Abstract
Children born to mothers living with HIV may experience greater risk of poor growth and development outcomes than their HIV-unexposed peers. Few studies have examined the relationship between maternal depression and social support with infant growth and development in the context of HIV. We conducted a prospective cohort study of 2,298 pregnant women living with HIV in Dar es Salaam, Tanzania, assessing antenatal depression (Hopkins Symptoms Checklist-25) and social support (Duke–UNC Functional Social Support Questionnaire) at 12–27 weeks of gestation. At one-year age, infant anthropometry and caregiver-reported infant development were assessed. Generalized estimating equations were used to assess mean differences (MD) and relative risks (RR) for growth and developmental outcomes. Symptoms consistent with maternal antenatal depression had 67% prevalence and were associated with infant wasting (RR 2.61; 95% confidence interval (CI) 1.03–6.65; z = 2.02; p = 0.04), but no other growth or developmental outcomes. Greater maternal social support was not associated with infant growth outcomes. Greater affective support was associated with better cognitive (MD 0.18; CI 0.01–0.35; z = 2.14; p = 0.03) and motor (MD 0.16; CI 0.01–0.31; z = 2.04; p = 0.04) development scores. Greater instrumental support was associated with better cognitive (MD 0.26; CI 0.10–0.42; z = 3.15; p < 0.01), motor (MD 0.17; CI 0.02–0.33; z = 2.22; p = 0.03), and overall (MD 0.19; CI 0.03–0.35; z = 2.35; p = 0.02) development scores. Depressive symptoms were associated with greater risk of wasting, while social support was associated with better infant development scores. Strategies to improve mental health and social support for mothers living with HIV during the antenatal period may benefit infant growth and development.
Keywords: Depression, Support, Growth, Development, Infant
Introduction
Globally, an estimated 44 million children experience stunting and an estimated 81 million preschool children aged 3–5 years are not reaching their full developmental potential [1, 2]. Multiple biological, social, economic, and environmental risk factors contribute to poor infant nutritional and developmental outcomes, and these risks may be more prevalent or carry greater significance for infants born to mothers living with HIV [3]. In Tanzania, the leading risk factors for poor child growth among the general population include unimproved sanitation, poor birth outcomes (preterm and small-for-gestational-age births), short maternal stature, use of biomass fuels for cooking, unimproved drinking water quality, and maternal depression [1, 4]. The relative contribution of risk factors for poor child development outcomes in Tanzania are not as well characterized; however, based on the Nurturing Care Framework it is expected that multiple factors, including inadequate nutrition, morbidity, inadequate safety and security, and suboptimal responsive caregiving and opportunities for learning, are major contributors [5]. Children who are HIV-exposed but uninfected (HEU), as compared to their HIV-unexposed and uninfected (HUU) peers, may be at greater risk of morbidity and mortality [6–8], growth faltering [9, 10], and poor developmental outcomes [11, 12]. Therefore, HEU children may experience greater burden or magnitude of risk associated with exposures affecting growth and development. Therefore, identifying modifiable risk factors for poor growth and developmental outcomes is a priority for HIV-exposed infants in Tanzania and countries with a high maternal HIV burden.
People living with HIV are estimated to have approximately twice the risk of depression [13]; moreover, in prior studies from Tanzania pregnant women living with HIV were found to be at higher risk of depression as compared to HIV-uninfected pregnant women [14]. Studies also suggest that maternal depression, both antenatal and postpartum, may be associated with lower child growth and development outcomes, though the evidence is mixed [15–17]. In Tanzania, maternal depression in non-HIV affected households has been linked to adverse growth and development outcomes [16], though the evidence specific to HEU children is lacking. Among the few studies conducted among mothers living with HIV, negative relationships between maternal depression and child development have been reported [18, 19], and few have evaluated its association with child growth [20].
In addition, maternal social support, a determinant of maternal mental health, has been shown to be positively associated with child nutrition status in low- and middle-income country (LMIC) settings [21, 22]. However, relationships between social support and child growth and development outcomes are difficult to characterise because measures of social support differ between studies and often attempt to capture perceived support received, either in the form of emotional support or financial/material support, which renders interpretation highly dependent on context and population. Social capital, which refers to the resources available to an individual and includes social support [23], has also been positively associated with different child developmental domains, though evidence specific to Tanzania and LMICs are less robust [24, 25]. Further, there remains paucity of studies that have examined the relationship between maternal social support and infant growth and development outcomes in the context of HIV.
To address evidence gaps on the relationship between maternal depression and social support in growth and development outcomes of HEU children, we conducted a prospective cohort study among pregnant women living with HIV and their infants in Dar es Salaam, Tanzania. We hypothesize that maternal depressive symptoms are negatively associated with growth and development outcomes, whereas social support is positively associate with growth and development outcomes.
Methods
Study Design and Population
We conducted a prospective cohort study among pregnant women and their infants who were enrolled in a randomized trial of vitamin D supplementation in Dar es Salaam, Tanzania (ClinicalTrials.gov identifier: NCT02305927). The trial protocol and main findings have been published elsewhere [26, 27]. Briefly, 2,300 pregnant women living with HIV, are ≥ 18 years of age, at 12–27 weeks gestation, and receiving anti-retroviral therapy (ART) were enrolled. Enrolment occurred from June 15, 2015, to April 17, 2018, with follow-up of all mothers and infants completed on October 20, 2019. During the trial, Tanzania was using the WHO Option B + approach, which provided lifelong triple-drug ART for all pregnant women living with HIV regardless of clinical or immunologic status [28]. Nearly all women (99%) received the first-line ART combination of tenofovir/lamivudine/efavirenz during pregnancy. Vitamin D supplementation was found to have no effect on the primary outcomes, including maternal HIV progression or death, small-for-gestational age (SGA) birth, and infant stunting (length-for-age z-score (LAZ) < −2) at one year of age [27].
Data Collection
At the enrolment visit at 12–27 weeks gestation, pregnant women were given a full clinical examination and nurses administered questionnaires on sociodemographic characteristics. Symptoms of maternal depression were assessed using the Hopkins Symptoms Checklist 25 (HSCL-25) that screens for 15 depressive and 10 anxiety symptoms experienced in the past 2 weeks and has been used in wide range of settings [29]. In high income settings, a cut-off of > 1.75 is applied to the average score (possible range: 1–4) to screen for symptomatic adults [30]. The HSCL-25 has been validated for use in pregnant women living with HIV in Tanzania, which identified a subscale of eight items (HSCL-8) with a recalibrated cut-off of > 1.06 of the average score to have a specificity of 89% and a sensitivity of 88% when compared to a Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) diagnosis of major depressive disorder (MDD) [14]. As a screening tool, neither HSCL-25 nor HSCL-8 is intended to distinguish between mild, moderate, and severe forms of depression. Mothers’ perceived social support was also measured at the enrolment visit using the Duke University–University of North Carolina Functional Social Support Questionnaire [31]. Following previous work in Tanzania, the social support questions were used to create two variables representing distinct constructs of support: affective support, representing different forms of emotional support received, and instrumental support, representing different forms of financial or material support received [32]. No post-enrolment visit assessments of depression or social support were conducted.
