Abstract
As an arousal hub region in the brain, the locus coeruleus (LC) has bidirectional connections with the autonomic nervous system. Magnetic resonance imaging (MRI)-based measures of LC structural integrity have been linked to cognition and arousal, but less is known about factors that influence LC structure and function across time. Here, we tested the effects of heart rate variability (HRV) biofeedback, an intervention targeting the autonomic nervous system, on LC MRI contrast and sympathetic activity. Younger and older participants completed daily HRV biofeedback training for five weeks. Those assigned to an experimental condition performed biofeedback involving slow, paced breathing designed to increase heart rate oscillations, whereas those assigned to a control condition performed biofeedback to decrease heart rate oscillations. At the pre- and post-training timepoints, LC contrast was assessed using turbo spin echo MRI scans, and RNA sequencing was used to assess cAMP-responsive element binding protein (CREB)-regulated gene expression in circulating blood cells, an index of sympathetic nervous system signaling. We found that left LC contrast decreased in younger participants in the experimental group, and across younger participants, decreases in left LC contrast were related to the extent to which participants increased their heart rate oscillations during training. Furthermore, decreases in left LC contrast were associated with decreased expression of CREB-associated gene transcripts. On the contrary, there were no effects of biofeedback on LC contrast among older participants in the experimental group. These findings provide novel evidence that in younger adults, HRV biofeedback involving slow, paced breathing can decrease both LC contrast and sympathetic nervous system signaling.
Keywords: biofeedback, CREB, heart rate variability, locus coeruleus, norepinephrine, sympathetic nervous system
1. Introduction
The locus coeruleus (LC), a small nucleus in the brainstem, helps coordinate the brain’s arousal system. Situated at the lateral floor of the fourth ventricle, the LC serves as the brain’s primary source of the neurotransmitter noradrenaline (Schwarz & Luo, 2015). Noradrenaline released from the LC to the brain and spinal cord regulates wakefulness, coordinates adaptive behavior, and modulates processes of learning and memory (Berridge & Waterhouse, 2003; Sara, 2009). Via β-adrenergic receptors, noradrenaline activates cAMP, which in turn activates cAMP-response-element-binding protein (CREB; Lorton & Bellinger, 2015; Roseboom & Klein, 1995; Thonberg et al., 2002). CREB is a stimulus-induced transcription factor that affects the expression of many different genes (Shaywitz & Greenberg, 1999). The LC is also implicated in the neuropathological progression of Alzheimer’s disease, with abnormal tau evident in the LC in younger adults before cortical tau tangles emerge (Braak et al., 2011; Harley et al., 2021). LC neurodegeneration is characteristic of Alzheimer’s disease (Chalermpalanupap et al., 2017), and older adults with relatively lower cell density within the LC exhibit faster rates of cognitive decline prior to death (Wilson et al., 2013).
In recent years, specialized magnetic resonance imaging (MRI) sequences have accelerated study of the human LC via their ability to quantify LC structure in vivo (Sasaki et al., 2006). In these sequences, the LC exhibits elevated MRI signal contrast relative to surrounding tissue. The properties of the LC that lead to its high MRI contrast are still under investigation (He et al., 2022; Priovoulos et al., 2020; Trujillo et al., 2017; Watanabe, 2022; Watanabe et al., 2019). MRI sequences that provide high LC contrast have been referred to as ‘neuromelanin MRI’ as high resolution post-mortem imaging followed by histology on the same tissue revealed that high MRI contrast corresponded spatially with LC neurons containing neuromelanin (Keren et al., 2015; see also Cassidy et al. 2019; Kitao et al. 2013). However, there is growing evidence that neuromelanin in LC neurons is not the factor driving LC MRI contrast. In mice, LC noradrenergic neurons produced high contrast with magnetization transfer MRI, even though no neuromelanin is observed in the LC of mice (Watanabe et al., 2019). Furthermore, neuromelanin on its own does not affect magnetization transfer (Priovoulos et al., 2020; Trujillo et al., 2017) and only leads to T1 shortening at levels that are higher than neuromelanin levels in the human brain (Priovoulos et al., 2020). Instead, it seems that the property driving MRI contrast is a lower macromolecular-to-free-water fraction within the LC (Priovoulos et al., 2020; Trujillo et al., 2017; Watanabe, 2022; Watanabe et al., 2019). LC neurons tend to have large cell bodies (e.g., see the “plump” LC neuron cell bodies in Fig. 1 of Ramon-Moliner, 1974): In humans, LC neuron somas are ~37 μm in diameter (Baker et al., 1989), whereas the somas of prefrontal neurons, for comparison, are 13-17 μm in diameter (Rajkowska & Goldman-Rakic, 1995). Insofar as it is the high water content in LC cells that drives LC MRI contrast, both the number of LC cells and their current cell body size should influence LC MRI contrast.
Higher LC MRI contrast is associated with better cognitive outcomes in older adults (Dahl et al., 2019; Hämmerer et al., 2018; Liu et al., 2020), higher subjective cognition (Bell et al., 2022), reduced risk of developing mild cognitive impairment (Elman et al., 2021) or Alzheimer’s disease (Galgani et al., 2023), and fewer preclinical Alzheimer’s disease indicators (Jacobs et al., 2021). However, the relationship between LC contrast and brain structure varies depending on age (Bachman et al., 2021). Specifically, we found that, whereas in older adults contrast of the rostral LC was positively associated with cortical thickness in various brain regions in younger adults caudal LC contrast showed negative associations with cortical thickness (Bachman et al., 2021).
Other findings also suggest that higher LC contrast is not always a positive indicator. For instance, one study found that LC volume - also quantified using an MRI sequence that yields elevated signal intensity in the LC - was positively correlated with anxious arousal and self-reported general distress in younger adults (Morris et al., 2020). Likewise, another study found that participants with higher LC contrast had lower heart rate variability (Mather et al., 2017). In animal studies, anxiety is associated with increased tonic LC activity (Harley & Yuan, 2021; McCall et al., 2015). The higher LC MRI contrast associated with lower HRV and higher anxiety (Mather et al., 2017; Morris et al, 2020) may be driven by influences of high tonic levels of LC activity on the macromolecular-to-free-water fraction within the LC in people who have lower parasympathetic activity or higher anxiety.
Therefore, the apparently discrepant findings in younger versus older adults may be explained by different factors contributing to LC contrast throughout the lifespan. In younger individuals, the tonic level of LC activity is the primary factor influencing individual differences in LC contrast, whereas later in life, neurodegeneration becomes the dominant factor influencing individual differences in LC contrast.
Tonic LC activity and neurodegeneration could each influence LC contrast by affecting LC cellular water content, but through different means. First, in early life, current anxiety/arousal states could influence the current water content of LC cells by influencing the tonic activity level of LC neurons. Stimulating neuronal activity shrinks the volume of the interstitial fluid-filled space around neurons by approximately 30% due to uptake of water by neurons and astrocytes (Østby et al., 2009). Transport of Na+ and other ions across the cell membrane during neuronal activity triggers water movement across the cell membrane against the osmotic gradient (MacAulay, 2021). In the cortex, researchers have observed that a β-adrenergic agonist decreases interstitial volume (Sherpa et al., 2016), while adrenergic antagonists increase interstitial volume (Xie et al., 2013). Thus, both general neuronal activity and specifically noradrenergic activity may influence the relative fluid volume of brain cells and the spaces around them, which could influence the magnetization transfer signal from the LC, although to date we lack studies tracking activity-based changes in cell size in the locus coeruleus. In later life, individual differences in neurodegeneration of LC neurons that affect free water concentrations (e.g., Chu et al., 2021) may account for a greater share of variance in individual differences in LC contrast than activity-related fluctuations in cell body volume.
Despite what has been learned about the LC from recent MRI studies, no published studies have assessed whether there are interventions that can change LC MRI contrast. What type of interventions might change LC contrast over time? Because the LC is a key player in the stress response and has bidirectional connections with the parasympathetic and sympathetic branches of the autonomic nervous system (Wood et al., 2017), we reasoned that an intervention targeting the autonomic nervous system could influence LC structure. One such intervention is heart rate variability (HRV) biofeedback (Lehrer & Gevirtz, 2014). Individuals with higher HRV, an index of parasympathetic control over heart rate and autonomic regulation (Mulcahy et al., 2019; Thayer & Lane, 2000), are better able to regulate their emotions and exhibit reduced physiological responses to stressors (Thayer & Lane, 2009; Weber et al., 2010), relative to those with lower HRV. HRV can be systematically manipulated through biofeedback that involves slow, paced breathing and simultaneous feedback on the coupling between heart rate oscillations and breathing (Lehrer & Gevirtz, 2014). Slow breathing, particularly at a pace around 10 seconds per breath, elicits high-amplitude oscillations in heart rate (Lehrer et al., 2003). Slow breathing also stimulates the vagus nerve (Brown & Gerbarg, 2005), which sends projections to the LC by way of the nucleus tractus solitarii (NTS; Badran et al., 2018; Fornai et al., 2011). Performing HRV biofeedback over a period of weeks has been shown to reduce levels of stress and anxiety (Goessl et al., 2017) in younger as well as older adults (Jester et al., 2019), but it is unknown whether HRV-biofeedback affects the LC’s structure and function.
Here, we examined whether HRV biofeedback affected LC MRI contrast and sympathetic activity. Younger and older participants completed 5 weeks of HRV biofeedback training as part of a clinical trial testing the effects of HRV biofeedback training on brain regions involved in emotion regulation (Clinicaltrials.gov NCT03458910 “Heart Rate Variability and Emotion Regulation,” Nashiro et al., 2023; Yoo et al., 2023). Participants randomized to an experimental condition completed daily biofeedback involving slow, paced breathing to increase heart rate oscillations and HRV, whereas participants randomized to an active control condition completed daily biofeedback training designed to decrease heart rate oscillations and HRV. We designed this novel active control condition in order to equate as many features of the intervention as possible while maximizing differences in heart rate oscillations. As an exploratory outcome, both before and after the 5-week training period, we assessed LC contrast in all participants using turbo spin echo (TSE) MRI scans that exhibit elevated signal intensity in the LC. Based on prior work demonstrating beneficial effects of HRV on emotional well-being, and in line with our hypotheses regarding LC contrast reflecting stress in younger adults, we expected that performing 5 weeks of HRV biofeedback training would decrease LC contrast in younger participants. Conversely, as we hypothesize that neurodegeneration, more so than tonic LC activity levels, contributes to variance in LC contrast in older adulthood, we predicted that HRV biofeedback would not decrease LC contrast in older participants. Furthermore, in a subset of younger participants, we collected blood samples before and after the training period to assess changes in a health-relevant index of sympathetic nervous system (SNS) activity – blood cell expression of genes regulated by the cAMP-responsive element binding protein (CREB) family of transcription factors, which mediates beta-adrenergic signaling from the SNS (Cole et al., 2010; Mayr & Montminy, 2001). In this subset, we predicted that training-related decreases in LC contrast would be coupled with decreases in SNS signaling and thereby reduce expression of CREB-regulated gene transcripts. Previous research has validated blood cell CREB-associated RNA expression levels as a measure of β-adrenergic signaling (Brown et al., 2010; Cole et al., 2005; Powell et al., 2013).
