Abstract
Background:
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) improves survival in select patients with peritoneal metastases (PM), but the impact of social determinants of health on CRS/HIPEC outcomes remains unclear.
Methods:
Retrospective review was conducted of a multi-institutional database of patients with PM who underwent CRS/HIPEC in the US between 2000–2017. The area deprivation index (ADI) was linked to the patient’s residential address. Patients were categorized as living in low (1–49) or high (50–100) ADI residences, with increasing scores indicating higher socioeconomic disadvantage. The primary outcome was overall survival (OS). Secondary outcomes included peri-operative complications, hospital/ICU length of stay (LOS), and disease-free survival (DFS).
Results:
Among 1,675 patients 1,061 (63.3%) resided in low ADI areas and 614 (36.7%) high ADI areas. Appendiceal tumors (n=1,102, 65.8%) and colon cancer (n=322, 19.2%) were the most common histologies. In multivariate analysis, high ADI was not associated with increased peri-operative complications, hospital/ICU LOS, or DFS. High ADI was associated with worse OS (median not reached vs 49 months; 5-year OS 61.0% vs 28.2%,P<0.0001). On multivariate Cox-regression analysis, high ADI (HR, 2.26; 95% CI 1.13–4.50;P<0.001), cancer recurrence (HR, 2.26; 95% CI 1.61–3.20;P<0.0001), increases in peritoneal carcinomatosis index (HR, 1.03; 95% CI 1.01–1.05;P<0.001) and incomplete cytoreduction (HR, 4.48; 95% CI 3.01–6.53;P<0.0001) were associated with worse OS.
Conclusions:
Even after controlling for cancer-specific variables, adverse outcomes persisted in association with neighborhood-level socioeconomic disadvantage. The individual and structural-level factors leading to these cancer disparities warrant further investigation to improve outcomes for all patients with peritoneal malignancies.
INTRODUCTION
Peritoneal metastases (PM) are common in late-stage gastrointestinal and gynecological cancers. Their presence often portends a poor prognosis without treatment. 1,2 Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has become the standard treatment for select patients with PM. 3–5 This treatment has been shown to improve survival in patients with PM from primary ovarian, colorectal, gastric, and appendiceal cancer. 6–8 However, these operations impose high morbidity, complex pre- and postoperative care, and high cost. 9,10
Social determinants of health have been shown to negatively impact cancer incidence, morbidity, mortality, and oncologic surgical outcomes. 11–13 Higher levels of socioeconomic disadvantage have been linked to decreased access to appropriate care and detrimental healthcare outcomes. 14 The Area Deprivation Index (ADI) is a validated metric of socioeconomic disadvantage developed by the Health Resources & Services Administration and was refined to the neighborhood level by Kind et al. in 2018. 15 The ADI provides neighborhood-specific state and national aggregate rankings of 17 socioeconomic variables including income, education, employment, and housing quality.
The influence of social determinants of health on outcomes of patients with nonmetastatic breast, prostate, lung, and colorectal cancer has shown worse survival in patients with low neighborhood socioeconomic status. 16 However, the effects of socioeconomic status on outcomes in patients with peritoneal metastases are poorly understood. Specifically, while high ADI has previously been linked to poor outcomes in many disease states, the association between the ADI and health outcomes has not been investigated in patients with PM undergoing CRS/HIPEC outside of small single institutional studies. 17–19 Since patients with peritoneal disease who receive CRS/HIPEC are particularly vulnerable to poor outcomes given the extent of their disease and the intensity of treatment they receive, it is important to understand which risk factors may predict a less favorable outcome, and work to mitigate their impact on morbidity and survival. Therefore, the objective of this study was to utilize a large, national, multi-institutional database to evaluate the impact of socioeconomic disadvantage on clinical outcomes in patients with PM undergoing CRS/HIPEC.
METHODS
Database
The U.S. HIPEC Collaborative Database is comprised of retrospectively identified patients who underwent CRS/HIPEC at 12 institutions from 2000 to 2017. 20 The following U.S.-based tertiary and quaternary academic referral centers are included in this study: Mayo Clinic, MD Anderson Cancer Center, University of Cincinnati, H. Lee Moffitt Cancer Center, University of California San Diego, The Ohio State University, the Medical College of Wisconsin, Johns Hopkins University, City of Hope, and the University of Wisconsin. Data were not available for this study from two institutions: University of Massachusetts Medical Center and Emory University. The database contains comprehensive information about the care of CRS/HIPEC patients at these institutions, including detailed demographic, preoperative, intraoperative, and postoperative data for each patient. Data on these patients from January 1, 2000, to December 31, 2017, were collected and submitted to the database. The database has institutional review board approval from all the participating centers.
