Abstract
Renal cell carcinoma (RCC) with fibromyomatous stroma (RCCFMS) include ELOC/TCEB1 mutated RCC and those with TSC1/2/MTOR alterations. Besides morphologic similarity, most of these tumors is known to be diffusely positive for CAIX and CK7 by immunohistochemistry. We previously showed strong and diffuse expression of GPNMB in translocation RCC and eosinophilic renal neoplasms with known TSC1/2/MTOR alterations. We retrospectively identified molecularly confirmed cases of TCEB1/ELOC mutated RCC (seven tumors from seven patients), and RCCFMS with alterations in TSC1/2/MTOR (six tumors from five patients, one patient with tuberous sclerosis syndrome). Additionally, we included seven clear cell papillary renal cell tumors (CCPRCT) and eight clear cell RCC (CCRCC), as they can also present morphologic overlap with RCCFMS. Morphologically, renal cell carcinomas with TSC1/2/MTOR alterations and those with TCEB1/ELOC mutations were indistinguishable and characterized by papillary, nested, or tubular architecture, with tumor cells with clear cytoplasm and low nuclear grade. By immunohistochemistry, CK7 was positive in 5/7 (71%) of TCEB1/ELOC mutated RCCs, 6/6 (100%) of RCCs with TSC1/2/mTOR alterations, and 7/7 (100%) of CCPRCTs (p=NS). CAIX was positive in 7/7 TCEB1/ELOC mutated RCCs, 6/6 (100%) of RCCs with TSC1/2/MTOR alterations, and 7/7 (100%) of CCPRCTs (p=NS). GPNMB was strongly and diffusely positive in all tumors with TSC1/2/MTOR alterations (6/6), while negative in all TCEB1/ELOC mutated RCCs (0/6), or CCPRCTs (0/7) (p=0.002). Two of eight CCRCC showed focal weak staining, while 6/8 were negative. In conclusion, the results support the use of GPNMB to distinguish RCCFMS with TSC1/2/MTOR alterations from others with similar morphology.
Keywords: Renal cell carcinoma with fibromyomatous stroma, ELOC, TCEB1, GPNMB, TSC, mTOR
Introduction
Renal cell tumors with clear cell morphology encompasses different tumor entities with various genetic alterations and prognoses, ranging from indolent/benign clear cell papillary renal cell tumor (CCPRCT) to aggressive carcinoma, such as conventional clear cell renal cell carcinoma (CCRCC) and MiT family translocation related renal cell carcinoma.1 Renal cell carcinoma (RCC) with fibromyomatous stroma (RCCFMS) are less common RCCs that include ELOC/TCEB1 mutated RCC and RCC associated with TSC1/2 or mTOR pathway alterations.2 ELOC/TCEB1 mutated RCC is a distinct entity with somatic mutations in ELOC/TCEB1 gene and loss of the alternate allele (8q21).3 Besides high morphologic similarity, the great majority of these tumors is also known to be diffusely positive for CAIX and CK7 by immunohistochemistry. It is not clear whether TSC1/2/MTOR and ELOC/TCEB1 mutated RCCFMS should be grouped together, despite distinct molecular alterations. Currently, definitive diagnosis of ELOC/TCEB1 RCC and TSC1/2/MTOR alteration associated RCC relies on molecular studies, which are expensive and time consuming.
GPNMB (glycoprotein nonmetastatic B) has been reported as a survival factor and protect endothelial cells against stress-induced premature senescence. Low expression of GPNMB can be found in most normal tissues, except for high expression in histiocytes.4, 5 High expression of GPNMB has been reported in aggressive malignancies in multiple organ systems with poor prognosis, including microphthalmia (MiT) translocation RCC, melanoma, triple negative breast cancer, and aggressive gliomas.4, 5 Our group has recently reported strong GPNMB expression in TFE3 and TFEB related RCCs, in eosinophilic solid and cystic renal cell carcinomas (ESC), in low grade oncocytic tumors (LOT), and in angiomyolipomas (AML), while only focally weakly positive or negative in conventional clear cell RCC, papillary RCC, chromophobe RCC, and oncocytoma.6
In this study, we explored the utility of GPNMB immunohistochemistry in the differential diagnosis of RCCFMS associated with TSC1/2/MTOR alterations or with TCEB1/ELOC mutations.
Materials and methods:
This study was approved by the institutional review board of Johns Hopkins University. We retrospectively reviewed the files of the Department of Pathology at Johns Hopkins University, National Institutes of Health, and Memorial Sloan Kettering Cancer center to identify molecularly confirmed cases of ELOC/TCEB1 mutated RCCs and RCCFMS with known TSC1/2/MTOR alterations.
