [1, 2]. However, faster decline in estimated glomerular filtration rate (eGFR) is often observed in high-risk subgroups, such as those with glomerular etiology, Black race, proteinuria, albuminuria, and high blood pressure (BP) [3–7]. Kidney failure (or stage 5 CKD) is not uncommon in this population; more than a half of children with chronic kidney disease (CKD) reach kidney failure by 19 years of age [3, 8]. Once children develop kidney failure, kidney transplantation is the preferred treatment modality as it confers superior survival and quality of life compared to dialysis [9, 10].
Whether the risk factors for faster eGFR decline have any lingering effect on the post-transplant kidney function remains unclear. On one hand, transplantation replaces the central part of the pathophysiology, the kidney, likely annulling the kidney-related risk factors such as glomerular etiology. However, on the other hand, some systemic factors (such as high BP) or unmodifiable factors (such as race/ethnicity) may have a prolonged effect even after transplantation. If any of these risk factors, which are readily measured well before developing kidney failure, could be used to better evaluate the prognosis after kidney transplantation, planning for kidney replacement therapy (KRT) and preparing post-transplant care could also be improved and tailored to the individual child’s risk profile.
The aim of this study was to assess whether the risk factors for faster pre-KRT eGFR decline may predict post-transplant eGFR trajectories in a well-studied cohort of children with CKD. We conducted a longitudinal analysis of the eGFR data among the kidney transplant recipients in the Chronic Kidney Disease in Children (CKiD) Study, which provides high-quality data on eGFR measures and pre-KRT clinical factors.
METHODS
The Chronic Kidney Disease in Children (CKiD) Study
The CKiD Study is an ongoing prospective cohort study of children and adolescents with CKD to which a total of 63 sites across the United States and Canada have contributed data to date (Supplemental Material). At the initial enrollment to the CKiD Study, participants were 16 years or younger with estimated glomerular filtration rate (eGFR) 30–90 ml/min/1.73m2 and without a previous history of kidney failure or KRT. Enrollment occurred in 3 phases in 2005–2009 (n= 586), 2011–2014 (n= 305), and 2016–2020 (n= 209). The CKiD Study conducts annual study visits of standardized protocols to collect longitudinal data focused on CKD progression and cardiovascular health, as well as growth and neurocognitive development. Full details on the study design have been described previously [11]. All study protocols were approved by local Institutional Review Boards. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism’.
Study Population and Objectives
Of the 1100 participants in the CKiD cohort, 246 were observed to have received a kidney transplant as of August 2021 and contributed data to estimate GFR at visits taking place before and after transplantation. All 246 participants were included in this study. The primary objectives of the study were to contrast the trajectories of eGFR before the initial KRT and after the transplant; and more importantly, to determine whether the risk factors for faster pre-KRT eGFR decline experienced by these participants were associated with faster post-transplant eGFR decline.
Modeling eGFR Trajectory
We used the U25 equations to calculate eGFR [12]. The U25 equations, developed using the data from the CKiD Study, estimate GFR based on serum creatinine and cystatin C levels. Serum creatinine and cystatin C were assayed at the CKiD central biochemistry laboratory using samples collected at each annual visit. These assays were calibrated to International Federation of Clinical Chemistry standards using isotope dilution mass spectroscopy-based reference materials [13, 14].
We studied the eGFR trajectory of the participants from their study enrollment to the last study visit, except for the period between the initiation of regular dialysis and the receipt of the first transplant. EGFR was not determined during regular dialysis as it represents neither the pre-KRT CKD progression nor the post-transplant eGFR trajectory [15].
The study time was centered at the date of transplant and scaled in years. As the person-time between the initiation of dialysis and the receipt of the first transplant in non-preemptive transplant cases was not pertinent (see above), a positive time value indicated the years since transplant, and a negative time value indicated the years before KRT. The KRT event would be the receipt of transplant in preemptive transplant cases and the initiation of dialysis in non-preemptive transplant cases. In summary, a visit years before KRT received the value of and a visit years after transplant received the value of in the time scale of the analysis. We modeled the eGFR trajectory within individual participants using mixed-effects models. We log-transformed eGFR as it showed a skewed distribution [12].
