Abstract
Background:
Although women are known to have a relatively higher left ventricular ejection fraction compared to men, a sex-neutral LVEF threshold continues to be used for clinical management. We sought to investigate the relationship among high (>65%), normal (55–65%) and low (<55%) LVEF and long-term all-cause mortality and major adverse cardiovascular events (MACE) in women presenting with suspected myocardial ischemia.
Methods:
A total of 734 women from the Women’s Ischemia Syndrome Evaluation (WISE) were analyzed. LVEF was calculated by invasive left ventriculography. The relationship between baseline characteristics, LVEF and outcomes were evaluated. A multivariable Cox regression model was used to assess the association of LVEF with outcomes, after adjusting for known risk factors.
Results:
Low LVEF was associated with higher rates of mortality and MACE compared to normal and high LVEF (p<0.0001). Normal LVEF was associated with higher mortality (p=0.047) and rate of myocardial infarctions (MI) compared to high LVEF (p=0.03). Low LVEF remained a significant predictor of mortality compared to high LVEF (p=0.013) in a multivariable regression model and normal compared to high LVEF trended towards higher mortality (p=0.16).
Conclusions:
Among women with suspected ischemia, women with LVEF above the defined normal threshold (>65%) had lower rates of all-cause mortality and non-fatal MI. Further investigation is needed to determine the optimal LVEF in women.
Keywords: WISE, Women, Ischemia, Left Ventricular Ejection Fraction, Outcomes
Introduction
Left ventricular ejection fraction (LVEF) is a measurement routinely used in clinical management, such as for the classification of heart failure with reduced ejection fraction (HFrEF) versus preserved ejection fraction (HFpEF), initiation of guideline-directed medical therapy, and determination of when an implantable cardiac defibrillator is needed. Multiple studies using various imaging modalities such as cardiac magnetic resonance imaging (MRI), computed tomography (CT) and echocardiography have shown that baseline LVEF is higher in women than men, and further increases with age.1,2,3 There is increasing recognition of sex differences in the presentation and outcomes associated with cardiovascular (CV) disease and awareness that women manifest CV disease in different ways, therefore it is unclear what a “normal” LVEF cut-off in women should be.4,5 Despite evidence of sex and age-related differences in LVEF, a sex neutral LVEF threshold continues to be used for diagnosis, risk stratification and treatment. The risk of adverse CV events and CV mortality has been shown to start at higher LVEF levels in women compared to men, and failure to recognize this can lead to insufficient evaluation and under-treatment of women with cardiac disease.6,7
Women have a higher prevalence of HFpEF compared to men, however a portion of these patients may actually have relatively reduced LVEF based on sex and age specific indices. Multiple therapeutic agents used in the treatment of HFrEF have failed to show similar benefit in HFpEF, however post-hoc analyses of some trials (TOPCAT, PARAGON-HF) showed that women may derive benefit from these treatments at higher LVEF levels compared to men.8,9,10 This suggests that there may need to be female-specific criteria with regards to drug and device therapy in women and further reiterates the lack of knowledge surrounding sex differences in the evaluation and management cardiac disease.
To address this current gap in knowledge and focus on creating a diagnostic and treatment algorithm specific to women, we analyzed women from the Women’s Ischemia Syndrome Evaluation. WISE consisted of a cohort of women presenting with suspected myocardial ischemia undergoing coronary angiography and represents a well-phenotyped population to study the prognostic implication of LVEF and further delineate the optimal threshold in symptomatic women. We investigated the relationship among high (>65%), normal (55–65%) and low LVEF (<55%) and outcomes including all-cause mortality and major adverse cardiovascular events (MACE).
Methods
The WISE study, which enrolled patients between 1996–2000 (NCT00000554), is a multicenter prospective study of a cohort of women >18 years of age with signs and symptoms suggestive of ischemia who underwent clinically indicated coronary angiography for evaluation of chest pain or suspected ischemia. The WISE protocol, which has been published in detail previously, was approved by institutional review boards of participating centers and all women provided written informed consent.11 Major exclusion criteria included pregnancy, contraindications to provocative diagnostic testing, cardiomyopathy, New York Heart Association class IV heart failure, recent myocardial infarction (MI), significant valvular or congenital heart disease, and a language barrier to questionnaire testing.
A total of 939 women were enrolled in the study and followed for adverse outcomes. Baseline demographics and medical history were obtained at enrollment and outcomes data, including mortality, nonfatal MI, hospitalization for heart failure and nonfatal stroke were collected. Coronary angiography was performed shortly after enrollment, and obstructive CAD was defined as presence of ≥50% diameter stenosis in ≥1 major epicardial coronary artery. LVEF was calculated by invasive contrast left ventriculography by the WISE angiographic core laboratory.12 Prior work has demonstrated comparability of core lab invasive ventriculography to echo and gated single-photon emission computed tomography, and superior to non-quantitative “eyeball” cineangiography.13,14,15 The LVEF cut-off values of low <55%, normal 55–65% and high >65% were chosen based on previously reported reference ranges.16,17,18 Outcomes data was collected annually by study site personnel and deaths were adjudicated by an Events Committee as cardiovascular or non-cardiovascular.11 MACE was defined as death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure (HF).
Statistical Analyses
Baseline characteristics were summarized by LVEF group using standard descriptive measures – frequency and percentage for categorical variables and median (IQR) for continuous variables. LVEF groups were then compared using Pearson’s chi-square test for categorical variables and Wilcoxon rank sum test or Kruskal-Wallis test for continuous variables. Kaplan-Meier analysis was used to estimate survival probabilities and the log-rank test was used to compare KM curves. Time-to-event was defined as the time from WISE study entry to last follow-up, death, or MACE. We also constructed a multivariable Cox proportional hazards regression model to further assess the association of LVEF with mortality. The chained equation approach for multiple imputation was used prior to performing the Cox regression to account for the missing LVEF measures in the data. The Cox model was adjusted for known risk factors of mortality as outlined in previous literature, including age, smoking status, statin use, history of diabetes, history of hypertension, history of HF, history of coronary artery disease (CAD), and CAD severity score. CAD severity score was determined using the Sharaf-Gensini system. Receiver operating characteristic curve (ROC) analysis and Youden’s index was used to determine the optimal LVEF cut-off for predicting mortality. All statistical analyses were performed using SAS software (SAS, version 9.2, Carey, N.C.) and a significance level of 0.05.
