Abstract
Introduction:
Olfactory impairment and Parkinson’s disease (PD) may share common genetic and environmental risk factors. This study investigates the association of a PD polygenic risk score (PRS) with olfaction, and whether the associations are modified by environmental exposures of PM2.5, NO2, or smoking.
Methods:
This analysis included 3,358 women (aged 50-80) from the Sister Study with genetic data and results from the Brief Smell Identification Test (B-SIT) administered in 2018-2019. PD PRS was calculated using 90 single nucleotide polymorphisms. Olfactory impairment was defined with different B-SIT cutoffs, and PD diagnosis was adjudicated via expert review. We report odds ratios (ORs) and 95% confidence intervals (CIs) from multivariable logistic regression.
Results:
As expected, PD PRS was strongly associated with the odds of having PD (OR highest vs. lowest quartile = 3.79 (1.64, 8.73)). The highest PRS quartile was also associated with olfactory impairment, with OR ranging from 1.24 (0.98, 1.56) for a B-SIT cutoff of 9 to 1.42 (1.04, 1.92) for a cutoff of 6. For individual B-SIT items, the highest PRS quartile was generally associated with lower odds of correctly identifying the odorant, albeit only statistically significant for pineapple (0.72 (0.56, 0.94), soap (0.76 (0.58, 0.99)) and rose (0.70 (0.54, 0.92)). The association of PD PRS with olfactory impairment was not modified by airborne environmental exposures or smoking.
Conclusion:
These preliminary data suggest that high PD genetic susceptibility is associated with olfactory impairment in middle-aged and older women.
Keywords: Parkinson, genetic susceptibility, olfactory impairment
Introduction
Olfactory impairment affects up to 80% of patients with Parkinson’s disease (PD), and may develop years if not decades before clinical diagnosis. However, little is known about the environmental and genetic causes of olfactory loss in the context of aging, nor their potential relevance to later PD development. Preliminary data, albeit inconsistent, suggest olfactory impairment may have shared genetic links with PD [1–3]. We hereby explored the possibility that PD genetic susceptibility, either alone or interacting with environmental factors, may contribute to olfactory impairment in older adults, which over time, may progress to clinical PD. We tested this possibility among selected participants of the Sister Study of the National Institute of Environmental Health Sciences (NIEHS) [4]. We used PD polygenic risk score (PRS) as an aggregate marker of genetic susceptibility to PD [5] and examined its relationship to olfactory impairment and PD. We also explored whether the association of PD PRS with olfactory impairment or PD was modified by exposures to air pollutants (fine particulate matter PM2.5 and nitrogen dioxide NO2) and smoking. These airborne exposures may directly gain access to the olfactory structures and potentially the brain itself via the nasal mucosa. Further, both exposures may potentially affect PD risk. The inverse association of cigarette smoking with PD is among the most robust epidemiological findings for PD [6]. Although data are far less consistent, ambient air pollutants have also been linked to PD risk [7].
Methods
Study population
The Sister Study is an ongoing nationwide cohort established by NIEHS scientists to investigate environmental and genetic risk factors for breast cancer and other chronic diseases [8]. In 2003-2009, the study enrolled a total of 50,884 women, aged 35-74, who had a sister with breast cancer from all 50 US states and Puerto Rico [8]. At enrollment, study participants completed a comprehensive Computer Assisted Telephone Interview, multiple mailed comprehensive questionnaire surveys, and a home visit. Nearly all participants (99.1%) provided blood samples at the home visit, and an additional 0.7% had saliva samples for DNA isolation. Since enrollment, the cohort has been followed with short annual health updates (AHUs) and detailed follow-ups (DFUs) every 2-3 years. In January 2018, we selected a sample of Sister study participants for the olfaction sub-study, as detailed elsewhere [4]. Eligible participants were those who answered the sense of smell question in the third DFU in 2014-2016, and who were alive and aged 50-79 in January 2018. We targeted this specific age group to cover the hypothetical life period that prodromal PD most likely developed. Of the 36,491 eligible, we selected a total of 4,019 subjects, including all who reported a poor sense of smell (n=2,819) and a random sample (n=1,200) who did not. We invited eligible participants to take the self-administered B-SIT test, distributed by mail. After removing 613 individuals who did not participate in the olfaction sub-study or did not complete the B-SIT test and 48 participants with missing genotype data, the primary analytical sample included 3,358 subjects. Details of the study design, sample selection, and study participation are provided in Supplemental Figure 1. The study was approved by the Institutional Review Boards at Michigan State University, NIEHS, and the US Department of Defense, and all Sister Study participants provided written consent.
Genotyping and PRS calculation
DNA genotyping was performed by the Neurogenetic laboratory of the National Institute on Aging, using Illumina Infinium platform on NeuroChip per manufacturers protocol (Illumina Inc., San Diego) [9]. Briefly, samples were removed if call rates were <95% and genotypic sex was confirmed. Individuals were checked for cryptic relatedness (PIHAT <0.05) and excess heterogeneity estimated by an F-statistic >0.15. Duplicate individuals included in the study for sample handling quality control were verified to be identical and one individual was removed from each pair. Filtered genotype data were imputed via Michigan imputation server [10] using TOPMed Version r2 reference panel [11] under default settings with the Eagle v2.4 option for phasing and Mixed setting for population. Variants passing the post-imputation quality criteria of R2 >0.3 and minor allele frequency (MAF) >1% were included. For the calculation of PD GRS, we included 90 single nucleotide polymorphisms (SNPs) that are significantly associated with PD from the latest PD genome-wide association studies (GWAS) meta-analysis [5]. PRS was calculated by multiplying the effect sizes for those 90 variants with the number of effect alleles [12] and then grouped by quartiles. Principal components (PCs) were created using PLINK. For the PCs calculation, variants were filtered for genotyping missing (<0.1), MAF (>0.05), and Hardy-Weinberg equilibrium test statistic (p≥10−6). The remaining variants were pruned (using a 50-kb window, with a 5 SNP shift per window and r2 threshold of 0.2), and the first five PCs were calculated using the pruned variants.
