SUMMARY
The symbioses that animals form with bacteria play important roles in health and disease, but the molecular details underlying how bacterial symbionts initially assemble within a host remain unclear.1–3 The bioluminescent bacterium Vibrio fischeri establishes a light-emitting symbiosis with the Hawaiian bobtail squid Euprymna scolopes by colonizing specific epithelium-lined crypt spaces within a symbiotic organ called the light organ.4 Competition for these colonization sites occurs between different strains of V. fischeri, with the lancet-like type VI secretion system (T6SS) facilitating strong competitive interference that results in strain incompatibility within a crypt space.5,6 Although recent studies have identified regulators of this T6SS, how the T6SS is controlled as symbionts assemble in vivo remains unknown.7,8 Here, we show that T6SS activity is suppressed by N-octanoyl-L-homoserine lactone (C8 HSL), which is a signaling molecule that facilitates quorum sensing in V. fischeri and is important for efficient symbiont assembly.9,10 We find that this signaling depends on the quorum-sensing regulator LitR, which lowers expression of the needle subunit Hcp, a key component of the T6SS, by repressing transcription of the T6SS regulator VasH. We show that LitR-dependent quorum sensing inhibits strain incompatibility within the squid light organ. Collectively, these results provide new insights into the mechanisms by which regulatory networks that promote symbiosis also control competition among symbionts, which in turn may affect the overall symbiont diversity that assembles within a host.
eTOC Blurb
Many bacterial symbionts encode interference competition mechanisms. Guckes et al. find that quorum sensing between bacterial symbionts suppresses an intercellular killing mechanism that promotes strain incompatibility within their squid host. This work provides insight into the multi-strain dynamics that take place during symbiosis establishment.
Graphical Abstract

RESULTS
Quorum sensing inhibits T6SS activity in V. fischeri
To establish symbiosis with E. scolopes, V. fischeri cells must access dedicated colonization sites (crypt spaces) within the light organ and then grow into light-emitting populations. Previous work has shown that the T6SS encoded on the second chromosome of some V. fischeri strains (T6SS2) can prevent populations comprising certain strain combinations from forming within individual crypt spaces.11 The main gene cluster encodes both the structural components of T6SS2 (Figure 1A) and at least one transcription factor that regulates its expression.7 However, how T6SS2 is regulated in vivo remains unclear, particularly as a population expands from a few founder cells to the carrying capacity of the crypt space.
Figure 1. C8 HSL-mediated quorum sensing suppresses T6SS2 activity in V. fischeri.

(A) T6SS2 structure. Mechanisms that control T6SS2 activity in V. fischeri are unknown.
(B) Left, fluorescence-based co-incubation assay with FQ-A001-derived test and CFP-labeled ES114 competitor strains. Right, CFP fluorescence of spots with test strains grown overnight (ON), to low cell density (LCD), or to high cell density (HCD). Bar = 1 mm
(C) Left, co-incubation assay with test and ermR ES114 competitor strains. Right, ermR ES114 abundance within spots with FQ-A001-derived test strains grown as in panel B.
(D) Left, co-incubation assay with FQ-A001 cells incubated in spent media from FQ-A001 cultures. Right, ermR ES114 abundance within spots with FQ-A001 grown in fresh or spent medium from cultures at LCD or HCD (OD600 = 0.3 and 2.5, respectively).
(E) ermR ES114 abundance in spots with FQ-A001 cells grown in spent media from HCD cultures of ES114 WT and ES114 luxI− ainS− ± indicated autoinducer (1 μM).
(F) hcp transcript levels in FQ-A001 cultures at LCD ± 1 μM C8 HSL. P-value from t-test.
C-E: Groups with identical (different) letters indicate same (different) statistical groups. See also Figure S1 and Data S1 for group statistics.
To test whether T6SS2 activity changes as a population expands, we cultured FQ-A001, which is a T6SS2-positive strain, in rich medium and sampled the culture at different cell densities to assess cellular T6SS2 activity. As a negative control for T6SS2 activity, we used a strain lacking both hcp within the T6SS2 gene cluster and hcp1 within an accessory T6SS2 gene cluster (Δhcp Δhcp1), which each encode identical copies of the Hcp subunit of the T6SS2 inner needle (Figure 1A).12 To initially assess T6SS2 activity, we sampled a culture of FQ-A001 and co-incubated those cells with CFP-labeled ES114, which is a T6SS2-negative strain susceptible to T6SS2-dependent killing. FQ-A001 cells derived from a culture grown overnight (ON) resulted in low CFP fluorescence in an Hcp-dependent manner (Figure 1B), consistent with FQ-A001 exhibiting T6SS activity as previously reported.7 While similar results were obtained with FQ-A001 cells harvested from a culture at low cell density (LCD; OD600 ~0.2), cells grown to high cell density (HCD; OD600 ~2.0) led to high CFP fluorescence that was comparable to the Δhcp Δhcp1 control (Figure 1B), which suggests low T6SS activity of FQ-A001 within the spot.
Previous work using an erythromycin-resistant (ermR) strain of ES114 demonstrated that FQ-A001 kills ES114 cells within the first hours of their co-incubation in a T6SS2-dependent manner.12 To investigate how growth physiology of FQ-A001 affects T6SS2-dependent killing, we tracked the abundance of ermR ES114 within spots generated with FQ-A001 cells harvested from cultures at different growth phases. After 4 h of incubation with FQ-A001 cells collected from either ON and LCD cultures, ermR ES114 CFU levels had decreased in a Hcp-dependent manner (Figures 1C and S1A). In contrast, ermR ES114 grew after being incubated with FQ-A001 cells harvested from HCD cultures (Figures 1C and S1A), which suggests the harvested FQ-A001 cells had low T6SS2 activity. Further examination of FQ-A001 revealed a strong inverse correlation between cell density and T6SS2 activity (Figure S1B), which suggests that T6SS2 activity is suppressed as a population grows.
The observations described above led us to consider whether T6SS2 activity depends on quorum sensing, which is known to regulate T6SS in other Vibrionaceae.13–16 Quorum sensing occurs when self-produced signaling molecules (autoinducers) achieve a sufficient concentration in the environment to induce specific cellular responses throughout a population. FQ-A001 produces autoinducer at levels comparable to ES114 (Figures S1C–D), which has been reported to produce as much as 1.1 μM C8 HSL.17 To test whether the T6SS2 suppression is linked to autoinducer, we first generated spent media by filter-sterilizing supernatants of FQ-A001 cultures, then briefly exposed naïve FQ-A001 cells to those spent media, and finally assessed their killing activity (Figure 1D). While spent media derived from LCD cultures had no effect on killing activity, the spent media generated from HCD cultures suppressed killing activity (Figure 1D). Spent media derived from LCD cultures of ES114 also suppressed FQ-A001 killing activity (Figure 1E), indicating T6SS2-negative strains also secrete the inhibitory product. In contrast, spent media generated from cultures of an ES114-derived mutant unable to produce HSL-based autoinducers (luxI− ainS−) failed to suppress FQ-A001 killing activity (Figure 1E). However, supplementation of that spent medium with C8 HSL but not N-3-oxohexanoyl-L-homoserine lactone (3-oxo-C6 HSL) suppressed FQ-A001 killing activity (Figure 1E). At 1 μM, C8 HSL lowered transcription levels of the T6SS2 Hcp needle subunit 6.9-fold in FQ-A001 cells grown to LCD (Figure 1F). Furthermore, promoter activity for each hcp gene decreased when FQ-A001 was grown on solid medium supplemented with C8 HSL (Figure S1E). Taken together, these data indicate that C8 HSL-mediated quorum sensing suppresses T6SS2 in V. fischeri.