EGFR Trajectory: Overall Model
The univariate (i.e., overall) model to describe the trajectories of eGFR before KRT and after transplant included two lines, one before KRT and another after transplant (Figure 1). Specifically, the log of eGFR in participant at visit taking place at time was modeled as:
where takes value 1 if the visit is after transplant and 0 otherwise. The line before KRT has an intercept (eGFR at KRT initiation) as and a slope (the rate of eGFR change on time prior to KRT) as ; and likewise, the line after transplant has an intercept whereby represents the mean increase in eGFR due to transplantation, and a slope whereby represents the difference of the rate of eGFR change due to kidney transplantation. The random effects terms ( and ) represent the departures of the lines of the individual from the population lines. We used an unstructured correlation matrix to estimate the random effect terms. The random effect terms were estimated using the empirical Bayes method [16]. The error term () represents the within-subject departures of the observed data from the lines of the individual. Of note, the annual change in the log of eGFR can be exponentiated to represent the annualized ratio in eGFR; in this manuscript, we present the annual decline in percentage, which was derived from the annualized ratio by for pre-KRT and by for the post-transplant periods.
Figure 1. Estimated Glomerular Filtration Rate (GFR) before kidney replacement therapy (KRT) and after transplant in 246 pediatric kidney transplant recipients in the CKiD study, 2005–2021.
Our data included 1602 pre-KRT and 592 post-transplant measurements. We used the U25 equations to estimate GFR [12]. The eGFR trajectory was characterized using a longitudinal model with 4 regression terms after a log transformation. Acronyms: KRT, kidney replacement therapy.
EGFR Trajectory: Subgroup Model
First, using the estimates from the overall model, we plotted the pre-KRT versus the post-transplant annual decline in eGFR within each participant to graphically assess their association. This plot was then stratified by 7 known risk factors for CKD progression (CKD etiology [glomerular vs. non-glomerular], race [Black vs. non-Black], urine protein-creatinine ratio [uPCR; >2 vs. ≤2 g/g], urine albumin-creatinine ratio [uACR; >1250 vs. ≤1250 mg/g], and systolic (SBP) and diastolic (DBP) blood pressure [>75 vs. ≤75 percentile], as well as pre-transplant dialysis history (non-preemptive transplant vs. preemptive transplant) [3–7, 17]. The cut-off values for the continuous variables were chosen such that each stratum included approximately half of the cohort (see Results).
Based on the findings from the graphical analysis, we expanded our longitudinal model above to allow the eGFR trajectory to differ between the subgroups of a predictor. Specifically, we added a subgroup indicator (e.g., for race, for Blacks and for non-Blacks) to all 4 regression terms as follows:
Using this approach, the intercept and slope of both pre-KRT and post-transplant periods are allowed to differ between subgroups whereby the regression coefficients determine the effect of being present on the level at KRT, the rate of eGFR change prior to KRT, the level right after transplant, and the rate of eGFR change after transplant, respectively. Of particular interest were: 1) the difference in the rate of eGFR change in the pre-KRT period between subgroups; and 2) : the difference in the rate of eGFR change in the post-transplant period between subgroups. The statistical significance of these differences was tested using the Wald test. All analyses were performed with R version 4.0.4 (R Foundation for Statistical Computing; Vienna, Austria). Mixed-effects models were estimated using the lme4 package (version 1.1–27.1).
RESULTS
The study included a total of 2194 eGFR measurements from 246 participants who received a kidney transplant during the follow-up (Table 1). The median time from the initial enrollment to transplantation was 4.4 years. The median age at transplant was 16.0 years. Among all participants, 88 (35.8%) were female, 64 (26.0%) had a glomerular etiology of CKD, 41 (16.7%) were Black, and 132 (53.7%) underwent a preemptive transplant. The medians of the last measurements prior to the initiation of kidney replacement therapy of uPCR, uACR, and the percentiles of SBP and DBP pre-KRT were 2.1 g/g, 1379 mg/g, 75 and 78, respectively.