Results
A total of 734 out of 939 women with LVEF, angiographic and outcomes data were included in this analysis with median follow-up occurring over 11.3 years. Baseline characteristics are listed in Table 1. The average LVEF of the total group was 65% and the majority of women had an LVEF of 60% or greater. Women with low LVEF were older with higher rates of comorbidities such as heart failure, diabetes, tobacco use and obstructive CAD, and had more elevated inflammatory markers. When comparing just normal and high LVEF groups, there were fewer baseline differences however high LVEF group was older, had higher high-density lipoprotein cholesterol levels, higher blood pressure parameters (including systolic blood pressure and mean arterial pressure) and a slightly lower rate of obstructive CAD (Table 1).
Table 1.
Baseline characteristics stratified by low (<55%), normal (55–65%) and high (>65%) LVEF
| Low LVEF (n=81) | Normal LVEF (n=355) | High LVEF (n=298) | p-value | p-value (normal v. high only) | |
|---|---|---|---|---|---|
| Age (years) | 61 (53–69) | 56 (48–66) | 59 (52–68) | 0.004 | 0.010 |
| BMI (kg/m2) | 28 (25–32) | 29 (25–33) | 29 (25–34) | 0.258 | 0.959 |
| LVEF (%) | 45 (35–50) | 65 (60–65) | 75 (70–76) | <0.001 | <0.001 |
| MAP (mmHg) | 95 (89–104) | 95 (87–103)) | 97 (89–107) | 0.069 | 0.022 |
| SBP (mmHg) | 132 (122–148) | 132 (120–149) | 138 (122–150) | 0.041 | 0.014 |
| DBP (mmHg) | 78 (68–81) | 76 (68–82) | 78 (70–85) | 0.160 | 0.060 |
| Hypertension | 52 (64%) | 190 (54%) | 174 (59%) | 0.189 | 0.239 |
| Diabetes | 33 (41%) | 78 (22%) | 57 (19%) | <0.001 | 0.373 |
| Dyslipidemia | 45 (61%) | 166 (50%) | 149 (54%) | 0.246 | 0.418 |
| CHF | 20 (25%) | 16 (5%) | 12 (4%) | <0.001 | 0.748 |
| Tobacco use | 55 (68%) | 183 (52%) | 156 (52%) | 0.030 | 0.168 |
| Current HRT use | 30 (38%) | 128 (37%) | 132 (45%) | 0.081 | 0.029 |
| HDL (mg/dL) | 47 (36–58) | 48 (40–58) | 51 (43–63) | 0.040 | 0.054 |
| LDL (mg/dL) | 129 (91–161) | 124 (95–152) | 119 (95–146) | 0.577 | 0.314 |
| Triglycerides (mg/dL) | 141 (103–225) | 141 (100–212) | 145 (105–231) | 0.801 | 0.517 |
| Glucose (mg/dL) | 105 (84–142) | 96 (83–119) | 94 (83–109) | 0.041 | 0.224 |
| Creatinine (mg/dL) | 0.8 (0.7–1.0) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.847 | 0.984 |
| CRP (mg/L) | 0.6 (0.3–1.4) | 0.4 (0.1–0.8) | 0.4 (0.2–0.7) | 0.018 | 0.537 |
| IL-6 (pg/mL) | 3.9 (2.6–6.5) | 2.9 (1.7–4.9) | 2.7 (1.6–5.1) | 0.015 | 0.974 |
| Obstructive CAD (>50%) | 48 (59%) | 129 (36%) | 84 (28%) | <0.001 | 0.027 |
| CAD severity score | 14 (5–79) | 8 (5–85) | 8 (5–73) | <0.001 | 0.749 |
Values reported as median (IQR) or n (%).
BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CRP, c-reactive protein; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HRT, hormone replacement therapy; IL-6, interleukin-6; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure
Over median 11 years of follow-up, all-cause mortality occurred in 18% of total patients (41%, 17% and 12% respectively in the low, normal and high LVEF groups). LVEF was associated with mortality among the 3 groups (p<0.0001, Figure 1A), with low LVEF demonstrating the highest rate of mortality. When comparing normal and high LVEF only, normal LVEF was associated with a significantly higher rate of mortality (p<0.05, Figure 2A). When the predictive value of LVEF was assessed in a multivariable Cox regression model after imputation adjusted for age, CV risk factors and CAD severity, low LVEF remained a significant predictor of mortality compared to high LVEF (HR 1.88, CI 1.14–3.11, p=0.01) and normal LVEF compared to high LVEF trended towards higher mortality (HR 1.33, CI 0.89–1.99, p=0.2, Table 2A).
Figure 1.
