SUMMARY
Pathogenic fungi populate a wide range of environments and infect a diversity of host species. Despite this substantial biological flexibility, the impact of interactions between fungi and their hosts on the evolution of pathogenicity remains unclear. We studied how repeated interactions between the fungus Cryptococcus neoformans and relevant environmental and mammalian host cells—amoeba and mouse macrophages—shape the evolution of this model fungal pathogen. First, using a collection of clinical and environmental isolates of C. neoformans, we characterized a range of survival phenotypes for these strains when exposed to host cells of different species. We then performed serial passages of an environmentally isolated C. neoformans strain through either amoeba or macrophages for ~75 generations to observe how these interactions select for improved replication within hosts. In one adapted population, we identified a single point mutation in the adenylyl cyclase gene, CAC1, that swept to fixation and confers a strong competitive advantage for growth inside macrophages. Strikingly, this growth advantage in macrophages is inversely correlated with disease severity during mouse infections, suggesting that adaptation to specific host niches can markedly reduce the pathogenicity of these fungi. These results raise intriguing questions about the influence of cyclic AMP (cAMP) signaling on pathogenicity and highlight the role of seemingly small adaptive changes in promoting fundamental shifts in the intracellular behavior and virulence of these important human pathogens.
In brief
Interactions between environmental microbes, like many fungi, and diverse hosts can fundamentally alter the biology and pathogenicity of these organisms. Using natural genetic variation and laboratory-based evolution, Hilbert et al. reveal the tempo, mode, and mechanisms by which adaptive evolution occurs in a clinically relevant fungal pathogen.
INTRODUCTION
The ability to thrive in different environments is critical for the evolutionary success of most organisms. For pathogenic microbes, adaptive traits that facilitate survival within host cells or tissues evolve through repeated interactions with host immune factors or exposure to host-like conditions. However, for opportunistic environmental pathogens, including many pathogenic fungi, the mechanisms by which these organisms gain the ability to infect mammalian hosts are not well understood. Furthermore, our understanding of how fungi adapt over short timescales within animal hosts in response to pressures from host immune cells, such as macrophages, is similarly limited.
The human fungal pathogen Cryptococcus neoformans is a globally distributed haploid yeast ubiquitous in the environment and often found associated with trees or bird guano.1 Human infection with C. neoformans occurs primarily through inhalation of fungal spores or desiccated yeasts into the lungs where, in immunocompetent patients, infection is either cleared or contained in a persistent state.2 Symptomatic disease is observed mostly in immunocompromised patients, where dissemination to the brain leads to cryptococcal meningitis, which is lethal unless treated.3 Although C. neoformans is an effective and important human pathogen, the evolutionary pressures and genomic adaptations that led to the emergence of pathogenicity in this species remain elusive.
Some evolutionary studies of virulence in C. neoformans focused on the characterization of large collections of isolates obtained from both clinical and environmental sampling.4–10 Genome-wide association studies identified genomic changes that may underlie the increased success of some of these strains as pathogens.4,9,10 Although such studies are critical in identifying key genes and pathways associated with increased pathogenicity, they are less useful in identifying selective pressures driving acquisition of these traits. Instead, complementary studies have focused on the role of environmental hosts, such as amoebae and nematodes, in shaping the evolution of virulence in Cryptococcus species.11,12
Amoebae are well studied as a potential source of environmental selective pressure for diverse microbes, given similarities to macrophages of animal immune systems. Like macrophages, amoebae can phagocytose microorganisms and undergo many similar processes following phagocytosis.13 Studies of bacterial species, like Legionella pneumophila, identified virulence factors required for success in both amoeba species and mammalian hosts, providing strong evidence that similarities across host-cell environments could select for broadly effective virulence strategies.14–17 For C. neoformans, the polysaccharide capsule has been shown to have protective roles against both amoeba and mammalian hosts, and other features of the intracellular behavior of C. neoformans appear conserved across these evolutionarily diverse species.18–21 However, whether pathogenicity can be evolved through repeated interactions with amoebae or other environmental hosts and the mechanisms through which this occurs have not been well studied.
Adaptation within mammalian hosts also has the potential to drive selection within fungal populations with important implications for disease progression. “Microevolution” studies performed by analyzing serial samples from human patients revealed numerous adaptive phenotypic changes in these strains over long time periods, although correlation between genetic changes and adaptive phenotypes has proven challenging to assess.22–24 Serial in vitro passaging schemes, on the other hand, provide a powerful system to identify phenotypic changes involved in host adaptation and identify the molecular mechanisms associated with such changes.25–28 Few studies, though, have compared the adaptive changes that occur in response to exposure to different host species. In addition, most serial passaging experiments focus on changes that occur in commonly used laboratory strains or closely related clinical isolates, which may not capture the evolutionary trajectories taken by divergent isolates of these fungal species.
Here, we used clinical and environmental isolates of C. neoformans to identify strain-level variation in survival in either environmental (amoeba) or mammalian (mouse macrophage) hosts. We serially passaged one of these environmental isolates to isolate host-adapted strains with enhanced abilities to survive in the intracellular niches of highly diverged host organisms. Remarkably, this approach revealed a single missense mutation in the CAC1 adenylyl cyclase gene sufficient to confer enhanced macrophage replication, which modulates the balance between host adaptation and pathogenicity in this important human fungal pathogen.
RESULTS
Natural variation in C. neoformans strain replication in amoebae and macrophages
We compared the replication of 14 unique C. neoformans isolates in cells of the amoeba, Acanthamoeba castellanii, or in the J774A.1 mouse macrophage-like cell line (Figure 1). The strains represent three of the four major non-recombining C. neoformans sub-lineages—VNI, VNBI, and VNBII—and are predominantly environmental isolates, although several clinical isolates were also included (Figure 1A).4
Figure 1. Phenotypic variation of C. neoformans clinical and environmental isolate survival in amoebae or macrophage hosts.

(A) Phylogenetic tree of clinical and environmental C. neoformans strains used in this paper. Tree was extracted from a larger published phylogeny using the R package Treehouse.4,29 Lineages are indicated by colored boxes and labels, and black dots indicate clinical isolates. All other strains were isolated from environmental sources. Scale bar, 0.1 substitutions/site.
(B and C) Replication of C. neoformans strains in co-incubation experiments with A. castellanii.
(D and E) Replication of the same strains in co-incubation experiments with J774A.1 macrophage cells.
For (B)–(E), bars show the average values ± SEM across 2–3 independent experiments on different days. Dots indicate the average values for 3 replicates for each independent experiment. Significance was assessed by comparison to the H99O reference strain by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Pairwise comparison of NRHc5004 and Muc458-2 in (D) was assessed by unpaired t test with Welch’s correction. *p < 0.05; **p < 0.01; ***p < 0.0001; ns, not significant.
See also Figures S1 and S5.
When incubated with A. castellanii for 24 h, we observed robust killing of cryptococcal cells for nearly all strains tested, despite roughly similar growth in the absence of amoebae and comparable phagocytosis rates (Figures 1B, 1C, S1A, and S1B). Notably, we identified two strains with enhanced replication in amoebae—NRHc5004 and NRHc5029—both of which were isolated from clinical sources. In contrast, all C. neoformans isolates tested were able to replicate to some extent when incubated with mouse macrophages (Figures 1D and 1E). The clinical isolate NRHc5004 also exhibits enhanced replication in macrophages compared with other closely related strains, underscoring the possibility that there are strategies for intracellular replication that confer success across evolutionarily diverse hosts. Again, we observed no significant differences in phagocytosis by macrophages, suggesting that differences in intracellular replication are primarily responsible for the strain variation we observe in these experiments (Figures S1C and S1D).
In addition to strain-specific variation in macrophage replication, we noted even more variable growth of these strains under tissue culture conditions in the absence of macrophages (Figures S1E and S1F). Intriguingly, the environmental isolate Ftc555-1 was killed by incubation in tissue culture media alone, but growth could be partially rescued through the addition of macrophage cells to the culture. We reasoned that we could exploit the preference of Ftc555-1 for the macrophage intracellular environment for serial passaging and host adaptation studies without excessive growth outside of these cells.
Serial passaging selects for host-adapted strains
We devised a serial-passaging scheme where Ftc555-1 was repeatedly subjected to phagocytosis by either A. castellanii amoebae or J774A.1 macrophages (Figure 2A; STAR Methods). Three independent populations were evolved in parallel in each cell type and are hereafter referred to as lines M1–M3 (for macrophage-passaged) and A1–A3 (for amoeba-passaged). We ran courses of experimental evolution for a total of 12 passages over the span of roughly 1 month of continuous culturing. At the end of the experiment, we collected each population and froze down pooled cultures. Importantly, aliquots of each passage were frozen over the course of the experiment to create a “fossil record,” which allowed for high-resolution tracking of adaptive mutations over time and assessment of intermediate phenotypes.
Figure 2. Serial passaging of C. neoformans through macrophages or amoeba cells selects for host-adapted strains.

(A) Schematic of the serial-passaging setup used in this study. See also STAR Methods.
(B) Replication of passaged strains in co-culture with A. castellanii. Single colonies from each individually evolved population were tested for growth in amoeba. Strains M1–M3 were passaged through macrophages; A1–A3 were passaged through amoeba.
(C) Replication of passaged strains in co-culture with mouse macrophage-like J774A.1 cells. The same single colonies were tested as in (B), and data are plotted identically.
For (B) and (C), plotted data are from one representative experiment. Error bars indicate SD and each dot indicates the value for one biological replicate from that experiment. Significance was assessed between the parental strain values and all evolved strains by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. **p < 0.01; ***p < 0.0001; ns, not significant.
See also Figure S2.
Following the final passage, we analyzed the ability of individual colonies from each population to replicate in either amoebae or macrophage hosts (Figures 2B and 2C). In amoebae, only the A1 population showed significant increases in replication (Figure 2B). Enhanced replication of this strain is specific to growth in culture with host cells, as media-only replication rates remained unchanged from the parental strain (Figure S2C). In macrophages, we observed enhanced replication in two of the three macrophage-passaged isolates (Figure 2C). Notably, in both macrophage-adapted strains (M1 and M3), we also observed a marked improvement in growth under tissue culture conditions in the absence of macrophages (Figure S2C). Importantly, there were minimal differences in phagocytosis of these strains compared with the parental strain, indicating that the phenotypes we observe are independent of changes in phagocytosis (Figures S2A and S2B).
A macrophage-evolved strain outcompetes the parental for growth in macrophages
Of the strains recovered from our serial-passaging approach, the M1 strain stood out with a nearly 5-fold increase in macrophage replication compared with the parental strain (Figure 2C).
We sought to determine the relative fitness advantage of this M1 isolate compared with parental Ftc555-1 through competition experiments in which we co-incubated a nourseothricin-resistant (NATR) version of the parental strain with unlabeled strains of interest and assessed growth in macrophages. Differential plating of the recovered populations from these experiments allowed us to determine the relative growth advantage of each strain.
The M1 strain outcompeted the NATR parental by over 5-fold in macrophages (Figure 3A). In contrast, when the parental Ftc555-1 was instead competed against the NATR-labeled version of itself as a control, we observed a competitive index of ~1. Therefore, the evolved M1 isolate has a strong fitness advantage over parental Ftc555-1 for growth in the macrophage environment.
Figure 3. The evolved M1 strain outcompetes the parental strain in macrophages.
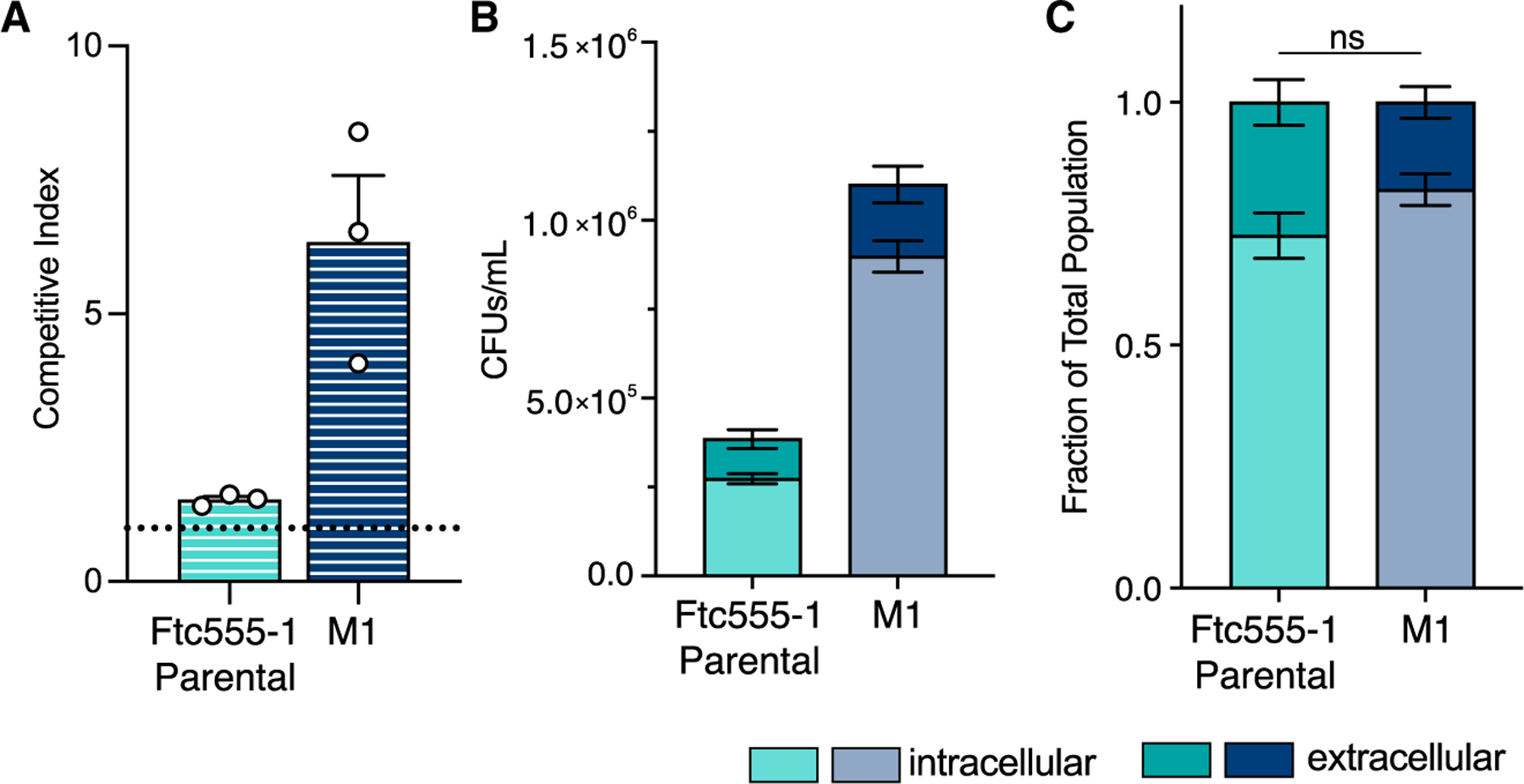
(A) Competition experiments between the NATR-labeled parental Ftc555-1 and unlabeled parental (teal), and M1 (navy blue) strains in macrophage cells. Competitive indices (CIs) are calculated as indicated in STAR Methods. A CI = 1 (dotted line) indicates equal growth of the two strains. Plotted values indicate the average value ± SEM of three replicate experiments carried out on three separate days; dots indicate the average value of replicates from a single experiment.
