Abstract
The greater efficacy of DNA-damaging drugs for pancreatic adenocarcinoma (PDAC) relies on targeting cancer-specific vulnerabilities while sparing normal organs and tissues due to their inherent toxicities. We tested LP-184, a novel acylfulvene analog, for its activity in preclinical models of PDAC carrying mutations in the DNA damage repair (DDR) pathways.
Cytotoxicity of LP-184 is solely dependent on prostaglandin reductase 1 (PTGR1), so that PTGR1 expression robustly correlates with LP-184 cytotoxicity in vitro and in vivo. Low-passage patient-derived PDAC xenografts with DDR deficiencies treated ex vivo are more sensitive to LP-184 compared to DDR-proficient tumors. Additional in vivo testing of PDAC xenografts for their sensitivity to LP-184 demonstrates marked tumor growth inhibition in models harboring pathogenic mutations in ATR, BRCA1 and BRCA2. Depletion of PTGR1, however, completely abrogates the anti-tumor effect of LP-184. Testing combinatorial strategies for LP-184 aimed at deregulation of nucleotide excision repair proteins ERCC3 and ERCC4 established synergy.
Our results provide valuable biomarkers for clinical testing of LP-184 in a large subset of genetically defined characterized refractory carcinomas. High PTGR1 expression and deleterious DDR mutations are present in approximately one third of PDAC making these patients ideal candidates for clinical trials of LP-184.
Keywords: LP-184, pancreatic cancer, PTGR1, DNA repair deficiency
Introduction
The American Cancer Society estimates 62,210 new diagnoses of pancreatic adenocarcinoma (PDAC) in the USA in 2021[1, 2]. Although PDAC accounts for about 3% of all cancers in the USA and worldwide, it underlies 12.7% and became the 4th leading cause of all cancer deaths in 2021 with all-stage 3-year survival rates at 11%[1, 2]. The continuously rising incidence of PDAC is projected to become the second leading cause of cancer-related deaths by 2040, surpassing lung cancer mortality[3]. The alarmingly poor outcomes of PDAC are due to its increased propensity for early metastatic dissemination and limited response to chemotherapy or radiation (RT). The currently available treatment options for the majority of patients with advanced-stage PDAC are limited to combination chemotherapy with 5-fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX)[4], gemcitabine and nab-paclitaxel[5]. Widespread implementation of molecular profiling of PDAC has demonstrated its genetic diversity, with homologous recombination and other DNA damage repair (DDR) pathway deficiencies found in 10–15% of all patients[6, 7]. The presence of germline HR deficiency in a subset of PDAC has recently demonstrated the clinical efficacy of the poly ADP-ribose polymerase inhibitor, olaparib. However, even in this subset of PDAC patients, the development of platinum agents and PARP inhibitor resistance is commonly seen[8] necessitating the development of new treatment options.
Here, we demonstrate the lethality of a fully synthetic acylfulvene derivative, LP-184[9, 10] against PDAC and other solid tumors with DNA repair deficiency. The acylfulvene cytotoxin illudin S was isolated as a racemic mixture of stereoisomers from Jack-o-Lantern (Omphalotus illudens) and other poisonous mushrooms. The mechanisms of acylfulvene cytotoxicity are related to the formation of alkylated adducts with nucleotides of DNA, resulting in cell-cycle arrest and apoptosis[11], generation of reactive oxygen species, chemical modification of intracellular proteins, and inhibition of cytosolic redox-regulating thiol-containing proteins such as glutathione reductase and thioredoxin[11]. Previous studies from our group[12, 13] and others[14] demonstrated that acylfulvene anti-tumor activity correlates with the expression of prostaglandin reductase 1 (PTGR1), thus supporting the idea that acylfulvene is a prodrug that requires catalytic conversion by an intracellular oxidoreductase. This study has established preclinical biomarkers of LP-184 activity for immediate exploration in clinical trials of PDAC and other recalcitrant solid tumors.
Materials and Methods
Reagents
Chemicals and chemotherapy drugs.
LP-184 and LP-284 were provided by Lantern Pharma. Synthesis of LP-184 and LP-284 is described in figure 3 of the United States Patent US 2021/0198191 A1(10): compound 5, (−)LP-184 refers to LP-184 in the manuscript and (+)LP-184 refers to LP-284 in the manuscript. Gemcitabine, irinotecan, oxaliplatin, and 5-fluorouracil were purchased from Fox Chase Cancer Center Pharmacy; spirinolactone was purchased from MedChem Express (cat. HY-B0561).
Figure 3. Nucleotide excision repair deficiency sensitizes pancreatic cancer cells to LP-184.
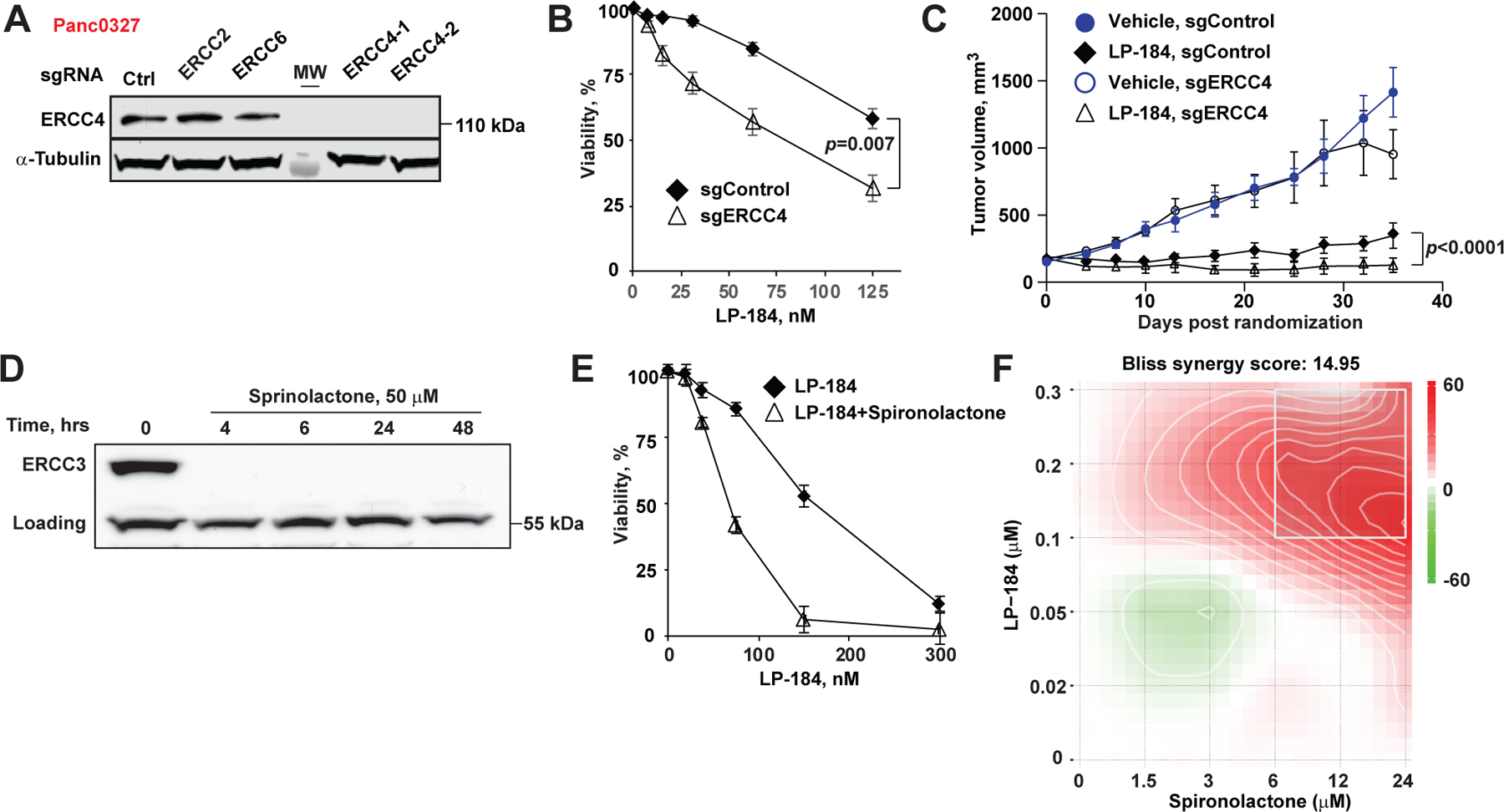
(A) Depletion of ERCC4 by CRISPRi in Panc 03.27 cells validation by Western blot. (B) Depletion of ERCC4 in Panc 03.27 cells sensitizes to LP-184 in vitro. (C) More effective growth inhibition of ERCC4-depleted Panc 03.27 xenografts by weekly intraperitoneal LP-184 at 3 mg/kg. (D) ERCC3 depletion in Capan 1 cells treated with spironolactone 50 µM in vitro. Equivalent concentration of DMSO was used as control. (E) Synergistic cytotoxicity of spironolactone and LP-184. (F) In vitro Bliss synergy score profile of spironolactone and LP-184 combinations.