Mother-infant pairs were followed-up at delivery and until trial discharge when the infant was 12 months of age. Infant anthropometry was obtained at trial discharge when the infant was one year of age. Infant recumbent length and weight were measured in triplicate using standardized procedures [33]. Infant length was measured using a rigid length board with a precision of 0.1 cm (SECA, Hamburg, Germany) and weight was measured with a precision of 5 g using a digital scale (SECA, Hamburg, Germany). Infant development was assessed at one year of age using the Caregiver-Reported Early Development Instruments (CREDI) [34, 35]. The CREDI module was added during the conduct of the trial with the first assessment occurring on 3 April 2017 with all subsequent visits being eligible for CREDI assessment; 187 infants completed their 1-year endline visit before this date and therefore do not have CREDI data. Briefly, CREDI is a caregiver reported measure of early childhood development (ECD) for children under three years of age and has been shown to have high validity, reliability, and acceptability across a range of social and cultural contexts, including Tanzania [34, 35]. The long form of CREDI was used that assessed cognitive, language, and motor development. The CREDI questionnaire was administered by trained study nurses one-on-one and took approximately 20 min to administer per participant.
Statistical Analysis
In the primary analysis, we defined symptoms consistent with maternal depression using the Tanzanian-adapted HSCL-25 cut-off and conducted a sensitivity analysis using the standard HSCL-25 cut-off. Maternal affective and instrumental support were analyzed in tertiles within the population due to lack of standard cut-offs to define levels of social support. The distribution of infant growth outcomes at one year of age (LAZ, weight for age (WAZ), and weight for length (WLZ) z-scores) were determined using World Health Organization (WHO) growth standards [33]. Infant stunting, underweight, and wasting were defined as having a score of −2 SDs below the mean for LAZ, WAZ, and WLZ, respectively. The developmental outcomes as assessed by CREDI were converted to normalised z-scores using standard CREDI methodology against a reference population using the CREDI scoring application (https://credi.shinyapps.io/Scoring_App/) [34].
Generalized estimating equations were used to assess the relationship between symptoms consistent with maternal depression and affective and instrumental social support (exposures) with infant growth and development (outcomes). Compound symmetry matrices were used to account for clustering for twin infants. For continuous outcomes, GEEs with identity links and gaussian distributions were used to estimate mean differences while GEEs with log links and poisson distributions were used for binomial outcomes to estimate relative risks. We conducted two sensitivity analyses: (1) using the standard HSCL-25 cut-off to define symptoms consistent with depression as a proxy for more severe depression symptoms and (2) restricting the sample to singletons to address differences by twin status. Missing indicators were used to account for missing data > 1%.
Regression models were adjusted for potential confounders based on a literature review of factors that may be associated with both maternal mental health and social support as well as infant growth and development outcomes. Confounders included in multivariable models were maternal age (< 25, 25–34, or > 34 years), maternal education (did not complete primary school, completed primary school, completed secondary school, or completed high school or higher education), household wealth quintiles, marital status (married or cohabitating, single, or widowed, divorced, or separated), maternal employment status (unemployed, informal employment, or formal employment), parity (firstborn, 2–3, or > 3), CD4 cell count category (< 200, 200–349, 350–499, or ≥ 500), WHO HIV disease stage (WHO Stage I, Stage II, or Stage III/IV), taking ART (yes/no), site of trial enrolment, and randomized vitamin D or placebo regimen. Women who were not taking ART at the time of baseline assessment, primarily because they were unaware of their HIV-infection status, were prescribed standard of care ART treatment as part of the pregnancy trial they were enrolled in. We also adjusted for child sex, age, and twin status to reduce extraneous variability in infant growth and development outcomes. Statistical analyses were completed using Stata 15.1 (StataCorp, Texas, USA) and SAS 9.3 (SAS Institute, North Carolina, USA).
Ethical Considerations
The parent trial was approved by the institutional review boards of Harvard T.H. Chan School of Public Health (IRB13–0231), the Muhimbili University of Health and Allied Sciences (2016–05-25/AEC/Vol.X/01), the Ethics Sub-Committee of the National Institute of Medical Research in Tanzania (NIMR/HQ/R.8a/Vol.IX/1658), and the Tanzania Food and Drugs Authority (TFDA13/CTR/0005/3).
Results
A total of 2,300 women were enrolled in the parent trial and 2,298 women (99.9%) had data on depressive symptoms and social support (Fig. 1). Baseline characteristics of mothers during pregnancy are presented in Table 1. Around half of the eligible women were of ages 25–34 years (58.2%), completed primary school (58.5%), and were employed either formally or informally (52.8%). More than half the women were not previously taking ART and newly initiated ART during the current pregnancy at study enrolment (59.3%). The prevalence of symptoms consistent with maternal antenatal depression was 67.1% using the Tanzania adapted HSCL-8 while the prevalence was lower at 15% when calculated using the standard HSCL-25 cut-off. Responses to each of the HSCL-25 questions are detailed in Supplemental Table 2. The four most commonly reported depressive symptoms were loss of sexual pleasure, self-blame for issues, feeling low energy, and crying easily (45–49% of respondents), along with 11% participants reporting suicidal ideation. As for maternal social support at baseline, participants’ responses to individual Duke University – University of North Carolina Functional Social Support Questionnaire items are detailed in Supplemental Table 3.
Fig. 1.
