Abstract

The structure and mechanism of the bacterial enzyme β-lactamase have been well-studied due to its clinical role in antibiotic resistance. β-Lactamase is known to hydrolyze the β-lactam ring of the cephalosporin scaffold, allowing a spontaneous self-immolation to occur. Previously, cephalosporin-based sensors have been developed to evaluate β-lactamase expression in both mammalian cells and zebrafish embryos. Here, we present a circular caged morpholino oligonucleotide (cMO) activated by β-lactamase-mediated cleavage of a cephalosporin motif capable of silencing the expression of T-box transcription factor Ta (tbxta), also referred to as no tail a (ntla), eliciting a distinct, observable phenotype. We explore the use of β-lactamase to elicit a biological response in aquatic embryos for the first time and expand the utility of cephalosporin as a cleavable linker beyond targeting antibiotic-resistant bacteria. The addition of β-lactamase to the current suite of enzymatic triggers presents unique opportunities for robust, orthogonal control over endogenous gene expression in a spatially resolved manner.
Introduction
Nucleic acid-based tools have found widespread application in the study of gene function, e.g., as antisense agents, due to their ease of synthesis, programmability, and specificity.1−6 A drawback of oligonucleotides can be their susceptibility to nuclease-mediated degradation, which can be addressed through the incorporation of chemical modifications.1 Morpholino oligonucleotides (MOs) are one class of modified nucleic acid analogs, containing a six-membered morpholine ring in place of the ribose and a neutral phosphoramidite linkage within the backbone. Despite the structural differences from natural nucleic acids, MOs maintain their high specificity and affinity to hybridize to the corresponding target RNA sequences.7,8 MOs allow for gene silencing by blocking translation or mRNA splicing.9−12
MOs are frequently used to probe both maternal and zygotic gene function during embryonic development in zebrafish embryos, as delivery into a fertilized oocyte results in immediate and global gene silencing with effects persisting up to 5 days post-fertilization.13−15 The development of caged MOs (cMOs) that can be activated with external triggers has broadened the utility of these tools for analysis of dynamic cellular processes with precise spatial and temporal control. This is particularly important for investigations of gene function during later developmental stages or tissue-specific cellular processes, both of which are impeded by immediate, global silencing. Upon delivery, cMOs are functionally inert and unable to engage their target sequences until activated with an external trigger, imparting conditional control over MO-induced gene silencing.16 We and others have devised various MO caging strategies by incorporating light-,17−22 enzyme-,23 and more recently, small molecule-responsive elements24 within the MO structure. Multiple designs have resulted in successful caging and subsequent conditional control of silencing function, including the incorporation of cleavable groups onto nucleobases,18,19 short, complementary blocking oligonucleotides that form hairpins or duplexes,17,25−27 and linkers for macrocyclization.20−24,28−33 While all of these designs have demonstrated varying degrees of success in aquatic embryo systems, end-to-end macrocyclization has proven to be a highly modular approach that can be used with a wide range of immolative linkers34 and commercially available MOs bearing suitable reactive handles. The curvature induced by the cyclization (and more recently bicyclization)20 of the MO impedes target binding until a single cleavage event restores the active, linear species, which can then hybridize to its mRNA target and induce gene silencing.
Enzymatic triggers provide unique opportunities for conditional control that complement current light- and small molecule-activated cMOs. As an example, precise activation by light exposure can be complicated by the inherent structural complexity, opacity, and physical movement of individual cells within developing organisms. In the case of small molecule delivery, controlled activation of the MO can be limited by diffusion of the small molecule trigger. These shortcomings can be addressed through enzymatic activation, as the triggering enzyme can be expressed in specific cells and at controlled time points through the use of inducible or tissue-specific promoters.35,36 The Chen lab has demonstrated this previously using an Escherichia coli nitroreductase, Nfsb, to control the activity of a cyclic cMO tethered with a 4-nitrobenzyl linker in live zebrafish embryos.23 Additionally, the Dmochowski group has developed a caspase-3 activatable peptide nucleic acid (PNA)-based oligonucleotide sensor to probe apoptosis in cells.37 Taken together, these reports demonstrate the versatility of enzyme-mediated strategies that can be applied to nucleic acid-based tools.
Herein, we expand upon this previous work and demonstrate for the first time the use of the bacterial enzyme β-lactamase to control oligonucleotide function. The use of β-lactamase as a triggering enzyme is particularly appealing since the structure, mechanism, and substrate specificity are well-established due to the enzyme’s prominent role in antibiotic resistance.38,39 β-Lactamases also possess many other favorable characteristics, including stability, catalytic efficiency, and the fact that their enzymatic function is unique to bacterial systems. As a result of this biorthogonality to the mammalian cellular environment, β-lactamases have been used to spatially control the activity of various drugs in directed enzyme prodrug therapy (DEPT) strategies.38−42 Cephalosporin motifs have been previously functionalized for β-lactamase-mediated activation of prodrugs against antibiotic resistant bacterial strains43,44 and fluorescent sensors probing β-lactamase expression in mammalian cells and zebrafish embryos.45,46 Here, we leverage β-lactamase-mediated antibiotic resistance mechanisms to control MO silencing function in vivo by designing and synthesizing a cyclic cMO tethered by a cephalosporin-based linker that is efficiently cleaved via β-lactamase-mediated hydrolysis.