Variables and Outcomes
The primary endpoint was overall survival (OS) from the date of surgery and secondary endpoints were the incidence of any peri-operative complications, hospital length of stay (LOS) and intensive care unit (ICU) LOS, and disease-free survival (DFS) from the date of surgery. Demographic data and tumor characteristics, along with baseline, perioperative, DFS, and OS data, were extracted from the database. The analyzed variables were sex, age, race (White, Black, Asian, Latino, and Other), body mass index (BMI), primary insurance, Charlson Comorbidity Index (CCI), American Society of Anesthesiologists (ASA) classification, tumor histology and area deprivation index (ADI). Peri-operative complications were assessed by the incidence of one or more of the following: superficial or deep surgical site infection, intraabdominal abscess, wound disruption, stroke, cardiac arrest, myocardial infarction, pneumonia, unplanned intubation, extended ventilatory support, tracheostomy, deep vein thrombosis, pulmonary embolism, pancreatitis, colonic ileus, anastomotic leak, urinary tract infection, acute renal failure, re-operation, pneumothorax, and pleural effusion. Using addresses at the time of surgery, the national ADI was obtained for each patient using the Neighborhood Atlas® (v2.0), based on 2011–2015 estimates from the ACS. 15 Patients were classified into a low or high ADI cohort based upon national rankings, with increasing levels indicating higher socioeconomic disadvantage (Low: 1–49, High: 50–100). 15,21 Patients without ADI data were excluded from this study.
Statistical Analysis
Descriptive statistics were used to examine the study cohort and compare characteristics between ADI cohorts. Continuous data was reported as mean ± standard deviation (SD) unless otherwise indicated. Categorical data was reported as frequencies and percentages and compared using chi-squared test or Fisher’s exact test.
Predictors of perioperative complications, hospital and ICU LOS were assessed by logistic and linear regression within the whole cohort. All patient and tumor characteristics were initially assessed by univariate logistic regression. Variables with a p-value < 0.30 on univariate analysis were included in an initial backwards stepwise elimination multivariable logistic regression model. Using backwards elimination, variables were sequentially removed from the model until all remaining predictors reached p < 0.05.
Kaplan–Meier analysis was used to examine survival by ADI. Significance was determined using the log-rank test. To assess the impact of ADI on OS, hazard ratios (HR) were examined using multivariate Cox proportional hazard models. Variables with a statistical significance of p < 0.30 on univariate analysis were evaluated in an initial multivariable regression analysis. Variables were sequentially removed via backwards elimination with a prespecified p-value cut-off of 0.05 a priori adjusting for age, gender, comorbidities, administration of neoadjuvant chemotherapy and primary tumor location.
An alpha cut-off of 0.05 was used for all significance tests. The data were analyzed using GraphPad Prism version 9.1.2 (GraphPad Software, San Diego, CA) and R software (version 4.1.0).
RESULTS
Patient Demographics
A total of 1,675 patients met the inclusion criteria for this study: 1,061 in the low ADI group (66.7%) and 614 in the high ADI group (33.3%). A summary of baseline patient demographic and clinical characteristics is presented in Table 1. The mean age of our cohort was 54.9 (± 12.7) years and 964 (57.6%) of the patients were women. The majority of our patients were White (n=1,380, 82.4%), with private primary insurance (n=1,050, 62.7%), ASA class of 3 (n=1,153 73.3%) and a mean CCI of 2.1 (± 1.6).
Table 1.