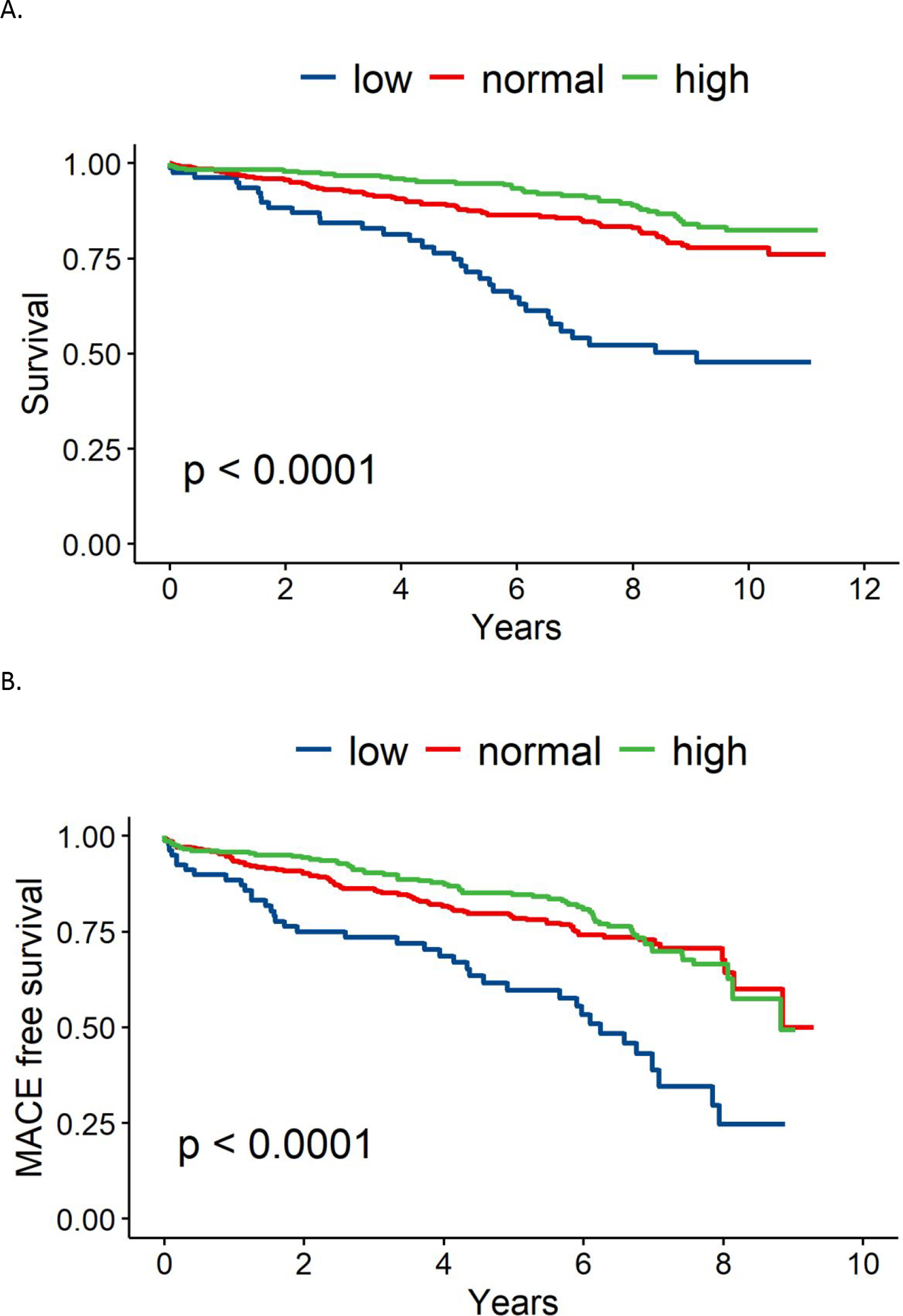
(A) 10-year all-cause mortality stratified by LVEF. (B) 10-year major adverse cardiovascular events (MACE) stratified by LVEF.
Figure 2.
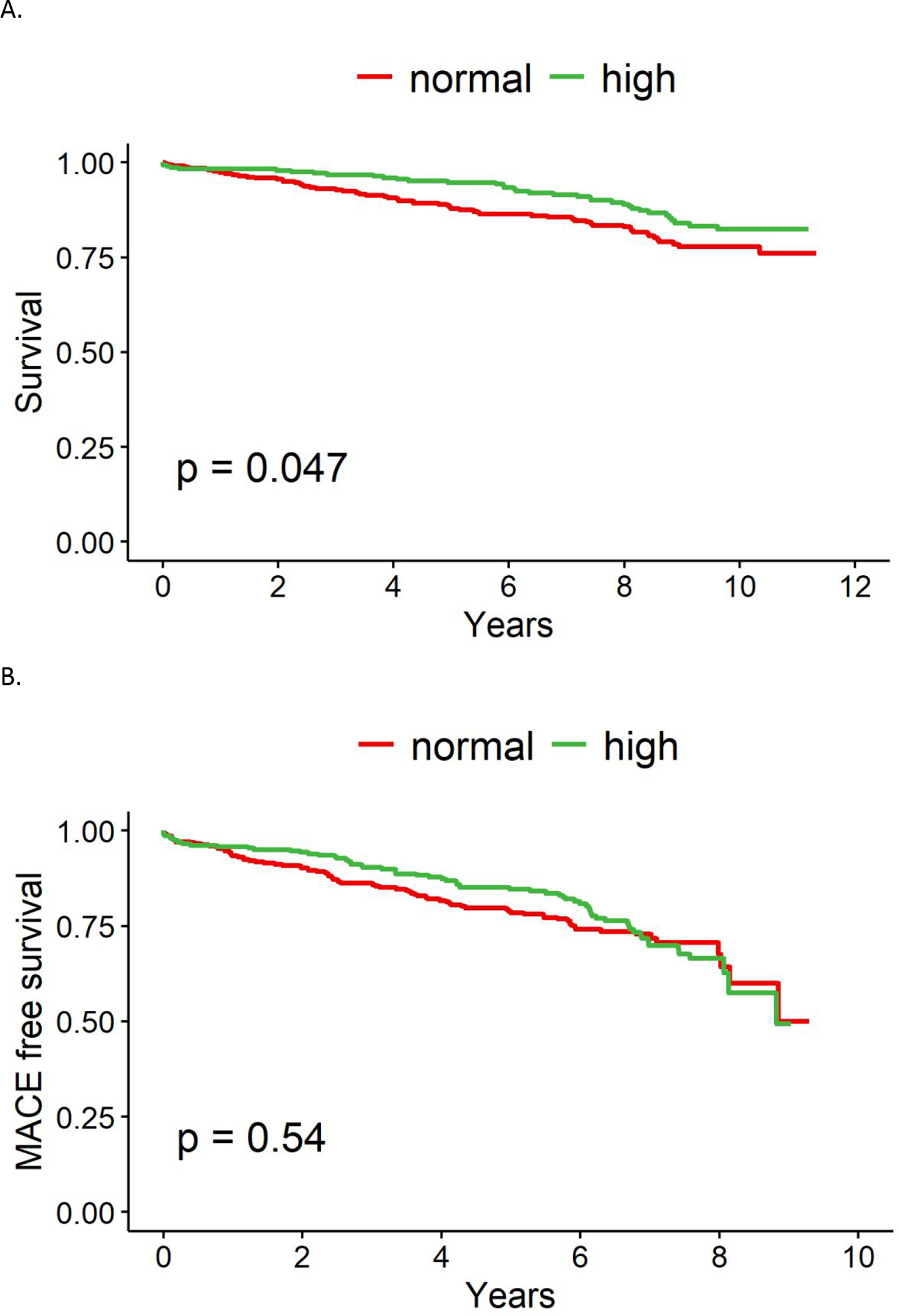
(A) 10-year all-cause mortality stratified by LVEF. (B) 10-year major adverse cardiovascular events (MACE) stratified by LVEF
Table 2.