Results and Discussion
Upon β-lactamase catalysis, the core β-lactam ring of the linker is hydrolyzed, generating the corresponding amine, which then undergoes self-immolation and spontaneous elimination via the carbamate linkage. This elimination reaction releases the 5′-terminus of the MO to generate the active, linear MO capable of hybridizing to the mRNA target and subsequently inducing gene silencing (Figure 1).
Figure 1.
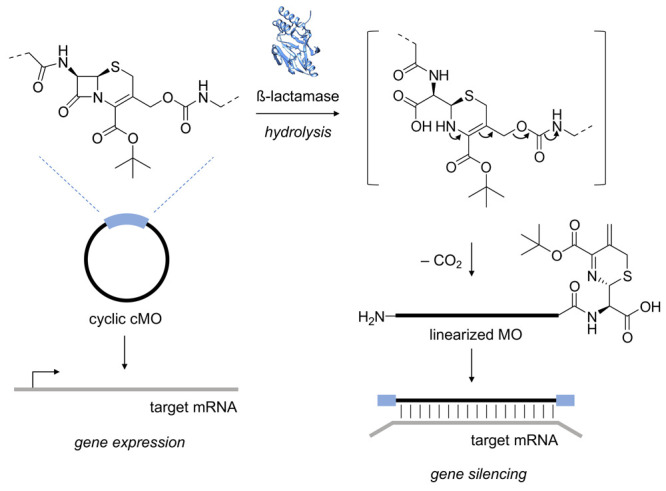
Following enzyme-mediated hydrolysis of the β-lactam ring by β-lactamase (PDB ID 4F6H), the resulting amine intermediate undergoes self-immolation. The release of the 5′-end generates the active, linearized MO capable of hybridizing to its respective target mRNA and silencing gene expression.
To synthesize the β-lactamase-activated cMO, the linker 8 was designed using a cephalosporin core scaffold. The C-3 and C-7 positions of the core cephalosporin scaffold were synthetically modified with orthogonal azido and chloroacetamide handles, respectively, necessary for bioconjugation to the terminally modified linear MO.39 The carbamate linkage was strategically installed at the C-3 position as it has been previously shown that leaving groups attached to the exocyclic methylene can undergo spontaneous elimination following hydrolysis of the β-lactam ring.47,48
The linker 8 was synthesized in four steps from commercially available 7-aminocephalosporanic acid (4) (Figure 2A). The tert-butyl ester 5 was obtained by treating 4 with tert-butyl acetate in the presence of p-toluenesulfonic acid (TsOH) and concentrated sulfuric acid.49 The chloroacetamide at the C-7 position was installed by reacting the free amine 5 with 2-chloroacetyl chloride to form the corresponding amide 6. Selective cleavage of the acetate at the C-3 position was achieved chemoenzymatically through lipase-mediated hydrolysis of 6 using the serine hydrolase Candida antarctica lipase B (CAL B) to generate the free alcohol 7.49 To install the azide handle, 3-azidopropan-1-amine (2) was synthesized from the corresponding alkyl chloride 1 as previously reported.50 The amine 2 was converted to the corresponding isocyanate 3 through treatment with diphosgene. The isocyanate 3 was then added to a solution of the alcohol 7 in the presence of dibutyltin dilaurate to generate the corresponding carbamate-containing cephalosporin linker 8. It should be noted that while the canonical cephalosporin scaffold contains a carboxylic acid moiety at the C-4 position, there have been no direct studies of enzyme reactivity with any synthetic C-4-protected analogs. To test that the bulky tert-butyl ester in 8 still presents a suitable enzyme substrate, the linker (1 mM) was incubated with recombinant β-lactamase (0.5 μg), and the reaction progression was monitored by LCMS (Supporting Figure S1). Gratifyingly, nearly full cleavage of the linker 8 was observed after 3 h, and the expected cleavage product was generated. These results suggest that the presence of the tert-butyl ester at the C-4 position does not impede enzymatic cleavage of the lactam ring. With knowledge that the linker can be cleaved by β-lactamase, we moved forward with the macrocyclization of our desired MO with the functionalized linker 8.
Figure 2.