Patient Demographics
| Variable | All (N = 1675) | Low ADI (N = 1061) | High ADI (N = 614) | P-Value |
|---|---|---|---|---|
|
| ||||
| Age | 54.9 (± 12.7) | 55.2 (± 12.89) | 54.7 (± 12.6) | 0.54 |
| Female | 964 (57.6%) | 609 (57.4%) | 355 (57.8%) | 0.90 |
| BMI | 27.8 (± 6.5) | 26.9 (± 5.9) | 29.4 (± 7.0) | <0.001 |
| Race | ||||
| White | 1380 (82.4%) | 839 (79.7%) | 541 (88.3%) | <0.001 |
| Black | 86 (5.1%) | 42 (3.9%) | 44 (7.2%) | |
| Asian | 84 (5.0%) | 81 (7.7%) | 3 (0.5%) | |
| Latino | 72 (4.3%) | 54 (5.1%) | 18 (2.9%) | |
| Other | 44 (2.6%) | 37 (3.5%) | 7 (1.1%) | |
| Health Insurance | <0.05 | |||
| Government | 505 (30.1%) | 321 (30.9%) | 184 (31.7%) | |
| Private | 1050 (62.7%) | 685 (66.1%) | 365 (62.8%) | |
| Self-pay | 2 (0.1%) | 2 (0.2%) | 0 (0.0%) | |
| Uninsured | 61 (3.6%) | 29 (2.8%) | 32 (5.5%) | |
| ASA Class | <0.05 | |||
| 1 | 7 (0.4%) | 4 (0.42%) | 3 (0.5%) | |
| 2 | 308 (19.6%) | 200 (21.1%) | 108 (18.3%) | |
| 3 | 1153 (73.3%) | 692 (73.1%) | 461 (78.1%) | |
| 4 | 69 (4.4%) | 51 (5.4%) | 18 (3.1%) | |
| Charlson Comorbidity Index | 2.1 (± 1.6) | 2.3 (± 1.6) | 1.8 (± 1.6) | <0.001 |
| Primary Cancer Type | <0.001 | |||
| Appendiceal | 1102 (65.8%) | 677 (64.1%) | 425 (69.3%) | |
| Colorectal | 322 (19.2%) | 232 (21.9%) | 90 (14.7%) | |
| Mesothelioma | 116 (6.9%) | 76 (7.2%) | 40 (6.5%) | |
| Gastric | 37 (2.2%) | 20 (1.9%) | 17 (2.8%) | |
| Small Bowel | 22 (1.3%) | 10 (0.9%) | 12 (1.9%) | |
| Ovarian | 7 (0.4%) | 7 (0.7%) | 0 (0.0%) | |
| Sarcoma | 6 (0.3%) | 3 (0.3%) | 3 (0.5%) | |
| Other | 58 (3.4%) | 32 (2.9%) | 26 (4.2%) | |
BMI, Body Mass Index; ASA, American Society of Anesthesiologists Classification
The two ADI groups differed in terms of patient demographics as well as oncologic characteristics including BMI, race, primary insurance type, ASA class, CCI, and primary cancer type. However, age and gender were not significantly different between the two ADI groups (Table 1).
Pre-Operative and Operative Characteristics
Nutrition status, measured by preoperative albumin level, was significantly higher in low ADI patients (Table 2). Pre-operative levels of tumor markers did not differ between groups. Compared to curative intent and completeness of cytoreduction (CC) level 0, palliative operative intent, and CC levels of 1, 2 and 3 were higher in the high ADI group. Operative time and rates of extubation in the OR differed by ADI group, whereas EBL and PCI scores did not.
Table 2.
Pre-operative and Operative Characteristics
| Variable | All (N = 1675) | Low ADI (N = 1061) | High ADI (N = 614) | P-Value |
|---|---|---|---|---|
|
| ||||
| Pre-operative Labs | ||||
| Albumin (g/dL) | 4.1 (± 0.5) | 4.2 (± 0.5) | 4.1 (±0.5) | <0.001 |
| Creatinine (mg/dL) | 0.9 (± 2.9) | 0.8 (± 0.3) | 1.1 (± 4.7) | <0.05 |
| CEA | 19.9 (± 105.8) | 19.6 (± 62.3) | 30.7 (± 178.1) | 0.20 |
| CA 19–9 | 102.4 (± 489.9) | 116.9 (± 570.3) | 69.6 (± 170.2) | 0.30 |
| CA 125 | 54.2 (± 105.7) | 53.9 (±96.6) | 54.3 (± 140.6) | 0.14 |
| Pre-operative Imaging | ||||
| CT Scan | 1262 (94.4%) | 876 (95.6%) | 539 (93.4%) | 0.07 |
| MRI | 202(18.2%) | 121 (14.6%) | 91 (20.8%) | <0.001 |
| DW-MRI | 116(10.5%) | 42 (5.2%) | 76(18.2%) | <0.001 |
| PET | 223 (23.1%) | 189 (24.8%) | 64(17.8%) | <0.001 |
| Diagnostic Laparoscopy | 342 (21.2%) | 236 (28.8%) | 137 (31.9%) | 0.25 |
| Operative Variables | ||||
| Previous CRS | 321 (19.2%) | 199(18.8%) | 122 (19.9%) | 0.61 |
| Previous HIPEC | 100 (5.9%) | 52 (4.9%) | 48 (7.9%) | <0.05 |
| Operative Intent | <0.01 | |||
| Curative | 1611 (96.2%) | 1030(97.1%) | 581 (94.6%) | |
| Palliative | 48 (2.9%) | 26 (2.5%) | 22 (3.6%) | |
| Operative Time (Hours) | 7.9 (± 3.2) | 8.17 (±3.4) | 7.41 (± 2.9) | <0.001 |