Table 1. Population Characteristics.
Continuous variables are shown in median [interquartile range] and binary variables are in N (%). The summary statistics of urine protein-creatinine ratio, urine albumin-creatinine ratio, and blood pressure were taken from the last measurements prior to the initiation of kidney replacement therapy. Acronyms: eGFR, estimated glomerular filtration rate.
| Characteristic | Overall (n= 246) |
|---|---|
|
| |
| Age at transplant (y) | 16.0 [13.0–18.1] |
| Female sex | 88 (35.8%) |
| Glomerular etiology | 64 (26.0%) |
| Black race | 41 (16.7%) |
| Preemptive transplant | 132 (53.7%) |
| Urine protein-creatinine ratio (g/g) | 2.1 [0.9–3.9] |
| Urine albumin-creatinine ratio (mg/g) | 1379.1 [428.0–2432.0] |
| Systolic blood pressure (percentile)a | 75.0 [47.5–94.0] |
| Diastolic blood pressure (percentile)a | 78.0 [50.0–94.0] |
| Number of eGFR measurements | |
| Total | 2194 |
| Pre-KRT | 1602 (73.0%) |
| Post-Tx | 592 (27.0%) |
| Within each participant | 9 [6–11] |
Blood pressure percentiles were standardized for age, sex, and height according to the 2017 American Academy of Pediatrics guidelines [23].
The median number of eGFR measurements within each participant was 9 (interquartile range [IQR]= 6, 11). Of the 2194 eGFR measurements, 1602 (73.0%) were conducted pre-KRT at a median of 2.8 (IQR= 1.0, 5.4) years before KRT and 592 (27.0%) were post-transplant at a median of 2.9 (IQR= 1.1, 5.2) years after transplant so that the time spans pre-KRT and post-transplant were very similar. Examining individual participants longitudinally, the final pre-KRT eGFR measurement was performed at a median of 13 (IQR= 1, 111) days before KRT. The first post-transplant eGFR measurement was performed at a median of 308 (IQR= 142, 779) days after transplant, and the final post-transplant eGFR measurement was at a median of 1482 (IQR= 665, 2297) days after transplant.
Graft failures and deaths were uncommon. The Kaplan-Meier cumulative incidences for the composite outcome of graft failure and death were 1.8% at 1-year post-transplant, 5.9% at 3-year post-transplant, and 11.3% at 5-year post-transplant.
EGFR Trajectory: Overall Model
Figure 1 depicts the eGFR data pre-KRT and post-transplant and the overall model. The mean eGFR was 18.5 ml/min/1.73 m2 at the initiation of KRT (= ) and increased to 70.5 ml/min/1.73 m2 after kidney transplantation (= ). As expected, the post-transplant eGFR annual decline (= ) was substantially slower compared to the pre-KRT eGFR annual decline (= ) (post-transplant= 5.0% [95% CI; 3.3%, 6.7%] vs. pre-KRT= 18.0% [95% CI; 16.1%, 19.8%]; p<0.001). Figure 2 compares the pre-KRT versus post-transplant eGFR annual decline for each participant. Compared to the pre-KRT decline, the post-transplant eGFR annual decline was less heterogeneous across the participants. Post-transplant eGFR annual decline was below 10% in 228 (92.7%) participants, whereas pre-KRT eGFR annual decline was below 10% in only 70 (28.5%). There was little correlation between the pre-KRT and post-transplant eGFR annual decline (r= 0.14; Figure 2), suggesting that rapid pre-KRT eGFR decline does not necessarily predict rapid post-transplant eGFR decline.
Figure 2. Annual Decline in Estimated Glomerular Filtration Rate (eGFR), Pre-Kidney Replacement Therapy versus Post-Transplant.
Solid curve line represents a loess model between pre-KRT and post-Tx eGFR annual decline. Grey band represents the 95% confidence band. There was little correlation between the pre-KRT and post-Tx eGFR annual decline (r= 0.14). Acronyms: Tx, transplant; and KRT, kidney replacement therapy.
