Abstract
Background:
Drinking water is a common source of exposure to inorganic arsenic. In the US, the Safe Drinking Water Act (SDWA) was enacted to protect consumers from exposure to contaminants, including arsenic, in public water systems (PWS). The reproductive effects of preconception and prenatal arsenic exposure in regions with low to moderate arsenic concentrations are not well understood.
Objectives:
This study examined associations between preconception and prenatal exposure to arsenic violations in water, measured via residence in a county with an arsenic violation in a regulated PWS during pregnancy, and five birth outcomes: birth weight, gestational age at birth, preterm birth, small for gestational age (SGA), and large for gestational age (LGA).
Methods:
Data for arsenic violations in PWS, defined as concentrations exceeding 10 parts per billion, were obtained from the Safe Drinking Water Information System. Participants of the Environmental influences on Child Health Outcomes Cohort Study were matched to arsenic violations by time and location based on residential history data. Multivariable, mixed effects regression models were used to assess the relationship between preconception and prenatal exposure to arsenic violations in drinking water and birth outcomes.
Results:
Compared to unexposed infants, continuous exposure to arsenic from three months prior to conception through birth was associated with 88.8 grams higher mean birth weight (95% CI: 8.2, 169.5), after adjusting for individual-level confounders. No statistically significant associations were observed between any preconception or prenatal violations exposure and gestational age at birth, preterm birth, SGA, or LGA.
Conclusions:
Our study did not identify associations between preconception and prenatal arsenic exposure, defined by drinking water exceedances, and adverse birth outcomes. Exposure to arsenic violations in drinking water was associated with higher birth weight. Future studies would benefit from more precise geodata of water system service areas, direct household drinking water measurements, and exposure biomarkers.
Keywords: arsenic, drinking water, contamination, reproductive health, water violations, public water systems
1. Introduction
A growing body of literature has focused on associations between environmental chemical exposures and birth outcomes. Arsenic, a non-essential metalloid, is one such exposure of focus. Inorganic arsenic is a naturally occurring, ubiquitous toxicant that is released into the environment from both natural and anthropogenic activities (Villaescusa & Bollinger, 2008). Inorganic arsenic compounds can dissolve in and contaminate drinking water (Chung et al., 2014). Most arsenic in groundwater and surface water comes from natural erosion and mineral deposition, making it expensive and challenging to regulate (Keshavarzi et al., 2011; Villaescusa & Bollinger, 2008). Drinking water, as a result, is one of the most prominent routes of exposure to arsenic in the US and globally (CDC, 2017; World Health Organization, 2022).
The Safe Drinking Water Act (SDWA), enacted in 1974, gave the Environmental Protection Agency (EPA) authorization to protect and regulate public water systems (PWS) (Weinmeyer et al., 2017). An estimated 90% of the US population is served by PWS (US EPA, 2015). As part of the SDWA, the EPA sets treatment techniques and maximum contaminant levels (MCL) for more than 90 contaminants, including arsenic, in PWS. In 2001, the EPA passed the Final Arsenic Rule in response to the National Research Council’s 1999 report, Arsenic in Drinking Water, which classified inorganic arsenic as a human carcinogen (National Research Council, 1999). The ruling reduced the MCL for arsenic in drinking water from 50 parts per billion (ppb) to 10 ppb. The ruling was supported by three independent, expert panels tasked by the EPA to review the science, financial implications, and health benefits of lowering the arsenic MCL (US EPA, n.d.). Since the adoption of the Final Arsenic Rule, the number of arsenic violations among PWS in the US has decreased by more than 50% from its peak of 883 violations in 2008 to 348 violations in 2017 (Foster et al., 2019). Nevertheless, few studies have explored associations between arsenic exceedances and birth outcomes in the US (Almberg et al., 2017; Young et al., 2023).
Heavy metals such as arsenic cross the placenta and accumulate in fetal tissue, creating a potential for in utero complications and adverse birth outcomes (Gundacker & Hengstschlager, 2012; Punshon et al., 2015). Preterm birth, low birth weight, and other adverse birth outcomes have significant impacts on child well-being and mortality (Raju et al., 2017). The existing literature on the relationship between arsenic exposure in drinking water and birth outcomes is limited and has yielded inconsistent findings (Bloom et al., 2014; Milton et al., 2017). It is worth noting that most of the existing literature was conducted in international settings with unregulated water systems, high average exposure levels (>50 ppb), and small sample sizes (Bloom et al., 2014; Milton et al., 2017). Further research is needed to explore the relationship between low- to moderate-levels of arsenic exposure and birth outcomes in the US, where PWS are regulated.
This analysis aims to assess the relationship between preconception and prenatal arsenic exposure, measured via proxy of residing in a county with an active arsenic violation in a PWS between three months prior to conception and birth, and birth outcomes. We used data from the geographically diverse Environmental influences on Child health Outcomes (ECHO) Cohort Study. We tested the hypothesis that exposure to an arsenic violation in drinking water is associated with birth weight, gestational age at birth, preterm birth, small for gestational age (SGA), and large for gestational age (LGA).
2. Methods
2.1. Study Population
We used data from 51 cohorts contributing to the national ECHO-wide Cohort Study, a consortium of 69 diverse cohorts in the US and Puerto Rico. The ECHO-wide cohort has been previously described in detail (Knapp et al., 2023). Briefly, the aim of the ECHO-wide cohort is to explore the intersection of early life environment and child health outcomes, including perinatal outcomes. A rich collection of extant and new data collected under the ECHO-wide Data Collection Protocol is available for analysis (ECHO Program Materials, 2022). All data collection and research methods were IRB approved. All participants provide written informed consent at enrollment.
Study inclusion and exclusion criteria are summarized in Supplemental Figure 1. There were 59,246 ECHO pregnancies as of the August 2022 data lock. For the present analysis, we excluded mother-child dyads with no available prenatal residential history and birth outcomes data. Over 35% of pregnancies from the contributing cohorts were not enrolled to the full ECHO-wide protocol and therefore were ineligible to be included in the analysis. We excluded mother-child dyads without residential history data beginning three months prior to the date of conception (estimated from gestational age at birth and date of birth) and continuing through delivery, as well as participants missing any outcome data (birth weight, gestational age at birth, preterm birth, SGA, or LGA). We focused our analysis on ECHO participants in the contiguous US, therefore excluding mother-child dyads with prenatal residential addresses in Hawaii, Alaska, and Puerto Rico. We limited our analysis to singleton births because multiple gestations are associated with the birth outcomes examined in this study (Heino et al., 2016; Warner et al., 2000). We excluded children born before 2006, the year the EPA arsenic standard for drinking water was reduced to 10 ppb. Four cohorts with fewer than 30 participants in the analytic sample were also removed to improve model convergence. Our final analytic sample included 15,342 mother-child dyads.
2.2. Birth Outcomes
The primary outcomes of interest were birth weight (in grams), gestational age at birth (in weeks), preterm birth (binary), SGA (binary), and LGA (binary). Birth weight was ascertained from medical records (n=9,886; 64.4%), parent or caregiver report (n=5,300; 34.5%), or study staff measurements (n=156; 1.0%). Preterm birth was defined as infants delivered less than 37 completed weeks of gestation. Gestational age at birth was ascertained from obstetrical estimates based on the date of last menstrual period and ultrasound findings (n=7,840; 51.1%), maternal self-report (n=6,014; 39.2%), or other means (n=1,488; 9.7%). Age- and sex-specific birth weight percentiles were used to classify children as SGA (<10th percentile) and LGA (>90th percentile) based on INTERGROWTH-21st fetal growth standards (Papageorghiou et al., 2018).