PD ascertainment
The Sister Study routinely queries about the diagnosis of chronic diseases as part of the cohort’s AHUs, DFUs, and helpdesk contacts, consistently over the years. For deceased individuals, the underlying cause of death was obtained via linkage to National Death Index (NDI). DFU surveys collect further details about PD: 1) PD clinical diagnosis, the year of diagnosis, and PD medication uses; 2) separately on the questionnaire, all current uses of prescribed or over-the-counter medications using the inventory method; 3) the presence of four motor symptoms in the past year, including hand tremor, slow walking or movements, small handwriting, and difficulty getting started when walking or moving. In 2018-2019, we contacted potential cases or the next-of-kin of deceased cases who reported a physician-made PD diagnosis for the study participants. We asked them to 1) confirm their diagnosis of PD, whether the diagnosis was current (if not, alternative diagnosis if available), the year or age of diagnosis, and the specialty of the diagnostic physician; 2) major PD signs/symptoms and whether they ever improved with medications and whether they were ever asymmetric; and 3) use of PD medications, whether they helped with PD symptoms and were still being used. For patients consenting to contact their treating physicians, we reached out to the physicians and asked them to fill out a short confirmation form about PD signs and symptoms and diagnostic history, neurological comorbidity, and alternative diagnosis, and to provide a copy of the relevant medical records. These data were reviewed by a movement disorder specialist (XH) and a neuroepidemiologist (HC). Our final PD adjudication was confirmed if 1) both the treating physician and the patient confirmed their PD diagnosis and the expert review agreed; or 2) if patient/physician data were not available, experts review of cohort data over the 15 years of follow-up supported the patient-reported physician diagnosis of PD. Of the 371 self-reported PD diagnoses, 242 were confirmed, and 72 were included in the olfaction sub-study with valid genetic data. Details of the PD adjudication process and findings were published elsewhere [13].
The Brief Smell Identification Test (B-SIT)
B-SIT is the short version of the 40-item University of Pennsylvania Smell Identification Test and has been widely used to screen for olfactory impairment in large population-based epidemiological studies [14]. The test was designed to be self-administered and contains 12 commonly-experienced odorants [14], one embedded on each page of a small booklet. Participants are instructed to scratch and smell each of these odorants, one at a time, and to identify the correct odorant from four possible answers in a forced multiple-choice format [14]. Every correct answer is awarded one point, summing to a final score ranging from 0-12, with a higher score indicating a better sense of smell [14]. Because this is one of the first analyses on this topic and there is no clinically validated B-SIT cutoff to define olfactory impairment, we used the scores of 9, 8, 7, and 6 as the cutoff to determine robustness of our results. All these cutoffs have been used in previous epidemiological studies [15–17], and represent varying degrees of olfaction loss from hyposmia to anosmia. In addition to the overall B-SIT performance, we also examined PD PRS in relation to the odds of correctly identifying each of the 12 individual odorants.
Covariate Assessments
The Sister Study has comprehensively collected data on demographics, lifestyle, environmental exposures, residential history, and health status at enrollment which were periodically updated at follow-up surveys. We considered the following covariates obtained at the time of study enrollment in the analysis, age (<50, 50-55, 56-60, ≥60 years), race and ethnicity (non-Hispanic White, non-Hispanic Black, and others), educational attainment (below college versus some college or higher degree), smoking status (ever versus never smokers), and self-reported health status (fair or poor versus good, very good, or excellent). Additionally, self-reported sense of smell (normal versus poor) from the third DFU, age at the olfaction sub-study (continuous), and exposures to the major ambient air pollutants of PM2.5 and NO2 from year of 2006 (continuous, scaled by inter-quartile range/IQR) were included. Details of the exposure assessment approach for ambient air pollutants have been published elsewhere [18].
Statistical Analyses
We analyzed the primary exposure of interest (PD PRS) by quartiles and as a continuous variable for a per-IQR increment of the PRS. In all analyses, we accounted for the sampling design that the sub-study participants were selected based on their self-reported olfaction in the third DFU by treating the self-report as a covariate in the outcome regression models. Under the assumption that B-SIT tested olfaction and sample selection were independent conditioning on the genetic risk of PD and adjusted covariates, the odds ratios (ORs) of PD PRS can be consistently estimated by our analytical strategy and interpreted as the association with B-SIT tested olfaction conditioning on the adjusted covariates [19]. In the analyses, we further accounted for the non-response in the outcome (B-SIT tested olfaction) by inverse probability weighting (IPW). For non-response, we assumed missing at random (MAR) and performed logistic regression for the missingness of the outcome on variables that were potentially related to the missingness. We used the best subset method based on Bayesian information criterion (BIC) for covariate selection (selected covariates: age, race, education, smoking status, exposure to PM2.5 and NO2 in 2006). For weighted analysis, each individual’s weight was calculated by the inverse of the predicted probability of not being missing in B-SIT score. Robust standard errors were used for the logistic regressions with IPW. We then tested and could not reject the null hypothesis that PD PRS was not related to the self-reported sense of smell (Supplemental Table 1). In the primary analyses, we first confirmed the relation between PD PRS and PD. Then we defined the B-SIT tested olfaction as a dummy variable (olfactory impairment or not) with different cutoff values (≤9, ≤8, ≤7, or ≤6) and analyzed the relation between PD PRS and olfactory impairment. As the PD PRS was derived from participants almost exclusively of European descent [5], we further restricted analyses to non-Hispanic Whites. Next, we examined PD PRS in relation to each of the 12 B-SIT odorants. Further, we examined whether the relation between PD PRS and olfaction was modified by air pollutants (below/above median) or smoking status (never/ever). In this secondary analysis, we defined PD PRS exposure as the highest quartile versus the rest, based on findings from the primary analysis. Lastly, we repeated the above analyses using PD as the analytic outcome. ORs and 95% confidence intervals (CIs) were reported. When applicable, we tested for the linear trend using the exposure quartile medians as a continuous outcome. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary NC) or PLINK (version 1.9), and all tests were two-sided at the significance level 0.05 with Sister Study data release 9.1.
Results
Population characteristic patterns were remarkedly consistent, regardless of the choice of B-SIT cutoffs (Table 1). Compared to women without olfactory impairment, those with olfactory impairment were older and more likely to be non-Hispanic blacks, and to self-report poor olfaction. They were also slightly more likely to have a lower than college education, to be ever smokers, and to self-report fair or poor health status. The two groups were however almost identical in their exposures to ambient air pollutants.
Table 1.