Cell lines.
Pancreatic cancer cell lines Capan-1, Panc1, MiaPaCa2, Panc03.27, and Hs766t were acquired from the American Type Culture Collection and maintained at Fox Chase Cancer Center Cell Culture Facility as validated stocks. Cell lines reported in this paper were tested negative for mycoplasma after thawing and retested thereafter approximately bi-monthly with the Lonza MycoAlert® Mycoplasma Detection Kit #LT07-318. tested for mycoplasma. Additionally, prior to engraftment, cell lines used in xenograft studies were tested negative for pathogens- Corynebacterium bovis, Hepatitis A, Hepatitis B, Hepatitis C, HIV1, HIV2 and mycoplasma (Idexx Bioanalytics). Experiments were completed within approximately 12–15 passages in DMEM or RPMI supplemented with 10% FBS, 1% penicillin/streptomycin, and l-glutamine. Capan-1 or Panc0327 sgControl, sgPTGR1, and sgERCC4 cell lines were grown in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, l-glutamine and 2ug/mL puromycin.
Gene depletion with CRISPRi.
Single-guide RNAs were selected and cloned as previously described in [15] and [16]. Single-guide RNAs (sgRNAs) were synthesized using Integrated DNA Technologies. Lenti Crispri V2 plasmid was a gift from Ralph Francescone (Francesco, Vendramini-Costa, et al., Cancer Discovery 2021).
Antibodies.
Commercially available antibodies were ERCC3/XPB (Cell Signaling, cat.# 8746, RRID: AB_10940109); ERCC4/XPF (Bethyl, cat. A301-315A, RRID: RRID:AB_938089); alpha-tubulin (Cell Signaling, cat.# 3873, RRID:AB_1904178); NRF2/NFE2L2 (Proteintech, cat.# 16396-1-AP, RRID:AB_2782956); PTGR1 (Proteintech, cat.# 13374-1-AP, RRID: AB_2173213).
Cell viability assays.
Capan-1, CFPAC-1, Panc1, MiaPaCa2, Panc03.27, and Hs766t were treated in vitro with LP-184 in a 96 well format in triplicate across seven concentrations ranging from 15.625 nM to 1 µM over 4 days using Promega’s Cell Titer Blue or CellTiter-Glo reagent. Drug sensitivity was measured in terms of IC50 values generated from dose-response curves plotted in GraphPad Prism.
Ex vivo testing.
Patient derived PDAC xenografts were freshly excised, fragmented and treated with LP-184 in a 96 well format in triplicate well across 9 concentrations ranging from 5.5 nM to 36.45 uM over 5 days. Viable cells were metabolically labeled with CellTracker Green, and DNA damage and proliferation were estimated using phosphorylated histone 2AX (pH2AX) and 5-ethynyl-2’-deoxyuridine uridine (EdU) incorporation, respectively. The results were normalized to the nuclei count (Hoechst stain). Drug sensitivity was measured in terms of IC50 values generated from dose-response curves plotted in GraphPad Prism.
RT-PCR.
To evaluate target gene expression, total RNA was extracted using an RNeasy Mini Kit (#74104, Qiagen). RNA was reverse-transcribed (RT) using Moloney murine leukemia virus (MMLV) reverse transcriptase (#28025013, Ambion) and a mixture of anchored proteins. oligo-dT and random decamers (IDT). Two reverse-transcription reactions were performed for each sample using either 100 or 25ng of input RNA in a final volume of 50 ml. Taqman or SYBR Green assays were used in combination with the Life Technologies Universal Master Mix and run on a 7900 HT sequence detection system (Life Technologies). Cycling conditions were 95C for 15 min, followed by 40 (two-step) cycles (95C for 15s; 60C for 60s). The cycle threshold (Ct) values were converted to quantities (in arbitrary units) using a standard curve (five points, four-fold dilutions) established with a calibrator sample.
Western blot.
For Western blot analysis, dispersed tissue or cultured cells were homogenized in T-Per (Thermo Fisher Scientific) or RIPA buffer (Santa Cruz Biotechnology) with phosphatase and protease inhibitors (#1862495, #1861278, Thermo Fisher Scientific) on ice and cleared by centrifugation. The protein concentration was measured using a Pierce BCA Protein Assay Kit (#23225, Thermo Fisher Scientific). Proteins were separated on 4–12% Bis-Tris Protein gels (Invitrogen) and then horizontally transferred to Immobilon-FL PVDF membranes (#IPFL00010, Millipore). For detection of phospho-H2AX in cell lysates, Panc03.27 cells were plated to 6-well plates and 8-well slide chambers in full growth media and treated on the following day with vehicle (0.5% DMSO), LP-184 0.5 µM or LP-284 1 µM for 24 hours.
Immunofluorescent labeling of phosphorylated histone H2AX.
Panc03.27 cells were plated to 8-well slide chambers in full growth media and treated on the following day with vehicle (0.5% DMSO), LP-184 0.5 µM or LP-284 1 µM for 24 hours. After 2 washes with PBS, cells were fixed with 4% PFA in PBS for 15 minutes at room temperature, permeabilized with 1% Triton X-100 for 10 minutes at room temperature, blocked for one hour with 3% BSA in PBS, then stained with pH2ax primary antibody overnight at +4ºC. The following day, cells were washed and incubated with secondary antibody for one hour at room temperature, with one well serving as a secondary-only negative control. During the final washing steps, cells were incubated with AlexaFluor 488 phalloidin (Invitrogen, Cat. A12379); mounting was accomplished with Prolong Gold antifade reagent with DAPI (Invitrogen, Cat. P36935). Images were acquired on a Nikon Eclipse Ti2 confocal microscope with the Z-stack setting. Representative areas were selected by the blue (DAPI) channel only. Image analysis was performed using Fiji (doi:10.1038/nmeth.2019). After automatic thresholding was applied, the integrated density of the TRITC channel (561 nM) was measured and normalized to the number of nuclei in the DAPI channel (405 nM).
Drug synergy testing.
cells were seeded in 96 well plates on day 1. On day 2, the indicated drugs and/or vehicle were added, and the cells were incubated at 37C for 72 h. Spironolactone was added and incubated for 30 min before the addition of LP-184. All other drugs were added at approximately the same time as the LP-184. After 72 h of incubation, cell viability was assayed using Cell Titer Glo. Synergy was calculated using SynergyFinder 3.0.[17].
Tumor xenograft studies.
The animal studies were approved by the Institutional Animal Care & Use Committee of Fox Chase Cancer Center. Capan-1 cells transduced with CRISPRi lentiviral vectors carrying control sgRNA or PTGR1 sgRNA were injected into the flanks of SCID mice and allowed to grow to approximately 100 mm3, at which time they were assigned either a vehicle (normal saline/0.5% ethanol) or LP-184 3 mg/kg. Mice were administered weekly intraperitoneal injections of vehicle or drug for approximately eight weeks. For LP-184 and gemcitabine efficacy testing in Capan-1 cells, xenografts were established in the flanks of C.B-17.ICRscid mice. When they reached approximately 250 mm3, animals were randomized to receive either vehicle (normal saline/0.5% ethanol), gemcitabine 50 mg/kg, or LP-184 3 mg/kg administered weekly intraperitoneal injections for 4 weeks.