Quorum-sensing regulator LitR inhibits T6SS activity through VasH
The TetR-like transcription factor LitR is expressed when V. fischeri engages in C8 HSL-mediated quorum sensing (Figure 2A).18 In ES114, LitR is a global regulator that controls bioluminescence production, motility, and colony opacity, and it also contributes to the initial colonization of the light organ.19,20 To determine whether LitR also controls T6SS2 in V. fischeri, we tested the killing activity of a ΔlitR strain of FQ-A001 and found it exhibited increased killing of ES114 even under HCD conditions (Figure 2B). Induction of litR expression in ΔlitR in trans suppressed T6SS2 activity thereby demonstrating genetic complementation (Figure 2B). Based on qRT-PCR, transcript levels of hcp were ~60-fold higher in ΔlitR, which could be complemented by expressing litR in trans (Figure 2C). In addition, promoter activity for each hcp gene was elevated in the ΔlitR mutant (Figure S2A). Together, these results suggest LitR represses T6SS2 expression.
Figure 2. LitR inhibits T6SS activity via VasH.
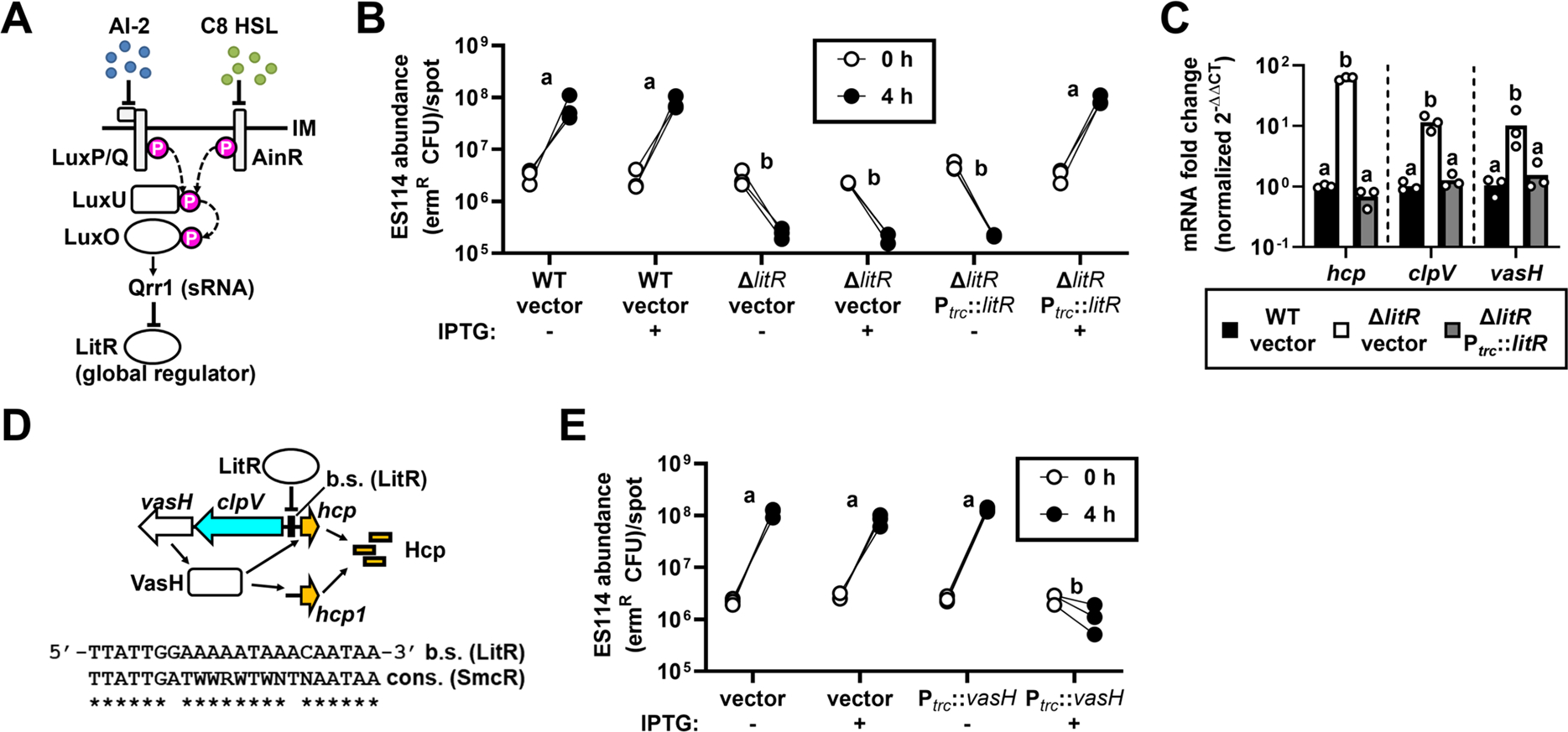
(A) C8 HSL-mediated quorum sensing promotes LitR expression by inhibiting the indicated phosphorelay.
(B) ermR ES114 abundance in spots with cells of indicated FQ-A001-derived strains grown in media ± IPTG to HCD.
(C) Levels of T6SS2 transcripts normalized to control rpoD in HCD-grown cells. Bars =mean, with samples shown by points.
(D) Schematic of LitR regulatory control over T6SS2 expression, with sequence of putative LitR binding site within the clpV-hcp intergenic region displaye€(E) ermR ES114 abundance in spots with cells of FQ-A001 with IPTG-inducible vasH grown to HCD ± IPTG.
B, C & E: Groups with identical (different) letters indicate same (different) statistical groups.
To determine how LitR represses T6SS2 expression, we inspected the main and auxiliary T6SS2 gene clusters for putative LitR binding sites but only identified one centered 106 bp upstream of the hcp promoter (Phcp) (Figure 2D).21 However, Phcp is divergently transcribed from clpV, which indicates that the putative LitR binding site occurs within the intergenic region containing the promoter for clpV (PclpV) (Figure 2D). This arrangement is notable because vasH, which encodes the bacterial enhancer binding protein that promotes σ54-dependent transcription of both hcp genes,7 is located downstream of and is co-transcribed with clpV (Figures 2D and S2B). Similar to hcp, transcript levels of clpV and vasH are elevated in ΔlitR (~10-fold), which suggests that LitR downregulates transcriptional expression of the VasH regulator (Figure 2C). To test whether ectopic expression of VasH could overcome T6SS2 suppression, we induced expression of vasH in FQ-A001 cells grown to high cell density and observed increased killing of ES114 (Figure 2E), which suggests that the suppression of T6SS2 by LitR is due to low levels of VasH. Taken together, these results suggest that the T6SS2 of V. fischeri is suppressed by quorum sensing due to LitR repression of VasH.