2.3. Preconception and Prenatal Arsenic Violation Exposure
Arsenic violations in drinking water were defined as occurrences of arsenic concentrations in PWS exceeding 10 ppb. Data on arsenic violations and attributes of PWS were obtained from the Safe Drinking Water Information System (SDWIS), a federal database monitored by the EPA, following the 2022 Quarter 4 update (EPA, n.d.). This system contains drinking water violations for all PWS in the US, as required by the SDWA. By definition, PWS regularly serve drinking water to at least 15 service connections or an average of 25 people per day for 60 or more days each year (US EPA, 2015). PWS were categorized by system type. Community water systems (CWS) supply water to a consistent population year-round. Transient- and non-transient non-community water systems often do not serve consistent populations or do not serve populations year-round. Additionally, transient non-community water systems are not subject to Final Arsenic Rule regulations. Therefore, we only included CWS in our analysis. Public water systems were further categorized by the number of people served based on EPA guidelines: very small (<500 served), small (501 – 3,300 served), medium (3,301 – 10,000 served), large (10,001 – 100,000 served), and very large (>100,000 served). There are concerns of inadequate reporting practices among very small PWS (Rubin, 2013). Therefore, to ensure reliable data, very small systems were excluded from the analysis.
Geographic areas served by PWS were obtained from SDWIS. The names of the counties served by each system were matched to corresponding Federal Information Processing Standards (FIPS) codes using the tigris package in RStudio. PWS with missing data regarding the counties served by the system were excluded from the analysis. Water system inclusion and exclusion criteria are summarized in Supplemental Figure 2.
Residential address history data were geocoded using ArcGIS, with more than 85% of observations geocoded as high quality. Prenatal residential addresses were available for ECHO participants for each month beginning three months prior to conception and continuing until birth. We matched participants with arsenic violations by time and location. Participants were classified as exposed to arsenic violations in public drinking water if they resided in a county with an active violation between three months prior to conception and birth. Four exposure windows were defined: (1) preconception (three months prior to conception to estimated date of conception); (2) first trimester (1st-13th week of pregnancy); (3) second trimester (14th-26th week of pregnancy); and (4) third trimester (27th week of pregnancy to delivery). Participants could be exposed during multiple exposure windows. Therefore, we had two exposure variables: a binary exposure variable (any exposure vs. no exposure) and a count exposure variable, calculated as the number of exposure windows in which a participant was exposed, ranging from zero to four.
2.4. Covariates
Covariate selection was completed a priori and based on prior literature and data availability (Almberg et al., 2017; Shih et al., 2020). Selected potential maternal confounders included: age at delivery (in years), year of delivery (in years), infant sex (male, female), maternal race (White, Black, other), maternal ethnicity (Hispanic, non-Hispanic), pre-pregnancy body mass index (underweight, normal weight, overweight, obese), any nicotine use during pregnancy (yes/no), education (less than high school, high school degree or equivalent, some college, bachelor’s degree, master’s degree and above), gestational diabetes (yes/no), and hypertensive disorders of pregnancy (yes/no). We controlled for year of delivery to account for temporal trends in both arsenic violations and birth outcomes (Foster et al., 2019; Freaney et al., 2022). Self-reported race and ethnicity were collected at baseline recognizing that these social constructs are strong predictors of environmental exposures (Flanagin et al., 2021; Geron et al., 2022). Hypertensive disorders of pregnancy included gestational hypertension, preeclampsia, eclampsia, and chronic hypertension.
2.5. Statistical Analysis
We calculated the number of water systems with violations in each contiguous US county and produced a choropleth map of these counts in relation to preconception and prenatal residential histories. We computed and compared the differences in the incidence of birth outcomes in exposed and unexposed mother-child dyads. We calculated intraclass correlation coefficients (ICC) to evaluate clustering of participants by (1) county and (2) original cohort site. The ICCs revealed non-ignorable cohort site clustering. Therefore, multivariable, mixed effects regression models, with a random intercept for each cohort, were applied to estimate associations between county-level arsenic violations in drinking water and birth outcomes. Analyses were performed using linear regression for continuous outcomes (birth weight and gestational age at birth) and logistic regression for dichotomous outcomes (preterm birth, SGA, and LGA). Arsenic exposure was treated as a dichotomous variable (exposed vs. unexposed) in the binary model and a categorical variable (unexposed vs. 1-3 windows vs. 4 windows) in the count model. Multiple imputations by chained equations (MICE), using 10 iterations and 10 imputations, were implemented to account for missing data using the mice package in RStudio.
Several sensitivity analyses were conducted to test the robustness of our findings. First, we implemented a leave-one-out analysis to evaluate any potential unexpected influence of individual cohorts. In our main analysis, we only included PWS that served more than 500 persons. We repeated our analysis to compare our findings after modifying the cutoff size for population served by CWS to assess the robustness of our results to this decision. We also excluded cohorts with inclusion criteria that were related to our exposure or outcome. Five cohorts in this analysis exclusively enrolled preterm infants. One cohort exclusively enrolled participants who accessed drinking water from private wells. We re-ran analyses excluding preterm cohorts, and further excluding the cohort that enrolled participants who accessed drinking water from private wells. Since arsenic exposure is associated with diabetes, we also ran analyses excluding participants who reported gestational diabetes (Kirkley et al., 2018).
Statistical analyses were performed using R (Version 4.1.0, R Foundation for Statistical Computing). Maps were created using ArcGIS Pro (Release 2.7.0, Environmental Systems Research Institute). All statistical tests were two-sided, with a p-value of less than 0.05 indicating statistical significance.
3. Results
Among the 15,342 mother-child dyads in the analytic sample, 794 (5.2%) resided in a county that had an active arsenic violation in a regulated PWS between three months prior to conception and birth. The remaining 14,548 (94.8%) mother-child dyads were not exposed to arsenic violations in drinking water during this time frame. Among the exposed mother-child dyads, 667 (84.0%) were exposed during the preconception period, 668 (84.1%) during the 1st trimester, 670 (84.4%) during the 2nd trimester, and 599 (75.4%) during the 3rd trimester (Supplemental Figure 3). More than half of the exposed mother-child dyads were exposed during all four exposure windows (n=491; 61.8%). An additional 118 (14.9%), 101 (12.7%), and 84 (10.6%) mother-child dyads were exposed during three, two, and one exposure windows, respectively.
Maternal socio-demographics and risk factors are presented in Table 1. Most mothers reported White race (n=9,990; 68.2%), non-Hispanic ethnicity (n=12,256; 82.0%), and attained a bachelor’s degree or higher (n=5,157; 51.2%). The average age of mothers at birth was 30 years. Mothers with exposure to arsenic violations were more likely to be Hispanic than unexposed mothers (49.2% vs. 16.3%). Additionally, mothers exposed to arsenic violations were more likely to be diagnosed with gestational diabetes (18.1% vs. 8.2%) and hypertensive disorders of pregnancy (15.1% vs. 12.8%), and less likely to report tobacco or cigarette use during pregnancy (4.4% vs. 9.2%).
