Abstract
Background:
Organophosphate esters (OPEs), used as flame retardants and plasticizers, are chemicals of concern for maternal and infant health. Prior studies examining temporal trends and predictors of OPE exposure are primarily limited by small sample sizes.
Objectives:
Characterize temporal trends and predictors of OPE exposure biomarkers.
Methods:
We determined urinary concentrations of eight biomarkers of OPE exposure at three timepoints during pregnancy for participants in the LIFECODES Fetal Growth Study (n = 900), a nested case-cohort recruited between 2007–2018. We examined biomarker concentrations, their variability during pregnancy, and temporal trends over the study period. In addition, we identified sociodemographic and pregnancy characteristics associated with biomarker concentrations. Analyses were conducted using both the within-subject pregnancy geometric means and biomarker concentrations measured at individual study visits.
Results:
Five OPE biomarkers were detected in at least 60% of the study participants. Biomarkers were not strongly correlated with one another and intraclass correlation coefficients, measuring within-subject variability during pregnancy, ranged from 0.27–0.51. Biomarkers exhibited varying temporal trends across study years. For example, bis(1-chloro-2-propyl) phosphate (BCIPP) increased monotonically, whereas bis(1,3-dichloro-2-propyl) phosphate (BDCIPP) and diphenyl phosphate (DPHP), displayed non-monotonic trends with concentrations that peaked between 2011–2014. We observed associations between sociodemographic characteristics and OPE biomarkers. In general, concentrations of most OPE biomarkers were higher among participants from racial and ethnic minority populations, participants who were younger, had higher pre-pregnancy body mass index (BMI), and less than a college degree. We observed consistent results using either averaged or visit-specific biomarker concentrations.
Significance:
We observed widespread exposure to several OPEs and OPE biomarkers displayed varying temporal trends in pregnant people from 2007–2018. Concentrations of most OPE biomarkers varied according to sociodemographic factors, suggesting higher burdens of exposure among participants with higher pre-pregnancy BMI, those belonging to racial and ethnic minority populations, and lower educational attainment.
Keywords: Organophosphate ester, Firemaster® 550, flame retardant, plasticizer, pregnancy, biomonitoring
INTRODUCTION
Since the 1970s, chemicals have been routinely added to consumer products such as furniture, electronics, building materials, and clothing, to serve as flame retardants. Following the phase out of polybrominated diphenyl ethers (PBDEs) in the early 2000s due to their environmental persistence, bioaccumulation and toxicity, organophosphate esters (OPEs) were introduced as alternatives (Dodson et al. 2012; Sharkey et al. 2020; United States Environmental Protection Agency 2005). In addition to their use as flame retardants, OPEs are used as plasticizers and lubricants. As a result, these chemicals are found commonly in household environments (e.g., electronics, food packaging, paint, furniture, children’s toys) (Li et al. 2019; Ma et al. 2021) and some personal care products (e.g., nail polish) (Mendelsohn et al. 2016). Like other flame retardants, OPEs are not chemically bound to products and therefore easily leach into the environment (Hoffman et al. 2017a; Stapleton et al. 2009).
Given their increased use in consumer products, the detection of OPEs in environmental samples (e.g., indoor dust, air) has also been on the rise since the early 2000s, which is reflected in human biomonitoring studies (Dodson et al. 2012; Hoffman et al. 2017a; Romano et al. 2017; Varshavsky et al. 2021). However, most studies to date have had limited ability to explore detailed temporal trends in OPE exposure due to short collection periods. In a recent thought-provoking study, Hoffman and colleagues combined data from 14 epidemiological studies with measures of OPE biomarkers in urine (Hoffman et al. 2017a). In their analysis, they observed an increase in the urinary concentrations of metabolites of both tris(1,3-dichloro-2-propyl) phosphate (TDCIPP) and triphenyl phosphate (TPHP) between 2002 and 2015. For example, the concentration of bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), a metabolite of TDCIPP, was over 15 times higher in 2015 than in 2002. However, this study was limited to investigating two metabolites measured at a single timepoint within each of the cohorts that were pooled for the analysis. Further as a pooled study, data from each underlying cohort were collected for differing research aims and the measured OPE biomarker concentrations may not be directly comparable to each other or generalizable to other populations.
As flame retardants, OPEs were assumed to be less harmful than the PBDEs they replaced because of reduced environmental persistence (Blum et al. 2019). Nevertheless, OPEs have been associated with neurotoxicity, developmental and reproductive toxicity, endocrine disruption, and carcinogenicity (Gan et al. 2023; Wang et al. 2020a). Pregnant people and their developing fetuses may be especially susceptible to their effects (Doherty et al. 2019). Further, studies that have quantified OPE biomarkers in urine have observed higher concentrations in pregnant people compared to non-pregnant people, which may suggest increased vulnerability or differences in metabolism and excretion (Hoffman et al. 2017a). For example, OPE biomarkers can accumulate in the placenta (Ding et al. 2016; Wang et al. 2021) and have been linked to placental toxicity (Baldwin et al. 2017; Rock et al. 2020; Varshavsky et al. 2021; Xu et al. 2022). Given the widespread use of OPEs and that pregnant people and their developing fetuses may be more susceptible to their effects, characterizing exposures in these populations is critical (Hoffman et al. 2017a; Varshavsky et al. 2021).
We measured biomarkers of exposure to OPE flame retardants and plasticizers in urine from up to three timepoints in pregnancy among 900 participants from the LIFECODES Fetal Growth Study, which, to date, is among the largest studies to examine predictors of OPE metabolites in pregnancy. Using these measures, we summarized the prevalence of OPE biomarkers, characterized temporal trends in biomarker concentrations during the study period (i.e., 2007–2018), and examined biomarker variability during pregnancy. In addition, we examined predictors of biomarker concentrations based on participant sociodemographic and pregnancy characteristics.
METHODS
Study design
This analysis uses data from participants in the LIFECODES Fetal Growth Study, a case-cohort nested within the prospective LIFECODES pregnancy cohort (Bommarito et al. 2022). The LIFECODES cohort is based at Brigham and Women’s Hospital in Boston, MA, and employs convenience sampling for recruitment, which has been described in depth elsewhere (McElrath et al. 2012). Briefly, participants are eligible for enrollment into the LIFECODES cohort if they are at least 18 years of age, seeking prenatal care prior to 16 weeks gestation, do not have a higher order pregnancy beyond twins, and plan on delivering at Brigham and Women’s Hospital. Participants provide written and informed consent at the time of enrollment (approximately 10 weeks of gestation) and attend two additional study visits during pregnancy (approximately 24 and 35 weeks of gestation). Study visits are integrated into routine prenatal care appointments. Participants complete detailed demographics questionnaires and provide urine samples at each visit. This study was approved by the Institutional Review Board at Brigham and Women’s Hospital and deemed exempt by the National Institute of Environmental Health Sciences. In addition, analysis of de-identified samples at the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research.
Participants were eligible for selection into the Fetal Growth Study if they had a singleton pregnancy that resulted in a live birth between 2008–2018 and had a birthweight available in their medical record. Using this information, birthweight-for-gestational age was determined using an internal growth reference based on the underlying Brigham and Women’s clinic population (Cantonwine et al. 2016). Selection into the Fetal Growth Study occurred in two phases. First, a random subcohort (n = 504) was selected from all eligible participants in the LIFECODES cohort (N = 3,330). Second, two additional enrichment sets of small-for-gestational age (SGA < 10th percentile birthweight-for-gestational age; n = 199) and large-for-gestational age (LGA > 90th percentile birthweight-for-gestational age; n = 198) births were sampled. The sampling scheme resulted in 901 participants and is shown in Supplemental Figure 1. We excluded one participant due to no available urine sample, providing us with 900 participants for the measurement of OPE biomarkers. Because the present analysis does not assess fetal growth outcomes, we incorporated survey weights to account for sampling into the cohort according to guidelines for secondary analyses of case-cohort data (O’Brien et al. 2022; Richardson et al. 2007).