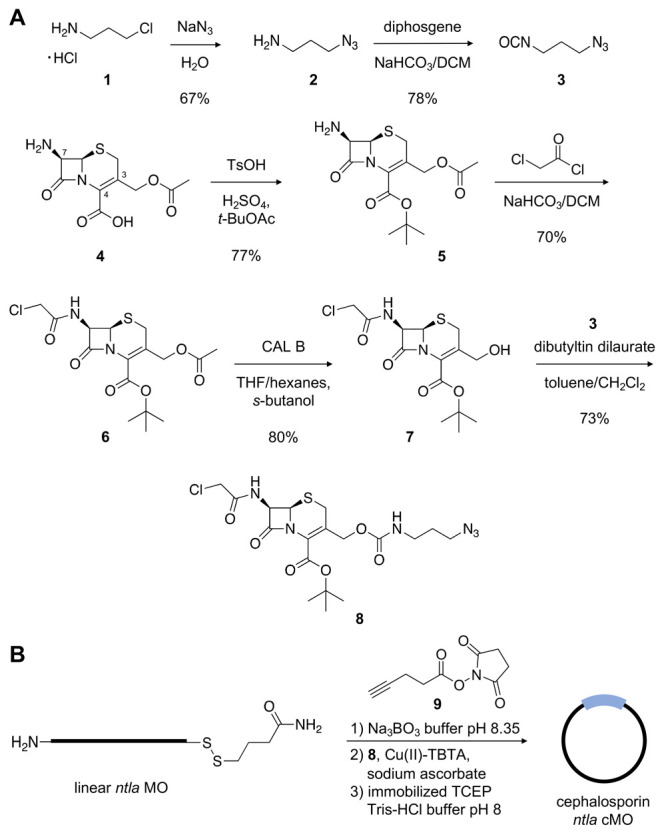
(A) Synthetic scheme of the cephalosporin-containing linker 8. (B) Synthesis of the cephalosporin ntla cMO.
A cyclic cephalosporin cMO targeting the T-box transcription factor Ta (tbxta), also known as no tail a (ntla, 5′-GACTTGAGGCAGCATATTTCCGAT-3′, anti-start codon underlined) was prepared in three steps using the synthesized linker 8 (Figure 2B). MO-mediated silencing of ntla during early embryogenesis induces an obvious and distinct morphant phenotype that includes truncation of the embryo tail and misshapen somites.51,52 Further, we and others have previously utilized the ntla MO as a model gene target for validating other conditionally activated cMO technologies.17,22−24,27−29,31 The ntla MO, containing 5′-amine and 3′-disulfide handles, was subjected to macrocyclization28,29 by first acylating the terminal amine with the NHS ester 9 to yield the corresponding alkyne suitable for bioconjugation to the azide-containing linker. The linker 8 was clicked to the alkyne-functionalized ntla MO via a copper(I)-catalyzed alkyne–azide cycloaddition (CuAAC).53 Lastly, the 3′-disulfide was reduced in the presence of resin-immobilized tris(2-carboxyethyl)phosphine (TCEP) to generate the free thiol, which then undergoes a spontaneous intramolecular cyclization via thioether formation with the chloroacetamide handle of 8 to generate the cyclic cephalosporin ntla cMO. Reaction progress for each step was analyzed via MALDI-TOF MS and purified by high-performance liquid chromatography (HPLC) (Supporting Figure S2). To ensure the removal of any remaining linear MO species, the final cMO product was subject to additional purification steps using iodoacetyl- and NHS-functionalized resins, as previously described.24
After confirmation of successful macrocyclization, we next sought to evaluate enzyme-mediated linearization and activation of the cephalosporin ntla cMO. Linearization was performed by incubating the cMO (20 μΜ) with recombinant β-lactamase for 3 h. We utilized a reticulocyte lysate-based translation system to analyze activation of the cMO.24 The consensus ntla binding sequence (ntlaBS) was cloned directly upstream of and in frame with the coding sequence of the firefly luciferase reporter gene. Hybridization of the MO to this target sequence blocks the translational machinery from accessing the start site, thereby repressing luciferase expression (Figure 3A). Following treatment with linear ntla MO (1 μM), luciferase expression is reduced by 50%, whereas treatment with the cyclic cephalosporin ntla cMO did not impact luciferase levels. However, MO silencing function is fully restored following linearization of the cyclic cMO with β-lactamase, as evidenced by a nearly 65% reduction of reporter gene expression (Figure 3B). Taken together, this data suggests that enzymatic catalysis by β-lactamase can be used as an efficient off to on switch to conditionally control MO gene silencing activity.
Figure 3.
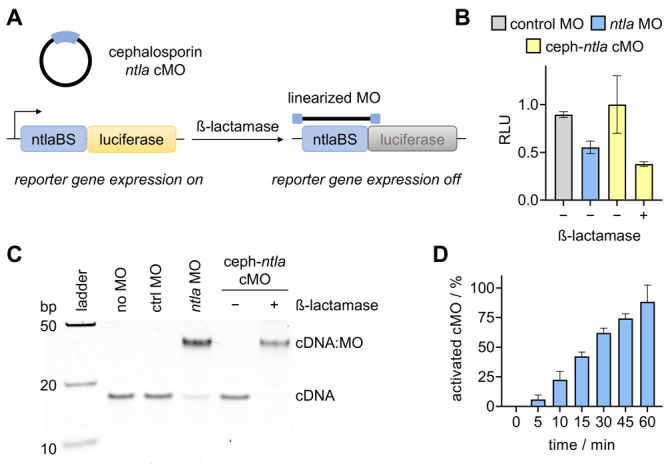
(A) Luciferase reporter assay in which luciferase expression is silenced in the presence of linearized MO. (B) Luminescence signal after treatment of the indicated MO. Data represent average ± SEM from three independent experiments. (C) Gel-shift assay showing activation of the circular morpholino sequestering its complementary target DNA (cDNA) in the presence of β-lactamase. (D) Quantification of a time course analysis of cyclic cephalosporin ntla cMO cleavage following incubation with recombinant β-lactamase. Bars represent averages and error bars represent standard deviations from three independent experiments.