Table 1:
Distribution of demographic factors for ECHO-wide Cohort Study participants, by exposure status.
| Characteristic | Overall (N=15,342) |
Exposed (N=794) |
Unexposed (N=14,548) |
|---|---|---|---|
| Maternal age at birth | 30.25 (5.54) | 30.24 (5.94) | 30.25 (5.52) |
| Missing | 97 | 1 | 96 |
| Year of birth | 2014 (3.72) | 2014 (4.35) | 2014 (3.68) |
| Infant sex | |||
| Male | 7,968 (51.94%) | 421 (53.02%) | 7,547 (51.88%) |
| Female | 7,374 (48.06%) | 373 (46.98%) | 7,001 (48.12%) |
| Maternal race | |||
| White | 9,990 (68.15%) | 580 (74.74%) | 9,410 (67.79%) |
| Black | 2,257 (15.40%) | 67 (8.63%) | 2,190 (15.78%) |
| Other | 2,411 (16.45%) | 129 (16.62%) | 2,282 (16.44%) |
| Missing | 684 | 18 | 666 |
| Maternal ethnicity | |||
| Hispanic | 2,700 (18.05%) | 387 (49.24%) | 2,313 (16.32%) |
| non-Hispanic | 12,256 (81.95%) | 399 (50.76%) | 11,857 (83.68%) |
| Missing | 386 | 8 | 378 |
| Pre-pregnancy BMI | |||
| Underweight | 364 (2.79%) | 19 (2.56%) | 345 (2.81%) |
| Normal weight | 5,933 (45.54%) | 276 (37.25%) | 5,657 (46.04%) |
| Overweight | 3,254 (24.98%) | 214 (28.88%) | 3,040 (24.74%) |
| Obese | 3,477 (26.69%) | 232 (31.31%) | 3,245 (26.41%) |
| Missing | 2,314 | 53 | 2,261 |
| Any tobacco or nicotine use | 1,176 (8.98%) | 31 (4.43%) | 1,145 (9.24%) |
| Missing | 2,250 | 94 | 2,156 |
| Maternal education | |||
| Less than high school | 839 (8.33%) | 106 (19.27%) | 733 (7.70%) |
| High school or equivalent | 1,730 (17.18%) | 123 (22.36%) | 1,607 (16.89%) |
| Some college; Trade school | 2,341 (23.25%) | 175 (31.82%) | 2,166 (22.76%) |
| Bachelor's | 2,723 (27.05%) | 82 (14.91%) | 2,641 (27.75%) |
| Master's or above | 2,434 (24.18%) | 64 (11.64%) | 2,370 (24.90%) |
| Missing | 5,275 | 244 | 5,031 |
| Gestational diabetes | 1,053 (8.77%) | 128 (18.10%) | 925 (8.19%) |
| Missing | 3,337 | 87 | 3,250 |
| Hypertensive disorder | 1,824 (12.89%) | 109 (15.08%) | 1,715 (12.77%) |
| Missing | 1,193 | 71 | 1,122 |
Abbreviations: BMI: body mass index.
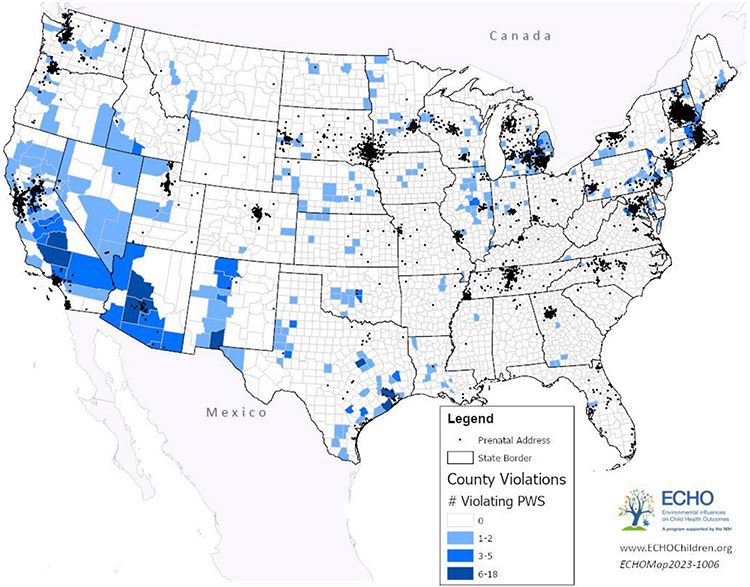
Figure 1 shows the number of CWS per county, serving more than 500 persons, with arsenic violations in the contiguous US from 2006 to 2022, and residential histories of study participants. A majority of counties were served by only one or two water systems with arsenic violations (n=227; 84.1%). There were 498 CWS with arsenic violations in the contiguous US between 2006 and 2022, for a total of 7,037 violations, and a median of 6 violations per system (IQR: 1-16.8). ECHO participants were served by 91 (18.3%) of the 498 CWS with arsenic violations. In the contiguous US, there were 270 counties served by at least one CWS, serving more than 500 persons, with an arsenic violation between 2006 and 2022.
Figure 1: Number of violating public water systems, serving 500 or more persons, per county, from 2006 to 2022, and ECHO-wide Cohort Study participant’s residential histories.
Abbreviations: PWS: public water systems.
The mean birth weight in the study population was 3,265.2 grams, and the mean gestational age at birth was 38.4 weeks (Table 2). Overall, the incidence of preterm birth, SGA, and LGA were 11.0% (n=1,684), 6.2% (n=947), and 16.7% (n=2,555), respectively. The incidence of preterm birth was greater among exposed infants compared with unexposed infants (15.0% vs. 10.8%). Incidences of SGA and LGA were similar between exposed and unexposed infants (5.3% vs. 6.2%; 18.4% vs. 16.6%, respectively). When comparing infants exposed during 1-3 exposure windows versus 4 exposure windows, the average birth weight and gestational age at birth was lowest among children with 1-3 windows of exposure (3,054.1 vs. 3,286.0 grams; 37.2 vs. 38.4 weeks, respectively). The incidence of preterm birth was higher among infants exposed during 1-3 windows of exposure (19.8% vs. 12.0%). The incidence of SGA and LGA were similar between infants exposed during 1-3 windows of exposure and infants exposed during 4 windows of exposure (5.9% vs. 4.9%; 19.8% vs. 17.5%, respectively).
Table 2:
Mean and incidence of perinatal outcomes among ECHO-wide Cohort Study participants, by exposure status.
| N | Birth weight mean (SD) |
Gestational age mean (SD) |
Preterm birth n (%) |
SGA n (%) |
LGA n (%) |
|
|---|---|---|---|---|---|---|
| Overall | 15,342 | 3,265.24 (683.87) | 38.42 (2.79) | 1,684 (10.98%) | 947 (6.17%) | 2,555 (16.65%) |
| Exposed | 794 | 3,197.50 (791.72) | 37.93 (3.50) | 119 (14.99%) | 42 (5.29%) | 146 (18.39%) |
| 1-3 Windows | 303 | 3,054.05 (991.39) | 37.15 (4.73) | 60 (19.80%) | 18 (5.94%) | 60 (19.80%) |
| 4 Windows | 491 | 3,286.02 (622.97) | 38.41 (2.33) | 59 (12.02%) | 24 (4.89%) | 86 (17.52%) |
| Unexposed | 14,548 | 3,268.94 (677.33) | 38.45 (2.74) | 1,565 (10.76%) | 905 (6.22%) | 2,409 (16.56%) |
Abbreviations: SGA: small for gestational age; LGA: large for gestational age; SD: standard deviation.