Quantification of urine OPE biomarkers
Measurements of OPE metabolites were used as an index of OPE exposure in this study. Specifically, we measured seven metabolites of OPE flame retardants and plasticizers and one biomarker of exposure to an OPE-containing flame retardant mixture, Firemaster ® 550 (FM550), in urine samples collected at the three study visits, roughly corresponding to the three trimesters of pregnancy. The OPE metabolites measured were: bis(2-chloroethyl) phosphate (BCEP), bis(1-chloro-2-propyl) phosphate (BCIPP), BDCIPP, di-n-butyl phosphate (DNBP), diphenyl phosphate (DPHP), 2-((isopropyl)phenyl)phenyl phosphate (iPPPP) and 4-((tert-butyl)phenyl) phenyl phosphate (tBPPP). We also measured 2,3,4,5-tetrabromobenzoic acid (TBBA), which is a metabolite of 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB), a component of FM550 and other flame retardant mixtures. After collection, urine samples were stored at −80 °C until analysis, which was carried out at CDC according to the method described by Jayatilaka et al. (Jayatilaka et al. 2019). Briefly, samples underwent enzymatic hydrolysis with β-glucuronidase/sulfatase (Helix Pomatia, type H-1) to release the conjugated metabolites, followed by off-line solid phase extraction (SPE) with a weak anion exchange cartridge to extract and concentrate the metabolites. Separation and detection of the target metabolites was obtained by reversed-phase high-performance liquid chromatography coupled with isotope dilution tandem mass spectrometry. Specific quantitation and confirmation ion transitions measured are reported elsewhere (Jayatilaka et al., 2019). Method precision, calculated as percent coefficient of variation from repeat measurements (N =40) of two quality control (QC) materials, was below 10% for all analytes. Accuracy was calculated by spiking urine at three different concentrations (0.5, 5, 20 ng/mL) in triplicate; accuracy ranged from 90 to 118%, depending on the analyte. SPE recoveries were calculated as the ratio of response ratios (native/label) for pre- and post-spiked extractions and ranged from 94 to 110% for most analytes except for iPPPP (87%) and tBPPP (69%). The limit of detection (LOD) for all metabolites was 0.10 ng/mL, except for iPPPP, tBPPP, and TBBA. The LOD for these metabolites was 0.05 ng/mL. Each analytical batch included calibration standards, two low- and two-concentration high QC materials, and several blanks. Analyte concentrations in duplicate QC materials were averaged and evaluated using standard statistical probability rules to ensure statistical control of the analytical batch (Caudill et al., 2008). If the QC samples failed the statistical evaluation, all of the study samples in the batch were re-extracted.
Urine dilution was accounted for using specific gravity (SG), which was measured using a digital handheld refractometer (AtagoCo., Ltd., Tokyo, Japan). We corrected concentrations of OPE biomarkers for urine dilution using the Boeniger method: , where ESG is the SG-corrected exposure measurement, Eo is the observed exposure measurement, SGmedian is the population’s overall median SG (1.015), and SGo is the observed specific gravity (Boeniger et al. 1993; Kuiper et al. 2021). We next calculated the geometric mean of biomarker concentrations measured at each study visit to estimate average exposure during pregnancy. We operationalized biomarkers that were detected in fewer than 60% of the participants as dichotomous variables for the remainder of the analysis based on whether any of the measured concentrations were above or below the LOD.
Covariate collection
Participants completed questionnaires at the time of enrollment in the LIFECODES cohort. The questionnaire inquired about sociodemographic characteristics (e.g., participant age, educational attainment, or self-reported race and ethnicity), behavioral factors (e.g., smoking or alcohol use during pregnancy), and health information (e.g., pre-pregnancy weight, parity, or health conditions). In addition, information about diagnoses occurring during pregnancy (e.g., gestational diabetes or pregnancy-induced hypertension) and birth outcomes (e.g., gestational age at delivery) were abstracted from medical records and validated by medical record review carried out by at least two maternal fetal medicine specialists. Calendar year of conception was determined based on the date of the enrollment visit and estimated gestational age at that visit. We collapsed year of conception into two-year intervals due to sparse data across some calendar years. Season of clinic visit was determined by date of visit and categorized as Summer (June – August), Fall (September – November), Winter (December – February), or Spring (March – May). With respect to race and ethnicity, the questionnaire allowed participants to select from several categories, including “Caucasian”, “Black”, “South Asian”, “East Asian”, “Native American or Pacific Islander”, “More than one race”, “Other”, “Unsure”, and “N/A”. Participants were allowed to select multiple categories and were provided with a write-in option. Hispanic ethnicity was included in a separate question. For the purposes of this analysis, we categorized race and ethnicity as “non-Hispanic White”, “non-Hispanic Black”, “Hispanic”, “Asian”, and “Multiple Race/Other” due to sample size considerations. Participant height was measured at the first study visit (m) and used along with self-reported pre-pregnancy weight (kg) to calculate pre-pregnancy body mass index (BMI, kg/m2).
Imputations
We used multiple imputation by chained equations (MICE) to impute biomarker concentrations below the LOD and missing covariates. The percent of samples with biomarker concentrations below the LOD ranged from 2.0% - 96.7% and the percent of missing covariate data ranged from 0.1 – 1.6%. We imputed biomarker concentrations below the LOD using tobit regression and assumed that the distribution of each metabolite was constrained between zero and the LOD (Keil et al. 2020). After imputing, we dichotomized biomarkers detected in fewer than 60% of the study participants, as described above, for the remainder of the analysis.
We imputed missing covariates using either predictive mean matching, logistic regression, or multinomial logistic regression depending on whether the covariate was continuous, binary, or categorical, respectively. Covariates with missing data that were imputed include pre-pregnancy weight (kg), alcohol consumption (yes vs. no), use of assisted reproductive technologies (ART) to conceive the current pregnancy (yes vs. no), maternal educational attainment (high school or less vs. some college or technical school vs. college or greater), insurance status (Private vs. self-pay/Medicaid/none), and infant sex (male vs. female) Imputed values of pre-pregnancy weight were then used to calculate pre-pregnancy BMI. We included additional covariates in in the MICE algorithms that were predictive of our exposure (i.e., sociodemographic and pregnancy characteristics) or outcomes (i.e., OPE biomarker concentrations), including: smoking (yes vs. no), race and ethnicity (non-Hispanic White vs. non-Hispanic Black vs. Hispanic vs. Asian vs. Multiple Race vs. Other), parity (1–7 children), year of conception, age (years), pre-pregnancy height (m), season of enrollment (Summer vs. Fall vs. Winter vs. Spring), maternal comorbidities (i.e., chronic hypertension, gestational hypertension, preeclampsia, gestational diabetes, diabetes mellitus) and birth outcomes (i.e., gestational age at delivery, birthweight, birth length).
We generated 10 imputed datasets using 20 iterations per dataset. Imputations were performed using the mice (version 3.14.0) (van Buuren and Groothuis-Oudshoorn 2011) and qgcomp (version 2.8.6) (Keil 2022) packages in R version 4.2.2.
Statistical analysis
First, we inspected the distribution of demographic and pregnancy characteristics by calculating the observed n (survey-weighted %). We also examined the distribution of both the averaged biomarker concentrations and concentrations measured at individual study visits by calculating the 5th, 25th, 50th, 75th, and 95th percentiles and the geometric mean. Differences in the distribution of OPE biomarkers by study visit were determined using multivariate Wald tests. We reported these values alongside the n (%) of participants with biomarker concentrations above the LOD. We calculated these basic descriptive statistics using the observed, not imputed, data.
Second, we examined both variability and the correlation structure of OPE biomarkers that were detected in at least 60% of the study participants. To determine variability, we calculated an intraclass correlation coefficient (ICC) using a mixed effects model with a random intercept for each participant. We examined the correlation structure by calculating the Pearson correlation coefficients (ρ) using the natural log-transformed concentrations of the averaged biomarkers. We used a heat map to display correlation coefficients.
Third, we examined temporal trends in average exposure biomarker concentrations and concentrations measured at individual study visits. To examine temporal trends in biomarkers, we calculated the geometric mean and 95% confidence interval (CI) for each exposure biomarker according to year of conception and visualized these data using line plots. For exposure biomarkers that were detected in fewer than 60% of the study participants, we tabulated the percentage of participants with detectable concentrations according to year of conception and present these findings supplementally. Differences in concentrations by year of conception were determined using multivariate Wald tests. As a sensitivity analyses, we examined whether temporal trends could be explained by changes in cohort composition over time, we calculated the marginal geometric mean (95% CI) biomarker concentration using a generalized linear model adjusted for any factors that change over the study period. Specifically, race and ethnicity, insurance provider, and season at study enrollment changed significantly across the study period (Supplemental Table 1).
To contextualize the temporal trends in exposure biomarkers observed in our study against a reference population, we calculated the geometric mean concentrations of the same biomarkers measured during equivalent years as our study among reproductive aged female participants (e.g., 18 – 49 years) in the National Health and Nutrition Examination Survey (NHANES). Calculations were performed with survey weights to account for the complex study design. Data were from the following NHANES cycles: 2011–2012, 2013–2014, 2015–2016 and 2017–2018 and information about cycle, sample size and geometric means are available in Supplemental Table 2. These comparisons were only carried out for exposure biomarkers that were detected in at least one study visit in over 60% of the participants. Notably, urine SG is not measured in NHANES. Thus, to correct for urine dilution in NHANES data, we used a modified Boeniger formula for urine creatinine (Cr) to allow comparisons of urine biomarker measurements across studies that have different measures of urine dilution (Kuiper et al. 2022). The modified Boeniger formula was , where ECr is the Cr-corrected exposure biomarker measurement, Eo is the observed exposure biomarker measurement, and Crmedian is the population’s median Cr, and Cro is the observed Cr.