| EBL (mL) | 473.2 (± 705.3) | 477.9 (± 736.6) | 464.6 (± 645.8) | 0.60 |
| PCI | 13.4 (± 8.8) | 13.5 (±8.4) | 13.2 (± 9.5) | 0.14 |
| CC | ||||
| 0 | 1024 (64.2%) | 694 (68.7%) | 330 (57.7%) | <0.001 |
| 1 | 351 (22.2%) | 211 (20.9%) | 140 (24.5%) | |
| 2 | 106 (6.7%) | 58 (5.7%) | 48 (8.4%) | |
| 3 | 101 (6.4%) | 47 (4.7%) | 54 (9.4%) | |
| Drain Placement | 357 (45.5%) | 204 (48.2%) | 153 (42.4%) | 0.14 |
| Extubated in OR | 1033 (86.5%) | 535 (81.7%) | 498 (92.6%) | <0.001 |
CEA, Carcinoembryonic Antigen; CA 19–9, Carbohydrate Antigen 19–9; CA 125, Cancer Antigen 125; CT, Computed Tomography; MRI, Magnetic Resonance Imaging; PET, Positron Emission Tomography; CRS, Cytoreductive Surgery; HIPEC, Hyperthermic Intraperitoneal Chemotherapy; EBL, Estimated Blood Loss; PCI, Peritoneal Cancer Index; CCR, Completeness of Cytoreduction
Post-Operative Course
Hospital LOS was similar between ADI groups while ICU LOS, incidence of any complication, and total number of complications was significantly higher in the low ADI group (Table 3). Discharge destination was similar between groups; however, rates of readmission were higher in the low ADI group and surveillance imaging every 3 months was more common than in high ADI patients. Mean duration of post-operative follow-up and rates of disease recurrence did not differ significantly between ADI cohorts (Table 3). In multivariate analysis, ADI was not associated with post-operative complications, hospital, or ICU LOS (Table 4A-C). Increasing CCI, operative time, and PCI were associated with a higher risk of any post-operative complication (Table 4A). Hospital LOS was higher with increasing age at the time of surgery, male gender, EBL, and the incidence of any complication (Table 4B). ICU LOS was longer in patients with a higher CCI or EBL and in those who incurred complications (Table 4C).
Table 3.
Post-Operative Course
| Variable | All (N = 1675) | Low ADI (N = 1061) | High ADI (N = 614) | P-Value |
|---|---|---|---|---|
|
| ||||
| Hospital LOS | 13.0 (± 55.5) | 13.0 (± 15.5) | 17.8 (± 90.9) | 0.17 |
| ICU LOS | 3.5 (± 6.1) | 3.8 (± 6.8) | 3.22 (± 5.6) | 0.01 |
| Any Complication | 904 (53.9%) | 600 (57.2%) | 304 (50.6%) | 0.01 |
| Total Complications | 1.4 (± 1.5) | 1.5 (± 1.5) | 0.9 (± 1.3) | <0.001 |
| Highest Clavien-Dindo Grade | ||||
| I | 140 (8.4%) | 77 (14.5%) | 63 (22.1%) | <0.001 |
| II | 403 (24.1%) | 285 (53.8%) | 118 (41.4%) | |
| III | 52 (3.1%) | 50 (9.4%) | 2 (0.7%) | |
| IIIA | 102 (6.1%) | 53 (10.0%) | 49 (17.2%) | |
| IIIB | 53 (3.2%) | 26 (4.9%) | 27 (9.5%) | |
| IV | 11 (0.7%) | 10 (1.9%) | 1 (0.4%) | |
| IVA | 24 (1.4%) | 17 (3.2%) | 7 (2.5%) | |
| IVB | 14 (0.8%) | 6 (1.1%) | 8 (2.8%) | |
| V | 16 (0.9%) | 6 (1.1%) | 10 (3.5%) | |
| Discharge Destination | 0.60 | |||
| Acute Rehab | 17 (1.4%) | 10 (1.5%) | 7 (1.3%) | |
| Home | 1065 (89.3%) | 585 (89.3%) | 480 (89.2%) | |
| Home-Health | 42 (3.5%) | 27 (4.12%) | 15 (2.8%) | |
| Hospice | 1 (0.1%) | 0 (0.0%) | 1 (0.2%) | |
| Other | 10 (0.8%) | 5 (0.8%) | 5 (0.9%) | |
| Skilled Nursing Facility | 58 (4.9%) | 28 (4.3%) | 30 (5.6%) | |
| Readmission | 342 (20.4%) | 237 (22.5%) | 105 (17.4%) | <0.001 |
| Duration of Follow-up (Months) | 29.3 (± 28.2) | 30.6 (± 29.0) | 28.3 (± 27.5) | 0.17 |
| Frequency of Surveillance Imaging | <0.001 | |||
| q2 months | 4 (0.5%) | 3 (0.7%) | 1 (0.2%) | |
| q3 months | 271 (31.3%) | 144 (34.3%) | 127 (28.5%) | |
| q4 months | 29 (3.3%) | 19 (4.5%) | 10 (2.2%) | |
| q6 months | 532 (61.4%) | 233 (55.5%) | 299 (67.0%) | |
| q12 months | 30 (3.5%) | 21 (5.0%) | 9 (2.0%) | |
| Cancer Recurrence | 613 (42.1%) | 402 (42.6%) | 211 (41.2%) | 0.65 |
LOS, Length of Stay
Table 4A.