(A) Univariate and multivariate analysis of baseline variables for all-cause mortality (B) Univariate and multivariate analysis of baseline variables for MACE
| A. | ||||
|---|---|---|---|---|
| Hazard Ratio | 95% CI of HR | Univariate p-value | Multivariable p-value | |
|
| ||||
| Age | 1.03 | 1.02–1.05 | <0.001 | <0.001 |
|
| ||||
| History of smoking | ||||
|
| ||||
| Former smoker | 1.68 | 1.20–2.37 | 0.017 | 0.003 |
|
| ||||
| Current smoker | 2.07 | 1.38–3.11 | 0.033 | <0.001 |
|
| ||||
| Statin use | 1.63 | 1.20–2.21 | <0.001 | 0.002 |
|
| ||||
| Diabetes | 1.78 | 1.30–2.43 | <0.001 | <0.001 |
|
| ||||
| Hypertension | 1.65 | 1.15–2.35 | <0.001 | 0.006 |
|
| ||||
| CHF | 2.25 | 1.52–3.33 | <0.001 | <0.001 |
|
| ||||
| CAD | 0.83 | 0.61–1.14 | 0.143 | 0.252 |
|
| ||||
| CAD severity score | 1.01 | 1.01–1.02 | <0.001 | <0.001 |
|
| ||||
| Low LVEF (v. high) | 1.88 | 1.14–3.11 | <0.001 | 0.013 |
|
| ||||
| Normal LVEF (v. high) | 1.33 | 0.89–1.99 | 0.197 | 0.159 |
|
| ||||
| B. | ||||
| Hazard Ratio | 95% CI of HR | Univariate p-value | Multivariable p-value | |
|
| ||||
| Age | 1.02 | 1.01–1.03 | <0.001 | 0.005 |
|
| ||||
| History of smoking | ||||
|
| ||||
| Former smoker | 1.88 | 1.40–2.52 | <0.001 | <0.001 |
|
| ||||
| Current smoker | 2.29 | 1.62–3.24 | <0.001 | <0.001 |
|
| ||||
| Statin use | 1.52 | 1.16–1.98 | <0.001 | 0.002 |
|
| ||||
| Diabetes | 1.88 | 1.43–2.47 | <0.001 | <0.001 |
|
| ||||
| Hypertension | 1.23 | 0.92–1.64 | <0.001 | 0.157 |
|
| ||||
| CHF | 2.57 | 1.81–3.65 | <0.001 | <0.001 |
|
| ||||
| CAD | 0.92 | 0.70–1.20 | 0.327 | 0.522 |
|
| ||||
| CAD severity score | 1.02 | 1.01–1.02 | <0.001 | <0.001 |
|
| ||||
| Low LVEF (v. high) | 1.28 | 0.82–2.00 | <0.001 | 0.274 |
|
| ||||
| Normal LVEF (v. high) | 1.03 | 0.74–1.43 | 0.714 | 0.854 |
CHF, congestive heart failure, CAD, coronary artery disease
LVEF was also associated with MACE among groups, with highest rate of MACE in the low LVEF group (p<0.0001, Figure 1B). There was no significant difference in MACE when comparing normal and high LVEF alone (Figure 2B). Upon analysis of individual MACE components, high LVEF was associated with a significantly lower risk of nonfatal MI compared to normal LVEF (1% vs 4%, p=0.03, Table 3), however the remainder of MACE components, including death, HF hospitalization, and non-fatal stroke were not significantly different. ROC curve analysis demonstrated that an LVEF of <58% was the optimal cut-off point for predicting mortality based upon Youden index (Figure 4).
Table 3.
Comparison of individual MACE components between normal and high LVEF
| Normal EF (n=355) | High EF (n=298) | Total (n=653) | p-value | |
|---|---|---|---|---|
|
| ||||
| All-cause mortality | 61 (17%) | 36 (12%) | 97 (15%) | 0.068 |
|
| ||||
| HF hospitalization | 17 (5%) | 18 (6%) | 35 (5%) | 0.483 |
|
| ||||
| Non-fatal MI | 15 (4%) | 4 (1%) | 19 (3%) | 0.029 |
|
| ||||
| Non-fatal stroke | 16 (5%) | 17 (6%) | 33 (5%) | 0.490 |
HF, heart failure hospitalization, MI, myocardial infarction
Figure 4.