Last, to examine predictors of exposure biomarkers, we contrasted exposure biomarker concentrations across categories of sociodemographic and pregnancy characteristics. Given the presence of temporal trends in biomarker concentrations, we calculated population marginal geometric mean concentrations using a generalized linear model that adjusted for year of conception for biomarkers that were detected in over 60% of the study population. For infrequently detected biomarkers, we calculated the population marginal percent with detectable concentrations using a regression model that adjusted for year of conception and present these results supplementally. Calendar year of conception was categorized into two-year intervals, as described above, and treated categorically to account for non-linear temporal trends in biomarker concentrations. We tested for differences in exposure biomarker concentrations by sociodemographic and pregnancy characteristics using multivariate Wald tests. Given that season of study visit was time-varying, we calculated the marginal geometric mean or percent detected in each season using the repeated exposure biomarker measurements from all study visits using a generalized estimating equation that adjusted for year of conception. For visualization purposes, we additionally calculated the percent change in biomarker concentrations relative to referent levels for each sociodemographic or pregnancy characteristic and displayed them using a heat map. To examine whether the associations between predictors and exposure biomarkers varied during pregnancy, we tested for interactions between each predictor and study visit. Because no interactions were observed (all p-values > 0.10), we did not stratify biomarker concentrations across sociodemographic and pregnancy characteristics by study visit.
Unless stated otherwise, we performed all analyses using inverse probability weights to account for sampling into the case-cohort using the survey (version 4.1.1) package (Lumley 2004) in R version 4.2.2 and combined estimates from imputed datasets according to Rubin’s rules. Sampling weights were generated for secondary analyses of case-cohort data (O’Brien et al. 2022): SGA case weight = 1.35; LGA case weight = 1.18; AGA case weight = 6.59.
RESULTS
Participant information
Demographic and pregnancy characteristics of study participants are shown in Table 1. Participants had a median age of 32.4 years and a pre-pregnancy BMI of 24.8 kg/m2. The study participants were primarily non-Hispanic White (58%), while approximately 14% and 16% were non-Hispanic Black and Hispanic, respectively. In addition, most participants had completed a college education or greater (66%) and had private health insurance (73%). Few reported drinking alcohol (7%) or smoking (7%) during pregnancy. Twelve percent used ART to conceive their pregnancy, and most were multiparas (62%). Study visits were evenly distributed across seasons (Supplemental Table 3). Participants who contributed samples for analysis at all three study visits were similar to those missing one or more samples, though there were some differences in the year of conception (Supplemental Table 4).
Table 1.
N (%) of demographic characteristics among participants in the LIFECODES Fetal Growth Study (n = 900)
| Variable | n (%) |
|---|---|
| Age at enrollment | |
| < 25 yrs | 102 (11) |
| 25 0 – 29.9 yrs | 187 (21) |
| 30.0 – 34.0 yrs | 304 (35) |
| ≥ 35.0 yrs | 307 (34) |
| Pre-pregnancy BMI | |
| < 25.0 kg/m2 | 462 (53) |
| 25.0 – 29.9 kg/m2 | 211 (25) |
| ≥ 30.0 kg/m2 | 216 (23) |
| Education | |
| High school or less | 120 (13) |
| Some college or technical school | 179 (21) |
| College or greater | 587 (66) |
| Race and ethnicity | |
| NH White | 517 (58) |
| NH Black | 136 (14) |
| Hispanic | 147 (16) |
| Asian | 56 (6) |
| Multiple race/Other | 44 (6) |
| Insurance provider | |
| Private/HMO | 638 (73) |
| Public/Self-pay/None | 252 (27) |
| Alcohol consumption during pregnancy | |
| No | 837 (93) |
| Yes | 55 (7) |
| Smoking during pregnancy | |
| No | 843 (93) |
| Yes | 57 (7) |
| Use of ART to conceive | |
| No | 790 (88) |
| Yes | 110 (12) |
| Parity | |
| 0 | 344 (38) |
| 1 | 353 (40) |
| 2 | 142 (16) |
| 3+ | 61 (6) |
| Infant sex | |
| Female | 422 (48) |
| Male | 478 (52) |
| Year of conception | |
| 2007 – 2008 | 217 (25) |
| 2009 – 2010 | 139 (17) |
| 2011 – 2012 | 267 (30) |
| 2013 – 2014 | 129 (15) |
| 2015 – 2016 | 94 (8) |
| 2017 – 2018 | 54 (5) |
Abbreviations: ART = assisted reproductive technologies, BMI = body mass index, NH = non-Hispanic
Note: number of missing observations (%) per variable: pre-pregnancy BMI, n = 11 (1%); education, n =14 (2%); insurance provider, n = 10 (1%), alcohol consumption, n = 8 (1%).
OPE biomarker characteristics
Of the OPE biomarkers examined, we detected five in at least 60% of the study participants: BCEP, BCIPP, BDCIPP, DNBP, and DPHP (Table 2). We reported detection rates and biomarker concentrations stratified by study visit in Supplemental Table 5. Ninety-nine percent of participants had a sample at study visit 1, while 90% and 86% had samples at study visits 2 and 3, respectively. The median (range) gestational age at each study visit was 11 (5–24 weeks gestation), 26 (19–30 weeks) and 35 (31–40 weeks gestation), at study visit 1, 2, and 3 respectively. We observed significant differences in BCIPP (p-value = 0.02) and BDCIPP (p-value = 0.03) concentrations by study visit, although the distributions of the biomarkers were similar across visits. For example, the median (IQR) concentration of BCIPP was 0.12 ng/mL (0.07 – 0.24), 0.15 ng/mL (0.08 – 0.34) and 0.13 ng/mL (0.08 – 0.29) at study visits 1, 2, and 3, respectively (Supplemental Table 5). There were no other notable trends of OPE biomarker concentrations during pregnancy.
Table 2.
Detection frequencies, limits of detection, and distributions of pregnancy-average OPE biomarker concentrations (ng/mL), and intraclass correlation coefficients in the LIFECODES Fetal Growth Study (n = 900, 2007–2018)
| Abbreviation | Chemical Name | n (%) > LODa | LOD | GM | Percentiles | ICCb | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | ||||||
| BCEP | Bis(2-chloroethyl) phosphate | 812 (90) | 0.10 | 0.32 | 0.09 | 0.17 | 0.28 | 0.54 | 1.61 | 0.50 |
| BCIPP | Bis(1-chloro-2-propyl) phosphate | 577 (64) | 0.10 | 0.17 | 0.05 | 0.09 | 0.14 | 0.24 | 1.02 | 0.51 |
| BDCIPP | Bis(1,3-dichloro-2-propyl) phosphate | 892 (99) | 0.10 | 0.75 | 0.20 | 0.39 | 0.71 | 1.34 | 3.46 | 0.50 |
| DNBP | Di-n-butyl phosphate | 568 (63) | 0.10 | 0.11 | 0.05 | 0.08 | 0.11 | 0.15 | 0.26 | 0.27 |
| DPHP | Diphenyl phosphate | 900 (100) | 0.10 | 1.05 | 0.38 | 0.65 | 1.00 | 1.60 | 3.42 | 0.33 |
| iPPPP | 2-((isopropyl)phenyl)phenyl phosphate | 135 (15) | 0.05 | - | - | - | - | - | - | - |
| tBPPP | 4-((tert-butyl)phenyl)phenyl phosphate | 350 (39) | 0.05 | - | - | - | - | - | - | - |
| TBBAc | 2,3,4,5-tetrabromobenzoic acid | 58 (6) | 0.05 | - | - | - | - | - | - | - |
Abbreviations: GM = geometric mean; ICC = intraclass correlation coefficient; LOD = limit of detection; OPE = organophosphate ester. The numbers (%) correspond to the of participants for each biomarker with at least one detectable concentration. Percentages are rounded to the nearest integer.
Computed using a survey-weighted linear mixed model with random intercept for each participant.
TBBA is a biomarker of exposure to 2-ethylhexyl-2,3,4,5-tetrabromobenzoate.
Overall, we observed that OPE biomarkers were weakly correlated with one another, with ρ ranging from −0.13 to 0.28 (Figure 1). The correlation structure between the pregnancy-averaged biomarker concentrations was consistent with the correlation structures observed for the biomarker concentrations measured at each individual study visit (data not shown). Intraclass correlation coefficients for each metabolite suggested low-to-moderate reliability over pregnancy (ICC range: 0.27 – 0.51; Table 2).
Figure 1.