Panc03.27 cells were injected into the hind legs of NSG mice and allowed to reach a size of approximately 250 mm3 at which time animals were randomly assigned to receive either vehicle, vehicle plus RT, LP-184 3 mg/kg alone, or LP-184 with radiation for 4 weeks. Radiation was delivered once weekly for three weeks using an LA 45 Microtron (Top Grade Healthcare). The mice were anesthetized with isoflurane, placed in a prone position, and maintained on 3% isoflurane/oxygen for the duration of treatment. Radiation was delivered at a gantry angle of zero degrees and at a source-to-surface distance (SSD) of 100 cm, with the treatment consisting of a single dose of approximately 4 Gy localized to the tumors, two hours prior to an IP injection of vehicle or LP-184.
Patient-derived xenografts.
The dosing solutions of LP-184 were freshly prepared from powder material by dissolving in ethanol and then adding sterile saline (final concentrations of 5% ethanol and 95% saline). Patient-derived PC models CTG-1522 and CTG-1643 (Champions TumorGraft® models) were grown as xenografts in immunocompromised athymic nude mice (Nude-Foxn1nu) procured from Envigo. The mice were fed a Teklad-irradiated (sterilized) mouse diet and bedded with Teklad-irradiated (sterilized) corncob bedding. When sufficient stock animals reached 1000–1500 mm³, the tumors were harvested for re-implantation into the pre-study animals. Pre-study animals were implanted unilaterally on the left flank with tumor fragments harvested from stock animals. When tumors reached an average tumor volume of 150 – 300 mm³ animals were matched by tumor volume into treatment or control groups used for dosing and dosing initiated on day 0. The tumors were measured using a digital caliper for the duration of the study. Tumors were measured in two dimensions using calipers, and volume was calculated using the following formula: Tumor Volume (mm3) = w2 × l/2, where w = width and l = length (mm) of the tumor. In this study, the calipers were aligned to the tumor edges (the tumors were not squeezed with a caliper). The resulting tumors were monitored by caliper twice weekly. Animal weights were measured twice per week. Animal behavior was monitored daily. All the mice were maintained in isolated housing at constant temperature and humidity. For each model, animals were randomly divided into two groups (n = 6 in each group) and administered intravenously on days 0, 2, 4, 6, 8, 16, 18, 20, 22, and 24: (a) control group, 5% ethanol in saline, and (b) LP-184, 4 mg/kg in vehicle. Tumor volume and body weight were measured 3 times per weeks until study termination on day 52. Statistical analyses were performed using GraphPad Prism version 9. Data were processed for Two-Way ANOVA using Geisser-Greenhouse correction and Sidak’s post-hoc analysis for group comparisons.
Data Availability.
Cell line gene expression data were obtained from the Cancer Cell Line Encyclopedia (CCLE). The Pearson correlation coefficient between PTGR1 or ERCC8 expression in log2 array counts and LP-184 activity in terms of -log10 IC50[M], indicated in Figure 2A, was calculated using the Microsoft Excel function PEARSON. Associated statistics were run considering a significance level of 0.05 and two-tailed hypothesis testing, using the web tool provided in the link: https://www.socscistatistics.com/tests/studentttest/default2.aspx.
Figure 2. LP-184 inhibits growth of pancreatic adenocarcinoma xenografts carrying DNA repair pathway mutations in PTGR1-dependnet manner.
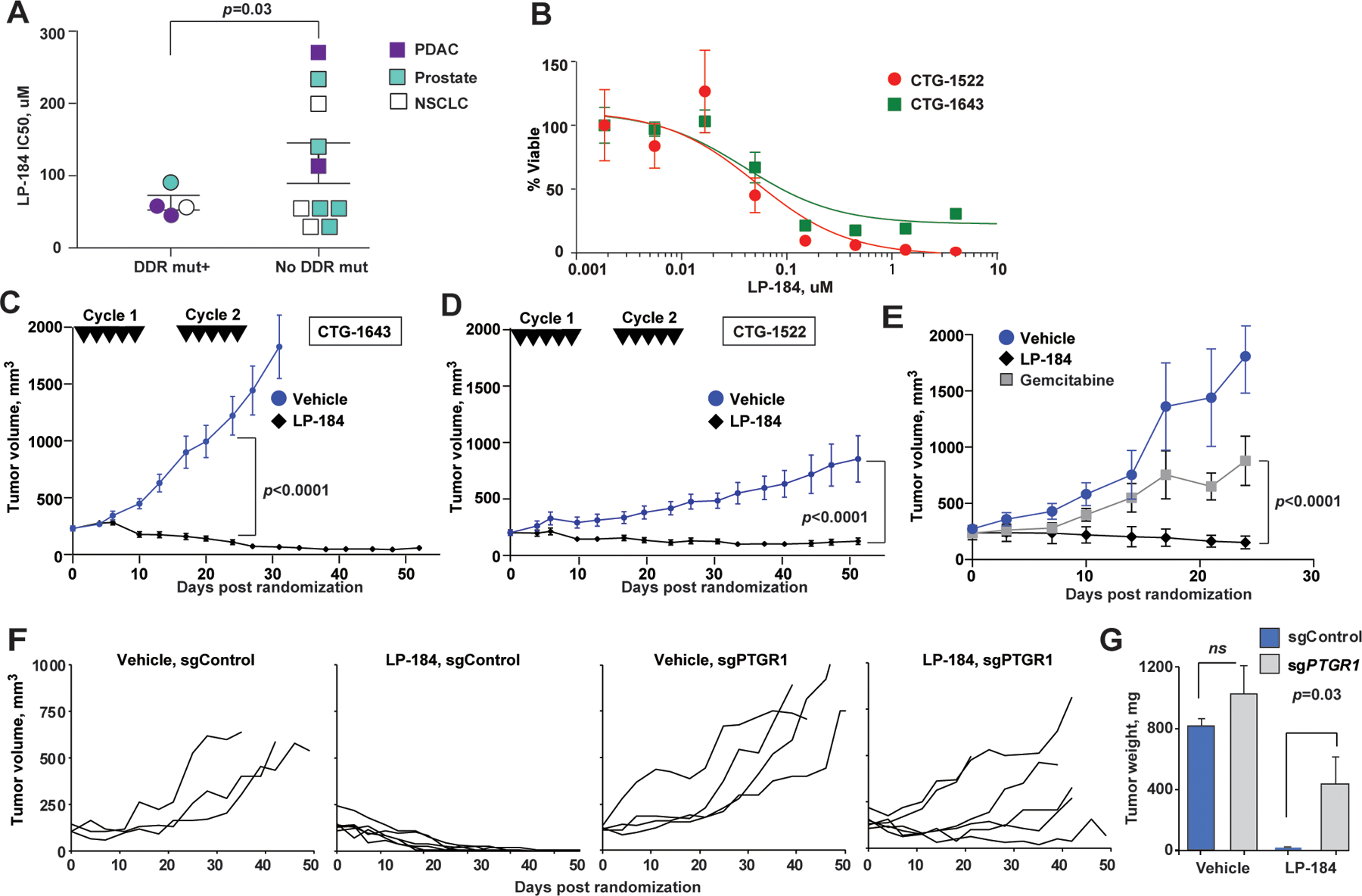
(A) Ex vivo LP-184 cytotoxicity in patient-derived xenografts correlates with presence of DDR pathway mutations. p=0.03, unpaired two-way t-test. (B) Representative LP-184 ex vivo cytotoxicity against PDX tumors of PDAC carrying pathogenic DDR pathway mutations (red circles, CTG-1522 carrying ATR I774fs; green squares, CTG-1643 carrying BRCA1 Q1460fs). (C, D) Regression of CTG-1643 (C) and CTG-1522 (D) xenografts following two cycles of LP-184 (each cycle consisted of 5 intravenous doses of LP-814 at 4 mg/kg on days 0, 2, 4, 6, 8, 16, 18, 20, 22, 24). (E) LP-184 (once weekly intraperitoneal doses of 3 mg/kg for 3 weeks) demonstrates superior efficacy compared to gemcitabine given at maximum tolerated dose (once weekly intraperitoneal dose of 50 mg/kg for 3 weeks). (F) Individual volumes of subcutaneous Capan-1 xenografts CRISPRi-depleted of PTGR1 (sgPTGR1) or sgControl-modified and treated with LP-184 at 3 mg/kg, or vehicle; p<0.0001, for tumor volume slopes of sgPTGR1 and sgControl. The schedule of intraperitoneal administration of LP-184 is shown in Figure S2B. All animals received 8–9 doses of LP-184. (G) Tumors weights at the end of experiment. p-values by unpaired two-way t-test. In C-F, linear mixed effects model was used to test differences in slopes of tumor volumes.