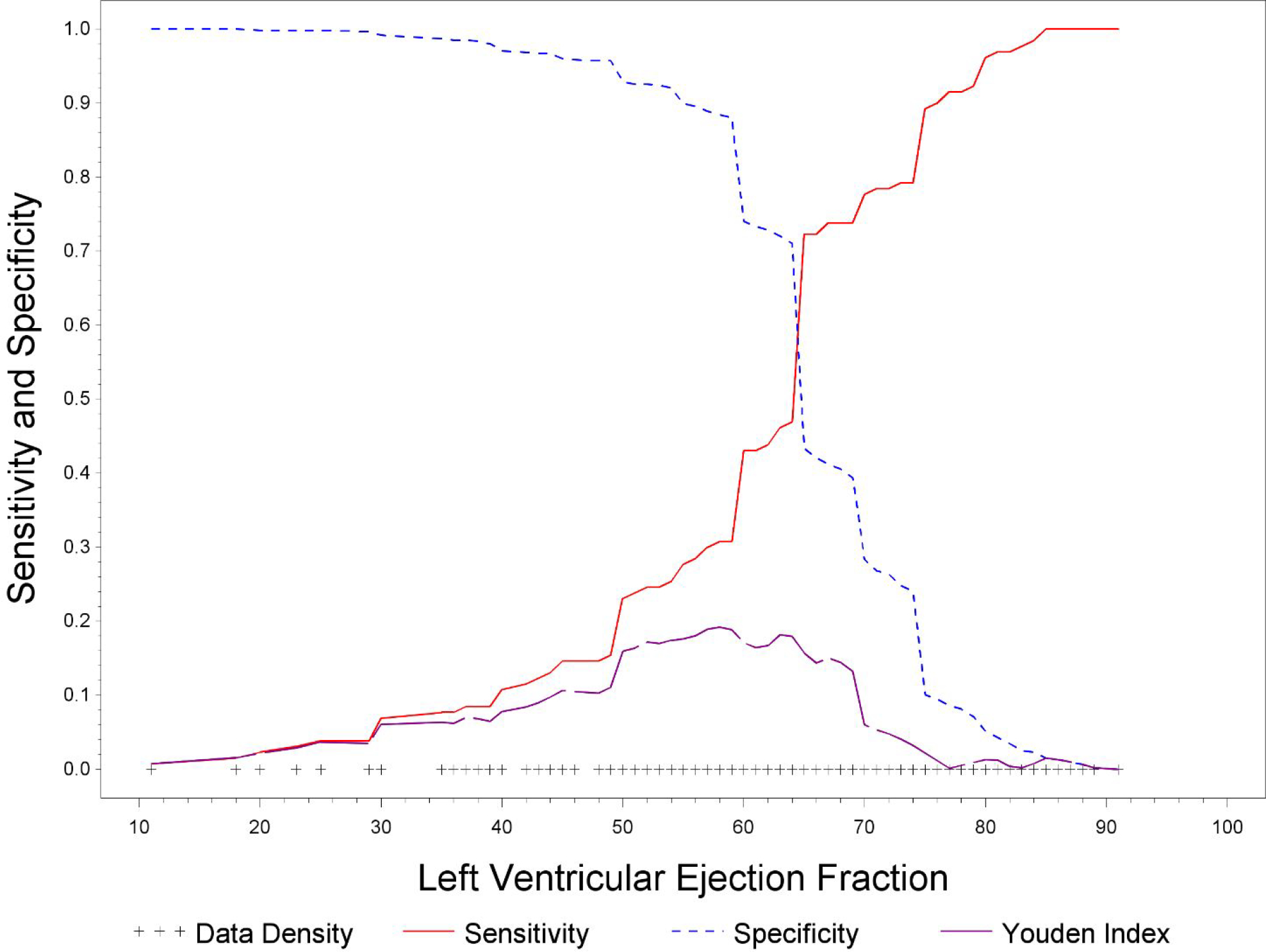
Receiver operating characteristic curve for Left Ventricular Ejection Fraction
Discussion
In this study, we examined LVEF and outcomes in women presenting with signs and symptoms of ischemia who are referred for coronary angiography. Our study focuses on an at-risk group of women with a 20% all-cause mortality and 12% CV mortality rate over a 10-year follow-up period, which is substantially higher than the average population.19 Our analysis shows that women with low LVEF (<55%) had the highest rates of all-cause mortality. Women with normal LVEF (55–65%) had a significantly higher rate of mortality compared to women with high LVEF (>65%), and an LVEF of 58% was determined to be the optimal cut-off point for predicting mortality.
There is an increasing awareness of sex specific presentation of ischemic heart disease (IHD). Women have unique risk factors contributing to IHD such as pregnancy related complications, autoimmune disease and radiation/chemotherapy for breast cancer and also present with an expanded spectrum of coronary disease related not only to epicardial CAD but also coronary microvascular dysfunction, vasospasm and spontaneous coronary artery dissection.20 In patients presenting with signs and symptoms of myocardial ischemia, women have a higher prevalence of non-obstructive CAD on coronary angiography as well as ischemia with no obstructive CAD (INOCA) and myocardial infarction with no obstructive CAD (MINOCA).21 These factors, in addition to current risk stratification tools based upon predominantly male populations, contribute to a lower recognition of IHD in women, which leads to less aggressive treatment, lower rates of diagnostic and interventional procedures, and suboptimal use of medical therapy for primary and secondary prevention.20,22
Of note, our data showed that 36% of women presenting with signs and symptoms of myocardial ischemia were found to have obstructive CAD on angiography, with the highest rate seen in the low LVEF group. Patients with normal LVEF had higher rates of obstructive CAD and MI compared to high LVEF. The risk of adverse cardiac events such as non-fatal MI has been shown to increase with low LVEF in both men and women, however prior data has shown that women have a relatively higher risk of adverse cardiac events for each 1% incremental decrease in LVEF compared to men (4.3% vs. 2.8%) and have a higher optimal prognostic LVEF for predicting hard cardiac events (52% vs. 47%).6 These findings along with our results illustrate sex differences in risk stratification associated with LVEF and suggest that despite having a “normal” LVEF, aggressive evaluation and treatment is necessary to prevent adverse cardiac events especially in those with known CAD.
