Mounting evidence supports the connection between coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and neurological manifestations, such as cognitive dysfunction (“brain fog”), headache, and neuropsychiatric disorders [2, 6]. Autopsy studies on limited available cases have reported a spectrum of neuropathological changes in COVID-19 patients, in particular neuroinflammation and microvascular injury [3, 5, 11, 14]. However, there have been many caveats to these findings, including difficulty in determining whether these pathologies are a direct consequence of cerebral viral infection, or arise due to systemic complications of COVID-19 such as coagulopathy or ischemia [8, 14], or are associated with comorbidities such as neurodegenerative disease (NDD) [7]. Indeed, data thus far provide little evidence of SARS-CoV-2 in the brain in association with systemic infection [12, 14]. In particular, a recent comprehensive autopsy study of 44 patients died of COVID-19 observed limited evidence of inflammation or direct viral cytopathology in the central nervous system [12]. Accordingly, we performed post-mortem neuropathological studies to identify cerebral SARS-CoV-2 in cases with or without NDD and determine whether there was evidence of direct vascular injury in the form of blood-brain barrier (BBB) disruption.
Consecutive research brain donations fulfilling the requirements for each study group were identified within the archive holdings of the multi-center Collaborative Neuropathology Network Characterizing Outcomes from TBI (CONNECT-TBI) [10] as either: 1) COVID-19 infection (COVID+, identified as patients with a known history of COVID-19 infection and/or confirmed at autopsy with positive SARS-CoV-2 qualitative PCR nasopharyngeal/oropharyngeal swabs) and history of a known NDD clinical diagnosis (COVID+ NDD+, n=10); 2) COVID+ with no NDD (COVID+ NDD-, n=2); 3) no COVID-19 (COVID-, identified as patients died prior to October 2019) with NDD (COVID- NDD+, n=6), and 4) controls of similar age with no COVID and no NDD (COVID- NDD-, n=5) (Supplemental Table 1, Supplemental Table 2, and Supplemental Materials and Methods, online resource). A standard set of tissue sections was selected for microscopic evaluation, including cingulate gyrus, hippocampus, thalamus, and medulla. Using hybridization chain reaction (HCR) RNA fluorescence in situ hybridization (RNA-FISH), no SARS-CoV-2 viral RNA was detected in any brain region examined across all study groups, including those who were COVID+ (Fig. 1). These findings are consistent with recent RT-PCR and RNA-FISH based studies which, similarly, report no detectable SARS-CoV-2 RNA in brain tissue homogenates or brain sections [3, 5]. While these and our observations might suggest there is no penetration of SARS-CoV-2 into the brain, we still do not rule out the neuroinvasive capacity of SARS-CoV-2 due to the possibility of viral RNA replication clearance at the time of death [16].
Fig. 1. No SARS-CoV-2 viral RNA detectable in the brain tissue.