Analysis of PTGR1 expression and mutation association in TCGA tumor samples was done using data obtained via the TCGA Firehouse publicly available access. RNA-sequencing data were log-normalized (RSEM v2). Mutations data was downloaded with in R using R-TCGA Toolbox package. Silent mutations were removed, and remaining mutations were categorized as a binary mutant or non-mutant group for either NFE2L2 and KEAP1. TCGA barcodes were used to match mutation and log2 RSEM RNA-seq data. A total of 6767 tumor samples across 30 tumor types (TCGA Projects) retained in the final data resented in Fig. 4A.
Figure 4. Irradiation induces PTGR1 expression and sensitizes tumors to LP-184.
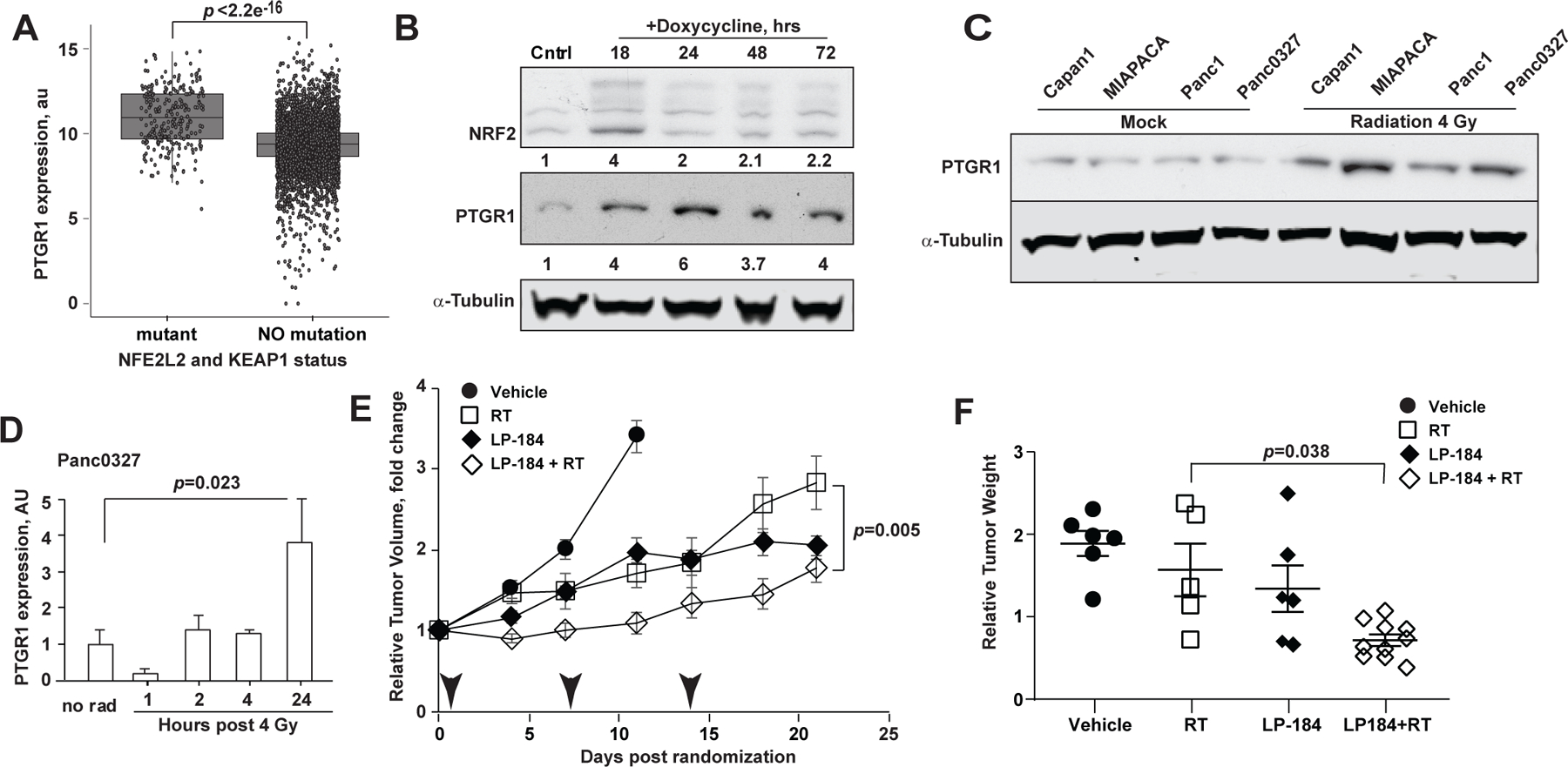
(A) Higher PTGR1 mRNA expression in KEAP1-mutated lung cancer cell lines. p-value, t-test. (B) Doxycycline-induced NRF2 expression is associated with increased PTGR1 in Panc1 cells as assessed by Western blot of total cell lysates collected at the indicated time points. (C) PTGR1 expression 2 hours following a single dose of 8 Gy of irradiation. Mock, cells were handled under the identical condition, except not irradiated. (D) Timing of PTGR1 expression in vivo in Panc 03.27 xenografts following a single dose of 4 Gy of irradiation. (E) Relative Tumor Volumes (RTV) normalized to the baseline of Panc 03.27 hind limb xenografts treated with 3 weekly intraperitoneal doses of LP-184 at 3 mg/kg or PBS vehicle, either alone, or in combination with 4 Gy of radiation (arrowheads). (F) Weights of xenografted tumors on Day 21 normalized to the initial pre-treatment estimated volumes; p-values: a linear mixed effects model was used to test difference in slopes of volume and the tumor weights.
Results.
PTGR1 is critical for cytotoxicity of a negative stereoisomer of LP-184.
LP-184 is a hydroxyurea methylacylfulvene derivative of irofulven, a previously clinically tested alkylating drug[18, 19]. We synthesized negative and positive enantiomers of hydroxyurea methylacylfulvene using chiral chromatography[20] further referred to as LP-184 and LP-284, respectively (Fig. 1A). Testing the cytotoxic activity of LP-184 and LP-284 against a panel of NCI-60 cancer cell lines in vitro demonstrated a strong correlation between LP-184 cytotoxicity and the expression of PTGR1 across a broad spectrum of epithelial cancer cell lines (Fig. 1B, Table S1).
Figure 1. LP-184 cytotoxicity is dependent on prostaglandin reductase 1 (PTGR1).