Detailed results from bivariate analyses of associations between exposure to arsenic violations and birth outcomes are found in Table 3. Bivariate analyses identified negative associations between preconception and prenatal exposure to arsenic violations in drinking water and both birth weight (−71.4 grams; 95% CI: −120.3, −22.6) and gestational age at birth (−0.5 weeks; 95% CI: −0.7, −0.3), and increased odds of preterm birth (1.5; 95% CI: 1.2, 1.8). All individual-level confounders were significantly associated with multiple birth outcomes. Hispanic ethnicity, for example, was significantly associated with decreased birth weight (−36.7 grams; 95% CI: −65.2, −8.2 and decreased odds for LGA (0.9, 95% CI: 0.8, 1.0).
Table 3:
Bivariable associations between prenatal arsenic exposure in drinking water and demographic factors with perinatal outcomes among participants in the ECHO-wide Cohort Study.
| N | Birth weight Est. (95% CI) |
Gestational age Est. (95% CI) |
Preterm birth OR (95% CI) |
SGA OR (95% CI) |
LGA OR (95% CI) |
|
|---|---|---|---|---|---|---|
| Exposure status | 15,342 | |||||
| Unexposed | Ref | Ref | Ref | Ref | Ref | |
| Exposed | −71.44 (−120.28, −22.60) | −0.52 (−0.72, −0.32) | 1.46 (1.19, 1.78) | 0.84 (0.60, 1.14) | 1.14 (0.94, 1.36) | |
| Maternal age at birth | 15,245 | 8.91 (6.97, 10.85) | 0.01 (0.00, 0.02) | 0.99 (0.99, 1.00) | 0.97 (0.96, 0.98) | 1.03 (1.02, 1.04) |
| Year of birth | 15,342 | −1.26 (−4.17, 1.65) | −0.02 (−0.03, 0.00) | 0.99 (0.98, 1.01) | 0.98 (0.96, 0.99) | 0.99 (0.98, 1.01) |
| Infant sex | 15,342 | |||||
| Male | Ref | Ref | Ref | Ref | Ref | |
| Female | −102.66 (−124.26, −81.06) | 0.15 (0.06, 0.24) | 0.91 (0.82, 1.01) | 1.03 (0.90, 1.18) | 0.97 (0.89, 1.05) | |
| Maternal race | 14,658 | |||||
| White | Ref | Ref | Ref | Ref | Ref | |
| Black | 341.70 (−372.63, −310.77) | −0.82 (−1.00, −0.69) | 1.77 (1.55, 2.01) | 2.48 (2.11, 2.91) | 0.39 (0.33, 0.46) | |
| Other | −119.15 (−149.26, −89.04) | −0.19 (−0.32, −0.07) | 1.19 (1.03, 1.37) | 1.47 (1.23, 1.76) | 0.69 (0.61, 0.78) | |
| Maternal ethnicity | 14,956 | |||||
| non-Hispanic | Ref | Ref | Ref | Ref | Ref | |
| Hispanic | −36.71 (−65.21, −8.22) | −0.10 (−0.21, 0.02) | 1.09 (0.96, 1.25) | 0.95 (0.79, 1.13) | 0.86 (0.76, 0.96) | |
| Pre-pregnancy BMI | 13,028 | |||||
| Underweight | Ref | Ref | Ref | Ref | Ref | |
| Normal weight | 198.38 (126.78, 269.99) | 0.50 (0.21, 0.79) | 0.66 (0.48, 0.92) | 0.58 (0.41, 0.84) | 1.85 (1.28, 2.78) | |
| Overweight | 230.72 (157.43, 304.01) | 0.25 (−0.04, 0.55) | 0.88 (0.64, 1.23) | 0.57 (0.40, 0.84) | 2.80 (1.93, 4.21) | |
| Obese | 200.08 (127.03, 273.13) | −0.09 (−0.39, 0.20) | 1.08 (0.79, 1.51) | 0.50 (0.35, 0.73) | 2.99 (2.06, 4.49) | |
| Any tobacco or nicotine use | 13,092 | |||||
| No | Ref | Ref | Ref | Ref | Ref | |
| Yes | −137.53 (−178.77, −96.29) | −0.44 (−0.61, −0.27) | 1.38 (1.15, 1.63) | 1.71 (1.38, 2.10) | 0.87 (0.73, 1.03) | |
| Maternal education | 10,067 | |||||
| Less than high school | Ref | Ref | Ref | Ref | Ref | |
| High school or equivalent | 26.25 (−28.51, 81.00) | 0.03 (−0.19, 0.26) | 0.96 (0.75, 1.22) | 0.83 (0.62, 1.12) | 0.94 (0.73, 1.20) | |
| Some college; Trade school | 79.67 (27.30, 132.04) | 0.01 (−0.21, 0.22) | 0.99 (0.79, 1.25) | 0.68 (0.51, 0.92) | 1.22 (0.97, 1.54) | |
| Bachelor's | 240.26 (188.87, 291.65) | 0.74 (0.53, 0.95) | 0.52 (0.41, 0.66) | 0.54 (0.40, 0.72) | 1.46 (1.17, 1.84) | |
| Master's or above | 240.31 (188.21, 292.42) | 0.82 (0.61, 1.00) | 0.47 (0.37, 0.61) | 0.55 (0.41, 0.75) | 1.29 (1.03, 1.63) | |
| Gestational diabetes | 12,005 | |||||
| No | Ref | Ref | Ref | Ref | Ref | |
| Yes | 3.20 (−39.13, 45.52) | −0.52 (−0.69, −0.35) | 1.38 (1.14, 1.65) | 0.62 (0.45, 0.84) | 1.41 (1.20, 1.65) | |
| Hypertensive disorder | 14,149 | |||||
| No | Ref | Ref | Ref | Ref | Ref | |
| Yes | −371.87 (−405.37, −338.37) | −1.70 (−1.80, −1.60) | 3.24 (2.86, 3.65) | 2.30 (1.95, 2.70) | 0.78 (0.68, 0.90) |
Abbreviations: SGA: small for gestational age; LGA: large for gestational age; Est.: estimate; CI: confidence interval; OR: odds ratio; BMI: body mass index.
Note: Estimates in bold are statistically significant.
Multivariable adjusted estimates for the five perinatal outcomes are presented in Table 4. When using a binary exposure variable, there was no evidence of an association between any preconception or prenatal exposure to arsenic in drinking water and birth weight, after adjusting for individual-level confounders (34.7 grams; 95% CI: −18.9, 88.3). Similarly, when using a count exposure variable, there was no evidence of a difference in birth weight among infants with 1-3 windows of exposure compared with unexposed infants (5.4 grams; 95% CI: −57.4, 68.2). There was, however, a statistically significant increase in birth weight among infants with continuous exposure (from three months prior to conception through birth) compared with unexposed infants (88.8 grams; 95% CI: 8.2, 169.5). In models using either a binary exposure variable or an ordinal exposure variable, there were no statistically significant associations between preconception and prenatal arsenic exposure and gestational age at birth, preterm birth, SGA, or LGA. Fully adjusted models are available in Supplemental Table 3 and Supplemental Table 4.
Table 4:
Covariate-adjusted associations between prenatal arsenic exposure in drinking water and perinatal outcomes in the ECHO-wide Cohort Study, using multivariable mixed models.
| Birth weight Est. (95% CI) |
Gestational age Est. (95% CI) |
Preterm birth OR (95% CI) |
SGA OR (95% CI) |
LGA OR (95% CI) |
|
|---|---|---|---|---|---|
| Binary Model | |||||
| Unexposed | Ref | Ref | Ref | Ref | Ref |
| Exposed | 34.71 (−18.92, 88.34) | −0.02 (−0.20, 0.17) | 0.95 (0.65, 1.39) | 0.80 (0.54, 1.18) | 1.15 (0.91, 1.47) |
| Count Model | |||||
| Unexposed | Ref | Ref | Ref | Ref | Ref |
| 1-3 Windows | 5.40 (−57.35, 68.15) | −0.09 (−0.31, 0.13) | 0.95 (0.60, 1.50) | 0.88 (0.53, 1.46) | 1.16 (0.86, 1.57) |
| 4 Windows | 88.84 (8.22, 169.46) | 0.11 (−0.17, 0.39) | 0.95 (0.56, 1.63) | 0.72 (0.42, 1.23) | 1.15 (0.81, 1.63) |
Abbreviations: SGA: small for gestational age; LGA: large for gestational age; Est.: estimate; CI: confidence interval; OR: odds ratio.