(B) Total counts of Ftc555-1 and M1 strains following macrophage co-incubation. Intracellular (light shades) and extracellular (dark shades) populations were collected and plated separately. Bars show the average values ± SEM from two independent experiments on separate days.
(C) Intracellular and extracellular growth following macrophage co-incubation plotted as a fraction of the total population. The same data from (B) but presented as a fraction rather than as raw counts. Significance was assessed by unpaired t test with Welch’s correction. ns, not significant.
See also Figure S3.
In addition to revealing the fitness advantage of the M1 strain in macrophages, our competition experiments revealed that M1 was able to outcompete the parental Ftc555-1 strain by nearly 40-fold in tissue culture media alone (Figure S3A). To address the possibility that increased macrophage replication in M1 reflects enhanced extracellular growth following escape from macrophages, either through killing of the host cell or non-lytic exocytosis, we assessed the role of extracellular growth in contributing to the M1 phenotype through several methods.30–32
First, we determined the amount of extracellular growth that occurs under normal experimental conditions. We performed co-incubation experiments with macrophages where we separated intracellular and extracellular populations and enumerated colony-forming units () from each sub-population. The M1 were significantly higher than the ancestral strain for both the intracellular and extracellular populations (Figure 3B). However, when assessed as a fraction of the total recovered , there was no significant difference in the percentage of the population that was extracellular at the end of the experiment (Figure 3C). This demonstrates that the M1 strain is not escaping macrophages at a noticeably higher rate than the parental strain, and extracellular replication is not a confounding issue.
We also assessed extracellular growth differences by using the antifungal drug fluconazole to limit C. neoformans replication in media (Figures S3B–S3D). Following phagocytosis by macrophages, Cryptococcus cells are somewhat protected from the effects of fluconazole, which allowed us to perform competition experiments where replication was limited to the macrophage intracellular environment (Figures S3C and S3D). The M1 strain had a nearly identical competitive index (>5-fold advantage) when grown in the presence or absence of fluconazole (Figures 3A and S3D), demonstrating that the fitness advantage in M1 is due to enhanced replication in the intracellular niche. Although we observed no difference in the minimum inhibitory concentration (MIC) for fluconazole between the two strains, the evolved M1 strain maintained a modest fitness advantage in media with fluconazole present (Figures S3B and S3D). Combined, these data demonstrate that experimental evolution selected for a strong intracellular fitness advantage in the M1 macrophage-passaged strain.
Enhanced growth of M1 is caused by a single-nucleotide polymorphism in the adenylyl cylase gene
To identify the genetic changes underlying the adaptive phenotype of the M1 strain, we used a combination of Nanopore and Illumina sequencing to assemble a high-quality reference genome of the Ftc555-1 starting isolate. We then performed whole-genome sequencing on several individual colonies from the M1 population and on a pooled culture from the final passage of our evolution experiment. Surprisingly, we found only a single-nucleotide change differentiating the M1 strain from the originating Ftc555-1 isolate. This mutation, which was fixed in both single colonies and the pooled culture, changes a single amino acid—Arg1227-Pro—in the gene CAC1 (CNAG_03202). CAC1 encodes the adenylyl cyclase gene in C. neoformans, which is required to produce cyclic adenosine monophosphate (cAMP) and for the regulation of numerous aspects of Cryptococcus biology (Figure 4A).33–37
Figure 4. A single-nucleotide change in the CAC1 adenylyl cyclase gene is necessary and sufficient for the enhanced growth of M1.

(A) Schematic of the cAMP signaling pathway in C. neoformans as worked out in the KN99 genetic background. CAC1, indicated with the yellow star, regulates the production of cAMP downstream of a number of host signals including nutrients and carbon dioxide. Production of cAMP leads to the activation of the Pka1 protein kinase, which functions to regulate many key aspects of C. neoformans biology and virulence, including capsule and melanin production, mating, titan cell formation, and others. Activation of Cac1 and modulation of cAMP levels is achieved through the interactions with a number of other genes, as indicated.
(B) Replication phenotypes of allele-swap strains in macrophage co-culture experiments. Allele-swap samples tested are two independently derived strains from CRISPR-mediated allele-swap transformations (STAR Methods).
(C) Allele frequency of the cac1-evo allele over the course of the evolution experiment. The number of reads containing the R1227P mutation in sequencing data from each passage population were counted and normalized to the read depth to calculate allele frequencies.
(D) Growth of intermediate passage populations in co-incubation experiments with macrophages. Each tested sample represents a non-homogeneous population of cells from the indicated passage.
For (B) and (D), data shown are the average value ± SD from one representative experiment with each dot representing a single replicate from that experiment. Significance in these experiments was assessed compared with the parental strain by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. ***p < 0.0001; ns, not significant.
See also Figure S4.
The importance of CAC1 for C. neoformans biology combined with the lack of other mutations in the M1 strain strongly suggested that the Arg1227Pro mutation was causative for the adaptive phenotypes in this strain. We swapped this single nucleotide in both the parental Ftc555-1 and M1 strains using CRISPR-mediated gene editing and tested allele-swapped strains for growth in our macrophage co-culture experiments (Figure 4B). When the evolved CAC1 allele—referred to from here on as cac1-evo—was introduced into the parental Ftc555-1 background, this was sufficient to cause a striking increase in replication in macrophage cells as well as in tissue culture media alone (Figures 4B and S4A). In the reciprocal swap, where the cac1-evo allele was replaced with the parental version (CAC1-parent), we observed a complete loss of the enhanced growth phenotypes. These data indicate that this single point mutation in the CAC1 adenylyl cyclase gene is both necessary and sufficient to confer the drastically altered macrophage growth phenotype that defines our evolved M1 strain.
Evolutionary dynamics of the CAC1 polymorphism reveal a strong fitness advantage for the evolved allele
We were curious about the emergence and dynamics of the cac1-evo allele over this short course of evolution. Taking advantage of the fossil record created during the experiment, we sequenced all intermediate passage populations and analyzed the CAC1 locus. By passage four, a very low number of reads (ten total reads, allele frequency = ~5%) contained the evolved allele, suggesting that the mutation arose de novo around this time (Figure 4C). The cac1-evo allele frequency rose quickly over subsequent passages: greater than 90% of reads carried the mutation at passage six, and it had fully swept through the population by passage seven. This suggests that the cac1-evo allele confers a strong fitness advantage, in alignment with the competitive advantage we had observed in earlier experiments (Figure 3).
To examine the correlation between the cac1-evo allele and adaptation to macrophage host cells, we compared the growth of intermediate passage populations in macrophage co-incubation experiments (Figures 4D and S4B). In agreement with the sequencing data, we see no significant difference in growth of the early passage populations. By passage four, replication in macrophages trended upward and by passage six was indistinguishable from the clonal M1 strain.
We also assessed the competitive advantage of each passage population in head-to-head competition experiments with the parental strain (Figures S4C and S4D). We noted an increase in the competitive index for growth in macrophages beginning around passage six and reaching maximal levels at roughly passage 8, consistent with individual population tests (Figure S4C). Media-only competition experiments revealed a marked increase in competitive advantage beginning in passage five, which remained largely unchanged across subsequent passage populations (Figure S4D). Together, these results link the emergence of macrophage adaptation in the M1 strain to the mutation of CAC1 and highlight the strength of the fitness advantage conferred by the cac1-evo allele, which rapidly swept through the population.
The cac1-evo allele causes phenotypic changes in pathogenicity-associated traits
cAMP signaling downstream of Cac1 regulates several key pathways important for the pathogenicity of C. neoformans.38 Paired with the high fitness consequences associated with the cac1-evo allele, this raises the question of how the Arg1227Pro mutation affects cryptococcal biology to promote replication in macrophages. Structural modeling of the Ftc555-1 Cac1 protein using AlphaFold revealed that the Arg1227Pro mutation falls outside of the catalytic domain and sits at the edge of the concave surface of the leucine-rich repeat (LRR) domain (Figure 5A). Although little is currently known about this region of the LRR, this domain is a conserved feature of all fungal adenylyl cyclases and has been shown in other species to mediate interactions with regulatory proteins such as Ras.39–41
Figure 5. The R1227P mutation falls into the LRR domain of Cac1 and affects pathogenicity-associated traits of C. neoformans.

(A) AlphaFold2 generated structural model of the Ftc555-1 parental Cac1. The catalytic domain is indicated in light purple shading, LRR indicated by blue shading, and other protein regions are shown in gray. Inset shows the position of the mutation in the cac1-evo allele (pink).
(B) An arginine at amino acid position 1227 is conserved across fungi. Sequence alignments of adenylyl cyclase orthologs from a panel of fungal species; only the region surrounding the isolated mutation is shown. Position 1227, the site of the cac1-evo mutation, is indicated by the black arrow, and species with the conserved arginine at this site are shaded in blue. Other residues conserved among fungi are indicated with colored shading toward the 3′ end of this region.
(C) Measurements of capsule size under two different inducing conditions: tissue culture conditions (left side) and 10% Sab’s (right side).
(D) Representative images of cells from (C). All images are at the same scale. Scale bar, 5 μm.
(E) Capsule size measurements under tissue culture conditions with or without the addition of 20 mM exogenous cAMP.
(F) Melanization of all strains at 30°C (left) and 37°C (right) on L-DOPA plates with or without 2.5 mM cAMP. The dark color of the yeast indicates melanization.
In (C) and (E), violin plots show the distribution of measurements for 50 cells from one representative experiment. For all violin plots, dotted lines indicate the median and quartiles. For (C), significance was assessed compared with the Ftc555-1 parental strain via Kruskal-Wallis test with Dunn’s multiple comparisons test. Significance between the M1 and Ftc555-1 cac1Δ in (C) and all comparisons in (E) were determined by Mann-Whitney test. *p < 0.05; ***p < 0.0001; ns, not significant.
See also Figure S5.
Interestingly, sequence comparisons of the adenylyl cyclase orthologs of other pathogenic and nonpathogenic fungi revealed conservation of an arginine at the corresponding amino acid position in most fungal species examined, despite overall low sequence conservation (Figure 5B). Tellingly, in species where arginine is not conserved, we see no evidence for substitutions as potentially disruptive as a proline at this site. Furthermore, we looked for variation at this position across the >500 clinical and environmental isolates of C. neoformans with sequence information available within public databases. We saw 100% conservation of the arginine at amino acid position 1227 across all CAC1 sequences in these isolates, although there was coding sequence variation in other regions of the gene (Figure S5A). Together, the conservation of this site within Cryptococcus and across fungi is highly suggestive that it is functionally important for the activity of Cac1.
We next probed the impact of this mutation through a series of assays analyzing its effects on downstream pathogenicity-associated phenotypes, including capsule production and melanization. Both traits have previously been shown to require cAMP signaling and play important roles during infection.33,42 Capsule is induced in vitro by specific culturing conditions that mimic host-like environments.43–45 We tested the ability of our strains to induce capsule using two different induction protocols (STAR Methods); mutants in the cAMP signaling pathway can be sensitive to specific capsule induction conditions, motivating our decision to test multiple protocols.44 We observed both strain-specific and condition-specific differences in capsule induction in the Ftc555-1 parental and M1 evolved strains (Figures 5C and 5D). Cells from the parental strain produced significantly larger capsules than M1 cells, whereas capsule sizes were roughly comparable between Ftc555-1 parental cells and those of the laboratory strain, KN99α. M1 cells produced limited capsule under tissue culture conditions (Figure 5C, left). However, capsule production was markedly reduced in M1 cells incubated in the Sab’s inducing media (Figures 5C and 5D, right).
A cac1Δ mutant in the Ftc555-1 background produced very little capsule in either condition, consistent with Cac1 activity being required for capsule production in the Ftc555-1 background, as it is in reference strains.33 Additional genetic analysis of upstream genes in the cAMP pathway, GPA1 and ACA1, revealed conserved roles in the regulation of cAMP and capsule induction in the Ftc555-1 background (Figure S5B). In contrast, deletion of the G protein-coupled receptor (GPCR), GPR4, which is required in reference strains for capsule induction upstream of Cac1 activity, had no effect in the Ftc555-1 background, regardless of CAC1 allele.46 This shows that cAMP signaling is altered in Ftc555-1-derived strains with potentially fundamental impacts on how environmental inputs are transduced by GPCRs to influence Cac1 activation.
