Abstract
Positron emission tomography (PET) can be used as a non-invasive method to longitudinally monitor and quantify the expression of proteins in the brain in vivo. It can be used to monitor changes in biomarkers of mental health disorders, and to assess therapeutic interventions, such as stem cell and molecular genetic therapies. The utility of PET monitoring depends on the availability of a radiotracer with good central nervous system (CNS) penetration and high selectivity for the target protein. This review evaluates existing methods for the visualization of reporter proteins and/or protein function using PET imaging, focusing on engineered systems, and discusses possible approaches for future success in the development of high-sensitivity and high-specificity PET reporter systems for the brain.
Keywords: PET tracer, PET imaging, reporter probes, central nervous system, gene therapies, stem cell therapies
The value of PET reporter systems to basic and clinical neuroscience
PET is a noninvasive imaging technique used to visualize physiological processes that occur at the molecular level in living subjects [1]. Among many applications, PET reporter systems (see Glossary) have demonstrated utility in the monitoring of disease progression and response to treatment in the periphery [2]. PET reporter systems allow quantitative assessment of transduced genes and cells in vivo, with a sensitivity of approximately 10−12 M, making it possible to monitor the expression of therapeutic genes or survival and trafficking of cells for gene therapy [3]. The ability to visualize stem cell/transgene expression without the need for invasive biopsies makes PET imaging well suited to the rapidly expanding field of neurotherapeutic interventions. A brain PET reporter gene may not confer a therapeutic effect directly, but it can be expressed alongside a therapeutic gene, thus providing an indirect measure of therapeutic gene expression [4]. Although there is a growing need for imaging and monitoring of the expression of reporter genes in the brain, there is currently no single PET reporter system that is suitable for the visualization of therapeutic transgene expression in the brain. This review summarizes the qualities of the PET reporter systems most commonly used in research and therapeutic contexts, comments on their suitability for PET imaging of the brain and provides considerations for the development of the next generation of brain PET reporter systems.
PET reporter systems
The main PET reporter systems currently available are listed in Table-1 and are discussed in more detail in this review. The characteristic features of a good PET reporter system for the mammalian brain include the following: the reporter gene should be small enough to be packaged in a viral vector and should not significantly disturb cell biology; the reporter probe should have high brain accessibility through passive diffusion across the blood-brain barrier without efflux transporter liability, low background binding (to maximize signal-to-noise), and absence of radiometabolites in brain (to maximize the accuracy of quantitative measures) [5, 6]. Usually, radiotracers are bioactive molecules labeled with a short-lived positron emitting radionuclide, carbon-11 (t1/2 = 20.4 min) or fluorine-18 (t1/2 = 109.8 min). Carbon-11 can be inserted into a wide array of bioactive compounds by with several accessible C-11 labeled synthons. Fluorine-18 is also a widely used radionuclide because of its longer half-life providing an option to transport the F-18 labeled radiotracers to nearby non-cyclotron centered medical or research facilities.
Table-1:
List of PET reporter systems.
| Reporter system | Reporter gene(s) | Reporter probe(s) |
|---|---|---|
| Intracellular entrapment: | HSV-1 TK HSV-1-sr39TK hΔTK2 hDTK2 hdCK VZV-tk | [18F]FIAU, [18F]FMAU, [18F]FEAU, [18F]FHBG |
| Pyruvate Kinase M2 (PKM2) | [18F]DASA-23 | |
| AADC TH/GAD1 | 6-[18F]fluoro-L-m-tyrosine, [11C]raclopride | |
| Protein binding: | hSSTR2 | [68Ga]DOTATOC, [68Ga]DOTATATE |
| hD2R | [11C]raclopride, [18F]fallypride, [18F]spiperone derivative. | |
| Cannabinoid (CB2) receptor | [11C]GW405833 | |
| ecDHFR | [18F]FE-TMP | |
| Optogenetics - ChRERa | [18F]FES | |
| Chemogenetics - DREADDs | [11C]CLZ, [11C]DCZ, and [18F]JHU37107 | |
| Chemogenetics - PSAMs | [18F]ASEM | |
| Transporter-based: | hNIS | [18F]tetrafluoroborate, [18F]fluorosulfate. |
| hNET | [11C]ephedrine, [124I]meta-iodobenzylguanidine (MIBG), and [18F]meta-fluorobenzylguanidine (MFBG), [11C]MeNER, and [11C]MRB | |
| hSERT | [11C]DASB, [11C]McN 5652, [11C]ADAM, [11C]DAPA, and [11C]AFM. |
PET reporter systems: applications and design
Molecular genetic tools have revolutionized basic systems neuroscience research and have tremendous potential for applications in medical diagnosis and the treatment of human diseases. For example, several advances have been made in gene therapy in the past two decades; genetically engineered stem cells have been used to deliver proteins to neighboring cells, kill cancer cells, and reduce graft-host rejection [7]. PET reporter genes can be engineered into stem cells to allow in vivo tracking of cell delivery and migration in both the periphery and in the brain [8]. PET reporter probes can target the therapeutic gene of interest, a co-expressed reporter gene, or an endogenous protein that the therapeutic intervention is designed to modulate. In pre-clinical studies, it is common for a therapeutic gene and reporter gene to be cloned with a regulatory sequence, such as a promoter or enhancer, that targets expression to the cell type of interest [9]. A radiolabeled reporter probe can bind to the reporter protein with high affinity, and hence provide a quantitative measure of protein expression in cells of interest using PET imaging. The imaging of reporter gene expression has been used in several biological applications, including the monitoring of disease progression such as cancer and degenerative neurological conditions, monitoring of gene therapy, and tracking cell migration [10]. PET reporter probes interact with gene products to image their expression via one of three different mechanisms (Fig. 1B):
Intracellular entrapment of radiotracers after phosphorylation by enzymes encoded by reporter genes [11]. Enzyme-based reporter gene systems include herpes simplex virus 1 thymidine kinase (HSV1-tk) and its mutant forms such as human Δ-mitochondrial thymidine kinase type 2 (hΔtk2), as well as a mutant of human deoxycytidine kinase (hdCK).
Radiotracers bind protein receptors encoded by reporter genes [12]. Many receptor-based reporter genes systems are available, including human somatostatin receptor type 2 (hSSTR2), dopamine D2 receptor (D2R), and optogenetic-based and chemogenetic-based reporters.
Accumulation of radiotracers into cells mediated by membrane transporters encoded by reporter genes [13]. Transporter-based reporter genes include the human sodium–iodide symporter (hNIS), and human norepinephrine transporter (hNET).
Figure 1: PET imaging: an overview.