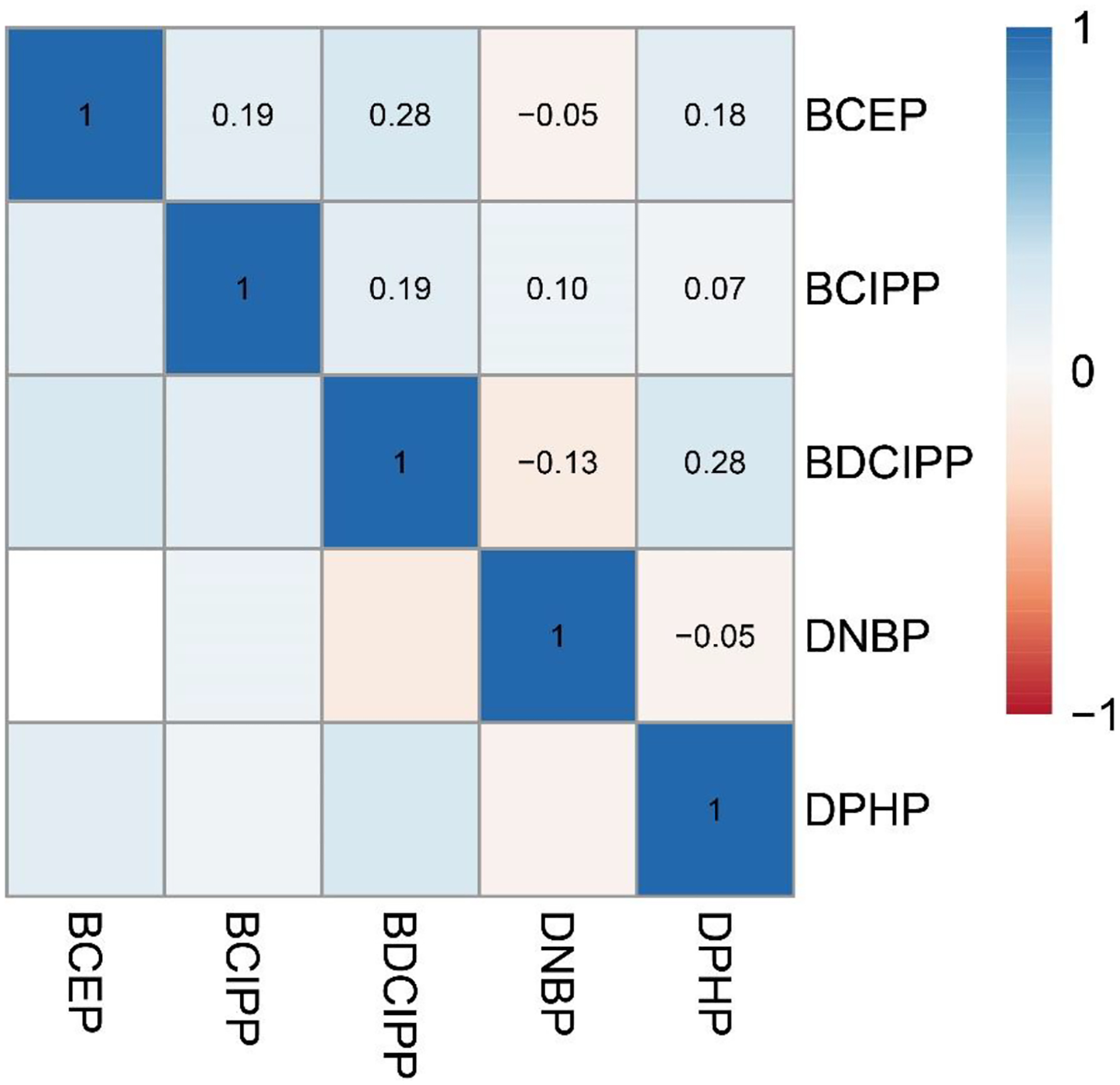
Pearson correlation coefficients for averaged exposure biomarkers in the LIFECODES Fetal Growth Study (n = 900). Abbreviations: BCEP = Bis(2-chloroethyl) phosphate, BCIPP = Bis(1-chloro-2-propyl) phosphate, BDCIPP = Bis(1,3-dichloro-2-propyl) phosphate, DNBP = Di-n-butyl phosphate, DPHP = Diphenyl phosphate.
Temporal trends in OPE exposure biomarkers
We noted significant changes in the concentration of exposure biomarkers over the study period (Figure 2; Supplemental Table 6). Concentrations of BCIPP increased from 0.10 ng/mL (95% CI: 0.08, 0.11) to 0.21 ng/mL (95% CI: 0.13, 0.32) in participants who conceived during 2007 – 2008 and 2017 – 2018, respectively. Other metabolites displayed non-monotonic temporal trends. For example, BDCIPP increased over the study period, from 0.52 ng/mL (95% CI: 0.45, 0.59) in 2007 – 2008 to 0.96 ng/mL (95% CI: 0.78, 1.17) in 2013 – 2014, followed by a decline to 0.74 ng/mL (95% CI: 0.54, 1.01) in 2017 – 2018. Concentrations of DPHP showed a similar trend: increasing from 2007–2008 to 2011–2012, decreasing thereafter. Concentrations of other biomarkers, including BCEP and DNBP and the percent of the population with detectable iPPPP (Supplemental Table 4), declined across the study period. We did not observe significant trends in detectable levels of TBBA (p-value = 0.25) or tBPPP (p-value = 0.20). Temporal trends were consistent after adjusting for factors that change in the population (i.e., race and ethnicity, insurance status, and season) over the study period (Supplemental Table 7).
Figure 2.
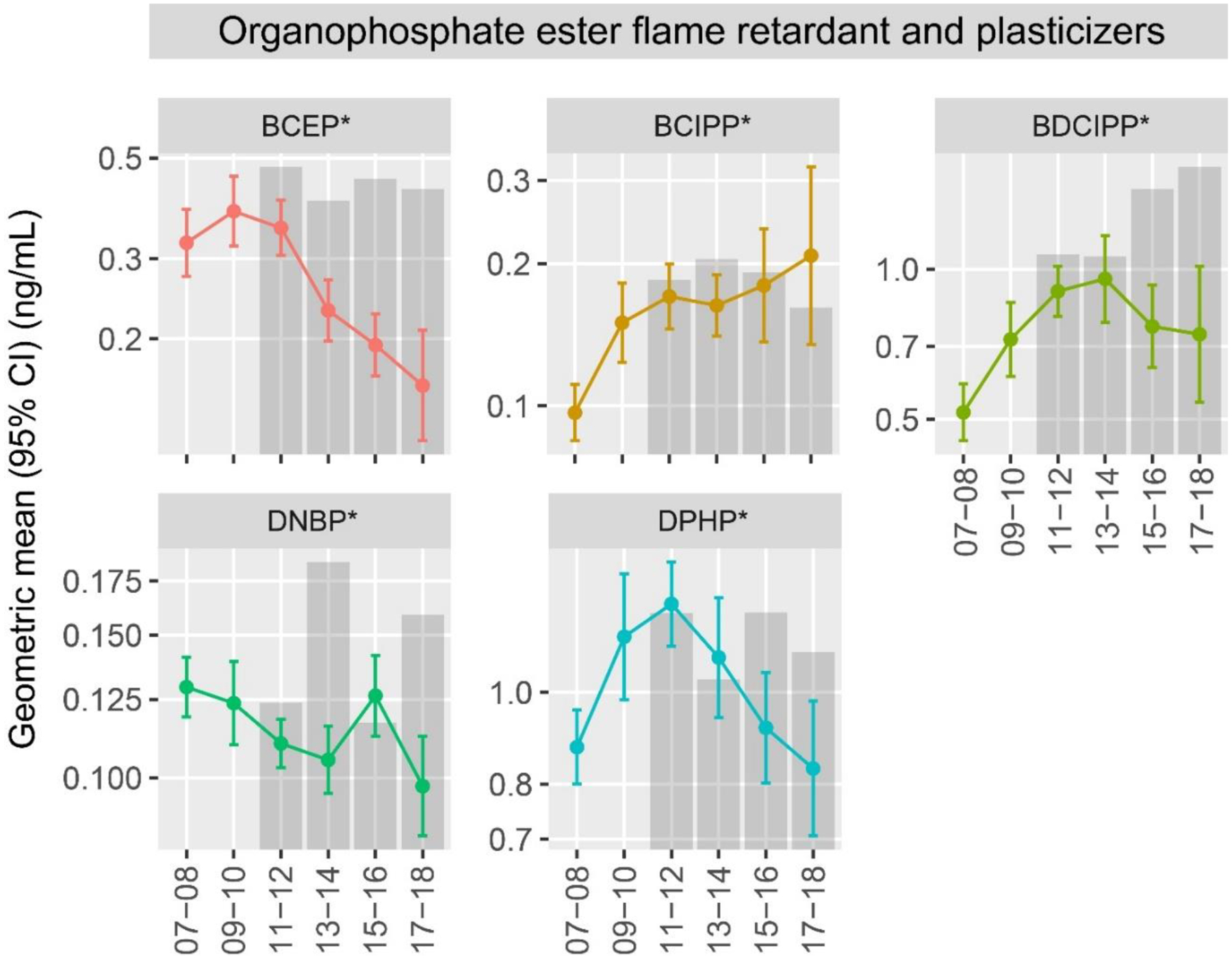
Temporal trends in averaged chemical biomarker concentrations (ng/mL) according to year of conception in the LIFECODES Fetal Growth Study (n = 900) and in NHANES. Note: Gray bars represent reference levels (geometric means) from NHANES reproductive aged female participants calculated using survey weights to account for the complex study design. Corresponding numbers for these figures can be found in Supplemental Tables 1 and 4. Asterisks (*) indicate multivariate Wald p-value < 0.05. Abbreviations: BCEP = Bis(2-chloroethyl) phosphate, BCIPP = Bis(1-chloro-2-propyl) phosphate, BDCIPP = Bis(1,3-dichloro-2-propyl) phosphate, DNBP = Di-n-butyl phosphate, DPHP = Diphenyl phosphate.