To further probe the nature of the cac1-evo allele in M1, we asked whether exogenous cAMP added to the media could rescue defects in capsule size that we observed in these experiments (Figures 5E, S5C, and S5D). Addition of high levels (20 mM) of cAMP partially rescued capsule production of both the M1 strain and the cac1Δ mutant in the Ftc555-1 background (Figures 5E and S5C). However, exogenous cAMP did not fully rescue the phenotypes of these strains, in contrast to the KN99α cac1 deletion that exhibits a robust rescue of capsule defects when supplemental cAMP is provided (Figure S5D).
In addition to capsule, we assessed melanization phenotypes of our strains and observed no differences across the M1, Ftc555-1 parental, and KN99α strains at 30°C (Figure 5F, left). Because the ability to melanize is preserved in strains carrying the cac1-evo allele but abolished in strains with CAC1 deletions, this confirms that the Arg1227Pro mutation does not confer a complete loss of function of Cac1 activity. Surprisingly, when we tested melanization at 37°C, both the Ftc555-1 parental and M1 strains had severe melanization defects (Figure 5F, right). Addition of exogenous cAMP was sufficient to rescue the defect of the parental strain at 37°C but had no effect on melanization of M1 or the cac1Δ mutants in any genetic background. At 30°C, exogenous cAMP differentially affected melanization across the cac1Δ mutants in different genetic backgrounds.
Although these data suggest cac1-evo represents a partial loss-of-function allele, the varying effects of this allele on different traits, across differing conditions and with varying responsiveness to cAMP supplementation reveals hidden complexity in the regulation of essential pathogenicity-associated traits. Collectively, these results point to different activity requirements and/or regulatory partners for the Cac1 pathway under distinct nutritional and environmental conditions among different strains.
CAC1 mutation restricts cell size modulation
We next tested the impact of the cac1-evo allele on modulation of cell size. The ability of C. neoformans to undergo morphological shifts and generate heterogeneously sized populations plays an important role in the success of in vivo infections. The formation of large polyploid titan cells (>10 μm diameter) allows for evasion of phagocytosis by immune cells and is crucial for establishing infection in the lungs; on the other end of the size spectrum, the recently described small seed cells facilitate dissemination of C. neoformans to extrapulmonary sites.37,47,48 cAMP signaling has previously been implicated in titan cell formation, but whether this pathway plays a general role in controlling all morphological shifts is unknown.
Titan cells can be induced in vitro by a variety of methods.37,47,49,50 Using two separate protocols, we observed significant differences in titanization between the parental and M1 strains (Figures 6A and S6A). Although the parental strain produced large numbers of titan cells, the M1 strain failed to produce any larger cells and was indistinguishable from the cac1Δ mutant strain. Addition of 20 mM cAMP to the media was sufficient to drive an increase in cell size in the M1 strain, but none crossed the 10 μm threshold for titan cell classification (Figures 6B and S6B). M1 cells were, however, significantly more responsive to exogenous cAMP treatment than the Ftc555-1 cac1Δ mutants, further demonstrating differences between the cac1-evo allele and complete loss-of-function alleles.
Figure 6. CAC1 mutations impact the ability of cells to modulate size.

(A and B) Measurements of cell body diameter from cells grown under titan-cell-inducing conditions (RPMI for 7 days) without cAMP supplementation (A) or with 20 mM cAMP added to the media (B). 50 cells were measured per strain. Percentages above each strain/condition indicates the percent of cells with a diameter >10 μm (indicated by the dotted line). Error bars show mean ± SD for one representative experiment.
(C) Total cell diameter of CAP-grown cells after exposure to PGM. Violin plots show measurements for >100 cells from one representative experiment.
For (A), significance was assessed compared with the Ftc555-1 parental strain via Kruskal-Wallis test with Dunn’s multiple comparisons test. For (B) and (C), pairwise significance between matched strains under different conditions was determined by Mann-Whitney test. *p < 0.05; ***p < 0.0001; ns, not significant.
See also Figure S6.
We next assessed the ability of our strains to produce smaller seed-cell populations using an in vitro protocol consisting of a series of media shifts, culminating in 24 h of growth in pigeon guano medium (PGM), a robust, environmentally relevant inducer of seed cells (STAR Methods).48 The Ftc555-1 parental strain exhibited a drastic downward shift in cell size upon transfer from capsule-inducing medium (CAP; STAR Methods) into PGM, a phenotype similar to the reference strain KN99α (Figure 6C).48 M1 cells exhibited a subtle but significant downward shift upon transfer from CAP to PGM more similar to the cac1Δ mutant, which maintained a consistent cell size across the tested conditions. These titan and seed-cell experiments demonstrate that the M1 strain, carrying just a single-nucleotide mutation in the CAC1 gene, is severely impaired in its ability to modulate cell size in response to both host-like and environmentally relevant cues.
The cac1-evo allele causes decreased mortality in murine infection
Mutation of genes in the cAMP pathway, including CAC1, are associated with pathogenicity phenotypes in vivo.33,35,36 We used a murine model of disseminated cryptococcosis by inoculating C57BL/6NJ mice intranasally with either Ftc555-1 or the derived M1 strain to assess pathogenicity. The competitive advantages in intracellular macrophage replication we observe from in vitro assays with our evolved strain suggest a model where M1 might similarly replicate and disseminate to higher levels in the context of animal infection. In contrast, other phenotypic differences observed for the M1 strain predict a potential trade-off involving decreased fitness for this strain in vivo due to enhanced recognition and control by the immune system. Consistent with the second possibility, we observed a striking difference in survival between the animals infected with the two strains (Figure 7A). Animals infected with the parental strain, Ftc555-1, declined steadily after infection and had a median time to endpoint of 34 days, whereas the M1 strain failed to cause lethal infection or symptomatic disease in any animals during the experiment (Figures S7A and S7B).
Figure 7. The cac1-evo allele decreases pathogenicity in murine infection models.

(A) Survival of C57BL/6NJ mice following intranasal inoculation with Ftc555-1 (teal) or M1 (navy blue). Ten animals were inoculated per strain (five male, five female) and were weighed daily until they reached predetermined endpoint criteria. Significance was determined by a log-rank test.
(B) Fungal burden in the lungs (L) and brains (B) of infected mice from (A). Ftc555-1 parental values represent fungal burden at infection endpoint and M1 values are from organs harvested on day 56 post-infection. Each data point indicates the count from an individual animal. Only a subset of the parental animals were analyzed for (four out of ten total infected mice). Boxes in box-and-whisker plots show the 25th to 75th percentiles. Whiskers show the full range of values (minimum to maximum) and the line indicates the median value.
(C) Histopathological analysis reveals cell-size differences between animals infected with parental and M1 strains. Infected mouse lungs were harvested on day 22 post-infection, fixed, and stained with H&E. Positions of 40× images are indicated by the black box in 10× images. Black arrows indicate C. neoformans cells. Lungs from five animals per C. neoformans strain were analyzed and one representative image is shown. Scale bars, 100 μm (10× images) and 20 mm (40× images).
(D) Quantification of C. neoformans cell size from H&E-stained lung sections. 100 cells per animal were analyzed and plotted. Line and error bars indicate mean ± SD. Significance was assessed by Mann-Whitney test. ***p < 0.0001.
See also Figure S7.
To address whether the lack of disease in animals infected with M1 was due to clearance of C. neoformans after inoculation, we sacrificed animals 56 days post-infection and assessed fungal burden in both lung and brain tissue (Figure 7B). Intriguingly, we recovered from both the lungs and the brains of M1-infected mice; levels from the lungs were, on average, higher than the starting inoculum (2.5 × 104 fungi/mouse), although still much lower than in animals infected with the parental strain at infection endpoint. recovered from the brain were also low, although we observed dissemination to the brain in seven out of ten of the M1-infected animals. These results indicate that mice were persistently infected with the M1 strain, which maintains its potential for dissemination.
We subsequently performed histopathological analysis of the lungs from infected mice at 22 days post-infection—a time point prior to the first endpoints in animals infected with the parental strain (Figure 7C). Abundant cryptococcal cells were easily identified in the lungs, and there was not a pronounced difference in the distribution of these cells or the amount of tissue inflammation between mice infected with the parental or M1 strains. However, we observed significant cell-size variation between C. neoformans strains in these tissues (Figures 7C, 7D, and S7C). Consistent with in vitro observations, cells of the Ftc555-1 parental strain in fixed lung sections were nearly uniformly greater than 10 μm in all animals, whereas very few M1 cells crossed this threshold. Intriguingly, the smaller cell size of the M1 cells does not seem to be correlated, as predicted, with obvious decreases in cell number or with gross changes in host immune responses within the lungs. Furthermore, immune suppression of animals infected with the M1 strain by administration of the steroid dexamethasone daily for 14 days did not lead to any animals reaching endpoint, suggesting that fungal growth, rather than the host immune system, may be the limiting factor in M1 infection (Figure S7D).
These seemingly paradoxical results show the remarkable effect a single-nucleotide change in the genome of a pathogen can have on the disease-causing potential of the organism. This surprising trade-off in adaptation to macrophages leading to decreased pathogenicity in mouse infections raises new questions about the role different environments play in shaping the evolution of fungal pathogens and how this may impact the course and outcome of an infection.
DISCUSSION
An emerging body of work examining strain-level variation both in vitro and in vivo reveals that even closely related C. neoformans strains can have divergent phenotypes.4,5,7,9,10,47,51,52 Our study provides a new perspective on phenotypic variation by directly comparing the growth of the same panel of clinicaland environmental isolates in host cells from two different species (Figures 1B–1E). We noted a correlation between strains that were able to withstand killing by amoebae and those that replicated well in macrophages, which suggests similarities in the intracellular environments of diverse host species that favor the growth of certain C. neoformans isolates over others. In contrast, experimental evolution revealed adaptive phenotypes that appear host specific, conferring an advantage in either amoebae hosts or in mammalian macrophages, but not both (Figure 2). Parallel studies of amoeba resistance, also in the Ftc555-1 background, similarly showed a lack of correlation between growth success in different host cells.52,53 Collectively, this suggests that although there may be some overlap in genetic pathways required for growth in evolutionarily diverse hosts, such overlap may be challenging to identify. The host-adapted isolates we recovered here along with future studies of non-laboratory strainswillhelpshed lighton conservation and divergence in host selective pressures that have shaped C. neoformans adaptive evolution.
Our courses of experimental evolution revealed that adaptation can happen over extremely short timescales in C. neoformans (Figure 2). Rapid adaptation of pathogens to new hosts or new environments is well documented for viral and bacterial pathogens.54–57 However, most previous studies of host adaptation in pathogenic fungi have focused on evolution occurring over much longer timescales.25–27,58 We estimate that the macrophage-passaged lines generated here were evolved for roughly 75–100 total generations, whereas amoeba-passaged C. neoformans strains experienced fewer generations due to potent killing by amoeba in early passages. Preliminary sequencing data revealed unique adaptive changes in our host-adapted populations, whereas other populations failed to adapt at all, highlighting the power of serial-passaging approaches to reveal a range of evolutionary trajectories.
The exact effect of the single point mutation we discovered in Cac1 and the mechanism by which this contributes to the growth phenotypes observed in M1 remains an exciting open question. Dissection of the functional impact of the cac1-evo allele in our adapted strain revealed unforeseen complexity in the regulation of cAMP signaling and downstream pathogenicity-related phenotypes. Our analysis of phenotypic impacts of the cac1-evo allele and the responsiveness of different strains to cAMP supplementation reveals two important findings: first, the Arg1227Pro mutation likely results in decreased cAMP production. Second, cAMP production differs across both the strains and experimental conditions tested, suggesting that even subtle changes in this signaling pathway may have profound effects on phenotypic outcome (Figures 5, 6, S5, and S6). How exactly environmental cues get integrated and through what machinery to affect cAMP and strain behavior in vitro and in vivo are important outstanding questions. Future genetic and biochemical analyses of the cac1-evo allele will explore the complex impact of this point mutation in CAC1.
It is also important to note that previous studies of cAMP signaling generally and CAC1 specifically were carried out almost exclusively in the context of the laboratory strains H99 and KN99. Two notable exceptions are studies of PKR1 and PDE2 variation in clinical isolates of C. neoformans, with demonstrated impacts on titan-cell formation and virulence.47,59 Work here revealed both functional differences in the cAMP signaling pathway components and substantial sequence variation across CAC1 sequences from diverse C. neoformans isolates, despite conservation at Arg1227 (Figure S5). In the Ftc555-1 parental strain alone, there are 17 coding changes in CAC1 compared with the most studied H99 lineage strains. Gene trees of CAC1 are incongruent with strain-level phylogenies, hinting at differences in the evolutionary pressures and histories of this gene across diverse strains (Figure S5A). Therefore, there may be functionally significant differences in the activity of this critical signaling pathway due to sequence changes in CAC1 itself or through epistasis with other genes with altered sequences or activity among strains. Curiously, PKR1 mutations have also been identified in serially sampled isolates from human patients, as well as in amoebae-adapted strains, although the impact of those mutations on adaptive phenotypes remains to be tested.24,53 Regardless, the convergence of multiple studies, including ours, on the cAMP signaling pathway hint that it is a hotspot of genetic variation within this species, with functional implications for a range of pathogenicity-associated phenotypes. This underscores the power and importance of using naturally occurring genetic variation among non-reference strains to gain insights into key virulence pathways that have previously only been studied in a single genetic context.60
Our results raise intriguing questions about the relative trade-offs between adaptation to specific host-cell environments and the ability to cause disease. Mouse infections revealed a striking difference in the pathogenicity of the serially passaged M1 strain when compared with the ancestral Ftc555-1 (Figure 7). Given its enhanced ability to replicate in mouse macrophages, we were surprised that M1 failed to cause lethal disease. However, the data are consistent with the important role of cell-size variation in modulating infection. Our analyses of cell-size changes in the M1 strain revealed that the cac1-evo allele functionally locks cells as a single morphotype (Figures 6, 7, S6, and S7). The inability of the M1 strain to generate cell-size heterogeneity partially explains the lack of severe disease in animals infected with this strain. However, further characterization of the M1 cells and their macrophage interactions may help inform our understanding of persistence and dissemination during animal infections, despite this lack of cell-size plasticity.