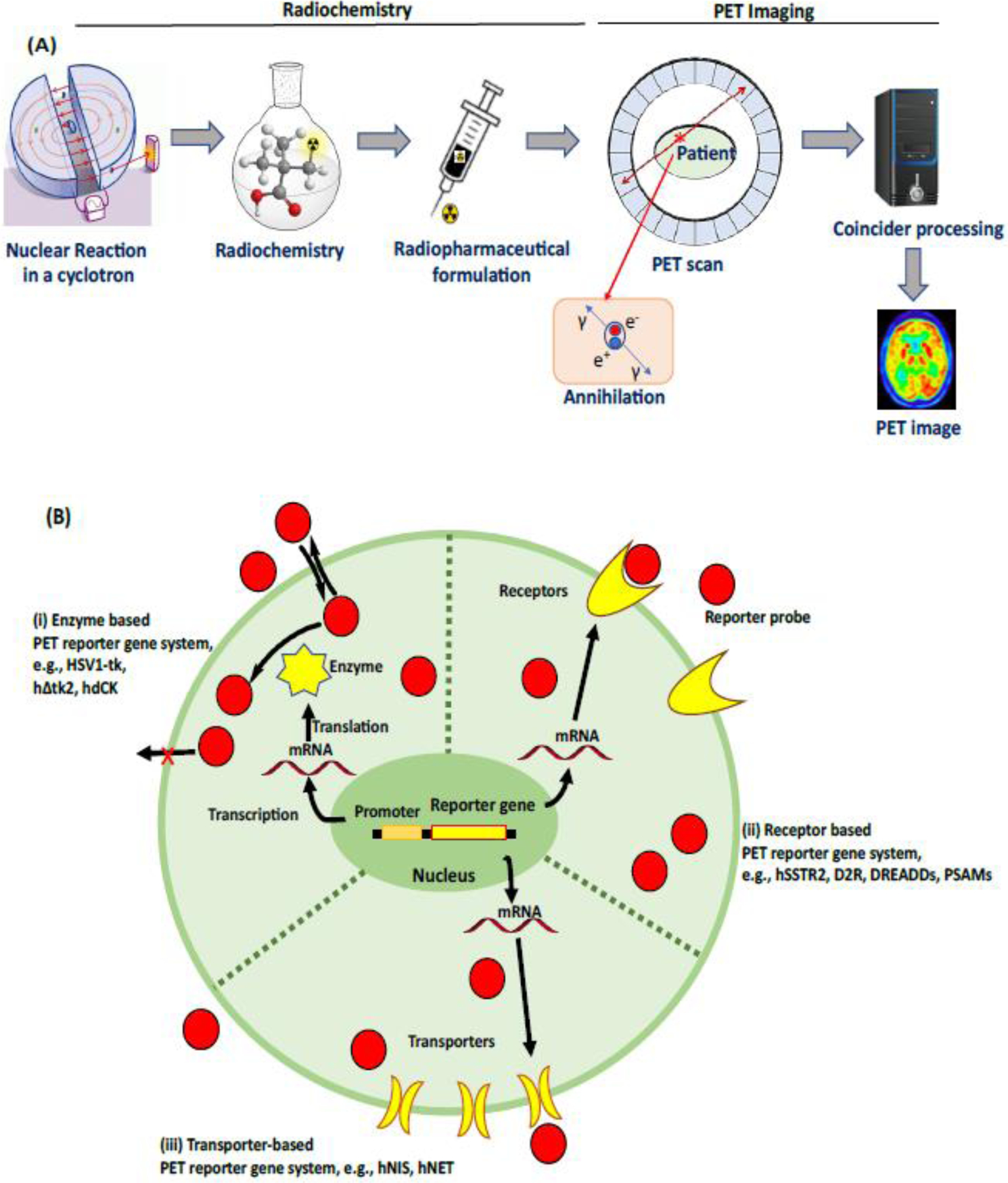
A) Schematic representation of brain imaging with PET. A cyclotron generates radionuclides which are used for the production of PET reporter probes via one or more chemical reactions (radiochemistry). A final radiopharmaceutical formulation is prepared with a suitable vehicle after the isolation of the PET reporter probe using HPLC, and then injected into living subjects. PET imaging involves the reconstruction of the location of reporter probe within the body, via data acquisition and post-processing (see Text box 1 for details). B) Overview of the expression of reporter gene systems; three different mechanisms by which PET radiotracers image reporter gene expression based on (i) enzymes: intracellular entrapment of radiotracers after phosphorylation by enzymes encoded by reporter genes, e.g. HSV1-tk phosphorylates pyrimidine-based radiotracers, such as [18F]FIAU, trapping the phospohorylated ligand inside the cell (ii) receptors: radiotracers bind protein receptors encoded by reporter genes, e.g. the human somatostatin receptor type 2 (hSSTR2), dopamine D2 receptor (D2R), and optogenetic-based receptors, encoded by a reporter gene (iii) transporters: accumulation of radiotracers into cells mediated by membrane transporters encoded by reporter genes, e.g. the human sodium–iodide symporter (hNIS), and human norepinephrine transporter (hNET) encoded by reporter gene.
A reporter probe is associated with each reporter gene. The probe will concentrate at the site of reporter gene expression and/or function. Thus, the measured radioactivity concentration of the reporter probe in the region of interest reflects the level of reporter gene expression/function. Expression level can be influenced by several factors including level of transcription, regulation of translation, and post-translational regulation of gene product. Thus, PET monitoring can provide valuable information regarding the level and stability of protein expression.
PET radiotracers do not generally alter biological processes because they are injected at tracer mass doses. Some PET radiotracers are substrates for efflux transporters at the Blood-Brain-Barrier (BBB), therefore systemic injections do not result in good brain uptake. A direct intrathecal infusion can overcome this issue; however, this approach produces additional risks and technical challenges [14]. PET is widely used in medical diagnosis because it can provide quantitative measurements of receptor density, target engagement, occupancy, volume of distribution, and binding potential using different models such as the ‘2-tissue compartmental model’ or ‘reference tissue model’ [15]. Several PET radioligands and their potential applications for molecular brain imaging have been discussed in recent literature [12, 16].
Intracellular entrapment PET reporter systems
In these systems, the reporter probe concentration reflects both gene expression level and function i.e., enzyme activity. There are several enzyme-based PET reporter systems, including the ones discussed next.
Herpes Simplex Virus – thymidine kinase (HSV1-tk)
The HSV-1-TK reporter system [11] has been used extensively in the periphery over the past 20 years to investigate protein-protein interactions, assess the temporal dynamics of different promoters, monitor embryonic stem (ES) cells after transplantation, assess anticancer therapy paradigms, and to assess gene and cell therapies. HSV1-tk causes phosphorylation of specific pyrimidine-based radiotracers, a mechanism like that of [18F]FDG trapping inside the cell after phosphorylation [17]. The resulting phosphorylated compounds do not cross the cell membrane readily, and hence accumulate within enzyme-expressing cells. The accumulated radioactivity within the cell reflects the level of HSV1-tk enzyme activity [18]. The radiotracers initially developed to undergo phosphorylation and entrapment by this enzyme reporter gene system include [14C]FIAU, [124I]FIAU, [131I]FIAU [8-3H]/[8-14C]ganciclovir, and [8-18F]fluoroganciclovir [19]. A mutant HSV1-tk reporter gene, named HSV1-sr39tk, was developed for applications in animal models and human gene therapy [20]. The mutant enzyme, combined with new radiolabeled bicyclic nucleoside analogues (BCNAs) such as 8-[18F]fluoropenciclovir (FPCV) and 9-(4-[18F]fluoro-3-(hydroxymethyl)butyl]guanine) ([18F]FHBG), significantly improved sensitivity for imaging of reporter gene expression in vivo [21]. However, the low lipophilicity of the PET radiotracers for this system results in poor permeability across the blood brain barrier.
Other enzyme-based PET reporter systems
Several other enzyme-based reporter genes have been developed, including human mitochondrial thymidine kinase-2 (hΔtk2) [22] and its associated mutants such as hTK2-N93D/ L109F [23], human deoxycytidine kinase (hdCK) [24], and a human D-mitochondrial thymidine kinase type 2 (hDTK2) [25], using specific pyrimidine-based radiotracers [26]. The varicella zoster virus-thymidine kinase (VZV-tk) gene and its nucleoside reporter probes are an alternative neuro-PET reporter system with radiolabeled bicyclic nucleoside analogues as reporter probes. VZV-tk expression in xenografts produced up to 60% higher uptake than HSV1-tk in vivo [27]. However, these alternative enzyme-based PET reporter systems also use BCNA probes, thus the issue of poor brain accessibility is likely to limit their utility for PET imaging in the CNS.