In general, the concentrations measured in LIFECODES participants were similar to or lower than the average concentrations reported for NHANES reproductive aged female participants, although temporal trends did not always clearly align. For example, while geometric mean concentrations of BDCIPP declined after 2014 in LIFECODES participants, they increased in NHANES participants (Figure 2; Supplemental Table 2). Temporal trends in exposure biomarkers did not differ when stratified by study visit compared to those observed using the pregnancy-averaged biomarker concentrations (data not shown).
Predictors of OPE biomarker concentrations
We observed differences in the concentrations of OPE exposure biomarkers according to sociodemographic and pregnancy characteristics (Figure 3; Supplemental Table 8). The concentration of several OPE biomarkers, including BCEP, BDCIPP, and DPHP, was generally higher among younger participants (< 25 years old), those with a pre-pregnancy BMI at or above 30 kg/m2, with less than a college degree education, and those without private health insurance. With respect to race and ethnicity, concentrations of the same biomarkers were highest among participants from racial and ethnic minority populations compared to non-Hispanic White participants. For example, concentrations of BCEP were 100% (0.46 ng/mL) higher in non-Hispanic Black, 91% (0.44 ng/mL) in Hispanic, 91% (0.44 ng/mL) in Asian, and 74% (0.40 ng/mL) in Multiple race/Other participants compared to non-Hispanic White (reference; 0.23 ng/mL) participants. Similar observations were noted for the detection frequency of iPPPP, tBPPP, and TBBA (Supplemental Table 8).
Figure 3.
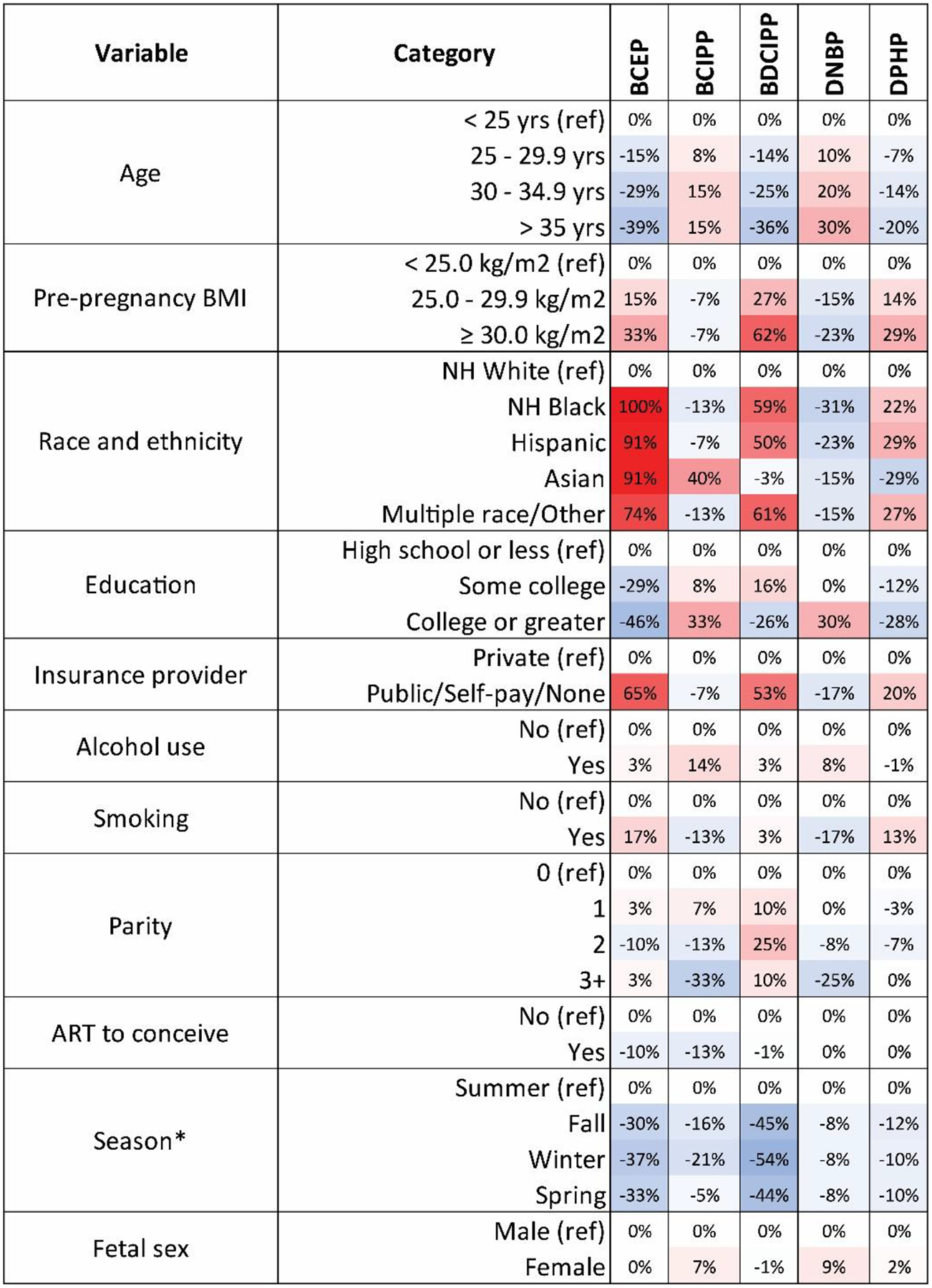
Heat map displaying the percent-change in the population marginal GM biomarker concentrations across sociodemographic and pregnancy characteristics in the LIFECODES Fetal Growth Study (n = 900). Abbreviations: ART = assisted reproductive technologies, BCEP = Bis(2-chloroethyl) phosphate, BCIPP = Bis(1-chloro-2-propyl) phosphate, BDCIPP = Bis(1,3-dichloro-2-propyl) phosphate, BMI = body mass index, DNBP = Di-n-butyl phosphate, DPHP = Diphenyl phosphate, GM = geometric mean, NH = non-Hispanic. Note: Population marginal means calculated using linear regression models adjusted for year of conception. Asterisks (*) indicate population marginal means calculated using generalized estimating equation where study visit specific biomarker concentrations were used, adjusted for year of conception.
Although they were not strongly correlated (ρ = 0.10), concentrations of BCIPP and DNBP showed similar patterns of association with sociodemographic and pregnancy variables. Both were generally higher among older participants, those with a pre-pregnancy BMI below 25.0 kg/m2, those with a college degree or greater, and those who had private health insurance. Non-Hispanic White participants also had the highest urinary concentrations of these OPE biomarkers compared to participants from racial and ethnic minority populations, except for Asian participants who had the highest concentrations of BCIPP.
We observed few associations between OPE biomarkers and alcohol use or smoking during pregnancy, parity, use of ART, or infant sex. However, we noted associations between some OPE biomarkers and the season of study visit. Biomarker concentrations or detection frequencies were generally highest in the summer compared to other seasons, especially for BCEP (p-value < 0.01), BCIPP (p-value < 0.01), BDCIPP (p-value < 0.01), and DPHP (p-value = 0.05). For example, compared to the summer (reference; 1.25 ng/mL), urinary concentrations of BDCIPP were 45% (0.69 ng/mL), 54% (0.58 ng/mL) and 44% (0.70 ng/mL) lower when study visits occurred in fall, winter, and spring, respectively.
DISCUSSION
In this study, we measured and examined biomarkers of exposure to OPE flame retardants and plasticizers in pregnant people participating in the LIFECODES Fetal Growth Study. Urinary concentrations of repeated exposure biomarkers showed low-to-moderate reliability over pregnancy, similar to other non-biologically persistent chemicals with intermittent exposure scenarios. Patterns of OPE biomarkers over calendar time were not consistent across all OPE biomarkers. Several exposure biomarkers showed monotonic or non-monotonic downward trends from 2007 to 2018, including BDCIPP and DPHP, which had inverse U-shaped patterns with peak concentrations occurring midway through the study period. BCIPP was the only biomarker with a monotonic increasing trend over the study period. Concentrations of OPE biomarkers also differed according to several sociodemographic characteristics. Specifically, we found higher concentrations for most OPE biomarkers among participants from racial and ethnic minority populations, as well as participants who were younger, had higher pre-pregnancy BMI, and had lower educational attainment. Overall, we frequently detected several OPE biomarkers, highlighting the need for continued study of OPE exposures.