EGFR Trajectory: Subgroup Model
Table 2 shows our eGFR trajectory models which compared the eGFR decline rates by each risk factor separately in the pre-KRT and post-transplant periods. The deleterious effects of the risk factors for accelerated pre-KRT eGFR decline were diminished or eliminated post-transplant. For example, those with glomerular CKD etiology showed significantly faster annual eGFR decline (30.8% [95% CI; 27.7%, 33.8%]) compared to those with non-glomerular etiology (13.5% [95% CI; 11.6%, 15.4%]; p<0.001) during the pre-KRT period. In contrast, during the post-transplant period, there was practically no difference in eGFR decline between the two groups (glomerular, 5.1% [95% CI; 1.8%, 8.3%] vs. non-glomerular, 5.0% [95% CI; 2.9%, 7.0%]; p= 0.935) congruent with the expectation that the transplanted kidney would be free from the primary glomerular or non-glomerular etiology. Of note, both glomerular and non-glomerular groups showed substantially slower eGFR decline post-transplant compared to pre-KRT. Similarly, while the pre-KRT eGFR decline was faster in Black participants (23.9% [95% CI; 19.4%, 28.1%]) compared to non-Black participants (16.7% [95% CI; 14.7% vs. 18.7%]), the post-transplant eGFR declines were very similar between the racial groups (4.6% [95% CI; −0.2%, 9.2%] vs. 5.1% [95% CI; 3.2%, 6.9%]).
Table 2. Comparison of Annual Decline with 95% confidence intervals in Estimated Glomerular Filtration Rate (eGFR) by Various Predictors.
Urine protein-creatinine ratio, urine albumin-creatinine ratio, and blood pressure were taken from the last measurements prior to the initiation of kidney replacement therapy. Acronyms: CKD, chronic kidney disease; NG, non-glomerular; G, glomerular; uPCR, urine protein-creatinine ratio; uACR, urine albumin-creatinine ratio; BP, blood pressure; pct, percentile (for age, gender, and height); and Tx, transplant.
| Subgroup | No. of participants | Pre-Kidney Replacement Therapy | Post-Transplant | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| No. of eGFR measurementsa | Annualized Decline in eGFR (%)b | P-value | No. of eGFR measurementsc | Annualized Decline in eGFR (%)d | P-value | |||
|
| ||||||||
| CKD etiology | NG | 182 | 1284 | 13.5 (11.6, 15.4) | <0.001 | 417 | 5.0 (2.9, 7.0) | 0.94 |
| G | 64 | 318 | 30.8 (27.7, 33.8) | 175 | 5.1 (1.8, 8.3) | |||
| Race | Non-Black | 205 | 1366 | 16.7 (14.7, 18.7) | 0.005 | 518 | 5.1 (3.2, 6.9) | 0.86 |
| Black | 41 | 236 | 23.9 (19.4, 28.1) | 74 | 4.6 (−0.2, 9.2) | |||
| uPCR (g/g) | ≤2 | 121 | 872 | 13.2 (10.6, 15.7) | <0.001 | 289 | 3.7 (1.1, 6.2) | 0.12 |
| >2 | 122 | 723 | 22.5 (20.0, 24.9) | 299 | 6.5 (4.0, 8.8) | |||
| uACR (mg/g) | ≤1250 | 116 | 807 | 14.0 (11.2, 16.6) | <0.001 | 275 | 2.8 (0.3, 5.3) | 0.01 |
| >1250 | 127 | 788 | 21.3 (18.8, 23.7) | 313 | 7.2 (4.8, 9.5) | |||
| Systolic BP (pct) | ≤75 | 123 | 879 | 14.9 (12.2, 17.6) | 0.001 | 281 | 2.5 (0.0, 5.0) | 0.02 |
| >75 | 119 | 708 | 21.3 (18.6, 23.9) | 307 | 6.5 (4.3, 8.6) | |||
| Diastolic BP (pct) | ≤75 | 115 | 819 | 15.6 (12.7, 18.3) | 0.02 | 285 | 3.1 (0.7, 5.4) | 0.047 |
| >75 | 126 | 763 | 20.2 (17.5, 22.7) | 300 | 6.4 (4.1, 8.6) | |||
| Preemptive Tx | Yes | 132 | 927 | 14.7 (12.1, 17.2) | <0.001 | 260 | 4.1 (1.8, 6.4) | 0.21 |
| No | 114 | 675 | 22.1 (19.4, 24.8) | 332 | 6.3 (3.7, 8.9) | |||
Measurements excluded for missing data: 7 for uPCR, 7 for uACR, 15 for SBP, and 20 for DBP.