Population characteristics by the Brief Smell Identification Test (B-SIT) results
| Characteristics | Olfactory impairment using various B-SIT cutoffs a b | |||||||
|---|---|---|---|---|---|---|---|---|
| B-SIT score ≤9 | B-SIT score ≤8 | B-SIT score ≤7 | B-SIT score ≤6 | |||||
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Age at enrollment, N (%) | ||||||||
| <50 | 124 (11.7) | 635 (26.2) | 83 (10.6) | 676 (25.0) | 59 (9.9) | 701 (24.3) | 43 (9.5) | 718 (23.7) |
| 50-54 | 180 (17.0) | 581 (24.0) | 122 (15.7) | 639 (23.7) | 92 (15.4) | 668 (23.1) | 66 (14.6) | 694 (22.9) |
| 55-59 | 257 (24.3) | 568 (23.4) | 196 (25.1) | 628 (23.3) | 146 (24.4) | 678 (23.5) | 112 (24.7) | 710 (23.5) |
| ≥60 | 496 (46.9) | 640 (26.4) | 379 (48.6) | 758 (28.0) | 301 (50.3) | 837 (29.0) | 234 (51.3) | 905 (29.9) |
| Race, N (%) | ||||||||
| Non-Hispanic White | 928 (87.8) | 2168 (89.4) | 686 (87.8) | 2410 (89.2) | 527 (88.1) | 2569 (89.1) | 401 (88.0) | 2695 (89.1) |
| Non-Hispanic Black | 77 (7.3) | 106 (4.4) | 56 (7.1) | 127 (4.7) | 41 (6.9) | 142 (4.9) | 32 (7.0) | 151 (5.0) |
| Others | 52 (4.9) | 151 (6.2) | 39 (5.1) | 163 (6.1) | 30 (5.0) | 173 (6.0) | 23 (5.0) | 180 (6.0) |
| Education, N (%) | ||||||||
| Some college or higher | 892 (84.3) | 2158 (89.0) | 660 (84.5) | 2390 (88.5) | 507 (84.7) | 2544 (88.2) | 387 (85.0) | 2663 (88.0) |
| Below college | 166 (15.7) | 266 (11.0) | 121 (15.5) | 311 (11.5) | 91 (15.3) | 340 (11.8) | 68 (15.0) | 363 (12.0) |
| Smoking status, N (%) | ||||||||
| Never smoker | 566 (53.5) | 1376 (56.8) | 422 (54.0) | 1520 (56.3) | 319 (53.4) | 1623 (56.3) | 245 (53.7) | 1697 (56.1) |
| Ever smoker | 492 (46.5) | 1048 (43.2) | 359 (46.0) | 1181 (43.7) | 279 (46.6) | 1261 (43.7) | 211 (46.3) | 1329 (43.9) |
| Self-reported olfaction impairment, N (%) | ||||||||
| No | 117 (11.0) | 930 (38.4) | 54 (6.9) | 993 (36.8) | 28 (4.7) | 1019 (35.3) | 17 (3.8) | 1030 (34.0) |
| Yes | 941 (89.0) | 1494 (61.6) | 727 (93.1) | 1708 (63.2) | 570 (95.3) | 1865 (64.7) | 439 (96.2) | 1996 (66.0) |
| Self-reported health status, N (%) | ||||||||
| Excellent, very good or good | 976 (92.3) | 2297 (94.8) | 727 (93.1) | 2546 (94.3) | 560 (93.7) | 2713 (94.1) | 428 (94.0) | 2846 (94.0) |
| Fair or poor | 82 (7.7) | 127 (5.2) | 54 (6.9) | 155 (5.7) | 38 (6.3) | 171 (5.9) | 28 (6.0) | 180 (6.0) |
| PM2.5 2006, (μg/m3) | 10.2±2.5 | 10.1±2.5 | 10.1±2.6 | 10.1±2.5 | 10.1±2.6 | 10.1±2.5 | 10.1±2.6 | 10.1±2.5 |
| NO2 2006, (ppb) | 9.1±5.1 | 8.9±4.9 | 9.1±5.2 | 9.0±4.9 | 9.2±5.4 | 8.9±4.9 | 9.1±5.4 | 9.0±4.9 |
Number may not add up to total due to weighting.
Weighted number (percentage) for categorical variables and weighted mean ± standard deviation for continuous variables.
The PRS was strongly associated with the odd of having PD in a dose-response manner (Table 2). Compared with the lowest PRS quartile, the multivariable OR was 1.81 for the second, 2.31 for the third, and 3.79 for the fourth quartile, with a clear dose-response relationship (OR per IQR = 2.24 (1.59, 3.15), p for trend=0.0005). We found similar results when limiting analyses to non-Hispanic White women. Olfactory impairment was also strongly associated with the odds of having PD (Supplemental Table 2). Using B-SIT cutoffs (9 or 8) indicative of hyposmia, the OR for PD was 7.19 and 7.89, respectively. Using cutoffs (7 or 6) for anosmia, the corresponding OR was 3.77 and 3.53. Finally, correctly identifying individual B-SIT items was uniformly associated with lower odds of having PD, with OR ranging from 0.28 (0.16, 0.48) for cherry to 0.56 (0.31, 1.04) for clove (Supplemental Table 3).
Table 2.
Polygenic risk score in relation to Parkinson’s disease in middle-aged and older women
| Polygenic risk score | Parkinson’s disease | Odds ratio (95% confidence interval) | ||
|---|---|---|---|---|
| Yes | No | Model 1 a | Model 2 b | |
| All subjects, N (%) | ||||
| Quartile 1 | 9 (12.5) | 875 (25.7) | Reference | Reference |
| Quartile 2 | 15 (20.8) | 866 (25.4) | 1.83 (0.75, 4.47) | 1.81 (0.74, 4.41) |
| Quartile 3 | 19 (26.4) | 843 (24.7) | 2.28 (0.95, 5.45) | 2.31 (0.96, 5.53) |
| Quartile 4 | 29 (40.3) | 826 (24.2) | 3.74 (1.63, 8.60) | 3.79 (1.64, 8.73) |
| Per inter-quartile range | 2.19 (1.56, 3.07) | 2.24 (1.59, 3.15) | ||
| P for trend | 0.0006 | 0.0005 | ||
| Non-Hispanic Whites, N (%) | ||||
| Quartile 1 | 7 (10.6) | 670 (22.1) | Reference | Reference |
| Quartile 2 | 13 (19.7) | 804 (26.5) | 1.53 (0.60, 3.88) | 1.53 (0.60, 3.90) |
| Quartile 3 | 19 (28.8) | 783 (25.8) | 2.13 (0.89, 5.14) | 2.18 (0.90, 5.26) |
| Quartile 4 | 27 (40.9) | 773 (25.5) | 3.21 (1.38, 7.47) | 3.27 (1.40, 7.64) |
| Per inter-quartile range | 1.95 (1.38, 2.76) | 1.98 (1.40, 2.82) | ||
| P for trend | 0.0020 | 0.0017 | ||
Model 1 adjusted for age, race, the first 5 principal components, and self-reported sense of smell.