While switching of MO activity looked excellent, we were surprised by the overall limited silencing of luciferase expression in the in vitro translation assay and thus conducted a gel-shift analysis of the MO:target interaction. A 25-mer DNA oligonucleotide that is fully complementary to the ntla MO (cDNA, Supporting Table S2) was used as an mRNA mimic. Heteroduplex formation with the MO or cMO was visualized by native PAGE (Figure 3C). No heteroduplex formation was observed with the cMO in the absence of β-lactamase, confirming the results of the in vitro translation assay, as the curvature induced by macrocyclization of the cMO prevents target binding. It is only after incubation with the triggering enzyme that nearly full sequestration of the target cDNA is observed, as evidenced by the shift in mobility of the heteroduplex in the gel. This shift indicates successful cleavage of the linker and subsequent target hybridization by the decaged linear MO. To quantitatively determine the activated amount of cMO, the circular antisense agent was incubated with recombinant β-lactamase for up to 1 h. At defined time points, the enzyme was heat-inactivated and the MO was hybridized with an excess of cDNA. The band densities in a gel-shift assay were quantified using a standard curve (Supporting Figure S3) to determine the percentage of activated cMO. After 1 h, the cMO was 88% linearized (Figure 3D and Supporting Figure S4). The nearly complete heteroduplex formation observed by the decaged MO suggested promise for in vivo gene silencing.
Next, we sought to investigate whether β-lactamase could be used to control MO-mediated gene silencing of an endogenous gene target during early zebrafish development. β-lactamase activity can be easily evaluated using nitrocefin (10), a cephalosporin-based chromogenic substrate that changes in color from yellow to red following β-lactam hydrolysis (yielding 11; Figure 4A).54 The utility of this substrate was validated using recombinant β-lactamase (Supporting Figure S5). We generated a β-lactamase expression construct by cloning an N-terminally HA-tagged mammalian codon-optimized β-lactamase into the pCS2+ vector, which can be used for enzyme expression in mammalian cells and as a template for in vitro mRNA production (Supporting Table S2).55 The expression of β-lactamase was confirmed in HEK293T cells and enzymatic function was validated by monitoring cleavage of the nitrocefin substrate (Supporting Figure S6).
Figure 4.
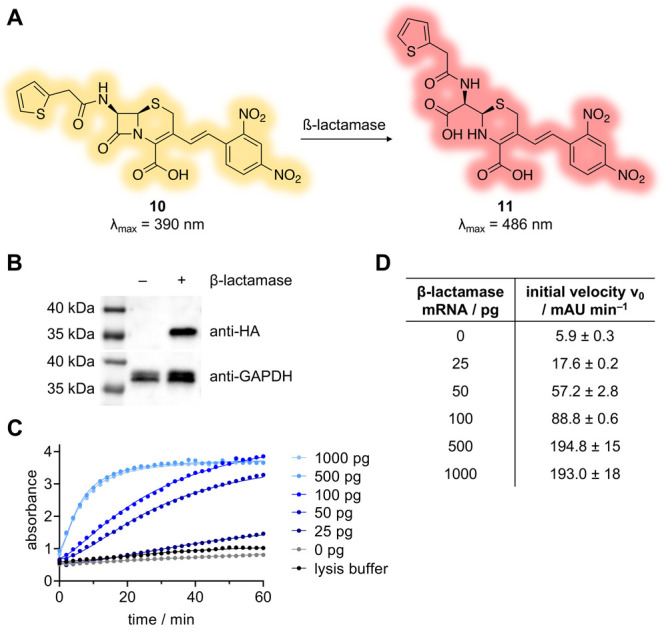
(A) Structure of the chromogenic β-lactamase substrate nitrocefin (10), which is hydrolyzed to 11 following β-lactamase catalysis. (B) Western blot analysis of β-lactamase expression in embryos injected with 400 pg of mRNA. (C) Zebrafish lysates analyzed for β-lactamase activity by monitoring production of 11 (absorbance at 486 nm) over time. (D) Initial velocities were determined through a linear regression analysis of the first 10 min of the data presented in (C). The error values indicate the standard deviation of values for the best fit parameters from the linear regression analysis.
In order to evaluate β-lactamase expression and activity in vivo, we next generated synthetic β-lactamase mRNA via in vitro transcription (Supporting Figure S7). The synthetic β-lactamase mRNA was injected into the yolk sac of 1- to 4-cell-stage zebrafish embryos. At 24 h post-fertilization (hpf), the injected embryos were collected, lysed,56 and β-lactamase expression was confirmed by Western blot (Figure 4B). To confirm that β-lactamase also maintained hydrolase activity, embryos were injected with increasing amounts of mRNA, lysed, and then incubated with nitrocefin. Hydrolysis of the substrate was monitored over time. An obvious dose-dependent correlation in β-lactamase activity was observed (Figure 4C). Further, quantification of the initial rates of nitrocefin hydrolysis demonstrated a clear positive correlation between the amount of mRNA injected into the embryos and the rate of enzymatic activity (Figure 4D). These results suggest that the extent of enzymatic activity can be finely tuned with the mRNA injection amount, with maximal activity plateauing following injection of approximately 500 pg of β-lactamase mRNA.