Quorum sensing inhibits strain incompatibility during symbiosis establishment
In the E. scolopes-V. fischeri symbiosis, strain incompatibility describes the inability of different strains to colonize the same crypt space within a light organ,22 and previous work has established a dual-strain squid-colonization assay based on FQ-A001 and ES114 to experimentally study this phenomenon.7,11,12 Briefly, juvenile squid are first exposed to an inoculum containing FQ-A001 and ES114 labeled with different fluorescent proteins, and then their light organs are assessed by fluorescence microscopy at 24 h post-inoculation (p.i.) to score the crypt spaces for each strain (Figures 3A–B). To determine whether quorum sensing impacts strain incompatibility, we treated juvenile squid with 1 μM C8 HSL directly after completion of the 3.5-h inoculation stage and found that 4/26 (15%) animals featured at least one crypt space containing both strains in contrast to none of the animals in the control group (p = 0.04136, two-tailed two-proportion Z-test) (Figure 3C), which suggests that C8 HSL can inhibit strain incompatibility within crypt spaces. To test whether this effect depends on LitR, we examined animals treated with 1 μM C8 HSL following exposure to an inoculum containing FQ-A001 ΔlitR and ES114. We found that none of the animals had co-colonized crypts in contrast to the 13/21 (62%) animals in the control group featuring FQ-A001 (p < 0.00001, two-tailed, two-proportion Z-test) (Figure 3D), which suggests that strain incompatibility occurs with ΔlitR and ES114 despite the presence of C8 HSL. Taken together, these results suggest that the ability of C8 HSL to inhibit strain incompatibility within the light organ depends on FQ-A001 encoding LitR.
Figure 3. Quorum sensing inhibits strain incompatibility during symbiosis establishment.

(A) Juvenile E. scolopes, with box highlighting light organ. Bar = 0.5 mm.
(B) Left, experimental design to assess T6SS2-dependent strain incompatibility between FQ-A001 (YFP) and ES114 (CFP) in vivo. Right, images of light organ featuring colonized crypt spaces, with corresponding scoring metric. Bar = 100 μm.
(C) Colonization assay performed ± 1μM C8 HSL, with animals featuring ≥ 1 co-colonized crypt space labeled with asterisk.
(D) Colonization assay performed with 1μM C8 HSL with FQ-A001-derived WT or ΔlitR strains, with animals featuring ≥ 1 co-colonized crypt space labeled with asterisk.
(E) Colonization assay performed with a 1-h delay in FQ-A001 inoculation, with animals featuring ≥ 1 co-colonized crypt space labeled with asterisk.
(F) Colonization assay performed with ES114-derived WT or luxI− ainS− strains and a 1-h delay in FQ-A001 inoculation, with animals featuring ≥ 1 co-colonized crypt space labeled with asterisk.
(C-F) Each P-value corresponds to an unpaired Mann-Whitney test.
Previous work has shown that strains related to FQ-A001 tend to enter crypt spaces more quickly than ES114.23 Therefore, we hypothesized that in a crypt accessed by both strains, strain incompatibility occurs before the population grows sufficiently large to engage in C8 HSL-mediated quorum sensing. To test this hypothesis, we exposed animals first to ES114 and then to FQ-A001 after a 1-h delay and found that 15/26 (58%) animals had at least one crypt space containing both strains in contrast to the control group that featured no co-colonized crypts (p < 0.0001, two-tailed, two-proportion Z-test) (Figure 3E), which suggests that early entry of ES114 to a crypt space can inhibit strain incompatibility with FQ-A001. To determine whether the ability of ES114 to produce HSL-based autoinducers impacts this effect, we used the luxI− ainS− mutant of ES114 and again delayed the introduction of FQ-A001 into the inoculum by 1 h. In contrast to the WT control that featured 12/16 (75%) animals with co-colonized crypt spaces, the group involving the ES114-derived mutant had only one animal with a single co-colonized crypt space (p = 0.00008, two-tailed, two-proportion Z-test) (Figure 3F), which suggests that the ability to produce HSL-based autoinducers is important for ES114 to occupy the same crypt space as FQ-A001. Taken together, these experiments suggest that C8 HSL-mediated quorum sensing can inhibit strain incompatibility in V. fischeri and that the initial assembly dynamics play a critical role in determining how different strains interact while establishing symbiosis.
DISCUSSION
Recent findings indicate that interbacterial competition plays an important role in animal-bacterial symbioses by dictating which symbionts are sufficiently abundant to function in vivo.11,24–26 However, the mechanisms by which bacterial symbionts regulate the genetic factors that govern competition have remained unclear. In this study, we have shown that C8 HSL-based quorum sensing inhibits expression of the VasH regulator via the transcription factor LitR in V. fischeri. This regulation lowers expression of Hcp, which prevents T6SS-dependent bacterial killing of other symbiotic strains.
We observed an anticorrelation between cell density and T6SS2 activity in V. fischeri that is due to regulation of Hcp expression at the level of transcription. A previous study suggested that transcriptional expression of Hcp is elevated when V. fischeri is grown on solid surfaces or in high-viscosity liquid environments but not in low-viscosity liquid environments.27 Notably, those experiments required the liquid-based cultures to grow for 12 h. Based on the findings reported here (Figures 1C, 1F, & 2C), those cultures had likely established a quorum so that LitR repressed transcription of the hcp genes, inadvertently leading to the incorrect conclusion that T6SS2 activity does not occur in low-viscosity liquid. Instead, our results suggest that V. fischeri expresses the T6SS2 system in aqueous environments unless Hcp expression is suppressed by cells engaging in C8 HSL-based quorum sensing. How C8 HSL-based quorum sensing affects viscosity-dependent intercellular interactions remains unknown and is an important direction for future studies.
Our previous studies that investigated the molecular underpinnings of T6SS2 benefited from the fact that ON cultures exhibit T6SS2 activity, which is curious given the discovery reported here that HCD conditions suppress T6SS2.7,12 One possibility is that the environmental conditions associated with ON cultures lower the stability of autoinducers as observed for other bacteria.28,29 The low levels of C8 HSL would promote expression of the small regulatory RNA Qrr1 that post-transcriptionally inhibits LitR, 30,31 thereby permitting T6SS2 to be transcribed. Alternatively, V. fischeri may encode another regulatory factor that overrides LitR repression to activate the T6SS2 gene expression during late stationary phase. Further studies are necessary to address how the differences in cell physiology between ON and HCD conditions alter T6SS2 activity.
High Hcp expression is paramount for cells to maintain T6SS-mediated aggression.32 In general, the stoichiometry of a T6SS requires up to 700 copies of Hcp per complex.33 Furthermore, because the inner tube is lost to the environment upon each firing event, Hcp must be synthesized de novo for an individual complex to function again. Among T6SS gene clusters in V. fischeri, only the hcp operons depend on σ54 for transcriptional activation,5,7 which is a regulatory architecture that has been shown in other T6SS-positive bacteria to permit the differential expression of secreted components relative to the components of the membrane complex and sheath.34 In V. fischeri, the main gene cluster contains one of the hcp genes,12 which is different than in some other Vibrionaceae that possess hcp genes only within auxiliary gene clusters with nearly identical promoter regions, e.g., V. cholerae.34 How the specific arrangement of the two hcp genes in V. fischeri impacts the dynamics of T6SS will be the focus of future investigations.