Protein binding-based PET reporter genes
Somatostatin receptor (SSTR)
The somatostatin receptors (SSTR) belong to the G-protein coupled receptor family and cause inhibitory effects on cell proliferation as well as release of active hormones. Initially, several reporter probes were developed for SPECT imaging of hSSTR2 such as [111In]diethylenetriamine pentaacetic acid–octreotide, [99mTc]P829, [99mTc]P2045, and [188Re]P829, but these ligands failed to provide adequate selectivity. Subsequently, the first PET probes, [68Ga]DOTATATE and [68Ga]DOTATOC, have superseded the SPECT reporter systems on account of their improved specific uptake, low background activity, and rapid clearance. AAV-mediated gene transduction into muscle and liver cells has been visualized using the aforementioned PET probes [28]. In addition, these radiotracers are used clinically for imaging neuroendocrine tumors [29, 30]. However, high expression of endogenous SSTR2 in the neocortex, amygdala, claustrum, and several other regions [31] is expected to limit the sensitivity and specificity of this system in the brain.
Dopamine D2 receptor (D2R)
In animal models, the expression of wild type and mutated dopamine type 2 receptors (D2Rs) has been quantified with PET using the specific D2R binding reporter probes [11C]raclopride, [18F]fallypride, and 3-(20-[18F]fluoroethyl)-spiperone ([18F]FESP) [32, 33]. A major caveat in this system is the potential for activation of transgenic D2Rs by endogenous dopamine ligands, perturbing transduced cells by changing cyclic AMP levels. To avoid such problems, the D2R mutant gene, D2R80A, was developed, with reduced affinity for the endogenous ligand, dopamine (DA). The D2R194A gene mutation further substantially reduced the effect of DA-mediated changes in cAMP levels and enhanced the affinity of [18F]FESP ligand binding [34]. The D2R80A receptor-based gene system employs radiotracers that can cross the BBB. However, high expression of endogenous D2R in the striatum, substantia nigra, frontal neocortex, and several other regions [35] is likely to limit the sensitivity and specificity of this system in the brain.
Cannabinoid type 2 receptor
The human type 2 cannabinoid receptor (hCB2) has been used as a reporter gene because of its low baseline expression in the brain. The reporter probe is a 11C-labeled CB2 ligand, [11C]GW405833. Expression of hCB2(D80N), a non-signaling form of CB2, permits visualization with [11C]GW405833 PET imaging, without producing a physiological effect [36]. One concern regarding this system is that brain uptake of [11C]GW405833 is relatively low. In rats, PET reporter imaging of hCB2 demonstrated longitudinal reporter gene expression up to 96 days after transduction [36]. A major concern with this system is that CB2 receptor expression is upregulated in activated microglia, as occurs in several CNS disorders associated with neuroinflammation [37], thus the utility of this system in monitoring the effect of treatments associated with such disorders is limited.
Optogenetic-chimeric receptors
Optogenetic systems allow targeted control of neuronal activity in animal models via administration of light. With recent advances in cell-type and pathway selectivity, these tools have widespread utility in preclinical animal models with potential to translate into human therapeutic applications [38, 39]. Several optical tools have been developed to activate or inhibit neuronal circuits, such as channelrhodopsin-2, halorhodopsin, and optoXRs [40, 41]. However, major challenges for use in large animals include monitoring transgenic receptor location, expression level, and function in vivo, noninvasively and longitudinally [12]. A PET reporter system for optogenetics allows the longitudinal visualization of opsin expression in mice and squirrel monkeys [42]. This reporter system, named ‘ChRERa’, consists of chimeric opsins tagged with a small human estrogen alpha protein epitope. The complementary reporter probe, [18F]fluoro-estradiol ([18F]FES), permits longitudinal noninvasive detection of opsins in the brain. The ChRERa reporter gene system is currently undergoing evaluation in old-world NHP.
Chemogenetic receptors
Chemogenetics offers a minimally invasive approach that can be used to control neuronal activity in the mammalian brain via systemic injections of activator ligand [43]. Chemogenetic technology has been used in animals to modulate specific neuronal populations by targeting them to express an exogenous receptor that has been engineered to be activated only by a selective agonist. Because it requires no implanted device, and can manipulate large regions of the brain for long periods of time (hours~), chemogenetics holds promise for therapeutic treatment of human brain diseases [44–46], although extensive tests of safety and efficacy will be needed prior to routine clinical use. Two classes of chemogenetic receptors have been widely adopted in the research community: Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) and Pharmacologically Selective Actuator Modules activated by Pharmacologically Selective Effector Molecules (PSAM/PSEM) [47]. The localization of muscarinic DREADDs using PET in rodent and nonhuman primate (NHP) brain was first established with [11C]clozapine-N-oxide ([11C]CNO) and [11C]clozapine ([11C]CLZ)[50, 51]. PET reporter imaging of DREADDs demonstrated longitudinal monitoring of neuronal grafts differentiated from induced pluripotent stem cells in mice [49] and virally expressed DREADDs in macaque monkeys [48]. Recently, a ligand with higher selectivity for DREADDs, [11C]deschloroclozapine ([11C]DCZ), was developed for NHP imaging [50, 51] (Fig. 2A–B). Additional PET radiotracers for DREADD visualization labeled with fluorine-18, [18F]JHU37152 and [18F]JHU37107, also exhibit high-affinity DREADD binding in vivo, and have a longer half-life, permitting use in research facilities that do not have a cyclotron on-site [52]. A follow up study demonstrated receptor quantification with [11C]DCZ by establishing the radiometabolite-corrected input function, measured from concurrent blood sampling, which enabled an estimation of total volume of distribution (VT) using a 2-tissue compartmental model [51]. The same study confirmed that reference tissue models provide a useful (albeit slightly underestimated) measure of DREADD expression.
Figure 2. Brain PET reporter systems.