The concentrations of OPE biomarkers we observed are within the ranges reported in prior studies of pregnant people (Bommarito et al. 2021; Castorina et al. 2017; Hoffman et al. 2017b; Luan et al. 2023; Luo et al. 2021; Percy et al. 2020; Romano et al. 2017; Tao et al. 2021; Zhao et al. 2021), though direct comparisons are difficult given differences in the calendar years of sample collection. Several biomarkers were highly detected in our study population, including BDCIPP (99%) and DPHP (100%). However, we infrequently detected TBBA (6%) and tBPPP (39%) which is consistent with other studies, although these biomarkers have not been reported as frequently as others (Ashley-Martin et al. 2023). We also observed low detection of iPPPP (15%; LOD = 0.05 ng/mL), which contrasts with some other study populations (Hoffman et al. 2017b; Ingle et al. 2020a; Kuiper et al. 2020). For instance, a study of 349 pregnant people in the Pregnancy, Infection, and Nutrition (PIN) Study reported detectable concentrations of iPPPP in 99.4% (LOD = 0.213–0.846 ng/mL) of study participants, with a geometric mean concentration of 6.8 ng/mL (Hoffman et al. 2017b). This discrepancy could result from differences in study periods between previous studies (i.e., early 2000s) and ours (i.e., 2007 – 2018), or differences in study population, location, or analytical methods. Notably, most prior studies that have offered detailed characterizations of OPE metabolites in pregnant people have been located in North America, though a few studies have measured OPE metabolites in pregnant populations located in Asia (Luan et al. 2023; Luo et al. 2021; Yao et al. 2021; Zhao et al. 2021) and Europe (Choi et al. 2021). Of those studies outside of North America, some metabolites overlapped with those that we have measured in our study, mainly BCIPP, BDCIPP, and DPHP. With one exception, all studies outside North America reported lower concentrations of BCIPP, BDCIPP, and DPHP. A study in Shanghai that recruited 272 pregnant women between 2019–2020 reported the geometric mean of DPHP was twice as high as we observed in our study (geometric mean in Zhao et al., 2021: 2.06 ng/mL vs. 1.05 ng/mL in present study) whereas all other studies in Asia and Europe reported lower levels of BCIPP, BDCIPP and DPHP (Choi et al. 2021; Luan et al. 2023; Luo et al. 2021; Tao et al. 2021; Yao et al. 2021; Zhao et al. 2021).
We observed that BCIPP and BDCIPP concentrations differed significantly by study visit, although consistent with prior studies, the differences were not large in magnitude (Buckley et al. 2022; Kuiper et al. 2020; Luo et al. 2021; Percy et al. 2020; Tao et al. 2021). Other studies have also reported changes in OPE biomarker concentrations over pregnancy, with several finding increased concentrations of DPHP or BDCIPP later in pregnancy (Buckley et al. 2022; Kuiper et al. 2020; Percy et al. 2020). Like prior studies (Bommarito et al. 2021; Ingle et al. 2020b; Kuiper et al. 2020; Percy et al. 2020; Romano et al. 2017; Tao et al. 2021), we also observed low to moderate reliability between concentrations in repeated spot urine samples collected across 3 trimesters (ICCs ranged from 0.27 for DNBP to 0.51 for BCIPP).
We observed temporal trends in OPE biomarkers between 2007 and 2018. There was a consistent increase in the concentration of BCIPP over this time period, but for other metabolites, we observed monotonic or non-monotonic declines in concentrations or detection. For example, concentrations of BCEP and DPHP peaked in 2011–2012 and BDCIPP peaked in 2013–2014, after which we observed lower concentrations for these metabolites. These trends align with previous reports of the chemical content of some consumer products (Cooper et al. 2016; Dodson et al. 2017; Stapleton et al. 2009; Stapleton et al. 2012). For example, in a study of polyurethane foam-containing furniture purchased in the United States, researchers found increases in the detection of TDCIPP in furniture until 2014, whereby detection rates fell sharply (Cooper et al. 2016). These trends also align with legislative changes designed to impact the use of chemical flame retardants. First, the phase out of PBDEs resulted in increased usage of alternative flame retardants, including OPEs (Blum et al. 2019; Charbonnet et al. 2020). However, changes to California’s residential furniture standards implemented in 2015 (e.g., Technical Bulletin 117–2013) were designed to reduce reliance on chemical flame retardants in favor of barrier fabrics and to require manufacturers to label furniture containing flame retardant chemicals (Charbonnet et al. 2020).
To date, few prior studies have examined detailed temporal trends in OPE biomarkers (Buckley et al. 2022; Hoffman et al. 2017a; Percy et al. 2020) and of those that have, all have been focused on North American populations. Hoffman et al. (2017a) combined data from 14 epidemiological studies in the United States and reported on temporal trends of DPHP and BDCIPP. Authors reported that concentrations of BDCIPP were over 15 times higher in 2015 than those observed in 2002. Concentrations of DPHP also increased, though the change was smaller in magnitude compared to BDCIPP. However, the pooled nature of the data from the analysis by Hoffman and colleagues limits the ability to accurately assess temporal trends as data from each study were collected for various reasons and data availability differed by cohort. Other studies of OPE exposure in pregnancy have tested for temporal trends in concentrations of OPE biomarkers, although they examined shorter periods of observation, had smaller sample sizes, and primarily reported no significant temporal trends (Buckley et al. 2022; Hoffman et al. 2017b; Percy et al. 2020). In addition, we did not find examples from studies located outside of the United States. For example, a study in a subset of the Environmental influences on Child Health Outcome (ECHO; n = 171) program reported no association between study year (i.e., 2008–2018) and OPE biomarkers. However, only linear temporal trends were considered (Buckley et al. 2022). Nevertheless, our findings suggest increasing exposure to the precursors of certain OPE biomarkers (e.g., BCIPP) and not others (e.g., BDCIPP and DPHP) and may help identify additional OPEs of targeted interest for future studies.
We observed similar patterns in the concentration of multiple OPE biomarkers across sociodemographic and pregnancy characteristics. Specifically, we observed higher geometric mean concentrations of BCEP, BDCIPP, and DPHP among younger participants, those with lower educational attainment, higher pre-pregnancy BMI, participants from racial and ethnic minority groups, and those without private health insurance. We also noted similar trends for iPPPP, TBBA, and tBPPP. However, many of these comparisons did not reach statistical significance due to low detection frequencies of these chemicals in participant urine. The observed differences in OPE metabolite concentrations by sociodemographic factors, such as categories of educational attainment or race and ethnicity, could relate to differences in underlying exposure sources (e.g., diet, furniture, electronics). In addition, others have suggested that both age and adiposity may result in differences in the metabolism and/or excretion of OPEs and could explain observations that OPE biomarkers differ according to those factors (Boyle et al. 2019; Van den Eede et al. 2015). However, we are unable to explore potential differences in exposure sources or metabolism among participants in the present study.
These observed differences in metabolite concentrations by sociodemographic characteristics are largely consistent with previous studies examining OPE exposure in pregnant populations. For example, a study in the ORigins of Child Health and Resilience in Development (ORCHARD) cohort (n = 90) reported higher concentrations of BDCIPP, iPPPP, and tBPPP among those with higher pre-pregnancy BMI, participants from racial and ethnic minority populations, and those with lower educational attainment (Kuiper et al. 2020). Likewise, both a small Rhode Island-based cohort (n = 59) and the ECHO program have reported that DPHP and BDCIPP concentrations were lower among older participants (Buckley et al. 2022; Romano et al. 2017). Notably, we also observed seasonal differences in BDCIPP, BCIPP, BCEP, and DPHP concentrations, which were all highest in the summer and lowest in the fall or winter. Similar seasonal trends for both DPHP and BDCIPP have been reported in the ECHO program, the PIN Study, the Health Outcomes and Measures of the Environment (HOME) cohort, and the Maternal-Infant Research on Environmental Chemicals (MIREC) study (n = 1,865) (Ashley-Martin et al. 2023; Buckley et al. 2022; Hoffman et al. 2017b; Ingle et al. 2018; Percy et al. 2020). Collectively, these findings suggest that exposure to OPEs may relate to temperature. For example, studies have shown seasonal variation in OPE concentrations in both indoor and outdoor environments (Wong et al. 2018; Wu et al. 2020; Xu et al. 2022; Zhao et al. 2021), which may relate to increased volatilization of OPEs in warmer environments. Seasonal differences in exposure may also reflect changes in exposure sources between seasons. For example, OPEs have been widely detected in indoor environments (Castorina et al. 2017; Dodson et al. 2012; Stapleton et al. 2009) and season-dependent changes in urinary biomarker concentrations may reflect shifts in time spent inside versus outside. In contrast, we reported opposite concentration patterns for BCIPP and DNBP across sociodemographic and pregnancy characteristics. Urinary concentrations of these biomarkers were higher among older participants, those with smaller pre-pregnancy BMI, greater educational attainment, and private health insurance. However, due to low detection frequencies in other studies, predictors of these biomarkers have not been previously reported among pregnant people (Bommarito et al. 2021; Buckley et al. 2022; Kuiper et al. 2020; Percy et al. 2020; Romano et al. 2017).