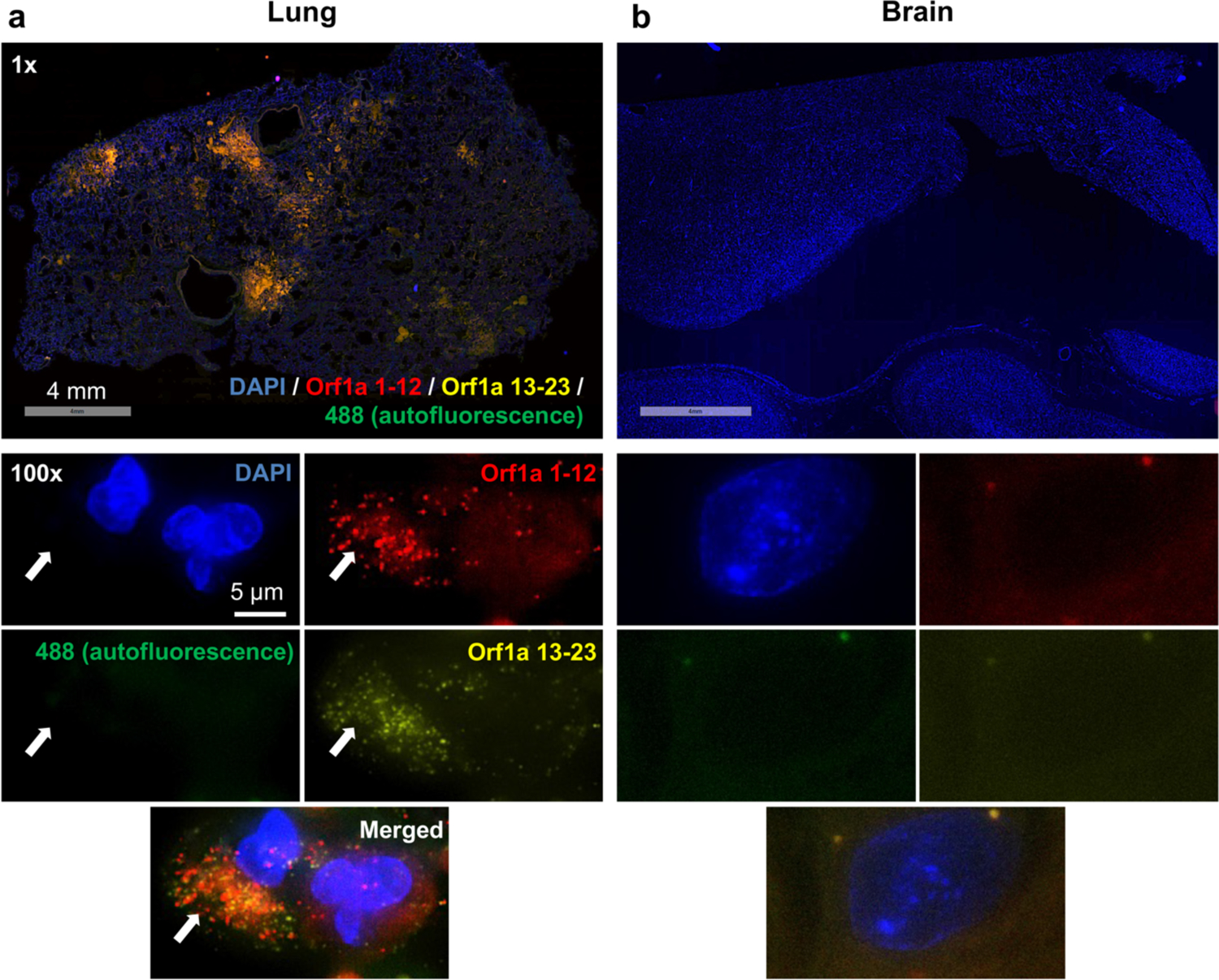
(a) The presence of SARS-CoV-2 viral RNA in the lung tissue, potentially associated with epithelial cell infection, was revealed through hybridization with probes of SARS-CoV-2 Orf1a (coupled with amplifier labeling Alexa Fluor 647 to Orf1a 1–12 and Alexa Fluor 546 to Orf1a 13–23). Amplifier labeling Alexa Fluor 488 green was used as the negative control marking tissue autofluorescence to minimize false positivity. (b) In contrast, no SARS-CoV-2 viral RNA was detected in the brain tissue. Scale bars 4 mm for the top low power images, 5 μm for the below high power images.
The consistent observation of brain microvascular injury in patients with COVID-19 [5] led us to suspect that blood-brain barrier (BBB) disruption might contribute to cerebral consequences of infection. To investigate this, we used immunohistochemistry to examine and evaluate potential extravasation of the serum protein fibrinogen, which does not normally cross the BBB [1]. Immunostaining of fibrinogen in each case was assessed and semi-quantitatively scored in line with published experience [1]. In all cases with COVID-19 infection (COVID+ NDD+ and COVID+ NDD-), we observed evidence of widespread BBB disruption. Specifically, in all regions examined, widespread perivascular and parenchymal fibrinogen staining was present (Fig. 2 and Supplemental Table 1, online resource). In comparison, in non-infected control cases (COVID-NDD-) there was typically no or at most minimal fibrinogen staining.