Immunohistochemical stains for CAIX (Leica, NCL-L-CAIX, 1:100), CK7 (Dako Agilent, M7018, 1:500), and GPNMB (Cell Signaling, 38313S, 1:1500) were performed on selected whole slide with 4 μm sections from formalin-fixed paraffin-embedded tissue blocks as previously reported.6, 7
Cytoplasmic staining of CK7 and GPNMB, and membranous staining for CAIX was considered positive if more than 10% of tumor cells showed moderate or strong staining, reported as diffuse is more than 90% of tumor cells were positive, and patchy if staining was seen in 10–90% of tumor cells. Non-neoplastic renal tubules were used as internal positive controls for CK7 and histiocytes was used as internal control for GPNMB in all cases. CCRCC was used as positive control for CAIX.
Sample preparation, sequencing, and analysis workflow
DNA was processed and library-prepped using the KAPA Hyper Prep chemistry (KAPA Biosystems) workflow as previously described, but with an updated JHOP IDT bait set, v5.2. 8 NGS was performed on a NovaSeq 6000 (Illumina Biotechnology) using 2 × 100 paired-end chemistry with a clinically validated laboratory-developed test at the Clinical Laboratory Improvement Amendments–certified Molecular Diagnostics Laboratory at Johns Hopkins, as previously described.9, 10
Results:
ELOC/TCEB1 mutant renal cell carcinoma.
Table 1 summarizes the results of immunohistochemistry. The study included seven ELOC/TCEB1 mutant RCC. Cases 1–4 were from a previous study from one of the authors.3 The average age was 62 years old (range 50–71). Cases 5–7 were not previously reported and were identified based on the molecular findings from NGS test performed for clinical purposes. Microscopically these tumors displayed lobulated, nodular, and cystic growth patterns and prominent papillary structures. Tumor cells had clear cytoplasm with nucleolar grade 2 in 6/7 (86%) and grade 3 in 1/7 (14%). The case with nucleolar grade 3 had areas of geographic necrosis. The fibromyomatous stroma was evident in all cases at the periphery of the tumor, and in intratumoral septae in 6/7 (86%). The clear cytoplasm ranged from scant to voluminous, and nuclear grade varied from low to high grade (Figure 1). Molecular studies of the three cases not previously published included alterations the following alterations in the ELOC gene: (i) c.236A>G resulting in amino acid p.Tyr79Cys, (ii) an exonic single nucleotide variation leading to the amino acid change Y795, (iii) c.275A>G leading to amino acid change p.Glu92Gly. The first of these three cases also had a likely pathogenic alteration in the gene KMT2A and the third showed a likely pathogenic mutation in the gene MPL. None of the cases showed any 3p or VHL gene alterations. By immunohistochemistry, all 7 cases showed diffuse membranous staining for CAIX and five were diffusely positive for CK7 while 2 were completely negative. One of the CK7-negative cases was the tumor with geographic necrosis and high nuclear grade, the other negative case did not have any distinctive morphologic feature. All cases were completely negative for GPNMB.
Table 1.
Immunohistochemistry results.
| TCEB1/ELOC mutated RCC | TSC1/2/MTOR mutated RCC | CCRCC | CCPRCT | P value | |
|---|---|---|---|---|---|
| CK7 | 5/7 (71%) | 6/6 (100%; 5 diffuse, 1 patchy) | 3/4 (75%, focal) | 7/7 (100%, all diffuse) | NS |
| CAIX | 7/7 (100%) | 6/6 (100%; 4 diffuse, 2 patchy) | 4/4 (100%, diffuse) | 7/7 (100%, all diffuse) | NS |
| GPNMB | 0/7 (0%) | 6/6 (100%; all strong and diffuse) | 2/8 (25%, weak and focal) | 0/7 (0%) | 0.002 |
CAIX: carbonic anhydrase IX; CCRCC: clear cell renal cell carcinoma; CCPRCT: clear cell papillary renal cell tumor; CK7: cytokeratin 7; GPNMB: glycoprotein nonmetastatic B; NS: not significant.
Figure 1. TCEB1/ELOC mutated renal cell carcinoma.