Model 2 further adjusted for education, smoking status, self-reported health status, and PM2.5 and NO2 in 2006.
Only the highest quartile of PD PRS was associated with modestly higher odds of having olfactory impairment (Table 3). The OR comparing the highest vs. lowest PD PRS quartile was 1.24 (0.98, 1.56) with the B-SIT cutoff of 9, 1.34 (1.03, 1.73) with the cutoff of 8, 1.31 (1.00, 1.73) with the cutoff of 7, and 1.42 (1.04, 1.92) with the cutoff of 6. Results were nearly the same in the analysis limited to non-Hispanic White women. Overall, the highest PRS quartile was associated with lower odds of correctly identifying individual B-SIT items as compared with the lowest quartile, although statistical significance was found only for pineapple (OR=0.72 (0.56, 0.94)), soap (OR=0.76 (0.58, 0.99)), and rose (OR=0.70 (0.54, 0.92)) (Table 4). When analyzing PRS as IQR increment, statistical significance was additionally found for leather (OR per IQR=0.85 (0.72, 0.99)).
Table 3.
PD polygenic risk score and olfactory impairment in middle-aged and older women, using different B-SIT cutoffsa
| Polygenic risk score | OR (95% CI) | |||
|---|---|---|---|---|
| B-SIT score ≤ 9 | B-SIT score ≤ 8 | B-SIT score ≤ 7 | B-SIT score ≤ 6 | |
| All subjects | ||||
| Quartile 1 | Reference | Reference | Reference | Reference |
| Quartile 2 | 0.99 (0.78, 1.25) | 1.06 (0.82, 1.38) | 0.92 (0.69, 1.22) | 0.97 (0.71, 1.34) |
| Quartile 3 | 1.04 (0.82, 1.32) | 1.04 (0.80, 1.35) | 0.82 (0.62, 1.09) | 0.94 (0.69, 1.30) |
| Quartile 4 | 1.24 (0.98, 1.56) | 1.34 (1.03, 1.73) | 1.31 (1.00, 1.73) | 1.42 (1.04, 1.92) |
| Per IQR | 1.12 (1.00, 1.25) | 1.13 (1.00, 1.28) | 1.12 (0.97, 1.28) | 1.18 (1.02, 1.37) |
| P for trend | 0.0541 | 0.0269 | 0.0554 | 0.0205 |
| Non-Hispanic Whites | ||||
| Quartile 1 | Reference | Reference | Reference | Reference |
| Quartile 2 | 0.96 (0.75, 1.24) | 1.03 (0.78, 1.37) | 0.89 (0.66, 1.20) | 0.94 (0.67, 1.33) |
| Quartile 3 | 1.00 (0.78, 1.28) | 1.01 (0.77, 1.33) | 0.81 (0.60, 1.10) | 0.97 (0.70, 1.35) |
| Quartile 4 | 1.25 (0.98, 1.60) | 1.33 (1.01, 1.74) | 1.31 (0.98, 1.74) | 1.42 (1.03, 1.95) |
| Per IQR | 1.11 (0.99, 1.25) | 1.11 (0.98, 1.27) | 1.11 (0.96, 1.28) | 1.20 (1.02, 1.40) |
| P for trend | 0.0466 | 0.0339 | 0.0547 | 0.0185 |
PD: Parkinson’s disease; B-SIT: Brief Smell Identification Test; OR: odds ratio; CI: confidence interval; IQR: inter-quartile range.
Adjusted for age, race, the first 5 principal components, self-reported sense of smell, education, smoking status, self-reported health status, and PM2.5 and NO2 in 2006.
Table 4:
Parkinson’s disease polygenic risk score and correctly identifying individual odorants in middle-aged and older women
| Polygenic risk score | Odds Ratio (95% Confidence Interval) a | |||||
|---|---|---|---|---|---|---|
| menthol | cherry | clove | leather | strawberry | lilac | |
| Quartile 1 | Reference | Reference | Reference | Reference | Reference | Reference |
| Quartile 2 | 0.87 (0.66, 1.14) | 1.09 (0.81, 1.46) | 1.03 (0.76, 1.39) | 0.90 (0.65, 1.25) | 1.05 (0.81, 1.35) | 1.04 (0.81, 1.34) |
| Quartile 3 | 0.89 (0.68, 1.18) | 1.20 (0.89, 1.63) | 1.07 (0.79, 1.45) | 0.91 (0.66, 1.27) | 1.16 (0.89, 1.51) | 1.17 (0.90, 1.51) |
| Quartile 4 | 0.96 (0.72, 1.27) | 0.79 (0.59, 1.06) | 0.85 (0.63, 1.14) | 0.77 (0.56, 1.07) | 0.89 (0.69, 1.15) | 0.91 (0.71, 1.17) |
| Per inter-quartile range | 0.98 (0.86, 1.12) | 0.87 (0.76, 1.01) | 0.92 (0.79, 1.07) | 0.85 (0.72, 0.99) | 0.97 (0.85, 1.10) | 0.97 (0.86, 1.10) |
| P for trend | 0.8503 | 0.1275 | 0.2771 | 0.1294 | 0.4442 | 0.5727 |
| Polygenic risk score | pineapple | smoke | lemon | soap | natural gas | rose |
| Quartile 1 | Reference | Reference | Reference | Reference | Reference | Reference |
| Quartile 2 | 0.81 (0.62, 1.05) | 1.15 (0.84, 1.58) | 1.04 (0.82, 1.31) | 1.05 (0.80, 1.38) | 0.91 (0.71, 1.17) | 0.87 (0.66, 1.14) |
| Quartile 3 | 0.83 (0.63, 1.08) | 1.04 (0.77, 1.42) | 0.86 (0.68, 1.08) | 0.84 (0.64, 1.09) | 0.94 (0.73, 1.22) | 0.89 (0.68, 1.17) |
| Quartile 4 | 0.72 (0.56, 0.94) | 0.87 (0.64, 1.19) | 0.88 (0.69, 1.11) | 0.76 (0.58, 0.99) | 0.81 (0.63, 1.05) | 0.70 (0.54, 0.92) |
| Per inter-quartile range | 0.89 (0.79, 1.01) | 0.96 (0.83, 1.12) | 0.90 (0.81, 1.01) | 0.82 (0.72, 0.93) | 0.90 (0.80, 1.02) | 0.85 (0.75, 0.97) |
| P for trend | 0.0227 | 0.2961 | 0.1434 | 0.0148 | 0.1386 | 0.0139 |
Adjusted for age, race, the first 5 principal components, self-reported sense of smell, education, smoking status, self-reported health status, and PM2.5 and NO2 in 2006.