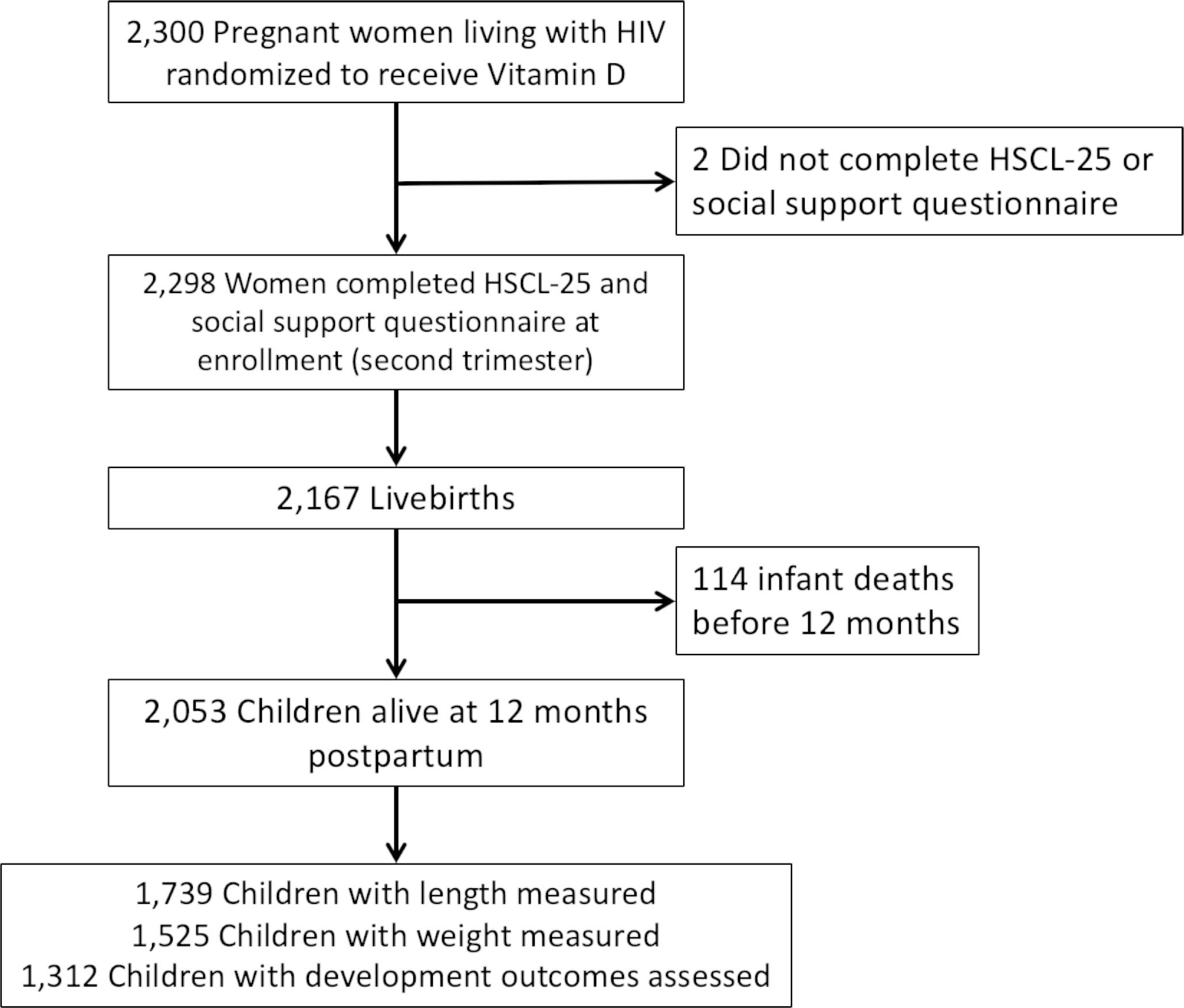
Flow chart of mother-infant pairs included in the analysis
Table 1.
Baseline demographics and clinical characteristics of pregnant women living with HIV who had depressive symptoms assessed (n = 2,298 pregnant women)
| Maternal Characteristics n = 2,298 | N (%) |
|---|---|
|
| |
| Age (years) | |
| < 25 | 369 (16.0) |
| 25 to 34 | 1,337 (58.2) |
| > 35 | 592 (25.8) |
| Education | |
| Did not complete primary school | 250 (10.9) |
| Completed primary school | 1,344 (58.5) |
| Completed secondary school | 544 (23.7) |
| Completed high school or other higher education | 160 (7.0) |
| Marital status | |
| Married or cohabitating | 1,739 (75.8) |
| Single | 492 (21.4) |
| Widowed, divorced, or separated | 63 (2.7) |
| Employed | |
| Unemployed | 1,078 (47.2) |
| Informally employed | 632 (27.7) |
| Formally employed | 575 (25.2) |
| Parity | |
| Firstborn | 560 (24.4) |
| Parity 2–3 | 1,355 (59.0) |
| Parity > 3 | 383 (16.7) |
| CD4 T-Cell count, cells/pL | |
| < 200 | 196 (8.5) |
| 200–349 | 278 (12.1) |
| 350–499 | 311 (13.5) |
| ≥ 500 | 297 (12.9) |
| Missing | 1,216 (52.9) |
| WHO Stage | |
| I | 1,973 (85.9) |
| II | 141 (6.1) |
| III or IV | 184 (8.0) |
| Timing of ART start | |
| During this pregnancy | 1,362 (59.3) |
| Preconception | 936 (40.7) |
| Depression - HSCL-25 standard cut-off | |
| No | 1,953 (85.0) |
| Yes | 345 (15.0) |
| Depression - HSCL-8 Tanzania-adapted cut-off | |
| No | 757 (32.9) |
| Yes | 1,541 (67.1) |
Figure 1 presents the flow of mother-child pairs that were included in the analysis. Of the 2,167 livebirths born to mothers in the trial, 2,053 were alive at trial discharge at one year of age and infant growth and developmental characteristics are presented in Supplemental Table 1. At one year of age, the mean infant LAZ was − 1.85 (SD: 1.46) with a stunting prevalence of 47.2%, mean WAZ was − 0.39 (SD: 1.18) with underweight prevalence of 8.1%, and mean WLZ was 0.72 (SD: 1.38) with wasting prevalence of 2.1%. Among 1,866 infants eligible for CREDI assessment, a total of 1,312 infants (70.3%) completed the assessment during study exit. In comparison to the CREDI reference population, the mean cognitive domain z-score was − 0.14 (SD: 1.15), the mean language domain z-score was − 0.18 (SD: 1.34), the mean motor domain z-score was 0.16 (SD: 1.07), and the mean overall developmental z-score was 0.12 (SD: 1.14).
The association between symptoms of maternal antenatal depression, as defined by both the Tanzania-adapted HSCL-25 and the standard HSCL-25, with child growth outcomes are reported in Table 2. Maternal depression was associated with the risk of infant wasting (Relative risk (RR): 2.61, CI: 1.03–6.65, z = 2.02, p-value = 0.04) but not with infant LAZ, stunting, WAZ, underweight, or WLZ (p > 0.05 for all regression models). Maternal depression was also not associated with any of developmental cognitive, language, or motor outcome domains, nor with overall developmental (p-values > 0.05). In sensitivity analyses using the standard HSCL-25 cut-off there was also no association for any growth or development outcome (Table 2). There were no differences in findings when restricting the analysis to singletons (results not shown).
Table 2.