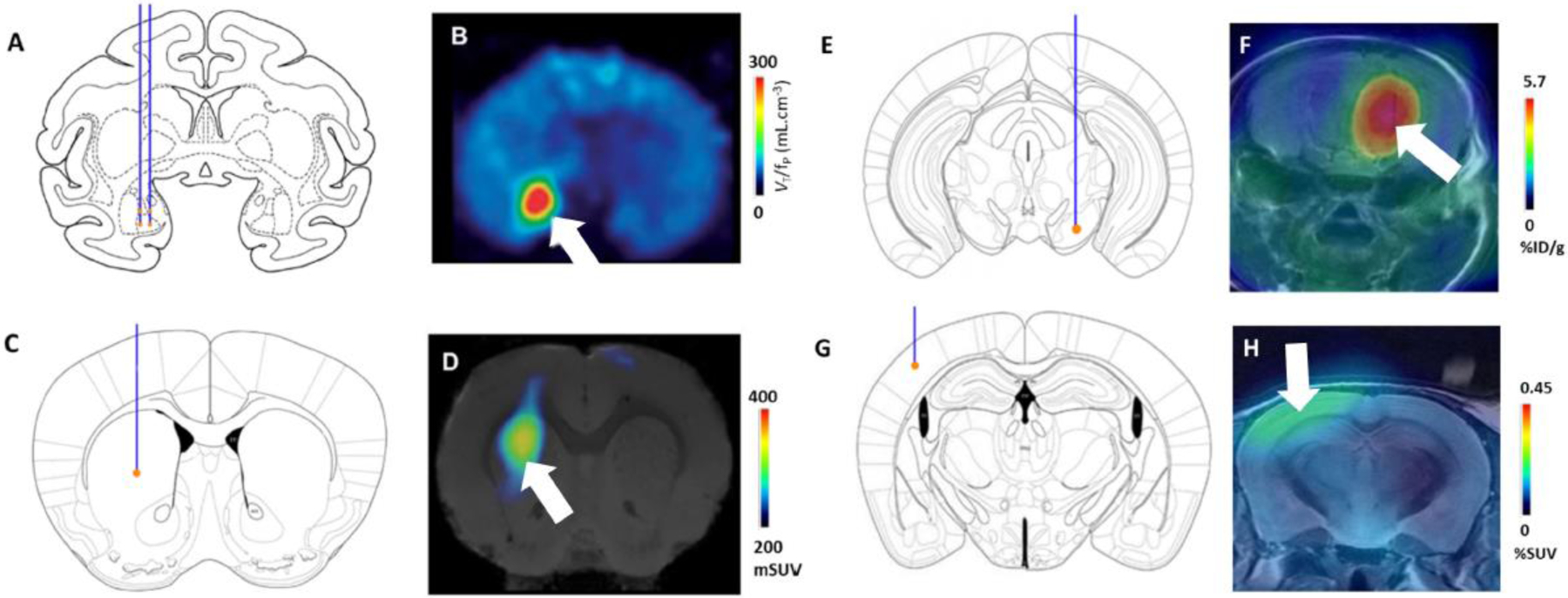
(A, B) Chemogenetic - DREADD reporter system. Lenti-HA-hM4Di injections targeted to right amygdala in monkey. A: Injection location depicted on a coronal section. B: Baseline [11C]DCZ binding (SUV) reveals contrast between the injection site (marked with an arrow) and the surrounding brain. (C, D) Chemogenetic - PSAM reporter system. AAV injection site in mouse left dorsal striatum (AAV-PSAM4-GlyR-EGFP). C: approximate injection location. D: PET image of mouse brain after [18F]ASEM injection, showing striatal localization of PSAM4-GlyR. (E,F) PKM2 reporter system. AAV9-EF1A-PKM2 targeted to the ventral tegmental area / substantia nigra of mouse brain. E: injection location. F: [18F]DASA-23 binding; arrow indicates region of radiotracer uptake. (G, H) ecDHFR reporter system. Recombinant ecDHFR-EGFP reporter protein was expressed in mouse somatosensory cortex after transduction with AAV-DJ. G: injection location. H: [18F]FE-TMP binding; arrow indicates area of accumulation of the radiotracer. Figures B, D, F and H were adapted from [51], [53], [56] and [57] respectively.
PSAM expression has been visualized in vivo in rodents and monkeys via the binding of [18F]ASEM, a radiolabeled α7 nAChR antagonist, as this ligand also binds with relatively high affinity to the PSAM4 - GlyR (Fig. 2C–D) [53, 54]. Competitive binding experiments with PET using [18F]ASEM confirmed the brain penetrance and PSAM4 receptor occupancy, in vivo, of a new generation of activating ligands – the ultrapotent PSEMs (uPSEMs) [53].
The development of a PET radioligand for the DREADD chemogenetic receptors has facilitated the adoption of this technology in NHPs, a critical step towards clinical translation. Promising alternative chemogenetic systems, such as the PSAM/PSEM system have been designed with clinical applications in mind [55] and would likely benefit from improved probes (see Outstanding Questions).
Outstanding questions.
PET reporter systems for brain applications have the potential to rapidly advance several technologies for basic systems neuroscience and therapeutic applications. What is the best approach to engineering systems with high selectivity and specificity?
How can we maximize the translational potential of PET reporter systems developed in lower order mammals? Engineering proteins with known cross-species homology, and/or reporter probes with existing approval for use in humans may be a good starting point.
The bioavailability of PET reporter probes is critical to the utility of the system. How can we optimize methods to maximize blood brain barrier penetrance? Of particular value would be a theoretical grounding for better understanding probe susceptibility to efflux transporters.
Can a ‘universal’ brain PET reporter system be developed? Several attempts have been made to create such a system (e.g. PKM2, ecDHFR, and the ligand binding domain of estrogen receptor α in ChRERa), but all require further optimization before they can be universally adopted.
Pyruvate kinase muscle isoenzyme 2 (hPKM2) receptors
The human pyruvate kinase muscle isoenzyme 2 (hPKM2) has been used as a PET reporter gene with [18F]DASA-23 as a reporter probe for imaging transduced tissue in the CNS because of favorable reporter probe pharmacokinetics, including BBB permeability. The reporter gene was delivered into the brain of mice using adeno-associated virus 9 (AAV9). PET imaging studies, performed over 8 weeks to evaluate the time course of PKM2 expression, revealed an increase in the brain uptake of [18F]DASA-23 in the AAV transfected mice (Fig. 2E–F) compared to healthy controls [56]. This approach can be modified such that the functional gene is coupled with a PKM2 reporter gene to allow indirect evaluation of functional gene expression using [18F]DASA-23. The PKM2 system has been tested in mice but does not yet have proven efficacy in larger mammals. PKM2 plays a role in cell proliferation, which may limit the suitability of this system for clinical applications.
Bacterial dihydrofolate reductase (ecDHFR) receptors
Unlike the other enzyme-based reporter systems, the ecDHFR-based reporter system relies on the direct visualization of protein expression, rather than protein products, and can be fused with a protein of interest to permit detection via the reporter probe, [18F]FE-TMP (Fig. 2G–H). The ecDHFR/TMP reporter system enables non-invasive visualization of neuronal ensemble activity thorough ecDHFR expression under an activity-dependent promotor in mice and neuronal tracts in the deep brain regions of NHPs [57]. However, this system is still in its first generation and has areas that need to be improved in terms of reporter probe characteristics.
Transporter based PET reporter genes
Human sodium-iodide symporter (hNIS)
The hNIS gene encodes a transmembrane glycoprotein, belonging to the family of sodium-mediated symporters. It is widely recognized as a biomarker and therapeutic target for thyroid cancer due to its high expression in the thyroid gland. This reporter system, utilizing iodine-131 or iodine-124, has been translated into clinical gene therapy for thyroid cancer and has been used as a reporter gene in preclinical studies for monitoring transduced immune cells and pluripotent stem cells in vivo, imaging the transcriptional activity of several specific promoter systems, and muscular dystrophy [58, 59]. The hNIS has also been considered as a therapeutic gene in targeted radiotherapy using [131I]iodide [60]. New 18F-labeled derivatives have been developed as radiotracers for imaging hNIS, which include [18F]tetrafluoroborate, and [18F]fluorosulfate. [18F]Tetrafluoroborate has been tested in clinical trials and in thyroid cancer patients [61]. The hNIS reporter system is not well suited to brain imaging because quantification of gene expression is not possible with radiolabeled iodide because it is not metabolically trapped within transduced cells.
Human norepinephrine transporter (hNET)
The hNET gene produces a transmembrane protein that facilitates transportation of norepinephrine, epinephrine, and dopamine from the synapse to presynaptic neurons. Several PET radiotracers have been developed for hNET imaging, including [11C]ephedrine, [124I]meta-iodobenzylguanidine (MIGB), and [18F]meta-fluorobenzylguanidine (MFBG) [62]. PET imaging of hNET has been translated into the clinic for the visualization of neuroendocrine tumors and heart disease [63]. However, the blood–brain barrier permeability appears to be poor in practice due to the presence of highly polar guanidine moieties in their structures [64]. More recently developed hNET radiotracers, with better CNS penetrance, have been used to demonstrate that noradrenergic projections undergo degeneration in PD - as quantified with [11C]MeNER PET [65], and in NHP to assess the occupancy of norepinephrine transporters – as quantified with [11C]MRB [66]. Both radiotracers have good brain penetrance. However, hNET is distributed throughout the brain, with especially high levels of expression in the thalamus, midbrain, and amygdala, thus limiting the sensitivity and specificity of this system [67].