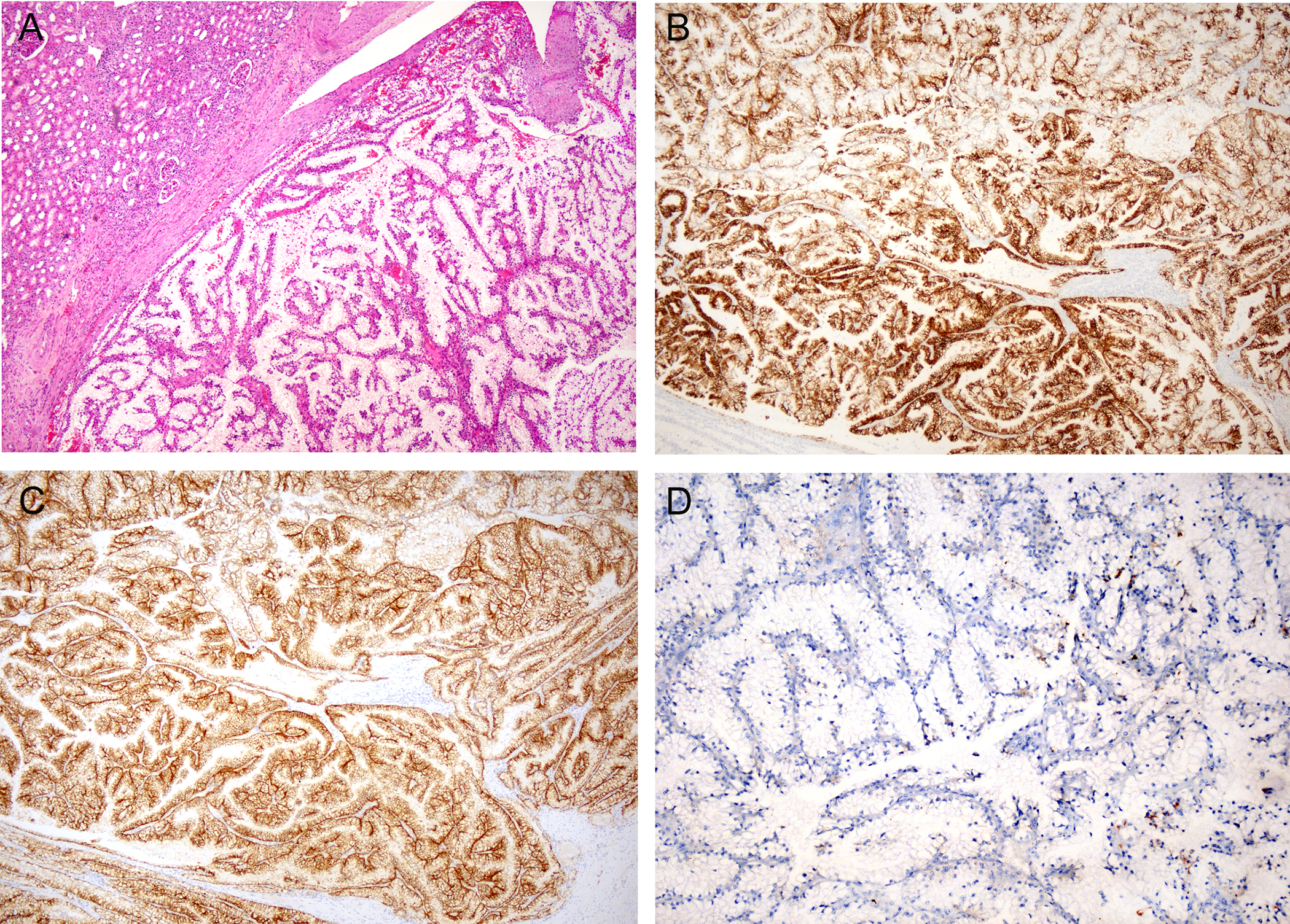
A. H&E-stained section shows a low power view of a tumor with papillary architecture and tumor cells with clear cytoplasm. B&C. Immunohistochemistry for CK7 (B) and CAIX (C) show strong and diffuse staining in tumor cells. D. Immunohistochemistry for GPNMB is negative.
Renal cell carcinoma with fibromyomatous stroma and TSC1/2/MTOR alterations
There were six tumors from five patients in this group. The mean age at presentation was 23 years old (range 19–30). There were two males and four females. The specimens included three radical nephrectomies, two partial nephrectomies and one needle biopsy. The tumor size was available in three patients, ranging from 1.9 to 4.3 cm (average 3.0 cm). One patient, a 30-year-old female, had confirmed tuberous sclerosis syndrome and presented with three separate masses that included one low grade oncocytic tumor, and two separate RCCFMS, the latter two included in this study. Microscopically these tumors displayed lobulated, nodular, or cystic growth patterns with prominent papillary structures in six of seven cases, while one case showed a nested architecture with delicate vascular septa like CCRCC. Tumor cells had abundant clear cytoplasm in all seven tumors and nucleolar grade 2 in three cases and grade 3 in three cases. The fibromyomatous stroma was prominent in all 7 cases (Figure 2). One case showed lymphovascular invasion and had metastasis in one regional lymph node. Molecular studies showed the following findings: (i) MTOR mutation p.L242Q; (ii) TSC1 frameshift mutation pN837fs; (iii) two TSC1 mutations, p.E839fs p.S331fs; (iv) TSC1 mutation pN837fs. Immunohistochemistry for CK7 was positive in 6/6 (100%), including diffusely positive in 5 and patchy in one. Expression of CAIX was positive in 6/6 (100%), including diffusely positive in four and patchy in two. All seven tumors were strongly and diffusely positive for GPNMB.