2. Materials and Methods
2.1. Participants
Data were collected as part of an intervention study testing the effects of 5 weeks of HRV biofeedback training in younger and older adults (ClinicalTrials.gov NCT03458910; for a full description of the study, see Yoo et al., 2023). Eligible participants were healthy, MRI-eligible younger adults between the ages of 18 and 35 and older adults between the ages of 55 to 80 (for more details including determination of sample size see Yoo et al, 2023). Potential participants were recruited via the USC Healthy Minds community subject pool, a USC online bulletin board, Facebook, and flyers between February 2018 and March 2020 (enrollment was cut short by the COVID pandemic). Individuals who regularly practiced biofeedback training or breathing techniques were excluded from participation. Older adults were screened for cognitive dysfunction by telephone using the TELE interview (Gatz et al., 1995); individuals scoring below 16 were excluded from participation.
2.2. Study Procedures Overview
Participants were scheduled in waves in small groups (each group meeting weekly in our lab on the USC campus on a different day of the week). After scheduling for a wave was complete, each group was randomized to one of the two conditions (for more information, see Yoo et al., 2023). Participants were blinded to researcher hypotheses regarding differential outcomes by condition. Those in an increase-oscillations (Osc+) condition completed 20-40 minutes of daily biofeedback training involving slow, paced breathing which was designed to increase heart rate oscillations and HRV. Participants in a decrease-oscillations (Osc−) condition completed 20-40 minutes of biofeedback training per day designed to decrease heart rate oscillations and HRV.
As part of the intervention study which lasted 7 weeks, MRI assessments were conducted at a pre-training timepoint (second study week), before participants learned about or practiced the intervention, and following 5 weeks of biofeedback training (seventh study week). A total of 175 participants (115 younger, 60 older) completed pre- and/or post-training MRI assessments, yielding a total of 325 TSE scans (detailed breakdown in the Supplementary Methods, Section 1). A flow chart detailing enrollment and dropouts is provided in Yoo et al., (2023, Fig. 1). Following exclusions for artifact or motion on native TSE scans (Section 2.3.1), 287 scans were used for LC delineation. Additional exclusions were applied due to artifact after warping TSE scans to MNI152 space (Section 2.3.1). In addition, blood samples were collected from a subset of 54 younger participants at pre- and post-training timepoints (first and sixth study weeks, respectively) to assess change in expression of genes regulated by CREB. A total of 129 participants (93 younger, 36 older) with LC contrast values and/or blood-based measures available at both timepoints were included for analysis. Characteristics of this sample are presented in Table 1. The University of Southern California Institutional Review Board approved the study. All participants provided written, informed consent prior to participation and received monetary compensation for their participation.
Table 1.
Sample characteristics.
| Age group | Condition | N | N (%) Female |
Age, mean (SD) |
Age, range |
Education, mean (SD) |
Education, range |
|---|---|---|---|---|---|---|---|
| Younger | Osc+ | 47 | 26 (55.3%) | 22.64 (2.57) | 18-28 | 15.99 (1.87) | 12-20 |
| Younger | Osc− | 46 | 24 (52.2%) | 22.57 (3.24) | 18-31 | 15.74 (2.64) | 12-24 |
| Older | Osc+ | 17 | 12 (70.6%) | 65 (6.86) | 55-80 | 17.12 (2.71) | 14-25 |
| Older | Osc− | 19 | 15 (78.9%) | 65.21 (5.61) | 57-77 | 16.53 (2.39) | 14-22 |
Note. Age and education are expressed in years. Osc+ = increase-oscillations condition; Osc− = decrease-oscillations condition; SD = standard deviation.
2.3. Heart Rate Biofeedback and Pulse Measurement
During the home training sessions, participants received biofeedback, seeing a continuously updating line indicating heart rate over time. Osc+ participants tried to make their heart rate oscillate, tracking their breathing, and Osc− participants tried to keep their heart rate steady. Heart rate was measured using an infrared pulse plethysmograph sensor clipped to the participant’s earlobe and attached via USB to a small laptop running emWave Pro (HeartMath®Institute, 2020) software (for more details see Nashiro et al., 2023). As already reported, we computed autoregressive spectral power for each training session (Yoo et al., 2022). For each participant, we averaged the spectral power across all home training sessions and extracted the summed power within the 0.063– 0.125 Hz (8-16s) range to provide a measure of training oscillatory power in a frequency range encompassing the breathing rates used by the Osc+ participants. The resulting value of training oscillatory power reflected how much each participant increased their heart rate oscillations on average during biofeedback training. Training oscillatory power values were log transformed prior to statistical analysis. A total of 2 Osc− participants included in analyses of LC contrast change were missing values of training oscillatory power.
2.4. MRI data collection
MRI data were collected at the University of Southern California David and Dana Dornsife Neuroimaging Center, on a Siemens Magnetom Trio 3T MRI scanner with a 32-channel head coil. Sequences relevant to the present analyses are described below.
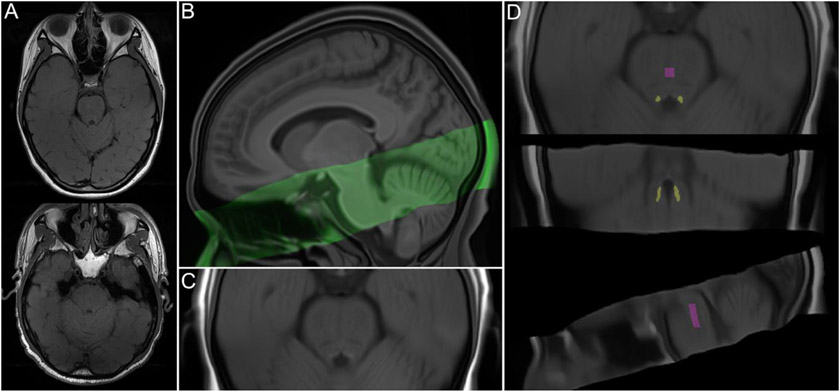
A high-resolution, T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) scan was acquired (TR = 2300ms, TE = 2.26 ms, flip angle = 9°, bandwidth = 200 Hz/Px, isometric voxel size = 1.0mm3, no gap between slices, 175 volumes). Based on the MPRAGE scan, a two-dimensional, multi-slice TSE scan was collected by aligning the field of view perpendicular to the respective participant’s brainstem. Parameters of this TSE sequence were as follows: TR = 750ms, TE = 12ms, flip angle = 120°, bandwidth = 287 Hz/Px, voxel size = 0.43 x 0.43 x 2.5mm, gap between slices = 1mm. The TSE sequence included 11 axial slices and covered the entire pons. TSE scans from randomly selected participants are shown in Figure 1A.
Figure 1.
(A) Turbo spin echo (TSE) scans from randomly selected younger (top) and older (bottom) participants. (B) Sagittal view of TSE template (green) overlaid onto whole-brain template, both warped to MNI152 0.5mm (linear) space. (C) Detailed axial view of TSE template, warped to MNI152 space. (D) TSE template, warped to MNI152 space, overlaid with locus coeruleus meta-mask and pontine reference region from Dahl et al. (2022), which were used for calculation of LC contrast ratios.
2.5. MRI data analysis
2.5.1. LC delineation.
We used a semi-automated method to delineate the LC on all available pre- and post-training TSE scans based on approaches described by Dahl et al. (2019) and Ye et al. (2021). LC delineation steps were performed using Advanced Normalization Tools (ANTs; Version 2.3.4; Avants et al., 2011; http://stnava.github.io/ANTs/). Visualization steps were performed using ITK-SNAP (Version 3.8.0; Yushkevich et al., 2006; http://www.itksnap.org). Parameters for each step are described in the Supplementary Methods (Section 2).
All TSE scans were first visually inspected; scans with excessive motion or susceptibility artifact overlapping the LC or pons (n = 34), incorrect positioning (n = 3), or different resolution (n = 1) were excluded from LC delineation (Supplementary Methods, Section 1). The remaining TSE and corresponding MPRAGE scans were upsampled to twice their native resolution using the ResampleImage ANTs routine. Upsampled MPRAGE scans were used to generate a whole-brain template with the antsMultivariateTemplateConstruction.sh routine (Figure 1B; see Supplementary Methods Section 2 for a description of template-building procedures). Each TSE scan was then coregistered to its corresponding whole-brain template-coregistered MPRAGE scan, using the antsRegistrationSyNQuick.sh routine. All coregistered TSE scans were used to build a TSE template (Figures 1B and 1C). Using the antsRegistrationSyN.sh routine, the resulting TSE template was coregistered to the whole-brain template to ensure spatial alignment. The whole-brain template was then coregistered to MNI152 0.5mm (linear) standard space, in order to facilitate comparison with previously-published LC maps. Transforms from all template-building and coregistration steps described above were applied in a single step to warp upsampled TSE scans to MNI152 space, using the antsApplyTransforms.sh routine. In addition, transforms from the final coregistration steps were applied to warp the TSE template to MNI152 space (Figure 1C). As a validation step, we examined whether locations of hyperintensity on the TSE template in MNI152 space aligned with the location of the Dahl et al. (2022) meta-map, which was generated by aggregating across published LC maps and thus reflected a plausible LC volume of interest with high agreement across studies. (For discussion of a template-space approach to LC localization, see Giorgi et al., 2022.) We found high correspondence between hyperintensities on the TSE template and the LC meta-map (Supplementary Figure S1).