Association of antenatal depression with infant growth and development outcomes
| Outcomes | Multivariable* associations for maternal depression defined by Tanzanian adapted HSCL-8 cut-off |
Sensitivity analysis* - multivariable associations for maternal depression defined by standard HSCL-25 cut-off |
||||
|---|---|---|---|---|---|---|
| Mean difference or Relative risk (CI) | z# | p-value | Mean difference or Relative risk (CI) | z# | p-value | |
|
| ||||||
| Anthropometric growth outcomes | ||||||
| Mean difference LAZ | 0.03 (−0.06–0.23) | 1.14 | 0.25 | −0.04 (−0.23–0.15) | −0.42 | 0.67 |
| Relative risk stunting (LAZ < -2) | 0.92 (0.83–1.02) | −1.54 | 0.12 | 1.06 (0.91–1.24) | 0.78 | 0.44 |
| Mean difference WAZ | −0.003 (−0.13–0.13) | −0.05 | 0.96 | −0.07 (−0.24–0.10) | −0.80 | 0.43 |
| Relative risk underweight (WAZ < -2) | 0.80 (0.56–1.16) | −1.17 | 0.25 | 0.63 (0.35–1.14) | −1.54 | 0.13 |
| Mean difference WLZ | −0.01 (−0.16–0.14) | −0.12 | 0.90 | −0.03 (−0.23–0.16) | −0.36 | 0.72 |
| Relative risk wasting (WLZ < -2) | 2.61 (1.03–6.65) | 2.02 | 0.04 | 1.79 (0.80–3.98) | 1.42 | 0.16 |
| CREDI developmental domain outcomes | ||||||
| Cognitive | −0.04 (−0.18–0.09) | −0.59 | 0.55 | −0.09 (−0.28–0.09) | −1.01 | 0.31 |
| Language | −0.04 (−0.20–0.11) | −0.56 | 0.58 | −0.06 (−0.27–0.15) | −0.57 | 0.57 |
| Motor | −0.04 (−0.17–0.08) | −0.70 | 0.49 | −0.07 (−0.24–0.10) | −0.78 | 0.44 |
| Overall | −0.05 (−0.19–0.08) | −0.78 | 0.43 | −0.06 (−0.24–0.12) | −0.65 | 0.51 |
Adjusted for: maternal age, maternal education, household wealth quintile, marital status, maternal employment status, parity, maternal CD4 category, maternal WHO HIV disease stage, taking ART, trial site, child sex, child age, child twin status, and randomized regimen
Mean z-score in SD units
The relationships between maternal social support with child growth and development outcomes are reported in Table 3. Neither affective nor instrumental social support was associated with any infant growth outcome; however, significant associations were found for infant development outcomes. In multivariable models, the highest tertile of affective support was associated with better cognitive (MD: 0.18, CI: 0.01–0.35, z = 2.14, p-value for trend = 0.03) and motor (MD: 0.16, CI: 0.01–0.31, z = 2.04, p-value for trend = 0.04) development scores as compared to the lowest tertile. Affective support was not associated with language domains or overall development (p-values for trend > 0.05). Greater instrumental support was associated with better cognitive development scores (highest vs. lowest tertiles of support MD: 0.26, CI: 0.10–0.42, z = 3.15, p-value for trend = 0.002), motor development scores (highest vs. lowest tertiles of support MD: 0.17, CI: 0.02–0.33, z = 2.22, p-value for trend = 0.03), and overall development scores (highest vs. lowest tertiles of support MD: 0.19, CI: 0.03–0.35, z = 2.35, p-value for trend = 0.02). In sensitivity analyses restricted to singletons, the association between affective support and cognitive (MD: 0.15, CI: −0.02–0.33, t = 1.72, p-value for trend = 0.09) and motor (MD: 0.14, CI: −0.03–0.30, t = 1.61, p-value for trend = 0.11) development were not significant. Similarly, the associations between instrumental support and motor development (MD: 0.16, CI: 0.00–0.32, t = 1.86, p-value for trend = 0.06) and overall development (MD: 0.17, CI: 0.00–0.34, t = 1.98, p-value for trend = 0.05) were not significant. There were no other differences in the findings for growth and other developmental outcomes (results not shown).
Table 3.
Association of maternal affective and instrumental social support tertiles with infant growth and development outcomes
| Outcomes | Multivariable* associations for maternal affective support |
Multivariable* associations for maternal instrumental support |
||||
|---|---|---|---|---|---|---|
| Mean difference or Relative risk (CI) | z# | p-value for trend | Mean difference or Relative risk (CI) | z# | p-value for trend | |
|
| ||||||
| Anthropometric growth outcomes | ||||||
| Mean difference LAZ | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | 0.20 (0.03–0.36) | 2.35 | 0.07 (−0.10–0.23) | 0.82 | ||
| Highest tertile | 0.05 (−0.13–0.22) | 0.52 | 0.56 | 0.01 (−0.16–0.19) | 0.13 | 0.93 |
| Relative risk stunting (LAZ < -2) | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | 0.88 (0.78–1.00) | −1.90 | 0.94 (0.83–1.08) | −0.84 | ||
| Highest tertile | 0.96 (0.85–1.10) | −0.53 | 0.68 | 1.02 (0.89–1.16) | 0.28 | 0.67 |
| Mean difference WAZ | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | 0.02 (−0.13–0.17) | 0.21 | −0.01 (−0.16–0.14) | −0.17 | ||
| Highest tertile | 0.10 (−0.06–0.26) | 1.22 | 0.23 | 0.07 (−0.09–0.23) | 0.85 | 0.37 |
| Relative risk underweight (WAZ < -2) | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | 1.04 (0.68–1.58) | 0.16 | 0.86 (0.57–1.29) | −0.74 | ||
| Highest tertile | 0.90 (0.57–1.42) | −0.46 | 0.66 | 0.88 (0.56–1.37) | −0.58 | 0.58 |
| Mean difference WLZ | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | −0.09 (−0.27–0.08) | −1.08 | −0.05 (−0.22–0.12) | −0.59 | ||
| Highest tertile | 0.05 (−0.13–0.23) | 0.57 | 0.59 | 0.06 (−0.12–0.24) | 0.64 | 0.47 |
| Relative risk wasting (WLZ < -2) | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | 1.63 (0.73–3.64) | 1.20 | 1.90 (0.81–4.44) | 1.48 | ||
| Highest tertile | 0.26 (0.05–1.24) | −1.69 | 0.17 | 1.10 (0.41–2.96) | 0.19 | 0.75 |
| CREDI developmental domain outcomes | ||||||
| Cognitive mean difference | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | 0.12 (−0.04–0.27) | 1.51 | 0.16 (0.004–0.31) | 2.01 | ||
| Highest tertile | 0.18 (0.01–0.35) | 2.14 | 0.03 | 0.26 (0.10–0.42) | 3.15 | 0.002 |
| Language | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | 0.03 (−0.14–0.21) | 0.36 | 0.22 (0.04–0.39) | 2.47 | ||
| Highest tertile | 0.06 (−0.13–0.24) | 0.62 | 0.53 | 0.16 (−0.02–0.35) | 1.73 | 0.11 |
| Motor | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | 0.09 (−0.05–0.23) | 1.23 | 0.08 (−0.07–0.22) | 1.04 | ||
| Highest tertile | 0.16 (0.01–0.31) | 2.04 | 0.04 | 0.17 (0.02–0.33) | 2.22 | 0.03 |
| Overall | ||||||
| Lowest tertile | Ref. | Ref. | Ref. | Ref. | ||
| Middle tertile | 0.09 (−0.06–0.24) | 1.18 | 0.15 (0.001–0.30) | 1.97 | ||
| Highest tertile | 0.12 (−0.04–0.28) | 1.49 | 0.13 | 0.19 (0.03–0.35) | 2.35 | 0.02 |
Adjusted for: maternal age, maternal education, household wealth quintile, marital status, maternal employment status, parity, maternal CD4 category, maternal WHO HIV disease stage, taking ART, trial site, child sex, child age, child twin status, and randomized regimen
Mean z-score in SD units
Discussion
We examined the relationships between symptoms consistent with antenatal depression and social support among pregnant women living with HIV with infant growth and development outcomes at one year of age in urban Tanzania. We found symptoms of maternal depression, as measured by the HSCL-25 screening tool, to be associated with wasting but not with any other infant growth or development outcomes. We also found no relationship between maternal social support and infant growth; however, higher affective social support was associated with higher cognitive development z-scores and instrumental social support was associated with higher cognitive, motor, and overall development z-scores.