In terms of the equation in methods section: for the first row and for the second row of each factor and the p-value is for testing whether .
Measurements excluded for missing data: 4 for uPCR, 4 for uACR, 4 for SBP, and 7 for DBP.
In terms of the equation in methods section: for the first row and for the second row of each factor and the p-value is for testing whether .
Certain pre-KRT risk factors such as uACR, SBP, and DBP continued to show statistically significant associations with faster eGFR decline even after transplant (Table 2). However, the differences in eGFR decline rates between the high- and low-risk groups were smaller post-transplant compared to pre-KRT, and the eGFR decline was substantially slower post-transplant compared to pre-KRT in both high- and low-risk groups. For example, those with SBP>75th percentile showed significantly faster annual eGFR decline (21.3% [95% CI; 18.6%, 23.9%]) compared to those with SBP≤75th percentile (14.9% [95% CI; 12.2%, 17.6%]; p= 0.001) during the pre-KRT period. This difference was less pronounced in the post-transplant period (>75th percentile, 6.5% [95% CI; 4.3%, 8.6%] vs. ≤75th percentile, 2.5% [95% CI; 0.0%, 5.0%]), although it was still statistically significant (p= 0.019). In addition, those with uPCR>2 g/g showed slightly faster annual eGFR decline (6.5% [95% CI; 4.0%, 8.8%]) compared to those with uPCR≤2 g/g (3.7% [95% CI; 1.1%, 6.2%]), although the association did not reach statistical significance (p= 0.117).
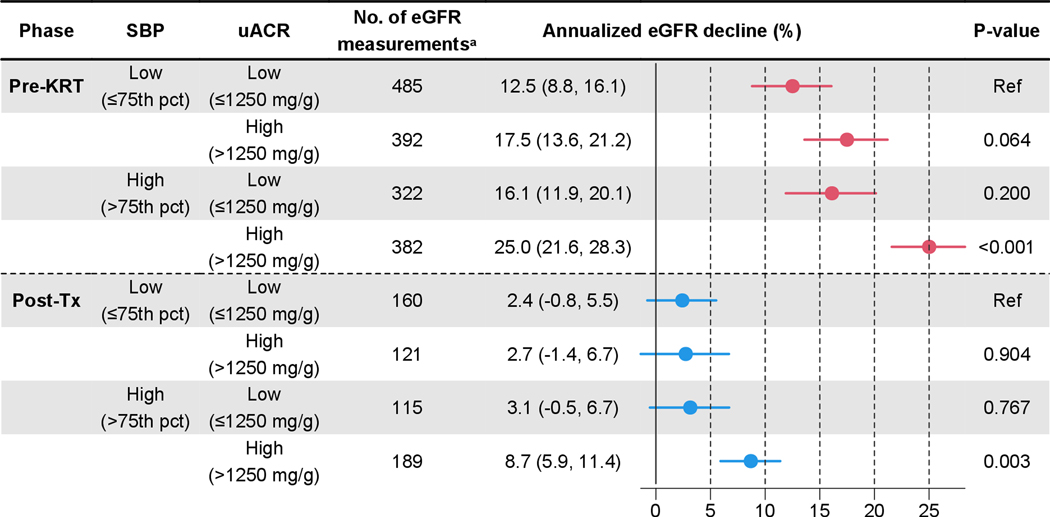
We further explored the multivariate association of uACR and blood pressure, and opted for a bivariate model of uACR and SBP as this model demonstrated superior model fit measured by the Akaike information criterion (AIC) compared to others that included DBP. More importantly, we observed a synergistic interaction between uACR and SBP, as Figure 3 shows. This interaction existed in both pre-KRT and post-transplant phases. Furthermore, participants who showed high uACR and high SBP before KRT continued to experience more rapid eGFR decline even after transplant, but those who showed only one of the risk factors did not experience faster post-transplant eGFR decline compared to those who showed neither. Specifically, the pre-KRT high-uACR & high-SBP group showed significantly faster decline in post-transplant eGFR (8.7% [95% CI; 5.9%, 11.4%]) compared to the low-uACR & low-SBP group (2.4% [95% CI; −0.8%, 5.5%]) (p=0.003). In contrast, the high-uACR & low-SBP group (2.7% [95% CI; −1.4%, 6.7%]) or the low-uACR & high-SBP group (3.1% [95% CI; −0.5%, 6.7%]) showed similar declines to the low-uACR & low-SBP group.