Note: Models adjusted for maternal age at birth (scaled), year of birth (scaled), infant sex, maternal race, maternal ethnicity, pre-pregnancy BMI, any tobacco or nicotine use, maternal education, gestational diabetes, and gestational hypertension. Estimates in bold are statistically significant.
Sensitivity analyses revealed that findings differed when comparing models with different cutoff criteria for the size of the populations served by the PWS (Supplemental Table 5). When there was no cutoff criterion (i.e., when the water systems serving fewer than 500 persons are included), we found statistically significant associations between any arsenic violations exposure and increased birth weight (51.1 grams; 95% CI: 20.9, 81.2), lower odds of SGA (OR=0.7; 95% CI: 0.6, 0.9), and higher odds of LGA (OR=1.2; 95% CI: 1.0, 1.4). Similarly, when we raised the cutoff criterion for water systems to 3,300 persons served, there was a statistically significant association with higher birth weight among exposed infants (77.8 grams; 95% CI: 3.5, 152.0). When we only included large and very large PWS (serving more than 10,000 persons), the odds ratio of SGA comparing exposed with unexposed infants was 0.4 (95% CI: 0.1, 0.9). Findings were similar to our main results when we excluded the five cohorts that exclusively enrolled preterm infants (Supplemental Table 6). The observed trends in perinatal outcomes were similar when we additionally excluded the cohort with well-water usage (Supplemental Table 7). When excluding participants reporting gestational diabetes, effect estimates were slightly attenuated towards the null (Supplemental Table 8). The leave-one-out analysis did not reveal any cohorts that skewed effect estimates (Supplemental Figure 6).
4. Discussion
We evaluated associations of preconception and prenatal exposure to arsenic violations in regulated PWS with five perinatal outcomes: birth weight, gestational age at birth, preterm birth, SGA, and LGA, using a sample of mother-child dyads from the ECHO-wide Cohort Study. Residence in a county served by a PWS serving more than 500 persons with an arsenic violation during the three months prior to conception or any trimester of pregnancy was not significantly associated with any of the five birth outcomes after adjusting for individual-level confounders. However, living in a county served by a PWS with an arsenic violation for the entire prenatal period (preconception through 3rd trimester) was associated with a higher birth weight compared with unexposed participants in our adjusted models.
The toxicokinetic mechanism by which arsenic affects birth outcomes is not well understood (Vahter, 2009). It is hypothesized that exposure to arsenic during pregnancy triggers inflammatory response via increased oxidative stress (Ahmed et al., 2011). Inflammation may serve as a catalyst for preterm birth through the release of prostaglandin and other inflammatory mediators which induce uterine contractions (Romero et al., 2006). Studies have found that placental inflammatory response is associated with poor neonatal growth (Mestan et al., 2010). One hypothesized pathway regarding this association is that inflammation may alter the placenta’s capacity to transport essential nutrients that promote fetal development (Gaccioli & Lager, 2016). Additionally, there is some evidence that inflammation increases the risk of placental abruption, which can result in preterm births (Nath et al., 2007).
We observed higher birth weight among infants of mothers with prenatal arsenic exposure. Arsenic exposure has been linked to both metabolic syndrome and diabetes (Kirkley et al., 2018; Pánico et al., 2022). Therefore, it is plausible that this association may have been mediated by the high prevalence of gestational diabetes in the exposed ECHO population. This is supported by our finding of attenuated estimates after excluding participants with gestational diabetes from the study population, but the overall effect estimate for birth weight remained positive. It is also possible that unmeasured confounders, such as maternal diet, well water usage, and bottled water consumption, could have biased our results. Nevertheless, higher term birth weight in association with arsenic exposure has been observed in prior literature. One study in Inner Mongolia, China (N = 9,890) reported higher birth weight (50.0 grams; 95% CI: 20.0, 80.0) among participants exposed to very high levels of arsenic (>100 ppb) in well water (Myers et al., 2010). Another study in Tenerife, Spain (N = 76) found elevated arsenic concentrations in meconium biomarkers to be associated with birth weight (223.8 grams; p-value=0.043) (Vall et al., 2012). Still, there are several studies indicating that infants born to mothers with prenatal arsenic exposure have lower birth weight (Hopenhayn et al., 2003; Kile et al., 2015; Yang et al., 2003). Further research is needed to understand these differences and the underlying mechanisms by which arsenic may influence fetal growth.
We observed non-significant differences in gestational duration and odds of preterm birth among the exposed infants in our study. Previous studies have also reported null findings regarding associations between arsenic exposure and preterm birth, including the studies in Inner Mongolia, China and Tenerife, Spain (Myers et al., 2010; Vall et al., 2012). Other studies, including one conducted in Bangladesh, found that arsenic exposure was associated with reductions in gestational age at birth (Kile et al., 2015). However, the study in Bangladesh was conducted in a region with high elevated average exposures and almost exclusively well water consumption.
We observed lower odds of SGA and higher odds of LGA among the infants exposed to arsenic violations in our adjusted models, although these associations were not statistically significant. SGA and LGA are not as frequently explored in the epidemiologic literature as birth weight, gestational age at birth, and preterm birth. One study in Mexico City found that maternal blood arsenic concentrations at delivery were associated with significantly higher odds of both SGA and LGA; however, these associations were not statistically significant when using 2nd and 3rd trimester blood arsenic concentrations (Mullin et al., 2019). Nevertheless, our findings have important public health implications as both SGA and LGA are linked to various health consequences for the mother and infant throughout the life-course, including cardiovascular disease, obesity, and kidney health (Mishra et al., 2014; Nam & Lee, 2018; Sjöholm et al., 2021).
To the best of our knowledge, this is the first epidemiologic study to explore the relation between arsenic violations in regulated PWS and birth outcomes at a national level. There have been smaller-scale US-based studies that have utilized SDWIS data, and they have found similar results. One study based in Virginia examined associations between multiple SDWA violations, including arsenic, and birth outcomes, including preterm birth and low birth weight. Similar to our study, the authors found no associations between health-based arsenic violations and any birth outcomes (Young et al., 2023). Another study based in Ohio investigated associations between arsenic violations in drinking water and five birth outcomes, including preterm birth and SGA. While the authors did identify higher odds of preterm birth, they found no association with SGA (Almberg et al., 2017). However, this study was not able to track participants who moved during pregnancy, and did not exclude very small PWS from the analysis.
Hispanic individuals had significantly higher rates of exposure to arsenic violations in drinking water compared to non-Hispanic individuals. We also found statistically significant differences in birth outcomes based on maternal race and ethnicity. Similar environmental justice implications have been noted in previous literature (Balazs et al., 2012; McDonald & Jones, 2018), with evidence that low levels of exposure to arsenic may have adverse birth consequences on disparate populations (Howe et al., 2020). Of note, when we revised the population served cutoff criteria for PWS to include very small systems, we found significant differences in birth weight, odds of SGA, and odds of LGA. It is possible that including smaller systems may capture more rural areas that are categorized by disparities in socio-demographics and infrastructures, as well as additional marginalized populations that are vulnerable to environmental contamination. Furthermore, the Southwest region of the U.S. experienced a large number of arsenic violations, and the proportion of individuals of Hispanic ethnicity in the population is higher in many Southwest counties. However, it should be noted that the ECHO cohorts do not fully represent the geographic distribution of racial and ethnic groups throughout the US (Knapp et al., 2023).