Our finding of 67.1% prevalence of symptoms consistent with depression using the Tanzanian-adapted HSCL-8 cut-off indicated the prevalence of antenatal depression is high but mostly consistent with other studies investigating the prevalence of depression among adult men and women living with HIV in Tanzania, which ranges from 45.4 to 57.8% [36, 37]. Despite a high prevalence, we only found an association between symptoms of maternal depression with infant wasting but no other growth outcomes. The mechanism hypothesized to link maternal depression and child growth is that the mother’s quality of parenting may be compromised, which leads to decreased responsive care and poorer growth outcomes [38]. We previously identified greater maternal education, initiation of ART prior to conception, and low social support as factors associated with increased risk of depression, while there was no association between maternal age, employment, or wealth with depression in the study sample [39]. Approximately 60% of mothers were recently diagnosed with HIV and initiated ART in pregnancy, suggesting that the stress associated with both pregnancy and recent HIV diagnosis may be major contributors to the high prevalence of depression in the study cohort. One in ten women reported suicidal ideation which is strikingly high as compared to studies in high-income countries which generally find less than a 5% prevalence [40, 41], but is less than a study in South Africa that found a 39% prevalence of suicide ideation among pregnant women living with HIV [42]. Further, affective and instrumental social support were also generally low in the study population with half to three-quarters of women stating they received less support than they would like on each item in the social support questionnaire. Overall, both maternal depression and suboptimal social support were common in the study cohort, which highlights the need for interventions that may improve maternal mental health and increase social support, especially during pregnancy and after HIV diagnosis.
A meta-analysis by Surkan et al. found that children born to mothers with maternal depression were more likely to be underweight or stunted, but the majority of evidence were not in the context of maternal HIV [15]. In contrast, a study of HIV-unexposed children conducted in rural Tanzania found no association between maternal depression and child stunting [16]. In addition, a study by Kaaya et al. conducted among HEU children in Tanzania also found no association between antenatal depression with child growth, but infants born to mothers who had both antenatal and postpartum depression had increased risk of wasting and underweight [20]. The consistency of our findings with other studies from Tanzania suggests that the relationship between maternal depression and infant growth may differ by the timing of depression, population, and context. It is possible that in the context of pregnant Tanzanian women living with HIV, antenatal depression may affect birth outcomes or short-term responsive caregiving. This may explain why an association was only found between antenatal depression and wasting, an indicator of acute undernutrition. It is possible that postpartum depression, which was not measured in our study, when present, may affect child stunting, an indicator of chronic undernutrition, through longer-term mechanisms, including child feeding practices and household food security [43, 44]. The existence of these two separate but interlinked pathways may explain why we found an association between antenatal depression with only wasting, in contrast to studies from other contexts and meta-analyses [15]. A study conducted amongst HIV-unexposed children in rural Nepal found maternal depression to be associated with decreased child dietary diversity [43]. Mothers’ ability to engage in responsive caregiving, as demonstrated by child dietary diversity, fits well within the Nurturing Care Framework [5], suggesting that there may exist many intermediary factors between maternal depression and child growth that would be sensitive to interventions. The provision of antenatal care should be leveraged to identify specific time-points where individuals can receive increased support to improve child outcomes, such as growth. Future studies should evaluate the relationship of timing and chronicity of depression, along with responsive caregiving, with child growth in the context of HIV in diverse populations.
We also found no association between symptoms of antenatal maternal depression and infant development outcomes. A systematic review by Liu et al. found that maternal depressive symptoms are associated with poorer cognitive development in early infancy up to one year in age [17], though few studies have evaluated this in the antenatal period in the context of HIV. A 2018 cross-sectional study among South African HEU children aged two years found that postpartum depression was associated with delays in child cognitive development at 12 months [18]. Similarly, a 2018 study among Zimbabwean HEU children two years of age demonstrated an association of maternal postpartum depression with lower child cognitive, expressive language, fine motor, gross, and visual reception development domains [19]. Antenatal depression is hypothesized to be associated with adverse child development through multiple pathways, including physiologic fetal development, whereby maternal depression is linked to an overactive stress response in the fetus, compromising learning ability and development later in life [45]. There may be distinct mechanisms linking postpartum antenatal depression to postpartum depression, which may in turn both adversely affect child developmental outcomes [46]. Postpartum depression is hypothesized to affect the mother’s relationship with her child and her ability to provide adequate stimulation as outlined in the Nurturing Care Framework [5, 38]. We did not have data on postpartum depression in our study, but our findings for the relationship between antennal depression with child growth and development remain valid as it would be inappropriate to adjust for a potential mediator, postpartum depression, due to the risk of overadjustment bias. Future studies that evaluate postpartum depression as a mediator between antenatal depression and child outcomes should be pursued.