Finally, histopathologic analysis of the lungs from infected animals showed less pronounced differences than expected considering the distinct outcomes of infections caused by parental and evolved strains. Future work more closely examining host cytokine production, immune cell recruitment, and disease progression during infection with this strain will help shed light on interactions with the host immune system and how this contributes to persistent infection in the absence of obvious disease. Regardless of the exact mechanism, our work shows the complex impact of a single-nucleotide change in the genome of this pathogenic fungus and highlights the long reach of simple adaptive changes to fundamentally change the course of evolution.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Nels Elde (nelde@genetics.utah.edu).
Materials availability
All strains and materials generated for this paper are available without restriction. Please request from the lead contact.
Data and code availability
All DNA sequencing datasets have been deposited in the NCBI SRA and are publicly available. The BioProject accession number is listed in the key resources table. The paper also analyzes existing, publicly available data. These accession numbers are also listed in the key resources table. Raw histopathology data from this study are hosted on the HistoWiz website and are publicly available as of the date of this publication. Accession link is provided in the key resources table. All other data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code. Existing bioinformatic packages were used as specified in the method details and the key resources table lists version numbers.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Mouse Anti-Glucuronoxylomannan (GXM) Antibody, clone 18B7 | Sigma | Cat#MABF2069 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| cAMP | Cayman Chemical | Cat#18820 |
| Phorbol myristate acetate (PMA) | Sigma | Cat#P1585 |
| Phusion Flash DNA Polymerase | Thermo Fisher | Cat# F548S |
| HiFi DNA Master Mix | New England Biolabs | Cat#E2621 |
| Fluconazole | Sigma | Cat#F8929 |
| Hexadecyltrimethylammonium bromide (CTAB) | Sigma | Cat#H9151 |
| 3,4-dihydroxy-L-phenylalanine | Sigma | Cat#D9628 |
| L-Asparagine monohydrate | Sigma | Cat#A7094 |
| Thiamine hydrochloride | Sigma | Cat#T4625 |
| Biotin | Sigma | Cat#B4639 |
| Yeast Extract | Gibco | Cat#212750 |
| Peptone | Gibco | Cat#211677 |
| Yeast nitrogen base without amino acids | Sigma | Cat#Y0626 |
| Sabouraud Dextrose Broth | Difco | Cat#238230 |
| DMEM with high glucose and L-glutamine | VWR | Cat#16777-129 |
| Fetal Bovine Serum | Thermo Fisher | Cat#26140079 |
| RPMI 1640 | VWR | Cat#16777-145 |
| RPMI 1640 with L-glutamine w/o sodium bicarbonate | Gibco | Cat#31800089 |
| Dexdomitor | Zoetis | Cat#122692-5 |
| Antisedan | Zoetis | Cat#87219-02296-2 |
| Dexamethasone | Sigma | Cat#D4902 |
|
| ||
| Critical commercial assays | ||
|
| ||
| NEBNext Ultra II DNA Library Prep | New England Biolabs | Cat#E7634L |
| Ligation Sequencing Kit | Oxford Nanopore | Cat#SQK-LSK110 |
| Fluconazole Etest Strips | BioMerieux | Cat#412349 |
| ChromaSpin 1000 | Takara | Cat#636093 |
|
| ||
| Deposited data | ||
|
| ||
| Nanopore and Illumina sequencing data | Sequence Read Archive | NCBI BioProject: PRJNA941895 |
| Histopathology images from mice infected with Ftc555-1 Parental and M1 strains | HistoWiz | HistoWiz Data:https://app.histowiz.com/shared_orders/897b4aa6-bf6c-49da-9009-7113f766aee9/slides/ |
| M. sympodalis adenylyl cyclase sequence | NCBI Genbank | GenBank: SHO76694.1 |
| U. maydis adenylyl cyclase sequence | NCBI Genbank | GenBank: XP_011388269 |
| C. neoformans adenylyl cylase sequence | NCBI Genbank | GenBank: XP_012050872 |
| C. gattii adenylyl cyclase sequence | NCBI Genbank | GenBank: XP_003195134.1 |
| S. pombe adenylyl cyclase sequence | NCBI Genbank | GenBank: NP_596159.1 |
| N. crassa adenylyl cyclase sequence | NCBI Genbank | GenBank: BAA00755.1 |
| B. cinerea adenylyl cyclase sequence | NCBI Genbank | GenBank: XP_024553314.1 |
| T. rubrum adenylyl cyclase sequence | NCBI Genbank | GenBank: KDB32528.1 |
| A. niger adenylyl cyclase sequence | NCBI Genbank | GenBank:GAq42063.1 |
| C. albicans adenylyl cyclase sequence | NCBI Genbank | GenBank:XP_716974.2 |
| S. cerevisiae adenylyl cyclase sequence | NCBI Genbank | GenBank:NP_012529 |
| CAC1 sequences from all C. neoformans isolates | FungiDB | FungiDB: https://fungidb.org/fungidb/app/record/gene/CNAG_03202#category:dna-polymorphism |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| J774A.1 | ATCC | Cat# TIB-67; RRID: CVCL_0358 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| Mice: C57BL/6NJ | Jackson Laboratory | RRID: IMSR_JAX:005304 |
| Amoeba: Acanthamoeba castellanii | ATCC | Cat# 30234 |
| C. neoformans: H99O | Gift from J. Perfect | N/A |
| C. neoformans: KN99α | Fungal Genetics Stock Center | Cat#10369 |
| C. neoformans: KN99α cac1Δ::NAT | Fungal Genetics Stock Center, Madhani Knockout Collection (2020 Plates) | N/A |
| C. neoformans: Bt46 | Gift from J. Perfect, Litvintseva et al.61 | N/A |
| C. neoformans: Ze90-1 | Gift from J. Perfect, Litvintseva et al.62 | N/A |
| C. neoformans: Ftc217-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc239-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc209-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc209-3 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: NRHc5004.ENR | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Muc458-2 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: NRHc5026.ENR.CLIN.ISO | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc555-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc158-2 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: NRHc5029.ENR.CLIN.ISO | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Gbc39-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc555-1 M1-1 | This study | N/A |
| C. neoformans: Ftc555-1 M2-1 | This study | N/A |
| C. neoformans: Ftc555-1 M3-2 | This study | N/A |
| C. neoformans: Ftc555-1 A1-1 | This study | N/A |
| C. neoformans: Ftc555-1 A2-1 | This study | N/A |
| C. neoformans: Ftc555-1 A3-1 | This study | N/A |
| C. neoformans: Ftc555-1 SH2-NAT | This study | ZAH_CN01 |
| C. neoformans: Ftc555-1 Parental cac1-evo(R1227P); SH2-NAT isolate H1 | This study | ZAH_CN03 |
| C. neoformans: Ftc555-1 Parental cac1-evo(R1227P); SH2-NAT isolate H7 | This study | ZAH_CN04 |
| C. neoformans: Ftc555-1 M1 CAC1-parent (P1227R); SH2-NAT isolate M2 | This study | ZAH_CN05 |
| C. neoformans: Ftc555-1 M1 CAC1-parent (P1227R); SH2-NAT isolate H11 | This study | ZAH_CN06 |
| C. neoformans: Ftc555-1 Parental cac1Δ::HYG | This study | ZAH_CN07 |
| C. neoformans: Ftc555-1 M1 cac1Δ::HYG; cac1-evo(R1227P) | This study | ZAH_CN08 |
| C. neoformans: Ftc555-1 Parental aca1Δ::NAT | This study | ZAH_CN09 |
| C. neoformans: Ftc555-1 Parental gpa1Δ::NAT | This study | ZAH_CN10 |
| C. neoformans: Ftc555-1 Parental gpr4Δ::NAT | This study | ZAH_CN11 |
| C. neoformans: Ftc555-1 M1 aca1Δ::NAT; cac1-evo(R1227P) | This study | ZAH_CN12 |
| C. neoformans: Ftc555-1 M1 gpa1Δ::NAT; cac1-evo(R1227P) | This study | ZAH_CN13 |
| C. neoformans: Ftc555-1 M1 gpr4Δ::NAT; cac1-evo(R1227P) | This study | ZAH_CN14 |
|
| ||
| Oligonucleotides | ||
|
| ||
| See Table S1 | N/A | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| SH2::NAT plasmid for CRISPR repair amplification | This study | pZAH31 |
| cac1Δ::HYG plasmid for CRISPR KO repair template | This study | pZAH40 |
| aca1Δ::NAT plasmid for CRISPR KO repair template | This study | pMWS05 |
| gpa1Δ::NAT plasmid for CRISPR KO repair template | This study | pMWS06 |
| gpr4Δ::NAT plasmid for CRISPR KO repair template | This study | pMWS07 |
| pRS316-PCnU6-scaffold for sgRNA Fusion PCRs | Huang et al.63 | AddGene Cat#171686; pBHM2329 |
| pRS316-PTEF1-CnoCas9 Codon Optimized C. neoformans Cas9 for CRISPR | Huang et al.63 | AddGene Cat#171687; pBHM2403 |
|
| ||
| Software and algorithms | ||
|
| ||
| Guppy-gpu basecaller (v 6.0.1) | Oxford Nanopore | RRID: SCR_023196 |
| Canu (v 2.1.1) | Koren et al.64 | RRID: SCR_015880; https://github.com/marbl/canu |
| Medaka (v 1.5.0) | Oxford Nanopore | https://github.com/nanoporetech/medaka |
| Pilon (v 1.24) | Walker et al.65 | RRID: SCR_014731; https://github.com/broadinstitute/pilon/ |
| GetOrganelle (v 1.7.5.3) | Jin et al.66 | https://github.com/Kinggerm/GetOrganelle |
| Liftoff (v 1.6.3) | Shumate and Salzberg67 | https://github.com/agshumate/Liftoff |
| Trimmomatic (v 0.39) | Bolger et al.68 | RRID: SCR_011848; http://www.usadellab.org/cms/index.php?page=trimmomatic |
| bwa-mem2 (v 2.2.1) | Vasimuddin et al.69 | RRID: SCR_022192; https://github.com/bwa-mem2/bwa-mem2 |
| Picard | Broad Institute | RRID: SCR_006525; https://github.com/broadinstitute/picard |
| Genome Analysis Tool Kit (GATK, v 3.8) | McKenna et al.70 | RRID: SCR_001876; https://software.broadinstitute.org/gatk/ |
| SAMtools (v 1.15.1) | Danecek et al.71 | RRID: SCR_002105; https://www.htslib.org/ |
| SnpEff (v 4.3t) | Cingolani et al.72 | RRID: SCR_005191; https://pcingola.github.io/SnpEff/ |
| Integrated Genomics Viewer (IGV) | Thorvaldsdóttir et al.73 | RRID: SCR_011793; http://www.broadinstitute.org/igv/ |
| AlphaFold (v 2.1.2) | Jumper et al.74 | https://github.com/deepmind/alphafold |
| Geneious | Geneious | RRID: SCR_010519; http://www.geneious.com/ |
| IQ-Tree | Nguyen et al.75 | RRID: SCR_017254; http://www.iqtree.org/ |
| FIJI | Schindelin et al.76 | RRID: SCR_002285; https://fiji.sc/ |
| QCA-Quigly’s Circle App | Dragotakes and Casadevall77 | https://github.com/quigly555/QCA |
| Prism 9 | GraphPad | RRID: SCR_002798; https://www.graphpad.com/ |
| WebSNAPER | Drenkard et al.78 | https://pga.mgh.harvard.edu/cgi-bin/snap3/websnaper3.cgi |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fungal strains and growth media
C. neoformans strains used in this study are listed in the key resources table. Strains were stored at −80°C in yeast extract peptone dextrose (YPD) media supplemented with 20% glycerol. Strains were inoculated by streaking onto YPD plates and incubated for 2–3 days at 30°C before use in experiments. Strains were stored at 4°C for <10 days and reinoculated as needed. Unless otherwise noted, all overnight cultures were grown for 16–18 hours in YPD at 30°C with shaking at 225 rpm.
The following media types were used for C. neoformans growth in this study: YPD (Yeast Extract [Gibco Cat# 212750], Proteose Peptone [Gibco Cat# 211677], 2% glucose), YNB (yeast nitrogen base without amino acids [Sigma Cat# Y0626], 2% glucose), 10% Sab’s/CAP media (10% Sabouraud Dextrose Broth [Difco Cat# 238230], buffered with 50 mM HEPES to pH=7.4), tissue culture media (DMEM with high glucose and L-glutamine [VWR Cat# 16777-129] supplemented with 10% heat-inactivated FBS, non-essential amino acids and penicillin-streptomycin), RPMI 1640 (VWR Cat# 16777-145), and RPMI 1640 with L-glutamine and without sodium bicarbonate (Gibco Cat# 31800089).