At this stage, a total of 6 warped TSE scans were excluded from LC delineation after visually confirming that, once warped to MNI152 space, they contained artifacts overlapping the LC or central pons. This left data from 78 younger (39 Osc+, 39 Osc−) and 36 older (17 Osc+, 19 Osc−) participants included for LC delineation and analyses of change in LC contrast1. We proceeded to delineate the LC for individual participants and timepoints by applying the Dahl et al. (2022) LC meta-map as a mask on all warped TSE scans (Figure 1D). Within the masked region of each scan, we extracted the intensity and location of the peak-intensity LC voxel in each z-slice and hemisphere. As another validation step, we compared the resulting intensity values to intensity values determined through manual delineation of the LC on native-resolution TSE scans (Supplementary Methods, Section 3). Two-way mixed-effects intraclass correlation analyses based on consistency indicated high correspondence between peak LC intensity values from the semi-automated and manual methods for the left LC (ICC(3, 1) = 0.939, 95% CI = 0.921 - 0.953, p < .001) and right LC (ICC(3, 1) = 0.924, 95% CI = 0.902 - 0.941, p < .001). To compute LC contrast ratios reflecting peak LC intensity relative to that of surrounding tissue, we also extracted intensity values from a central pontine region (Figure 1D). Specifically, we applied the central pontine reference map from Dahl et al. (2022) as a mask on individual TSE scans that had been warped to MNI space and extracted the peak intensity value within the masked region.
2.5.2. Calculation of LC MRI contrast.
LC MRI contrast is typically calculated as a ratio reflecting peak signal intensity in the LC relative to peak intensity within a pontine reference region (Liu et al., 2017):
No published studies have examined the stability of peak LC signal intensity locations across time, or factors which influence locations of peak LC intensity, thus we performed an exploratory step to guide our calculation of LC contrast. Specifically, we assessed whether, for each participant, locations of peak intensity in each left and right LC shifted from pre- to post-training (peak LC intensity locations are depicted in Supplementary Figure S2A). To do so, we calculated for each participant the 3-dimensional distance between peak LC intensity locations at the pre- and post-training timepoints, for left and right LC separately (Supplementary Figure S2B). A linear mixed effects analysis indicated that these distances differed from 0 across training conditions, age groups and hemispheres (p <. 001; Supplementary Results, Section 1), suggesting that locations of peak LC intensity were not consistent within individuals across time. This is not surprising in that the LC voxel with peak intensity is not necessarily an outlier and there may be other voxels with similar intensity values. In this type of situation, random noise may lead to different high contrast voxels to be identified as the peak voxel in different scan sessions. We therefore aimed to calculate LC contrast in a way that was not biased by peak LC signal intensity location at either the pre- or post-training timepoint. Specifically, for each participant, we calculated LC contrast at each timepoint as an average of LC contrast at the locations of pre- and post-training peak LC signal intensity.
In addition to calculating LC contrast based on locations of peak LC intensity, we calculated values of rostral and caudal LC contrast. For this step, we used the locations of rostral and caudal clusters along the LC’s rostrocaudal axis where we previously found age differences in LC contrast (Dahl et al., 2019; rostral: MNI z = 101-104; caudal: MNI z = 87-95). For each participant and timepoint, contrast ratios in each z-slice and hemisphere were calculated, then ratios were averaged across hemispheres for each z-slice. Finally, ratios were averaged across the caudal and rostral clusters of slices to obtain a value of rostral and caudal LC contrast, respectively, for each participant.
2.6. Blood sampling and RNA sequencing analysis
For a subset of participants (N = 54 younger adults: 30 Osc+, 24 Osc−), peripheral blood samples were collected under resting conditions at the pre- and post-training timepoints by antecubital venipuncture into PAXgene RNA tubes. Following collection, samples were gently inverted ten times and kept at room temperature for between 2.00 and 70.20 hours (mean = 6.95 hours). Samples were then stored frozen at −80°C at the USC School of Gerontology before they were transferred and assayed in a single batch at the UCLA Social Genomics Core Laboratory, as previously described (Cole et al., 2020). Briefly, total RNA was extracted from 2.5 ml blood samples using an automated nucleic acid processing system (QIAcube; Qiagen), checked for suitable RNA integrity and mass (>50 ng by NanoDrop One spectrophotometry; achieved mean = 4497 ng) and assayed by RNA sequencing in the UCLA Neuroscience Genomics Core Laboratory using Lexogen QuantSeq 3’ FWD cDNA library synthesis and multiplex DNA sequencing on an Illumina HiSeq 4000 instrument with single-strand 65-nt sequence reads (all following the manufacturer’s standard protocol). Analyses targeted >10 million sequence reads per sample (achieved mean 15.1 million), each of which was mapped to the RefSeq human transcriptome sequence using the STAR aligner (achieved average 94% mapping rate) to generate transcript counts per million total transcripts (TPM). TPM values were floored at 1 TPM to reduce spurious variability, log2-transformed to reduce heteroscedasticity, and analyzed by linear statistical models with promoter sequence-based bioinformatics analyses of CREB activity as described below.
2.7. Statistical analysis
We fit a linear mixed effects model to assess the fixed effects of timepoint, training condition, age group and hemisphere on LC contrast. Mixed models were also fit for each age group separately to examine the fixed effects of timepoint, training condition, hemisphere and their interactions on LC contrast. Significant timepoint x condition interactions were supplemented with post hoc comparisons of pre- versus post-training LC contrast for each training condition and hemisphere.
Next, we tested whether changes in LC contrast were related to the extent to which participants increased their heart rate oscillations during biofeedback training. For each participant, values of change in left and right LC contrast were calculated as the difference between pre- and post-training LC contrast values. We then fit another mixed model testing the fixed effects of training oscillatory power (see Section 2.3 for details of this measure), hemisphere, age group, and their interactions on LC contrast. For each age group and hemisphere separately, we also performed planned Pearson correlation analyses to test associations between change in LC contrast and training oscillatory power. Supplementary analyses were performed to assess training effects on rostral and caudal LC contrast (Supplementary Methods, Section 4).
Based on previous findings of sex differences in LC contrast (Bachman et al., 2021; but see Betts et al., 2017, Liu et al., 2019, Trujillo et al., 2019, and Ye et al., 2022), sex differences in the responsiveness of the LC to stress (Bangasser et al., 2016), sex differences in potentially LC-related cognitive strategies (Ycaza Herrera et al., 2019), and sensitivity of the LC to sex hormones (Bangasser et al., 2016; Luckey et al., 2021), we tested for sex differences in LC contrast change and its relationship with training oscillatory power by fitting the previously described mixed models including sex and its interactions as fixed effects (Supplementary Methods, Section 5). These analyses were performed only for younger participants (Osc+: 24 female, 15 male; Osc−: 18 female, 21 male) because we were underpowered to detect sex differences among older participants.
These analyses were performed in R (Version 4.1.0; R Core Team, 2021). Linear mixed effects models were fit using the R package `lme4` (Version 1.1-27.1; Bates et al., 2015), and significance of fixed effects was assessed with Satterthwaite’s method as implemented in the R package `lmerTest` (Version 3.1-3; Kuznetsova et al., 2017). All models included random intercepts for participants. A sum coding contrast scheme was applied to factor variables (condition: Osc+ = 0.5, Osc− = 0.5; timepoint: post-training = 0.5, pre-training = 0.5; age group: older = 0.5, younger = −0.5; hemisphere: left = 0.5, right = −0.5; sex: female = 0.5, male = −0.5). Post hoc comparisons of model-estimated marginal means were performed with a Bonferroni correction for multiple comparisons, as implemented in the `emmeans` R package (Version 1.6.2-1; Lenth, 2021). Effect sizes were calculated using the R package `effectsize` (Version 0.5; Ben-Shachar et al., 2020) and reported as d.
We also tested whether training condition (Osc+ or Osc−) or change in LC contrast was associated with change in CREB activity from pre- to post-training using an established bioinformatic measure of CREB gene regulation employed in previous research (Cole et al., 2020). Data from 54 younger participants (30 Osc+, 24 Osc−) with available blood-based measures at both timepoints were included for analysis of CREB activity change by training condition, and data from 39 younger participants (22 Osc+, 17 Osc−) with available blood-based measures and LC contrast values at both timepoints were included for analysis of associations between CREB activity change and LC contrast change. In these analyses, whole transcriptome profiling data were screened to identify genes that showed > 1.5-fold differential change over time between conditions or > 1.5-fold differential change in expression per standard deviation (SD) of pre- to post-training LC contrast change, and the core promoter DNA sequences of those genes were scanned for the prevalence of CREB-binding motifs using the TELiS database (Cole et al., 2020; Cole et al., 2005). Analyses were conducted as previously described (Cole et al., 2020), with CREB activity quantified by the ratio of CREB-binding site prevalence (defined by TRANSFAC position-specific weight matrix V$CREB_Q4) in genes up-regulated in association with condition differences in change or LC contrast change (i.e., >1.5-fold upregulation from pre- to post-training timepoint per SD of LC contrast change) vs. down-regulated (>1.5-fold down-regulated), and log2-ratios averaged over 9 parametric combinations of promoter sequence length (−300, −600, and −1000 to +200 bp relative to the RefSeq transcription start site) and detection stringency (TRANSFAC mat_sim = .80, .90, and .95). Statistical significance was assessed using standard errors derived from bootstrap resampling of linear model residual vectors in underlying gene expression data, which controls for correlation across genes. For additional details on analytic methods, see Cole et al. (2005) and Cole et al. (2020).