Our findings of lack of associations between maternal perceived social support and infant growth outcomes provides new evidence for HEU children. Studies conducted among HIV-unexposed children in LMIC settings suggest mixed findings on the role of social support in child growth outcomes [21, 22]. Ickes et al.’s 2018 cross-sectional study in Uganda found maternal social support to be associated with positive child-feeding practices, but not with child growth outcomes [21]. In the Young Lives cohort in Vietnam, Ethiopia, India, and Peru, Lee et al. found that social support, including financial support, was positively associated with child growth in Peru and India at ages 8 to 12 years [22]. This literature suggests that even when social support can be leveraged into productive child-rearing inputs, there remain multiple steps before that translates to improved growth or development outcomes.
How social support is defined and interpreted by participants likely differs by population as well as social contexts and there are likely significant differences in the social environments of mothers living with HIV and those without. Amongst people living with HIV, social support is a significant contributor to health maintenance and ART adherence [47]. This may translate to a mother’s ability to provide improved nutrition and an environment supportive of child growth. As social support is a contributor to a mother’s overall mental health status [14], social support itself may mediate mothers’ ability to leverage social support into productive child-rearing inputs [43]. This may reflect separate but related dimensions of support, first distinguishing social support received by mothers from social support that specifically targets child well-being, of which only the former was examined in our study. Second, we were unable to distinguish perceived social support from “actual” or “real” social support. Both dimensions of support may individually affect child outcomes or may do so in tandem and study designs need to account for these distinctions [21]. Further studies are needed to unpack the nuances and mechanisms linking maternal mental health, social support, and child growth outcomes in the context of HIV-affected households, paying close attention to the context and definition of support, the type of support received, and how it is used in child-rearing activities.
We found that maternal affective support was positively associated with cognitive and motor development scores, whereas maternal instrumental support was positively associated with cognitive, motor, and overall development scores. In sensitivity analyses restricting to singletons, the associations between affective support and cognitive and motor development, as well as between instrumental support and motor and overall development, did not reach significance. Our study was conducted amongst HEU children and while the evidence base among HEU children is lacking, our findings are consistent with those conducted among HUU children. A cross-sectional study in Brazil found that amongst HUU children ages 4–6 years, caregiver social support and social capital were positively associated with socio-emotional development [24]. A community-based randomized trial in rural Uganda by Singla et al. also demonstrated that parenting interventions targeting caregiver social support was positively associated with language development and cognitive development amongst children aged 1–3 years [25]. This study demonstrated a plausible mechanistic link between social support and child development as being that of parenting techniques and responsive care [5], similar to the hypothesized mechanism linking maternal depression and infant growth [38]. While our study examined different developmental domains, age group, and HIV context, our work demonstrates consistent positive association between maternal or caregiver support and child development. The consistent literature of the positive association between maternal social support and child development outcomes among HEU and HUU children suggests that development outcomes are more sensitive to changes in social support rather than infant growth outcomes. As a result, interventions aimed to improve social support may be promising in terms of potential impacts on supporting child developmental outcomes, but they may have more limited effects on child growth. This also implies a need for observational and intervention research to determine specific pathways that social support affects child development and broader health, nutrition, and well-being.
This study has several limitations. First, we only measured depression and social support at a single time point in the second trimester of pregnancy and our study did not characterise the progression of depression over time, and we did not capture postpartum depression. Antenatal and postpartum depression have been suggested to have additive adverse effects on child development and the chronicity depression of may affect child nutrition outcomes [20]. Therefore, future studies should look to untangle the links between antenatal and postpartum depression and child growth and development outcomes. Second, our measure of depression was based on maternal-reported symptoms and observations, as opposed to a formal clinical diagnosis obtained through a clinical examination. Some participants may therefore not accurately report their symptoms and observations, which in the context of prospectively measured infant growth and development would likely be non-differential and bias the measures of association to the null. Additionally, we did not use gestational age-corrected age for preterm born children in the application of WHO growth standards to assess growth at one year of age, which may overestimate the degree of undernutrition in preterm-born infants [48]; however, measures of associations are unlikely to be affected substantially at one year of age. Lastly, this study was conducted in urban Dar es Salaam in Tanzania in the context of HIV and therefore the results may not be directly generalizable to other populations and contexts.
Overall, the high prevalence of symptoms consistent with antenatal depression among pregnant women living with HIV in Tanzania warrants attention, irrespective of its association with child growth and development outcomes. We found maternal depression was associated with increased risk of infant wasting, an important risk factor for morbidity and mortality outcomes, which further supports the need to pursue mental health interventions for pregnant women living with HIV. We also found robust associations of both affective and instrumental support with child development outcomes which suggests that social support interventions for pregnant women living with HIV may also improve child development outcomes. Our findings highlight the need for interventions to support maternal mental health that consider the interconnectedness between antenatal depression, social support, and how these facets change through the course of pregnancy and in the postpartum period. In designing studies, researchers should also consider the nuances of measurement of social support and whether it distinguishes between support for the mother or for the child, and between “real” and perceived support. Lastly, given that maternal depression and social support are upstream of child outcomes with many intermediary steps, mediation and other analyses are needed to better understand the mechanisms through which depression and its timing affect child outcomes. With these considerations, randomized trials and implementation research of interventions targeting the mental health and social support of mothers living with HIV have the potential to improve child nutrition and development outcomes.
Supplementary Material
Funding
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under Award Number R01 HD83113. CPD was partially funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grants K24 DK104676 and 2P30 DK040561. AS was supported by the National Institutes of Health Fogarty International Center and the NICHD under Award Number D43 TW010543. NP was supported by the Canadian Institutes of Health Research Fellowship Award and the Thrasher Research Foundation Early Career Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Footnotes
Competing Interests The authors have no competing interests to declare.
Ethics Approval The parent trial was approved by the institutional review boards of Harvard T.H. Chan School of Public Health (IRB13–0231), the Muhimbili University of Health and Allied Sciences (2016–05-25/AEC/Vol.X/01), the Ethics Sub-Committee of the National Institute of Medical Research in Tanzania (NIMR/HQ/R.8a/Vol.IX/1658), and the Tanzania Food and Drugs Authority (TFDA13/CTR/0005/3).
Consent to Participant Written informed consent was obtained from every participant.
Code Availability Available for review upon reasonable request.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10461-023-04073-5.
Data Availability
Data cannot be shared publicly because of requirement for ethical approval and data transfer agreement. The deidentified dataset supporting this research may be made available following a submitted request to ghp@hsph.harvard.edu and completion of ethical approval and data transfer agreement from the Tanzania National Institute of Medical Research (http://reims.nimr.or.tz:8010/guides/DTA.pdf).