Figure 2. Two examples of renal cell carcinoma with fibromyomatous stroma and associated TSC1/2/mTOR alterations.
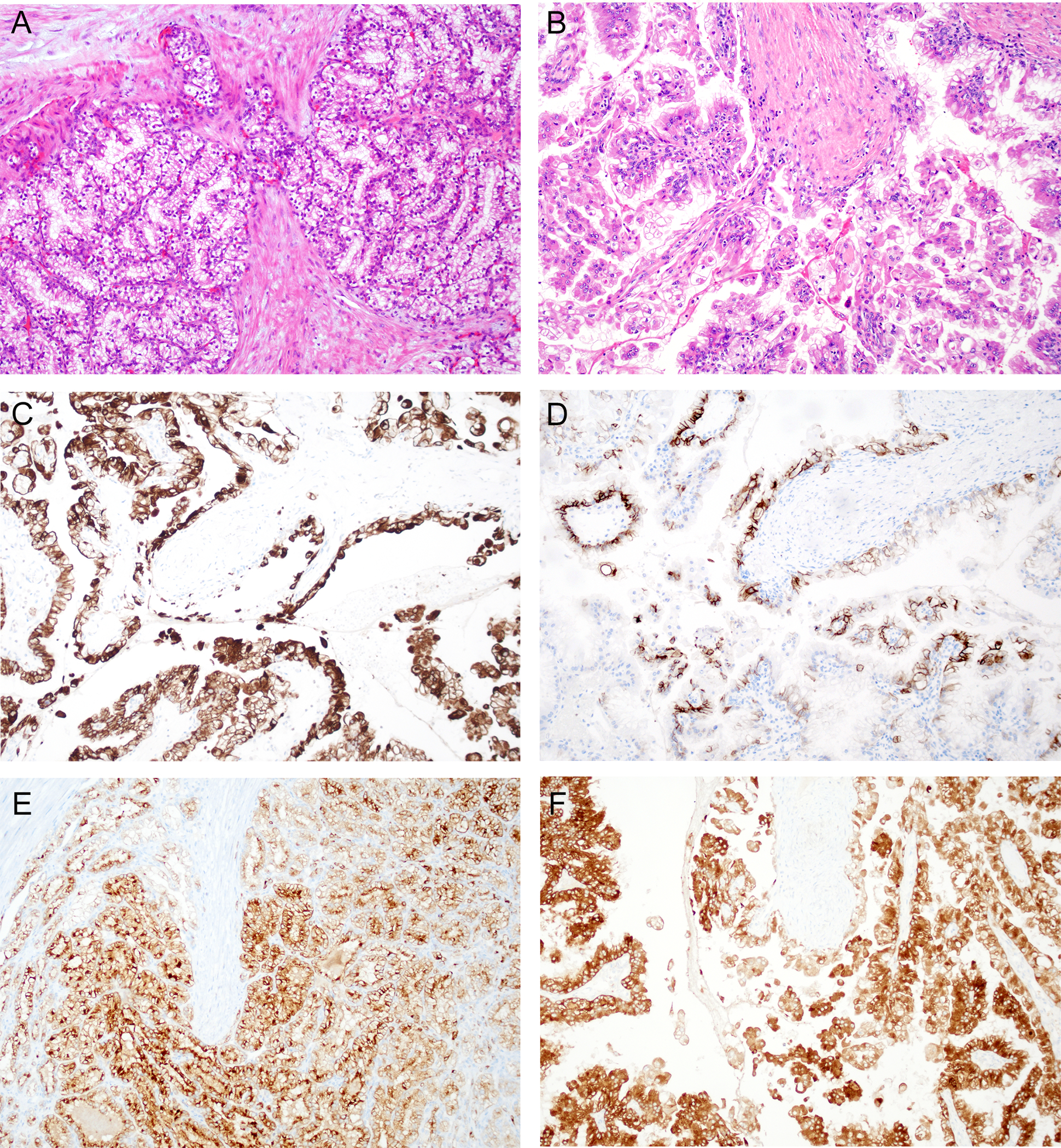
A&B. Both tumors show similar morphology characterized by papillary structures lined by tumor cells with clear cytoplasm and surrounded by fibromyomatous stroma. C. Immunohistochemistry of case in “A” shows positive staining for CK7 in tumor cells. D. Immunohistochemistry of case in “A” shows patchy staining for CAIX in tumor cells. E. Immunostaining for GPNMB on case in “A” shows strong and diffuse staining in tumor cells. F. Immunostaining for GPNMB on case in “B” shows strong and diffuse staining in tumor cells.