Our discovery that quorum sensing can lower T6SS activity in V. fischeri has major implications on whether strain incompatibility will occur within the squid light organ. For instance, if a strain can establish a quorum within a crypt space prior to the entry of an incompatible T6SS-positive cell, then the corresponding C8 HSL will likely dampen T6SS activity of the secondary colonizer, which inhibits interference competition to permit the occurrence of a mixed population. Consequently, our finding of conditions that result in crypt spaces co-colonized with ES114 and FQ-A001 illustrates a striking example of an inhibitory priority effect, in which the production of C8 HSL by the primary colonizer alters the habitat in a way that allows greater strain diversity during the initial colonization of the light organ. Colonization of a crypt space leads to that space becoming recalcitrant to subsequent colonization events due to constriction of the crypt-specific duct within 6–24 hours, so that the strains that successfully colonize the host during the first few hours after hatching are the only ones that can be maintained throughout the life history of the squid.35 Thus, this regulatory mechanism that operates within this critical window of time may explain how both T6SS-positive and T6SS-negative strains are frequently recovered from individual wild-caught squid.11 Future work will hone in on this period of time to directly test the extent to which LitR controls T6SS2 expression in vivo.
The ability of V. fischeri to regulate T6SS activity by quorum sensing enables a specific bacterial signal to alter how microbes interact with one another in specific environments. Our observation is reminiscent of how quorum sensing influences the interactions among V. cholerae cells that are growing on the chitin-rich exoskeletons of zooplankton.36,37 By expressing chitinases that breakdown the otherwise insoluble chitin polymers, V. cholerae releases smaller chains of N-acetylglucosamine that stimulate the natural transformation pathway.38 Under conditions of high cell density, quorum sensing upregulates Hcp expression via HapR, which contributes to increased T6SS activity.13 Consequently, quorum sensing on chitinaceous surfaces enables V. cholerae to lyse neighboring cells via effectors delivered by T6SS, which releases large fragments of DNA that can be incorporated by natural transformation.39 Our discovery that V. fischeri responds to quorum sensing in an opposite manner, i.e., by suppressing T6SS expression, underscores how the ecological niche of a microbe drives the evolution of its T6SS. Thus, an understanding of the spatiotemporal dynamics of T6SS activity for the V. fischeri populations within the light organ will be an important direction for future research. Recent work has shown that the different populations within the light organ can interact via quorum sensing such that 3-oxo-C6 HSL synthesized by one population can induce bioluminescence production by cells in neighboring crypt spaces.40 Whether C8 HSL can also facilitate interpopulation signaling and perhaps mediate the suppression of T6SS activity from a distance remains to be seen.
While we have shown that LitR downregulates T6SS activity through VasH, we expect other traits of FQ-A001 that affect the symbiosis to be regulated by quorum sensing independent of VasH. In ES114, it is well established that LitR regulates bioluminescence production, motility, and colony opacity, and this global regulator also contributes to the initial colonization of the light organ.19,20 Our finding that the LitR regulon can include T6SS-related genes suggests that this regulatory network can be re-purposed in different symbiotic strains. Further work is needed to determine the extent to which the regulatory network of LitR has been re-wired in different strains and how these modifications impact symbiosis establishment.
The ability of LitR to suppress T6SS activity may highlight a cost to fitness if T6SS expression is left unchecked. In addition to cells needing to resynthesize the inner tube de novo after each firing event as described above, they must also unfold the sheath subunits through a dedicated ATPase to recycle the T6SS membrane complex.41 One possibility is that when coupled with bioluminescence production, the energetics associated with a constitutive T6SS would be too high for the energy captured from fermentation of chitin-derived oligosaccharides supplied by the squid host to maintain the symbiont population at night.42 Conversely, there may be a benefit of a multi-strain population inhabiting a crypt space that impacts the symbiosis. The remarkable amenability of the E. scolopes-V. fischeri system to experimental manipulation makes the pursuit of such inquiries feasible. In summary, an understanding of the regulatory mechanisms of cellular aggression in a bacterial symbiont, such as the example reported here, provides important insight into the role of intercellular interactions on the dynamics of symbiont assembly during the initial steps of host colonization.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further information and reasonable requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Tim I. Miyashiro (tim14@psu.edu).
Materials availability
Strains and plasmids generated for this study are available upon reasonable request from the lead contact.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
E. scolopes hatchlings were generated using the mariculture facility described previously.47,48 Briefly, egg clutches that were produced by female E. scolopes were maintained in individual chambers with circulating water. Upon hatching, individual hatchlings were transferred to tumblers for experimentation. All adult animals associated with the mariculture facility were collected in offshore seawater in Oahu, HI. Collection, care, and research of all laboratory animals was completed under the program’s Institutional Animal Care and Use Committee (IACUC). IACUC protocol #PROTO202101789.
All V. fischeri strains used in this study were derived from either ES114 or FQ-A001, and regularly maintained on LBS medium (defined below) at 28°C.44,45 Glycerol stocks of V. fischeri strains were maintained at −80°C. Strains were regularly authenticated by testing for the presence of specific mutations, e.g., ΔlitR, by colony PCR using primers that flank the mutation.
Growth conditions
The V. fischeri strains and plasmids used in this report are listed in the Key Resources Table. V. fischeri strains were grown aerobically in LBS medium [1% (w/v) tryptone, 0.5% (w/v) yeast extract, 2% (w/v) NaCl, 50 mM Tris–HCl (pH 7.5)] or SWTO medium [0.5% (w/v) tryptone, 0.3% (w/v) yeast extract, 0.3% of glycerol in Instant Ocean mixed to 35 ppt].49,50 For solid medium, agar was added to a final concentration of 1.5% w/v. When growing strains harboring plasmids, the medium was supplemented with chloramphenicol (Cm) at a final concentration of 2.5 μg ml−1 and/or erythromycin (Erm) at a final concentration of 5 μg ml−1. When appropriate, IPTG was dissolved in water as a vehicle and used at a final concentration of 100 μM. Autoinducers (C8 HSL and 3-oxo-C6 HSL) were dissolved in DMSO as a vehicle and used at a final concentration of 1 μM.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Wild-type V. fischeri | 43,44 | ES114 |
| T6SS2+ Wild-type V. fischeri | 22,45 | FQ-A001 |
| FQ-A001 Δhcp Δhcp1 | 12 | NPW58 |
| FQ-A001 ΔlitR | This study | KRG001 |
| ES114 ΔainS ΔluxIR Plux-luxCDABEG | 30 | JHK007 |
| C8 HSL bioreporter strain of V. fischeri | 46 | DC22 |