References
- 1.Smith Fawzi MC, Andrews KG, Fink G, Danaei G, McCoy DC, Sudfeld CR, et al. Lifetime economic impact of the burden of childhood stunting attributable to maternal psychosocial risk factors in 137 low/middle-income countries. BMJ Glob Heal. 2019;4(1):e001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCoy DC, Peet ED, Ezzati M, Danaei G, Black MM, Sudfeld CR, et al. Early Childhood Developmental Status in Low- and Middle-Income Countries: National, Regional, and global prevalence estimates using Predictive modeling. PLoS Med. 2016. Jun;13(6):e1002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherr L, Croome N, Parra Castaneda K, Bradshaw K, Herrero Romero R. Developmental challenges in HIV infected children—An updated systematic review. Child Youth Serv Rev [Internet]. 2014;45:74–89. Available from: https://www.sciencedirect.com/science/article/pii/S0190740914001467. [Google Scholar]
- 4.Danaei G, Andrews KG, Sudfeld CR, Fink G, McCoy DC, Peet E et al. Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels. PLOS Med [Internet]. 2016. Nov 1 [cited 2023 Mar 1];13(11):e1002164. Available from: 10.1371/journal.pmed.1002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britto PR, Lye SJ, Proulx K, Yousafzai AK, Matthews SG, Vaivada T, et al. Nurturing care: promoting early childhood development. Lancet (London England). 2017. Jan;389(10064):91–102. [DOI] [PubMed] [Google Scholar]
- 6.Filteau S The HIV-exposed, uninfected African child. Trop Med Int Health [Internet]. 2009. Mar [cited 2022 Jun 21];14(3):276–87. Available from: https://pubmed.ncbi.nlm.nih.gov/19171011/. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro RL, Lockman S, Kim S, Smeaton L, Rahkola JT, Thior I et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis [Internet]. 2007. Aug 15 [cited 2022 Jun 21];196(4):562–9. Available from: https://pubmed.ncbi.nlm.nih.gov/17624842/. [DOI] [PubMed] [Google Scholar]
- 8.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol [Internet]. 2014. Apr [cited 2022 Jun 21];176(1):11–22. Available from: https://pubmed.ncbi.nlm.nih.gov/24325737/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Growth TCI, Team N, CIGNIS) I. S. Micronutrient Fortification to Improve Growth and Health of Maternally HIV-Unexposed and Exposed Zambian Infants: A Randomised Controlled Trial. PLoS One [Internet]. 2010. [cited 2022 Jun 21];5(6):e11165. Available from: 10.1371/journal.pone.0011165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudfeld CR, Lei Q, Chinyanga Y, Tumbare E, Khan N, Dapaah-Siakwan F et al. Linear Growth Faltering Among HIV-Exposed Uninfected Children. J Acquir Immune Defic Syndr [Internet]. 2016. Oct 1 [cited 2022 Jun 21];73(2):182–9. Available from: https://pubmed.ncbi.nlm.nih.gov/27116046/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ntozini R, Chandna J, Evans C, Chasekwa B, Majo FD, Kanda-wasvika G, et al. Early child development in children who are HIV-exposed uninfected compared to children who are HIV-unexposed: observational sub-study of a cluster-randomized trial in rural Zimbabwe. J Int AIDS Soc. 2020. May;23(5):e25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wedderburn CJ, Weldon E, Bertran-Cobo C, Rehman AM, Stein DJ, Gibb DM et al. Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: a systematic review and meta-analysis. Lancet Child Adolesc Heal [Internet]. 2022. Jun 1 [cited 2022 Aug 21];6(6):393–408. Available from: https://pubmed.ncbi.nlm.nih.gov/35483380/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–30. [DOI] [PubMed] [Google Scholar]
- 14.Kaaya SF, Fawzi MCS, Mbwambo JK, Lee B, Msamanga GI, Fawzi W. Validity of the Hopkins Symptom Checklist-25 amongst HIV-positive pregnant women in Tanzania. Acta Psychiatr Scand. 2002;106(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull World Health Organ. 2011. Aug;89(8):608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neamah HH, Sudfeld C, McCoy DC, Fink G, Fawzi WW, Masanja H et al. Intimate partner violence, depression, and child growth and development. Pediatrics. 2018;142(1). [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Kaaya S, Chai J, McCoy DC, Surkan PJ, Black MM, et al. Maternal depressive symptoms and early childhood cognitive development: a meta-analysis. Psychol Med. 2017;47(4):680–9. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez VJ, Matseke G, Cook R, Bellinger S, Weiss SM, Alcaide ML, et al. Infant development and pre- and Post-partum Depression in Rural South African HIV-Infected Women. AIDS Behav. 2018. Jun;22(6):1766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mebrahtu H, Simms V, Chingono R, Mupambireyi Z, Weiss HA, Ndlovu P, et al. Postpartum maternal mental health is associated with cognitive development of HIV-exposed infants in Zimbabwe: a cross-sectional study. AIDS Care - Psychol Socio-Medical Asp AIDS/HIV. 2018;30:74–82. [DOI] [PubMed] [Google Scholar]
- 20.Kaaya S, Garcia ME, Li N, Lienert J, Twayigize W, Spiegelman D, et al. Association of maternal depression and infant nutritional status among women living with HIV in Tanzania. Matern Child Nutr. 2016;12(3):603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ickes SB, Wu M, Mandel MP, Roberts AC. Associations between social support, psychological well-being, decision making, empowerment, infant and young child feeding, and nutritional status in Ugandan children ages 0 to 24 months. Matern Child Nutr [Internet]. 2018. Jan 1 [cited 2022 Oct 5];14(1):e12483. Available from: 10.1111/mcn.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HY, Song IH, Kawachi I. Maternal and child social support and food availability in relation to child growth in four low- and middle-income countries. Sci Rep [Internet]. 2022. Dec 1 [cited 2022 Jun 28];12(1). Available from: https://pubmed.ncbi.nlm.nih.gov/35396562/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porta MS, Greenland S, Hernán M, Silva I dos S, Last JM. A dictionary of epidemiology. Six edition / edi... Porta MS, Greenland S, Hernán M, Silva I dos S, Last JM, editors. Oxford: Oxford University Press; 2014. [Google Scholar]
- 24.Surkan PJ, Park S, Ridgeway K, Ribeiro M, Fidalgo TM, Martins SS et al. Caregiver Social Capital and Supportive Relationships are Associated with Better Child Social-Emotional Development. Child Psychiatry Hum Dev [Internet]. 2022. [cited 2022 Aug 23]; Available from: https://pubmed.ncbi.nlm.nih.gov/35088156/. [DOI] [PubMed] [Google Scholar]
- 25.Singla DR, Kumbakumba E, Aboud FE. Effects of a parenting intervention to address maternal psychological wellbeing and child development and growth in rural Uganda: a community-based, cluster randomised trial. Lancet Glob Heal [Internet]. 2015. [cited 2022 Aug 23];3(8):e458–69. Available from: https://pubmed.ncbi.nlm.nih.gov/26144389/. [DOI] [PubMed] [Google Scholar]