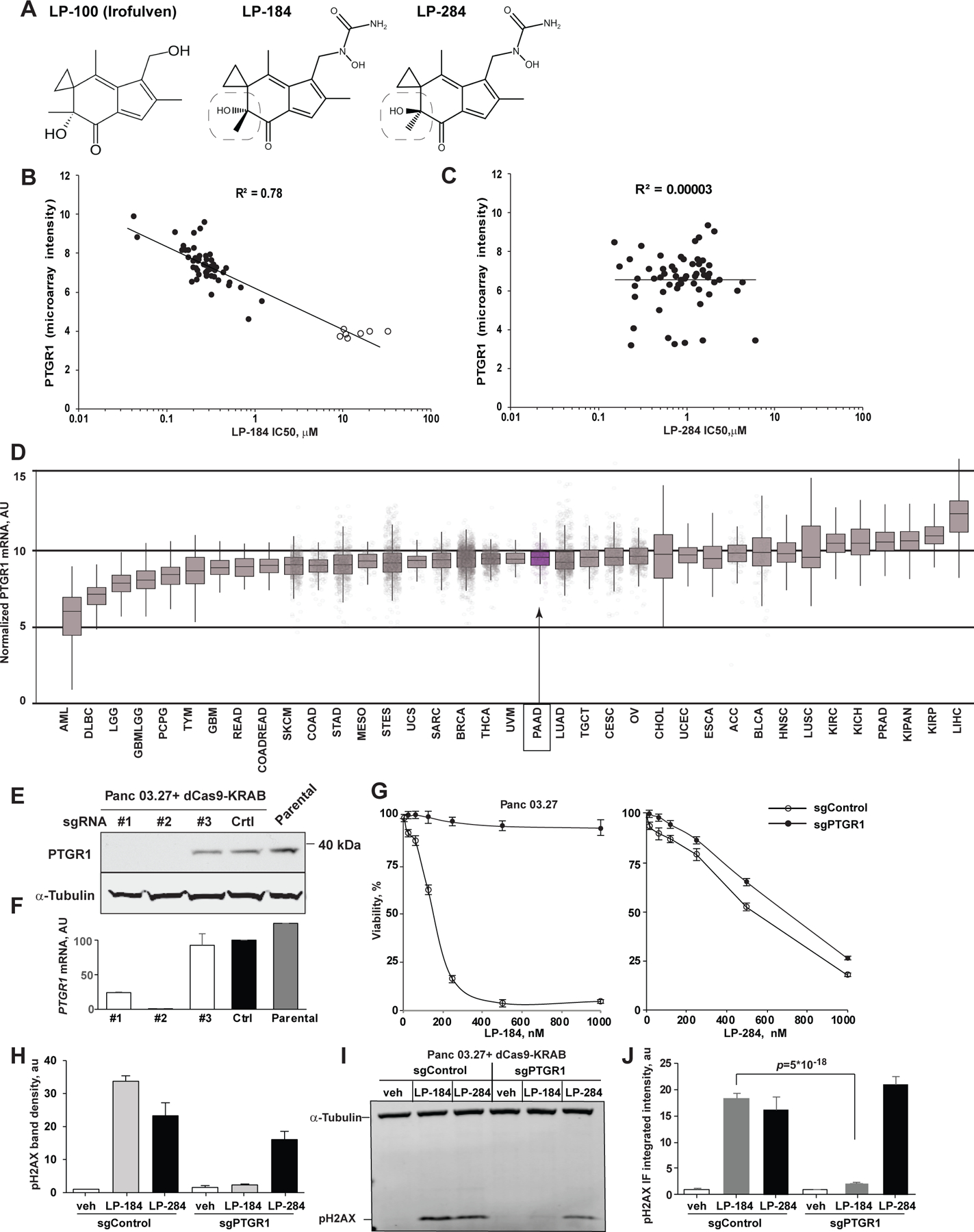
(A) Chemical structure of irofulven and its derivative stereoisomers LP-184 and LP-284. Box highlights the critical isomerism of the hydroxyl moiety. (B, C) Correlation of PTGR1 expression and cytotoxicity (IC50, µM) of LP-184 (B) and LP-284 (C) tested in vitro against a panel of NCI-60 cell lines. See also Table S1. Leukemic cell lines with low expression of PTGR1 are colored in red. (D) Normalized (RSEM) expression of PTGR1 mRNA in human cancers (Pan-Cancer Atlas, TCGA). Note lower expression in hematological malignancies (e.g., AML, DLBCL). Purple, pancreatic adenocarcinoma (PAAD). (E, F) PTGR1 depletion with CRISPRi targeting PTGR1 promoter in Panc 03.27 by Western blot (E) and qRT-PCR (F) cell lines. (G) LP-184 exhibits cytotoxicity in strict dependency on PTGR1 expression in Panc 03.27 cells, whereas cytotoxicity of LP-284 stereoisomer is independent of the PTGR1 status. Shown are averaged results of 3 independent repeats; error bars, standard deviations. (H, I) Induction of DNA damage as assessed by phosphorylated form of histone H2AX in Panc 03.27 cells treated with LP-184 (24 hours at 0.5 µM) or its stereoisomer LP-284 (24 hours at 1 µM). Note absence of pH2AX expression in sgPTGR1-depleted cells. Shown, pH2AX bands density normalized to alpha-tubulin in two repeats of Western blot (H) and a representative Western blot membrane (I). (J) Immunofluorecent detection of pH2AX (integrated intensity) in Panc 03.27 cells modified with non-targeting sgRNA (sgControl) or depleted of PTGR1 and treated with LP-184 and LP-284 as in H. See also Supplementary Figure S1.
However, its stereoisomer LP-284 showed no correlation with PTGR1 expression, supporting the possibility that LP-184 is activated from a prodrug to a highly reactive DNA alkylator[14] by PTGR1 oxidoreductase. The dependency LP-184 cytotoxicity on PTGR1 is further supported by a higher activity of LP-184 in cell lines derived from solid tumors, whereas low cytotoxicity in leukemic cell lines correlates with low levels of PTGR1 (Fig. 1B). Notably, such a pattern of higher PTGR1 expression is carcinomas is observed in human Pan-Cancer Atlas (Fig. 1D, S1A). To validate the dependency of LP-184 on PTGR1 expression, we depleted PTGR1 in two pancreatic adenocarcinoma cell lines using the CRISPRi approach[15]. PTGR1 protein and transcript levels were undetectable in both the Panc 03.27 (Fig. 1E, F) and Capan-1 (Fig. S2B–C) cell lines. Consistently, in both cell lines, loss of PTGR1 expression resulted reduced sensitivity to LP-184 even at concentrations as high as 1 µM. In contrast, the cytotoxicity of the positive stereoisomer LP-284 was unaffected by PTGR1 depletion in both Panc 03.27 and Capan-1 cells (Fig. 1G, S1D). A strict dependency of LP-184 on the catalytic conversion to an active DNA-damaging alkylating agent by PTGR1 was further validated by induction of phosphorylated form of histone H2AX only in PTGR1-proficient cells, but not in PTGR1-null cells, whereas PTGR1-depleted Panc 03.27 cells treated with LP-284 showed robust pH2AX expression (Fig. 1H–J).
LP-184 exhibit greater anti-tumor activity in the presence of DNA damage repair mutations.
We next tested LP-184 activity in patient-derived xenograft models characterized by a broad range of pathogenic mutations in DNA damage repair (DDR) genes. Notably, in ex vivo 3D tumor tissue cultures of PDAC, prostate, and non-small cell lung cancers, we found that the half-maximal inhibitory concentration (IC50) of LP-184 was significantly lower in PDX models carrying pathogenic mutations in the DDR genes (Fig. 2A, and Table S2).
Further detailed ex vivo testing of PDAC models CTG-1522 (ATR I774fs) and CTG-1643 (BRCA1 Q1460fs), both of which harbor pathogenic DDR mutations, showed a high LP-184 sensitivity below 50 nM (Fig. 2B, S2A). We observed rapid and sustained regression of established PDX tumors implanted into scid mice following two cycles of treatment (Fig. 2C,D). The activity of LP-184 was markedly superior to that of gemcitabine (Fig. 2E) when tested against established subcutaneous xenografts of Capan-1 cells carrying a pathogenic mutation in BRCA2 (S1982Rfs*22). To discern the contribution of PTGR1 expression to LP-184 antitumor activity in vivo, we treated the C-B17.scid mice carrying established subcutaneous Capan-1 xenografts with weekly intraperitoneal injections of LP-184 at 3 mg/kg (Fig. S2B,C) and demonstrated regression of PTGR1-competent tumors (sgControl, n=5, Fig. 2F), whereas five out of six sgPTGR1-depleted tumors progressed on therapy. At study termination, the tumor growth inhibition (TGI) reached 109%, and in 3 out of 5 LP-184 treated sgControl mice, tumors were not measurable, while 2 remaining mice had residual tumors 10% of the original tumor volume (Fig. 2G).
Development of synergistic targeting of DDR pathway for LP-184 combinations.
The transcription-coupled nucleotide excision repair (TC-NER) pathway has been implicated in the repair of DNA lesions caused by alkylating agents such as acylfulvenes and LP-184[21]. To prove this relationship in PDAC, we introduced partial deficiency in the TC-NER pathway by depleting ERCC4 in Panc 03.27 cells using CRIPSRi (Fig. 3A). Depletion of ERCC4 in Panc 03.27 cells increased in vitro LP-184 cytotoxicity by nearly 2-fold (Fig. 3B) and increased the sensitivity of ERCC4-depleted Panc 03.27 xenografts to LP-184 treatment (Fig. 3C). These findings may be relevant to a rare subset of PDAC with deleterious mutations in TC-NER (estimated 5.5% in TCGA, source: cbioportal.org, link: https://bit.ly/3Vr298w), or to PDAC with low expression of TC-NER pathway components. Depletion of ERCC3, a critical helicase in the TFIIH complex, can be achieved in vitro using the common aldosterone antagonist spironolactone[22, 23]. Indeed, in vitro spironolactone treatment of Capan-1 cells induced a rapid and sustained depletion of ERCC3 (Fig. 3D). Notably, while spironolactone had no appreciable effect on the viability of multiple PDAC cell lines, the combination of spironolactone and LP-184 resulted in synergistic cytotoxicity, as estimated by the Bliss score (Fig. 3E–F, Table 1).