Macrophage cell culture
J774A.1 cells (female) were purchased from ATCC (ATCC Cat# TIB-67, RRID: CVCL_0358). Cells were cultured in a humidified incubator at 37°C with 5% CO2 in DMEM with high glucose and L-glutamine (VWR Cat# 16777-129) supplemented with 10% heat inactivated FBS, non-essential amino acids and penicillin-streptomycin. J774A.1 macrophages were used between passages 5 and 20 from thaw for all experiments. Cells were routinely tested for mycoplasma contamination. Cell lines were not authenticated.
Amoeba cell culture
The Acanthamoeba castellanii amoeba strain ATCC 30234 was purchased from ATCC and cultured axenically in Peptone-Yeast-Glucose (PYG) Media (2% proteose peptone, 0.1% yeast extract, 0.1 M glucose, 4 mM MgSO4, 0.4 M CaCl2, 0.1% sodium citrate dihydrate, 0.05 mM Fe(NH4)2(SO4)2• 6H2O, 2.5 mM NaH2PO3, 2.5 mM K2HPO3, pH 6.5). Unless otherwise noted, all chemicals were purchased from Sigma. Amoebae were routinely cultured in T175 tissue culture flasks at 28°C and were used for assays between passages 5 and 20 from thaw.
Mouse infection models
For survival experiments, ~8-week-old C57BL/6NJ mice were ordered from Jackson Laboratory (RRID: IMSR_JAX:005304) and randomly assigned to experimental groups, with 5 male and 5 female mice tested per condition. Mice were anesthetized with ketamine/dexmedetomidine hydrochloride (Dexdomitor, Zoetis) delivered intraperitoneally. They were then suspended by their front incisors on a horizontal strand of thread. Mice were inoculated intranasally with 2.5x104 Cryptococcus cells in 50 μl of PBS using a micropipette. The inoculum was placed dropwise onto a nasal flare before being inhaled by mice. Ten minutes later, mice were intraperitoneally administered the reversal agent atipamezole (Antisedan, Zoetis). Mice were weighed daily and euthanized by CO2 asphyxiation and cervical dislocation when they lost 15% of their initial mass.
For histopathology analysis, 5 ~8-week-old C57BL/6NJ female mice were infected as above. At 22 d.p.i., all animals were humanely euthanized.
For the immune suppression experiment, 10 ~8-week-old C57BL/6NJ female mice were infected with the M1 macrophage-adapted strain as above. Mice were monitored for 36 days and were then treated with either PBS (n=5) or 1mg/kg/day dexamethasone (n=5, Sigma Cat# D4902). PBS and dexamethasone were delivered intraperitoneally daily and animals were monitored for weight loss for 14 days and were then euthanized as above.
All animal procedures were approved by the University of Utah Institutional Animal Care and Use Committee.
METHOD DETAILS
Co-culture of fungi and macrophage or amoeba
Macrophages were seeded into two replicate 96-well plates 18–24 hours before each assay at a density of 104 cells/well in 100 μL normal macrophage growth media and incubated at 37°C and 5% CO2. Overnight cultures of each C. neoformans strains were subcultured down to an OD of 0.2 and allowed to reach mid-log phase (4–5 hours, OD=0.6–1.0) before use in experiments. Each strain to be tested was then washed twice with sterile PBS and adjusted to a concentration of ~2x105 cells/mL in macrophage growth media containing 1 μg/mL of the anti-GXM antibody 18B7 (Sigma Cat# MABF2069). Cells were opsonized in this media for 1h at 37°C. While C. neoformans cells were opsonizing, macrophages were activated for 1 hour by replacing the media with 100 μL of growth media with 10 nM phorbol myristate acetate (PMA, Sigma Cat# P1585). Media was removed from activated macrophages and 100 μL of opsonized C. neoformans suspensions were added to each well (2x104 yeast cells/well for an MOI ~1). 100 μL of opsonized cell suspensions were also added to empty wells in the plate as controls for media growth. C. neoformans cells were allowed to be phagocytosed for 1 hour with incubation at 37°C with 5% CO2. After 1 hour, supernatant and extracellular yeasts were removed and macrophages were gently washed 1–2 times with warm PBS before replacing with fresh macrophage growth media. One replicate plate was immediately lysed with 200 μL of 0.1% sodium deoxycholate to determine starting and percent phagocytosis counts. Wells were washed twice with sterile water to collect all C. neoformans cells and combined with lysates. The remaining plate was incubated for an additional 24 hours before lysis. Lysed cultures were serially diluted and plated onto YPD. Plates were incubated at 30°C for 2–3 days before were enumerated.
Fold change in was calculated by comparing the counts after 1 hour of phagocytosis to the 24 hour counts. Media only fold changes were calculated by comparing counts from the media only wells at 1 hour vs. the media only wells at 24 hours. Percent phagocytosis was determined by comparing the 1 hour counts from wells with macrophages to the control wells with media only.
Amoeba co-culture experiments were carried out similarly to the macrophage experiments with a few modifications. Amoeba were seeded at a density of 5x104 cells/well into replicate 96-well plates and C. neoformans cell suspensions were prepared at 1x106 cells/mL in Ac Buffer (4 mM MgSO4, 0.4 M CaCl2, 0.1% sodium citrate dihydrate, 0.05 mM Fe(NH4)2(SO4)2• 6H2O, 2.5 mM NaH2PO3, 2.5 mM K2HPO3, pH 6.5) to maintain an MOI of ~1. Amoeba were starved of nutrients in Ac Buffer for 1 hour at 28°C before 100 μL of yeast cell suspensions were added, and the cultures remained in Ac Buffer throughout the duration of the experiment. All incubations were performed at 28°C. Timings, washes, and enumeration was carried out as described above. For all co-incubation experiments, each condition and strain was tested in triplicate and experiments were repeated 2–3 times on different days.
Serial passaging of fungi through host cells
8.5–9 x 105 macrophage or A. castellanii cells were seeded into T25 flasks 16–18 hours before each passage and incubated under their respective normal growth conditions. For the first passage, an overnight culture of Ftc555-1 was grown with shaking for 16 hours with shaking at 30°C. This culture was centrifuged at 3000 x g for 5 minutes and washed twice with PBS before the OD was measured. Cells were normalized to an OD of 1 (~1.5 x 107 cells/mL) in 3.5 mL of either macrophage growth media containing 1 μg/mL 18B7 anti-capsule antibody (for macrophage passaging) or in Ac Buffer (for amoeba passaging). Cell suspensions were incubated at 37°C (macrophage passaged) or 28°C (amoeba passaged) for 1 hour. The remaining starting inoculum culture was mixed 1:1 with 40% glycerol and aliquots were frozen at −80°C.
While C. neoformans cells were incubating, the media from macrophage containing flasks was removed and replaced with macrophage growth media containing 10 nM PMA. PYG media was removed from amoeba flasks and replaced with Ac buffer and cells were incubated at their standard temperatures for 1 hour. Following this 1 hour incubation, 1 mL of the appropriate C. neoformans cell suspension was added to each flask and gently rocked to ensure the entire host cell layer was covered. This small volume ensured that yeast cells settled onto the host cell monolayer and facilitated phagocytosis. Phagocytosis was allowed to proceed for 2 hours before extracellular yeasts were removed. Cells were washed 1–2 times with pre-warmed PBS and media was replaced with 2 mL of growth media. Phagocytosed yeasts and host cells were incubated for 24 hours.
After 24 hours of incubation, the media containing extracellular yeasts was discarded from lines 1 and 2 of both amoeba- and macrophage-containing flasks (A1-A2 and M1-M2). One mL of 0.1% sodium deoxycholate was added to all of the flasks to lyse host cells and lysates were collected. Flasks were washed twice with PBS. Lysates and washes were combined and centrifuged at 3000 x g for 5 min and the supernatant was discarded. C. neoformans cell pellets were resuspended in YPD media, vortexed to mix, and incubated at 30°C with shaking (225 rpm) for 24 hours.
Following outgrowth, ODs were measured and each individual culture was washed and adjusted to an OD of 0.425 in 1.5 mL of the appropriate growth media. The remaining culture was frozen as above to create a fossil record for the experiment. Passaging proceeded as above from this point. All macrophage passages were performed at 37°C with 5% CO2. Amoeba passages were largely carried out at 28°C, but passages 5 and 10 were performed at 37°C with 5% CO2 to avoid losing thermotolerance in these strains. All outgrowth steps were carried out at 30°C. Following the outgrowth of the final passage, multiple aliquots were frozen. Samples from each population were also serially diluted and plated onto YPD plates for determination. Individual colonies from these plates were picked into 5 mL of YPD, grown overnight and frozen for use in phenotyping experiments.
Strain construction and molecular biology
Primers used in this work are listed in Table S1. All genetic manipulations were done using CRISPR-mediated gene editing and the TRACE system following previously published protocols.63,79 Briefly, gRNAs were generated using guide-specific primers and amplification from the pBHM2329 plasmid (gift from H. Madhani). CAS9 was amplified for transformation from the pBHM2403 plasmid. All DNA used for CRISPR transformations was generated by PCR and purified using the Zymo DNA Clean and Concentrator 25 kit. If necessary, ethanol precipitations were performed to further concentrate DNA. Cells were grown as described for transformations and DNA was introduced by electroporation with an Eppendorf Eporator at 2 kV. DNA concentrations used were as follows, unless otherwise noted: 250 ng Cas9, 100 ng gRNA, 2.5 μg Repair Template.
For insertion of a the nourseothricin cassette at the Safe Haven 2 locus (SH2), we designed a VNB lineage-specific guide targeting this locus.80 A repair template plasmid carrying the ~1 kb of sequence immediately 5’ and 3’ to this gRNA sequence from the H99 genome was constructed using HiFi DNA Master Mix (NEB Cat#E2621) with a standard NATR cassette inserted between the two flanks. Repair templates were then PCR amplified from this plasmid (pZAH31) for CRISPR transformations.
For allele swap strains, the M1-derived mutation in CAC1 conveniently generates a new PAM site in this strain, so we used strain-specific gRNAs that overlap or terminate at this mutation, wherein the PAM and guide sequences would then be disrupted by introduction of the alternate allele. Repair constructs containing the site of the mutation and ~900 bp upstream and downstream of this position were amplified directly from genomic DNA from either Ftc555-1 or M1. We used a co-CRISPR approach, simultaneously targeting CAC1 and the SH2 locus in order to be able to select for successful transformation. For allele swap transformations, we simultaneously transformed the following amounts of DNA: 250 ng M1- or Ftc555-1-specific gRNA, 250 ng VNB SH2 gRNA, 1 mg Cas9, 2 mg CAC1 gDNA Repair Template, 2 mg SH2-NAT Repair Template.
For generating cAMP pathway gene mutants in the Ftc555-1 and M1 backgrounds, gRNAs targeting the 5’ and 3’ ends of the gene were generated and both were used in transformations to ensure deletion of the entire open-reading frame. A repair template plasmid was created by amplifying the ~1kb regions upstream and downstream of the ORF of CAC1, ACA1, GPA1 or GPR4 from Ftc555-1 gDNA. HiFi DNA master mix was used to insert a hygromycin or nourseothricin resistance cassette (HYGR or NATR) between these flanking gDNA sequences and ligate it into pUC19. Repair template was amplified from the resulting plasmid for use in CRISPR transformations. Plasmids generated are listed in the key resources table.
Transformant selection and genotyping
Following electroporation of CRISPR reagents, strains were plated onto selective media (either YPAD+NAT or YPAD+HYG) and allowed to grow for 2–3 days at 30°C. Individual colonies were then patched onto non-selective YPD media for 2–3 passages before being patched back onto selective media in order to identify stable genomic integrants.
Colonies were genotyped by colony PCR: small amounts of cells were picked into PCR strip tubes and microwaved for 2.5 minutes before a Phusion-Flash (Thermo Cat# F548S) PCR mix containing the appropriate primers was added directly to these cells. For SH2 and gene knockout genotyping, a primer sitting inside of the inserted drug cassette on both 5’ and 3’ ends (ZAH_G40 and G01) were paired with primers in the flanking genomic regions outside of the repair template constructs (see Table S1). Allele swap colonies were genotyped using primers 5’ and 3’ to the repair construct (ZAH_H20 and H21) paired with destabilized primers designed with the WebSNAPER tool to bind specifically to either the parental or evolved CAC1 allele (ZAH_H22 for the parental allele and ZAH_H30 for the evolved allele).78 Allele swap colonies were first genotyped for the correct CAC1 allele, and were then confirmed to also carry the NATR cassette at the SH2 locus.
Genomic sequences were amplified from gDNA extracted from candidate colonies and integration and allele identity was confirmed by Sanger sequencing. PCRs were also performed to ensure that no strains had integrated the gRNA or CAS9 constructs.
Competition experiments
For competition experiments, cell suspensions were made identically for test strains and the NATR-labeled parental Ftc555-1. These cell suspensions were mixed 1:1 before adding 100 μL to wells for co-culture experiments, which were carried out as described above. To determine the for test strain vs. the parental strain, serial dilutions were plated onto both YPD plates as well as YPAD+NAT plates. Competitive index here is calculated as:
Intra- and extracellular determinations
To determine intracellular and extracellular counts, co-incubation experiments were set up as described above. After 24 hours of incubation with macrophages, the media supernatant containing extracellular yeasts was removed to an empty well of the 96-well plate and replaced with fresh macrophage media. If there were still substantial unassociated yeasts observed, this step was repeated for a second time. Otherwise, macrophages were lysed as above to collect intracellular yeasts. Both intracellular and extracellular wells were washed, serially diluted, and plated for enumeration as described.
Fluconazole MICs and competition
Overnight cultures of C. neoformans strains were adjusted to a density of ~5x 106 cells/mL and were spread onto either YPD-agar or RPMI agar medium with L-glutamine and without sodium bicarbonate. Fluconazole Etest strips (bioMérieux) were placed on the agar surface of the RPMI plates once cultures had dried. All plates were placed at 37°C and imaged after 48 and 72 hours. Only strains with robust growth on control plates (-Etest strip) were imaged and used to determine MIC values.