Risk Factors for Post-Operative Complications
| Variable | Odds Ratio | P-value |
|---|---|---|
|
| ||
| ADI class | 0.92 [0.73; 1.19] | 0.54 |
| Charlson Comorbidity Index* | 1.13 [1.05; 1.22] | <0.001 |
| Operative Time (hours)* | 1.15 [1.10; 1.20] | <0.001 |
| PCI* | 1.03 [1.01 ;1.04] | <0.001 |
Risk for each 1-unit increase
Table 4B.
Risk Factors for Increasing Hospital LOS
| Variable | Coefficient of Effect | P-value |
|---|---|---|
|
| ||
| ADI class | 0.03 [−0.98; 1.04] | 0.95 |
| Age (per 10 years)* | 0.58 [0.08;1.07] | <0.05 |
| Male | 1.33 [0.31;2.35] | <0.01 |
| EBL (L)* | 4.21 [2.78;5.65] | <0.001 |
| Any Complication | 6.21 [5.33;7.09] | <0.001 |
Risk for each 1-unit increase
Table 4C.
Risk Factors for Increasing ICU LOS
| Variable | Coefficient of Effect | P-value |
|---|---|---|
|
| ||
| ADI class | −0.52 [−1.22;0.19] | 0.15 |
| Charlson Comorbidity Index* | 0.54 [0.28;0.79] | <0.001 |
| EBL (L)* | 2.60 [1.75;3.44] | <0.001 |
| Any Complication | 1.20 [0.48; 1.91] | <0.001 |
Risk for each 1-unit increase
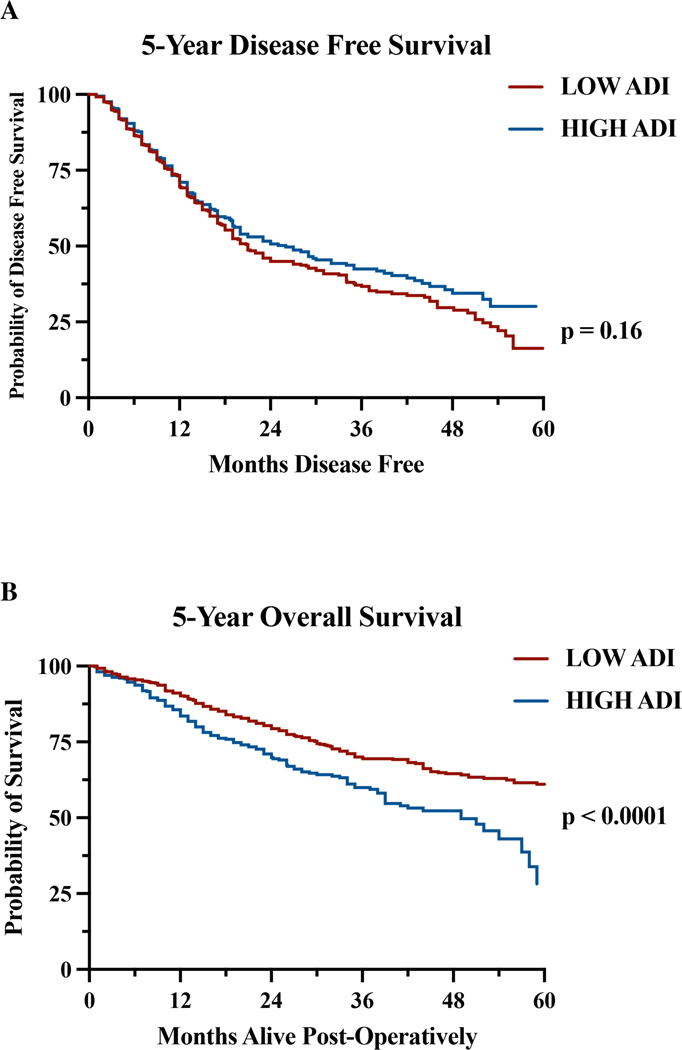
Disease-free and Overall Survival
DFS was not significantly different between ADI groups. The median DFS was 21 and 26 months, and the 5-year DFS rates were 16.3% and 30.1% in the low and high ADI groups, respectively (Figure 1A). Median and 5-year OS was significantly worse for high ADI patients (median survival 49 months, 5-year OS: 28.2%) than low ADI patients (median survival not reached, 5-year OS: 61.0%) (Figure 1B).