| E. coli for cloning | Lab stock | Top10 |
| E. coli for cloning and conjugation into V. fischeri | Lab stock | S17-1 λ-pir |
| ES114 Tn7::erm | 18 | TIM313 |
| FQ-A001 ΔvasH | 7 | KRG005 |
| Chemicals, peptides, and recombinant proteins | ||
| Yeast Extract | Fisher, Hampton, NH, USA | Cat#BP1422-500 |
| Tryptone | Fisher, Hampton, NH, USA | Cat#BP1421-500 |
| Sodium Chloride | Fisher, Hampton, NH, USA | Cat#BP358-10 |
| Agar | Fisher, Hampton, NH, USA | Cat#BP1423-500 |
| Tris Base | Fisher, Hampton, NH, USA | Cat#BP152-1 |
| N-octanoyl-L-homoserine lactone | Cayman Chemical, Ann Arbor, MI, USA | Cat#10011199 |
| N-(3-Oxohexanoyl)-L-homoserine lactone | Sigma-Aldrich St. Louis, MO, USA | Cat#K3007-10MG |
| Instant Ocean Sea Salt | Fisher, Hampton, NH, USA | Cat#NC1023135 |
| KpnI-HF | NEB, Ipswich, MA, USA | Cat#R3142S |
| SacI-HF | NEB, Ipswich, MA, USA | Cat#R3156S |
| XbaI-HF | NEB, Ipswich, MA, USA | Cat#R0145S |
| XmaI-HF | NEB, Ipswich, MA, USA | Cat#R0180L |
| SalI-HF | NEB, Ipswich, MA, USA | Cat#R3138S |
| T4 DNA Ligase | NEB, Ipswich, MA, USA | Cat#M0202L |
| Chloramphenicol | Fisher, Hampton, NH, USA | Cat#BP904100 |
| PFU Ultra Polymerase | Agilent, Santa Clara, CA, USA | Cat#600385-51 |
| EconoTaq Plus 2x Master Mix | Fisher, Hampton, NH, USA | Cat#NC0421795 |
| Isopropyl-β-D-thiogalactopyranoside (IPTG) | Fisher, Hampton, NH, USA | Cat#BP162010 |
| MuLV Reverse Transcriptase | NEB, Ipswich, MA, USA | Cat#M0253S |
| Oligonucleotides are listed in Table S1 | ||
| Plasmids are listed in Table S2 | ||
| Critical commercial assays | ||
| Zero Blunt PCR Cloning Kit | Invitrogen, Waltham, MA, USA | Cat#K270020 |
| EZNA Miniprep Kit | Omega Bio-tek, Norcross, GA, USA | Cat#D6943-02 |
| Gel Extraction Kit | Omega Bio-tek, Norcross, GA, USA | Cat#D2500-01 |
| Cycle Pure Kit | Omega Bio-tek, Norcross, GA, USA | Cat#D6492-01 |
| RNeasy Kit | Qiagen, Hilden, Germany | Cat#74004 |
| Turbo DNA-free Kit | Invitrogen, Waltham, MA, USA | Cat# AM 1907 |
| Software and algorithms | ||
| Prism 9 | GraphPad | Version 9.3.1 |
| ImageJ | NIH (Public Domain) | FIJI version 2.0.0 |
Unless stated otherwise, starter cultures for the experiments described in this report began by inoculating for each strain 3 mL LBS containing antibiotic as appropriate with an isolated colony of the indicated strain and incubating cultures at 28°C overnight with shaking (200 rpm). Cell suspensions were then generated by normalizing culture samples to OD600 = 1.0 using LBS as a diluent. In the assays described below, these cell suspensions were used either directly in the assay or to generate cultures by diluting 1:100 into the indicated medium in either culture tubes or Erlenmeyer flasks. In this study, LCD and HCD correspond to OD600 ranging from 0.1–0.3 and 2.0–2.6, respectively. To generate spent media, culture samples were cooled quickly on ice and centrifuged at 4°C at 3,220 × g for 7 minutes. The resulting supernatant was filtered through a 0.22-μm filter and stored at 4°C until being used.
METHOD DETAILS
Molecular Biology
The deletion allele ΔlitR was generated using FQ-A001 genomic DNA by PCR amplification of 1.5-kb regions flanking VFFQA001_RS16675 and cloning the products into pEVS79, generating the mutagenesis construct pKRG002. Primers and restriction sites used in the cloning process are listed in Table S2. To generate the knockout mutant, this plasmid was introduced into FQ-A001 by conjugation and screening by PCR for the double-crossover event, according to a previously established protocol.18
Construction of promoter reporters was accomplished by amplifying specific promoter regions from FQ-A001 genomic DNA using the primers listed in the Key Resources Table. The amplicon was cloned into the pCR-Blunt vector (Invitrogen) and verified by sequencing. The insert was subcloned into pTM267 using XbaI and XmaI to generate a GFP transcriptional fusion.18
The inducible litR construct was generated by amplifying the litR gene from FQ-A001 using the primers listed in the Key Resources Table. The amplicon was cloned into the pCR-Blunt vector (Invitrogen), confirmed by sequencing, and isolated using XmaI and SalI (NEB) restriction enzymes. The insert was subsequently cloned downstream of the Ptrc IPTG-inducible promoter using the XmaI/SalI-derived vector fragment of pTM214.51
Co-incubation assays
Co-incubation assays consisted of mixtures of FQ-A001-derived cells with either ES114 harboring pYS112, which expresses CFP, or the ES114-derived strain TIM313, which contains a chromosomally integrated ermR marker at the Tn7 site. To control for potential variation among the ES114-derived strains in co-incubation assays, cultures of pYS112/ES114 and TIM313 were grown in SWTO-Cm and SWTO, respectively, to OD600 = 1.0, at which point they were chilled on ice, mixed with glycerol to 25% (v/v), dispensed as 0.5-mL aliquots, and frozen at −80°C. Prior to initiating an assay, aliquots were thawed on ice.
To initiate a co-incubation assay, a 50-μL cell suspension of an FQ-A001-derived culture was combined with 50 μL of an ES114-derived strain in a microfuge tube and briefly vortexed. A 10-μL volume was placed onto the surface of LBS agar, with up to six different samples per petri dish, with biological replicates on different petri dishes. Petri dishes were incubated at 24°C and assessed at the indicated time. For experiments involving multiple time points, one sample was prepared for each time point.
To assess the CFP fluorescence within a sample that included pYS112/ES114, an image of the spot was acquired in RAW format using a Rebel T5 Camera (Canon) mounted on a SZX16 fluorescence dissecting microscope (Olympus) equipped with an SDF PLFL 0.3x objective and a CFP filter set. Images were processed by converting the RAW image format to TIFF using ImageJ (v. 1.52a (NIH)) digital camera raw (DCRaw macro) with the following settings: use_temporary_directory, white_balance = [Camera white balance], do_not_automatically_brighten, output_colorspace = [sRGB], read_as = [8-bit], interpolation = [High-speed, low-quality bilinear], and half_size. The blue channel of each image was used to show the CFP fluorescence associated with each spot.
To assess the abundance of ermR CFU within a sample that included TIM313, an agar plug containing the spot was excised using flame-sterilized forceps, suspended in 1 mL LBS medium, serially diluted, plated onto LBS-Erm, and incubated at 28°C. The resulting CFU counts were used to calculate the abundance of TIM313 at the time of harvest.
Bioluminescence assay
Strain DC22 was used as a biosensor for C8 HSL. A culture of DC22 was grown by diluting a starter culture 1:1000 into a 500-mL Erlenmeyer flask containing 100 mL SWTO and growing it at 28°C at 200 rpm. At OD600 = 0.8, the culture was centrifuged at 4,000 × g for 15 min. The supernatant was removed by pipetting, and the pellet was resuspended in SWTO at 1:10 the original culture volume. Each sample was prepared by combining 1.8 mL of a cell-free supernatant with 0.2 mL of the concentrated biosensor cell suspension and incubated at 28°C shaking at 200 rpm. After 20 min, a 100-μL sample was transferred to a cuvette and the corresponding bioluminescence was measured using a GloMax 20/20 luminometer (Promega). The turbidity was determined by adding 0.9 mL SWTO to the cuvette and measuring OD600 using a BioPhotometer spectrophotometer (Eppendorf). To construct a standard curve for using DC22 as a biosensor for C8 HSL, spent medium was generated from a culture of DC22 was first grown in 20 mL SWTO to OD600 = 2.0 as described above.