3. Results
3.1. LC contrast decreased in younger participants in the Osc+ condition
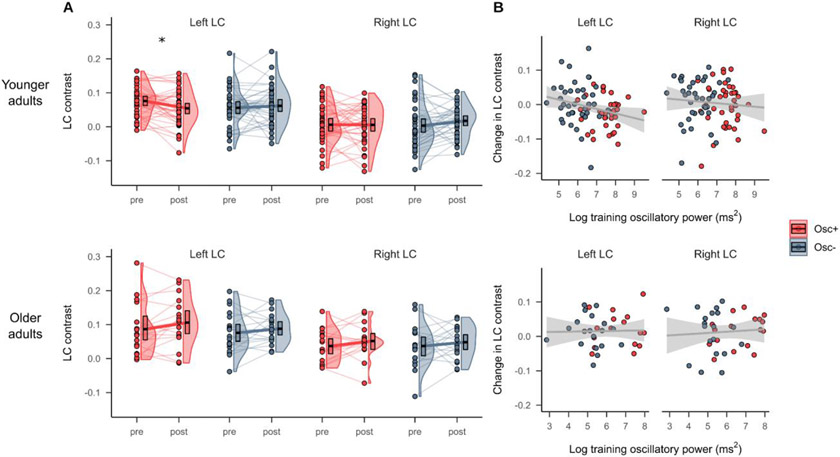
LC contrast at the pre- and post-training timepoints is shown in Figure 2A. Using independent samples t-tests, we first confirmed that there were no significant baseline differences in the LC contrast among either younger, t(76) = 1.73, p = .09, d = 0.39 (left) and t(76) = 0.19, p = .85, d = 0.04 (right), or older adults, t(34) = .46, p = .65, d = 0.15 (left) and t(34) = 0.003, p = . 997, d = 0.001 (right). Then, using a linear mixed effects analysis testing the fixed effects of condition, timepoint and hemisphere on LC contrast in younger participants (Table 2A), we found a significant training condition x timepoint interaction on LC contrast (p = .034, d = −0.283). Post hoc comparisons of estimated marginal means indicated that at the post- relative to the pre-training timepoint, LC contrast was numerically lower among younger participants in the Osc+ condition, left: t(228) = −2.193, p = .029, d = −0.497; right: t(228) = −0.059, p = .953, d = −0.013 and numerically higher among younger participants in the Osc− condition, left: t(228) = 0.599, p = .550, d = 0.136; right: t(228) = 1.423, p = .156, d = 0.322. For younger participants, we also found a significant fixed effect of hemisphere on LC contrast (p < .001, d = 1.462), with left LC contrast being higher than right LC contrast, but no other fixed effects were significant. For older participants (Table 2B), we did not find a significant training condition x timepoint interaction (p = .713, d = 0.073), but we did observe a significant fixed effect of timepoint (p = .046, d = 0.400), with LC contrast being higher at the post- compared to the pre-training timepoint. As in the younger sample, we observed a significant effect of hemisphere on LC contrast in older participants (p < .001, d = 1.261), driven by higher contrast for the left relative to the right LC. Notably, in a model including data from both age groups, we did not observe significant timepoint x condition, age x timepoint x condition, or age x timepoint interactions on LC contrast (p’s > .05; Supplementary Results, Section 2).
Figure 2.
(A) LC MRI contrast at the pre- and post-training timepoints for the Osc+ and Osc− conditions, for younger (top) and older (bottom) participants. (B) Associations between pre- to post-training change in LC contrast and training oscillatory power, a measure of how much participants increased their heart rate oscillations across practice sessions, for younger (top) and older (bottom) participants. Linear regression lines with 95% confidence intervals are shown in gray. Significance labels (*p < .05) refer to pairwise comparisons between pre- and post-training LC contrast.
Table 2:
Results of linear mixed effects analysis testing the fixed effects of timepoint, training condition, and hemisphere on LC MRI contrast, in younger adults (A) and older adults (B).
| Predictor | Estimate | SE | 95% CI | t | p |
|---|---|---|---|---|---|
| A. Younger adults | |||||
| Intercept | 0.035 | 0.005 | 0.026, 0.044 | 7.657 | <.001 |
| Timepoint | −0.001 | 0.005 | −0.01, 0.009 | −0.115 | 0.909 |
| Condition | 0.001 | 0.009 | −0.017, 0.019 | 0.085 | 0.932 |
| Hemisphere | 0.054 | 0.005 | 0.044, 0.063 | 11.036 | <.001 |
| Timepoint x Condition | −0.021 | 0.010 | −0.04, −0.002 | −2.137 | 0.034 |
| Timepoint x Hemisphere | −0.014 | 0.010 | −0.033, 0.005 | −1.480 | 0.140 |
| Condition x Hemisphere | 0.011 | 0.010 | −0.008, 0.03 | 1.106 | 0.270 |
| Timepoint x Condition x Hemisphere | −0.013 | 0.019 | −0.051, 0.025 | −0.655 | 0.513 |
| B. Older adults | |||||
| Intercept | 0.066 | 0.008 | 0.051, 0.081 | 8.630 | <.001 |
| Timepoint | 0.014 | 0.007 | 0, 0.029 | 2.021 | 0.046 |
| Condition | 0.008 | 0.015 | −0.022, 0.038 | 0.515 | 0.610 |
| Hemisphere | 0.046 | 0.007 | 0.032, 0.06 | 6.366 | <.001 |
| Timepoint x Condition | 0.005 | 0.014 | −0.023, 0.033 | 0.368 | 0.713 |
| Timepoint x Hemisphere | 0.002 | 0.014 | −0.026, 0.031 | 0.170 | 0.865 |
| Condition x Hemisphere | 0.012 | 0.014 | −0.016, 0.041 | 0.867 | 0.388 |
| Timepoint x Condition x Hemisphere | 0.004 | 0.029 | −0.053, 0.06 | 0.122 | 0.903 |
Note. Models included random intercepts for participants. Factors were coded as: timepoint (post-training = 0.5, pre-training = −0.5), condition (Osc+ = 0.5, Osc− = −0.5), hemisphere (left = 0.5, right = −0.5).
Supplementary analyses indicated no significant training condition x timepoint or training condition x timepoint x age group interaction effects on caudal LC contrast (p’s >= .421). For rostral LC contrast, there was a significant training condition x timepoint x age group interaction effect (p = .044) but no significant training condition x timepoint interaction effect (p = 0.764). This 3-way interaction effect was driven by decreases in rostral LC contrast for younger participants in the Osc+ condition and increases for younger participants in the Osc− condition, and the opposite pattern in older participants, but no pairwise comparisons of rostral LC contrast were significant. These results are fully described in the Supplementary Results (Section 3).
3.2. Training oscillatory power was associated with decreases in left LC contrast
Associations between training oscillatory power and change in LC contrast are depicted in Figure 2B. In younger participants, we found a significant negative correlation between training oscillatory power and change in left LC contrast, r(74) = −0.249, 95% CI = −0.449 - −0.025, p = .030, but no significant correlation between training oscillatory power and change in right LC contrast, r(74) = −0.085, 95% CI = −0.305 - 0.143, p = .463. In older participants, training power was not correlated with change in either left LC contrast, r(34) = 0.024, 95% CI = −0.307 - 0.35, p = .889, or right LC contrast, r(34) = 0.076, 95% CI = −0.259 - 0.395, p = .660. We note that the negative association between training power and left LC contrast change in younger adults did not emerge in a linear mixed effects analysis testing the fixed effects of training power, age group, hemisphere and their interactions on LC contrast; specifically, this analysis indicated no significant fixed effects of training power or interaction effects involving training power (p’s > .05; Supplementary Results, Section 4). There were no significant correlations between change in either rostral or caudal LC contrast and training oscillatory power, in either younger or older adults (p’s >= .363; Supplementary Results, Section 4).
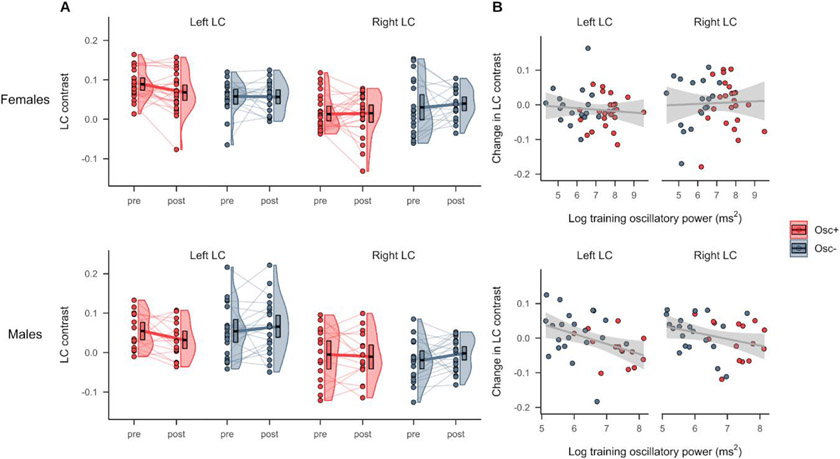
3.3. The association between training oscillatory power and change in left LC contrast was more negative in males
Pre- and post-training LC contrast for younger males and females is shown in Figure 3A. A linear mixed effects analysis testing fixed effects of timepoint, condition, hemisphere, sex and their interactions in younger adults indicated no significant timepoint x condition x sex or timepoint x condition x hemisphere x sex interactions on LC contrast (p’s > 0.05; Supplementary Results, Section 5). We note that this analysis indicated a significant fixed effect of sex on LC contrast (p = .007, d = 0.650), driven by greater LC contrast for females than males, as well as a significant timepoint x condition interaction on LC contrast (p = .032, d = −0.290), in line with what we observed above (Section 3.1). When we next added sex as a fixed effect to the model testing the effects of training oscillatory power and hemisphere on change in LC contrast (Table 3), we found a marginally significant interaction between training power and sex (p = .050, d = 0.470). This was driven by younger males having a more negative association between training power and change in LC contrast than younger females (Figure 3B). Notably, this analysis also indicated a significant fixed effect of sex (p = .048, d = −0.473), with females exhibiting greater decreases in LC contrast relative to males, and a significant fixed effect of training oscillatory power on change in LC contrast (p = .036, d= −0.505), after accounting for the effects of sex and hemisphere.
Figure 3.
(A) LC MRI contrast at the pre- and post-training timepoints for younger participants in the Osc+ and Osc− conditions, stratified by sex (top = females, bottom = males). (B) Associations between change in LC contrast and training oscillatory power among younger participants, stratified by sex (top = females, bottom = males). Linear regression lines with 95% confidence intervals are shown in gray.
Table 3:
Results of linear mixed effects analysis testing the fixed effects of training oscillatory power, hemisphere, and sex on change in LC MRI contrast in younger adults.
| Predictor | Estimate | SE | 95% CI | t | p |
|---|---|---|---|---|---|
| Intercept | 0.080 | 0.039 | 0.004, 0.156 | 2.050 | 0.044 |
| Training power | −0.012 | 0.006 | −0.024, −0.001 | −2.142 | 0.036 |
| Hemisphere | 0.041 | 0.049 | −0.055, 0.137 | 0.838 | 0.405 |
| Sex | −0.157 | 0.078 | −0.309, −0.004 | −2.008 | 0.048 |
| Training power x Hemisphere | −0.008 | 0.007 | −0.022, 0.006 | −1.132 | 0.261 |
| Training power x Sex | 0.023 | 0.012 | 0, 0.046 | 1.993 | 0.050 |
| Hemisphere x Sex | −0.027 | 0.098 | −0.218, 0.165 | −0.273 | 0.785 |
| Training power x Hemisphere x Sex | 0.003 | 0.015 | −0.025, 0.032 | 0.215 | 0.830 |
Note. Models included random intercepts for participants. Factors were coded as: hemisphere (left = 0.5, right = −0.5), sex (female = 0.5, male = −0.5).