We then utilized our cyclic cephalosporin ntla cMO to determine whether we could induce enzymatic activation of MO function in vivo. As mentioned previously, ntla has been established as an excellent candidate gene target for silencing using conditionally controlled MO technologies.17,23,27−29 Silencing of ntla expression during early embryogenesis results in a distinct phenotype observable at 24 hpf, including truncation of tail length, loss of the notochord, and U-shaped somites (Figure 5A).51,52 The quantification of embryo body length was used to evaluate the strength of the morphant phenotype (Supporting Figure S8).24 Zebrafish embryos were injected in the yolk with 200 pg of either a negative control MO, the linear ntla MO, or our cyclic cephalosporin ntla cMO following previously established methods for zebrafish microinjections.14,56,57 Based on the diameter (0.7 mm) and cytoplasmic bridging of early-stage embryos, we estimate the final concentration of the morpholino to be approximately 120 nM in the injected embryos.58,59 Microinjection into the embryo yolk sac, as opposed to directly into the cell, enables a higher throughput workflow (200-300 embryos from one mating)60 and reduces injection errors that could result in embryo-to-embryo variation. However, a caveat of this injection technique is that precise assessment of the intracellular MO concentration is difficult to discern.59 Where indicated, 400 pg of β-lactamase mRNA was coinjected with the specified MO or cMO, and embryos were incubated until 24 hpf (Figure 5B). Embryos that were not injected or injected with the control MO developed as expected and displayed minimal signs of toxicity. However, we observed a slight increase in toxicity in embryos coinjected with MO and mRNA. To further investigate this toxicity, control MO (200 pg) was injected with increasing amounts of β-lactamase mRNA (up to 1000 pg). No trend was detected and no increase in phenotypic defects or toxicity was observed (Supporting Figure S9). Importantly, embryos injected with the cyclic cephalosporin ntla cMO alone also exhibited minimal phenotypic defects, confirming the inactivity of the cyclic cMO in vivo. However, upon coinjection of the cephalosporin ntla cMO with β-lactamase mRNA, full rescue of the ntla morphant phenotype was observed, comparable to the frequency of phenotype observed with the linear ntla MO. Gratifyingly, zebrafish coinjected with the cyclic cephalosporin ntla cMO and synthetic Nfsb mRNA did not show any ntla morphant phenotypes (Supporting Figure S10A). The same observation was made when injected embryos were exposed to 365 or 405 nm light (Supporting Figure S10B). Together, these results demonstrate the orthogonality of the two enzymatic cMO activation approaches and to the optical stimulation used for photocaged cMOs. There was a higher frequency of nonspecific phenotypic defects in Nfsb-mRNA injected embryos, showing lower toxicity of the β-lactamase expression (Supporting Figures 9A and 10A).52 Taken together, these results support the utility of the β-lactamase enzyme for conditionally controlling cephalosporin-modified antisense oligonucleotides to investigate endogenous gene function in vivo.
Figure 5.
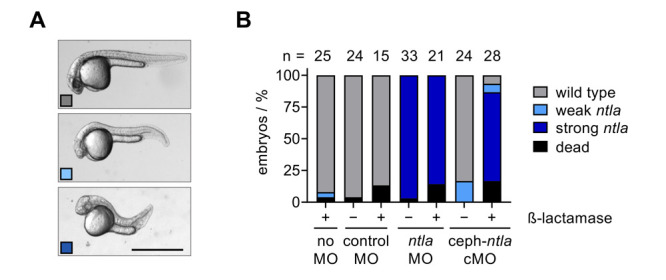
(A) Representative images of ntla morphant phenotypes in zebrafish embryos at 24 hpf. Scale bar equals 1 mm. (B) Phenotypic scoring of embryos injected with the indicated morpholino and amount of β-lactamase mRNA.
Conclusion
In summary, we leveraged enzyme-mediated antibiotic resistance mechanisms to develop a β-lactamase-triggered antisense agent. A novel linker containing a cephalosporin core-scaffold was synthesized and used to cyclize a MO. The curvature of the cyclic cMO successfully abrogated hybridization to and silencing of its mRNA target. β-lactamase-mediated cleavage of the cMO linker, MO linearization, and restoration of gene silencing were confirmed in vitro by gel-shift assay and silencing of reporter gene expression. Efficient enzyme-triggered silencing of endogenous gene expression was demonstrated in live zebrafish embryos coinjected with the cyclic cephalosporin ntla cMO and mRNA expressing β-lactamase. These results complement the current suite of conditionally controlled nucleic acids.17−20,22−24,27−29,31 Despite only one prior example of β-lactamase use in zebrafish embryos,45 the work presented herein demonstrates that the highly specific interaction between β-lactamase and cephalosporin provides a novel and robust means to control gene expression in a potentially tunable manner. Future studies placing β-lactamase expression under the control of tissue-specific promoters (similar to what has been shown previously with nitroreductase to enable targeted cell ablation experiments)23 will provide spatial and temporal control of enzyme function. While numerous fish lines expressing nitroreductase have been reported,61 transgenic fish lines stably expressing β-lactamase have not yet been generated, thereby precluding these studies. We anticipate that the enzyme activation presented here could be multiplexable with the other available orthogonal approaches for the conditional control of MO function.19,20,22−24,28,29 Lastly, the activation of MOs demonstrates that β-lactamase expression has potential for being a broadly applicable trigger for molecular function in zebrafish, significantly expanding its utility.