The association of PRS with olfactory impairment was similar for environmental exposures in interaction analysis (Figure 1), albeit the association seemed to be more evident among women with higher than median PM2.5 exposure regardless of the B-SIT cutoff used. For example, with a B-SIT cutoff of 8, the OR comparing the highest versus other PRS quartiles was 1.47 (1.10, 1.98) among women with higher than median PM2.5 exposures, versus 1.15 (0.88, 1.51) for women with lower than median exposures. When using the B-SIT cutoff of 6, the contrast is more evident with corresponding ORs of 1.83 (1.30, 2.59) versus 1.20 (0.87, 1.67). In analyzing PD as the outcome (Supplemental Figure 2), the association of PRS with PD appears to be slightly stronger in the women with higher than median exposures to PM2.5 or NO2 and in never smokers, although none of the interaction terms were statistically different.
Figure 1. Parkinson’s disease Polygenic risk score and olfactory impairment in middle-aged and older women, stratified by airborne exposures.
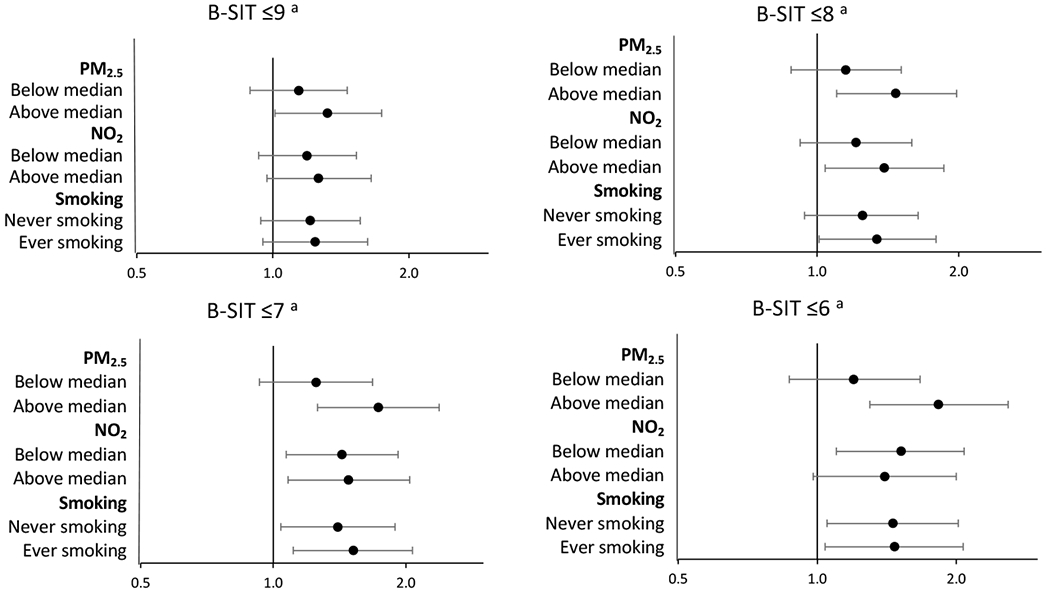
B-SIT: Brief Smell Identification Test
Odds ratios and 95% confidence intervals are presented comparing the fourth quartile of the Parkinson’s disease polygenic risk score vs. lower quartiles combined.
a Models were adjusted for age, race, the first 5 principal components, self-reported sense of smell, education, smoking status, self-reported health status, and PM2.5 and NO2 in 2006.
Discussion
The past two decades have experienced multiple breakthroughs in understanding the genetic complexity of late-onset sporadic PD. The latest GWAS study identified 90 common genetic variants that are independently associated with sporadic PD with genome-wide significance [5]. Based on these findings, a PD PRS was developed as a single composite measure of the overall genetic susceptibility to sporadic PD, and it has been associated with PD risk, age at onset, motor progression, and cognitive decline in recent studies [20, 21]. The derivation of a single measure of PD genetic susceptibility may also facilitate analyses to explore gene-environment interactions [22], which have been hypothesized to play important roles in the development of late-onset sporadic PD [23].
In this sample of a nationwide cohort of middle-aged and older women, we independently confirmed that this risk score is strongly associated with PD risk in a dose-response manner, supporting the validity and broad generalizability of this PD PRS. Although we did not find statistical evidence for interactions with major ambient air pollutants or smoking, the association of PRS with PD appears to be slightly more evident in never smokers and women exposed to higher levels of air pollutants, consistent with the hypothetical roles of these airborne environmental exposures in PD etiology [24]. We also found women in the highest quartile of PD PRS might have a higher risk of olfactory impairment. Taken together, this study provides provocative, albeit preliminary, evidence that PD and olfactory impairment may have shared genetic susceptibility.
To the best of our knowledge, this is one of the first studies to investigate the etiological links incorporating genetic susceptibility, environmental exposures, prodromal symptoms, and PD clinical diagnosis. Despite little empirical evidence to date, there are strong biological rationales to examine these potential interconnections, given the length and complexity of PD prodromal etiology. Olfactory loss is one of the most robust and earliest PD prodromal symptoms [25–27]. The Braak hypothesis [28] further posits that PD pathogenesis may start in the olfactory structures, years if not decades, before spreading to the substantia nigra where dopaminergic neuron death occurs, and PD motor dysfunction arises. This controversial hypothesis provides a theoretical platform and a practical noninvasive approach to studying olfactory impairment as an intermediate phenotype to explore potential triggers and accelerators of PD pathogenesis [29]. Comparing potential risk factors for PD with those for olfactory impairment and identifying potential causes that first lead to olfactory impairment and then to PD motor dysfunction may allow us to develop interventions that may modify these processes.