Reverse transcriptase-Quantitative PCR (RT-qPCR)
For the experiment shown in Figure 1F, cultures of FQ-A001 were grown in SWTO supplemented with C8 HSL or vehicle to LCD. The volume of sample that was harvested from each culture was equivalent to the volume of cells needed to generate an OD600 = 1.0 in 1 mL. To accomplish this, the cells were centrifuged at 3,320 × g for 7 minutes and all but 1 mL of the supernatant was removed. The pellet was re-suspended and centrifuged again at 9,000 × g for 2.5 minutes, after which all but 50 μL of supernatant was removed. Cell pellets were snap frozen in a 70 percent ethanol bath supercooled by dry ice and then stored at −80°C.
For the experiment shown in Figure 2C, cultures of FQ-A001 (WT) or KRG001 (ΔlitR) harboring either pTM214 (vector) or pKRG023 (Ptrc::litR) were grown in SWTO-Cm supplemented with 100 μM IPTG to HCD. The volume of sample that was harvested from each culture was equivalent to the volume of cells needed to generate an OD600 = 1.0 in 1 mL. These cells were centrifuged at 9,000 xg for 2.5 minutes, after which all but 50 μL of supernatant was aspirated. Cell pellets prepared for storage at −80°C as described above.
RNA extraction was performed using the Rneasy mini kit (Qiagen) according to manufacturer instructions. For each sample, 1 μg of RNA was treated with DNase using the TURBO DNA-free kit (Invitrogen) according to manufacturer instructions, and reaction products were confirmed to be free of DNA by PCR using primers specific for rpoD. cDNA was generated by treating 700 ng of RNA with MuLV reverse transcriptase (NEB) and random hexamers (ThermoFisher) according to manufacturer instructions. For each gene target, 50 ng of cDNA template was amplified by qPCR with an AriaMx real-time thermocycler in 20-μL reactions containing Evagreen dye (Biotium), EconoTaq Plus 2x master mix (Lucigen), and primer sets listed in Table S2. The thermocycler profile for the amplification included a 10-min hot start at 95°C followed by 40 cycles of incubation at 95°C for 15 s, 50.5°C for 45 s, and 72°C for 30 s. PCR amplicon identity was verified by heating from 55°C to 95°C with ramp time of 15 s. Relative fold changes were determined using the ΔΔCT method, with expression normalized using housekeeping gene rpoD.52 The ability to distinguish between hcp and hcp1 is limited by the sequence similarity between the two genes.
GFP-based promoter reporter assays
To initiate the assay, a 2.5-μL volume of a cell suspension derived from a starter culture was placed on LBS-Cm agar and incubated at 24°C for 24 h. Images of each spot of growth were acquired in RAW format using a Rebel T5 Camera (Canon) mounted on a SZX16 fluorescence dissecting microscope (Olympus) equipped with an SDF PLFL 0.3x objective and both GFP and mCherry filter sets. Images were processed by converting the RAW image format to TIFF using ImageJ (v. 1.52a (NIH)) digital camera raw (DCRaw macro) with the following settings: use_temporary_directory, white_balance = [Camera white balance], do_not_automatically_brighten, output_colorspace = [sRGB], read_as = [8-bit], interpolation = [High-speed, low-quality bilinear], and half_size. The red channel of the image taken using the mCherry filter was used to determine the mCherry fluorescence. mCherry signal was used to define the region of interest (ROI) via thresholding. The mean green fluorescence level within each ROI was determined using the green channel for the image taken with the GFP filter. Autofluorescence levels were determined from the pixel values associated with the green channel of an image of a non-fluorescent strain, FQ-A001 harboring plasmid pVSV105 taken using the GFP filter set.
Endpoint RT-PCR
To initiate the assay, three 10-μL volumes of cell suspension derived from a starter culture were placed on LBS and incubated at 24°C. After 22 h, the spots of growth were scraped up from the surface using a sterile wooden stick and collectively resuspended in 1 mL LBS. A 0.9-mL volume of the cell suspension was subjected to RNA extraction and DNase treatment using the RNA Extraction Kit (Epicentre). To generate cDNA, 1.0 μg of RNA was subjected to reverse transcription using MuLV-RT and random hexamer primers from the cDNA Synthesis Kit (ThermoFisher). For each 50-μL PCR, 1 μL of cDNA was used as template with PFU Ultra Polymerase and a thermocycler profile initiating with a 2-min 95°C step, followed by 30 cycles of incubation at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, and ending with 72°C for 5 min. Primers are listed in the Oligonucleotides table. PCR products were evaluated using gel electrophoresis with 2.0% agarose gel.
Squid-colonization assays
Cultures of the indicated strains were harvested at OD600 ~ 1.0, and cells were washed twice by centrifugation at 5,000 × g for 2 minutes, removal of the supernatant, and re-suspension with filter-sterilized seawater (FSSW) with each wash. The cell suspension was further diluted into 50 mL FSSW, which was poured into a tumbler with 50 mL FSSW and hatchlings, with targeted inoculum sizes of 5,000 CFU/mL for FQ-A001 and 50,000 CFU/mL for ES114. Relative to parental strains, inoculums for KRG001 (FQ-A001 ΔlitR) and JHK007 (ES114 luxI− ainS−) were increased 5-fold and 1.5-fold, respectively, due to colonization defects associated with each strain. Hatchlings used in these experiments were less than 24 h old and generated by a mariculture facility as described elsewhere.47 Delayed inoculation experiments were performed by generating inoculums initially with only ES114-derived strains and then adding FQ-A001-derived cells 1 h later. After 3.5 h of exposure to ES114 cells, animals were washed of the inoculum by being transferred to 100 mL fresh FSSW twice. After washing, each squid was singly housed in an individual vial containing 4 mL FSSW. For animals that were exposed to C8 HSL treatment, 1 μM C8 HSL, which was diluted in dimethylsulfoxide (DMSO) as a solvent, was added to the 4 mL FSSW in which animals were housed overnight. This concentration of C8 HSL is less than the 5 μM used to complement the ainS mutant of ES114 in vivo as previously described.53 DMSO was used as a vehicle-only control and previous work has shown 0.25% DMSO does not impact squid physiology.53 At 24 h post-inoculation (pi), animals were transferred to new vials containing 4 mL FSSW and evaluated for bioluminescence using GloMax 20/20 luminometer (Promega). Immediately afterward, animals were placed on ice for 10 minutes, fixed in 4% paraformaldehyde, and imaged by fluorescence microscopy with a Zeiss 780 confocal microscope (Carl Zeiss AG, Jena, Germany).11
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical details of experiments, including the statistical test(s) used to determine significance and how significance is represented in each plot, can be found in the figure legends and in the main text. For culture-based experiments, each circle represents an independent biological replicate. For animal experiments, samples were randomized prior to imaging and analysis. Each plot displayed in this manuscript is representative of at least two experimental trials. Plots were generated and statistical tests were performed using GraphPad Prism (v 9.5.1).