Our study results are subject to several limitations. First, arsenic exposure was measured via a proxy of residing in a county with an arsenic water violation during preconception or pregnancy. We recognize that most water systems do not serve all persons within a county and that multiple systems may serve the same county. However, due to the nature of SDWIS data, aggregating to a county-level was the finest granularity we could achieve. We attempted to mitigate the degree of exposure misclassification by excluding very small water systems from our analysis. However, differences in effect estimates when applying different population served cutoff criteria for PWS revealed that our exposure classification was not robust to this analytic decision, which may be attributable to the fact that a large majority of arsenic MCL exceedances occur in very small water systems (Foster et al., 2019). Our study would have benefited from more detailed water system service boundaries. However, very few states have publicly available data on water system service areas. The structure of ECHO data gives rise to the potential of measurement error in our classification of exposure windows. Residential history data are collected monthly, and trimesters are based on weeks of gestation instead of days. We also had limited knowledge on the external dose of arsenic in drinking water. For systems with arsenic violations, we knew that the measured concentration of source water used by the PWS surpassed the MCL of 10 ppb. However, we had no continuous measures of the concentration of arsenic in water systems, so we were unable to assess a dose-response relationship between arsenic concentrations and birth outcomes. We also had no information on the true arsenic concentrations of the individual households of our study participants and thus non-differential misclassification was likely. Additionally, violations are assigned for a specified duration – often quarterly – and are based on the running average of concentrations measured from each site. Therefore, it is possible that a PWS was in violation of the arsenic MCL before the violation was in effect, and concentrations may have fallen below the MCL before the violation was considered corrected. We had insufficient information on private well usage, bottled water usage, and diet for our study participants. In many regions of the US, private drinking-water wells frequently exceed the MCL of 10 ppb (Water Resources, 2019). Rural areas have a higher proportion of private well users compared to urban areas. Therefore, it is possible that our estimates of association may be biased and that rural areas may be underrepresented in this study. Our results are conservative because participants are exposed to multiple water sources, including in homes, schools, and workplaces, that may be in counties different from their residential address. Furthermore, this study only looked at a single exposure – arsenic in drinking water. Environmental exposures most often occur as complex mixtures. Co-exposure to multiple contaminants can influence the toxicity of a single metal (Rechtman et al., 2020). Future analyses could utilize SDWIS data to assess exposure mixtures, which may uncover associations that were not present in this single-contaminate study.
Despite the aforementioned limitations, our study had several important strengths. First, our study benefited from the quality and depth of our residential history data, which allowed us to capture exposure to arsenic violations in PWS over different periods of preconception and pregnancy. There are critical periods to fetal growth, and environmental influences during these periods can disrupt fetal development (Colombo et al., 2019). Additionally, our analysis was able to account for mothers who moved during pregnancy. Residential mobility is common during pregnancy; an estimated 22% of mothers move during pregnancy, and 49% of those move between counties (Miller et al., 2010). In our sample, 1,403 (9.1%) mothers moved at least once between preconception and birth. Failure to capture residential mobility could lead to misclassification of the exposure, which would bias the association between arsenic exposure and birth outcomes. Relatedly, our knowledge of participant movement during the study period allowed us to more accurately define the timing of exposures at various gestational milestones. Our ability to study the duration of exposures to arsenic violations in drinking water is unique to our analysis and helps inform the need for more stringent monitoring of PWS. Our study expands upon the existing research due to its large sample size. We were able to study associations between arsenic exposure and birth outcomes among a geographically, temporally, and sociodemographically diverse population. Our large sample size allowed for greater statistical power to detect associations between arsenic exposure in drinking water and perinatal outcomes, compared with previous studies conducted in smaller samples.
5. Conclusion
To our knowledge, this is the first nationwide study of the association between arsenic violations and perinatal outcomes in a region with regulated PWS and low- to moderate-levels of arsenic exposure. Overall, arsenic violations in drinking water are uncommon in the US. Residing in a county with a water system in violation of the arsenic MCL of 10 ppb during pregnancy did not appear to be associated with increased risk for select birth outcomes. These findings may be partially attributable to protective effects of SDWA regulations. However, this study and future studies of similar nature would benefit from improved exposure characterization through more precise geodata of water system service areas, information on household drinking water sources, and exposure biomarkers. Further epidemiologic research is needed to corroborate these findings using both continuous measures of arsenic concentrations in drinking water and individual bioloads.
Supplementary Material
Arsenic violations in US public water systems were uncommon between 2006 and 2022
Continuous exposure to arsenic violations was associated with increased birth weight
Exposure to arsenic violations was not associated with adverse birth outcomes
Safe Drinking Water Act regulations may be protective of public health
Funding
This work was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health, under award numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), UH3OD023320 (J.L.A.), UH3OD023253 (C.A.C), UH3OD023248 (D.D), UH3OD023313 (S.D.), UH3OD023328 (C.S.D.), UH3OD023318 (A.L.D.), UH3OD023279 (A.J.E.), UH3OD023289 (A.F.), UH3OD023282 (J.E.G.), UH3OD023287 (C.V.B.), UH3OD023365 (I.H.P.), UH3OD023275 (M.R.K.), UH3OD023271 (C.J.K.), UH3OD023347 (B.M.L.), UH3OD023389 (L.D.L.), UH3OD023344 (D.M.), UH3OD023268 (S.T.W.), UH3OD023288 (C.T.M.), UH3OD023342 (K.L.), UH3OD023349 (T.G.O.C.), UH3OD023348 (M.O.S.), UH3OD023285 (J.M.K.), UH3OD023290 (J.B.H.), UH3OD023272 (S.L.S.), UH3OD023249 (J.B.S.), UH3OD023305 (L.T), UH3OD023337 (R.J.W.), K01ES032046 (A.S.D.), 5U19A1104317 and 5UG3OD023282 (C.B.), R00ES030400 (C.G.H.), and R00ES030403 (L.G.K.).
Abbreviations
- PWS
Public water system
- CWS
Community water system
- EPA
Environmental Protection Agency
- SDWA
Safe Drinking Water Act
- SDWIS
Safe Drinking Water Information System
- MCL
Maximum contaminant level
- ppb
Parts per billion
- SGA
Small for gestational age
- LGA
Large for gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Sharing
Deidentified data from the ECHO Program are available through NICHD’s Data and Specimen Hub (DASH), https://dash.nichd.nih.gov/. DASH is a centralized resource that allows researchers to access data from various studies via a controlled-access mechanism. Researchers can now request access to these data by creating a DASH account and submitting a Data Request Form. The NICHD DASH Data Access Committee will review the request and provide a response in approximately two to three weeks. Once granted access, researchers will be able to use the data for three years. See the DASH Tutorial for more detailed information on the process.