Acknowledgments
This work was supported by the National Institutes of Health (R01GM112728) and the University of Pittsburgh.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.3c00027.
Detailed experimental protocols and supporting data and figures (PDF)
The authors declare no competing financial interest.
Special Issue
Published as part of ACS Chemical Biologyvirtual special issue “Nucleic Acid Regulation”.
Supplementary Material
References
- Khvorova A.; Watts J. K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35 (3), 238–248. 10.1038/nbt.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S.; Mehtab S.; Krishnan Y. The predictive power of synthetic nucleic acid technologies in RNA biology. Acc. Chem. Res. 2014, 47 (6), 1710–9. 10.1021/ar400323d. [DOI] [PubMed] [Google Scholar]
- Havens M. A.; Hastings M. L. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 2016, 44 (14), 6549–63. 10.1093/nar/gkw533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.; Corey D. R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018, 46 (4), 1584–1600. 10.1093/nar/gkx1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setten R. L.; Rossi J. J.; Han S. P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discovery 2019, 18 (6), 421–446. 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- Rinaldi C.; Wood M. J. A. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14 (1), 9–21. 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy A. R.; Karchner S. I.; Hahn M. E. Gene knockdown by morpholino-modified oligonucleotides in the zebrafish (Danio rerio) model: applications for developmental toxicology. Methods Mol. Biol. 2012, 889, 51–71. 10.1007/978-1-61779-867-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov I. A.; Chen J. K. Oligonucleotide-based tools for studying zebrafish development. Zebrafish 2010, 7 (1), 31–40. 10.1089/zeb.2010.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerton J.; Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997, 7 (3), 187–95. 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- Hardy S.; Legagneux V.; Audic Y.; Paillard L. Reverse genetics in eukaryotes. Biol. Cell 2010, 102 (10), 561–80. 10.1042/BC20100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole R.; Krainer A. R.; Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discovery 2012, 11 (2), 125–40. 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. R.; Abrams J. M. Morpholino antisense oligonucleotides: tools for investigating vertebrate development. Genome Biol. 2001, 2 (5), reviews1015. 10.1186/gb-2001-2-5-reviews1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B.; Weidinger G.; Knaut H.; Thisse B.; Thisse C.; Raz E.; Schier A. F. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (23), 14919–24. 10.1073/pnas.222459999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill B. R.; Petzold A. M.; Clark K. J.; Schimmenti L. A.; Ekker S. C. A primer for morpholino use in zebrafish. Zebrafish 2009, 6 (1), 69–77. 10.1089/zeb.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov I. A.; Chen J. K. Chemical technologies for probing embryonic development. Chem. Soc. Rev. 2008, 37 (7), 1294–307. 10.1039/b703023c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah K. E.; Deiters A. Translational control of gene function through optically regulated nucleic acids. Chem. Soc. Rev. 2021, 50 (23), 13253–13267. 10.1039/D1CS00257K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov I. A.; Sinha S.; Chen J. K. Light-controlled gene silencing in zebrafish embryos. Nat. Chem. Biol. 2007, 3 (10), 650–1. 10.1038/nchembio.2007.30. [DOI] [PubMed] [Google Scholar]
- Deiters A.; Garner R. A.; Lusic H.; Govan J. M.; Dush M.; Nascone-Yoder N. M.; Yoder J. A. Photocaged morpholino oligomers for the light-regulation of gene function in zebrafish and Xenopus embryos. J. Am. Chem. Soc. 2010, 132 (44), 15644–50. 10.1021/ja1053863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardhan A.; Deiters A.; Ettensohn C. A. Conditional gene knockdowns in sea urchins using caged morpholinos. Dev. Biol. 2021, 475, 21–29. 10.1016/j.ydbio.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak S.; Sarode B. R.; Deiters A.; Chen J. K. Bicyclic Caged Morpholino Oligonucleotides for Optical Gene Silencing. ChemBioChem 2022, 23 (21), e202200374. 10.1002/cbic.202200374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Kim H. B.; Sul J. Y.; Yeldell S. B.; Eberwine J. H.; Dmochowski I. J. Efficient Synthesis of Light-Triggered Circular Antisense Oligonucleotides Targeting Cellular Protein Expression. ChemBioChem 2018, 19 (12), 1250–1254. 10.1002/cbic.201800012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W.; Bardhan A.; Darrah K.; Tsang M.; Deiters A. Optical Control of MicroRNA Function in Zebrafish Embryos. J. Am. Chem. Soc. 2022, 144 (37), 16819–16826. 