Human serotonin transporter (hSERT)
The hSERT gene encodes a transmembrane protein that regulates extracellular fluid serotonin concentrations. Several PET radiotracers have been developed for hSERT imaging, including [11C]DASB, [11C]McN 5652, [11C]ADAM, [11C]DAPA, and [11C]AFM. Of these radiotracers, [11C]DASB penetrates the blood–brain barrier most quickly, and hence can be used to visualize hSERT using shorter scan times; [11C]AFM has the highest signal-to-noise ratio, and hence provides the most spatially accurate report of hSERT expression. In human brain, hSERT expression is somewhat denser in medial temporal lobe structures and ventral pallidum but is generally lower throughout the brain than hDAT and hNET [68]. [11C]DASB and [11C]AFM display high signal-to-noise ratios in vivo, in part due to the relatively low endogenous expression levels of hSERT [69]. Thus, a non-functional hSERT mutant, combined with [11C]DASB or [11C]AFM might have potential for use as a PET brain reporter system.
Imaging therapeutic transgenes and stem cells in the brain
The translation of preclinical gene therapy studies into the clinic can be facilitated by the monitoring of therapeutic transgene expression in the brain using PET. For example, gene therapy has been used in the treatment of Parkinson’s disease (PD) in clinical trials; the enzyme aromatic L-amino acid decarboxylase (AADC) improves the clinical symptoms of PD by synthesizing DA via the catalysis of systemically administered levodopa or carbidopa [70]. AADC transgene-based gene therapy for PD patients is currently in phase I/II clinical trials [70]. Many candidate gene therapies for PD patients are focused on increasing the expression of enzymes for the synthesis of DA such as AADC, tyrosine hydroxylase (TH), or GTP cyclohydroxylase 1 (GCH1) to increase DA bioavailability in the nigrostriatal synapses.
PET imaging of the DA transporter has been used to assess the survival and growth of ES cells genetically modified to develop into DA synthesizing neurons in a rat model of PD [71]. [18F]FE-PE2I is used in the clinic to quantify presynaptic dopamine transporter (DAT) expression [72]. In a mouse model of PD, PET measurements revealed that ES cell treatment caused an increase in the expression of striatal presynaptic dopamine transporters and of postsynaptic DA D2Rs [73] (Fig. 3A–D). PET imaging with [18F]FECNT has shown partial recovery of DA transporter in the lesioned striatum after transplantation of ES cells. [18F]FECNT shows good brain kinetics and has high selectivity and affinity to the DAT. It should be noted, however, that radiometabolites that enter the brain confound measurements based on reference tissue models [74]. In another PD model, PET imaging with [18F]FBCTT-PET/CT revealed the restoration of the expression of pre-synaptic DAT, and [18F]fallypride-PET/CT demonstrated functional DA release in the lesioned striatum after transplantation of ES cells [75]. In sum, longitudinal and noninvasive PET imaging of ES cells can be used to assess the regulation, survival, and function of DA neurons, providing valuable insights for the study of therapeutic applications of human ES cells.
Figure 3: Recovery of dopamine transporter binding six months after DA stem cell transplantation in the rat striatum in a 6-hydroxy-dopamine model of PD.
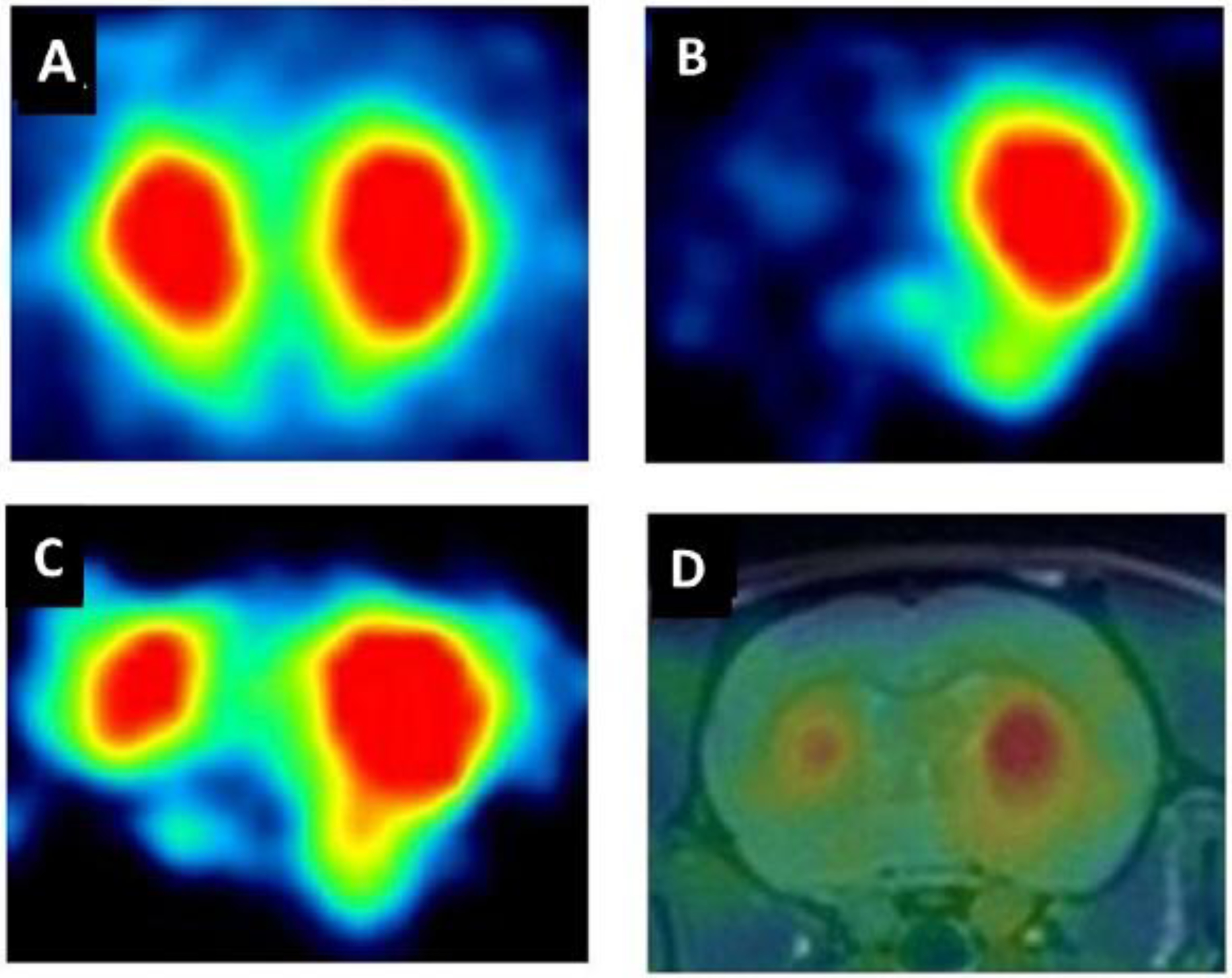
(A) A naive rat showed bilaterally symmetrical uptake of [18F]FECNT, a radioligand for the dopamine transporter. (B) Unilateral injection of a dopaminergic toxin created a hemiparkinsonian rat, which displayed negligible [18F]FECNT uptake in the lesioned striatum. (C) ES cell transplantation partially restored uptake of [18F]FECNT in the lesioned striatum. (D) Coregistered PET and MR images after ES-cell transplantation. In this example, PET imaging of the dopamine transporter was used as a PET reporter system to monitor the survival and growth of transplanted cells. Figure adapted from [71].