Women are known to have higher baseline LVEFs compared to men due to smaller left ventricular volumes and threshold values to define low LVEF have been demonstrated to be several points higher in women, however they continue to be risk stratified using the same LVEF cut-offs for treatment decisions and prognostication.1,2 A recent large registry-based cohort study, which included over 400,000 patients, again showed that women had a higher average EF while twice as many men presented with LVEF<50%, and all-cause mortality and CV mortality were the lowest at an LVEF of 65–69.9% for both men and women. While mortality was significantly higher below an LVEF of 55% for both sexes, only women had a continued increase risk of death at a threshold of 60–64.9%.7 Additionally there appeared to be an increase in mortality (mostly non-CV related) associated with hyperdynamic LV function, particularly in younger women, although the number of patients in this range was small. Our study demonstrated that all-cause mortality (predominantly adjudicated CV deaths) was lower in patients with LVEF>65% compared to 55–65% and reached a nadir at an LVEF of 70–75%, further suggesting that the optimal LVEF in women is still unknown. Women are more predisposed to developing HFpEF due to intrinsic sex differences and epidemiological factors such as differences in cardiac remodeling, microvascular dysfunction, stronger immunity/increased inflammation and higher rates of hypertension and obesity.12,23 It is possible that a portion of these women may actually have relatively reduced LVEF when using sex neutral cut-offs as there has been evidence showing contractile dysfunction despite an LVEF that falls within a “normal” range.24,21
Although our study showed lower mortality in LVEF>65%, there have been studies showing that high LVEF is associated with worse outcomes including results from the recent CONFIRM trial which showed a higher long-term mortality in women with LVEF>65%, particularly with obstructive CAD.17,18 However only 33% of women in this study had high LVEF, whereas over 60% of women in our study were classified as having a high LVEF and heterogeneity in clinical characteristics may account for the different results.
Focusing on a female-specific approach for the recognition, diagnosis and risk assessment of CV disease will lead to more optimal management and initiation of therapeutics. Lifestyle intervention and risk factor modification in women can have a significant impact in the reduction of CV disease. Medications such as aspirin and statins are a crucial component of primary prevention and are known to improve mortality in patients with IHD however previous WISE data showed that there was suboptimal use of these therapies, even in women with known obstructive and non-obstructive CAD.26 Additional delineation of sex specific risk assessment in addition to existing risk factors is crucial and closer attention may need to be paid to women presenting with so called “normal” LVEFs. Further supporting the idea of sex-specific LVEF cut-offs are results from the PARAGON-HF and TOPCAT trials, which evaluated the use of angiotensin receptor neprilysin inhibitor and mineralocorticoid receptor agonists. While these medications did not show any benefit in HFpEF patients, post-hoc analyses show that women may derive benefit at a higher LVEF compared to men.9,10 If a subset of women with HFpEF actually have relatively reduced function based on age and sex, then earlier initiation of routine heart failure medications needs to be considered. With the US Food and Drug Administration’s recent expanded labeling for use of angiotensin receptor neprilysin inhibitors in patients with higher LVEF, it is even more important to determine sex-specific LVEF cutoffs to further increase the number of women who may benefit from its use.27 Evaluation of LVEF is also important when considering primary prevention for sudden cardiac death and severe LVEF dysfunction is an indication for an implantable cardiac defibrillator. Sudden cardiac death was shown to be a substantial contributor to mortality in the WISE cohort despite preserved LVEF, and would not qualify for primary prevention devices, again highlighting the potential issue of using a sex-neutral LVEF threshold to determine therapeutics.28,29
Limitations
There are several limitations in our study that deserve comment. This study focused on a high-risk group of women presenting with signs and symptoms of myocardial ischemia, therefore the results are not applicable to men and may not be generalizable to all women. LVEF was calculated with invasive left ventriculography and therefore echocardiographic parameters such as classification of diastolic dysfunction were not available. Further, prior work has demonstrated comparability of core lab interpreted invasive ventriculography to echo and gated single-photon emission computed tomography, and superior to non-quantitative “eyeball” cineangiography13,14,15.
Conclusions and Implications
Our study demonstrates that women with LVEF above a defined normal threshold (>65%) had lower rates of all-cause mortality and non-fatal MI compared to lower levels. Current guidelines do not address female-specific differences in management of heart disease and there remains a deficit of large studies focusing on women to establish reference standards. Further investigation is needed to confirm optimal female-specific LVEF indices in order to most effectively improve heart health with preventive and treatment strategies in women.
Figure 3.