Figure 1.
Kaplan-Meier estimates of A 5-year disease-free survival (Low ADI: 16.3% and High ADI: 30.1%; P = 0.16) and B 5-year overall survival rates (Low ADI: 61.0% and High ADI: 28.2%; P < 0.0001)
The results of the multivariate Cox regression analysis for factors associated with OS are reported in Table 5. On multivariate Cox regression analysis, high ADI (HR, 2.26; 95% CI 1.13–4.50; P<0.001), cancer recurrence (HR, 2.26; 95% CI 1.61–3.20; P<0.0001), increases in PCI (HR, 1.03; 95% CI 1.01–1.05; P<0.001), and incomplete cytoreduction ((CC-1 HR, 1.48; 95% CI 1.06–2.06; P<0.01); (CC-2 HR, 2.08; 95% CI 1.30–3.18; P<0.001); (CC-3 HR, 4.48; 95% CI 3.01–6.53; P<0.0001)) were associated with worse OS.
Table 5.
Multivariate Cox-Regression Analysis for Overall Survival
| Variable | Hazard Ratio | P-Value |
|---|---|---|
|
| ||
| High ADI | 2.26 [1.13–4.50] | <0.001 |
| CCR (ref. CC0) | ||
| CC-1 | 1.48 [1.06–2.06] | <0.01 |
| CC-2 | 2.08 [1.30–3.18] | <0.001 |
| CC-3 | 4.48 [3.01–6.53] | <0.0001 |
| PCI* | 1.03 [1.01–1.05] | <0.01 |
| Recurrence | 2.26 [1.61–3.20] | <0.0001 |
Risk for each 1-unit increase; Model includes adjustments for patient age, gender, comorbidities, administration of neoadjuvant chemotherapy and primary tumor location.
DISCUSSION
Socioeconomic factors have been previously linked to cancer incidence and mortality,22 but clinical outcomes for patients with PM who undergo CRS/HIPEC have not been examined outside of small, single institutional studies. 23,24 The major finding of this national, multi-center, retrospective study is that despite adjustments for cancer specific variables such as patient age, gender, comorbidities, administration of neoadjuvant chemotherapy and primary tumor site, patients with PM from socioeconomically disadvantaged neighborhoods experienced significantly lower overall survival after CRS/HIPEC.
Previous studies in colorectal, gastric, and ovarian cancer have shown that patients who are more socioeconomically disadvantaged experience barriers to therapy, present with more advanced disease, and exhibit lower overall survival. 13,19,25 In appropriately selected patients with PM, CRS/HIPEC has improved median overall survival. 26 However, insurance authorization, proximity to a specialized treatment center, and lack of access to a peritoneal surface malignancy specialist have been cited as major barriers for referring physicians and patients seeking treatment. 24,27,28 Our cohort representation correlates with these previous studies as only one-third of our patients came from low-socioeconomic neighborhoods.
Lower socioeconomic status has previously been associated with more comorbid conditions at presentation, fewer elective procedures and worse peri-operative outcomes.29,30 It was interesting that in our cohort, unlike previous studies, 13,24 patients in the low ADI class presented with a higher comorbidity burden. However, our study aligns with recent findings that showed that these differences in presentation and perioperative outcomes subsided after adjusting for comorbidities. 23,31
Patients with low socioeconomic status have been shown to have decreased overall survival in patients with ovarian, cervical, colorectal, and breast cancer. 17,19,32 Our study similarly finds that high levels of socioeconomic deprivation are associated with a substantially increased risk of mortality among patients with an array of primary cancer types and peritoneal metastases. Indeed, the magnitude of this association is similar to that of the mortality risk associated with cancer recurrence and incomplete cytoreduction. However, patients who underwent CRS/HIPEC showed no difference in the incidence of recurrence or DFS based on socioeconomic status in our study and others. 23,24,32 While these findings are encouraging, previous studies have demonstrated that socioeconomically disadvantaged and non-White patients were less likely to undergo CRS/HIPEC. In turn, the patients who did not receive treatment exhibited a higher mortality rate. 23,24,32 Thus, further efforts to improve the awareness of malignancies that may benefit from CRS/HIPEC, specialized sites of care, and treatment benefits must be established to improve patient outcomes. 33
There are several limitations to this study. First, only patients who underwent CRS/HIPEC were included and therefore additional disparities may exist among patients with PM who are not referred for CRS/HIPEC. Second, as a database study, there may be additional patient characteristics that were not captured by the database, but that could have impacted patient outcomes. We do believe that this study is strengthened by its population representation and size because it allowed us to examine the associations of the ADI and clinical outcomes in 10 centers across the United States.