Fig. 2. Representative fibrinogen staining in COVID+ cases and in controls.
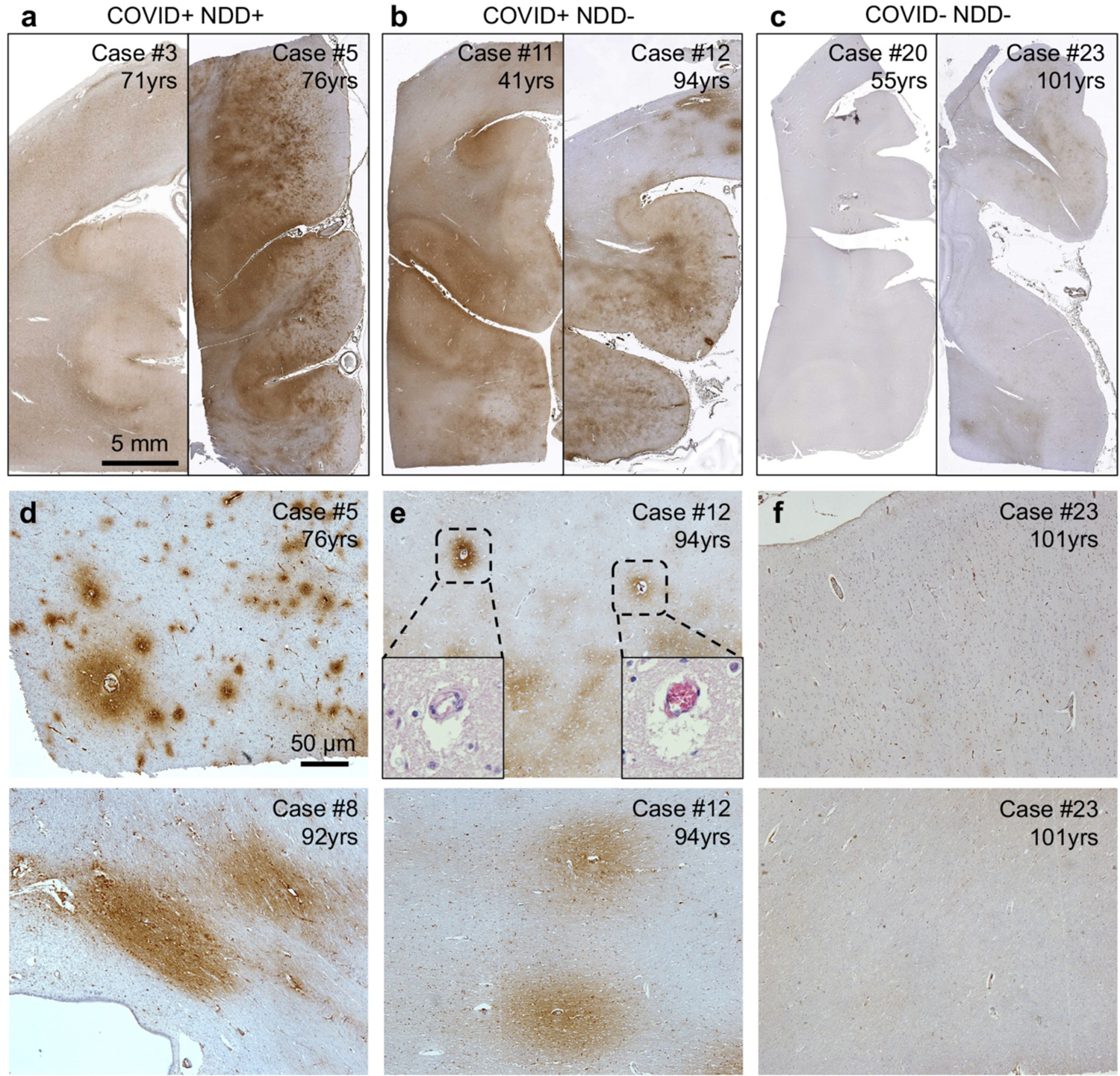
(a) Moderate (left, score of 2) to extensive (right, score of 3) fibrinogen immunoreactivity in cingulate gyrus was observed in COVID+ NDD+ cases. (b) Similarly, widespread, multifocal extensive fibrinogen immunoreactivity (score of 3) was identified in two COVID+ NDD- cases. (c) In contrast, absent (left, score of 0) to sparse (right, score of 1) fibrinogen immunoreactivity was observed in COVID- NDD-cases. (d) Substantial and widespread microscopic fibrinogen immunoreactivity in cingulate cortex (upper panel) and corpus callosal white matter (lower panel) was identified in COVID+ NDD+ cases. (e) In COVID+ NDD- cases, multifocal fibrinogen immunoreactivity was also evident in cingulate cortical layers (upper panel) and associated white matter (lower panel). H&E staining (inset) shows clumps of red blood cells in certain small vessels associated with fibrinogen extravasation. (f) Only limited fibrinogen immunoreactivity was observed in COVID- NDD- cases. Scale bars a-c 5 mm, d-f 50 μm.
Notably, disruption of BBB is a neuropathological feature in many NDDs (Supplemental Fig. 1) and even normal aging [13]. To address this, our study included both aged individuals and those with comorbid NDDs as controls when assessing COVID-19 related neuropathological changes. Nevertheless, the extensive fibrinogen extravasation we observed in context of SARS-CoV-2 infection was in excess of that observed in normal aging, with widespread and substantial fibrinogen extravasation in both brain gray and white matter in our youngest COVID+, aged just 41 years (Fig. 2b). While we observed limited evidence of microthrombi within some small vessels, most of the fibrinogen staining was present in regions without apparent microthrombi. Taken together, our findings suggest a plausible association between COVID-19 infection and BBB disruption. Nonetheless, our relatively small number of cases warrants a more extensive examination to confirm these findings.