Other renal tumors with clear cell morphology
We have previously reported absence of GPNMB expression in 87 cases of clear cell renal cell carcinoma (median H-score of 0).6 In this study, we stained eight cases of CCRCC, including four with molecular results. None of these tumors displayed prominent fibromyomatous stroma. The molecular findings in this group included: (i) mutations in VHL, BIRC3, ETV4, FANCE, FAT1, GATA1, MAGI2, PTCH1, RECQL4, SDHD, SPTA1; (ii) mutations in BRCA2, EMSY, FOXP1, KDR, LZTR1, MAGI2, PRKDC; (iii) mutations in SDHC, BBC3, BLM, BRCA1, CDKN1B, ETV6, FGFR4, RAD51, RIT1, ROS1, TMPRSS2, TSHR; (iv) mutations in VHL, BAP1, LRP1B, AR, ASXL2, CCN6, FANCC, MDM4, PARP1, PDGFRB, RECQL4, SETD2, SPTA1, TSHR. By immunohistochemistry, two of eight cases showed focal weak staining for GPNMB, while 6/8 were completely negative. Immunostaining for CK7 was focally positive in 3/4 (75%) cases and negative in two cases, while CAIX was diffusely positive in 4/4 (100%) cases. All four cases with the molecular results reported above were negative for GPNMB.
We also included seven cases of CCPRCT, which are also composed of tumor cells with clear cytoplasm and frequently diffusely positivity for CK7 and CAIX, and therefore they are included in the differential diagnosis of RCCFMS. A small subset of CCPRCT can have FMS stroma (previously referred to as RAT, but now accepted as same family of tumors). The average tumor size was 2.3 cm. All tumor displayed the solid and cystic architecture and tumor cells had clear cytoplasm with low nuclear grade. None of tumors displayed prominent fibromyomatous stroma. The linear arrangement of tumor cells and subnuclear vacuoles were see focally in all tumors. All cases were diffusely positive for CK7 and CAIX and completely negative for GPNMB (Figure 3).
Figure 3. Clear cell papillary renal cell tumor.
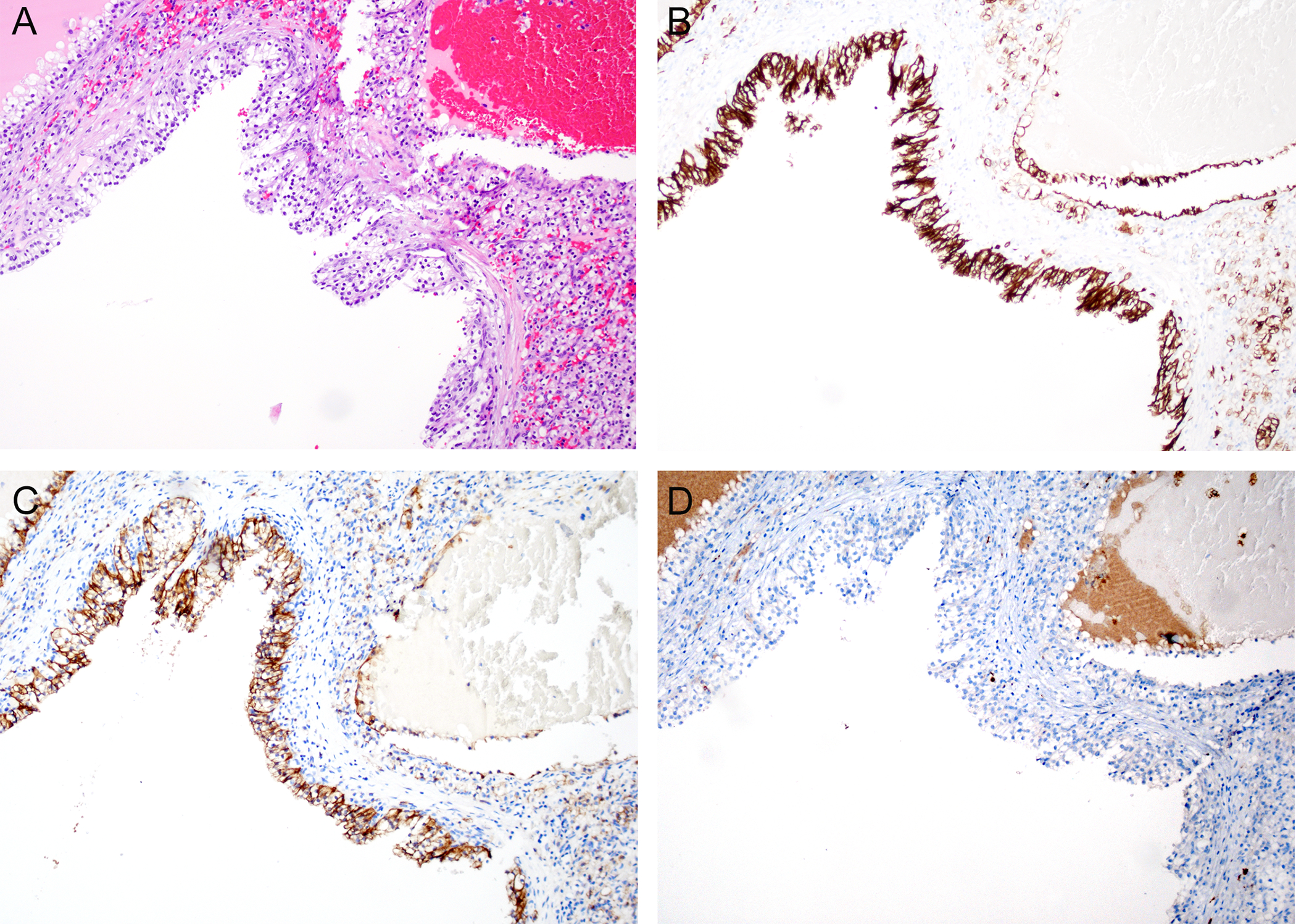
A. H&E-stained low power view of a clear cell papillary renal cell tumor showing a cystic and solid neoplasm with low grade tumor cells with clear cytoplasm. In contrast to the study cases, this tumor does not show prominent fibromyomatous stroma. B. Immunohistochemistry for CK7 shows diffuse staining of tumor cells. C. Immunohistochemistry for CAIX showing positive staining in tumor cells. D. Immunostaining for GPNMB is completely negative.