3.4. Decreases in left LC contrast were associated with decreases in CREB activity
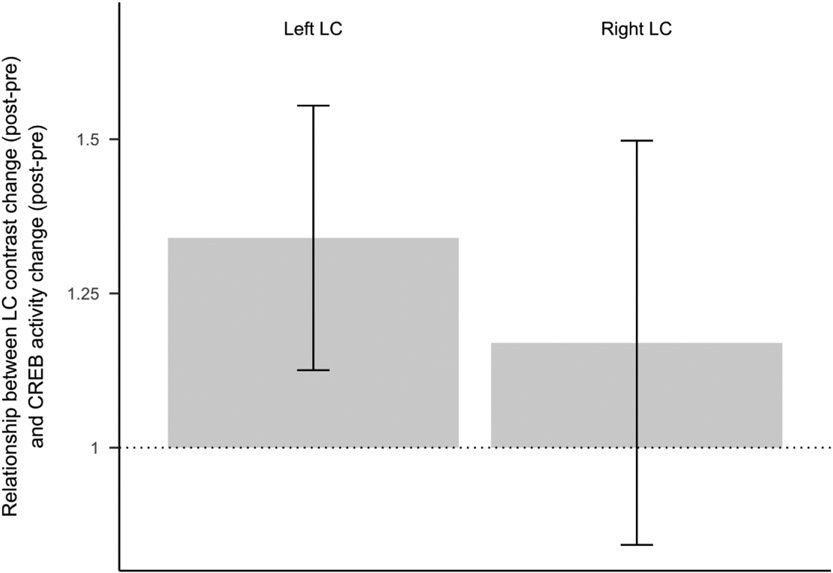
Results of RNA sequencing in younger participants with available blood-based measures indicated a significant interaction between timepoint and condition on expression of genes regulated by the SNS-responsive CREB transcription factor (bootstrap z = −3.30, p = .001). Younger participants in the Osc− condition showed what appears to be a secular trend, with increased CREB activity from pre- to post-training (z = 2.70, p = .008), whereas participants in the Osc+ condition were buffered against that trend, showing no significant change over time (z = −0.45, p = .650).
We also found that greater change in LC contrast was associated with greater change in CREB activity (Figure 4), selectively for left LC contrast (z = 1.97, p = .049), with no significant effect for right LC contrast (z = 0.63, p = .530). In other words, participants with larger decreases in left LC contrast had larger decreases in CREB activity.
Figure 4.
Regression coefficients reflecting the association between LC contrast change (post-training - pre-training) and CREB activity change (post-training - pre-training), for left and right LC separately). Error bars reflect regression coefficient standard errors derived from bootstrap resampling of linear model residual vectors in underlying gene expression data. Figure reflects data from a subset of 39 younger participants for whom both blood-based measures and LC contrast values were available (22 Osc+, 17 Osc−) .
4. Discussion
In recent years, much has been learned about how LC MRI contrast, a proxy for LC structural integrity, relates to cognition across the lifespan (Betts et al., 2019; Dahl et al., 2019; Elman et al., 2021; Liu et al., 2020). However, no published studies have examined factors that influence LC contrast across time. Here, we found that in younger adults, performing 5 weeks of HRV biofeedback training decreased LC contrast. This effect was larger for the left LC and scaled with the extent to which participants increased their heart rate oscillations during training. We also found that among younger participants with available blood-based measures, decreases in left LC contrast were coupled with decreases in activity of the CREB transcription factor that mediates SNS signaling through β-adrenergic receptors (Cole et al., 2010; Mayr & Montminy, 2001). On the contrary, among older adults who completed biofeedback training, we did not observe training effects on LC contrast. Thus, for younger adults, using biofeedback to increase heart rate oscillations in daily training sessions affected LC MRI contrast.
Why might HRV biofeedback training have decreased LC contrast in younger adults? The beneficial effects of HRV biofeedback involving slow breathing are thought to occur through multiple mechanisms, including stimulation of the vagus nerve (Huang et al., 2018; Lehrer & Gevirtz, 2014). The vagus nerve is a major component of the parasympathetic nervous system and sends inputs to the LC via the medullary NTS (Badran et al., 2018; Fornai et al., 2011). The NTS is affected by respiration, with NTS cell firing suppressed during inhalation and facilitated during exhalation (Miyazaki et al., 1998). Thus, the two respiratory phases may affect LC activity differently. In support of this idea, exhalation-gated auricular vagal afferent nerve stimulation elicits greater responses in NTS and LC compared to inhalation-gated stimulation in humans (Garcia et al., 2017; Sclocco et al., 2019). Slow-paced breathing increases the amplitude of oscillations in lung volume and blood pressure (Barnett et al., 2020; Russo et al., 2017) and should therefore increase the amplitude of oscillations in stretch receptor inputs from the lungs and blood vessels to the vagus nerve. Thus, one possibility is thus that the repeated practice of slow, paced breathing promotes phasic while disrupting tonic LC activity. In addition, a cluster of neurons in the medullary preBötzinger complex serves as a major breathing rhythm generator and provides excitatory input to the LC; when breathing is slow, the preBötzinger cluster provides less excitatory input to the LC, promoting lower tonic levels of arousal (Yackle et al., 2017). These slow-breathing effects would have the net effect of shifting LC activity to a higher phasic and lower tonic level, which would manifest as lower LC MRI signal contrast and reduced sympathetic activity. The association we observed between decreases in LC contrast and decreases in activity of the CREB transcription factor are consistent with the notion of decreased LC contrast in younger adults reflecting decreased cumulative noradrenergic activity during the intervention time frame.
More broadly, our effects may be accounted for by an overall shift to parasympathetic dominance that occurs with the repeated practice of HRV biofeedback training. The LC receives projections from the medulla’s nucleus paragigantocellularis (Aston-Jones et al., 1986; Aston-Jones et al., 1991), which itself receives widespread autonomic inputs and has been implicated in the regulation and control of sympathetic activity and respiration (Van Bockstaele & Aston-Jones, 1995). Parasympathetic/sympathetic balance is then expected to directly impact the LC. As correlational evidence for this idea in humans, we previously found that HRV was negatively associated with LC MRI contrast in younger adults (Mather et al., 2017). In addition, LC efferent projections provide excitatory control over preganglionic sympathetic neurons and inhibitory control over the parasympathetic dorsal motor vagal nucleus and nucleus ambiguus (Samuels & Szabadi, 2008). Having relatively lower LC structural integrity would therefore give rise to less excitatory input to sympathetic centers and reduced inhibition of parasympathetic centers, as well as reduced excitatory input to the central nucleus of the amygdala by LC neurons, which also contribute to sympathetic activation (Wood et al., 2017).
We found that the effects of biofeedback were larger for the left than the right LC. Decreases in LC contrast for participants in the Osc+ condition were greater for the left than the right LC, and significant associations with training oscillatory power and CREB activity were observed for the left, but not the right, LC. Previous studies have reported higher MRI-assessed LC integrity in the left compared to the right LC (Betts et al., 2017; Dahl et al., 2019; Galgani et al., 2023; Giorgi et al., 2022; Liu et al., 2019). Our findings are also in line with reports of more positive associations between LC contrast and cortical thickness for left relative to right LC (Bachman et al., 2021), as well as hemispheric differences in functional connectivity of the LC (Jacobs et al., 2018).
We also observed sex differences in how training oscillatory power related to change in LC contrast among younger participants, with males exhibiting a more negative association between training power and change in LC contrast than females. Relative to that of males, the female LC exhibits morphological and functional differences: LC neurons are more sensitive to corticotropin releasing factor (Bangasser et al., 2016) and exhibit greater dendritic density and branching (Bangasser et al., 2011; Ross & Van Bockstaele, 2020) in females. In line with previous reports of higher MRI-assessed measures of LC integrity in females than males (Bachman et al., 2021; Galgani et al., 2023; Riphagen et al., 2020), we found that younger females had higher LC contrast than males across conditions, hemispheres and timepoints. Our findings of change being more coupled with training oscillatory power in younger males than females suggests that there are sex differences in the factors that shape LC contrast over time, which warrants further investigation.
Although we observed differential effects of the two HRV biofeedback training conditions on LC contrast among younger participants, this was not the case among older participants. Instead, among older participants, there was an overall increase in LC contrast from pre- to post-training. The similar changes seen across the two conditions raise the possibility that older adults’ LC contrast levels were sensitive to an aspect of the intervention not explored here and present in both training conditions. Yet the older cohort was small in size and mostly female and may not have been sufficiently powered to detect condition-specific effects. It is also possible that baseline differences in stress or emotional well-being between the younger and older adults contributed to the age differences in the impact of the interventions on LC contrast. The lack of condition effects we observed for older participants should therefore be interpreted with caution.
There are several other limitations to note. First, RNA sequencing analyses included only a subset of younger participants as we started collecting blood samples after some participants had completed the study. In addition, activity of the CREB transcription factor is not a direct measure of noradrenergic activity. Second, participants in this study included mostly university students, limiting the external validity of results and potentially introducing a secular trend towards greater SNS activity as the 7-week study progressed; we aimed to avoid semester breaks in the study and therefore, across conditions, enrolled most younger participants at the beginning of semesters, when there are usually fewer exams and deadlines relative to later weeks in the semester.
Another limitation is that we did not test the reliability of the LC contrast measure within the context of our study. However, we previously found an ICC of 0.87 for LC contrast measured with a similar sequence (Dahl et al., 2019, see Supplementary Information p. 15-16), and other published studies indicate moderate-to-high reliability of LC contrast (Betts et al., 2017; Langley et al., 2017; Tona et al., 2017). Another limitation is that, while the older adults in this study were living independently in the community and had TELE scores ≥16, we did not employ more sensitive cognitive screening. The TELE screens for possible dementia using a brief telephone-based assessment but it does not rule out potential mild cognitive impairment. Thus, our older cohort may have included participants with mild cognitive impairment, which could have affected results. For instance, greater levels of Alzheimer’s disease pathology in the LC may decrease its sensitivity to interventions such as heart rate variability biofeedback.