In the current study, we empirically tested this paradigm by examining PD PRS as a potential common cause for olfactory impairment and PD, and whether airborne pollutants may modify the associations. We chose to study PD PRS because it is the single strongest risk factor for late-onset sporadic PD with a plausible causality [5]. In addition, genetic susceptibility has a clear temporality as it precedes the development of both olfactory impairment and PD. Our previous GWAS analyses suggest potential genetic links of olfaction to the PD loci of LRRK2 and MAPT among of >6,000 older US adults of European ancestry [1, 2]. However, neither association reached genome-wide significance. A recent cross-sectional analysis in Australia (n=1,395 combined) directly examined a PRS derived from SNPs predicting PD age at onset in relation to B-SIT test performance overall and on individual odorants [3]. The authors did not find any significant associations except that participants who scored incorrectly on the odorant of chocolate tended to have a higher PD PRS score.
Compared with the Australian study [3], our study is much larger but only included women. In our analysis, we limited study participation to women aged 50-79 years, aiming to target the group at risk of prodromal neurodegeneration. The PD PRS was developed based on the genetic structures of participants of European descent; however, excluding women with race/ethnicity other than non-Hispanic White had little impact on our study findings. In our analysis, although we did not find a dose-response relationship between PD PRS and olfactory impairment as in PD, we found women in the highest PRS quartile had poor olfaction compared to the lowest quartile, results that were robust to different choices of cutoff. The analyses of individual odorants generally showed women with the highest PRS were less likely to identify the correct odorant, although only a few items reached statistical significance. Nevertheless, the observed association of PRS with olfactory impairment was modest compared to that with PD. This contrast is somewhat expected because this genetic score was derived to maximize the power of characterizing genetic susceptibility to PD [5], and only about 10% of older adults with olfactory impairment proceed to diagnosis with PD in the following decade or so [25]. Therefore, the vast majority of olfactory impairment in older adults may not be related to PD at all, or the pathogenesis may not progress to clinically recognizable PD during one’s lifetime. Future research is needed to characterize features of olfactory impairment that are specific to prodromal PD development and more likely to progress to clinical PD.
The study of olfactory impairment in the context of PD research may also illuminate environmental contributions to PD etiology. The olfactory structure is among the few anatomic sites where the human mucosal surfaces directly interact with the environment and its path to the brain is well established. In this study, we explored whether the genetic susceptibility to olfactory impairment or PD could be modified by airborne exposures. The analysis on PD was limited by sample size, but the results suggest stronger associations among women with higher levels of PM2.5 or NO2 and nonsmokers, consistent with the expected effects of these airborne exposures on PD. Results on olfactory impairment were less informative, although the association of PD PRS with olfactory impairment was statistically significant among women with higher than median PM2.5 exposure, but not among those with lower exposure levels, regardless of the B-SIT cutoff used.
Our study, while conceptually novel, has multiple limitations. First, the tested paradigm is an oversimplified sketch of PD prodromal development. Many factors may come into play in prodromal PD development and this study focuses only on the genetics, olfaction, and PD. Second, this paradigm should be best tested in prospective cohort studies with clearer temporal relationships among various components of the disease development process. Temporal relationships could not be specified in this study, with the exception that PD genetic susceptibility precedes all other components. Further, olfaction was only assessed in the current study after most PD cases had been identified, complicating the temporal inference in the analysis. While it is commonly believed that olfactory impairment develops in prodromal PD before its clinical diagnosis, the current study did not document this temporal order and therefore results must be interpreted cautiously. Third, although olfactory impairment is among the most informative prodromal markers of PD [30], as discussed above, evidence to date shows that this relationship lacks specificity. In addition, like most large epidemiological studies, we used a single brief screening test of smell identification to define olfactory impairment at a single time point, which is not ideal for an outcome that is known to decline with age. Repeated, thorough olfaction assessments may help to characterize olfactory impairment that differentiates PD or neurodegeneration destiny from others in the context of aging. Fourth, we identified PD cases first via self-reports, and we then conducted case adjudication and confirmed its known association with age, PD genetic susceptibility, and multiple nonmotor symptoms [13]. Despite our validation efforts, underreport of PD diagnosis and adjudication errors are inevitable. Fifth, our study participants are volunteers of predominantly non-Hispanic White women, highly educated and health conscious. While studies showed that PD PRS is equally associated with PD risk by sex, preliminary longitudinal evidence suggests the association of olfactory impairment with PD is modestly weaker in women than in men [25]. Further, compared to White men or Black men and women, White women are the least likely to develop poor olfaction. Therefore, our study findings in predominantly non-Hispanic White women may not be readily generalizable to men or women with other race/ethnicity backgrounds. Finally, our study aims to investigate the potential genetic links between poor olfaction and PD, using PD PRS as the surrogate of PD genetic susceptibility. A comprehensive investigation of the genetic causes of olfactory impairment in older adults is beyond our scope of work.
In conclusion, our study confirmed the validity and broad generalizability of PD PRS in characterizing the genetic risk of PD, and provided preliminary evidence that high PD genetic susceptibility may also contribute to olfactory impairment.
Supplementary Material
A novel study of potential genetic links between olfaction and Parkinson’s disease.
High Parkinson’s polygenic risk score is associated with olfactory impairment.
The association appears to be independent of major air pollutants and smoking.
Acknowledgements
This study is supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Parkinson’s Research Program (W81XWH-17-1-0536); the Parkinson’s Foundation (PF-IMP-1825); and in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES044005) and National Institute on Aging (ZO1 AG000949). The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office of the contract W81XWH-17-1-0536. Dr. Chen is also supported by grants from the National Institutes of Health (R01ES029227 and R01AG071517) and internal supports from Michigan State University. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense, the National Institutes of Health, or the authors’ employers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Statement
The data that support findings from this study are available from the Sister Study, following appropriate approval procedures as detailed at https://sisterstudy.niehs.nih.gov/English/collaboration.htm.
Declaration of Competing Interest
Dr. Pinto is on the speaker’s bureau for Sanofi and Regeneron; he also serves as a site investigator for these two companies. He has served on an advisory board for Connect Biopharma. Other authors declare no conflict of interests.