- 26.Sudfeld CR, Manji KP, Duggan CP, Aboud S, Muhihi A, Sando DM, et al. Effect of maternal vitamin D3 supplementation on maternal health, birth outcomes, and infant growth among HIV-infected Tanzanian pregnant women: study protocol for a randomized controlled trial. Trials. 2017;18(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudfeld CR, Manji KP, Muhihi A, Duggan CP, Aboud S, Alwy Al-Beity FM et al. Vitamin D3 supplementation during pregnancy and lactation for women living with HIV in Tanzania: A randomized controlled trial. PLOS Med [Internet]. 2022. Apr 15;19(4):e1003973. Available from: 10.1371/journal.pmed.1003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Organization WH. Programmatic update: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: executive summary. World Health Organization; 2012. p. 8. [PubMed] [Google Scholar]
- 29.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19(1):1–15. [DOI] [PubMed] [Google Scholar]
- 30.Winokur A, Winokur DF, Rickels K, Cox DS. Symptoms of Emotional Distress in a Family Planning Service: Stability over a Four-Week Period. Br J Psychiatry [Internet]. 2018/01/29. 1984;144(4):395–9. Available from: https://www.cambridge.org/core/article/symptoms-of-emotional-distressin-a-family-planning-service-stability-over-a-fourweek-period/1C0020B1432C2DDB2E3A7CD2DDFFBE4D. [DOI] [PubMed] [Google Scholar]
- 31.Broadhead WE, Gehlbach SH, De Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire: measurement of social support in family medicine patients. Med Care. 1988;26(7):709–23. [DOI] [PubMed] [Google Scholar]
- 32.Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44(4):470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enrolment and baseline characteristics in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl [Internet]. 2006. Apr [cited 2023 Mar 20];450:7–15. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.library.tufts.edu/16817674/. [DOI] [PubMed] [Google Scholar]
- 34.McCoy DC, Waldman M, Fink G. Measuring early childhood development at a global scale: Evidence from the Caregiver-Reported Early Development Instruments. Early Child Res Q [Internet]. 2018;45:58–68. Available from: https://www.sciencedirect.com/science/article/pii/S0885200618300449. [Google Scholar]
- 35.McCoy DC, Sudfeld CR, Bellinger DC, Muhihi A, Ashery G, Weary TE, et al. Development and validation of an early childhood development scale for use in low-resourced settings. Popul Health Metr. 2017. Feb;15(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regan M, Muhihi A, Nagu T, Aboud S, Ulenga N, Kaaya S, et al. Depression and viral suppression among adults living with HIV in Tanzania. AIDS Behav. 2021. Oct;25(10):3097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sudfeld CR, Kaaya S, Gunaratna NS, Mugusi F, Fawzi WW, Aboud S, et al. Depression at antiretroviral therapy initiation and clinical outcomes among a cohort of Tanzanian women living with HIV. AIDS. 2017. Jan;31(2):263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet (London England). 2014. Nov;384(9956):1800–19. [DOI] [PubMed] [Google Scholar]
- 39.Regan M, Muhihi A, Saleh A, Duggan C, Ulenga N, Alwy Al-Beity FM et al. [Under review] Antenatal depression and adverse birth outcomes among pregnant women living with HIV in Dar es Salaam, Tanzania. 2023. [DOI] [PMC free article] [PubMed]
- 40.Mikšić Å, Miškulin M, Juranić B, Rakošec Ž, Včev A, Degmečić D. Depression and Suicidality during Pregnancy. Psychiatr Danub [Internet]. 2018. [cited 2023 Mar 6];30(1):85–90. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.library.tufts.edu/29546863/. [DOI] [PubMed] [Google Scholar]
- 41.Tabb KM, Gavin AR, Faisal-Cury A, Nidey N, Chan YF, Malinga T et al. Prevalence of antenatal suicidal ideation among racially and ethnically diverse WIC enrolled women receiving care in a Midwestern public health clinic. J Affect Disord [Internet]. 2019. Sep 1 [cited 2023 Mar 6];256:278–81. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.library.tufts.edu/31195245/. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez VJ, Mandell LN, Babayigit S, Manohar RR, Weiss SM, Jones DL. Correlates of Suicidal Ideation During Pregnancy and Postpartum Among Women Living with HIV in Rural South Africa. AIDS Behav [Internet]. 2018. Oct 1 [cited 2023 Mar 6];22(10):3188–97. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.library.tufts.edu/29752621/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller LC, Neupane S, Sparling TM, Shrestha M, Joshi N, Lohani M et al. Maternal depression is associated with less dietary diversity among rural Nepali children. Matern Child Nutr [Internet]. 2021. Oct 1 [cited 2022 Jun 21];17(4). Available from: https://pubmed.ncbi.nlm.nih.gov/34132034/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gebreyesus SH, Endris BS, Hanlon C, Lindtjorn B. Maternal depression symptoms are highly prevalent among food-insecure households in Ethiopia. Public Health Nutr [Internet]. 2018. Apr 1 [cited 2023 Mar 19];21(5):849–56. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.library.tufts.edu/29151371/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sohr-Preston SL, Scaramella LV. Implications of Timing of Maternal Depressive Symptoms for Early Cognitive and Language Development. Clin Child Fam Psychol Rev 2006. 91 [Internet]. 2006 Apr 25 [cited 2022 Oct 5];9(1):65–83. Available from: 10.1007/s10567-006-0004-2. [DOI] [PubMed] [Google Scholar]
- 46.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: A synthesis of recent literature. Gen Hosp Psychiatry [Internet]. 2004. [cited 2023 Mar 6];26(4):289–95. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.library.tufts.edu/15234824/. [DOI] [PubMed] [Google Scholar]
- 47.Mukoswa GM, Charalambous S, Nelson G. The association between social capital and HIV treatment outcomes in South Africa. PLoS One [Internet]. 2017. Nov 1 [cited 2022 Aug 20];12(11):e0184140. Available from: 10.1371/journal.pone.0184140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perumal N, Roth DE, Cole DC, Zlotkin SH, Perdrizet J, Barros AJD et al. Effect of Correcting the Postnatal Age of Preterm-Born Children on Measures of Associations Between Infant Length-for-Age z Scores and Mid-Childhood Outcomes. Am J Epidemiol [Internet]. 2021. Mar 1 [cited 2022 Oct 30];190(3):477–86. Available from: https://academic.oup.com/aje/article/190/3/477/5893776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared publicly because of requirement for ethical approval and data transfer agreement. The deidentified dataset supporting this research may be made available following a submitted request to ghp@hsph.harvard.edu and completion of ethical approval and data transfer agreement from the Tanzania National Institute of Medical Research (http://reims.nimr.or.tz:8010/guides/DTA.pdf).