It is unclear why the BBB might be compromised by COVID-19 infection, but neuroinflammation may play a role in promoting this disruption, as BBB disruption and neuroinflammation are commonly observed as comorbidities [5]. With regard to a potential specific mechanism, a previous study suggests that the spike protein attached to brain endothelial cells could further exacerbate the BBB disruption by triggering a pro-inflammatory response [15]. Nevertheless, despite a disrupted BBB, there is no direct evidence of SARS-CoV-2 entry into the brain in infected humans [3, 5], including in our cases. This is in contrast with experimental data showing that the spike protein of SARS-CoV-2 could be absorbed across the BBB in a mouse model [9], possibly through adsorptive-mediated transcytosis of the spike protein involving angiotensin-converting enzyme 2 (ACE2). In addition, treatment with anti-spike or anti-ACE2 antibodies has been shown to reduce the entry of SARS-CoV-2 into the BBB [4].
Overall, we find autopsy evidence of widespread BBB disruption in the brains of individuals with history of COVID-19 infection, but no detectable virus in tissue sections. Conceivably, BBB dysfunction may contribute to the neurological impairment during disease progression and the long-lasting cerebral symptoms in survivors. Nevertheless, we must acknowledge limitations in case numbers and clinical information in this series. More comprehensive studies of COVID-19 related BBB disruption, including neuropathology and advanced imaging studies, are required to explore the contribution of this pathology to immediate and late neurological consequences of COVID-19 outcomes.
Supplementary Material
Acknowledgement
Here, we thank the NINDS/NIA-supported Center Without Walls, CONNECT-TBI, and Center for Neurodegenerative Disease Research (CNDR) for providing the brain tissue sections, particularly Aimee M. Schantz, John Campos, Theresa Schuck, and John L. Robinson for their coordination. We acknowledge the Penn Tumor Tissue and Biospecimen Bank for providing the lung tissue sections. We also appreciate Comparative Pathology Core at University of Pennsylvania and particularly Dr. Charles-Antoine Assenmacher for helping with whole slide scanning using Aperio VERSA 200 platform.