This study is not without limitations. First, participants are recruited into the LIFECODES study, in part, from the Maternal Fetal Medicine clinic at Brigham and Women’s Hospital and are living in or around Boston, MA. Given that this represents a higher obstetrical risk population in an urban area and with relatively high socioeconomic status, observations in this population may not be fully generalizable to the rest of the United States. In addition, while we used inverse probability weights throughout all statistical analyses, it may not fully account for the effects of sampling on the results in this study (O’Brien et al. 2022). Further, while we characterized trends across the study period, participants were not continuously followed over the years included in the study. Last, we had limited access to certain variables that may be important predictors of OPE biomarkers. Specifically, other studies have demonstrated that factors related to the indoor environment (e.g., types of furniture, electronics, and cleaning behaviors), personal care product use (e.g., nail polish, other cosmetics), or biospecimen collection (e.g., first morning void, time of collection) are associated with concentrations of OPE biomarkers in urine (Castorina et al. 2017; Ingle et al. 2020a; Ingle et al. 2019).
Yet, this analysis has several key strengths. First, this study is among the largest to date with detailed information on trends and predictors of OPE biomarkers among pregnant people. Specifically, we provide this information in a large, well-characterized, prospective pregnancy cohort (n = 900) with exposure biomarkers measured at up to three time points. Given that OPE biomarkers have relatively short half-lives within the body (e.g., on the order of days), multiple measures provide a more stable estimate of the average exposure during pregnancy (Wang et al. 2020b). Notably, most participants (78%) in this study provided urine samples at all three study visits. Second, we provided information on trends and predictors of eight biomarkers of OPE exposure, five of which were detected in > 60% of participants. This represents a larger number of OPE biomarkers than have been reported in many prior studies. Given the emerging interest in OPE biomarkers and their potential effects on reproductive and developmental outcomes, this is an important contribution to our understanding of these chemicals. Last, given the long period of time represented by the participants in this cohort (i.e., 2007 – 2018), we were able to report detailed temporal trends over a longer time frame than is currently available in many previous pregnancy cohorts.
CONCLUSION
We characterized distributions, temporal trends, and predictors of OPE exposure biomarkers in pregnant people participating in the LIFECODES Fetal Growth Study with exposure biomarkers quantified at three study visits. We observed varying temporal trends in exposure biomarker concentrations over the study period (i.e., 2007 – 2018), which may reflect changes in the use of certain OPE flame retardant and plasticizers in commerce. Given the frequent detection of several OPE biomarkers in the population, future studies can help better understand the potential health risks associated with exposure, especially for pregnant people and their infants.
Supplementary Material
Highlights.
Biomarkers of OPE exposure were highly detected, suggesting widespread OPE exposure
OPE biomarkers displayed varying temporal trends from 2007 to 2018
Demographic characteristics were associated with urinary OPE biomarker concentrations
FUNDING
This research was funded by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (ZIAES103321). The funder had no role in the conduct or reporting of this study.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
REFERENCES
- Ashley-Martin J; MacPherson S; Zhao Z; Gaudreau É; Provencher G; Fisher M; Borghese MM; Bouchard MF; Booij L; Arbuckle TE Descriptive analysis of organophosphate ester metabolites in a pan-Canadian pregnancy cohort. Sci Total Environ 2023:163327. [DOI] [PubMed] [Google Scholar]
- Baldwin KR; Phillips AL; Horman B; Arambula SE; Rebuli ME; Stapleton HM; Patisaul HB Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats. Sci Rep 2017;7:7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A; Behl M; Birnbaum L; Diamond ML; Phillips A; Singla V; Sipes NS; Stapleton HM; Venier M Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ Sci Technol Lett 2019;6:638–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF; Lowry LK; Rosenberg J INTERPRETATION OF URINE RESULTS USED TO ASSESS CHEMICAL EXPOSURE WITH EMPHASIS ON CREATININE ADJUSTMENTS: A REVIEW. American Industrial Hygiene Association Journal 1993;54:615–627 [DOI] [PubMed] [Google Scholar]
- Bommarito PA; Cantonwine DE; Stevens DR; Welch BM; Davalos AD; Zhao S; McElrath TF; Ferguson KK An application of group-based trajectory modeling to define fetal growth phenotypes among small-for-gestational age births in the LIFECODES Fetal Growth Study. Am J Obstet Gynecol 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommarito PA; Welch BM; Keil AP; Baker GP; Cantonwine DE; McElrath TF; Ferguson KK Prenatal exposure to consumer product chemical mixtures and size for gestational age at delivery. Environ Health 2021;20:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M; Buckley JP; Quirós-Alcalá L Associations between urinary organophosphate ester metabolites and measures of adiposity among U.S. children and adults: NHANES 2013–2014. Environ Int 2019;127:754–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP; Kuiper JR; Bennett DH; Barrett ES; Bastain T; Breton CV; Chinthakindi S; Dunlop AL; Farzan SF; Herbstman JB; Karagas MR; Marsit CJ; Meeker JD; Morello-Frosch R; O’Connor TG; Romano ME; Schantz S; Schmidt RJ; Watkins DJ; Zhu H; Pellizzari ED; Kannan K; Woodruff TJ Exposure to Contemporary and Emerging Chemicals in Commerce among Pregnant Women in the United States: The Environmental influences on Child Health Outcome (ECHO) Program. Environ Sci Technol 2022;56:6560–6573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE; Ferguson KK; Mukherjee B; Chen Y-H; Smith NA; Robinson JN; Doubilet PM; Meeker JD; McElrath TF Utilizing Longitudinal Measures of Fetal Growth to Create a Standard Method to Assess the Impacts of Maternal Disease and Environmental Exposure. Supporting Information. Means and standard deviations (SD) or ultrasound parameters outside the clinically proscribed window of gestation.. PLOS ONE 2016;11:e0146532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina R; Butt C; Stapleton HM; Avery D; Harley KG; Holland N; Eskenazi B; Bradman A Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort). Chemosphere 2017;179:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnet JA; Weber R; Blum A Flammability standards for furniture, building insulation and electronics: Benefit and risk. Emerging Contaminants 2020;6:432–441 [Google Scholar]
- Choi G; Keil AP; Villanger GD; Richardson DB; Daniels JL; Hoffman K; Sakhi AK; Thomsen C; Herring AH; Drover SSM; Nethery R; Aase H; Engel SM Pregnancy exposure to common-detect organophosphate esters and phthalates and maternal thyroid function. Science of The Total Environment 2021;782:146709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EM; Kroeger G; Davis K; Clark CR; Ferguson PL; Stapleton HM Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environ Sci Technol 2016;50:10653–10660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J; Xu Z; Huang W; Feng L; Yang F Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci Total Environ 2016;554–555:211–217 [DOI] [PubMed] [Google Scholar]
- Dodson RE; Perovich LJ; Covaci A; Van den Eede N; Ionas AC; Dirtu AC; Brody JG; Rudel RA After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol 2012;46:13056–13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE; Rodgers KM; Carey G; Cedeno Laurent JG; Covaci A; Poma G; Malarvannan G; Spengler JD; Rudel RA; Allen JG Flame Retardant Chemicals in College Dormitories: Flammability Standards Influence Dust Concentrations. Environmental Science & Technology 2017;51:4860–4869 [DOI] [PubMed] [Google Scholar]
- Doherty BT; Hammel SC; Daniels JL; Stapleton HM; Hoffman K Organophosphate Esters: Are These Flame Retardants and Plasticizers Affecting Children’s Health? Curr Environ Health Rep 2019;6:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan H; Zhang Y; Wang YF; Tao FB; Gao H Relationships of prenatal organophosphate ester exposure with pregnancy and birth outcomes: A systematic scoping review of epidemiological studies. Ecotoxicol Environ Saf 2023;252:114642. [DOI] [PubMed] [Google Scholar]