PET has been used to monitor the metabolic changes that occur after gene delivery in clinical trials of gene therapy for PD [76]. One study demonstrated 30% higher uptake of L-[18F]3-fluoro-α-methyl tyrosine ([18F]FMT) in the putamen region after AAV-AADC gene transfer in patients with moderate to advanced PD [77]. Another study demonstrated increases in enzyme activity assessed by PET in gene therapy using [18F]FDOPA [78]. An open label, phase 1 clinical trial of PD gene therapy using AAV-GAD1 (glutamate decarboxylase 1), suggested significant improvements in PD symptoms [79]. The presence of endogenous DAT limits the utility of the corresponding reporter probes for use in a universal brain PET reporter system.
Concluding remarks and future perspectives
PET imaging has been long used for the monitoring of stem cells and transgenes in the periphery, in both basic and clinical settings. Progress in the deployment of stem cell and transgene expression in pre-clinical models has been made in parallel with the development of improved methods for PET imaging in the brain. For example, longitudinal PET imaging in rats made it possible to monitor the loss of DA receptors in a model of Parkinson’s disease, and the recovery of dopaminergic function after treatment with stem cells [71]. PET imaging of a chemogenetic receptor in a nonhuman primate made it possible to determine that the absence of a behavioral effect resulted from a failed injection; a follow-up injection improved the expression and rescued the behavioral effect [44]. These examples make clear why brain PET imaging could be so valuable in clinical settings. Many new stem cell and transgene-based therapies are undergoing clinical trials for the treatment of neurological and neuropsychiatric disorders [80]. Brain PET reporter probes for monitoring reporter expression and/or inflammation in a longitudinal fashion would make it easier to evaluate both the efficacy of treatment and track potential complications associated with these novel treatments. The absence of suitable brain PET reporter probes for certain treatments hinders such monitoring. The development of a ‘universal’ brain PET reporter system – one that could be combined with any neurotherapeutic treatment, and would confer high sensitivity and selectivity – would facilitate the development and application of such treatments in both basic and clinical contexts. A universal PET reporter system could also expedite the translation of promising techniques in preclinical use, including optogenetics and chemogenetics.
When designing an ‘ideal’ neuro-PET system, one can seek to optimize the physiochemical properties of candidate radiotracers to maximize brain penetrance and select target proteins that are physiologically inert and suitably sized for packaging into viral vectors. One can also seek to design protein-radiotracer pairs with high on-target affinity and low background binding. However, the metabolic stability of new radiotracers is difficult to predict (see Outstanding Questions). Overcoming the challenges described above to produce universal brain PET reporter systems may expand the utility of PET monitoring to stem cell treatments and transgenic methods of neuromodulation, which will be of tremendous value to both researchers and clinicians.
Text Box 1: What is PET imaging?
Positron emission tomography (PET) is a method that takes advantage of the selective uptake of a radioactive compound (radiotracer) to infer the distribution of a molecular target within a living subject. The radiotracer emits two 511 MeV γ-rays in opposite directions when the emitted positron from radioactive decay annihilates with a nearby electron (Fig. 1A). These γ-rays are detected by a ring of detectors in the PET camera surrounding the subject, and the timing and locations of detection can be used to reconstruct the time and origin of radionuclide decay. This provides an approximate spatial measure of where the radiotracer was distributed when it decayed. If many decay events are clustered within a given region, it can be inferred that this region is rich in target receptor expression. PET cameras typically provide relatively low spatial resolution (maximum of ~2 mm for monkeys and smaller animals using microPET, and ~4 mm for humans). Therefore, multimodal imaging, such as PET-CT and PET-MRI, are valuable because they combine the high spatial resolution of CT/MRI (as low as 1 mm) with the sensitivity of PET (10−12 M), which is much higher than that or MRI (10−4 M). Due to its high sensitivity, PET can measure very low concentration (10−5-10−10 g) of radiotracers at a specific region of interest. PET, therefore, remains an invaluable molecular imaging modality in biomedical and clinical practice [81, 82].
Highlights.
Positron emission tomography (PET) is a non-invasive modality for in vivo assessment of pathophysiological processes in living subjects.
PET imaging detects γ-rays produced by decay of a radioactive compound (radiotracer) to infer the location of the binding of that compound within the brain, thus revealing the location of the receptor to which it bound.
PET reporter systems are used to monitor the progression of disease and the expression of therapeutic transgenes, for instance in the context of stem cell and molecular genetic therapies.
Successful PET reporter systems for the brain are improving the treatment of neurological diseases, and hold promise for the advancement of novel treatments for neuropsychiatric disorders.
Acknowledgments
This work was supported by the National Institutes of Health (NIH: NIMH & NIDA). This study was funded by the Intramural Research Programs of the National Institute of Mental Health (projects ZIAMH002795, ZIAMH002793) and the National Institute of Drug Abuse (ZIADA000069).
Declaration of interests
MM has received research funding from AstraZeneca, Redpin Therapeutics, Dompé farmaceutici, and Attune Neurosciences, and is named as an inventor on a patent describing novel DREADD ligands (WO2019/157083). TM is named as an inventor on a patent for the use of DCZ as a PET reporter probe (WO2019/245047). Other coauthors declare no conflict of interest.
Glossary
- Biomarker
A substance the presence of which is tightly correlated with the progression of an underlying phenomenon, e.g. a neurological or neuropsychiatric illness.
- Binding potential
The ratio of receptor density (Bmax) to radioligand equilibrium dissociation constant (KD).
- Bmax
Total density (concentration) of receptors in a sample of tissue in-vitro or concentration of available (free) receptors in-vivo.
- Cyclotron
An apparatus in which charged atomic and subatomic particles are accelerated by an alternating electric field while following an outward spiral or circular path in a magnetic field to generate isotopes.
- Enzyme-based PET reporter system
Intracellular entrapment of radiotracers after phosphorylation by enzymes encoded by reporter genes.
- KD
Radiotracer equilibrium dissociation constant.
- PET reporter gene
Gene whose product can be directly or indirectly imaged with a PET reporter probe.
- PET reporter probe
Radiotracer with selective binding to a PET reporter gene product.
- PET reporter system
Combination of both PET reporter gene and PET reporter probe.
- Positron emission tomography (PET)
Molecular imaging modality based on the use of chemical or biologic agents labeled with positron-emitting radionuclides.
- Radiotracer/Radioligand
Short-lived positron-emitting compound used for PET imaging.
- Receptor based PET reporter system
Radiotracers that bind protein receptors encoded by reporter genes.
- Reference tissue model
Analysis model that allows quantification of receptor kinetics without measuring the arterial input function, thus avoiding arterial cannulation and time-consuming metabolite measurements.
- Standardized uptake value (SUV)
The decay-corrected tissue concentration of a PET tracer in a specific region of interest on the PET image divided by the injected activity and then divided again by body weight—when all the tracer is distributed evenly throughout the body, the SUV would be 1 for every region.
- Standardized uptake value ratio (SUVR)
A quantitative method used in PET imaging to make regional comparisons within a subject as well as between subjects and computed as the degree of radiotracer uptake in a target region of interest with respect to a reference region.
- Synthon
A moiety of a chemical compound that can be formed by a known synthetic process.
- Transgene
A gene that is artificially introduced into the genome of an organism.
- Transporter based PET reporter system
Accumulation of radiotracers into cells mediated by membrane transporters encoded by reporter genes.