DATA AND CODE AVAILABILITY
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Supplementary Material
DATA S1. Statistical tests for indicated experimental results. Related to Figures 1 and 2.
(A) Statistical results for experiments shown in Figures 1C–E.
(B) Statistical results for experiments shown in Figures 2B, 2C, and 2E.
Highlights.
Quorum sensing inhibits type 6 secretion system (T6SS) activity in Vibrio fischeri.
Quorum-sensing regulator LitR downregulates the T6SS activator VasH
Quorum sensing inhibits strain incompatibility during symbiosis establishment
ACKNOWLEDGEMENTS
This work was supported by National Institute of General Medical Sciences Grant R01 GM129133 (to T.I.M.), National Institute of Allergy and Infectious Diseases Fellowship F32 AI147543 (to K.R.G.), and Beckman Scholars Program award (to T.A.Y.). The authors also thank the outstanding feedback of three anonymous reviewers during the peer-review process.
INCLUSION AND DIVERSITY STATEMENT
We support inclusive, diverse, and equitable conduct of research.
Footnotes
DECLARATIONS OF INTEREST
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Loso T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 110, 3229–3236. 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran NA (2006). Symbiosis. Curr. Biol. 16, R866–871. 10.1016/j.cub.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Ganesan R, Wierz JC, Kaltenpoth M, and Flórez LV (2022). How It All Begins: Bacterial Factors Mediating the Colonization of Invertebrate Hosts by Beneficial Symbionts. Microbiol. Mol. Biol. Rev. 86, e0012621. 10.1128/mmbr.00126-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visick KL, Stabb EV, and Ruby EG (2021). A lasting symbiosis: how Vibrio fischeri finds a squid partner and persists within its natural host. Nat. Rev. Microbiol. 19, 654–665. 10.1038/s41579-021-00557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guckes KR, and Miyashiro TI (2023). The type-VI secretion system of the beneficial symbiont Vibrio fischeri. Microbiology (Reading) 169. 10.1099/mic.0.001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bongrand C, and Ruby EG (2019). The impact of Vibrio fischeri strain variation on host colonization. Curr. Opin. Microbiol. 50, 15–19. 10.1016/j.mib.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guckes KR, Cecere AG, Williams AL, McNeil AE, and Miyashiro T (2020). The Bacterial Enhancer Binding Protein VasH Promotes Expression of a Type VI Secretion System in Vibrio fischeri during Symbiosis. J. Bacteriol. 202, e00777–19. 10.1128/JB.00777-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith S, Salvato F, Garikipati A, Kleiner M, and Septer AN (2021). Activation of the Type VI Secretion System in the Squid Symbiont Vibrio fischeri Requires the Transcriptional Regulator TasR and the Structural Proteins TssM and TssA. J. Bacteriol. 203, e0039921. 10.1128/JB.00399-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma SC, and Miyashiro T (2013). Quorum sensing in the squid-Vibrio symbiosis. Int. J. Mol. Sci. 14, 16386–16401. 10.3390/ijms140816386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyashiro T, and Ruby EG (2012). Shedding light on bioluminescence regulation in Vibrio fischeri. Mol. Microbiol. 84, 795–806. 10.1111/j.1365-2958.2012.08065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speare L, Cecere AG, Guckes KR, Smith S, Wollenberg MS, Mandel MJ, Miyashiro T, and Septer AN (2018). Bacterial symbionts use a type VI secretion system to eliminate competitors in their natural host. Proc. Natl. Acad. Sci. USA 115, E8528–E8537. 10.1073/pnas.1808302115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guckes KR, Cecere AG, Wasilko NP, Williams AL, Bultman KM, Mandel MJ, and Miyashiro T (2019). Incompatibility of Vibrio fischeri strains during symbiosis establishment depends on two functionally redundant hcp genes. J. Bacteriol. 201, e00221–19. 10.1128/JB.00221-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao Y, and Bassler BL (2014). Quorum regulatory small RNAs repress type VI secretion in Vibrio cholerae. Mol. Microbiol. 92, 921–930. 10.1111/mmi.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salomon D, Gonzalez H, Updegraff BL, and Orth K (2013). Vibrio parahaemolyticus type VI secretion system 1 is activated in marine conditions to target bacteria, and is differentially regulated from system 2. PloS one 8, e61086. 10.1371/journal.pone.0061086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng L, Gu D, Wang Q, Liu Q, and Zhang Y (2012). Quorum sensing and alternative sigma factor RpoN regulate type VI secretion system I (T6SSVA1) in fish pathogen Vibrio alginolyticus. Arch. Microbiol. 194, 379–390. 10.1007/s00203-011-0780-z. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Pan J, Gao H, Han Y, Zhang A, Huang Y, Liu P, Kan B, and Liang W (2021). CqsA/LuxS-HapR Quorum sensing circuit modulates type VI secretion system V fl T6SS2 in Vibrio fluvialis. Emerg. Microbes. Infect. 10, 589–601. 10.1080/22221751.2021.1902244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girard L, Blanchet E, Stien D, Baudart J, Suzuki M, and Lami R (2019). Evidence of a Large Diversity of N-acyl-Homoserine Lactones in Symbiotic Vibrio fischeri Strains Associated with the Squid Euprymna scolopes. Microbes. Environ. 34, 99–103. 10.1264/jsme2.ME18145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyashiro T, Wollenberg MS, Cao X, Oehlert D, and Ruby EG (2010). A single qrr gene is necessary and sufficient for LuxO-mediated regulation in Vibrio fischeri. Mol. Microbiol. 77, 1556–1567. 10.1111/j.1365-2958.2010.07309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fidopiastis PM, Miyamoto CM, Jobling MG, Meighen EA, and Ruby EG (2002). LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 45, 131–143. [DOI] [PubMed] [Google Scholar]
- 20.Lupp C, and Ruby EG (2005). Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J. Bacteriol. 187, 3620–3629. 10.1128/JB.187.11.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DH, Jeong HS, Jeong HG, Kim KM, Kim H, and Choi SH (2008). A consensus sequence for binding of SmcR, a Vibrio vulnificus LuxR homologue, and genome-wide identification of the SmcR regulon. J. Biol. Chem. 283, 23610–23618. 10.1074/jbc.M801480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, LaSota ED, Cecere AG, LaPenna KB, Larios-Valencia J, Wollenberg MS, and Miyashiro T (2016). Intraspecific competition impacts Vibrio fischeri strain diversity during initial colonization of the squid light organ. Appl. Environ. Microbiol. 82, 3082–3091. 10.1128/AEM.04143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bongrand C, and Ruby EG (2019). Achieving a multi-strain symbiosis: strain behavior and infection dynamics. ISME J. 13, 698–706. 10.1038/s41396-018-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steele MI, Kwong WK, Whiteley M, and Moran NA (2017). Diversification of Type VI Secretion System Toxins Reveals Ancient Antagonism among Bee Gut Microbes. mBio. 8. 10.1128/mBio.01630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verster AJ, Ross BD, Radey MC, Bao Y, Goodman AL, Mougous JD, and Borenstein E (2017). The Landscape of Type VI Secretion across Human Gut Microbiomes Reveals Its Role in Community Composition. Cell. Host. Microbe. 22, 411–419 e414. 10.1016/j.chom.