Figure 3. Comparison of Annual Decline in Estimated Glomerular Filtration Rate (eGFR) by Urine Albumin-Creatinine Ratio and Systolic Blood Pressure.
The low-uACR & low-SBP group (first row) was used as the reference group for each of the two phases. Urine albumin-creatinine ratio and blood pressure were taken from the last measurements prior to the initiation of kidney replacement therapy. Acronyms: KRT, kidney replacement therapy; Tx, transplant; uACR, urine albumin-creatinine ratio; SBP, systolic blood pressure; and pct, percentile (for age, gender, and height).
a 21 measurements during pre-KRT and 7 during post-Tx excluded for missing data in uACR or SBP.
Of note, these pre-KRT risk factors appear to have persisted after transplantation. The high-SBP group continued to show high SBP percentiles throughout post-KT measurements (median [IQR]= 85 [72, 96] percentiles), and the high-uACR group continued to show high uACR throughout post-KT measurements (median [IQR]= 2485.7 [1797.9, 4293.3] mg/g).
DISCUSSION
In this longitudinal study of 2194 eGFR measurements from 246 children and adolescents who received a kidney transplant, the rate of post-transplant eGFR decline was only gradual even among children who had showed rapidly progressing forms of CKD prior to KRT. The traditional pre-KRT risk factors for rapid CKD progression, such as Black race, glomerular etiology, and proteinuria showed weaker or no association with faster post-transplant eGFR decline. On the other hand, children who showed both uACR and SBP pre-KRT experienced faster eGFR decline post-transplant compared to those who showed only one or none of these risk factors.
Several pre-KRT clinical factors such as glomerular etiology, proteinuria, and high blood pressure have been well established as strongly associated with rapid CKD progression and pre-KRT eGFR decline. Unless there is disease recurrence in the transplanted kidney, one would not expect the post-transplant decline to reflect the underlying glomerular disease. As expected, the post-transplant eGFR decline did not differ by etiology, corroborating the results from the previous literature [18, 19]. Although a study using the UK transplant registry data (n= 5121) reported a statistically significant association of glomerular etiology with faster post-transplant eGFR decline among children and young adults (<30 years of age) [20], the reported difference in the annual eGFR decline was <1 mL/min/1.73m2 per year between the glomerular and non-glomerular groups, suggesting limited clinical implications. Additionally, other important risk factors for faster pre-KRT eGFR decline, namely proteinuria, albuminuria, and high blood pressure, were not discussed in the previous studies of post-transplant eGFR trajectory [18–21]. Our study shows that when both albuminuria and high blood pressure are present before KRT, they remained as significant risk factors for faster eGFR decline even after transplantation. Lastly, the UK registry study reported additional risk factors for faster post-transplant eGFR decline, including Black race [20]. In our study, Black race was not associated with post-transplant eGFR decline, although it was associated with pre-KRT eGFR decline. The reason for this discrepancy is unclear, while race as a social construct might have different operationalization and clinical implication across the geographical and cultural contexts. Nonetheless, at least in the North American clinical context, the lack of association between race and post-transplant eGFR decline lends support to removing any explicit or implicit racial disparities in the assessment of candidacy for kidney transplantation.