We hope that some of the questions raised by our study can be addressed in future research. For instance, our study was not well-suited to examine the relationship between changes in anxiety and changes in LC MRI contrast, as our previously published findings with younger participants indicated that participants expected both interventions to improve their well-being (Nashiro et al., 2023). While it was a strength of our study that the comparison group yielded similar expectations, the expectations that the interventions would have positive effects may have driven the decreases in negative mood and depression seen in both groups (see Nashiro et al., 2023) and may have obscured effects of the interventions themselves on self-reported mood, depression and anxiety. Meta analyses of previous studies indicate that HRV biofeedback reduces depression and anxiety (Goessl et al., 2017; Lehrer et al., 2020; Pizzoli et al., 2021), but previous studies have not had such well-matched expectations in their comparison groups so it remains to be seen how much previous findings were driven by participant expectations. In future studies, to be able to examine the role of LC contrast changes in anxiety changes, it would be helpful to avoid inducing expectations of emotional changes (by, for instance, examining these relationships in a study in which the main outcome is cognitive performance).
Another important question for future research is whether longer interventions might yield larger effects. Our study only encompassed 5 weeks of HRV biofeedback training, whereas training over longer time periods may yield larger effects on LC contrast in both hemispheres and in younger and older participants. With larger samples that provide sufficient power for mediation analyses (Fritz & MacKinnon, 2007), it would also be interesting to examine variables that might mediate changes in LC MRI contrast.
Of course, a major question to address in future research is what these changes in LC MRI contrast represent on a cellular level. As discussed in the Introduction, current research suggests that they represent some change in the macromolecular-to-free-water fraction within the LC (Priovoulos et al., 2020; Trujillo et al., 2017; Watanabe, 2022; Watanabe et al., 2019). It is possible that HRV biofeedback affects the macromolecular-to-free-water fraction within the LC by affecting LC tonic levels of activity or by affecting inflammation in this region. Future research should include additional measures to provide insight into these and other potential pathways.
5. Conclusions
In summary, in this study, we assessed the effect of performing 5 weeks of heart rate variability biofeedback training on LC contrast, a measure that has been linked to cognition in older adults and arousal and negative affect in younger adults. We found that training decreased left LC contrast among younger participants and this effect scaled with the extent to which participants increased their heart rate oscillations during training. Furthermore, decreases in left LC contrast were related to decreases in CREB activity, a marker of sympathetic nervous system activity. These results provide novel evidence that among younger adults, LC contrast can be changed through the daily practice of increasing heart rate oscillations. More generally, these results provide new evidence suggesting that autonomic activity can influence LC contrast. Understanding the relationships between autonomic activity and LC contrast is important for better understanding the underlying mechanisms that lead to low or high LC contrast in childhood and young adulthood, before Alzheimer’s-disease-related pathology has had an impact.
Supplementary Material
Highlights.
Heart rate variability (HRV) biofeedback decreased locus coeruleus (LC) MRI contrast in younger adults.
Increases in HRV during biofeedback were related to decreases in left LC contrast.
Left LC contrast decreases were related to CREB transcription factor activity decreases.
Acknowledgments
We are grateful to Sumedha Attanti and Juliana Lee who performed manual LC delineation and the following individuals who helped collect data for the project: Collin Amano, Kathryn Cassutt, Christine Cho, Paul Choi, Akanksha Jain, Katherine Jeung, Sophia Ling, Althea Wolfe, Michelle Wong, Yong Zhang, and Cyndy Zhuang. We also thank Martin Dahl for advice on LC delineation.
This work was supported by National Science Foundation grant number DGE-1842487, and by the National Institute on Aging of the National Institutes of Health under award numbers R01AG057184, P30AG017265, and T32AG000037. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- CREB
cAMP-responsive element binding protein
- HRV
heart rate variability
- LC
locus coeruleus
- MPRAGE
magnetization prepared rapid acquisition gradient echo
- MRI
magnetic resonance imaging
- NTS
nucleus tractus solitarii
- SD
standard deviation
- SNS
sympathetic nervous system
- TSE
turbo spin echo
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data and Code Availability
Data are available at: https://openneuro.org/datasets/ds003823. Code for statistical analysis is available at: https://github.com/EmotionCognitionLab/HRV-LC.
Declaration of Interest
Declarations of interest: none.
Welch’s t-tests indicated that participants who were included versus excluded from analysis did not differ significantly in terms of age, t(57.0) = 0.23, p = .817, or education level, t(64.8) = 0.57, p = .571. A Chi-squared test of independence indicated that the distribution of females versus males differed when comparing participants who were included versus excluded for the purpose of analysis, χ2(3) = 86.85, p < .001; in particular, 58.1% of participants included for analysis were females, 43.6% of participants excluded from analysis were females.
References
- Aston-Jones G, Ennis M, Pieribone V, Nickell W, & Shipley M (1986). The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science, 234(4777), 734–737. 10.1126/science.3775363 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, & Astier B (1991). Afferent regulation of locus coeruleus neurons: Anatomy, physiology and pharmacology. Progress in Brain Research, 88, 4775. 10.1016/s0079-6123(08)63799-1 [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, & Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–2044. 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman SL, Dahl MJ, Werkle-Bergner M, Düzel S, Forlim CG, Lindenberger U, Kühn S, & Mather M (2021). Locus coeruleus MRI contrast is associated with cortical thickness in older adults. Neurobiology of Aging, 100, 72–82. 10.1016/j.neurobiolaging.2020.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran BW, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, Brown JC, DeVries WH, Austelle CW, McTeague LM, & George MS (2018). Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimulation, 11(3), 492–500. 10.1016/j.brs.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KG, Trk I, Hornung J-P, & Halasz P (1989). The human locus coeruleus complex: An immunohistochemical and three dimensional reconstruction study. Experimental Brain Research, 77(2), 257–270. 10.1007/BF00274983 [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR, & Khantsis S (2016). Sex differences in the locus coeruleus- system and its regulation by stress. Brain Research, 1641, 177188. 10.1016/j.brainres.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, & Valentino RJ (2011). Sexual dimorphism in locus coeruleus dendritic morphology: A structural basis for sex differences in emotional arousal. Physiology & Behavior, 103(3-4), 342–351. 10.1016/j.physbeh.2011.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett WH, Latash EM, Capps RA, Dick TE, Wehrwein EA, & Molkov YI (2020). Traube–Hering waves are formed by interaction of respiratory sinus arrhythmia and pulse pressure modulation in healthy men. Journal of Applied Physiology, 129(5), 1193–1202. 10.1152/japplphysiol.00452.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1). 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bell TR, Elman JA, Beck A, Fennema-Notestine C, Gustavson DE, Hagler DJ, Jack AJ, Lyons MJ, Puckett OK, Toomey R, Franz CE, & Kremen WS (2022). Rostral-middle locus coeruleus integrity and subjective cognitive decline in early old age. Journal of the International Neuropsychological Society, 1–12. 10.1017/S1355617722000881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar MS, Lüdecke D, & Makowski D (2020). effectsize: Estimation of effect size indices and standardized parameters. Journal of Open Source Software, 5(56), 2815. 10.21105/joss.02815 [DOI] [Google Scholar]
- Berridge CW, & Waterhouse BD (2003). The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews, 42(1), 33–84. 10.1016/S0165-0173(03)00143-7 [DOI] [PubMed] [Google Scholar]
- Betts MJ, Cardenas-Blanco A, Kanowski M, Jessen F, & Düzel E (2017). In vivo MRI assessment of the human locus coeruleus along its rostrocaudal extent in young and older adults. NeuroImage, 163, 150–159. 10.1016/j.neuroimage.2017.09.042 [DOI] [PubMed] [Google Scholar]
- Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-Cabronero J, Callaghan MF, Lambert C, Cardenas-Blanco A, Pine K, Passamonti L, Loane C, Keuken MC, Trujillo P, Lüsebrink F, Mattern H, Liu KY, Priovoulos N, Fliessbach K, Dahl MJ, … Hämmerer D (2019). Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain, 142(9), 2558–2571. 10.1093/brain/awz193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, & Tredici KD (2011). Stages of the pathologic process in alzheimer disease: Age categories from 1 to 100 years. Journal of Neuropathology & Experimental Neurology, 70(11), 960969. [DOI] [PubMed] [Google Scholar]
- Brown HJ, Peng L, Harada JN, Walker JR, Cole S, Lin S-F, Zack JA, Chanda SK, & Sun R (2010). Gene Expression and Transcription Factor Profiling Reveal Inhibition of Transcription Factor cAMP-response Element-binding Protein by γ-Herpesvirus Replication and Transcription Activator. Journal of Biological Chemistry, 285(33), 25139–25153. 10.1074/jbc.M110.137737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RP, & Gerbarg PL (2005). Sudarshan Kriya Yogic Breathing in the Treatment of Stress, Anxiety, and Depression: Part I Neurophysiologic Model. The Journal of Alternative and Complementary Medicine, 11(1), 189–201. 10.1089/acm.2005.11.189 [DOI] [PubMed] [Google Scholar]