10.1021/jacs.2c04479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazoe S.; McQuade L. E.; Chen J. K. Nitroreductase-activatable morpholino oligonucleotides for in vivo gene silencing. ACS Chem. Biol. 2014, 9 (9), 1985–90. 10.1021/cb500429u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah K.; Wesalo J.; Lukasak B.; Tsang M.; Chen J. K.; Deiters A. Small Molecule Control of Morpholino Antisense Oligonucleotide Function through Staudinger Reduction. J. Am. Chem. Soc. 2021, 143 (44), 18665–18671. 10.1021/jacs.1c08723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini A. J.; Schuler A. D.; Zebala J. A.; Mayer A. N. PhotoMorphs: a novel light-activated reagent for controlling gene expression in zebrafish. Genesis 2009, 47 (11), 736–43. 10.1002/dvg.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallafuss A.; Gibson D.; Morcos P.; Li Y.; Seredick S.; Eisen J.; Washbourne P. Turning gene function ON and OFF using sense and antisense photo-morpholinos in zebrafish. Development 2012, 139 (9), 1691–9. 10.1242/dev.072702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X.; Shestopalov I. A.; Sinha S.; Zheng G.; Pitt C. L.; Li W. H.; Olson A. J.; Chen J. K. Versatile synthesis and rational design of caged morpholinos. J. Am. Chem. Soc. 2009, 131 (37), 13255–69. 10.1021/ja809933h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazoe S.; Shestopalov I. A.; Provost E.; Leach S. D.; Chen J. K. Cyclic caged morpholinos: conformationally gated probes of embryonic gene function. Angew. Chem., Int. Ed. 2012, 51 (28), 6908–11. 10.1002/anie.201201690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazoe S.; Liu Q.; McQuade L. E.; Deiters A.; Chen J. K. Sequential gene silencing using wavelength-selective caged morpholino oligonucleotides. Angew. Chem., Int. Ed. 2014, 53 (38), 10114–8. 10.1002/anie.201405355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Wu L.; Wang P.; Lv C.; Yang Z.; Tang X. Manipulation of gene expression in zebrafish using caged circular morpholino oligomers. Nucleic Acids Res. 2012, 40 (21), 11155–62. 10.1093/nar/gks840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griepenburg J. C.; Rapp T. L.; Carroll P. J.; Eberwine J.; Dmochowski I. J. Ruthenium-caged antisense morpholinos for regulating gene expression in zebrafish embryos. Chem. Sci. 2015, 6 (4), 2342–2346. 10.1039/C4SC03990D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M. J.; Beebe L. L.; Deodato D.; Ball R. E.; Page A. T.; VanLeuven A. J.; Harris K. T.; Park S.; Hariharan V.; Lauderdale J. D.; Dore T. M. Bypassing Glutamic Acid Decarboxylase 1 (Gad1) Induced Craniofacial Defects with a Photoactivatable Translation Blocker Morpholino. ACS Chem. Neurosci. 2019, 10 (1), 266–278. 10.1021/acschemneuro.8b00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Wang Y.; Wu J.; Lv C.; Wang J.; Tang X. Caged circular antisense oligonucleotides for photomodulation of RNA digestion and gene expression in cells. Nucleic Acids Res. 2013, 41 (1), 677–86. 10.1093/nar/gks996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alouane A.; Labruère R.; Le Saux T.; Schmidt F.; Jullien L. Self-Immolative Spacers: Kinetic Aspects, Structure-Property Relationships, and Applications. Angew. Chem., Int. Ed. 2015, 54 (26), 7492–7509. 10.1002/anie.201500088. [DOI] [PubMed] [Google Scholar]
- Yazawa R.; Hirono I.; Aoki T. Characterization of promoter activities of four different Japanese flounder promoters in transgenic zebrafish. Mar. Biotechnol. (NY) 2005, 7 (6), 625–33. 10.1007/s10126-005-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvadia A. J.; Linney E. Windows into development: historic, current, and future perspectives on transgenic zebrafish. Dev. Biol. 2003, 256 (1), 1–17. 10.1016/S0012-1606(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Yang L.; Eberwine J. H.; Dmochowski I. J. Caspase-Activated Oligonucleotide Probe. Bioconjugate Chem. 2020, 31 (9), 2172–2178. 10.1021/acs.bioconjchem.0c00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo R. A. β-Lactamases: A Focus on Current Challenges. Cold Spring Harbor Perspect. Med. 2017, 7 (1), a025239. 10.1101/cshperspect.a025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooke C. L.; Hinchliffe P.; Bragginton E. C.; Colenso C. K.; Hirvonen V. H. A.; Takebayashi Y.; Spencer J. beta-Lactamases and beta-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431 (18), 3472–3500. 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshawe K. D. Antibody-directed enzyme prodrug therapy (ADEPT) for cancer. Expert Rev. Anticancer Ther 2006, 6 (10), 1421–31. 10.1586/14737140.6.10.1421. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Kale V.; Chen M. Gene-directed enzyme prodrug therapy. AAPS J. 2015, 17 (1), 102–10. 10.1208/s12248-014-9675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellmann N.; Deckert P. M.; Bachran D.; Fuchs H.; Bachran C. Targeted enzyme prodrug therapies. Mini-Rev. Med. Chem. 2010, 10 (10), 887–904. 10.2174/138955710792007196. [DOI] [PubMed] [Google Scholar]