For macrophage experiments and competitions with fluconazole supplementation, co-incubation setup was performed as above through the one hour phagocytosis step. When extracellular yeasts and media were removed, media was replaced with standard macrophage growth media containing either 2 μg/mL Fluconazole (in DMSO, Sigma Cat#F8929) or media with an equal volume of DMSO. The remainder of the experiment was performed as described above.
Genomic DNA extraction and sequencing
Single colonies or 250 μL of pooled glycerol stocks for sequencing were inoculated into 50 mL of YPD and grown overnight with shaking at 30°C. Cells were collected by centrifugation and lyophilized overnight. High-molecular-weight genomic DNA was isolated following the CTAB extraction protocol as previously described. Following CTAB extraction, samples were further purified using the Takara ChromaSpin 1000 column. Concentrations were measured by Qubit broad range assay. For Nanopore sequencing of Ftc555-1, one microgram of HMW gDNA was prepared for sequencing using the SQK-LSK110 Kit and run on an R9 (FLO-MIN106) flow cell. For Illumina sequencing, samples were sheared with a Covaris S2 Focused-ultrasonicator and libraries were prepared using the New England Biolabs NEBNext Ultra II DNA Library Prep kit (Cat#E7634L) with an average insert size of 450 bp. 12–14 strains per run were barcoded and pooled, and 100 million 150x150 bp paired-end reads were collected on a NovaSeq S4 Flow Cell with the v1.5 Reagent Kit. Illumina library preparation and sequencing was performed by the University of Utah’s High Throughput Genomics core facility at the Huntsman Cancer Institute.
Sequencing analysis
Fast5 files from Nanopore sequencing of the parental strain were re-basecalled using guppy-gpu (v 6.0.1). The resulting fastq files were directly used for assembly using Canu (v 2.1.1) with an estimated genome size of 20 Megabases and default parameters.64 This assembly had 14 chromosome-scale contigs and a number of shorter contigs which mapped either to telomeric regions or mitochondrial DNA. These were manually trimmed from the assembly. The assembly was polished once with Nanopore reads using Medaka (v 1.5.0; https://github.com/nanoporetech/medaka) and then polished five times with Illumina reads from the parental strain using pilon (v 1.24) until no additional changes were being made to the assembly.65 A circular mitochondrial genome was assembled from Illumina reads using GetOrganelle (v1.7.5.3) with the fungus_mt setting and confirmed by comparison to the trimmed contigs assembled by Canu.66 This mitochondrial genome was manually added to the assembly. Chromosomes were named based on comparison to other reference assemblies and annotations were transferred using Liftoff (v1.6.3).67
For Illumina sequencing data of evolved colonies and passages, adapters were trimmed using Trimmomatic (v 0.39) and reads were aligned to our de novo assembly using bwa-mem2 (v 2.2.1).68,69 Duplicates were marked and read groups added with Picard and the resulting files were sorted and indexed with SAMtools (v1.15.1).71 Variants were called using sorted BAM files with the Genome Analysis Tool Kit (HaplotypeCaller and GenotypeGVCF, v3.8) with the ploidy set to 1.70 Variants were filtered out based on the following criteria using GATK VariantFiltration: QD<2.0, FS > 60, MQ <40, GQ <50, DP <10. To remove called SNPs in homopolymeric runs likely caused by sequencing errors, we used the BCFtools isec function to identify unique SNPs in each sample when compared to those found in the parental strain sequencing.71 The predicted impacts of called variants were assessed using SnpEff (v 4.3t) using our de novo assembly as reference72.
To analyze allele frequencies of the cac1-evo allele, sorted BAM files from each passage were visualized in IGV and the number of reads containing the Arg1227Pro (Chromosome 8 of our de novo assembly: 1057343 G>C, or Chromosome 8 of the H99 reference assembly: 333567 C>G ) were counted and normalized to the total sequencing coverage at that position.73 All sequencing analysis was performed using resources through the University of Utah Center for High Performance Computing. All of the raw sequence data has been deposited under NCBI BioProject:PRJNA941895.
Structure prediction and conservation analysis
The structure of the Ftc555-1 Cac1 protein was modeled using AlphaFold (v2.1.2).74 The predicted structure with the highest confidence (ranked_0.pdb) is shown. The catalytic domain was annotated based on sequence homology to the S. cerevisiae adenylyl cyclase homolog, Cyr1. The first 870 amino acids of Cac1 were unstructured in the model and were manually trimmed for display in Figure 5A.
Fungal adenylyl cyclase orthologs were downloaded from FungiDB or NCBI Genbank and aligned using MAFFT.81–84 Nucleotide sequences for the CAC1 genes from all publicly available C. neoformans strains were similarly downloaded from FungiDB. Introns were trimmed and the coding sequences were aligned in Geneious using the Translation Align function with the MUSCLE algorithm option. Alignments of the CAC1 sequences from the strains used in this paper were used as input to IQ-TREE for phylogenetic tree construction.75 The MGK+F3X3+I model was selected by the ModelFinder function, and the tree was estimated with 100 non-parametric bootstrap replicates.85 Some IQ-TREE analyses were performed with the IQ-Tree webserver.86 Bootstrap values for the tree shown in Figure S5A were uniformly greater than 95 indicating high confidence in the depicted tree.
Melanization assays
Melanization of different strains was analyzed following previously established protocols.87 Briefly, overnight cultures of C. neoformans strains were pelleted by centrifugation (3000xg, 5 minutes), washed twice in sterile water and adjusted to an OD of 0.25. Five microliters of each strain was spotted onto L-DOPA plates (7.6 mM L-asparagine monohydrate, 5.6 mM glucose, 10 mM MgSO4, 0.5 mM 3,4-dihydroxy-L-phenylalanine, 0.3 mM thiamine-hydrochloride, and 20 nM biotin) and onto YPD plates and incubated at 30°C or 37°C. Plates were checked every day until the WT control (KN99α) turned dark brown, usually day 3. For cAMP supplementation experiments, cAMP (Cayman Chemical) was added to plates to a final concentration of 2.5 mM. Strain preparations were spotted in parallel onto cAMP containing and control L-DOPA plates.
In vitro capsule induction assays
Capsule was induced using two different protocols.43 For both protocols, C. neoformans strains of interest were grown overnight (16–18 hours) in YPD media and OD600 was measured for each strain. In the first protocol, strains were subcultured to an OD of 0.3 in DMEM media containing 10% FBS, non-essential amino acids, and penicillin/streptomycin and incubated in a 6-well plate at 37°C with 5% CO2. In parallel, the same subcultures were subcultured to an OD of 0.3 in 10% Sabouraud’s Dextrose Media with 50 mM HEPES adjusted to pH 7.4. Strains in 10% Sab’s were incubated in 6 well plates at 37°C without CO2. As a control, the same strains were diluted in YPD media to the same OD and replicate plates were incubated at 37°C with or without CO2. Cell and capsule size measurements were made after 48 hours of incubation. Cells were centrifuged at 3000 x g, resuspended in PBS and fixed with formaldehyde. Samples were then mixed 1:1 with india ink to visualize capsule. 5–10 μL was pipetted onto a microscope slide and pictures were taken on an AxioObserver 7 (Zeiss) at 100x magnification. For each strain, measurements were made for 50 cells under each condition using a combination of manual measurement in FIJI and the automated QCA Matlab program.76,77 For cAMP supplementation experiments, cAMP was dissolved in the same media used above at a concentration of 20 mM and the final pH of the media with and without cAMP was equalized.
Titan and seed cell induction assays
Titan cells were induced using two different previously described protocols: by incubation in serum-free RPMI or in PBS with 10% heat-inactivated fetal bovine serum (HI-FBS).37,50 Briefly, overnight cultures were grown with shaking for 16–18 hours in YPD. Cells were washed three times in PBS and adjusted to a concentration of 5x103 cells/ml in each media type. 1 mL aliquots were dispensed into three replicate wells of a 24-well plate and incubated at 37°C with 5% CO2 for 7 days. Exogenous cAMP supplementation experiments were carried out as above.
Seed cell formation was assessed in vitro using previously described protocols.48 Strains of interest were grown in YNB media overnight with rotation at 37°C. YNB cultures were then diluted 1:50 in 10% Sabouraud’s Dextrose Media with 50 mM HEPES (pH 7.4, also referred to CAP media) and grown with rotation at 37°C for 24 hours. To induce seed cells, 2.5 mL of the Sab’s culture was subcultured into Pigeon Guano Media (PGM) and grown for 24h at 37°C with rotation. At every subculture step, aliquots of cells were fixed for cell size quantification as described above. For seed cell and titan formation assays, cell visualization, imaging and cell size quantifications were carried out as in the capsule induction experiments above.
Animal experiments
Animal infections were performed as described above (see mouse infection models). For fungal burden determination, organs were harvested either at infection endpoint (parental-infected animals) or at 56 days post infection (M1 infected animals), placed on ice, and homogenized in 5 ml of PBS. Serial dilutions of homogenates were plated on Sabouraud’s dextrose agar with 10 mg/mL gentamicin and 100 mg/mL carbenicillin. Plates were incubated at 30°C for 2–3 days before were counted to determine fungal burden per organ.
For histopathology analysis, all animals were humanely euthanized at 22 d.p.i. and their lungs were harvested and fixed in neutral buffered formalin for 72 hours before being transferred to 70% ethanol. Histology was performed by HistoWiz Inc. Samples were processed, paraffin-embedded, sectioned and 4 sections per animal were stained with hematoxylin and eosin (H&E). Whole slide scanning (40x) was performed on an Aperio AT2 (Leica Biosystems). Images from all processed slides can be accessed at the link in the key resources table. One slide per animal was analyzed and scored by a board-certified veterinary pathologist through HistoWiz Inc.
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses were performed with GraphPad Prism 9 software. Information about quantification, replication and statistical details of experiments can be found in the corresponding figure legends.
Supplementary Material
Highlights.
C. neoformans strains vary in their interactions with diverse host species
Serial passaging reveals that fungi adapt rapidly to distinct host-cell environments
A missense mutation in adenylyl cyclase confers increased growth in macrophages
Adaptation to macrophages comes with trade-offs for in vivo pathogenicity
ACKNOWLEDGMENTS
We thank John Perfect for providing the strains used in this study, Hiten Madhani for CRISPR reagents, members of the Elde and Brown labs and Kyla Ost for helpful conversations in developing this project, and Vikas Yadav for sequencing advice. This work was funded by a Helen Hay Whitney Foundation fellowship to Z.A.H., NIH T32 funding to K.Y.C. and J.M.B. (T32AI055434) and to C.T.M. (T32GM141848), an NIH R01 (R01AI130248) to J.C.S.B., a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease award to N.C.E., and an NIH R35 (R35GM134936) to N.C.E.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2023.08.054.
DECLARATION OF INTERESTS
The authors declare no competing interests.