- Cassidy CM, Zucca FA, Girgis RR, Baker SC, Weinstein JJ, Sharp ME, Bellei C, Valmadre A, Vanegas N, Kegeles LS, Brucato G, Kang UJ, Sulzer D, Zecca L, Abi-Dargham A, & Horga G (2019). Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proceedings of the National Academy of Sciences, 116(11), 5108–5117. 10.1073/pnas.1807983116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalermpalanupap T, Weinshenker D, & Rorabaugh JM (2017). Down but not out: The consequences of pretangle tau in the locus coeruleus. Neural Plasticity, 2017, 19. 10.1155/2017/7829507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WT, Wang W, Zaborszky L, Golde TE, DeKosky S, Duara R, Loewenstein DA, Adjouadi M, Coombes SA, & Vaillancourt DE (2022). Association of Cognitive Impairment With Free Water in the Nucleus Basalis of Meynert and Locus Coeruleus to Transentorhinal Cortex Tract. Neurology, 98(7), e700–e710. 10.1212/WNL.0000000000013206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, & Seeman TE (2010). Computational identification of gene-social environment interaction at the human IL6 locus. Proceedings of the National Academy of Sciences, 107(12), 5681–5686. 10.1073/pnas.0911515107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Shanahan MJ, Gaydosh L, & Harris KM (2020). Population-based RNA profiling in Add Health finds social disparities in inflammatory and antiviral gene regulation to emerge by young adulthood. Proceedings of the National Academy of Sciences, 117(9), 4601–4608. 10.1073/pnas.1821367117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, & Zack JA (2005). Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics (Oxford, England), 21(6), 803–810. 10.1093/bioinformatics/bti038 [DOI] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Düzel S, Bodammer NC, Lindenberger U, Kühn S, & Werkle-Bergner M (2019). Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nature Human Behaviour, 3(11), 1203–1214. 10.1038/s41562-019-0715-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Werkle-Bergner M, Kennedy BL, Guzman S, Hurth K, Miller CA, Qiao Y, Shi Y, Chui HC, & Ringman JM (2022). Locus coeruleus integrity is related to tau burden and memory loss in autosomal-dominant Alzheimer’s disease. Neurobiology of Aging, 112, 39–54. 10.1016/j.neurobiolaging.2021.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Puckett OK, Beck A, Fennema-Notestine C, Cross LK, Dale AM, Eglit GML, Eyler LT, Gillespie NA, Granholm EL, Gustavson DE, Hagler DJ, Hatton SN, Hauger R, Jak AJ, Logue MW, McEvoy LK, McKenzie RE, Neale MC, … Kremen WS (2021). MRI-assessed locus coeruleus integrity is heritable and associated with multiple cognitive domains, mild cognitive impairment, and daytime dysfunction. Alzheimer’s & Dementia, 17, 1017–1025. https://doi.org/ 10.1002/alz.12261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Ruffoli R, Giorgi FS, & Paparelli A (2011). The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation: Locus coeruleus and vagus nerve stimulation. European Journal of Neuroscience, 33(12), 2169–2178. 10.1111/j.1460-9568.2011.07707.x [DOI] [PubMed] [Google Scholar]
- Fritz MS, & MacKinnon DP (2007). Required sample size to detect the mediated effect. Psychological Science, 18(3), 233–239. 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgani A, Lombardo F, Martini N, Vergallo A, Bastiani L, Hampel H, Hlavata H, Baldacci F, Tognoni G, De Marchi D, Ghicopulos I, De Cori S, Biagioni F, Busceti CL, Ceravolo R, Bonuccelli U, Chiappino D, Siciliano G, Fornai F, … Giorgi FS (2023). Magnetic resonance imaging Locus Coeruleus abnormality in amnestic Mild Cognitive Impairment is associated with future progression to dementia. European Journal of Neurology, 30(1), 32–46. 10.1111/ene.15556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RG, Lin RL, Lee J, Kim J, Barbieri R, Sclocco R, Wasan AD, Edwards RR, Rosen BR, Hadjikhani N, & Napadow V (2017). Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain, 158(8), 1461–1472. 10.1097/j.pain.0000000000000930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, & Pedersen N (1995). An Empirical Test of Telephone Screening to Identify Potential Dementia Cases. International Psychogeriatrics, 7(3), 429–438. 10.1017/S1041610295002171 [DOI] [PubMed] [Google Scholar]
- Giorgi FS, Martini N, Lombardo F, Galgani A, Bastiani L, Della Latta D, Hlavata H, Busceti CL, Biagioni F, Puglisi-Allegra S, Pavese N, & Fornai F (2022). Locus Coeruleus magnetic resonance imaging: a comparison between native-space and template-space approach. Journal of Neural Transmission, 129(4), 387–394. 10.1007/s00702-022-02486-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessl VC, Curtiss JE, & Hofmann SG (2017). The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychological Medicine, 47(15), 25782586. 10.1017/S0033291717001003 [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Callaghan MF, Hopkins A, Kosciessa J, Betts M, Cardenas-Blanco A, Kanowski M, Weiskopf N, Dayan P, Dolan RJ, & Düzel E (2018). Locus coeruleus integrity in old age is selectively related to memories linked with salient negative events. Proceedings of the National Academy of Sciences, 115(9), 2228–2233. 10.1073/pnas.1712268115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW, Walling SG, Yuan Q, & Martin GM (2021). The ‘a, b, c’s of pretangle tau and their relation to aging and the risk of Alzheimer’s Disease. Seminars in Cell & Developmental Biology, 116, 125–134. 10.1016/j.semcdb.2020.12.010 [DOI] [PubMed] [Google Scholar]
- Harley CW, & Yuan Q (2021). Locus coeruleus optogenetic modulation: Lessons learned from temporal patterns. Brain Sciences, 11(12), 1624. 10.3390/brainsci11121624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Chen Y, LeWitt PA, Yan F, & Haacke EM (2022). Response to “Neuromelanin? MRI of Noradrenergic and Dopaminergic Neurons.” Journal of Magnetic Resonance Imaging, jmri.28481 10.1002/jmri.28481 [DOI] [PubMed] [Google Scholar]
- Huang C, Gevirtz RN, Onton J, & Criado JR (2018). Investigation of vagal afferent functioning using the Heartbeat Event Related Potential. International Journal of Psychophysiology, 131, 113–123. 10.1016/j.ijpsycho.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Becker JA, Kwong K, Engels-Domínguez N, Prokopiou PC, Papp KV, Properzi M, Hampton OL, d’Oleire Uquillas F, Sanchez JS, Rentz DM, El Fakhri G, Normandin MD, Price JC, Bennett DA, Sperling RA, & Johnson KA (2021). In vivo and neuropathology data support locus coeruleus integrity as indicator of alzheimer’s disease pathology and cognitive decline. Science Translational Medicine, 13(612), eabj2511. 10.1126/scitranslmed.abj2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Müller-Ehrenberg L, Priovoulos N, & Roebroeck A (2018). Curvilinear locus coeruleus functional connectivity trajectories over the adult lifespan: A 7T MRI study. Neurobiology of Aging, 69, 167–176. 10.1016/j.neurobiolaging.2018.05.021 [DOI] [PubMed] [Google Scholar]
- Jester DJ, Rozek EK, & McKelley RA (2019). Heart rate variability biofeedback: Implications for cognitive and psychiatric effects in older adults. Aging & Mental Health, 23(5), 574580. 10.1080/13607863.2018.1432031 [DOI] [PubMed] [Google Scholar]
- Keren NI, Taheri S, Vazey EM, Morgan PS, Granholm A-CE, Aston-Jones GS, & Eckert MA (2015). Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. NeuroImage, 113, 235–245. 10.1016/j.neuroimage.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao S, Matsusue E, Fujii S, Miyoshi F, Kaminou T, Kato S, Ito H, & Ogawa T (2013). Correlation between pathology and neuromelanin MR imaging in Parkinson’s disease and dementia with Lewy bodies. Neuroradiology, 55(8), 947–953. 10.1007/s00234-013-1199-9 [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13). 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Langley J, Huddleston DE, Liu CJ, & Hu X (2017). Reproducibility of locus coeruleus and substantia nigra imaging with neuromelanin sensitive MRI. Magnetic Resonance Materials in Physics, Biology and Medicine, 30(2), 121–125. 10.1007/s10334-016-0590-z [DOI] [PubMed] [Google Scholar]
- Lehrer PM, & Gevirtz R (2014). Heart rate variability biofeedback: How and why does it work? Frontiers in Psychology, 5. 10.3389/fpsyg.2014.00756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer P, Kaur K, Sharma A, Shah K, Huseby R, Bhavsar J, Sgobba P, & Zhang Y (2020). Heart Rate Variability Biofeedback Improves Emotional and Physical Health and Performance: A Systematic Review and Meta Analysis. Applied Psychophysiology and Biofeedback, 45(3), 109–129. 10.1007/s10484-020-09466-z [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu S-E, Eckberg DL, Edelberg R, Shih WJ, Lin Y, Kuusela TA, Tahvanainen KUO, & Hamer RM (2003). Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine, 65(5), 796805. 10.1097/01.PSY.0000089200.81962.19 [DOI] [PubMed] [Google Scholar]
- Lenth RV (2021). Emmeans: Estimated marginal means, aka least-squares means. https://CRAN.R-project.org/package=emmeans [Google Scholar]
- Liu KY, Acosta-Cabronero J, Cardenas-Blanco A, Loane C, Berry AJ, Betts MJ, Kievit RA, Henson RN, Düzel E, Howard R, & Hämmerer D (2019). In vivo visualization of age-related differences in the locus coeruleus. Neurobiology of Aging, 74, 101–111. 10.1016/j.neurobiolaging.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, Kievit RA, Tsvetanov KA, Betts MJ, Düzel E, Rowe JB, Howard R, & Hämmerer D (2020). Noradrenergic-dependent functions are associated with age-related locus coeruleus signal intensity differences. Nature Communications, 11(1), 1712. 10.1038/s41467-020-15410-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, Marijatta F, Hämmerer D, Acosta-Cabronero J, Düzel E, & Howard RJ (2017). Magnetic resonance imaging of the human locus coeruleus: A systematic review. Neuroscience & Biobehavioral Reviews, 83, 325–355. 10.1016/j.neubiorev.2017.10.023 [DOI] [PubMed] [Google Scholar]
- Lorton D, & Bellinger D (2015). Molecular mechanisms underlying β-Adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. International Journal of Molecular Sciences, 16(12), 5635–5665. 10.3390/ijms16035635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey AM, Robertson IH, Lawlor B, Mohan A, & Vanneste S (2021). Sex differences in locus coeruleus: A heuristic approach that may explain the increased risk of Alzheimer’s disease in females. Journal of Alzheimer’s Disease, 83(2), 505–522. 10.3233/JAD-210404 [DOI] [PubMed] [Google Scholar]
- MacAulay N. (2021). Molecular mechanisms of brain water transport. Nature Reviews Neuroscience, 22(6), 326–344. 10.1038/s41583-021-00454-8 [DOI] [PubMed] [Google Scholar]
- Mather M, Joo Yoo H, Clewett DV, Lee T-H, Greening SG, Ponzio A, Min J, & Thayer JF (2017). Higher locus coeruleus MRI contrast is associated with lower parasympathetic influence over heart rate variability. NeuroImage, 150, 329–335. 10.1016/j.neuroimage.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr B, & Montminy M (2001). Transcriptional regulation by the phosphorylation-dependent factor CREB. Nature Reviews Molecular Cell Biology, 2(8), 599–609. 10.1038/35085068 [DOI] [PubMed] [Google Scholar]