Table 1.
Testing LP-184 combinations for synergy
| LP-184 combinations (mean Bliiss +/− SD) | ||||||
|---|---|---|---|---|---|---|
| Cell line | Gemcitabine | Oxaliplatin | Irinotecan | 5FU | Spironolactone | Triptolide* |
| Capan-1 | 9.32 +/− 3.041 | 6.91 +/− 1.557 | 8.84 +/− 1.211 | 3.58 +/− 1.998 | 11.57 +/− 0.485 | 1.46 +/= 0.20 |
| Panc03.27 | 1.3 +/− 0.25 | 3.98 +/− 0.86 | 7.05 +/− 0.45 | −2.12 +/− 1.54 | 14.95 +/− 1.17 | 1.26 +/− 0.04 |
| Hs766t | 2.31 +/− 2.92 | 7.32 +/− 9.44 | 5.92 +/− 2.70 | −3.93 +/− 1.61 | 13.83 +/− 5.88 | ND |
Bliss method: < −10: likely to be antagonistic; −10 to 10: likely to be additive; >10: likely to be synergistic.
by Chou-Talalay method: <1: likely to be synergistic; =1: likely to be additive; >1: likely to be antagonistic.
We next tested combinations of LP-184, which is widely used in PDAC chemotherapy agents such as gemcitabine, 5-FU, irinotecan, oxaliplatin, in three PDAC cell lines. These cell lines represent the typical pattern of PDAC mutations: Capan-1 (KRAS G12V, TP53 A159V, BRCA2 S1982Rfs*22), Hs766T (KRAS G61H, SMAD4 null), and Panc 03.27 (KRAS G12V, TP53 c.375+5G>T). The overall Bliss synergy scores computed from in vitro cell viability data are presented in Table 1. Across the board, we observed high Bliss synergy scores (score > 10 indicates synergy) for the combination of LP-184 with ERCC3 degrader spironolactone, whereas combinations of LP-184 with other chemotherapeutic drugs showed only an additive effect. Gemcitabine showed synergy with LP-184 in HR-deficient Capan-1 cells.
Radiation-induced expression of PTGR1 synergizes with LP-184.
We next investigated mechanisms to induce higher expression of PTGR1 in tumors that could be poised for synergy with LP-184. We determined that PTGR1 expression in human cancers positively correlated with activating mutations in the NRF2 pathway (mutations in NFE2L2 or KEAP1) were associated with high PTGR1 RNA expression (Fig. 4A) in keeping with previous reports of transcriptional regulation of PTGR1[24, 25] in the context of an antioxidant response. Notably, NRF2-induced genes have been shown to neutralize radiation-induced free radicals and to confer radiation resistance in tumors[26, 27].
Radiation can thus provide an opportunity to selectively increase PTGR1 expression and the linked LP-184 anti-tumor cytotoxicity in irradiated tumors. Based on this rationale, doxycycline-induced NRF2 expression in pancreatic cancer cell lines was associated with increased PTGR1 expression (Fig. 4B). We next established that a single dose of 8 Gy radiation of PDAC cells cultured in vitro induced PTGR1 expression approximately 4-fold in all four tested PDAC cell lines (Fig. 4C). In vivo-irradiated Panc 03.27 tumors showed increased PTGR1 expression, peaking at 24 h (Fig. 4D). To validate the predicted impact of RT pre-treatment on pancreatic tumor response to subsequent LP-184 treatment in vivo, we established Panc03.27 xenografts in the hind limbs of scid mice[28, 29] and treated the animals with three weekly doses of 4 Gy radiation. Radiation was delivered 2 h before an intraperitoneal dose of LP-184 (3 mg/kg weekly). At the end of the experiment on day 21, the LP-184 + RT group showed statistically significant differences in mean tumor volume and weight relative to the RT alone groups (Fig. 4E,F). The tumor weights normalized to the initial volume was lower in LP-184 + RT animals than in the RT alone animals (p= 0.03), although the differences between LP-184 alone and the LP-184/RT combination group did not reach statistical significance. These results demonstrate that the combination of LP-184 and radiation can be used to treat localized pancreatic tumors, and the regimen can be further optimized to achieve complete tumor regression.
Discussion.
Chemically, LP-184 is an N-hydroxy-N-urea derivative of methylacylfulvene, with a molecularly defined mechanism of action. LP-184 has a favorable pharmacokinetic profile and a broader therapeutic window than its predecessor acylfulvenes related to the mushroom Illudin S. As LP-184 has been granted Orphan Drug Designation by the U.S. Food and Drug Administration for the treatment of pancreatic cancer, malignant gliomas, and atypical teratoid rhabdoid tumors, the presented work illustrates the unique features and clinically relevant biomarkers for this innovative drug and its dependency on PTGR1 for enzymatic activation. Remarkably, a stereoisomer LP-284 showed no dependence on PTGR1 for cytotoxicity. Elimination of PTGR1 using CRISPRi in PDAC models markedly reduced the cancer cell sensitivity to LP-184 treatment in vitro (Fig. 1E–J) and in vivo (Fig. 2F,G), although we could not rule out a possible bystander effect of PTGR1-positive non-cancer cells resulting in slower growth of LP-184-treated PTGR1-null cancer cells, This dependency implicates the possibility that LP-184 can be used as a targeted therapy in situations where the PTGR1 levels are above the threshold levels in the tumor but relatively lower in the surrounding healthy tissue. This pattern naturally occurs in pancreatic, lung, prostate, ovarian, thyroid, and liver cancers, based on analyses of Pan-Cancer Atlas data (Fig. 1D).
Acylfulvenes create alkyl DNA adduct in the minor groove of DNA which are repaired primarily by TC-NER[30], although evidence of double strand breaks in irofulven-treated cells suggest that the HR and NHEJ pathways may also have a role in repairing DNA damage caused by acylfulvene class molecules[31, 32]. Deficiency in either the TC-NER or HR pathways, either via knockout of key proteins or BRCA1 mutation in the PDX model of PDAC CTG-1643, significantly enhanced the sensitivity to LP-184 (Fig. 2). Depletion of ERCC4/XPF in Panc 03.27 cells resulted in a two-fold increase in sensitivity to LP-184 and regression of LP-184 treated ERCC4 depleted Panc03.27 tumor xenografts. It will be of interest to investigate in the clinical trial setting whether low expression of the core TC-NER pathway components sensitize tumors to LP-184 even in the absence of pathogenic TC-NER mutations. Our results also provide the rationale, based on the synthetic lethality of established mutations with LP-184, for the selection of tumors to likely benefit from the drug. Here, we demonstrated the role of PTGR1 gene expression and DDR mutation patterns as determinants of tumor responsiveness to LP-184. Analysis of TCGA data suggests that ~40% of pancreatic cancer patients have elevated PTGR1 transcript levels (above PanCancer median, Fig. S1A), and approximately one-third carry deleterious DDR mutations based on a panel of 135 well-known DDR genes: 3–5% of tumors are positive for mutations in the TC-NER pathway genes, and an additional 15% are positive for HR pathway mutations. These selected mutations in conjunction with PTGR1 expression will enrich PDAC and other solid tumors with the greatest potential for therapeutic benefit from LP-184 as a single agent in upcoming Phase I clinical trials.