Discussion
Although morphology is still the gold standard for pathological diagnosis, molecular studies in association with morphology and clinical behavior have remarkably advanced and improved the classification of renal cell tumors. Still, there is a need for additional ancillary tests that are easily accessible and inexpensive to help in the classification of renal tumors.11–13
In the past a few years, many studies have been focused on the renal cell tumor with eosinophilic features,13 including multiple entities with TSC1, TSC2 or MTOR alterations.14–16 Classification of renal cell tumors with clear cell morphology has also been notably expanded.1 Recently, Shah et al. published a case series of sporadic RCCFMS harboring sporadic mutations in TSC1, TSC2, MTOR, and TCEB1/ELOC mutations.17 In their study, tumors were grouped based on their overlapping morphologic and immunohistochemistry features. Here, we show that immunohistochemistry for GPNMB helps separate this group into those with TSC1/2 or MTOR alterations, GPNMB-positive, from those with TCEB1/ELOC alterations, GPNMB-negative.
GPNMB is a transmembrane protein that is transcriptionally activated by TFE3 and TFEB. Previous studies showed that MTORC1 activation secondary to TSC2 loss, leads to overactivity of TFE3 and TFEB. 18, 19 These observations lead to the hypothesis that GPNMB could be used as a marker of renal neoplasms driven by TSC1/2/MTOR or MiT family alterations. 6 In our previous study, we have shown expression of GPNMB was strong and diffuse in MiT family translocation RCC and renal tumors with eosinophilic features and known TSC1/2/MTOR alterations including eosinophilic solid and cystic RCC, and low grade oncocytic tumors.6
Immunohistochemistry for CAIX and CK7 are routinely used to differentiate various renal cell tumors 20, 21. Among the common differential diagnoses for tumors with clear cell morphology, diffuse CAIX labeling associated with focal or negative CK7 staining favors the diagnosis of CCRCC. Studies on RCCFMS and ELOC/TCEB1 mutated RCC show diffusely positive CAIX and CK7 labeling.3, 22 In contrast to the “cup shaped” CAIX immunohistochemistry pattern of CCPRCT, ELOC/TCEB1 mutant and TSC1/2/MTOR altered RCCs demonstrate “box shaped” CAIX membranous staining, a distinction that is often difficult to make on a given case. In our study, most of the ELOC/TCEB1 mutated RCCs and RCCFMS with TSC1/2/MTOR alterations were positive for both CAIX and CK7. The exceptions were two TCEB1/ELOC mutated RCCs that were negative for CK7, including the case with geographic necrosis. Therefore, while CAIX and CK7 are helpful for an initial immunophenotypic characterization of these tumors, they provide minimal differential value within the group of RCCFMS. Furthermore, our study shows that higher grade cases could be negative for CK7 and the traditional immunophenotype would not be helpful in this setting. High grade TCEB1/ELOC mutant RCC have been reported in the literature in cases with metastatic disease in which molecular testing was performed. Despite their more aggressive clinical course, these tumors shared morphologic features of lower grade TCEB1/ELOC mutant RCCs including papillary structures lined by clear cells arranged in large nests separated by fibromyomatous stroma.23
Management of renal tumors has evolved recently into considering non-surgical options in patients with small tumors and/or in patients with tumors with indolent clinical behavior where the potential gain of a surgical excision does not justify the surgical risk.24 Diagnosis of these tumors relies on needle biopsy specimens that often have limited amount of tissue. Judicial use of the tissue to obtain as much information needed for appropriate classification to guide therapy is essential. The findings in this study support the use of GPNMB in this setting, providing subclassification information that could be potentially useful for therapeutic options, should the genetic alterations be confirmed by molecular testing (specifically cases that might respond to MTOR pathway inhibition).25, 26
Our previous studies have demonstrated that diffusely strong GPNMB expression is identified in TFE3/TFEB translocation RCC and in TSC1/2/MTOR alteration associated renal tumors.6 GPNMB immunostain has been proposed to serve as a screening marker for MiT translocation RCC and TSC1/2/MTOR alteration associated renal tumors.6, 19, 27 Focal or weak GPNMB labeling is observed in renal tumor types (CCRCC, chromophobe RCC, etc) without either MiT translocation or TSC1/2/MTOR alteration.6 Therefore, for the purpose of screening translocation RCC or RCCFMS and TSC1/2/MTOR mutations, non-diffuse expression of GPNMB labeling should be interpreted as non-specific or negative result. The differential diagnosis between translocation RCC and RCCFMS with TSC1/2/MTOR alterations will rely on the presence of CK7 and CAIX expression in the latter.