- McCall Jordan G., Al-Hasani R, Siuda Edward R., Hong Daniel Y., Norris Aaron J., Ford Christopher P., & Bruchas Michael R. (2015). CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron, 87(3), 605–620. 10.1016/j.neuron.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Arata A, Tanaka I, & Ezure K (1998). Activity of rat pump neurons is modulated with central respiratory rhythm. Neuroscience Letters, 249(1), 61–64. 10.1016/S0304-3940(98)00402-9 [DOI] [PubMed] [Google Scholar]
- Morris LS, Tan A, Smith DA, Grehl M, Han-Huang K, Naidich TP, … Kundu P (2020). Sub-millimeter variation in human locus coeruleus is associated with dimensional measures of psychopathology: An in vivo ultra-high field 7-Tesla MRI study. NeuroImage: Clinical, 25(September 2019), 102148. 10.1016/j.nicl.2019.102148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy JS, Larsson DEO, Garfinkel SN, & Critchley HD (2019). Heart rate variability as a biomarker in health and affective disorders: A perspective on neuroimaging studies. NeuroImage, 202, 116072. 10.1016/j.neuroimage.2019.116072 [DOI] [PubMed] [Google Scholar]
- Nashiro K, Min J, Yoo HJ, Cho C, Bachman SL, Dutt S, Thayer JF, Lehrer PM, Feng T, Mercer N, Nasseri P, Wang D, Chang C, Marmarelis VZ, Narayanan S, Nation DA, & Mather M (2023). Increasing coordination and responsivity of emotion-related brain regions with a heart rate variability biofeedback randomized trial. Cognitive, Affective, & Behavioral Neuroscience, 23, 66–83. 10.3758/s13415-022-01032-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østby I, Øyehaug L, Einevoll GT, Nagelhus EA, Plahte E, Zeuthen T, Lloyd CM, Ottersen OP, & Omholt SW (2009). Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLoS Computational Biology, 5(1), e1000272. 10.1371/journal.pcbi.1000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzoli SFM, Marzorati C, Gatti D, Monzani D, Mazzocco K, & Pravettoni G (2021). A meta-analysis on heart rate variability biofeedback and depressive symptoms. Scientific Reports, 11(1), 6650. 10.1038/s41598-021-86149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, & Cole SW (2013). Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via -adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences, 110(41), 16574–16579. 10.1073/pnas.1310655110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priovoulos N, van Boxel SCJ, Jacobs HIL, Poser BA, Uludag K, Verhey FRJ, & Ivanov D (2020). Unraveling the contributions to the neuromelanin-MRI contrast. Brain Structure and Function, 225(9), 2757–2774. 10.1007/s00429-020-02153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Rajkowska G & Goldman-Rakic PS (1995). Cytoarchitectonic Definition of Prefrontal Areas in the Normal Human Cortex: I. Remapping of Areas 9 and 46 using Quantitative Criteria. Cerebral Cortex, 5(4), 307–322. 10.1093/cercor/5.4.307 [DOI] [PubMed] [Google Scholar]
- Ramon-Moliner E. (1974). The locus coeruleus of cat: III. Light and electron microscopic studies. Cell and Tissue Research, 149(2). 10.1007/BF00222274 [DOI] [PubMed] [Google Scholar]
- Riphagen JM, van Hooren RWE, Pagen LHG, Poser BA, & Jacobs HIL (2020). Rostro-caudal locus coeruleus integrity differences vary with age and sex using ultra-high field imaging: Neuroimaging / Optimal neuroimaging measures for early detection. Alzheimer’s & Dementia, 16(S5). 10.1002/alz.046722 [DOI] [Google Scholar]
- Roseboom PH, & Klein DC (1995). Norepinephrine stimulation of pineal cyclic AMP response element-binding protein phosphorylation: Primary role of a beta-adrenergic receptor/cyclic AMP mechanism. Molecular Pharmacology, 47(3), 439–449. [PubMed] [Google Scholar]
- Ross JA, & Van Bockstaele EJ (2020). The role of catecholamines in modulating responses to stress: Sex-specific patterns, implications, and therapeutic potential for post-traumatic stress disorder and opiate withdrawal. European Journal of Neuroscience, 215, ejn.14714. 10.1111/ejn.14714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MA, Santarelli DM, & O’Rourke D (2017). The physiological effects of slow breathing in the healthy human. Breathe, 13(4), 298–309. 10.1183/20734735.009817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels E, & Szabadi E (2008). Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part I: Principles of functional organisation. Current Neuropharmacology, 6(3), 235–253. 10.2174/157015908785777229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ (2009). The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews Neuroscience, 10(3), 211–223. 10.1038/nrn2573 [DOI] [PubMed] [Google Scholar]
- Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, Takahashi S, Ehara S, Terayama Y, & Sakai A (2006). Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in parkinson’s disease. Neuroreport, 17(11), 1215–1218. 10.1097/01.wnr.0000227984.84927.a7 [DOI] [PubMed] [Google Scholar]
- Schwarz LA, & Luo L (2015). Organization of the locus coeruleus-norepinephrine system. Current Biology, 25(21), R1051–6. 10.1016/j.cub.2015.09.039 [DOI] [PubMed] [Google Scholar]
- Sclocco R, Garcia RG, Kettner NW, Isenburg K, Fisher HP, Hubbard CS, Ay I, Polimeni JR, Goldstein J, Makris N, Toschi N, Barbieri R, & Napadow V (2019). The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: A multimodal ultrahigh-field (7T) fMRI study. Brain Stimulation, 12(4), 911–921. 10.1016/j.brs.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz AJ, & Greenberg ME (1999). CREB: A Stimulus-Induced Transcription Factor Activated by A Diverse Array of Extracellular Signals. Annual Review of Biochemistry, 68(1), 821–861. 10.1146/annurev.biochem.68.1.821 [DOI] [PubMed] [Google Scholar]
- Sherpa AD, Xiao F, Joseph N, Aoki C, & Hrabetova S (2016). Activation of β-adrenergic receptors in rat visual cortex expands astrocytic processes and reduces extracellular space volume: ECS AND ASTROCYTES DURING βAR ACTIVATION. Synapse, 70(8), 307–316. 10.1002/syn.21908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heartbrain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33(2), 8188. 10.1016/j.neubiorev.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 20116. 10.1016/s0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thonberg H, Fredriksson JM, Nedergaard J, & Cannon B (2002). A novel pathway for adrenergic stimulation of cAMP-response-element-binding protein (CREB) phosphorylation: Mediation via α1-adrenoceptors and protein kinase C activation. Biochemical Journal, 364(1), 73–79. 10.1042/bj3640073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona K-D, Keuken MC, de Rover M, Lakke E, Forstmann BU, Nieuwenhuis S, & van Osch MJP (2017). In vivo visualization of the locus coeruleus in humans: Quantifying the test–retest reliability. Brain Structure and Function, 222(9), 4203–4217. https://doi.org/czz [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo P, Summers PE, Ferrari E, Zucca FA, Sturini M, Mainardi LT, Cerutti S, Smith AK, Smith SA, Zecca L, & Costa A (2017). Contrast mechanisms associated with neuromelanin-MRI. Magnetic Resonance in Medicine, 78(5), 1790–1800. 10.1002/mrm.26584 [DOI] [PubMed] [Google Scholar]
- Trujillo P, Petersen KJ, Cronin MJ, Lin Y-C, Kang H, Donahue MJ, Smith SA, & Claassen DO (2019). Quantitative magnetization transfer imaging of the human locus coeruleus. NeuroImage, 200, 191–198. 10.1016/j.neuroimage.2019.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, & Aston-Jones G (1995). Integration in the ventral medulla and coordination of sympathetic, pain and arousal functions. Clinical and Experimental Hypertension (New York, N.Y.: 1993), 17(1-2), 153–165. 10.3109/10641969509087062 [DOI] [PubMed] [Google Scholar]
- Watanabe T. (2022). Neuromelanin? MRI of Noradrenergic and Dopaminergic Neurons. Journal of Magnetic Resonance Imaging, jmri.28479. 10.1002/jmri.28479 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Wang X, Tan Z, & Frahm J (2019). Magnetic resonance imaging of brain cell water. Scientific Reports, 9(1), 1–14. 10.1038/s41598-019-41587-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, Perschel FH, Arck PC, & Deter HC (2010). Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. European Journal of Applied Physiology, 109(2), 201211. 10.1007/s00421-009-1341-x [DOI] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, & Bennett DA (2013). Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology, 80(13), 1202–1208. 10.1212/WNL.0b013e3182897103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CS, Valentino RJ, & Wood SK (2017). Individual differences in the locus coeruleus-norepinephrine system: Relevance to stress-induced cardiovascular vulnerability. Physiology & Behavior, 172, 40–48. 10.1016/j.physbeh.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, & Nedergaard M (2013). Sleep Drives Metabolite Clearance from the Adult Brain. Science, 342(6156), 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, Luo L, & Krasnow MA (2017). Breathing control center neurons that promote arousal in mice. Science, 355(6332), 14111415. 10.1126/science.aai7984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ycaza Herrera A, Wang J, & Mather M (2019). The gist and details of sex differences in cognition and the brain: How parallels in sex differences across domains are shaped by the locus coeruleus and catecholamine systems. Progress in Neurobiology, 176, 120–133. 10.1016/j.pneurobio.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Rua C, O’Callaghan C, Jones PS, Hezemans FH, Kaalund SS, Tsvetanov KA, Rodgers CT, Williams G, Passamonti L, & Rowe JB (2021). An in vivo probabilistic atlas of the human locus coeruleus at ultra-high field. NeuroImage, 225, 117487. 10.1016/j.neuroimage.2020.117487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, O’Callaghan C, Rua C, Hezemans FH, Holland N, Malpetti M, Jones PS, Barker RA, Williams-Gray CH, Robbins TW, Passamonti L, & Rowe J (2022). Locus Coeruleus Integrity from 7 T MRI Relates to Apathy and Cognition in Parkinsonian Disorders. Movement Disorders, 37(8), 1663–1672. 10.1002/mds.29072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HJ, Nashiro K, Min J, Cho C, Bachman SL, Nasseri P, Porat S, Dutt S, Grigoryan V, Choi P, Thayer JF, Lehrer PM, Chang C, & Mather M (2022). Heart rate variability (HRV) changes and cortical volume changes in a randomized trial of five weeks of daily HRV biofeedback in younger and older adults. International Journal of Psychophysiology, 181, 50–63. 10.1016/j.ijpsycho.2022.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HJ, Nashiro K, Min J, Cho C, Mercer N, Bachman SL, Nasseri P, Dutt S, Porat S, Choi P, Zhang Y, Grigoryan V, Feng T, Thayer JF, Lehrer P, Chang C, Stanley JA, Head E, Rouanet J, … Mather M (2023). Multimodal neuroimaging data from a 5-week heart rate variability biofeedback randomized clinical trial. Scientific Data, 10(1), 503. 10.1038/s41597-023-02396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, & Gerig G (2006). User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage, 31(3), 1116–1128. 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.