References
- Ahmed S, Khoda SM, Rekha RS, Gardner RM, Ameer SS, Moore S, Ekström E-C, Vahter M, & Raqib R (2011). Arsenic-Associated Oxidative Stress, Inflammation, and Immune Disruption in Human Placenta and Cord Blood. Environmental Health Perspectives, 119(2), 258–264. 10.1289/ehp.1002086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almberg KS, Turyk ME, Jones RM, Rankin K, Freels S, Graber JM, & Stayner LT (2017). Arsenic in drinking water and adverse birth outcomes in Ohio. Environmental Research, 157, 52–59. 10.1016/j.envres.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Balazs CL, Morello-Frosch R, Hubbard AE, & Ray I (2012). Environmental justice implications of arsenic contamination in California’s San Joaquin Valley: A cross-sectional, cluster-design examining exposure and compliance in community drinking water systems. Environmental Health, 11(1), 84. 10.1186/1476-069X-11-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Surdu S, Neamtiu IA, & Gurzau ES (2014). Maternal arsenic exposure and birth outcomes: A comprehensive review of the epidemiologic literature focused on drinking water. International Journal of Hygiene and Environmental Health, 217(7), 709–719. 10.1016/j.ijheh.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2017, April 7). Arsenic Factsheet. https://www.cdc.gov/biomonitoring/Arsenic_FactSheet.html [Google Scholar]
- Chung J-Y, Yu S-D, & Hong Y-S (2014). Environmental Source of Arsenic Exposure. Journal of Preventive Medicine and Public Health, 47(5), 253–257. 10.3961/jpmph.14.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J, Gustafson KM, & Carlson SE (2019). Critical and Sensitive Periods in Development and Nutrition. Annals of Nutrition and Metabolism, 75(Suppl. 1), 34–42. 10.1159/000508053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHO Program Materials. (2022, August). Environmental Influences on Child Health Outcomes. https://dcricollab.dcri.duke.edu/sites/echomaterials/SitePages/Home.aspx [Google Scholar]
- EPA. (n.d.). Data Downloads / ECHO; [Data set]. Retrieved November 8, 2022, from https://echo.epa.gov/tools/data-downloads [Google Scholar]
- Flanagin A, Frey T, Christiansen SL, & AMA Manual of Style Committee. (2021). Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA, 326(7), 621–627. 10.1001/jama.2021.13304 [DOI] [PubMed] [Google Scholar]
- Foster SA, Pennino MJ, Compton JE, Leibowitz SG, & Kile ML (2019). Arsenic Drinking Water Violations Decreased across the United States Following Revision of the Maximum Contaminant Level. Environmental Science & Technology, 53(19), 11478–11485. 10.1021/acs.est.9b02358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freaney PM, Harrington K, Molsberry R, Perak AM, Wang MC, Grobman W, Greenland P, Allen NB, Capewell S, O’Flaherty M, Lloyd-Jones DM, & Khan SS (2022). Temporal Trends in Adverse Pregnancy Outcomes in Birthing Individuals Aged 15 to 44 Years in the United States, 2007 to 2019. Journal of the American Heart Association, 11(11), e025050. 10.1161/JAHA.121.025050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaccioli F, & Lager S (2016). Placental Nutrient Transport and Intrauterine Growth Restriction. Frontiers in Physiology, 7. 10.3389/fphys.2016.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geron M, Cowell W, Amarasiriwardena C, Andra SS, Carroll K, Kloog I, Wright RO, & Wright RJ (2022). Racial/ethnic and neighborhood disparities in metals exposure during pregnancy in the Northeastern United States. Science of The Total Environment, 820, 153249. 10.1016/j.scitotenv.2022.153249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundacker C, & Hengstschläger M (2012). The role of the placenta in fetal exposure to heavy metals. Wiener Medizinische Wochenschrift, 162(9–10), 201–206. 10.1007/s10354-012-0074-3 [DOI] [PubMed] [Google Scholar]
- Heino A, Gissler M, Hindori-Mohangoo AD, Blondel B, Klungsøyr K, Verdenik I, Mierzejewska E, Velebil P, Sól Ólafsdóttir H, Macfarlane A, Zeitlin J, & Euro-Peristat Scientific Committee. (2016). Variations in Multiple Birth Rates and Impact on Perinatal Outcomes in Europe. PLOS ONE, 11(3), e0149252. 10.1371/journal.pone.0149252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopenhayn C, Ferreccio C, Browning SR, Huang B, Peralta C, Gibb H, & Hertz-Picciotto I (2003). Arsenic Exposure from Drinking Water and Birth Weight: Epidemiology, 14(5), 593–602. 10.1097/01.ede.0000072104.65240.69 [DOI] [PubMed] [Google Scholar]
- Howe CG, Farzan SF, Garcia E, Jursa T, Iyer R, Berhane K, Chavez TA, Hodes TL, Grubbs BH, Funk WE, Smith DR, Bastain TM, & Breton CV (2020). Arsenic and birth outcomes in a predominately lower income Hispanic pregnancy cohort in Los Angeles. Environmental Research, 184, 109294. 10.1016/j.envres.2020.109294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi B, Moore F, Mosaferi M, & Rahmani F (2011). The Source of Natural Arsenic Contamination in Groundwater, West of Iran. Water Quality, Exposure and Health, 3(3–4), 135–147. 10.1007/s12403-011-0051-x [DOI] [Google Scholar]
- Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, Quamruzzaman Q, Rahman M, & Christiani DC (2015). Estimating effects of arsenic exposure during pregnancy on perinatal outcomes in a Bangladeshi cohort: Epidemiology, 1. 10.1097/EDE.0000000000000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkley AG, Carmean CM, Ruiz D, Ye H, Regnier SM, Poudel A, Hara M, Kamau W, Johnson DN, Roberts AA, Parsons PJ, Seino S, & Sargis RM (2018). Arsenic exposure induces glucose intolerance and alters global energy metabolism. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 314(2), R294–R303. 10.1152/ajpregu.00522.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp EA, Kress AM, Parker CB, Page GP, McArthur K, Gachigi KK, Alshawabkeh AN, Aschner JL, Bastain TM, Breton CV, Bendixsen CG, Brennan PA, Bush NR, Buss C, Camargo CA Jr, Catellier D, Cordero JF, Croen L, Dabelea D, … Environmental Influences On Child Health Outcomes, O. B. O. P. C. F. (2023). The Environmental influences on Child Health Outcomes (ECHO)-wide Cohort. American Journal of Epidemiology, kwad071. 10.1093/aje/kwad071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald YJ, & Jones NE (2018). Drinking Water Violations and Environmental Justice in the United States, 2011–2015. American Journal of Public Health, 108(10), 1401–1407. 10.2105/AJPH.2018.304621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestan K, Yu Y, Matoba N, Cerda S, Demmin B, Pearson C, Ortiz K, & Wang X (2010). Placental Inflammatory Response Is Associated With Poor Neonatal Growth: Preterm Birth Cohort Study. Pediatrics, 125(4), e891–e898. 10.1542/peds.2009-0313 [DOI] [PubMed] [Google Scholar]
- Miller A, Siffel C, & Correa A (2010). Residential Mobility During Pregnancy: Patterns and Correlates. Maternal and Child Health Journal, 14(4), 625–634. 10.1007/s10995-009-0492-z [DOI] [PubMed] [Google Scholar]
- Milton A, Hussain S, Akter S, Rahman M, Mouly T, & Mitchell K (2017). A Review of the Effects of Chronic Arsenic Exposure on Adverse Pregnancy Outcomes. International Journal of Environmental Research and Public Health, 14(6), 556. 10.3390/ijerph14060556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K, Datta V, Aarushi A, Kaur Narula M, Iyer RS, & Nangia S (2014). The Association between Weight for Gestational Age and Kidney Volume: A Study in Newborns in India. Iranian Journal of Pediatrics, 24(1), 93–99. [PMC free article] [PubMed] [Google Scholar]