Overall, our findings are encouraging in that the post-transplant eGFR trajectory showed only a gradual decline across the entire population, even among the children who had rapidly progressing forms of CKD. However, a potentially more important finding from our study is that albuminuria and high blood pressure before KRT may have a lingering effect even after transplantation, specifically when both risk factors are present. The synergistic interaction between the two factors may indicate that successfully managing at least one of the two factors would improve the post-transplant eGFR trajectory. These findings may possibly be explained by both clinical and non-clinical mechanisms. For example, albuminuria and high blood pressure before KRT might have been caused by a persistent and recurrent subtype of hypertension, which often continues to be a risk factor even after transplantation [22]. Alternatively, high albuminuria and blood pressure before KRT may be correlated with unobserved factors (e.g., ability to comply with care, availability of support systems) affecting the eGFR decline after transplant. More research is needed to examine robust management of albuminuria and hypertension via clinical interventions such as renin-angiotensin-aldosterone system (RAAS) blockers as well as non-clinical approaches such as improved education and support for patients and caregivers.
This study has limitations. As our dataset did not include transplant-specific variables such as immunosuppression regimen, donor type, or histological match in detail, this analysis did not account for such clinical factors. If our exposure (pre-KRT risk factors) influences the transplant-specific variables (e.g., immunosuppression regimen selection), which in turn would affect the post-transplant eGFR trajectory, accounting for the transplant-specific variables would have allowed us to dissect the direct versus indirect association between our exposure and the outcome. Additionally, our study used an observational design, and therefore our findings do not necessarily indicate causality.
To summarize, in our longitudinal study of 246 pediatric kidney transplant recipients, post-transplant eGFR decline was remarkably slower compared to their pre-KRT eGFR decline even among those with traditional risk factors for faster pre-KRT CKD progression such as glomerular etiology and Black race. Nonetheless, those with pre-KRT albuminuria and high blood pressure continued to show faster eGFR decline even after transplant, and specifically if they had both risk factors. Given this synergistic interaction, controlling at least one of the two risk factors pre-KRT may suffice to remove lingering deleterious effects on post-transplantation eGFR trajectory.
Supplementary Material
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD and Derek Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U24-DK-082194, U24-DK-66116). The CKID website is located at https://statepi.jhsph.edu/ckid and a list of CKiD collaborators can be found at https://statepi.jhsph.edu/ckid/site-investigators/. A full list of CKiD investigators is provided in the supplemental material.
SUPPORT
The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01 DK066143, U01 DK066174, U24 DK082194, U24 DK066116). The CKID website is located at http://www.statepi.jhsph.edu/ckid.
Footnotes
FINANCIAL DISCLOSURE
The authors declare that they have no relevant financial interests.
DATA AVAILABILITY STATEMENT
The CKiD study regularly updates data deposited for public use at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository (https://repository.niddk.nih.gov/studies/ckid/).