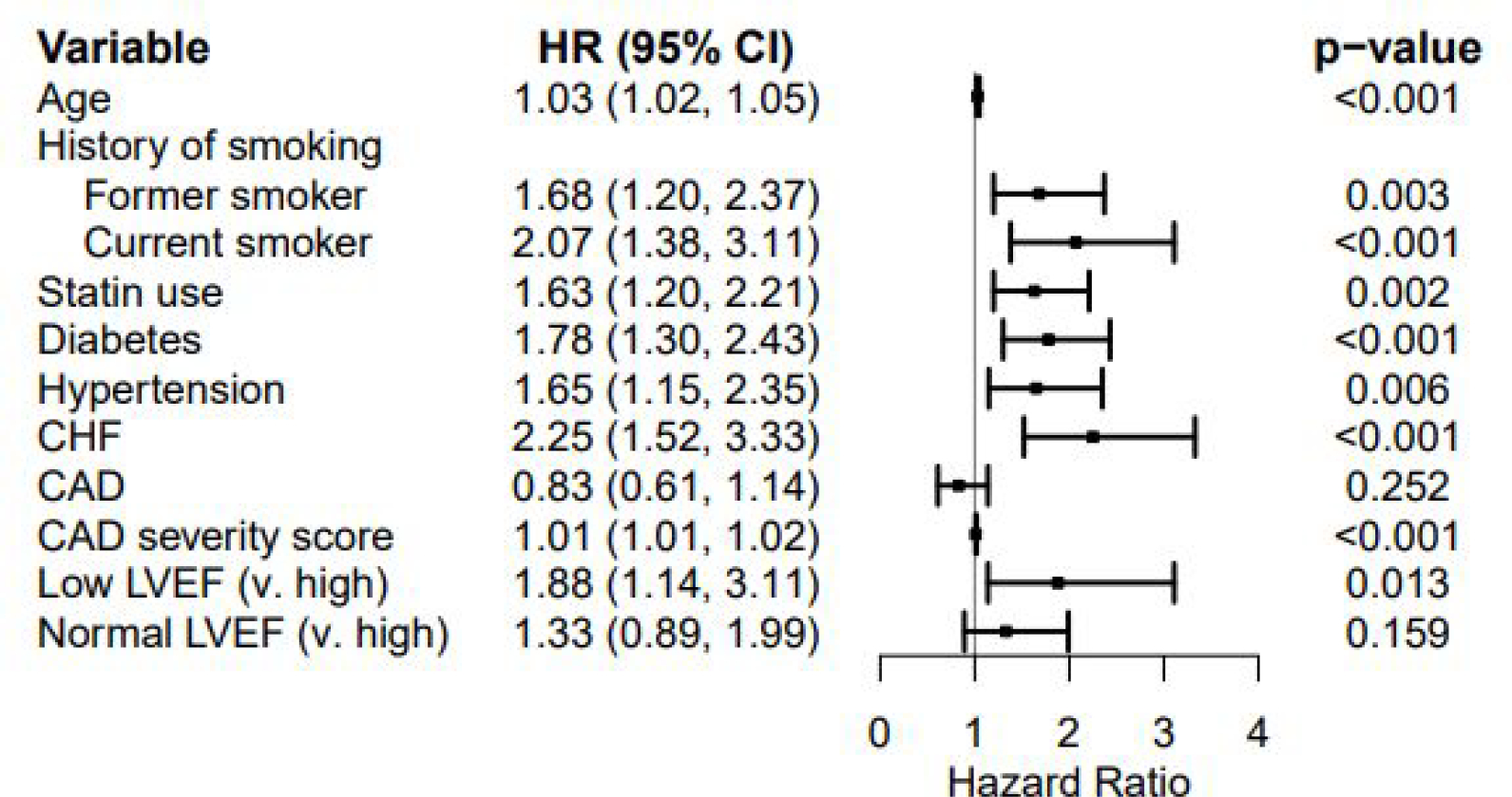
Forest plot for all-cause mortality
CHF, congestive heart failure, CAD, coronary artery disease
Key Messages.
What is already known on this topic:
Women are known to have different presentation and outcomes associated with cardiovascular disease compared to men.
Women have a higher left ventricular ejection fraction (LVEF) at baseline compared to men, however a sex-neutral LVEF threshold is commonly used in practice for risk stratification and clinical decision-making.
What this study adds:
In women presenting with signs and symptoms of ischemia, normal LVEF (55–65%) was associated with higher mortality compared to high LVEF (>65%).
Normal LVEF (55–65%) was associated with higher rate of myocardial infarctions compared to high LVEF (>65%).
An LVEF of 58% was demonstrated to be the optimal cut-off point for predicting mortality.
How this study might affect research, practice or policy:
These findings contribute to the gap of knowledge in gender-specific differences in the diagnosis and management of cardiovascular disease.
The current applied thresholds for interpreting LVEF and making clinical decisions may need to be re-visited in women, especially those at higher cardiovascular risk. Further research is needed to confirm optimal female-specific LVEF thresholds.
ACKNOWLEDGEMENTS
FUNDING
This work was supported by contracts from the National Heart, Lung and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, U54AG065141, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research (SWHR), Washington, D.C., the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California.
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the U.S. Department of Health and Human Services.