In conclusion, we found that in a national, muti-center study, even after controlling for cancer specific variables, adverse outcomes persisted in association with neighborhood level socioeconomic disadvantage. The individual and structural level factors leading to these cancer disparities warrant further investigation to improve outcomes for all patients with peritoneal malignancies.
SYNOPSIS.
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) improves outcomes in select patients with peritoneal metastases. In this multi-center study with 1,675 patients, we found that even after controlling for cancer specific variables, socioeconomic disadvantage adversely impacted overall survival in patients with peritoneal malignancies who underwent CRS/HIPEC.
Acknowledgments
Disclosures: Shannon N. Radomski received financial support from the National Cancer Institute (NCI), Grant 5T32CA126607-12.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Nagata H, Ishihara S, Hata K, et al. Survival and Prognostic Factors for Metachronous Peritoneal Metastasis in Patients with Colon Cancer. Annals of Surgical Oncology. 2017/05/01 2017;24(5):1269–1280. doi: 10.1245/s10434-016-5732-z [DOI] [PubMed] [Google Scholar]
- 2.Rijken A, Lurvink RJ, Luyer MDP, et al. The Burden of Peritoneal Metastases from Gastric Cancer: A Systematic Review on the Incidence, Risk Factors and Survival. Journal of Clinical Medicine. 2021;10(21):4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. The Lancet Oncology. 2004;5(4):219–228. doi: 10.1016/S1470-2045(04)01425-1 [DOI] [PubMed] [Google Scholar]
- 4.Harper MM, Kim J, Pandalai PK. Current Trends in Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Disease from Appendiceal and Colorectal Malignancies. J Clin Med. May 18 2022;11(10)doi: 10.3390/jcm11102840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Stein RM, Aalbers AGJ, Sonke GS, van Driel WJ. Hyperthermic Intraperitoneal Chemotherapy for Ovarian and Colorectal Cancer: A Review. JAMA Oncol. Aug 1 2021;7(8):1231–1238. doi: 10.1001/jamaoncol.2021.0580 [DOI] [PubMed] [Google Scholar]
- 6.Choudry HA, Bednar F, Shuai Y, et al. Repeat Cytoreductive Surgery-Hyperthermic Intraperitoneal Chemoperfusion is Feasible and Offers Survival Benefit in Select Patients with Peritoneal Metastases. Annals of Surgical Oncology. 2019/05/01 2019;26(5):1445–1453. doi: 10.1245/s10434-019-07218-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirnezami R, Mehta AM, Chandrakumaran K, et al. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. British Journal of Cancer. 2014/10/01 2014;111(8):1500–1508. doi: 10.1038/bjc.2014.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Sweringen HL, Hanseman DJ, Ahmad SA, Edwards MJ, Sussman JJ. Predictors of survival in patients with high-grade peritoneal metastases undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Surgery. Oct 2012;152(4):617–24; discussion 624–5. doi: 10.1016/j.surg.2012.07.027 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz PB, Stahl CC, Vande Walle KA, et al. What Drives High Costs of Cytoreductive Surgery and HIPEC: Patient, Provider or Tumor? Ann Surg Oncol. Dec 2020;27(13):4920–4928. doi: 10.1245/s10434-020-08583-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiseman JT, Abdel-Misih S, Beal EW, et al. A multi-institutional analysis of Textbook Outcomes among patients undergoing cytoreductive surgery for peritoneal surface malignancies. Surg Oncol. Jun 2021;37:101492. doi: 10.1016/j.suronc.2020.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Lam MB, Raphael K, Mehtsun WT, et al. Changes in Racial Disparities in Mortality After Cancer Surgery in the US, 2007–2016. JAMA Netw Open. Dec 1 2020;3(12):e2027415. doi: 10.1001/jamanetworkopen.2020.27415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. Mar-Apr 2004;54(2):78–93. doi: 10.3322/canjclin.54.2.78 [DOI] [PubMed] [Google Scholar]
- 13.Yu KX, Yuan WJ, Huang CH, et al. Socioeconomic deprivation and survival outcomes in patients with colorectal cancer. Am J Cancer Res. 2022;12(2):829–838. [PMC free article] [PubMed] [Google Scholar]