- Mullin AM, Amarasiriwardena C, Cantoral-Preciado A, Claus Henn B, Leon Hsu H-H, Sanders AP, Svensson K, Tamayo-Ortiz M, Téllez-Rojo MM, Wright RO, & Burris HH (2019). Maternal blood arsenic levels and associations with birth weight-for-gestational age. Environmental Research, 177, 108603. 10.1016/j.envres.2019.108603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, Kwok RK, Mumford JL, & Mendola P (2010). Maternal drinking water arsenic exposure and perinatal outcomes in Inner Mongolia, China. Journal of Epidemiology & Community Health, 64(4), 325–329. 10.1136/jech.2008.084392 [DOI] [PubMed] [Google Scholar]
- Nam H-K, & Lee K-H (2018). Small for gestational age and obesity: Epidemiology and general risks. Annals of Pediatric Endocrinology & Metabolism, 23(1), 9–13. 10.6065/apem.2018.23.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath CA, Ananth CV, Smulian JC, Shen-Schwarz S, & Kaminsky L (2007). Histologic evidence of inflammation and risk of placental abruption. American Journal of Obstetrics and Gynecology, 197(3), 319.e1–319.e6. 10.1016/j.ajog.2007.06.012 [DOI] [PubMed] [Google Scholar]
- National Research Council. (1999). Arsenic in Drinking Water (p. 6444). National Academies Press, 10.17226/6444 [DOI] [Google Scholar]
- Pánico P, Velasco M, Salazar AM, Picones A, Ortiz-Huidobro RI, Guerrero-Palomo G, Salgado-Bernabé ME, Ostrosky-Wegman P, & Hiriart M (2022). Is Arsenic Exposure a Risk Factor for Metabolic Syndrome? A Review of the Potential Mechanisms. Frontiers in Endocrinology, 13, 878280. 10.3389/fendo.2022.878280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorghiou AT, Kennedy SH, Salomon LJ, Altman DG, Ohuma EO, Stones W, Gravett MG, Barros FC, Victora C, Purwar M, Jaffer Y, Noble JA, Bertino E, Pang R, Cheikh Ismail L, Lambert A, Bhutta ZA, & Villar J (2018). The INTERGROWTH-21st fetal growth standards: Toward the global integration of pregnancy and pediatric care. American Journal of Obstetrics and Gynecology, 218(2), S630–S640. 10.1016/j.ajog.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Punshon T, Davis MA, Marsit CJ, Theiler SK, Baker ER, Jackson BP, Conway DC, & Karagas MR (2015). Placental arsenic concentrations in relation to both maternal and infant biomarkers of exposure in a US cohort. Journal of Exposure Science & Environmental Epidemiology, 25(6), 599–603. 10.1038/jes.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, & Saigal S (2017). Adults born preterm: A review of general health and system-specific outcomes. Acta Paediatrica, 106(9), 1409–1437. 10.1111/apa.13880 [DOI] [PubMed] [Google Scholar]
- Rechtman E, Curtin P, Papazaharias DM, Renzetti S, Cagna G, Peli M, Levin-Schwartz Y, Placidi D, Smith DR, Lucchini RG, Wright RO, & Horton MK (2020). Sex-specific associations between co-exposure to multiple metals and visuospatial learning in early adolescence. Translational Psychiatry, 10(1), 358. 10.1038/s41398-020-01041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Kusanovic J, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, & Mazor M (2006). The preterm parturition syndrome. BJOG: An International Journal of Obstetrics & Gynaecology, 113, 17–42. 10.1111/j.1471-0528.2006.01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin SJ (2013). Evaluating violations of drinking water regulations. Journal - American Water Works Association, 105(3), E137–E147. 10.5942/jawwa.2013.105.0024 [DOI] [Google Scholar]
- Shih Y-H, Scannell Bryan M, & Argos M (2020). Association between prenatal arsenic exposure, birth outcomes, and pregnancy complications: An observational study within the National Children’s Study cohort. Environmental Research, 183, 109182. 10.1016/j.envres.2020.109182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöholm P, Pahkala K, Davison B, Niinikoski H, Raitakari O, Juonala M, & Singh GR (2021). Birth weight for gestational age and later cardiovascular health: A comparison between longitudinal Finnish and indigenous Australian cohorts. Annals of Medicine, 53(1), 2060–2071. 10.1080/07853890.2021.1999491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA. (n.d.). Drinking Water Arsenic Rule History. Drinking Water Requirements for States and Public Water Systems, https://www.epa.gov/dwreginfo/drinking-water-arsenic-rule-history [Google Scholar]
- US EPA. (2015, September 21). Information about Public Water Systems [Collections and Lists]. https://www.epa.gov/dwreginfo/information-about-public-water-systems [Google Scholar]
- Vahter M. (2009). Effects of Arsenic on Maternal and Fetal Health. Annual Review of Nutrition, 29(1), 381–399. 10.1146/annurev-nutr-080508-141102 [DOI] [PubMed] [Google Scholar]
- Vall O, Gómez-Culebras M, Garcia-Algar O, Joya X, Velez D, Rodriguez-Carrasco E, & Puig C (2012). Assessment of Prenatal Exposure to Arsenic in Tenerife Island. PLoS ONE, 7(11), e50463. 10.1371/journal.pone.0050463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaescusa I, & Bollinger J-C (2008). Arsenic in drinking water: Sources, occurrence and health effects (a review). Reviews in Environmental Science and Bio/Technology, 7(4), 307–323. 10.1007/s11157-008-9138-7 [DOI] [Google Scholar]
- Warner BB, Kiely JL, & Donovan EF (2000). MULTIPLE BIRTHS AND OUTCOME. Clinics in Perinatology, 27(2), 347–361. 10.1016/S0095-5108(05)70025-7 [DOI] [PubMed] [Google Scholar]
- Water Resources. (2019, March 1). Arsenic and Drinking Water. https://www.usgs.gov/mission-areas/water-resources/science/arsenic-and-drinking-water [Google Scholar]
- Weinmeyer R, Norling A, Kawarski M, & Higgins E (2017). The Safe Drinking Water Act of 1974 and Its Role in Providing Access to Safe Drinking Water in the United States. AMA Journal of Ethics, 19(10), 1018–1026. 10.1001/journalofethics.2017.19.10.hlaw1-1710 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2022, December 7). Arsenic. Arsenic. https://www.who.int/news-room/fact-sheets/detail/arsenic [Google Scholar]
- Yang C-Y, Chang C-C, Tsai S-S, Chuang H-Y, Ho C-K, & Wu T-N (2003). Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environmental Research, 91(1), 29–34. 10.1016/S0013-9351(02)00015-4 [DOI] [PubMed] [Google Scholar]
- Young HA, Kolivras KN, Krometis L-AH, Marcillo CE, & Gohlke JM (2023). Examining the association between safe drinking water act violations and adverse birth outcomes in Virginia. Environmental Research, 218, 114977. 10.1016/j.envres.2022.114977 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data from the ECHO Program are available through NICHD’s Data and Specimen Hub (DASH), https://dash.nichd.nih.gov/. DASH is a centralized resource that allows researchers to access data from various studies via a controlled-access mechanism. Researchers can now request access to these data by creating a DASH account and submitting a Data Request Form. The NICHD DASH Data Access Committee will review the request and provide a response in approximately two to three weeks. Once granted access, researchers will be able to use the data for three years. See the DASH Tutorial for more detailed information on the process.