Funding
This research was made available with the following support from National Institutes of Health grant U54NS115322 (DHS and WS), National Institutes of Aging grants U19AG062418 (Virginia M.-Y. Lee), P30AG072979 (EBL) and P01AG066597 (EBL), and Nancy and Buster Alvord Endowment (CDK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: EBL is the member of the Editorial Board for Acta Neuropathologica but was not involved in the editorial handling of this article. Other authors declare no competing interests.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. Tissue was obtained from the following Institutions: University of Washington, University of Glasgow, and University of Pennsylvania. Tissue was acquired at routine diagnostic autopsy, and approval for its use was granted by the respective institutional review boards.
Data and materials availability:
All data are available in the main text and the supplementary materials.
References:
- 1.Hay JR, Johnson VE, Young AM, Smith DH, Stewart W (2015) Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans. J Neuropathol Exp Neurol 74: 1147–1157 Doi 10.1097/NEN.0000000000000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M et al. (2020) Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med 382: 2268–2270 Doi 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaunmuktane Z, Mahadeva U, Green A, Sekhawat V, Barrett NA, Childs L, Shankar-Hari M, Thom M, Jager HR, Brandner S (2020) Microvascular injury and hypoxic damage: emerging neuropathological signatures in COVID-19. Acta Neuropathol 140: 397–400 Doi 10.1007/s00401-020-02190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krasemann S, Haferkamp U, Pfefferle S, Woo MS, Heinrich F, Schweizer M, Appelt-Menzel A, Cubukova A, Barenberg J, Leu J et al. (2022) The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Reports 17: 307–320 Doi 10.1016/j.stemcr.2021.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V et al. (2021) Microvascular Injury in the Brains of Patients with Covid-19. N Engl J Med 384: 481–483 Doi 10.1056/NEJMc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D et al. (2020) Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 77: 683–690 Doi 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAlpine LS, Fesharaki-Zadeh A, Spudich S (2021) Coronavirus disease 2019 and neurodegenerative disease: what will the future bring? Curr Opin Psychiatry 34: 177–185 Doi 10.1097/YCO.0000000000000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortega-Paz L, Capodanno D, Montalescot G, Angiolillo DJ (2021) Coronavirus Disease 2019-Associated Thrombosis and Coagulopathy: Review of the Pathophysiological Characteristics and Implications for Antithrombotic Management. J Am Heart Assoc 10: e019650 Doi 10.1161/JAHA.120.019650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA, Erickson MA (2021) The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci 24: 368–378 Doi 10.1038/s41593-020-00771-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DH, Dolle JP, Ameen-Ali KE, Bretzin A, Cortes E, Crary JF, Dams-O’Connor K, Diaz-Arrastia R, Edlow BL, Folkerth Ret al (2021) COllaborative Neuropathology NEtwork Characterizing ouTcomes of TBI (CONNECT-TBI). Acta Neuropathol Commun 9: 32 Doi 10.1186/s40478-021-01122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF Jr., Sabeti P (2020) Neuropathological Features of Covid-19. N Engl J Med 383: 989–992 Doi 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, Winkler CW, Sun J, Dickey JM, Ylaya K et al. (2022) SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 612: 758–763 Doi 10.1038/s41586-022-05542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14: 133–150 Doi 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakur KT, Miller EH, Glendinning MD, Al-Dalahmah O, Banu MA, Boehme AK, Boubour AL, Bruce SS, Chong AM, Claassen J et al. (2021) COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 144: 2696–2708 Doi 10.1093/brain/awab148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Zhou L, Bao L, Liu J, Zhu H, Lv Q, Liu R, Chen W, Tong W, Wei Q et al. (2021) SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target Ther 6: 337 Doi 10.1038/s41392-021-00719-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062 Doi 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text and the supplementary materials.