- 14.Unger JM, Moseley AB, Cheung CK, et al. Persistent Disparity: Socioeconomic Deprivation and Cancer Outcomes in Patients Treated in Clinical Trials. J Clin Oncol. Apr 20 2021;39(12):1339–1348. doi: 10.1200/jco.20.02602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. N Engl J Med. Jun 28 2018;378(26):2456–2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng E, Soulos PR, Irwin ML, et al. Neighborhood and individual socioeconomic disadvantage and survival among patients with nonmetastatic common cancers. JAMA Network Open. 2021;4(12):e2139593-e2139593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurani SS, McCoy RG, Lampman MA, et al. Association of Neighborhood Measures of Social Determinants of Health With Breast, Cervical, and Colorectal Cancer Screening Rates in the US Midwest. JAMA Netw Open. Mar 2 2020;3(3):e200618. doi: 10.1001/jamanetworkopen.2020.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenzweig MQ, Althouse AD, Sabik L, et al. The Association Between Area Deprivation Index and Patient-Reported Outcomes in Patients with Advanced Cancer. Health Equity. 2021;5(1):8–16. doi: 10.1089/heq.2020.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hufnagel DH, Khabele D, Yull FE, et al. Increasing Area Deprivation Index negatively impacts ovarian cancer survival. Cancer Epidemiology. 2021/10/01/ 2021;74:102013. doi: 10.1016/j.canep.2021.102013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beal EW, Ahmed A, Grotz T, et al. Trends in the indications for and short-term outcomes of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Am J Surg. Mar 2020;219(3):478–483. doi: 10.1016/j.amjsurg.2019.09.017 [DOI] [PubMed] [Google Scholar]
- 21.Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMs. 2016;4(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hastert TA, Beresford SAA, Sheppard L, White E. Disparities in cancer incidence and mortality by area-level socioeconomic status: a multilevel analysis. Journal of Epidemiology and Community Health. 2015;69(2):168. doi: 10.1136/jech-2014-204417 [DOI] [PubMed] [Google Scholar]
- 23.Cantos A, Eguia E, Wang X, Abood G, Knab LM. Impact of sociodemographic factors on outcomes in patients with peritoneal malignancies following cytoreduction and chemoperfusion. Journal of Surgical Oncology. 2022;125(8):1285–1291. doi: 10.1002/jso.26843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieser C, Phelos H, Zureikat A, et al. Socioeconomic Barriers to CRS HIPEC for Appendiceal Cancer within a Regional Academic Hospital System. Annals of Surgical Oncology. 2022/10/01 2022;29(11):6593–6602. doi: 10.1245/s10434-022-11949-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens MR, Blackshaw GRJC, Lewis WG, et al. Influence of socio-economic deprivation on outcomes for patients diagnosed with gastric cancer. Scandinavian Journal of Gastroenterology. 2005/01/01 2005;40(11):1351–1357. doi: 10.1080/00365520510023666 [DOI] [PubMed] [Google Scholar]
- 26.Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol. May 2015;22(5):1686–93. doi: 10.1245/s10434-014-3978-x [DOI] [PubMed] [Google Scholar]
- 27.Bernaiche T, Emery E, Bijelic L. Practice patterns, attitudes, and knowledge among physicians regarding cytoreductive surgery and HIPEC for patients with peritoneal metastases. Pleura and Peritoneum. 2018;3(1)doi:doi: 10.1515/pp-2017-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong CT, Dhiman A, Smith A, et al. Insurance Authorization Barriers in Patients Undergoing Cytoreductive Surgery and HIPEC. Ann Surg Oncol. Sep 16 2022;doi: 10.1245/s10434-022-12437-9 [DOI] [PubMed] [Google Scholar]
- 29.K CM, Oral E, Rung AL, et al. Neighborhood deprivation and risk of mortality among men with prostate cancer: Findings from a long-term follow-up study. Prostate. May 2022;82(7):783–792. doi: 10.1002/pros.24320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bharathan B, Welfare M, Borowski DW, Mills SJ, Steen IN, Kelly SB. Impact of deprivation on short- and long-term outcomes after colorectal cancer surgery. Br J Surg. Jun 2011;98(6):854–65. doi: 10.1002/bjs.7427 [DOI] [PubMed] [Google Scholar]
- 31.Powers BD, Fulp W, Dhahri A, et al. The Impact of Socioeconomic Deprivation on Clinical Outcomes for Pancreatic Adenocarcinoma at a High-volume Cancer Center: A Retrospective Cohort Analysis. Ann Surg. Dec 1 2021;274(6):e564–e573. doi: 10.1097/sla.0000000000003706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rieser CJ, Hoehn RS, Zenati M, et al. Impact of Socioeconomic Status on Presentation and Outcomes in Colorectal Peritoneal Metastases Following Cytoreduction and Chemoperfusion: Persistent Inequalities in Outcomes at a High-Volume Center. Annals of Surgical Oncology. 2021/07/01 2021;28(7):3522–3531. doi: 10.1245/s10434-021-09627-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aquina CT, Brown ZJ, Beane JD, et al. Disparities in access to care among patients with appendiceal or colorectal cancer and peritoneal metastases: A medicare insurance-based study in the United States. Front Oncol. 2022;12:970237. doi: 10.3389/fonc.2022.970237 [DOI] [PMC free article] [PubMed] [Google Scholar]