- Pereira M. P.; Shi J.; Kelley S. O. Peptide Targeting of an Antibiotic Prodrug toward Phagosome-Entrapped Mycobacteria. ACS Infect Dis 2015, 1 (12), 586–92. 10.1021/acsinfecdis.5b00099. [DOI] [PubMed] [Google Scholar]
- Liu R.; Miller P. A.; Vakulenko S. B.; Stewart N. K.; Boggess W. C.; Miller M. J. A Synthetic Dual Drug Sideromycin Induces Gram-Negative Bacteria To Commit Suicide with a Gram-Positive Antibiotic. J. Med. Chem. 2018, 61 (9), 3845–3854. 10.1021/acs.jmedchem.8b00218. [DOI] [PubMed] [Google Scholar]
- Raz E.; Zlokarnik G.; Tsien R. Y.; Driever W. beta-lactamase as a marker for gene expression in live zebrafish embryos. Dev. Biol. 1998, 203 (2), 290–4. 10.1006/dbio.1998.8999. [DOI] [PubMed] [Google Scholar]
- Zlokarnik G.; Negulescu P. A.; Knapp T. E.; Mere L.; Burres N.; Feng L.; Whitney M.; Roemer K.; Tsien R. Y. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science 1998, 279 (5347), 84–8. 10.1126/science.279.5347.84. [DOI] [PubMed] [Google Scholar]
- Boyd D. B. Electronic structures of cephalosporins and penicillins. 15. Inductive effect of the 3-position side chain in cephalosporins. J. Med. Chem. 1984, 27 (1), 63–6. 10.1021/jm00367a012. [DOI] [PubMed] [Google Scholar]
- Faraci W. S.; Pratt R. F. Elimination of a good leaving group from the 3′-position of a cephalosporin need not be concerted with β-lactam ring opening: TEM-2 β-lactamase-catalyzed hydrolysis of pyridine-2-azo-4′-(N′,N′-dimethylaniline) cephalosporin (PADAC) and of cephaloridine. J. Am. Chem. Soc. 1984, 106 (5), 1489–1490. 10.1021/ja00317a053. [DOI] [Google Scholar]
- Patterson L. D.; Miller M. J. Enzymatic deprotection of the cephalosporin 3′-acetoxy group using Candida antarctica lipase B. J. Org. Chem. 2010, 75 (4), 1289–92. 10.1021/jo902406b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar K.; Madras G.; Chatterjee K. Dendron conjugation to graphene oxide using click chemistry for efficient gene delivery. RSC Adv. 2015, 5 (62), 50196–50211. 10.1039/C5RA07004J. [DOI] [Google Scholar]
- Halpern M. E.; Ho R. K.; Walker C.; Kimmel C. B. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell 1993, 75 (1), 99–111. 10.1016/S0092-8674(05)80087-X. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S.; van Eeden F. J. M.; Halpern M. E.; Kimmel C. B.; Nüsslein-Volhard C. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 1994, 120 (4), 1009–15. 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- Shabanpoor F.; Gait M. J. Development of a general methodology for labelling peptide-morpholino oligonucleotide conjugates using alkyne-azide click chemistry. Chem. Commun. 2013, 49 (87), 10260–2. 10.1039/C3CC46067C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan C. H.; Morris A.; Kirby S. M.; Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1972, 1 (4), 283–8. 10.1128/AAC.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Astolfo D. S.; Pagliero R. J.; Pras A.; Karthaus W. R.; Clevers H.; Prasad V.; Lebbink R. J.; Rehmann H.; Geijsen N. Efficient intracellular delivery of native proteins. Cell 2015, 161 (3), 674–690. 10.1016/j.cell.2015.03.028. [DOI] [PubMed] [Google Scholar]
- Yuan S.; Sun Z. Microinjection of mRNA and morpholino antisense oligonucleotides in zebrafish embryos. J. Visualized Exp. 2009, 27, e1113. 10.3791/1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. N.; Sweeney M. F.; Mably J. D. Microinjection of zebrafish embryos to analyze gene function. J. Visualized Exp. 2009, 25, 1115. 10.3791/1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B.; Ballard W. W.; Kimmel S. R.; Ullmann B.; Schilling T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203 (3), 253–310. 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B.; Law R. D. Cell lineage of zebrafish blastomeres. I. Cleavage pattern and cytoplasmic bridges between cells. Dev. Biol. 1985, 108 (1), 78–85. 10.1016/0012-1606(85)90010-7. [DOI] [PubMed] [Google Scholar]
- Hoo J. Y.; Kumari Y.; Shaikh M. F.; Hue S. M.; Goh B. H. Zebrafish: A Versatile Animal Model for Fertility Research. Biomed Res. Int. 2016, 2016, 9732780. 10.1155/2016/9732780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curado S.; Stainier D. Y.; Anderson R. M. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat. Protoc 2008, 3 (6), 948–54. 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