Although molecularly defined biomarkers are clinically important, the vast majority of PDAC and other solid cancers do not carry mutations in the DDR pathway. Thus, the development of synergistic therapeutic combinations could be a path to the broader clinical application of LP-184. Our findings illustrate the deregulation of ERCC3, a critical helicase in the NER pathway, as a strategy to induce synthetic lethality with a combination of spironolactone and LP-184 (Fig. 3). Another approach we tested was to precisely induce PTGR1 expression in the tumor through the effect of ionizing radiation (Fig. 4). This favorable combinatorial effect may represent an opportunity to develop LP-184 as a selective tumor radiosensitizer via the direct effect of radiation on PTGR1 expression. It will be of interest in the future studies to determine if tumors with activating mutations in the KEAP-NRF2 pathway could more susceptible to LP-184 cytotoxicity by virtue of elevated expression of PTGR1. The synergistic combination approaches of LP-184 with spironolactone and radiation will require further optimization in vivo for clinical translation. On the basis of our findings, we anticipate that LP-184 will extend therapeutic opportunities to a large subset of patients with genetically defined PDAC.
Supplementary Material
Acknowledgements:
This work was supported by NIH core grant CA06927, by the Pew Charitable Fund, and by a generous gift from Mrs. Concetta Greenberg to the M&C Greenberg Pancreatic Cancer Institute at Fox Chase Cancer Center. Some of the authors were supported by NIH R01 CA188430, R21 CA164205, R21 CA231252 (I.A.).
Footnotes
Conflict of interest statement: IA and DR received research funding from Lantern Pharma. AK, CS, JM, UK, KB and PS are employees or consultants of Lantern Pharma.
References:
- 1.Islami F, Ward EM, Sung H, Cronin KA, Tangka FKL, Sherman RL, Zhao J, Anderson RN, Henley SJ, Yabroff KR, Jemal A and Benard VB, Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J Natl Cancer Inst, 2021, https://www.ncbi.nlm.nih.gov/pubmed/34240195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE and Jemal A, Cancer Statistics, 2021. CA Cancer J Clin, 2021. 71(1): p. 7–33, https://www.ncbi.nlm.nih.gov/pubmed/33433946. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Wehner MR, Matrisian LM and Nead KT, Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw Open, 2021. 4(4): p. e214708, https://www.ncbi.nlm.nih.gov/pubmed/33825840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, U. Groupe Tumeurs Digestives of, and P. Intergroup, FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med, 2011. 364(19): p. 1817–25, https://www.ncbi.nlm.nih.gov/pubmed/21561347. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, and Renschler MF, Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med, 2013. 369(18): p. 1691–703, https://www.ncbi.nlm.nih.gov/pubmed/24131140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, and Petricoin EF 3rd, Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol, 2020. 21(4): p. 508–518, https://www.ncbi.nlm.nih.gov/pubmed/32135080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network. Electronic address, a.a.d.h.e. and N. Cancer Genome Atlas Research, Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell, 2017. 32(2): p. 185–203 e13, https://www.ncbi.nlm.nih.gov/pubmed/28810144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algul H, O’Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, and Kindler HL, Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med, 2019. 381(4): p. 317–327, https://www.ncbi.nlm.nih.gov/pubmed/31157963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staake MD, Kashinatham A, McMorris TC, Estes LA and Kelner MJ, Hydroxyurea derivatives of irofulven with improved antitumor efficacy. Bioorg Med Chem Lett, 2016. 26(7): p. 1836–8, https://www.ncbi.nlm.nih.gov/pubmed/26922141. [DOI] [PubMed] [Google Scholar]
- 10.Gregory J. Tobin, S.T.B., Malakhov Andrey D., Archer Varsin A., Illudin analogs, uses thereof, and methods for synthesizing the same, in US 2021/0198191A1 2019, Lantern Pharma: USA, https://patents.google.com/patent/US20210198191A1/ [Google Scholar]
- 11.Gong J, Vaidyanathan VG, Yu X, Kensler TW, Peterson LA and Sturla SJ, Depurinating acylfulvene-DNA adducts: characterizing cellular chemical reactions of a selective antitumor agent. J Am Chem Soc, 2007. 129(7): p. 2101–11, https://www.ncbi.nlm.nih.gov/pubmed/17256933. [DOI] [PubMed] [Google Scholar]
- 12.Kathad U, Kulkarni A, McDermott JR, Wegner J, Carr P, Biyani N, Modali R, Richard JP, Sharma P and Bhatia K, A machine learning-based gene signature of response to the novel alkylating agent LP-184 distinguishes its potential tumor indications. BMC Bioinformatics, 2021. 22(1): p. 102, https://www.ncbi.nlm.nih.gov/pubmed/33653269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni A, McDermott JR, Kathad U, Modali R, Richard JP, Sharma P and Bhatia K, The acylfulvene alkylating agent, LP-184, retains nanomolar potency in non-small cell lung cancer carrying otherwise therapy-refractory mutations. Oncotarget, 2021. 12(8): p. 791–806, https://www.ncbi.nlm.nih.gov/pubmed/33889302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu X, Erzinger MM, Pietsch KE, Cervoni-Curet FN, Whang J, Niederhuber J and Sturla SJ, Up-regulation of human prostaglandin reductase 1 improves the efficacy of hydroxymethylacylfulvene, an antitumor chemotherapeutic agent. J Pharmacol Exp Ther, 2012. 343(2): p. 426–33, https://www.ncbi.nlm.nih.gov/pubmed/22895897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP and Lim WA, Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell, 2013. 152(5): p. 1173–83, https://www.ncbi.nlm.nih.gov/pubmed/23452860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG and Zhang F, Genome-scale CRISPR-Cas9 knockout screening in human cells. Science, 2014. 343(6166): p. 84–87, https://www.ncbi.nlm.nih.gov/pubmed/24336571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ianevski A, Giri AK and Aittokallio T, SynergyFinder 3.0: an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res, 2022. 50(W1): p. W739–43, https://www.ncbi.nlm.nih.gov/pubmed/35580060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schilder RJ, Blessing JA, Shahin MS, Miller DS, Tewari KS, Muller CY, Warshal DP, McMeekin S and Rotmensch J, A phase 2 evaluation of irofulven as second-line treatment of recurrent or persistent intermediately platinum-sensitive ovarian or primary peritoneal cancer: a Gynecologic Oncology Group trial. Int J Gynecol Cancer, 2010. 20(7): p. 1137–41, https://www.ncbi.nlm.nih.gov/pubmed/21495215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo W, Boyer M, Chung HC, Ong SY, Lim R, Zee B, Ma B, Lam KC, Mo FK, Ng EK, Ho R, Clarke S, Roh JK, Beale P, Rha SY, Jeung HC, Soo R, Goh BC, Chan AT, and Cancer Therapeutics Research G, Irofulven as first line therapy in recurrent or metastatic gastric cancer: a phase II multicenter study by the Cancer Therapeutics Research Group (CTRG). Cancer Chemother Pharmacol, 2007. 59(3): p. 295–300, https://www.ncbi.nlm.nih.gov/pubmed/16783579. [DOI] [PubMed] [Google Scholar]
- 20.Gong J, Neels JF, Yu X, Kensler TW, Peterson LA and Sturla SJ, Investigating the role of stereochemistry in the activity of anticancer acylfulvenes: synthesis, reductase-mediated bioactivation, and cellular toxicity. J Med Chem, 2006. 49(8): p. 2593–9, https://www.ncbi.nlm.nih.gov/pubmed/16610802. [DOI] [PubMed] [Google Scholar]