- Hoffman K; Butt CM; Webster TF; Preston EV; Hammel SC; Makey C; Lorenzo AM; Cooper EM; Carignan C; Meeker JD; Hauser R; Soubry A; Murphy SK; Price TM; Hoyo C; Mendelsohn E; Congleton J; Daniels JL; Stapleton HM Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ Sci Technol Lett 2017a;4:112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K; Lorenzo A; Butt CM; Adair L; Herring AH; Stapleton HM; Daniels JL Predictors of urinary flame retardant concentration among pregnant women. Environ Int 2017b;98:96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle ME; Mínguez-Alarcón L; Carignan CC; Butt CM; Stapleton HM; Williams PL; Ford JB; Hauser R; Meeker JD The association between urinary concentrations of phosphorous-containing flame retardant metabolites and semen parameters among men from a fertility clinic. Int J Hyg Environ Health 2018;221:809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle ME; Mínguez-Alarcón L; Carignan CC; Butt CM; Stapleton HM; Williams PL; Ford JB; Hauser R; Meeker JD The association of urinary phosphorous-containing flame retardant metabolites and self-reported personal care and household product use among couples seeking fertility treatment. J Expo Sci Environ Epidemiol 2020a;30:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle ME; Watkins D; Rosario Z; Vélez Vega CM; Huerta-Montanez G; Calafat AM; Ospina M; Cordero JF; Alshawabkeh A; Meeker JD The association of urinary organophosphate ester metabolites and self-reported personal care and household product use among pregnant women in Puerto Rico. Environ Res 2019;179:108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle ME; Watkins D; Rosario Z; VélezVega CM; Calafat AM; Ospina M; Ferguson KK; Cordero JF; Alshawabkeh A; Meeker JD An exploratory analysis of urinary organophosphate ester metabolites and oxidative stress among pregnant women in Puerto Rico. Sci Total Environ 2020b;703:134798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatilaka NK; Restrepo P; Davis Z; Vidal M; Calafat AM; Ospina M Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere 2019;235:481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A qgcomp: Quantile G-Computation. 2022
- Keil AP; Buckley JP; O’Brien KM; Ferguson KK; Zhao S; White AJ A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environmental Health Perspectives 2020;128:047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JR; O’Brien KM; Ferguson KK; Buckley JP Urinary specific gravity measures in the U.S. population: Implications for the adjustment of non-persistent chemical urinary biomarker data. Environment International 2021;156:106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JR; O’Brien KM; Welch BM; Barrett ES; Nguyen RHN; Sathyanarayana S; Milne GL; Swan SH; Ferguson KK; Buckley JP Combining Urinary Biomarker Data From Studies With Different Measures of Urinary Dilution. Epidemiology 2022;33:533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JR; Stapleton HM; Wills-Karp M; Wang X; Burd I; Buckley JP Predictors and reproducibility of urinary organophosphate ester metabolite concentrations during pregnancy and associations with birth outcomes in an urban population. Environ Health 2020;19:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J; Zhao L; Letcher RJ; Zhang Y; Jian K; Zhang J; Su G A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environ Int 2019;127:35–51 [DOI] [PubMed] [Google Scholar]
- Luan M; Liang H; Chen Y; Chen D; Ji H; Chen H; Miao M; Yuan W Prenatal exposure to organophosphate esters is associated with decreased anogenital distance in offspring. Sci Total Environ 2023;856:159050. [DOI] [PubMed] [Google Scholar]
- Lumley T Analysis of Complex Survey Samples. Journal of Statistical Software 2004;9:1–19 [Google Scholar]
- Luo D; Liu W; Wu W; Tao Y; Hu L; Wang L; Yu M; Zhou A; Covaci A; Xia W; Xu S; Li Y; Mei S Trimester-specific effects of maternal exposure to organophosphate flame retardants on offspring size at birth: A prospective cohort study in China. J Hazard Mater 2021;406:124754. [DOI] [PubMed] [Google Scholar]
- Ma Y; Stubbings WA; Cline-Cole R; Harrad S Human exposure to halogenated and organophosphate flame retardants through informal e-waste handling activities - A critical review. Environ Pollut 2021;268:115727. [DOI] [PubMed] [Google Scholar]
- McElrath TF; Lim K-H; Pare E; Rich-Edwards J; Pucci D; Troisi R; Parry S Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. American journal of obstetrics and gynecology 2012;207:407. e401–407. e407 [DOI] [PubMed] [Google Scholar]
- Mendelsohn E; Hagopian A; Hoffman K; Butt CM; Lorenzo A; Congleton J; Webster TF; Stapleton HM Nail polish as a source of exposure to triphenyl phosphate. Environment International 2016;86:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM; Lawrence KG; Keil AP The Case for Case-Cohort: An Applied Epidemiologist’s Guide to Reframing Case-Cohort Studies to Improve Usability and Flexibility. Epidemiology 2022;33:354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy Z; Vuong AM; Ospina M; Calafat AM; La Guardia MJ; Xu Y; Hale RC; Dietrich KN; Xie C; Lanphear BP; Braun JM; Cecil KM; Yolton K; Chen A Organophosphate esters in a cohort of pregnant women: Variability and predictors of exposure. Environ Res 2020;184:109255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DB; Rzehak P; Klenk J; Weiland SK Analyses of case-control data for additional outcomes. Epidemiology 2007;18:441–445 [DOI] [PubMed] [Google Scholar]
- Rock KD; St Armour G; Horman B; Phillips A; Ruis M; Stewart AK; Jima D; Muddiman DC; Stapleton HM; Patisaul HB Effects of Prenatal Exposure to a Mixture of Organophosphate Flame Retardants on Placental Gene Expression and Serotonergic Innervation in the Fetal Rat Brain. Toxicol Sci 2020;176:203–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME; Hawley NL; Eliot M; Calafat AM; Jayatilaka NK; Kelsey K; McGarvey S; Phipps MG; Savitz DA; Werner EF; Braun JM Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ Health 2017;16:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey M; Harrad S; Abou-Elwafa Abdallah M; Drage DS; Berresheim H Phasing-out of legacy brominated flame retardants: The UNEP Stockholm Convention and other legislative action worldwide. Environment International 2020;144:106041. [DOI] [PubMed] [Google Scholar]
- Stapleton HM; Klosterhaus S; Eagle S; Fuh J; Meeker JD; Blum A; Webster TF Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol 2009;43:7490–7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM; Sharma S; Getzinger G; Ferguson PL; Gabriel M; Webster TF; Blum A Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environmental Science & Technology 2012;46:13432–13439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y; Hu L; Liu L; Yu M; Li Y; Li X; Liu W; Luo D; Covaci A; Xia W; Xu S; Li Y; Mei S Prenatal exposure to organophosphate esters and neonatal thyroid-stimulating hormone levels: A birth cohort study in Wuhan, China. Environment International 2021;156:106640. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. Furniture Flame Retardancy Partnership: Environmental Profiles of Chemical Flame-Retardant Alternatives for Low-Density Polyurethane Foam. 2005 [Google Scholar]
- van Buuren S; Groothuis-Oudshoorn K mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 2011;45:1–67 [Google Scholar]
- Van den Eede N; Heffernan AL; Aylward LL; Hobson P; Neels H; Mueller JF; Covaci A Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environment International 2015;74:1–8 [DOI] [PubMed] [Google Scholar]
- Varshavsky JR; Robinson JF; Zhou Y; Puckett KA; Kwan E; Buarpung S; Aburajab R; Gaw SL; Sen S; Gao S; Smith SC; Park JS; Zakharevich I; Gerona RR; Fisher SJ; Woodruff TJ Organophosphate Flame Retardants, Highly Fluorinated Chemicals, and Biomarkers of Placental Development and Disease During Mid-Gestation. Toxicol Sci 2021;181:215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C; Chen H; Li H; Yu J; Wang X; Liu Y Review of emerging contaminant tris(1,3-dichloro-2-propyl)phosphate: Environmental occurrence, exposure, and risks to organisms and human health. Environ Int 2020a;143:105946. [DOI] [PubMed] [Google Scholar]
- Wang X; Chen P; Zhao L; Zhu L; Wu F Transplacental Behaviors of Organophosphate Tri- and Diesters Based on Paired Human Maternal and Cord Whole Blood: Efficiencies and Impact Factors. Environ Sci Technol 2021;55:3091–3100 [DOI] [PubMed] [Google Scholar]
- Wang X; Liu Q; Zhong W; Yang L; Yang J; Covaci A; Zhu L Estimating renal and hepatic clearance rates of organophosphate esters in humans: Impacts of intrinsic metabolism and binding affinity with plasma proteins. Environment International 2020b;134:105321. [DOI] [PubMed] [Google Scholar]
- Wong F; de Wit CA; Newton SR Concentrations and variability of organophosphate esters, halogenated flame retardants, and polybrominated diphenyl ethers in indoor and outdoor air in Stockholm, Sweden. Environmental Pollution 2018;240:514–522 [DOI] [PubMed] [Google Scholar]
- Wu Y; Venier M; Salamova A Spatioseasonal Variations and Partitioning Behavior of Organophosphate Esters in the Great Lakes Atmosphere. Environ Sci Technol 2020;54:5400–5408 [DOI] [PubMed] [Google Scholar]
- Xu C; Ma H; Gao F; Zhang C; Hu W; Jia Y; Xu J; Hu J Screening of Organophosphate Flame Retardants with Placentation-Disrupting Effects in Human Trophoblast Organoid Model and Characterization of Adverse Pregnancy Outcomes in Mice. Environ Health Perspect 2022;130:57002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y; Li M; Pan L; Duan Y; Duan X; Li Y; Sun H Exposure to organophosphate ester flame retardants and plasticizers during pregnancy: Thyroid endocrine disruption and mediation role of oxidative stress. Environment International 2021;146:106215. [DOI] [PubMed] [Google Scholar]
- Zhao Y; Ding J; Lv L; Zhang H Exposure to organophosphate flame esters during early pregnancy and risk of spontaneous abortion: A case-control study. Chemosphere 2021;268:129375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