The differential diagnosis of CK7 and CAIX positive tumors also includes CCPRCT, especially given that CCPRCTs are benign and RCCFMS are malignant. While CCPRCTs are characterized by the reverse nuclear polarity with subnuclear vacuoles and the nuclear alignment, they can also display fibromyomatous stroma.2, 28, 29 Similarly, CCRCC could also present with fibromyomatous stroma and, from a practical standpoint, should be included in the differential diagnosis of RCCFMS.29 In this study, all seven cases of CPRCTs were negative for GPNMB. CCRCCs reported in our previous study were either completely negative or only focally weakly positive.6 Therefore, positive GPNMB staining in a tumor with clear cell morphology and fibromyomatous stroma is highly suggestive of TSC1/2/MTOR alterations, while the differential diagnosis of GPNMB negative cases still include all other tumors with clear cell morphology, in which emphasis in the morphologic features and immunophenotype remains critical.
A previous study of RCCFMS showed that mutations in TCEB/ELOC and TSC1/2/MTOR are not mutually exclusive, with one case showing both.17 We did not encounter any case with both genetic alterations and, therefore, this scenario appears to be infrequent based on published cases. Unfortunately, we are not able to confirm whether tumors with alterations in both gene groups would express GPNMB.
A main limitation of our study is the low number of cases, like other published studies with these tumor types.17 Still, the value of the results relies on the sharp contrast of the GPNMB expression being diffuse positive in all cases with TSC1/2/MTOR alteration and completely negative in those with TCEB1/ELOC mutations, and only weakly focal stain or negative result in CCRCC or CCPRCT.
In conclusion, our study demonstrated that positive GPNMB immunostain distinguishes RCCFMS and TSC1/2/MTOR alterations, while excludes its mimickers TCEB1/ELOC mutated RCC, CCRCC and CCPRCT.
Reference
- 1.Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. [DOI] [PubMed] [Google Scholar]
- 2.Shah RB. Renal Cell Carcinoma With Fibromyomatous Stroma-The Whole Story. Adv Anat Pathol. 2022;29:168–177. [DOI] [PubMed] [Google Scholar]
- 3.Hakimi AA, Tickoo SK, Jacobsen A, et al. TCEB1-mutated renal cell carcinoma: a distinct genomic and morphological subtype. Mod Pathol. 2015;28:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taya M, Hammes SR. Glycoprotein Non-Metastatic Melanoma Protein B (GPNMB) and Cancer: A Novel Potential Therapeutic Target. Steroids. 2018;133:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose AA, Grosset AA, Dong Z, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res. 2010;16:2147–2156. [DOI] [PubMed] [Google Scholar]
- 6.Salles DC, Asrani K, Woo J, et al. GPNMB expression identifies TSC1/2/mTOR-associated and MiT family translocation-driven renal neoplasms. J Pathol. 2022;257:158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matoso A, Chen YB, Rao V, et al. Atypical Renal Cysts: A Morphologic, Immunohistochemical, and Molecular Study. Am J Surg Pathol. 2016;40:202–211. [DOI] [PubMed] [Google Scholar]
- 8.Craven KE, Fischer CG, Jiang L, et al. Optimizing Insertion and Deletion Detection Using Next-Generation Sequencing in the Clinical Laboratory. J Mol Diagn. 2022;24:1217–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xian RR, Xie Y, Haley LM, et al. CREBBP and STAT6 co-mutation and 16p13 and 1p36 loss define the t(14;18)-negative diffuse variant of follicular lymphoma. Blood Cancer J. 2020;10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng G, Chen P, Pallavajjalla A, et al. The diagnostic utility of targeted gene panel sequencing in discriminating etiologies of cytopenia. Am J Hematol. 2019;94:1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon R, Argani P, Epstein JI, et al. Contemporary Characterization and Recategorization of Adult Unclassified Renal Cell Carcinoma. Am J Surg Pathol. 2021;45:450–462. [DOI] [PubMed] [Google Scholar]