- 21.Borcsok J, Sztupinszki Z, Bekele R, Gao SP, Diossy M, Samant AS, Dillon KM, Tisza V, Spisak S, Rusz O, Csabai I, Pappot H, Frazier ZJ, Konieczkowski DJ, Liu D, Vasani N, Rodrigues JA, Solit DB, Hoffman-Censits JH, Plimack ER, Rosenberg JE, Lazaro JB, Taplin ME, Iyer G, Brunak S, Lozsa R, Van Allen EM, Szuts D, Mouw KW, and Szallasi Z, Identification of a Synthetic Lethal Relationship between Nucleotide Excision Repair Deficiency and Irofulven Sensitivity in Urothelial Cancer. Clin Cancer Res, 2021. 27(7): p. 2011–2022, http://www.ncbi.nlm.nih.gov/pubmed/33208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alekseev S, Ayadi M, Brino L, Egly JM, Larsen AK and Coin F, A small molecule screen identifies an inhibitor of DNA repair inducing the degradation of TFIIH and the chemosensitization of tumor cells to platinum. Chem Biol, 2014. 21(3): p. 398–407, https://www.ncbi.nlm.nih.gov/pubmed/24508195. [DOI] [PubMed] [Google Scholar]
- 23.Xu D, Cao Q, Wang L, Wang J, Xu B, Attwood K, Wei L, Wu Y, Smith GJ, Katsuta E, Takabe K, Chatta G, Guru KA, Goodrich DW, and Li QJ, A Preclinical Study to Repurpose Spironolactone for Enhancing Chemotherapy Response in Bladder Cancer. Mol Cancer Ther, 2022. 21(5): p. 786–798, https://www.ncbi.nlm.nih.gov/pubmed/35247903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korytina GF, Akhmadishina LZ, Aznabaeva YG, Kochetova OV, Zagidullin NS, Kzhyshkowska JG, Zagidullin SZ and Viktorova TV, Associations of the NRF2/KEAP1 pathway and antioxidant defense gene polymorphisms with chronic obstructive pulmonary disease. Gene, 2019. 692: p. 102–112, https://www.ncbi.nlm.nih.gov/pubmed/30641209. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Rodriguez R, Torres-Mena JE, Quintanar-Jurado V, Chagoya-Hazas V, Rojas Del Castillo E, Del Pozo Yauner L, Villa-Trevino S and Perez-Carreon JI, Ptgr1 expression is regulated by NRF2 in rat hepatocarcinogenesis and promotes cell proliferation and resistance to oxidative stress. Free Radic Biol Med, 2017. 102: p. 87–99, https://www.ncbi.nlm.nih.gov/pubmed/27867096. [DOI] [PubMed] [Google Scholar]
- 26.Jeong Y, Hoang NT, Lovejoy A, Stehr H, Newman AM, Gentles AJ, Kong W, Truong D, Martin S, Chaudhuri A, Heiser D, Zhou L, Say C, Carter JN, Hiniker SM, Loo BW Jr., West RB, Beachy P, Alizadeh AA, and Diehn M, Role of KEAP1/NRF2 and TP53 Mutations in Lung Squamous Cell Carcinoma Development and Radiation Resistance. Cancer Discov, 2017. 7(1): p. 86–101, https://www.ncbi.nlm.nih.gov/pubmed/27663899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koppula P, Lei G, Zhang Y, Yan Y, Mao C, Kondiparthi L, Shi J, Liu X, Horbath A, Das M, Li W, Poyurovsky MV, Olszewski K, and Gan B, A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat Commun, 2022. 13(1): p. 2206, https://www.ncbi.nlm.nih.gov/pubmed/35459868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panetta JV, Cvetkovic D, Chen X, Chen L and Ma CC, Radiodynamic therapy using 15-MV radiation combined with 5-aminolevulinic acid and carbamide peroxide for prostate cancer in vivo. Phys Med Biol, 2020. 65(16): p. 165008, https://www.ncbi.nlm.nih.gov/pubmed/32464613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Wang B, Chen X, Cvetkovic D, Chen L, Lang J and Ma CM, Local Tumor Control and Normal Tissue Toxicity of Pulsed Low-Dose Rate Radiotherapy for Recurrent Lung Cancer: An In Vivo Animal Study. Dose Response, 2015. 13(2): p. 1559325815588507, https://www.ncbi.nlm.nih.gov/pubmed/26675811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaspers NG, Raams A, Kelner MJ, Ng JM, Yamashita YM, Takeda S, McMorris TC and Hoeijmakers JH, Anti-tumour compounds illudin S and Irofulven induce DNA lesions ignored by global repair and exclusively processed by transcription- and replication-coupled repair pathways. DNA Repair (Amst), 2002. 1(12): p. 1027–38, https://www.ncbi.nlm.nih.gov/pubmed/12531012. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Wiltshire T, Senft J, Wenger SL, Reed E and Wang W, Fanconi anemia D2 protein confers chemoresistance in response to the anticancer agent, irofulven. Mol Cancer Ther, 2006. 5(12): p. 3153–61, https://www.ncbi.nlm.nih.gov/pubmed/17172419. [DOI] [PubMed] [Google Scholar]
- 32.Wiltshire T, Senft J, Wang Y, Konat GW, Wenger SL, Reed E and Wang W, BRCA1 contributes to cell cycle arrest and chemoresistance in response to the anticancer agent irofulven. Mol Pharmacol, 2007. 71(4): p. 1051–60, https://www.ncbi.nlm.nih.gov/pubmed/17229870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cell line gene expression data were obtained from the Cancer Cell Line Encyclopedia (CCLE). The Pearson correlation coefficient between PTGR1 or ERCC8 expression in log2 array counts and LP-184 activity in terms of -log10 IC50[M], indicated in Figure 2A, was calculated using the Microsoft Excel function PEARSON. Associated statistics were run considering a significance level of 0.05 and two-tailed hypothesis testing, using the web tool provided in the link: https://www.socscistatistics.com/tests/studentttest/default2.aspx.
Figure 2. LP-184 inhibits growth of pancreatic adenocarcinoma xenografts carrying DNA repair pathway mutations in PTGR1-dependnet manner.

(A) Ex vivo LP-184 cytotoxicity in patient-derived xenografts correlates with presence of DDR pathway mutations. p=0.03, unpaired two-way t-test. (B) Representative LP-184 ex vivo cytotoxicity against PDX tumors of PDAC carrying pathogenic DDR pathway mutations (red circles, CTG-1522 carrying ATR I774fs; green squares, CTG-1643 carrying BRCA1 Q1460fs). (C, D) Regression of CTG-1643 (C) and CTG-1522 (D) xenografts following two cycles of LP-184 (each cycle consisted of 5 intravenous doses of LP-814 at 4 mg/kg on days 0, 2, 4, 6, 8, 16, 18, 20, 22, 24). (E) LP-184 (once weekly intraperitoneal doses of 3 mg/kg for 3 weeks) demonstrates superior efficacy compared to gemcitabine given at maximum tolerated dose (once weekly intraperitoneal dose of 50 mg/kg for 3 weeks). (F) Individual volumes of subcutaneous Capan-1 xenografts CRISPRi-depleted of PTGR1 (sgPTGR1) or sgControl-modified and treated with LP-184 at 3 mg/kg, or vehicle; p<0.0001, for tumor volume slopes of sgPTGR1 and sgControl. The schedule of intraperitoneal administration of LP-184 is shown in Figure S2B. All animals received 8–9 doses of LP-184. (G) Tumors weights at the end of experiment. p-values by unpaired two-way t-test. In C-F, linear mixed effects model was used to test differences in slopes of tumor volumes.
Analysis of PTGR1 expression and mutation association in TCGA tumor samples was done using data obtained via the TCGA Firehouse publicly available access. RNA-sequencing data were log-normalized (RSEM v2). Mutations data was downloaded with in R using R-TCGA Toolbox package. Silent mutations were removed, and remaining mutations were categorized as a binary mutant or non-mutant group for either NFE2L2 and KEAP1. TCGA barcodes were used to match mutation and log2 RSEM RNA-seq data. A total of 6767 tumor samples across 30 tumor types (TCGA Projects) retained in the final data resented in Fig. 4A.
Figure 4. Irradiation induces PTGR1 expression and sensitizes tumors to LP-184.

(A) Higher PTGR1 mRNA expression in KEAP1-mutated lung cancer cell lines. p-value, t-test. (B) Doxycycline-induced NRF2 expression is associated with increased PTGR1 in Panc1 cells as assessed by Western blot of total cell lysates collected at the indicated time points. (C) PTGR1 expression 2 hours following a single dose of 8 Gy of irradiation. Mock, cells were handled under the identical condition, except not irradiated. (D) Timing of PTGR1 expression in vivo in Panc 03.27 xenografts following a single dose of 4 Gy of irradiation. (E) Relative Tumor Volumes (RTV) normalized to the baseline of Panc 03.27 hind limb xenografts treated with 3 weekly intraperitoneal doses of LP-184 at 3 mg/kg or PBS vehicle, either alone, or in combination with 4 Gy of radiation (arrowheads). (F) Weights of xenografted tumors on Day 21 normalized to the initial pre-treatment estimated volumes; p-values: a linear mixed effects model was used to test difference in slopes of volume and the tumor weights.