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
REFERENCES
- 1.Idnurm A, Bahn YS, Nielsen K, Lin X, Fraser JA, and Heitman J (2005). Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol 3, 753–764. 10.1038/nrmicro1245. [DOI] [PubMed] [Google Scholar]
- 2.Montoya MC, Magwene PM, and Perfect JR (2021). Associations between Cryptococcus genotypes, phenotypes, and clinical parameters of human disease: a review. J. Fungi (Basel) 7, 260, 10.3390/jof7040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajasingham R, Govender NP, Jordan A, Loyse A, Shroufi A, Denning DW, Meya DB, Chiller TM, and Boulware DR (2022). The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect. Dis 22, 1748–1755. 10.1016/S1473-3099(22)00499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desjardins CA, Giamberardino C, Sykes SM, Yu CH, Tenor JL, Chen Y, Yang T, Jones AM, Sun S, Haverkamp MR, et al. (2017). Population genomics and the evolution of virulence in the fungal pathogen Cryptococcus neoformans. Genome Res 27, 1207–1219. 10.1101/gr.218727.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Litvintseva AP, Frazzitta AE, Haverkamp MR, Wang L, Fang C, Muthoga C, Mitchell TG, and Perfect JR (2015). Comparative analyses of clinical and environmental populations of Cryptococcus neoformans in Botswana. Mol. Ecol 24, 3559–3571. 10.1111/mec.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litvintseva AP, and Mitchell TG (2009). Most environmental isolates of Cryptococcus neoformans var. grubii (serotype A) are not lethal for mice. Infect. Immun 77, 3188–3195. 10.1128/IAI.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu CH, Chen Y, Desjardins CA, Tenor JL, Toffaletti DL, Giamberardino C, Litvintseva A, Perfect JR, and Cuomo CA (2020). Landscape of gene expression variation of natural isolates of Cryptococcus neoformans in response to biologically relevant stresses. Microb. Genom 6, e000319, 10.1099/mgen.0.000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu CH, Sephton-Clark P, Tenor JL, Toffaletti DL, Giamberardino C, Haverkamp M, Cuomo CA, and Perfect JR (2021). Gene expression of diverse Cryptococcus isolates during infection of the human central nervous system. mBio 12, e0231321, 10.1128/mBio.02313-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sephton-Clark P, Tenor JL, Toffaletti DL, Meyers N, Giamberardino C, Molloy SF, Palmucci JR, Chan A, Chikaonda T, Heyderman R, et al. (2022). Genomic variation across a clinical Cryptococcus population linked to disease outcome. mBio 13, e0262622, 10.1128/mbio.02626-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerstein AC, Jackson KM, McDonald TR, Wang Y, Lueck BD, Bohjanen S, Smith KD, Akampurira A, Meya DB, Xue C, et al. (2019). Identification of pathogen genomic differences that impact human immune response and disease during Cryptococcus neoformans infection. mBio 10, e01440–19, 10.1128/mBio.01440-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall A, Fu MS, Guimaraes AJ, and Albuquerque P (2019). The ‘amoeboid predator-fungal animal virulence’ hypothesis. J. Fungi (Basel) 5, 10, 10.3390/jof5010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadevall A, Steenbergen JN, and Nosanchuk JD (2003). ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi — the Cryptococcus neoformans paradigm. Curr. Opin. Microbiol 6, 332–337. 10.1016/s1369-5274(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 13.Rayamajhee B, Willcox MDP, Henriquez FL, Petsoglou C, Subedi D, and Carnt N (2022). Acanthamoeba, an environmental phagocyte enhancing survival and transmission of human pathogens. Trends Parasitol 38, 975–990. 10.1016/j.pt.2022.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Segal G, and Shuman HA (1999). Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun 67, 2117–2124. 10.1128/IAI.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Quadan T, Price CT, and Abu Kwaik YA (2012). Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella. Trends Microbiol 20, 299–306. 10.1016/j.tim.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JM, Ghosh S, and O’Connor TJ (2020). Combinatorial selection in amoebal hosts drives the evolution of the human pathogen Legionella pneumophila. Nat. Microbiol 5, 599–609. 10.1038/s41564-019-0663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor TJ, Adepoju Y, Boyd D, and Isberg RR (2011). Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proc. Natl. Acad. Sci. USA 108, 14733–14740. 10.1073/pnas.1111678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steenbergen JN, Shuman HA, and Casadevall A (2001). Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98, 15245–15250.. 10.1073/pnas.261418798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chrisman CJ, Alvarez M, and Casadevall A (2010). Phagocytosis of Cryptococcus neoformans by, and nonlytic exocytosis from, Acanthamoeba castellanii. Appl. Environ. Microbiol 76, 6056–6062. 10.1128/AEM.00812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, and Casadevall A (2011). Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog 7, e1002047, 10.1371/journal.ppat.1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derengowski L.da S., Paes HC, Albuquerque P, Tavares AHFP, Fernandes L, Silva-Pereira I, and Casadevall A (2013). The transcriptional response of Cryptococcus neoformans to ingestion by Acanthamoeba castellanii and macrophages provides insights into the evolutionary adaptation to the mammalian host. Eukaryot. Cell 12, 761–774. 10.1128/EC.00073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes J, Beale MA, Vanhove M, Jarvis JN, Kannambath S, Simpson JA, Ryan A, Meintjes G, Harrison TS, Fisher MC, and Bicanic T (2017). A population genomics approach to assessing the genetic basis of within-host microevolution underlying recurrent cryptococcal meningitis infection. G3 (Bethesda) 7, 1165–1176. 10.1534/g3.116.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ormerod KL, Morrow CA, Chow EWL, Lee IR, Arras SDM, Schirra HJ, Cox GM, Fries BC, and Fraser JA (2013). Comparative genomics of serial isolates of Cryptococcus neoformans reveals gene associated with carbon utilization and virulence. G3 (Bethesda) 3, 675–686. 10.1534/g3.113.005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Farrer RA, Giamberardino C, Sakthikumar S, Jones A, Yang T, Tenor JL, Wagih O, Van Wyk MV, Govender NP, et al. (2017). Microevolution of serial clinical isolates of Cryptococcus neoformans var. grubii and C. gattii. mBio 8, e00166–17, 10.1128/mBio.00166-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunke S, Seider K, Fischer D, Jacobsen ID, Kasper L, Jablonowski N, Wartenberg A, Bader O, Enache-Angoulvant A, Schaller M, et al. (2014). One small step for a yeast - microevolution within macrophages renders Candida glabrata hypervirulent due to a single point mutation. PLoS Pathog 10, e1004478, 10.1371/journal.ppat.1004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wartenberg A, Linde J, Martin R, Schreiner M, Horn F, Jacobsen ID, Jenull S, Wolf T, Kuchler K, Guthke R, et al. (2014). Microevolution of Candida albicans in macrophages restores filamentation in a nonfilamentous mutant. PLoS Genet 10, e1004824, 10.1371/journal.pgen.1004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu G, Chen SH, Qiu J, Bennett JE, Myers TG, and Williamson PR (2014). Microevolution during serial mouse passage demonstrates FRE3 as a virulence adaptation gene in Cryptococcus neoformans. mBio 5. e00941–e00914. 10.1128/mBio.00941-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sephton-Clark P, McConnell SA, Grossman N, Baker RP, Dragotakes Q, Fan Y, Fu MS, Gerbig G, Greengo S, Hardwick JM, et al. (2023). Similar evolutionary trajectories in an environmental Cryptococcus neoformans isolate after human and murine infection. Proc. Natl. Acad. Sci. USA 120, e2217111120, 10.1073/pnas.2217111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steenwyk JL, and Rokas A (2019). Treehouse: a user-friendly application to obtain subtrees from large phylogenies. BMC Res. Notes 12, 541, 10.1186/s13104-019-4577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez M, and Casadevall A (2006). Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol 16, 2161–2165. 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 31.Ma H, Croudace JE, Lammas DA, and May RC (2006). Expulsion of live pathogenic yeast by macrophages. Curr. Biol 16, 2156–2160. 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 32.Seoane PI, Taylor-Smith LM, Stirling D, Bell LCK, Noursadeghi M, Bailey D, and May RC (2020). Viral infection triggers interferon-induced expulsion of live Cryptococcus neoformans by macrophages. PLoS Pathog 16, e1008240, 10.1371/journal.ppat.1008240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alspaugh JA, Pukkila-Worley R, Harashima T, Cavallo LM, Funnell D, Cox GM, Perfect JR, Kronstad JW, and Heitman J (2002). Adenylyl cyclase functions downstream of the Ga protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1, 75–84. 10.1128/EC.1.1.75-84.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hicks JK, D’Souza CA, Cox GM, and Heitman J (2004). Cyclic AMP-dependent protein kinase catalytic subunits have divergent roles in virulence factor production in two varieties of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 3, 14–26. 10.1128/EC.3.1.14-26.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, and Heitman J (2001). Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol 21, 3179–3191. 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alspaugh JA, Perfect JR, and Heitman J (1997). Cryptococcus neoformans mating and virulence are regulated by the G-protein a subunit GPA1 and cAMP. Genes Dev 11, 3206–3217. 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dambuza IM, Drake T, Chapuis A, Zhou X, Correia J, Taylor-Smith L, LeGrave N, Rasmussen T, Fisher MC, Bicanic T, et al. (2018). The Cryptococcus neoformans titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog 14, e1006978, 10.1371/journal.ppat.1006978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caza M, and Kronstad JW (2019). The cAMP/protein kinase A pathway regulates virulence and adaptation to host conditions in Cryptococcus neoformans. Front. Cell. Infect. Microbiol 9, 212, 10.3389/fcimb.2019.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki N, Choe HR, Nishida Y, Yamawaki-Kataoka Y, Ohnishi S, Tamaoki T, and Kataoka T (1990). Leucine-rich repeats and carboxyl terminus are required for interaction of yeast adenylate cyclase with RAS proteins. Proc. Natl. Acad. Sci. USA 87, 8711–8715. 10.1073/pnas.87.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minato T, Wang J, Akasaka K, Okada T, Suzuki N, and Kataoka T (1994). Quantitative analysis of mutually competitive binding of human Raf-1 and yeast adenylyl cyclase to Ras proteins. J. Biol. Chem 269, 20845–20851. 10.1016/s0021-9258(17)31899-9. [DOI] [PubMed] [Google Scholar]
- 41.Dubacq C, Guerois R, Courbeyrette R, Kitagawa K, and Mann C (2002). Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cyr1p/Cdc35p in budding yeast. Eukaryot. Cell 1, 568–582. 10.1128/EC.1.4.568-582.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casadevall A, Rosas AL, and Nosanchuk JD (2000). Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol 3, 354–358. 10.1016/S1369-5274(00)00103-X. [DOI] [PubMed] [Google Scholar]
- 43.Zaragoza O, and Casadevall A (2004). Experimental modulation of capsule size in Cryptococcus neoformans. Biol. Proced. Online 6, 10–15. 10.1251/bpo68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaragoza O, Fries BC, and Casadevall A (2003). Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO(2). Infect. Immun 71, 6155–6164. 10.1128/IAI.71.11.6155-6164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Granger DL, Perfect JR, and Durack DT (1985). Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J. Clin. Invest 76, 508–516. 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue C, Bahn YS, Cox GM, and Heitman J (2006). G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol. Biol. Cell 17, 667–679. 10.1091/mbc.e05-07-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hommel B, Mukaremera L, Cordero RJB, Coelho C, Desjardins CA, Sturny-Leclère A, Janbon G, Perfect JR, Fraser JA, Casadevall A, et al. (2018). Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog 14, e1006982, 10.1371/journal.ppat.1006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denham ST, Brammer B, Chung KY, Wambaugh MA, Bednarek JM, Guo L, Moreau CT, and Brown JCS (2022). A disseminationprone morphotype enhances extrapulmonary organ entry by Cryptococcus neoformans. Cell Host Microbe 30, 1382–1400.e8. 10.1016/j.chom.2022.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trevijano-Contador N, de Oliveira HC, García-Rodas R, Rossi SA, Llorente I, Zaballos Á, Janbon G, Ariño J, and Zaragoza Ó (2018). Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog 14, e1007007, 10.1371/journal.ppat.1007007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saidykhan L, Correia J, Romanyuk A, Peacock AFA, Desanti GE, Taylor-Smith L, Makarova M, Ballou ER, and May RC (2022). An in vitro method for inducing titan cells reveals novel features of yeast-to-titan switching in the human fungal pathogen Cryptococcus gattii. PLoS Pathog 18, e1010321, 10.1371/journal.ppat.1010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukaremera L, McDonald TR, Nielsen JN, Molenaar CJ, Akampurira A, Schutz C, Taseera K, Muzoora C, Meintjes G, Meya DB, et al. (2019). The mouse inhalation model of Cryptococcus neoformans infection recapitulates strain virulence in humans and shows that closely related strains can possess differential virulence. Infect. Immun 87, e00046–19, 10.1128/IAI.00046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauters TJC, Roth C, Murray D, Sun S, Floyd-Averette A, Onyishi CU, May RC, Heitman J, and Magwene P (2022). Amoeba predation of Cryptococcus: a quantitative and population genomic evaluation of the accidental pathogen hypothesis. Preprint at bioRxiv 10.1101/2022.12.08.519367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu MS, Liporagi-Lopes LC, dos Santos SR, Tenor JL, Perfect JR, Cuomo CA, and Casadevall A (2021). Amoeba predation of Cryptococcus neoformans results in pleiotropic changes to traits associated with virulence. mBio 12, e00567–21, 10.1128/mBio.00567-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slev PR, and Potts WK (2002). Disease consequences of pathogen adaptation. Curr. Opin. Immunol 14, 609–614. 10.1016/s0952-7915(02)00381-3. [DOI] [PubMed] [Google Scholar]
- 55.Didelot X, Walker AS, Peto TE, Crook DW, and Wilson DJ (2016). Within-host evolution of bacterial pathogens. Nat. Rev. Microbiol 14, 150–162. 10.1038/nrmicro.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duffy S, Shackelton LA, and Holmes EC (2008). Rates of evolutionary change in viruses: patterns and determinants. Nat. Rev. Genet 9, 267–276. 10.1038/nrg2323. [DOI] [PubMed] [Google Scholar]