REFERENCES
- 1.Kula AJ, Somers MJG, on behalf of the American Society of Pediatric Nephrology (2021) Children with CKD Are Not Little Adults with CKD: Pediatric Considerations for the Advancing American Kidney Health Initiative. CJASN 16:470–472. 10.2215/CJN.11540720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg RJ, Furth S, Lemley KV, et al. (2003) National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics 111:1416–1421. 10.1542/peds.111.6.1416 [DOI] [PubMed] [Google Scholar]
- 3.Ng DK, Pierce CB (2021) Kidney Disease Progression in Children and Young Adults With Pediatric CKD: Epidemiologic Perspectives and Clinical Applications. Seminars in Nephrology 41:405–415. 10.1016/j.semnephrol.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warady BA, Abraham AG, Schwartz GJ, et al. (2015) Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. American Journal of Kidney Diseases 65:878–888. 10.1053/j.ajkd.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson MA, Ng DK, Warady BA, et al. (2021) The CKiD study: overview and summary of findings related to kidney disease progression. Pediatr Nephrol 36:527–538. 10.1007/s00467-019-04458-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuhrman DY, Schneider MF, Dell KM, et al. (2017) Albuminuria, Proteinuria, and Renal Disease Progression in Children with CKD. Clin J Am Soc Nephrol 12:912–920. 10.2215/CJN.11971116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fathallah-Shaykh SA, Flynn JT, Pierce CB, et al. (2015) Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol 10:571–577. 10.2215/CJN.07480714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng DK, Matheson MB, Warady BA, et al. (2019) Incidence of Initial Renal Replacement Therapy Over the Course of Kidney Disease in Children. Am J Epidemiol 188:2156–2164. 10.1093/aje/kwz220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillen DL, Stehman-Breen CO, Smith JM, et al. (2008) Survival Advantage of Pediatric Recipients of a First Kidney Transplant Among Children Awaiting Kidney Transplantation. American Journal of Transplantation 8:2600–2606. 10.1111/j.1600-6143.2008.02410.x [DOI] [PubMed] [Google Scholar]
- 10.Dharnidharka VR, Fiorina P, Harmon WE (2014) Kidney Transplantation in Children. N Engl J Med 371:549–558. 10.1056/NEJMra1314376 [DOI] [PubMed] [Google Scholar]
- 11.Furth SL, Cole SR, Moxey-Mims M, et al. (2006) Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1:1006–1015. 10.2215/CJN.01941205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce CB, Muñoz A, Ng DK, et al. (2021) Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney International 99:948–956. 10.1016/j.kint.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Kwong T, Erway B, et al. (2009) Validation of creatinine assays utilizing HPLC and IDMS traceable standards in sera of children. Pediatr Nephrol 24:113–119. 10.1007/s00467-008-0957-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Cox C, Seegmiller JC, et al. (2020) Recalibration of cystatin C using standardized material in Siemens nephelometers. Pediatr Nephrol 35:279–285. 10.1007/s00467-019-04389-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shafi T, Levey AS (2018) Measurement and Estimation of Residual Kidney Function in Patients on Dialysis. Adv Chronic Kidney Dis 25:93–104. 10.1053/j.ackd.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins HE (1992) An Empirical Bayes Approach to Statistics. In: Kotz S, Johnson NL (eds) Breakthroughs in Statistics. Springer; New York, New York, NY, pp 388–394 [Google Scholar]
- 17.Amaral S, Sayed BA, Kutner N, Patzer RE (2016) Preemptive kidney transplantation is associated with survival benefits among pediatric patients with end-stage renal disease. Kidney Int 90:1100–1108. 10.1016/j.kint.2016.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaboré R, Ferrer L, Couchoud C, et al. (2021) Dynamic prediction models for graft failure in paediatric kidney transplantation. Nephrology Dialysis Transplantation 36:927–935. 10.1093/ndt/gfaa180 [DOI] [PubMed] [Google Scholar]
- 19.Foster BJ, Dahhou M, Zhang X, et al. (2011) Association between age and graft failure rates in young kidney transplant recipients. Transplantation 92:1237–1243. 10.1097/TP.0b013e31823411d7 [DOI] [PubMed] [Google Scholar]
- 20.Hamilton AJ, Plumb LA, Casula A, Sinha MD (2020) Associations with kidney transplant survival and eGFR decline in children and young adults in the United Kingdom: a retrospective cohort study. BMC Nephrol 21:492. 10.1186/s12882-020-02156-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Souza VC, Rabilloud M, Cochat P, et al. (2017) Trajectories and Predictors of Allograft Dysfunction after Renal Transplantation in Children. Am J Nephrol 45:63–68. 10.1159/000453076 [DOI] [PubMed] [Google Scholar]
- 22.Brubaker AL, Stoltz DJ, Chaudhuri A, et al. (2018) Superior Hypertension Management in Pediatric Kidney Transplant Patients After Native Nephrectomy. Transplantation 102:1172–1178. 10.1097/TP.0000000000002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn JT, Kaelber DC, Baker-Smith CM, et al. (2017) Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 140:. 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The CKiD study regularly updates data deposited for public use at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository (https://repository.niddk.nih.gov/studies/ckid/).