- Volume of distribution (VT)
Defined as the ratio of the radioligand concentration in tissue target region (CT, kBq·cm−3) to that in plasma (CP, kBq·mL−1) at equilibrium.
- 2-tissue compartmental model
Follows two compartments that are for the two distinct kinetic compartments in the tissue (C1(t) and C2(t)), representing, for instance, the free and receptor-bound tracer concentrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Vaquero JJ and Kinahan P (2015) Positron Emission Tomography: Current Challenges and Opportunities for Technological Advances in Clinical and Preclinical Imaging Systems. Annu. Rev. Biomed. Eng 17, 385–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber WA (2006) Positron Emission Tomography as an Imaging Biomarker. JCO 24, 3282–3292 [DOI] [PubMed] [Google Scholar]
- 3.Jacobs AH et al. (2021) Imaging of Gene and Cell-Based Therapies: Basis and Clinical Trials. In Molecular Imaging, pp. 1539–1587 [Google Scholar]
- 4.Yaghoubi SS et al. (2012) Positron Emission Tomography Reporter Genes and Reporter Probes: Gene and Cell Therapy Applications. Theranostics 2, 374–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambhir SS et al. (2000) Imaging Transgene Expression with Radionuclide Imaging Technologies. Neoplasia 2, 118–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins Sara A. et al. (2012) PET Imaging for Gene & Cell Therapy. CGT 12, 20–32 [DOI] [PubMed] [Google Scholar]
- 7.Arabi F et al. (2022) Gene therapy clinical trials, where do we go? An overview. Biomedicine & Pharmacotherapy 153, 113324. [DOI] [PubMed] [Google Scholar]
- 8.Serganova I and Blasberg RG (2019) Molecular Imaging with Reporter Genes: Has Its Promise Been Delivered? J Nucl Med 60, 1665–1681 [DOI] [PubMed] [Google Scholar]
- 9.Shahryari A et al. (2021) Engineering Gene Therapy: Advances and Barriers. Adv. Therap 4, 2100040 [Google Scholar]
- 10.Gao T et al. (2022) Reporter Genes for Brain Imaging Using MRI, SPECT and PET. Int J Mol Sci 23, 8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaghoubi SS and Gambhir SS (2006) PET imaging of herpes simplex virus type 1 thymidine kinase (HSV1-tk) or mutant HSV1-sr39tk reporter gene expression in mice and humans using [18F]FHBG. Nat Protoc 1, 3069–3074 [DOI] [PubMed] [Google Scholar]
- 12.Boehm MA et al. (2021) Translational PET applications for brain circuit mapping with transgenic neuromodulation tools. Pharmacology Biochemistry and Behavior 204, 173147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan J et al. (2007) Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: problems and progress. Nuclear Medicine and Biology 34, 667–679 [DOI] [PubMed] [Google Scholar]
- 14.Shah N and Padalia D (2023) Intrathecal Delivery System. In StatPearls, StatPearls Publishing LLC; [PubMed] [Google Scholar]
- 15.Ametamey SM et al. (2008) Molecular Imaging with PET. Chem. Rev 108, 1501–1516 [DOI] [PubMed] [Google Scholar]
- 16.Slifstein M and Abi-Dargham A (2017) Recent Developments in Molecular Brain Imaging of Neuropsychiatric Disorders. Seminars in Nuclear Medicine 47, 54–63 [DOI] [PubMed] [Google Scholar]
- 17.Blasberg RG and Tjuvajev JG (2003) Molecular-genetic imaging: current and future perspectives. J. Clin. Invest 111, 1620–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F et al. (2014) A cyclic HSV1-TK reporter for real-time PET imaging of apoptosis. Proc. Natl. Acad. Sci. U.S.A 111, 5165–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjuvajev JG et al. (2002) Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J Nucl Med 43, 1072–1083 [PubMed] [Google Scholar]
- 20.Min J and Gambhir S (2004) Gene Therapy Progress and Prospects: Noninvasive imaging of gene therapy in living subjects. Gene Ther 11, 115–125 [DOI] [PubMed] [Google Scholar]
- 21.Gambhir SS et al. (2000) A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc. Natl. Acad. Sci. U.S.A 97, 2785–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponomarev V et al. (2007) A Human-Derived Reporter Gene for Noninvasive Imaging in Humans: Mitochondrial Thymidine Kinase Type 2. Journal of Nuclear Medicine 48, 819–826 [DOI] [PubMed] [Google Scholar]
- 23.Campbell DO et al. (2012) Structure-guided Engineering of Human Thymidine Kinase 2 as a Positron Emission Tomography Reporter Gene for Enhanced Phosphorylation of Non-natural Thymidine Analog Reporter Probe. Journal of Biological Chemistry 287, 446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu CJ et al. (2010) Novel PET Probes Specific for Deoxycytidine Kinase. J Nucl Med 51, 1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponomarev V et al. (2007) A Human-Derived Reporter Gene for Noninvasive Imaging in Humans: Mitochondrial Thymidine Kinase Type 2. Journal of Nuclear Medicine 48, 819–826 [DOI] [PubMed] [Google Scholar]
- 26.M. Alauddin M and G. Gelovani J (2010) Radiolabeled Nucleoside Analogues for PET Imaging of HSV1-tk Gene Expression. CTMC 10, 1617–1632 [DOI] [PubMed] [Google Scholar]
- 27.Deroose CM et al. (2012) Preliminary validation of varicella zoster virus thymidine kinase as a novel reporter gene for PET. Nuclear Medicine and Biology 39, 1266–1274 [DOI] [PubMed] [Google Scholar]
- 28.Cotugno G et al. (2011) Noninvasive Repetitive Imaging of Somatostatin Receptor 2 Gene Transfer with Positron Emission Tomography. Human Gene Therapy 22, 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawicki LM et al. (2017) Evaluation of 68Ga-DOTATOC PET/MRI for whole-body staging of neuroendocrine tumours in comparison with 68Ga-DOTATOC PET/CT. Eur Radiol 27, 4091–4099 [DOI] [PubMed] [Google Scholar]
- 30.Delpassand ES et al. (2020) 64 Cu-DOTATATE PET/CT for Imaging Patients with Known or Suspected Somatostatin Receptor–Positive Neuroendocrine Tumors: Results of the First U.S. Prospective, Reader-Masked Clinical Trial. J Nucl Med 61, 890–896 [DOI] [PubMed] [Google Scholar]
- 31.Breder C et al. (1992) Differential expression of somatostatin receptor subtypes in brain. J. Neurosci 12, 3920–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLaren DC et al. (1999) Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Ther 6, 785–791 [DOI] [PubMed] [Google Scholar]
- 33.MacLaren DC et al. (1999) Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Ther 6, 785–791 [DOI] [PubMed] [Google Scholar]
- 34.Liang Q et al. (2001) Noninvasive, quantitative imaging in living animals of a mutant dopamine D2 receptor reporter gene in which ligand binding is uncoupled from signal transduction. Gene Ther 8, 1490–1498 [DOI] [PubMed] [Google Scholar]
- 35.Camps M et al. (1989) Dopamine receptors in human brain: Autoradiographic distribution of D2 sites. Neuroscience 28, 275–290 [DOI] [PubMed] [Google Scholar]
- 36.Vandeputte C et al. (2011) A PET Brain Reporter Gene System Based on Type 2 Cannabinoid Receptors. J Nucl Med 52, 1102–1109 [DOI] [PubMed] [Google Scholar]
- 37.Maresz K et al. (2005) Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 95, 437–445 [DOI] [PubMed] [Google Scholar]
- 38.White M et al. (2020) Taking Optogenetics into the Human Brain: Opportunities and Challenges in Clinical Trial Design. Open Access J Clin Trials 2020, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen Y et al. (2020) Challenges for Therapeutic Applications of Opsin-Based Optogenetic Tools in Humans. Front. Neural Circuits 14, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenno L et al. (2011) The Development and Application of Optogenetics. Annu. Rev. Neurosci 34, 389–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y et al. (2022) Noninvasive Manipulation of Ion Channels for Neuromodulation and Theranostics. Acc. Mater. Res 3, 247–258 [Google Scholar]