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kochanowsky RM, Bradshaw C, Forlastro I, and Stock SP (2020). Xenorhabdus bovienii strain jolietti uses a type 6 secretion system to kill closely related Xenorhabdus strains. FEMS. Microbiol. Ecol. 96. 10.1093/femsec/fiaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speare L, Smith S, Salvato F, Kleiner M, and Septer AN (2020). Environmental Viscosity Modulates Interbacterial Killing during Habitat Transition. mBio. 11. 10.1128/mBio.03060-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yates EA, Philipp B, Buckley C, Atkinson S, Chhabra SR, Sockett RE, Goldner M, Dessaux Y, Camara M, Smith H, and Williams P (2002). N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 70, 5635–5646. 10.1128/IAI.70.10.5635-5646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byers JT, Lucas C, Salmond GP, and Welch M (2002). Nonenzymatic turnover of an Erwinia carotovora quorum-sensing signaling molecule. J. Bacteriol. 184, 1163–1171. 10.1128/jb.184.4.1163-1171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimbrough JH, and Stabb EV (2013). Substrate specificity and function of the pheromone receptor AinR in Vibrio fischeri ES114. J. Bacteriol. 195, 5223–5232. 10.1128/JB.00913-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surrett ED, Guckes KR, Cousins S, Ruskoski TB, Cecere AG, Ludvik DA, Okafor CD, Mandel MJ, and Miyashiro TI (2023). Two enhancer binding proteins activate sigma(54)-dependent transcription of a quorum regulatory RNA in a bacterial symbiont. Elife 12, e78544. 10.7554/eLife.78544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin L, Lezan E, Schmidt A, and Basler M (2019). Abundance of bacterial Type VI secretion system components measured by targeted proteomics. Nat. Commun. 10, 2584. 10.1038/s41467-019-10466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basler M (2015). Type VI secretion system: secretion by a contractile nanomachine. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 370, 20150021. 10.1098/rstb.2015.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seibt H, Aung KM, Ishikawa T, Sjostrom A, Gullberg M, Atkinson GC, Wai SN, and Shingler V (2020). Elevated levels of VCA0117 (VasH) in response to external signals activate the type VI secretion system of Vibrio cholerae O1 El Tor A1552. Environ. Microbiol. 22, 4409–4423. 10.1111/1462-2920.15141. [DOI] [PubMed] [Google Scholar]
- 35.Essock-Burns T, Bongrand C, Goldman WE, Ruby EG, and McFall-Ngai MJ (2020). Interactions of Symbiotic Partners Drive the Development of a Complex Biogeography in the Squid-Vibrio Symbiosis. mBio 11, e00853–20. 10.1128/mBio.00853-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crisan CV, and Hammer BK (2020). The Vibrio cholerae type VI secretion system: toxins, regulators and consequences. Environ. Microbiol. 22, 4112–4122. 10.1111/1462-2920.14976. [DOI] [PubMed] [Google Scholar]
- 37.Veening JW, and Blokesch M (2017). Interbacterial predation as a strategy for DNA acquisition in naturally competent bacteria. Nat. Rev. Microbiol. 15, 629. 10.1038/nrmicro.2017.89. [DOI] [PubMed] [Google Scholar]
- 38.Meibom KL, Blokesch M, Dolganov NA, Wu CY, and Schoolnik GK (2005). Chitin induces natural competence in Vibrio cholerae. Science. 310, 1824–1827. 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 39.Matthey N, Stutzmann S, Stoudmann C, Guex N, Iseli C, and Blokesch M (2019). Neighbor predation linked to natural competence fosters the transfer of large genomic regions in Vibrio cholerae. Elife. 8, e48212. 10.7554/eLife.48212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yount TA, Murtha AN, Cecere AG, and Miyashiro TI (2022). Quorum Sensing Facilitates Interpopulation Signaling by Vibrio fischeri within the Light Organ of Euprymna scolopes. Isr. J. Chem. 63, e202200061. 10.1002/ijch.202200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basler M, and Mekalanos JJ (2012). Type 6 secretion dynamics within and between bacterial cells. Science. 337, 815. 10.1126/science.1222901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartzman JA, Koch E, Heath-Heckman EA, Zhou L, Kremer N, McFall-Ngai MJ, and Ruby EG (2015). The chemistry of negotiation: rhythmic, glycan-driven acidification in a symbiotic conversation. Proc. Natl. Acad. Sci. USA 112, 566–571. 10.1073/pnas.1418580112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boettcher KJ, and Ruby EG (1990). Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 172, 3701–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandel MJ, Stabb EV, and Ruby EG (2008). Comparative genomics-based investigation of resequencing targets in Vibrio fischeri: focus on point miscalls and artefactual expansions. BMC. Genomics. 9, 138. 10.1186/1471-2164-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bultman KM, Cecere AG, Miyashiro T, Septer AN, and Mandel MJ (2019). Draft Genome Sequences of Type VI Secretion System-Encoding Vibrio fischeri Strains FQ-A001 and ES401. Microbiol. Resour. Announc. 8. 10.1128/MRA.00385-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyell NL, Colton DM, Bose JL, Tumen-Velasquez MP, Kimbrough JH, and Stabb EV (2013). Cyclic AMP receptor protein regulates pheromone-mediated bioluminescence at multiple levels in Vibrio fischeri ES114. J. Bacteriol. 195, 5051–5063. 10.1128/JB.00751-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cecere AG, and Miyashiro TI (2022). Impact of transit time on the reproductive capacity of Euprymna scolopes as a laboratory animal. Lab. Anim. Res. 38, 25. 10.1186/s42826-022-00135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cecere AG, Cook RA, and Miyashiro TI (2023). A case study assessing the impact of mating frequency on the reproductive performance of the Hawaiian bobtail squid Euprymna scolopes. Lab. Anim. Res. 39, 17. 10.1186/s42826-023-00168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wasilko NP, Larios-Valencia J, Steingard CH, Nunez BM, Verma SC, and Miyashiro T (2019). Sulfur availability for Vibrio fischeri growth during symbiosis establishment depends on biogeography within the squid light organ. Mol. Microbiol. 111, 621–636. 10.1111/mmi.14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyell NL, Dunn AK, Bose JL, and Stabb EV (2010). Bright mutants of Vibrio fischeri ES114 reveal conditions and regulators that control bioluminescence and expression of the lux operon. J. Bacteriol. 192, 5103–5114. 10.1128/JB.00524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyashiro T, Klein W, Oehlert D, Cao X, Schwartzman J, and Ruby EG (2011). The N-acetyl-D-glucosamine repressor NagC of Vibrio fischeri facilitates colonization of Euprymna scolopes. Mol. Microbiol. 82, 894–903. 10.1111/j.1365-2958.2011.07858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmittgen TD, and Livak KJ (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 53.Studer SV, Schwartzman JA, Ho JS, Geske GD, Blackwell HE, and Ruby EG (2014). Non-native acylated homoserine lactones reveal that LuxIR quorum sensing promotes symbiont stability. Environ. Microbiol. 16, 2623–2634. 10.1111/1462-2920.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DATA S1. Statistical tests for indicated experimental results. Related to Figures 1 and 2.
(A) Statistical results for experiments shown in Figures 1C–E.
(B) Statistical results for experiments shown in Figures 2B, 2C, and 2E.
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