References
- [1].Dong J, Wyss A, Yang J, Price TR, Nicolas A, Nalls M, Tranah G, Franceschini N, Xu Z, Schulte C, Alonso A, Cummings SR, Fornage M, Zaykin D, Li L, Huang X, Kritchevsky S, Liu Y, Gasser T, Wilson RS, De Jager PL, Singleton AB, Pinto JM, Harris T, Mosley TH Jr., Bennett DA, London S, Yu L, Chen H, Genome-wide association analysis of the sense of smell in U.S. older adults: identification of novel risk loci in African-Americans and European-Americans, Molecular Neurobiology 54(10) (2016) 8021–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dong J, Yang J, Tranah G, Franceschini N, Parimi N, Alkorta-Aranburu G, Xu Z, Alonso A, Cummings SR, Fornage M, Huang X, Kritchevsky S, Liu Y, London S, Niu L, Wilson RS, De Jager PL, Yu L, Singleton AB, Harris T, Mosley TH Jr., Pinto JM, Bennett DA, Chen H, Genome-wide meta-analysis on the sense of smell among US older adults, Medicine 94(47) (2015) e1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Raj S, Thalamuthu A, Armstrong NJ, Wright MJ, Kwok JB, Trollor JN, Ames D, Schofield PR, Brodaty H, Sachdev PS, Mather KA, Investigating olfactory gene variation and odour identification in older adults, Genes (Basel) 12(5) (2021). 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cao Z, Yang A, D’Aloisio AA, Suarez L, Deming-Halverson S, Li C, Luo Z, Pinto JM, Werder EJ, Sandler DP, Chen H, Assessment of self-reported sense of smell, objective testing, and associated factors in middle-aged and older women, JAMA Otolaryngology-- Head & Neck Surgery 148 (5) (2022) 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, Tan M, Kia DA, Noyce AJ, Xue A, Bras J, Young E, von Coelln R, Simon-Sanchez J, Schulte C, Sharma M, Krohn L, Pihlstrom L, Siitonen A, Iwaki H, Leonard H, Faghri F, Gibbs JR, Hernandez DG, Scholz SW, Botia JA, Martinez M, Corvol JC, Lesage S, Jankovic J, Shulman LM, Sutherland M, Tienari P, Majamaa K, Toft M, Andreassen OA, Bangale T, Brice A, Yang J, Gan-Or Z, Gasser T, Heutink P, Shulman JM, Wood NW, Hinds DA, Hardy JA, Morris HR, Gratten J, Visscher PM, Graham RR, Singleton AB, 23andMe Research Team, System Genomics of Parkinson’s Disease Consortium, International Parkinson’s Disease Genomics Consortium, Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies, The Lancet Neurology 18(12) (2019) 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen H, Huang X, Guo X, Mailman RB, Park Y, Kamel F, Umbach DM, Xu Q, Hollenbeck A, Schatzkin A, Blair A, Smoking duration, intensity, and risk of Parkinson disease, Neurology 74(11) (2010) 878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu CY, Fang Y, Li FL, Dong B, Hua XG, Jiang W, Zhang H, Lyu Y, Zhang XJ, Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis, Environmental Research 168 (2019) 448–459. [DOI] [PubMed] [Google Scholar]
- [8].Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, Kleeberger CA, Shore DL, DeRoo LA, Taylor JA, Weinberg CR, Sister Study Research Team, The Sister Study cohort: Baseline methods and participant characteristics, Environmental Health Perspectives 125(12) (2017) 127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Blauwendraat C, Faghri F, Pihlstrom L, Geiger JT, Elbaz A, Lesage S, Corvol JC, May P, Nicolas A, Abramzon Y, Murphy NA, Gibbs JR, Ryten M, Ferrari R, Bras J, Guerreiro R, Williams J, Sims R, Lubbe S, Hernandez DG, Mok KY, Robak L, Campbell RH, Rogaeva E, Traynor BJ, Chia R, Chung SJ, International Parkinson’s Disease Genomics Consortium (IPDGC), COURAGE-PD Consortium, Hardy JA, Brice A, Wood NW, Houlden H, Shulman JM, Morris HR, Gasser T, Kruger R, Heutink P, Sharma M, Simon-Sanchez J, Nalls MA, Singleton AB, Scholz SW, NeuroChip, an updated version of the NeuroX genotyping platform to rapidly screen for variants associated with neurological diseases, Neurobiology of Aging 57 (2017) 247 e9–247 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, Schlessinger D, Stambolian D, Loh PR, Iacono WG, Swaroop A, Scott LJ, Cucca F, Kronenberg F, Boehnke M, Abecasis GR, Fuchsberger C, Next-generation genotype imputation service and methods, Nature genetics 48(10) (2016) 1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Taliun D, Harris DN, Kessler MD, Carlson J, Szpiech ZA, Torres R, Taliun SAG, Corvelo A, Gogarten SM, Kang HM, Pitsillides AN, LeFaive J, Lee SB, Tian X, Browning BL, Das S, Emde AK, Clarke WE, Loesch DP, Shetty AC, Blackwell TW, Smith AV, Wong Q, Liu X, Conomos MP, Bobo DM, Aguet F, Albert C, Alonso A, Ardlie KG, Arking DE, Aslibekyan S, Auer PL, Barnard J, Barr RG, Barwick L, Becker LC, Beer RL, Benjamin EJ, Bielak LF, Blangero J, Boehnke M, Bowden DW, Brody JA, Burchard EG, Cade BE, Casella JF, Chalazan B, Chasman DI, Chen YI, Cho MH, Choi SH, Chung MK, Clish CB, Correa A, Curran JE, Custer B, Darbar D, Daya M, de Andrade M, DeMeo DL, Dutcher SK, Ellinor PT, Emery LS, Eng C, Fatkin D, Fingerlin T, Forer L, Fornage M, Franceschini N, Fuchsberger C, Fullerton SM, Germer S, Gladwin MT, Gottlieb DJ, Guo X, Hall ME, He J, Heard-Costa NL, Heckbert SR, Irvin MR, Johnsen JM, Johnson AD, Kaplan R, Kardia SLR, Kelly T, Kelly S, Kenny EE, Kiel DP, Klemmer R, Konkle BA, Kooperberg C, Kottgen A, Lange LA, Lasky-Su J, Levy D, Lin X, Lin KH, Liu C, Loos RJF, Garman L, Gerszten R, Lubitz SA, Lunetta KL, Mak ACY, Manichaikul A, Manning AK, Mathias RA, McManus DD, McGarvey ST, Meigs JB, Meyers DA, Mikulla JL, Minear MA, Mitchell BD, Mohanty S, Montasser ME, Montgomery C, Morrison AC, Murabito JM, Natale A, Natarajan P, Nelson SC, North KE, O’Connell JR, Palmer ND, Pankratz N, Peloso GM, Peyser PA, Pleiness J, Post WS, Psaty BM, Rao DC, Redline S, Reiner AP, Roden D, Rotter JI, Ruczinski I, Sarnowski C, Schoenherr S, Schwartz DA, Seo JS, Seshadri S, Sheehan VA, Sheu WH, Shoemaker MB, Smith NL, Smith