- 57.Lenski RE (2017). Experimental evolution and the dynamics of adaptation and genome evolution in microbial populations. ISME J 11, 2181–2194. 10.1038/ismej.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McClelland EE, Adler FR, Granger DL, and Potts WK (2004). Major histocompatibility complex controls the trajectory but not host-specific adaptation during virulence evolution of the pathogenic fungus Cryptococcus neoformans. Proc. Biol. Sci 271, 1557–1564. 10.1098/rspb.2004.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agustinho DP, Brown HL, Chen G, Brent MR, and Doering TL (2023). Unbiased discovery of natural sequence variants that influence fungal virulence. Preprint at bioRxiv 10.1101/2023.04.23.537984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson KM, Ding M, and Nielsen K (2023). Importance of clinical isolates in Cryptococcus neoformans research. J. Fungi (Basel) 9, 364, 10.3390/jof9030364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litvintseva AP, Marra RE, Nielsen K, Heitman J, Vilgalys R, and Mitchell TG (2003). Evidence of sexual recombination among Cryptococcus neoformans serotype A isolates in sub-Saharan Africa. Eukaryot. Cell 2, 1162–1168. 10.1128/EC.2.6.1162-1168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Litvintseva AP, Carbone I, Rossouw J, Thakur R, Govender NP, and Mitchell TG (2011). Evidence that the human pathogenic fungus Cryptococcus neoformans var. grubii may have evolved in Africa. PLoS One 6, e19688, 10.1371/journal.pone.0019688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang MY, Joshi MB, Boucher MJ, Lee S, Loza LC, Gaylord EA, Doering TL, and Madhani HD (2022). Short homology-directed repair using optimized Cas9 in the pathogen Cryptococcus neoformans enables rapid gene deletion and tagging. Genetics 220, iyab180, 10.1093/genetics/iyab180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, and Phillippy AM (2017). Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 27, 722–736. 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, et al. (2014). Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 9, e112963, 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, and Li DZ (2020). GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol 21, 241. 10.1186/s13059-020-02154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shumate A, and Salzberg SL (2021). Liftoff: accurate mapping of gene annotations. Bioinformatics 37, 1639–1643. 10.1093/bioin-formatics/btaa1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolger AM, Lohse M, and Usadel B (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Md, Misra S, Li H, and Aluru S (2019). Efficient architecture-aware acceleration of BWA-MEM for multicore systems. 2019 IEEE International Parallel Distributed Process Symposium (IPDPS), 314–324. 10.1109/ipdps.2019.00041. [DOI] [Google Scholar]
- 70.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, and DePristo MA (2010). The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20, 1297–1303. 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, and Li H (2021). Twelve years of SAMtools and BCFtools. Gigascience 10, giab008. 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, and Ruden DM (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6, 80–92. 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thorvaldsdóttir H, Robinson JT, and Mesirov JP (2013). Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform 14, 178–192. 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Ždek A, Potapenko A, et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen LT, Schmidt HA, von Haeseler A, and Minh BQ (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol 32, 268–274. 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dragotakes Q, and Casadevall A (2018). Automated measurement of cryptococcal species polysaccharide capsule and cell body. J. Vis. Exp 56957 10.3791/56957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drenkard E, Richter BG, Rozen S, Stutius LM, Angell NA, Mindrinos M, Cho RJ, Oefner PJ, Davis RW, and Ausubel FM (2000). A simple procedure for the analysis of single nucleotide polymorphisms facilitates map-based cloning in Arabidopsis. Plant Physiol 124, 1483–1492. 10.1104/pp.124.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fan Y, and Lin X (2018). Multiple applications of a transient CRISPR-Cas9 coupled with electroporation (TRACE) system in the Cryptococcus neoformans species complex. Genetics 208, 1357–1372. 10.1534/genetics.117.300656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Upadhya R, Lam WC, Maybruck BT, Donlin MJ, Chang AL, Kayode S, Ormerod KL, Fraser JA, Doering TL, and Lodge JK (2017). A fluorogenic C. neoformans reporter strain with a robust expression of m-cherry expressed from a safe haven site in the genome. Fungal Genet. Biol 108, 13–25. 10.1016/j.fgb.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Basenko EY, Pulman JA, Shanmugasundram A, Harb OS, Crouch K, Starns D, Warrenfeltz S, Aurrecoechea C, Stoeckert CJ, Kissinger JC, et al. (2018). FungiDB: an integrated bioinformatic resource for fungi and oomycetes. J. Fungi (Basel) 4, 39, 10.3390/jof4010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stajich JE, Harris T, Brunk BP, Brestelli J, Fischer S, Harb OS, Kissinger JC, Li W, Nayak V, Pinney DF, et al. (2012). FungiDB: an integrated functional genomics database for fungi. Nucleic Acids Res 40, D675–D681. 10.1093/nar/gkr918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katoh K, and Standley DM (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol 30, 772–780. 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katoh K, Misawa K, Kuma K, and Miyata T (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30, 3059–3066. 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, and Jermiin LS (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trifinopoulos J, Nguyen LT, von Haeseler A, and Minh BQ (2016). W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44, W232–W235. 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nichols CB (2021). Visualization and documentation of capsule and melanin production in Cryptococcus neoformans. Curr. Protoc 1, e27, 10.1002/cpz1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All DNA sequencing datasets have been deposited in the NCBI SRA and are publicly available. The BioProject accession number is listed in the key resources table. The paper also analyzes existing, publicly available data. These accession numbers are also listed in the key resources table. Raw histopathology data from this study are hosted on the HistoWiz website and are publicly available as of the date of this publication. Accession link is provided in the key resources table. All other data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code. Existing bioinformatic packages were used as specified in the method details and the key resources table lists version numbers.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Mouse Anti-Glucuronoxylomannan (GXM) Antibody, clone 18B7 | Sigma | Cat#MABF2069 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| cAMP | Cayman Chemical | Cat#18820 |
| Phorbol myristate acetate (PMA) | Sigma | Cat#P1585 |
| Phusion Flash DNA Polymerase | Thermo Fisher | Cat# F548S |
| HiFi DNA Master Mix | New England Biolabs | Cat#E2621 |
| Fluconazole | Sigma | Cat#F8929 |
| Hexadecyltrimethylammonium bromide (CTAB) | Sigma | Cat#H9151 |
| 3,4-dihydroxy-L-phenylalanine | Sigma | Cat#D9628 |
| L-Asparagine monohydrate | Sigma | Cat#A7094 |
| Thiamine hydrochloride | Sigma | Cat#T4625 |
| Biotin | Sigma | Cat#B4639 |
| Yeast Extract | Gibco | Cat#212750 |
| Peptone | Gibco | Cat#211677 |
| Yeast nitrogen base without amino acids | Sigma | Cat#Y0626 |
| Sabouraud Dextrose Broth | Difco | Cat#238230 |
| DMEM with high glucose and L-glutamine | VWR | Cat#16777-129 |
| Fetal Bovine Serum | Thermo Fisher | Cat#26140079 |
| RPMI 1640 | VWR | Cat#16777-145 |
| RPMI 1640 with L-glutamine w/o sodium bicarbonate | Gibco | Cat#31800089 |
| Dexdomitor | Zoetis | Cat#122692-5 |
| Antisedan | Zoetis | Cat#87219-02296-2 |
| Dexamethasone | Sigma | Cat#D4902 |
|
| ||
| Critical commercial assays | ||
|
| ||
| NEBNext Ultra II DNA Library Prep | New England Biolabs | Cat#E7634L |
| Ligation Sequencing Kit | Oxford Nanopore | Cat#SQK-LSK110 |
| Fluconazole Etest Strips | BioMerieux | Cat#412349 |
| ChromaSpin 1000 | Takara | Cat#636093 |
|
| ||
| Deposited data | ||
|
| ||
| Nanopore and Illumina sequencing data | Sequence Read Archive | NCBI BioProject: PRJNA941895 |
| Histopathology images from mice infected with Ftc555-1 Parental and M1 strains | HistoWiz | HistoWiz Data:https://app.histowiz.com/shared_orders/897b4aa6-bf6c-49da-9009-7113f766aee9/slides/ |
| M. sympodalis adenylyl cyclase sequence | NCBI Genbank | GenBank: SHO76694.1 |
| U. maydis adenylyl cyclase sequence | NCBI Genbank | GenBank: XP_011388269 |
| C. neoformans adenylyl cylase sequence | NCBI Genbank | GenBank: XP_012050872 |
| C. gattii adenylyl cyclase sequence | NCBI Genbank | GenBank: XP_003195134.1 |
| S. pombe adenylyl cyclase sequence | NCBI Genbank | GenBank: NP_596159.1 |
| N. crassa adenylyl cyclase sequence | NCBI Genbank | GenBank: BAA00755.1 |
| B. cinerea adenylyl cyclase sequence | NCBI Genbank | GenBank: XP_024553314.1 |
| T. rubrum adenylyl cyclase sequence | NCBI Genbank | GenBank: KDB32528.1 |
| A. niger adenylyl cyclase sequence | NCBI Genbank | GenBank:GAq42063.1 |
| C. albicans adenylyl cyclase sequence | NCBI Genbank | GenBank:XP_716974.2 |
| S. cerevisiae adenylyl cyclase sequence | NCBI Genbank | GenBank:NP_012529 |
| CAC1 sequences from all C. neoformans isolates | FungiDB | FungiDB: https://fungidb.org/fungidb/app/record/gene/CNAG_03202#category:dna-polymorphism |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| J774A.1 | ATCC | Cat# TIB-67; RRID: CVCL_0358 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| Mice: C57BL/6NJ | Jackson Laboratory | RRID: IMSR_JAX:005304 |
| Amoeba: Acanthamoeba castellanii | ATCC | Cat# 30234 |
| C. neoformans: H99O | Gift from J. Perfect | N/A |
| C. neoformans: KN99α | Fungal Genetics Stock Center | Cat#10369 |
| C. neoformans: KN99α cac1Δ::NAT | Fungal Genetics Stock Center, Madhani Knockout Collection (2020 Plates) | N/A |
| C. neoformans: Bt46 | Gift from J. Perfect, Litvintseva et al.61 | N/A |
| C. neoformans: Ze90-1 | Gift from J. Perfect, Litvintseva et al.62 | N/A |
| C. neoformans: Ftc217-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc239-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc209-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc209-3 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: NRHc5004.ENR | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Muc458-2 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: NRHc5026.ENR.CLIN.ISO | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc555-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc158-2 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: NRHc5029.ENR.CLIN.ISO | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Gbc39-1 | Gift from J. Perfect, Chen et al.5 | N/A |
| C. neoformans: Ftc555-1 M1-1 | This study | N/A |
| C. neoformans: Ftc555-1 M2-1 | This study | N/A |
| C. neoformans: Ftc555-1 M3-2 | This study | N/A |
| C. neoformans: Ftc555-1 A1-1 | This study | N/A |
| C. neoformans: Ftc555-1 A2-1 | This study | N/A |
| C. neoformans: Ftc555-1 A3-1 | This study | N/A |
| C. neoformans: Ftc555-1 SH2-NAT | This study | ZAH_CN01 |
| C. neoformans: Ftc555-1 Parental cac1-evo(R1227P); SH2-NAT isolate H1 | This study | ZAH_CN03 |
| C. neoformans: Ftc555-1 Parental cac1-evo(R1227P); SH2-NAT isolate H7 | This study | ZAH_CN04 |
| C. neoformans: Ftc555-1 M1 CAC1-parent (P1227R); SH2-NAT isolate M2 | This study | ZAH_CN05 |
| C. neoformans: Ftc555-1 M1 CAC1-parent (P1227R); SH2-NAT isolate H11 | This study | ZAH_CN06 |
| C. neoformans: Ftc555-1 Parental cac1Δ::HYG | This study | ZAH_CN07 |
| C. neoformans: Ftc555-1 M1 cac1Δ::HYG; cac1-evo(R1227P) | This study | ZAH_CN08 |
| C. neoformans: Ftc555-1 Parental aca1Δ::NAT | This study | ZAH_CN09 |
| C. neoformans: Ftc555-1 Parental gpa1Δ::NAT | This study | ZAH_CN10 |
| C. neoformans: Ftc555-1 Parental gpr4Δ::NAT | This study | ZAH_CN11 |
| C. neoformans: Ftc555-1 M1 aca1Δ::NAT; cac1-evo(R1227P) | This study | ZAH_CN12 |
| C. neoformans: Ftc555-1 M1 gpa1Δ::NAT; cac1-evo(R1227P) | This study | ZAH_CN13 |
| C. neoformans: Ftc555-1 M1 gpr4Δ::NAT; cac1-evo(R1227P) | This study | ZAH_CN14 |
|
| ||
| Oligonucleotides | ||
|
| ||
| See Table S1 | N/A | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| SH2::NAT plasmid for CRISPR repair amplification | This study | pZAH31 |
| cac1Δ::HYG plasmid for CRISPR KO repair template | This study | pZAH40 |
| aca1Δ::NAT plasmid for CRISPR KO repair template | This study | pMWS05 |
| gpa1Δ::NAT plasmid for CRISPR KO repair template | This study | pMWS06 |
| gpr4Δ::NAT plasmid for CRISPR KO repair template | This study | pMWS07 |
| pRS316-PCnU6-scaffold for sgRNA Fusion PCRs | Huang et al.63 | AddGene Cat#171686; pBHM2329 |
| pRS316-PTEF1-CnoCas9 Codon Optimized C. neoformans Cas9 for CRISPR | Huang et al.63 | AddGene Cat#171687; pBHM2403 |
|
| ||
| Software and algorithms | ||
|
| ||
| Guppy-gpu basecaller (v 6.0.1) | Oxford Nanopore | RRID: SCR_023196 |
| Canu (v 2.1.1) | Koren et al.64 | RRID: SCR_015880; https://github.com/marbl/canu |
| Medaka (v 1.5.0) | Oxford Nanopore | https://github.com/nanoporetech/medaka |
| Pilon (v 1.24) | Walker et al.65 | RRID: SCR_014731; https://github.com/broadinstitute/pilon/ |
| GetOrganelle (v 1.7.5.3) | Jin et al.66 | https://github.com/Kinggerm/GetOrganelle |
| Liftoff (v 1.6.3) | Shumate and Salzberg67 | https://github.com/agshumate/Liftoff |
| Trimmomatic (v 0.39) | Bolger et al.68 | RRID: SCR_011848; http://www.usadellab.org/cms/index.php?page=trimmomatic |
| bwa-mem2 (v 2.2.1) | Vasimuddin et al.69 | RRID: SCR_022192; https://github.com/bwa-mem2/bwa-mem2 |
| Picard | Broad Institute | RRID: SCR_006525; https://github.com/broadinstitute/picard |
| Genome Analysis Tool Kit (GATK, v 3.8) | McKenna et al.70 | RRID: SCR_001876; https://software.broadinstitute.org/gatk/ |
| SAMtools (v 1.15.1) | Danecek et al.71 | RRID: SCR_002105; https://www.htslib.org/ |
| SnpEff (v 4.3t) | Cingolani et al.72 | RRID: SCR_005191; https://pcingola.github.io/SnpEff/ |
| Integrated Genomics Viewer (IGV) | Thorvaldsdóttir et al.73 | RRID: SCR_011793; http://www.broadinstitute.org/igv/ |
| AlphaFold (v 2.1.2) | Jumper et al.74 | https://github.com/deepmind/alphafold |
| Geneious | Geneious | RRID: SCR_010519; http://www.geneious.com/ |
| IQ-Tree | Nguyen et al.75 | RRID: SCR_017254; http://www.iqtree.org/ |
| FIJI | Schindelin et al.76 | RRID: SCR_002285; https://fiji.sc/ |
| QCA-Quigly’s Circle App | Dragotakes and Casadevall77 | https://github.com/quigly555/QCA |
| Prism 9 | GraphPad | RRID: SCR_002798; https://www.graphpad.com/ |
| WebSNAPER | Drenkard et al.78 | https://pga.mgh.harvard.edu/cgi-bin/snap3/websnaper3.cgi |