- 42.Bonaventura J et al. (2023) Expression of the excitatory opsin ChRERα can be traced longitudinally in rat and nonhuman primate brains with PET imaging. Sci. Transl. Med 15, 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roth BL (2016) DREADDs for Neuroscientists. Neuron 89, 683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyakawa N et al. (2023) Chemogenetic attenuation of cortical seizures in nonhuman primates. Nat Commun 14, 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roseboom PH et al. (2021) Evidence in primates supporting the use of chemogenetics for the treatment of human refractory neuropsychiatric disorders. Molecular Therapy 29, 3484–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song J et al. (2022) Chemogenetics as a neuromodulatory approach to treating neuropsychiatric diseases and disorders. Mol Ther 30, 990–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miura Y et al. (2022) Chemogenetics of cell surface receptors: beyond genetic and pharmacological approaches. RSC Chem. Biol 3, 269–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagai Y et al. (2016) PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat Commun 7, 13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji B et al. (2016) Multimodal Imaging for DREADD-Expressing Neurons in Living Brain and Their Application to Implantation of iPSC-Derived Neural Progenitors. J. Neurosci 36, 11544–11558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagai Y et al. (2020) Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci 23, 1157–1167 [DOI] [PubMed] [Google Scholar]
- 51.Yan X et al. (2021) [11 C]deschloroclozapine is an improved PET radioligand for quantifying a human muscarinic DREADD expressed in monkey brain. J Cereb Blood Flow Metab 41, 2571–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonaventura J et al. (2019) High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat Commun 10, 4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magnus CJ et al. (2019) Ultrapotent chemogenetics for research and potential clinical applications. Science 364, eaav5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hori Y et al. (2023) Multimodal imaging for validation and optimization of ion channel-based chemogenetics in nonhuman primates, Neuroscience, bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yizhar O and Wiegert JS (2019) Designer Drugs for Designer Receptors: Unlocking the Translational Potential of Chemogenetics. Trends Pharmacol Sci 40, 362–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haywood T et al. (2019) Positron emission tomography reporter gene strategy for use in the central nervous system. Proc. Natl. Acad. Sci. U.S.A 116, 11402–11407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimojo M et al. (2021) A genetically targeted reporter for PET imaging of deep neuronal circuits in mammalian brains. The EMBO Journal 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groot-Wassink T et al. (2004) Quantitative Imaging of Na/I Symporter Transgene Expression Using Positron Emission Tomography in the Living Animal. Molecular Therapy 9, 436–442 [DOI] [PubMed] [Google Scholar]
- 59.Mandell RB et al. (1999) Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res 59, 661–668 [PubMed] [Google Scholar]
- 60.Konieczny P et al. (2013) Gene and cell-mediated therapies for muscular dystrophy: Therapy for Muscular Dystrophy. Muscle Nerve 47, 649–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samnick S et al. (2018) Initial Clinical Investigation of [18F]Tetrafluoroborate PET/CT in Comparison to [124I]Iodine PET/CT for Imaging Thyroid Cancer. Clin Nucl Med 43, 162–167 [DOI] [PubMed] [Google Scholar]
- 62.Zhang H et al. (2014) Imaging the Norepinephrine Transporter in Neuroblastoma: A Comparison of [18F]-MFBG and 123I-MIBG. Clinical Cancer Research 20, 2182–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maxwell JE and Howe JR (2015) Imaging in neuroendocrine tumors: an update for the clinician. International Journal of Endocrine Oncology 2, 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X et al. (2020) Recent advances in radiotracers targeting norepinephrine transporter: structural development and radiolabeling improvements. J Neural Transm 127, 851–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nahimi A et al. (2018) Noradrenergic Deficits in Parkinson Disease Imaged with 11 C-MeNER. J Nucl Med 59, 659–664 [DOI] [PubMed] [Google Scholar]
- 66.Gallezot J-D et al. (2011) Evaluation of [(11)C]MRB for assessment of occupancy of norepinephrine transporters: Studies with atomoxetine in non-human primates. Neuroimage 56, 268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith HR et al. (2006) Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience 138, 703–714 [DOI] [PubMed] [Google Scholar]
- 68.Huang Y et al. (2002) Comparative Evaluation in Nonhuman Primates of Five PET Radiotracers for Imaging the Serotonin Transporters: [11 C]McN 5652, [11 C]ADAM, [11 C]DASB, [11 C]DAPA, and [11 C]AFM. J Cereb Blood Flow Metab 22, 1377–1398 [DOI] [PubMed] [Google Scholar]
- 69.Murphy DL et al. (2004) Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv 4, 109–123 [DOI] [PubMed] [Google Scholar]
- 70.Hwu PW et al. (2021) Gene therapy in the putamen for curing AADC deficiency and Parkinson’s disease. EMBO Mol Med 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goggi JL et al. (2020) Dopamine transporter neuroimaging accurately assesses the maturation of dopamine neurons in a preclinical model of Parkinson’s disease. Stem Cell Res Ther 11, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marner L et al. (2022) [18F]FE-PE2I PET is a feasible alternative to [123I]FP-CIT SPECT for dopamine transporter imaging in clinically uncertain parkinsonism. EJNMMI Res 12, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodríguez-Gómez JA et al. (2007) Persistent Dopamine Functions of Neurons Derived from Embryonic Stem Cells in a Rodent Model of Parkinson Disease. Stem Cells 25, 918–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zoghbi SS et al. (2006) PET imaging of the dopamine transporter with 18F-FECNT: a polar radiometabolite confounds brain radioligand measurements. J Nucl Med 47, 520–527 [PubMed] [Google Scholar]
- 75.Goggi JL et al. (2020) Dopamine transporter neuroimaging accurately assesses the maturation of dopamine neurons in a preclinical model of Parkinson’s disease. Stem Cell Res Ther 11, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shalaby KE and El-Agnaf OMA (2022) Gene-Based Therapeutics for Parkinson’s Disease. Biomedicines 10, 1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eberling JL et al. (2008) Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 70, 1980–1983 [DOI] [PubMed] [Google Scholar]
- 78.Christine CW et al. (2019) Magnetic resonance imaging–guided phase 1 trial of putaminal AADC gene therapy for Parkinson’s disease. Ann Neurol 85, 704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaplitt MG et al. (2007) Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. The Lancet 369, 2097–2105 [DOI] [PubMed] [Google Scholar]
- 80.Kang L et al. (2023) AAV vectors applied to the treatment of CNS disorders: Clinical status and challenges. Journal of Controlled Release 355, 458–473 [DOI] [PubMed] [Google Scholar]
- 81.Goud NS et al. (2021) Carbon-11: Radiochemistry and Target-Based PET Molecular Imaging Applications in Oncology, Cardiology, and Neurology. J. Med. Chem 64, 1223–1259 [DOI] [PubMed] [Google Scholar]
- 82.Nerella SG et al. (2022) PET Molecular Imaging in Drug Development: The Imaging and Chemistry Perspective. Front. Med 9, 812270. [DOI] [PMC free article] [PubMed] [Google Scholar]