JA, Sotoodehnia N, Stilp AM, Tang W, Taylor KD, Telen M, Thornton TA, Tracy RP, Van Den Berg DJ, Vasan RS, Viaud-Martinez KA, Vrieze S, Weeks DE, Weir BS, Weiss ST, Weng LC, Willer CJ, Zhang Y, Zhao X, Arnett DK, Ashley-Koch AE, Barnes KC, Boerwinkle E, Gabriel S, Gibbs R, Rice KM, Rich SS, Silverman EK, Qasba P, Gan W, NHLBI Trans-Omic for Precision Medicine (TOPMed) Consortium, Papanicolaou GJ, Nickerson DA, Browning SR, Zody MC, Zollner S, Wilson JG, Cupples LA, Laurie CC, Jaquish CE, Hernandez RD, O’Connor TD, Abecasis GR, Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program, Nature 590(7845) (2021) 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Igo RP Jr., Kinzy TG, Cooke Bailey JN, Genetic risk scores, Current Protocols in Human Genetics 104(1) (2019) e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cao Z, Song S, Huang X, Li C, Luo Z, D’Aloisio AA, Suarez L, Hernandez DG, Singleton AB, Sandler DP, Chen H, Parkinson’s disease case ascertainment in the Sister Study: A cohort for environmental health research, Journal of Parkinson’s disease 13(5) (2023) 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Doty RL, Marcus A, Lee WW, Development of the 12-item cross-cultural smell identification test (CC-SIT), The Laryngoscope 106(3 Pt 1) (1996) 353–356. [DOI] [PubMed] [Google Scholar]
- [15].Joseph T, Auger SD, Peress L, Rack D, Cuzick J, Giovannoni G, Lees A, Schrag AE, Noyce AJ, Screening performance of abbreviated versions of the UPSIT smell test, Journal of Neurology 266(8) (2019) 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dong J, Pinto JM, Guo X, Alonso A, Tranah G, Cauley JA, Garcia M, Satterfield S, Huang X, Harris T, Mosley TH Jr., Chen H, The prevalence of anosmia and associated factors among U.S. Black and White older adults, The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 72(8) (2017) 1080–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cao Z, Luo Z, Huang X, Pinto JM, Simonsick EM, Shiroma EJ, Chen H, Self-reported versus objectively assessed olfaction and Parkinson’s disease risk, Journal of Parkinson’s disease 10(4) (2020) 1789–1795. [DOI] [PubMed] [Google Scholar]
- [18].Kirwa K, Szpiro AA, Sheppard L, Sampson PD, Wang M, Keller JP, Young MT, Kim SY, Larson TV, Kaufman JD, Fine-scale air pollution models for epidemiologic research: Insights from approaches developed in the Multi-ethnic Study of Atherosclerosis and Air Pollution (MESA Air), Current Environmental Health Reports 8(2) (2021) 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wild CJ, Secondary analysis of case-control data, Chapter 14, CRC Handbook of Statistical Methods for Case-Control Studies (2018) 251–260. [Google Scholar]
- [20].Maraki MI, Hatzimanolis A, Mourtzi N, Stefanis L, Yannakoulia M, Kosmidis MH, Dardiotis E, Hadjigeorgiou GM, Sakka P, Ramirez A, Grenier-Boley B, Lambert JC, Heilmann-Heimbach S, Stamelou M, Scarmeas N, Xiromerisiou G, Association of the polygenic risk score with the probability of prodromal Parkinson’s disease in older adults, Frontiers Molecular Neuroscience 14 (2021) 739571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Koch S, Laabs BH, Kasten M, Vollstedt EJ, Becktepe J, Bruggemann N, Franke A, Kramer UM, Kuhlenbaumer G, Lieb W, Mollenhauer B, Neis M, Trenkwalder C, Schaffer E, Usnich T, Wittig M, Klein C, Konig IR, Lohmann K, Krawczak M, Caliebe A, Validity and prognostic value of a polygenic risk score for Parkinson’s disease, Genes (Basel) 12(12) (2021) 1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jacobs BM, Belete D, Bestwick J, Blauwendraat C, Bandres-Ciga S, Heilbron K, Dobson R, Nalls MA, Singleton A, Hardy J, Giovannoni G, Lees AJ, Schrag AE, Noyce AJ, Parkinson’s disease determinants, prediction and gene-environment interactions in the UK Biobank, Journal of Neurology, Neurosurgery, and Psychiatry 91(10) (2020) 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tanner CM, Goldman SM, Ross GW, Grate SJ, The disease intersection of susceptibility and exposure: chemical exposures and neurodegenerative disease risk, Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 10(3 Suppl) (2014) S213–225. [DOI] [PubMed] [Google Scholar]
- [24].Dorsey ER, Okun MS, Tanner CM, Bad air and Parkinson disease - the fog may be lifting, JAMA Neurology 78(7) (2021) 793–795. [DOI] [PubMed] [Google Scholar]
- [25].Chen H, Shrestha S, Huang X, Jain S, Guo X, Tranah GJ, Garcia ME, Satterfield S, Phillips C, Harris TB; Health ABC Study, Olfaction and incident Parkinson disease in US White and Black older adults, Neurology 89 (14) (2017) 1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR, Association of olfactory dysfunction with risk for future Parkinson’s disease, Annals of Neurology 63(2) (2008) 167–173. [DOI] [PubMed] [Google Scholar]
- [27].Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB, Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study, Brain: A Journal of Neurology 142(7) (2019) 2051–2067. [DOI] [PubMed] [Google Scholar]
- [28].Braak H, Bohl JR, Müller CM, Rüb U, de Vos RAI, Tredici KD, Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered, Movement Disorders: Official Journal of the Movement Disorder Society 21(12) (2006) 2042–2051. [DOI] [PubMed] [Google Scholar]
- [29].Chen H, Wang K, Scheperjans F, Killinger B, Environmental triggers of Parkinson’s disease - Implications of the Braak and dual-hit hypotheses, Neurobiology of Disease 163 (2022) 105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB, MDS Task Force on the Definition of Parkinson’s Disease, Update of the MDS research criteria for prodromal Parkinson’s disease, Movement Disorders: Official Journal of the Movement Disorder Society 34(10) (2019) 1464–1470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