- 12.Collins K, Hwang M, Antic T, et al. Merlin immunohistochemistry is useful in diagnosis of tumours within the spectrum of biphasic hyalinizing psammomatous renal cell carcinoma. Histopathology. 2022;81:577–586. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Reuter VE, Matoso A, et al. Re-evaluation of 33 ‘unclassified’ eosinophilic renal cell carcinomas in young patients. Histopathology. 2018;72:588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia QY, Wang XT, Zhao M, et al. TSC/MTOR -associated Eosinophilic Renal Tumors Exhibit a Heterogeneous Clinicopathologic Spectrum : A Targeted Next-generation Sequencing and Gene Expression Profiling Study. Am J Surg Pathol. 2022;46:1562–1576. [DOI] [PubMed] [Google Scholar]
- 15.Tjota M, Chen H, Parilla M, et al. Eosinophilic Renal Cell Tumors With a TSC and MTOR Gene Mutations Are Morphologically and Immunohistochemically Heterogenous: Clinicopathologic and Molecular Study. Am J Surg Pathol. 2020;44:943–954. [DOI] [PubMed] [Google Scholar]
- 16.Chen YB, Mirsadraei L, Jayakumaran G, et al. Somatic Mutations of TSC2 or MTOR Characterize a Morphologically Distinct Subset of Sporadic Renal Cell Carcinoma With Eosinophilic and Vacuolated Cytoplasm. Am J Surg Pathol. 2019;43:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah RB, Stohr BA, Tu ZJ, et al. “Renal Cell Carcinoma With Leiomyomatous Stroma” Harbor Somatic Mutations of TSC1, TSC2, MTOR, and/or ELOC (TCEB1): Clinicopathologic and Molecular Characterization of 18 Sporadic Tumors Supports a Distinct Entity. Am J Surg Pathol. 2020;44:571–581. [DOI] [PubMed] [Google Scholar]
- 18.Asrani K, Murali S, Lam B, et al. mTORC1 feedback to AKT modulates lysosomal biogenesis through MiT/TFE regulation. J Clin Invest. 2019;129:5584–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baba M, Furuya M, Motoshima T, et al. TFE3 Xp11.2 Translocation Renal Cell Carcinoma Mouse Model Reveals Novel Therapeutic Targets and Identifies GPNMB as a Diagnostic Marker for Human Disease. Mol Cancer Res. 2019;17:1613–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez ML, Alaghehbandan R, Pivovarcikova K, et al. Reactivity of CK7 across the spectrum of renal cell carcinomas with clear cells. Histopathology. 2019;74:608–617. [DOI] [PubMed] [Google Scholar]
- 21.Alshenawy HA. Immunohistochemical Panel for Differentiating Renal Cell Carcinoma with Clear and Papillary Features. Pathol Oncol Res. 2015;21:893–899. [DOI] [PubMed] [Google Scholar]
- 22.Williamson SR, Cheng L, Eble JN, et al. Renal cell carcinoma with angioleiomyoma-like stroma: clinicopathological, immunohistochemical, and molecular features supporting classification as a distinct entity. Mod Pathol. 2015;28:279–294. [DOI] [PubMed] [Google Scholar]
- 23.DiNatale RG, Gorelick AN, Makarov V, et al. Putative Drivers of Aggressiveness in TCEB1-mutant Renal Cell Carcinoma: An Emerging Entity with Variable Clinical Course. Eur Urol Focus. 2021;7:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray S, Cheaib JG, Pierorazio PM. Active Surveillance for Small Renal Masses. Rev Urol. 2020;22:9–16. [PMC free article] [PubMed] [Google Scholar]
- 25.Hua H, Kong Q, Zhang H, et al. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battelli C, Cho DC. mTOR inhibitors in renal cell carcinoma. Therapy. 2011;8:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argani P Translocation carcinomas of the kidney. Genes Chromosomes Cancer. 2022;61:219–227. [DOI] [PubMed] [Google Scholar]
- 28.Aron M, Chang E, Herrera L, et al. Clear cell-papillary renal cell carcinoma of the kidney not associated with end-stage renal disease: clinicopathologic correlation with expanded immunophenotypic and molecular characterization of a large cohort with emphasis on relationship with renal angiomyoadenomatous tumor. Am J Surg Pathol. 2015;39:873–888. [DOI] [PubMed] [Google Scholar]
- 29.Deml KF, Schildhaus HU, Comperat E, et al. Clear cell papillary renal cell carcinoma and renal angiomyoadenomatous tumor: two variants of a morphologic, immunohistochemical, and genetic distinct entity of renal cell carcinoma. Am J Surg Pathol. 2015;39:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]