Footnotes
DISCLOSURES
Dr. Bairey Merz serves as Board of Director for iRhythm and SHL Telemedicine and receives personal fees paid through CSMC from Abbott Diagnostics and Phillips. All other authors report no disclosures. Dr. Handberg reports grants from NIH/NHLBI, during the conduct of the study; grants from Aastom Biosciences, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Brigham and Women’s Hospital, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Duke Clinical Research Institute, East Carolina University, Everyfit Inc, Gilead, Ionis, Medtronic, Merck & Co., Mesoblast, PCORI, Relypsa, Sanofi Aventis, outside the submitted work. Dr. Pepine reports grants from NIH/NHLBI, during the conduct of the study; grants from NIH/NCATS, grants from BioCardia BC-14–001-02; Mesoblast, Inc. MSB-MPC-CHF001; Ventrix, Inc.; Athersys Inc. AMI MultiStem; Verily Life Sciences LLC-Project Baseline OSMB; Ironwood MSB-MPC-CHF00-DMC, Imbria Pharmaceuticals Inc.; Milestone Pharmaceuticals Inc.; Caladrius Biosciences, Inc.; Gatorade Trust; and McJunkin Family Foundation, outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
REFERENCES
- 1.Chung AK, Das SR, Leonard D, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the dallas heart study. Circulation. 2006;113(12):1597–1604. [DOI] [PubMed] [Google Scholar]
- 2.Gebhard C, Buechel RR, Stähli BE, et al. Impact of age and sex on left ventricular function determined by coronary computed tomographic angiography: results from the prospective multicentre CONFIRM study. European Heart Journal - Cardiovascular Imaging. 2017;18(9):990–1000. [DOI] [PubMed] [Google Scholar]
- 3.Gebhard C, Stähli BE, Gebhard CE, et al. Age- and gender-dependent left ventricular remodeling. Echocardiography. 2013;30(10):1143–1150. [DOI] [PubMed] [Google Scholar]
- 4.Lam CSP, Arnott C, Beale AL, et al. Sex differences in heart failure. European Heart Journal. 2019;40(47):3859–3868c. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S, Fernandes VRS, Bluemke DA, McClelland RL, Kronmal RA, Lima JAC. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2009;2(3):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wexler O, Yoder SR, Elder JL, et al. Effect of gender on cardiovascular risk stratification with ECG gated SPECT left ventricular volume indices and ejection fraction. J Nucl Cardiol. 2009;16(1):28–37. [DOI] [PubMed] [Google Scholar]
- 7.Stewart S, Playford D, Scalia GM, et al. Ejection fraction and mortality: a nationwide register-based cohort study of 499 153 women and men. Eur J Heart Fail. Published online November 26, 2020:ejhf.2047. [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. [DOI] [PubMed] [Google Scholar]
- 9.Merrill M, Sweitzer NK, Lindenfeld J, Kao DP. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction. JACC: Heart Failure. 2019;7(3):228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMurray JJV, Jackson AM, Lam CSP, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from paragon-hf. Circulation. 2020;141(5):338–351. [DOI] [PubMed] [Google Scholar]
- 11.Bairey Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (Wise) Study: protocol design, methodology and feasibility report. Journal of the American College of Cardiology. 1999;33(6):1453–1461. [DOI] [PubMed] [Google Scholar]
- 12.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (Pilot phase data from the nhlbi-sponsored women’s ischemia syndrome evaluation [wise] study angiographic core laboratory). The American Journal of Cardiology. 2001;87(8):937–941. [DOI] [PubMed] [Google Scholar]
- 13.Garg N, Dresser T, Aggarwal K, Gupta V, Mittal MK, Alpert MA. Comparison of left ventricular ejection fraction values obtained using invasive contrast left ventriculography, two-dimensional echocardiography, and gated single-photon emission computed tomography. SAGE Open Med. 2016;4:2050312116655940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naik MM, Diamond GA, Pai T, Soffer A, Siegel RJ. Correspondence of left ventricular ejection fraction determinations from two-dimensional echocardiography, radionuclide angiography and contrast cineangiography. J Am Coll Cardiol. 1995;25(4):937–942. [DOI] [PubMed] [Google Scholar]
- 15.Bernard Y, Meneveau N, Boucher S, et al. Lack of agreement between left ventricular volumes and ejection fraction determined by two-dimensional echocardiography and contrast cineangiography in postinfarction patients. Echocardiography. 2001;18(2):113–122. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the european association of cardiovascular imaging. Journal of the American Society of Echocardiography. 2015;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- 17.Saab FA, Steg PG, Avezum A, Lopez-Sendon J, Anderson FA, Huang W et al. Can an elderly woman’s heart be too strong? Increased mortality with high ver- sus normal ejection fraction after an acute coronary syndrome. The Global Registry of Acute Coronary Events. Am Heart J 2010;160:849–54. [DOI] [PubMed] [Google Scholar]
- 18.Gebhard C, Maredziak M, Messerli M, et al. Increased long-term mortality in women with high left ventricular ejection fraction: data from the CONFIRM (Coronary ct angiography evaluation for clinical outcomes: an international multicenter) long-term registry. European Heart Journal - Cardiovascular Imaging. 2020;21(4):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenkre TS, Malhotra P, Johnson BD, et al. Ten-year mortality in the wise study(Women’s ischemia syndrome evaluation). Circ Cardiovasc Qual Outcomes. 2017;10(12). (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia M, Mulvagh SL, Merz CNB, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118(8):1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E, Bairey Merz CN. Ischemia and no obstructive coronary artery disease (Inoca): what is the risk? J Am Heart Assoc. 2018;7(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucholz EM, Strait KM, Dreyer RP, et al. Editor’s Choice-Sex differences in young patients with acute myocardial infarction: A VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6(7):610–622. doi: 10.1177/2048872616661847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunlay SM, Roger VL. Gender differences in the pathophysiology, clinical presentation, and outcomes of ischemic heart failure. Curr Heart Fail Rep. 2012;9(4):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138(2):198–205. [DOI] [PubMed] [Google Scholar]
- 25.Föll D, Jung B, Schilli E, et al. Magnetic resonance tissue phase mapping of myocardial motion: new insight in age and gender. Circ Cardiovasc Imaging. 2010;3(1):54–64. [DOI] [PubMed] [Google Scholar]
- 26.Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: Findings from the National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation (Wise) angiographic core laboratory. American Heart Journal. 2013;166(1):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaduganathan M, Claggett BL, Greene SJ, et al. Potential implications of expanded us food and drug administration labeling for sacubitril/valsartan in the us. JAMA Cardiol. 2021;6(12):1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta PK, Johnson BD, Kenkre TS, et al. Sudden cardiac death in women with suspected ischemic heart disease, preserved ejection fraction, and no obstructive coronary artery disease: a report from the women’s ischemia syndrome evaluation study. JAHA. 2017;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction. Journal of the American College of Cardiology. 2006;47(6):1161–1166. [DOI] [PubMed] [Google Scholar]


